Abstract
Lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1) is the major receptor for oxidized LDL (oxLDL), and plays a key role in the pathogenesis of atherosclerosis and cardiovascular diseases. Monoclonal antibodies (mAbs) specific for human LOX-1 (hLOX-1) were generated by a phage display technique using chickens immunized with recombinant hLOX-1 (rhLOX-1). A total of 53 independent scFv clones reactive for rhLOX-1 were obtained. Of the 53 clones, 49 recognized the C-type lectin-like domain (CTL domain), which contributes to the binding of oxLDL. Of these, 45 clones inhibited oxLDL-binding with LOX-1. Furthermore, some of these clones cross-reacted with rabbit, pig and/or mouse LOX-1. For possible application as therapeutic agents in the future, two cross-reactive mAbs were re-constructed as chicken-human chimeric antibodies. The chimeric antibodies showed similar characteristics compared to the original antibodies, and inhibited oxLDL binding to LOX-1 expressed on CHO cells. The results obtained in this study indicate that anti-LOX-1 mAbs might be useful tools for functional analyses and development of therapeutic agents for cardiovascular indications such as atherosclerosis.
Key words: LOX-1, oxLDL, chicken monoclonal antibody, chimeric antibody, neutralizing antibody
Introduction
LOX-1 was first identified in vascular endothelial cells, and has been characterized as the major receptor for oxLDL in endothelial cells.1 Studies have indicated that LOX-1 has a critical role in the pathogenesis of atherosclerosis and cardiovascular diseases.2 Recently, a soluble form of LOX-1 (sLOX-1) released by proteolytic cleavage was detected in serum from acute coronary syndrome (ACS) patients.3 This suggests that sLOX-1 might be a useful biomarker for early diagnosis of ACS.
LOX-1 is a 50 kDa type-II membrane protein that, as assessed by structure, belongs to the C-type lectin family. LOX-1 consists of four domains, the N-terminal intracellular domain, the transmembrane domain, the Neck domain, and the CTL domain.4 Among these, the CTL domain is critical for LOX-1 function, as the C-terminal residues and arginine residues in this domain are essential for oxLDL-binding.5–7
Although mAbs specific to LOX-1 are useful for expression and functional analyses of LOX-1,1,5,8–11 the number of anti-LOX-1 mAbs is insufficient1,5,9,11 at least in part because generation of mAbs against LOX-1 by immunization of mammalian species is difficult due to the high conservation of the CTL domain among mammals.5
However, the chicken is a useful animal for developing specific antibodies against conserved mammalian proteins because of the phylogenic differences between chickens and mammals.12–15 In fact, numerous chicken mAbs have been produced using cell fusion and phage-display techniques.12–15 Although a LOX-1 homolog has not yet been found in chickens, useful mAbs against mammalian LOX-1 can be produced by immunizing chickens. To study chicken mAbs against various LOX-1 epitopes, we generated 53 chicken mAbs specific to LOX-1 by a phage-display technique using chickens immunized with rhLOX-1. Here, we report data for 49 mAbs that recognized the CTL domain, of which 45 also inhibited oxLDL-binding with LOX-1.
Results
Production of recombinant LOX-1.
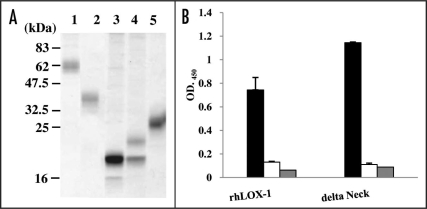
Recombinant human, mouse, rabbit and pig versions of LOX (rhLOX-1, rmLOX-1, rrLOX-1 and rpLOX-1) as well as delta Neck, were each produced in FreeStyle™ 293-F cells. These recombinant LOX-1 proteins were detected as approximately 62 kDa, 40 kDa, 20 kDa, 22 kDa and 30 kDa bands, respectively, on SDS-PAGE under non-reducing conditions (Fig. 1A). rhLOX-1 and delta Neck were each detectable as a half-molecule bands under reducing conditions (data not shown). These results confirmed that rhLOX-1 and delta Neck are cross-linked by a disulfide bond through Cys140.7 The proteins exhibited binding activity toward human oxLDL, but not the negative control LDL (Fig. 1B). The result suggests that the recombinant proteins maintained the correct structure and function. rmLOX-1, rrLOX-1 and rpLOX-1 were monomers; these proteins showed the same profiles under both reducing and non-reducing conditions. Recombinant LOX-1s (human, mouse, rabbit and pig) were detected as broad bands or two bands (Fig. 1A). LOX-1s contains putative N-glycosylation signals,5 so the differences in molecular weight (MW) between these bands are probably due to variation in glycosylation.
Figure 1.
SDS-PAGE profiles and reactivity of recombinant LOX-1s. (A) SDS-PAGE profiles of rhLOX-1 (lane 1), delta Neck (lane 2), rmLOX-1 (lane 3), rrLOX-1 (lane 4) and rpLOX-1 (lane 5). Recombinant proteins were purified from the supernatant of 293-F cells by nickel affinity chromatography. All samples were subjected to SDS-PAGE under non-reducing conditions and were stained with CBBR. Numbers on the right indicate apparent molecular masses in kDa. (B) Reactivity of rhLOX-1 and delta Neck to oxLDL (black), LDL (negative control, white) and BSA (control, gray) was measured by ELISA using biotin-labeled rhLOX-1- or delta Neck-coated plates. Data are means ± SD of three independent experiments.
Specific antibodies against LOX-1.
By using spleen cells from chickens immunized with rhLOX-1, the scFv phage library (5.0 × 108 cfu) was constructed. After the sixth round of panning selection against rhLOX-1, the specificity of the concentrated scFv phage library was examined by ELISA. Of 207 scFv phage clones from libraries of the fifth and sixth pannings, 113 were reactive for rhLOX-1 (data not shown). The results of nucleic acid sequencing in the positive clones showed that these clones could be subclassified to 51 independent clones (data not shown). In the panning selections against rmLOX-1 using the same phage-display library, two independent clones were selected from libraries of the third and fourth pannings. Finally, a total of 53 clones were constructed as rIgY for quantitative experiments.
Reactivity of recombinant antibodies against LOX-1s.
We investigated whether 53 chicken antibodies obtained by phage-display technique recognize the CTL or Neck domains and LOX-1 from other mammalian species.
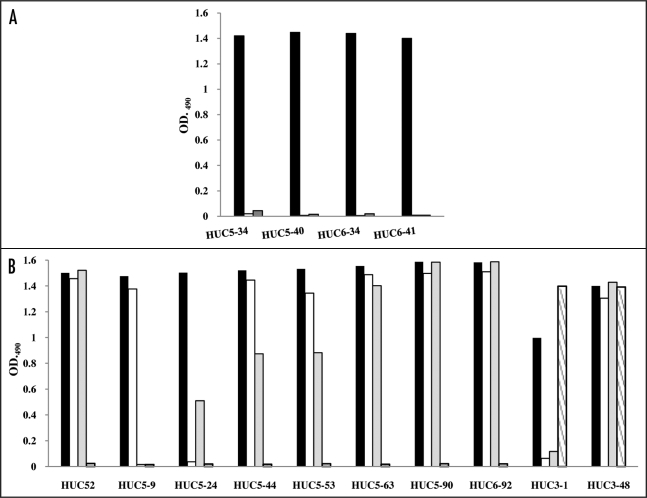
The reactivities of the 53 rIgY antibodies against LOX-1 were assessed by ELISA and FACS. Of 53 clones, HUC5–34, HUC5–40, HUC6–34 and HUC6–41, reacted with rhLOX-1, but not with delta Neck (Fig. 2A). The result suggests that these four clones recognize the Neck domain of LOX-1 (anti-Neck domain clones). The residual clones reacted with both rhLOX-1 and delta Neck with similar intensities (data not shown), indicating that they recognize the CTL domain.
Figure 2.
Reactivity of rIgY antibodies against recombinant LOX-1s. (A) Reactivity of rIgY antibodies against rhLOX-1 and delta NECK. rIgYs (1 µg/ml) were added to wells coated with biotinylated rhLOX-1 (black), delta Neck (white) or BSA (negative control, gray). (B) Cross-reactivity of 53 rIgY antibodies against LOX-1 from four species. The rIgYs (1 µg/ml) were added wells coated with biotinylated rhLOX-1 (black), rpLOX-1 (white), rrLOX-1 (gray) or rmLOX-1 (shaded). Of the 53 clones, eight cross-reacted with rrLOX-1 and/or rpLOX-1 and only two clones also recognized rmLOX-1.
Seven (HUC52, HUC5–44, HUC5–53, HUC5–63, HUC5–90, HUC6–92 and HUC3–48) of 53 clones were cross-reactive with both rabbit and pig LOX-1 (Fig. 2B). HUC5–24 cross-reacted with rrLOX-1 (Fig. 2B), and HUC5–9 reacted with rpLOX-1 (Fig. 2B). The two clones (HUC3-1 and HUC3–48) selected from panning against rmLOX-1 were reactive with rmLOX-1. Interestingly, HUC3–48 reacted with LOX-1s from all species tested (Fig. 2B). However, in western blotting these clones did not react with recombinant LOX-1s (data not shown), indicating that these clones recognize conformational epitopes. In fact, these clones also reacted with LOX-1-expressing cells in FACS analysis (data not shown). None of the clones tested reacted with BSA and wild type CHO cells (data not shown).
Neutralization activity of anti-LOX-1 antibodies.
LOX-1 is expressed in atherosclerosis and several cardiovascular diseases, such as myocardial ischemia.2 In a rat model, administration of anti-LOX-1 antibody effectively suppressed intimal hyperplasia.10 Thus, inhibition of LOX-1 activity may be a useful strategy to produce novel drugs for cardiovascular disorders. The neutralization activity of 53 anti-LOX-1 antibodies was examined using a modified method described previously.19 Forty-five of 53 clones showed neutralization activity (data not shown), suggesting that these clones recognized the CTL domain, which is essential for oxLDL-binding.5 Although the Neck domain of LOX-1 is not critical for oxLDL binding with LOX-1, anti-Neck domain clones HUC5–34, HUC5–40, HUC6–34 and HUC6–41 (Fig. 2A) slightly inhibited oxLDL-binding (data not shown). The MW of rhLOX-1 is about 62 kDa (Fig. 1A, lane 1), and that of rIgY is about 250 kDa.17 With MW four-fold greater than rhLOX-1, anti-Neck domain clones likely exhibited steric inhibition.
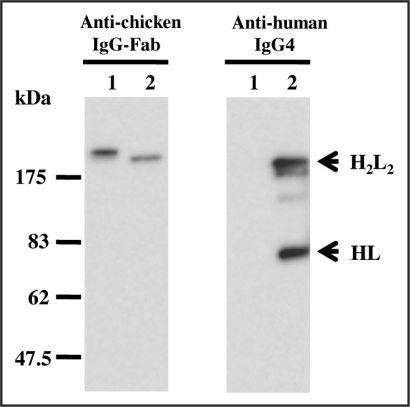
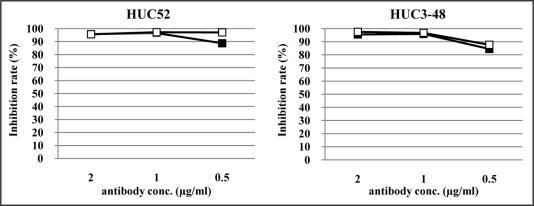
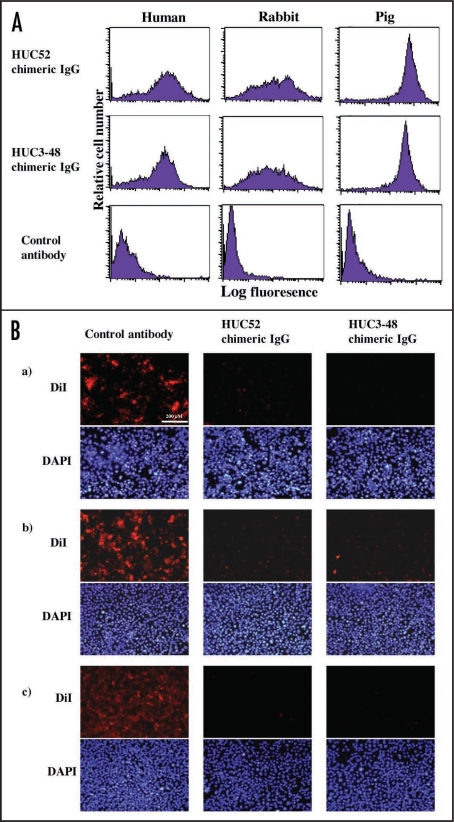
To have potential as therapeutic agents, two clones, HUC52, which cross-reacted with rabbit and pig LOX-1, and HUC3–48, which recognized LOX-1s from all species tested, were reconstructed as chimeric IgG antibodies (Fig. 3). In order to avoid Ig effector functions involving complement activation and antibody-dependent cell-mediated cytotoxicity, the IgG4 subclass was selected. The chimeric antibodies had similar reaction patterns compared to their original rIgY forms (Fig. 4). Inhibition studies were then performed using LOX-1-expressing CHO cells. Regardless of the animal species tested, the two chimeric IgGs reacted with LOX-1-expressing CHO cells (Fig. 5A), and blocked oxLDL-binding to LOX-1-expressing CHO cells (Fig. 5B). HUC3–48 also showed inhibition activity against mouse LOX-1-expressing cells (data not shown).
Figure 3.
Western blot analysis of recombinant chicken antibody and chicken-human chimeric antibody. The rIgY (lane 1) and chimeric IgG (lane 2) were each run on 10% SDS-polyacrylamide gels under non-reducing conditions. Anti-chicken IgG-Fab and anti-human IgG4 were used as a detection antibody. Numbers on the right indicate apparent molecular masses in kDa. H2L2 represents antibody full length and HL indicates the halved molecule.
Figure 4.
Inhibition of oxLDL-binding with LOX-1 by anti-LOX-1 antibodies. rhLOX-1 was used as the capture molecule. rIgY (closed square) or chimeric IgG (open square) indicated inhibition rate of oxLDL-binding with LOX-1 using anti-human ApoB antibody. Anti-DNP rIgY was used as a negative control and standard for comparison.
Figure 5.
Reactivities and blocking activities of chimeric IgGs against LOX-1-expressing cells. (A) LOX-1-expressing CHO cells were incubated with chimeric IgG (1 µg/ml) revealed with FITC-labeled anti-human IgG. Fluorescence was analyzed by FACS. (B) Human (a), rabbit (b) or pig (c) LOX-1-expressing CHO cells were incubated with chimeric IgG (5 µg/ml). DiI-labeled oxLDL (3 µg/ml) was added for 2 h. After that, the cells were fixed with 4% (v/v) paraformaldehyde. Then, the nuclei of the cells was counterstained with DAPI and subjected to observation with fluorescence microscopy.
Discussion
LOX-1 is the main receptor for oxLDL on endothelial cells, and it mediates the recognition and internalization of oxLDL.1 Recent studies on LOX-1 have shown that this molecule plays a critical role in the development of atherosclerosis and cardiovascular diseases.2 For further research into the function of LOX-1, LOX-1-specific mAbs are thought to be one of the most useful tools for basic analysis and clinical applications. However, a limited number of mAbs against human LOX-1 have been reported. This might be because the CTL domain is highly conserved among mammalian species5 and so anti-LOX1 mAbs are difficult to generate. We have successfully produced chicken mAbs against conserved mammalian molecules using cell fusion and phage-display techniques.12–15 In the present study, a total of 53 scFv chicken mAbs specific for LOX-1 were generated from only two panning selections. Most of the rIgY forms from these scFv antibodies recognized the CTL domain of LOX-1, and only 4 clones recognized the Neck domain (Fig. 2A). These results indicate that the chicken is a useful animal for producing antibodies specific for mammalian LOX-1, particularly the CTL domain.
Mice, rabbits and pig are typically used as models for atherosclerosis and cardiovascular diseases. For example, ApoE-knockout mice and Watanabe heritable hyperlipidemic rabbits are used as animal models of spontaneous hyperlipidemia and in the analysis of LOX-1 function.16,18–21 Therefore, we investigated whether the mAbs presented here cross-react with LOX-1s from these model animals. Six clones (HUC52, HUC5–44, HUC5–53, HUC5–63, HUC5–90 and HUC6–92) that reacted with rhLOX-1 also displayed cross-reactivity to both rrLOX-1 and rpLOX-1 (Fig. 2B). In contrast, no rmLOX-1 cross-reactive antibodies were obtained in the first antibody selection. We then selected antibodies using the scFv phage library from rhLOX-1-immunized chickens, and identified clones HUC3-1, which cross-reacted with rmLOX-1 and rhLOX-1, and HUC3–48, which cross-reacted with recombinant LOX-1s from all three species examined (Fig. 2B).
A total of 45 rIgYs including HUC3-48 mAb inhibited oxLDL-binding with LOX-1 (data not shown). The result indicates that these 45 mAbs are reactive for the CTL domain, which is critical for the binding of oxLDL.5
For possible utilization as therapeutic antibody agents for humans, we reconstructed two clones as chicken-human chimeric IgG by converting the chicken constant regions into human regions (Fig. 3). These antibodies were evaluated in inhibition assays using cells expressing LOX-1. HUC52 and HUC3–48 chimeric IgGs, which had similar LOX-1 reactivity compared to their respective parental antibodies (Fig. 4), blocked oxLDL binding to LOX-1 expressed on CHO cells (Fig. 5B). This evidence suggests that these antibodies should be further evaluated in animal models.
Since the variability of FR residues in chicken antibodies is very small compared to those in human and rodent, it is possible that the same human template can be used to humanize all chicken antibodies. Humanization of chicken mAbs has been achieved by CDR-grafting, followed by framework fine-tuning using a phage displayed combinatorial library.22 In the current study, chicken mAbs were successfully humanized as divalent IgG4 without loss of antibody affinity. Therefore, humanized chicken antibodies may have applications as treatments for human disease in the future.
In conclusion, we generated 53 mAbs against LOX-1 using phage-display techniques and 45 of these mAbs that recognized the CTL domain were neutralizing antibodies. In addition, two human chimeric mAbs from HUC52 and HUC3–48, cross-reacted with rabbit, pig or mouse LOX-1, also showed neutralization activitities against LOX-1 expressing cells. These results indicate that our mAb clones might be useful tools for the investigation of LOX-1 function, and might have clinical applications. Production of chicken-mouse, chicken-rabbit and chicken-pig chimeric mAbs for preclinical studies using animal models is in progress.
Materials and Methods
Antigens.
Recombinant human LOX-1 (amino acids 61–273; rhLOX-1) and its deletion mutant (amino acids 137–273; delta Neck), recombinant mouse LOX-1 (amino acids 188–363 of mouse LOX-1; rmLOX-1), recombinant rabbit LOX-1 (amino acids 101–278 of rabbit LOX-1; rrLOX-1) and recombinant pig LOX-1 (amino acids 61–274 of pig LOX-1; rpLOX-1) were generated with pcDNA4/myc-HisA (Invitrogen, USA) in order to synthesize each LOX-1 as a 6xHistidine tag (His tag) fusion protein as described previously.18 Proteins were produced in a FreeStyle™ 293 Expression System (Invitrogen) and were purified using a Probond protein purification kit (Invitrogen), and their molecular sizes were confirmed by SDS-PAGE. Purified proteins were also biotinylated using a Biotin Labeling Kit-NH2 (Dojindo, Japan). The PCR primers used in this study were shown in Table 1.
Table 1.
PCR primers used in this study
| Primer | Sequence |
| Leader-F | 5′-ATGCGGATCCGCCATGGCCTGGGCTCCTCTCCT |
| Leader-R | 5′-TGCCTGCACCAGGGAACCTG |
| hLOX-F | 5′-TCCCTGGTGCAGGCATCCCAGGTGTCTGACCTC |
| hLOX-R | 5′-ATGCACCGGTCTGTGCTCTTAGGTTTGCC |
| hLOX-Neck-F | 5′-TCCCTGGTGCAGGCAGTAGCAAATTGTTCAGCTC |
| mLOX-F | 5′-TCCCTGGTGCAGGCAGAGTCCCAGAGAGAACTC |
| mLOX-R | 5′-ATGCACCGGTAATTTGCAAATGATTTGTC |
| rLOX-F | 5′-TCCCTGGTGCAGGCAGAGTCACAAAGGGAACTC |
| rLOX-R | 5′-ATGCACCGGTCTCTGATCTCAGCAGATTTG |
| pLOX-F | 5′-TCCCTGGTGCAGGCATCCCAGGTGTCTGATCTCCTG |
| pLOX-R | 5′-GACTACCGGTCTGTGCTCTCAAGAGATTCGC |
Primers for generation of recombinant LOX-1 protein. Restriction sites are underlined.
Cells.
cDNA encoding the human LOX-1 was subcloned into a Tet-On Gene Expression vector pTRE2 hyg (Clontech Laboratories, USA). The plasmid was transfected into CHO-K1 Tet-On cells (Clontech Laboratories) by Lipofectamin2000 tansfection reagent (Invitrogen) according to the manufacture's instruction. Stably transformants were selected under Ham's F12/10% fetal bovine serum (FBS) supplemented with 400 µg/ml hygromycin B (Wako, Japan). The cells expressing LOX-1 in response to doxycycline (Wako) were selected. The LOX-1 expression was induced with 1 µg/ml doxycyline for 24 h before the experiments. Rabbit and pig cDNA were subcloned into a mammalian expression vector pEF6V5-HisA (Invitrogen), respectively. The plasmid was each transfected into CHO-K1 cells by FuGENE HD transfect reagent (Roche Diagnostics, Switzerland). Stably LOX-1-expressing cells were selected and maintained under Ham's F12/10% FBS supplemented with 8 µg/ml blasticidin S (Invitrogen).
Immunization and construction of phage-display library.
Chicken scFv mAbs were generated by the chicken phagedisplay technique.15 One-month-old H-B15 inbred chickens were immunized intraperitoneally (i.p.) with rhLOX-1 (50 µg/ml/chicken) in an equal volume of alum solution (ALUM). The chickens received three additional i.p. injections with the same antigen together with ALUM at 3-week intervals. Four days after the final injection, spleen cells were isolated from immunized chickens. RNA was extracted from spleen cells, immunoglobulin variable region (VH and VL) genes were amplified and a scFv phage library was constructed as described previously.15
Panning selection.
The phage-display scFv library from rhLOX-1-immunized chickens was panned against rhLOX-1 or rmLOX-1. For selection of rhLOX-1-specific antibodies, 100 µl (10 µg/ml) of rhLOX-1 was coated on a Maxisorp Nunc-Immuno™ module (NUNC, USA). An ELISA plate was then blocked with 2% (w/v) non-fat dried milk powder (EuroClon, Italia) in phosphate-buffered saline (PBS) at room temperature (RT) for 1 h. For selection against rmLOX-1, 100 µl (5 µg/ml) of biotin-labeled rmLOX-1 was coated on Nunc Immobilizer™ Streptavidin plates (NUNC). Panning selection was performed as described previously.15
Recombinant chicken IgY (rIgY) and chicken-human chimeric IgG4 antibody (chimeric IgG).
The rIgY and chimeric IgG were generated using the VH and VL genes from phage-displayed chicken antibodies obtained in this study, and plasmid vectors17,23 were used for construction of the light and heavy chains, respectively. Constructed plasmid DNAs were transfected into COS-7 cells or FreeStyle™ 293-F cells (Invitrogen) using FuGENE HD transfect reagent.
Western blotting.
Western blotting for detection of recombinant LOX-1s was carried out. Recombinant LOX-1s were subjected to SDS-PAGE under non-reducing conditions, and were then transferred to an Immun-Blot™ PVDF membrane (Bio-Rad, USA) at 350 mA for 1 h. Membranes were incubated for 1 h at room temperature with chicken anti-LOX-1 rIgYs and developed by ECL plus (GE Healthcare, UK). Chemiluminescent signals were then analyzed using a LAS-3000 (Fuji Film, Japan).
Western blotting for detection of rIgY and chimeric IgG was carried out as described previously.17,18 Horseradish peroxidase (HRP)-labeled anti-chicken IgG-Fab fragment (Bethyl, USA) was used for detection of rIgY and chimeric IgG. Mouse anti-human IgG4 antibody (BD Biosciences, USA) was used as the first antibody and HRP-labeled-mouse IgG antibody (Southern Biotech, USA) was used as the second antibody for detection of chimeric IgG. The rIgY and chimeric IgG were detected using ECL plus and LAS-3000.
ELISA for reactivity and cross-reactivity of rIgYs.
The wells of Nunc Immobilizer™ Streptavidin plates were coated with 50 µl (1 µg/ml) of biotin-labeled rhLOX-1, delta Neck, rmLOX-1, rrLOX-1, rpLOX-1 or BSA (control antigen) in carbonate buffer (pH 9.5) for 1 h at RT. After washing with PBS-T, the respective rIgYs were added at 1 µg/ml. Plates were incubated at 37°C for 1 h. After washing with PBS-T, bound antibodies were detected using a HRP-labeled goat anti-chicken IgG (H + L) (Kirkeggaard and Perry Laboratories, USA). After washing with PBS-T, o-phenylene diamine sulfate (OPD, Sigma, USA) was added and the optical density was measured at 490 nm using a Model 680 microplate reader (Bio-Rad). Human anti-human LOX-1 mAb TS-92,9 was used as a positive control.
FACS analysis.
The recombinant antibodies were each incubated with LOX-1-expressing CHO cells at 4°C for 1 h in PBS containing 0.1% FBS and 0.1% NaN3 (FACS buffer). After washing with FACS buffer, cells were incubated with FITC-labeled anti-chicken IgG (H + L) or anti-human IgG (H + L) (Southern Biotech, USA) for 30 min at 4°C. Fluorescence was analyzed by FACSCalibur (BD, USA).
Inhibition analysis by mAb in oxLDL-binding with recombinant LOX-1protein.
Inhibition analysis was performed using a modified method reported previously.16 Biotin-labeled rhLOX-1 (50 ng/well) was immobilized on Nunc Immobilizer™ Streptavidin plates by incubating at RT for 1 h in 50 µl of carbonate buffer. After washing with PBS, rIgY (1 µg/ml), TS-92 (positive control, 1 µg/ml) and anti-2,4-dinitrophenyl (DNP) rIgY (negative control, 1 µg/ml)18 were each added to rhLOX-1-coated wells, and plates were incubated at RT for 2 h. After washing with PBS, plates were incubated overnight at 4°C with 50 µl of human oxLDL (3 µg/ml) in PBS containing 20% (vol/vol) newborn calf serum (NBCS, Gibco, USA). Plates were then washed with PBS, and incubated for 2 h at RT with the HRP-sheep anti-human ApoB polyclonal antibody (The Binding Site, UK) diluted 1,000 times with PBS containing 1% (w/v) BSA for detection of oxLDL. After washing with PBS, the peroxidase reaction was initiated by incubating plates for 5 min at RT with 50 µl of SureBlue Reserve™ TMB Microwell Peroxidase Substrate (Kirkegaard & Perry Laboratories, USA). The reaction was terminated with 0.1 M hydrochloric acid and 0.3 M sulfuric acid. Peroxidase activity was determined by measuring absorbance at 450 nm.
Inhibition analysis by mAb in oxLDL-binding with LOX-1expression cells.
The LOX-1-expressing CHO cells were incubated with anti-LOX-1 antibody in Ham's F12 containing 10% NBCS for 1 h at 37°C. After 1 h, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Molecular Probes, USA)-labeled oxLDL (3 µg/ml) was added to the cells for 2 h. The cells were washed three times with PBS and fixed with 4% (v/v) paraformaldehyde in PBS for 20 min. Then, the nuclei of the cells were counterstained with 5 µg/ml DAPI (Sigma) and subjected to observation with fluorescence microscopy.
Acknowledgements
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
Abbreviations
- LOX-1
lectin-like oxidized low-density lipoprotein receptor-1
- LDL
low-density lipoprotein
- oxLDL
oxidized lowdensity lipoprotein
- CTL
C-type lectin-like
- DNP
2,4-dinitrophenyl
Footnotes
Previously published online as a mAbs E-publication: http://www.landesbioscience.com/journals/mabs/article/8919
References
- 1.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 2.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1). A critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Hayashida K, Kume N, Murase T, Minami M, Nakagawa D, Inada T, et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112:812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama T, Sawamura T, Furutani Y, Matsuoka R, Yoshida MC, Fujiwara H, Masaki T. Structure and chromosomal assignment of the human lectin-like oxidized low-densitylipoprotein receptor-1 (LOX-1) gene. Biochem J. 1999;339:177–184. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Narumiya S, Masaki T, Sawamura T. Conserved C-terminal residues within the lectin-like domain of LOX-1 are essential for oxidized low-density-lipoprotein binding. Biochem J. 2001;355:289–296. doi: 10.1042/0264-6021:3550289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Inoue K, Narumiya S, Masaki T, Sawamura T. Requirements of basic amino acid residues within the lectin-like domain of LOX-1 for the binding of oxidized low-density lipoprotein. FEBS Lett. 2001;499:215–219. doi: 10.1016/s0014-5793(01)02557-1. [DOI] [PubMed] [Google Scholar]
- 7.Ohki I, Ishigaki T, Oyama T, Matsunaga S, Xie Q, Ohnishi-Kameyama M, et al. Crystal structure of human lectin-like, oxidized low-density lipoprotein receptor 1 ligand binding domain and its ligand recognition mode to OxLDL. Structure. 2005;13:905–917. doi: 10.1016/j.str.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Williams V, Liu L, Chen H, Sawamura T, Antakli T, Mehta JL. LOX-1 inhibition in myocardial ischemia-reperfusion injury: modulation of MMP-1 and inflammation. Am J Physiol Heart Circ Physiol. 2002;283:1795–1801. doi: 10.1152/ajpheart.00382.2002. [DOI] [PubMed] [Google Scholar]
- 9.Hu B, Li D, Sawamura T, Mehta JL. Oxidized LDL through LOX-1 modulates LDL-receptor expression in human coronary artery endothelial cells. Biochem Biophys Res Commun. 2003;307:1008–1012. doi: 10.1016/s0006-291x(03)01295-6. [DOI] [PubMed] [Google Scholar]
- 10.Hinagata J, Kakutani M, Fujii T, Naruko T, Inoue N, Fujita Y, et al. Oxidized LDL receptor LOX-1 is involved in neointimal hyperplasia after balloon arterial injury in a rat model. Cardiovasc Res. 2006;69:263–271. doi: 10.1016/j.cardiores.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 12.Asaoka H, Nishinaka S, Wakamiya N, Matsuda H, Murata M. Two chicken monoclonal antibodies specific for heterophil Hanganutziu-Deicher antigen. Immunol Lett. 1992;32:91–96. doi: 10.1016/0165-2478(92)90205-3. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K, Horiuchi H, Furusawa S, Horiuchi M, Shinagawa M, Matsuda H. Chicken monoclonal antibodies against synthetic bovine prion protein peptide. J Vet Med Sci. 1998;60:777–779. doi: 10.1292/jvms.60.777. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda H, Mitsuda H, Nakamura N, Furusawa S, Mohri S, Kitamoto T. A chicken monoclonal antibody with specificity for the N-terminal of human prion protein. FEMS Immunol Med Microbiol. 1999;23:189–194. doi: 10.1111/j.1574-695X.1999.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura N, Shuyama A, Hojyo S, Shimokawa M, Miyamoto K, Kawashima T, et al. Establishment of chicken monoclonal antibody panel against prion protein. J Vet Med Sci. 2004;66:807–814. doi: 10.1292/jvms.66.807. [DOI] [PubMed] [Google Scholar]
- 16.Kakutani M, Ueda M, Naruko T, Masaki T, Sawamura T. Accumulation of LOX-1 ligand in plasma and atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits: identification by a novel enzyme immunoassay. Biochem Biophys Res Commun. 2001;282:180–185. doi: 10.1006/bbrc.2001.4508. [DOI] [PubMed] [Google Scholar]
- 17.Shimamoto T, Nishibori N, Aosasa M, Horiuchi H, Furusawa S, Matsuda H. Stable production of recombinant chicken antibody in CHO-K1 cell line. Biologicals. 2005;33:169–174. doi: 10.1016/j.biologicals.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Nishimichi N, Nakano A, Takikawa K, Inoue N, Matsuda H, Sawamura T. Determination of LOX-1-ligand activity in mouse plasma with a chicken monoclonal antibody for ApoB. Atherosclerosis. 2008;200:303–309. doi: 10.1016/j.atherosclerosis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Arai Y, Kurihara H, Kita T, Sawamura T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ Res. 2005;97:176–184. doi: 10.1161/01.RES.0000174286.73200.d4. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Kakutani M, Minami M, Kataoka H, Kume N, Narumiya S, et al. Increased expression of lectin-like oxidized low density lipoprotein receptor-1 in initial atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2000;20:1107–1115. doi: 10.1161/01.atv.20.4.1107. [DOI] [PubMed] [Google Scholar]
- 22.Nishibori N, Horiuchi H, Furusawa S, Matsuda H. Humanization of chicken monoclonal antibody using phage-display system. Mol Immunol. 2006;43:634–642. doi: 10.1016/j.molimm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Nishibori N, Shimamoto T, Nakamura N, Shimokawa M, Horiuchi H, Furusawa S, Matsuda H. Expression vectors for chicken-human chimeric antibodies. Biologicals. 2004;32:213–218. doi: 10.1016/j.biologicals.2004.09.002. [DOI] [PubMed] [Google Scholar]