Abstract
Cells undergoing apoptosis in vivo are rapidly detected and cleared by phagocytes. Swift recognition and removal of apoptotic cells is important for normal tissue homeostasis and failure in the underlying clearance mechanisms has pathological consequences associated with inflammatory and auto-immune diseases. Cell cultures in vitro usually lack the capacity for removal of nonviable cells because of the absence of phagocytes and, as such, fail to emulate the healthy in vivo micro-environment from which dead cells are absent. While a key objective in cell culture is to maintain viability at maximal levels, cell death is unavoidable and non-viable cells frequently contaminate cultures in significant numbers. Here we show that the presence of apoptotic cells in monoclonal antibody-producing hybridoma cultures has markedly detrimental effects on antibody productivity. Removal of apoptotic hybridoma cells by macrophages at the time of seeding resulted in 100% improved antibody productivity that was, surprisingly to us, most pronounced late on in the cultures. Furthermore, we were able to recapitulate this effect using novel super-paramagnetic Dead-Cert™ Nanoparticles to remove non-viable cells simply and effectively at culture seeding. These results (1) provide direct evidence that apoptotic cells have a profound influence on their non-phagocytic neighbors in culture and (2) demonstrate the effectiveness of a simple dead-cell removal strategy for improving antibody manufacture in vitro.
Key words: apoptosis, hybridoma, phagocytosis, viability, cell-culture, cell-death, antibody, nanoparticles
Introduction
Cell death occurs normally in all tissues via well-known physiological mechanisms, the most widely studied of which is apoptosis. A key feature of apoptosis is its non-phlogistic nature: apoptotic cells are rapidly removed by phagocytes in vivo without causing inflammatory responses or damage to neighboring cells. Failure in this clearance mechanism is linked to pathological events associated with autoimmune and inflammatory diseases. Although cells of several different lineages including fibroblasts, epithelial cells and endothelial cells have been shown to be capable of engulfing their apoptotic neighbors (reviewed in ref. 1), the best-known scavenger of apoptotic cells is the macrophage. This professional phagocyte is extremely proficient at seeking out sites of apoptosis, using receptors that bind chemoattractants including lysophosphatidylcholine1 and fractalkine (CX3CL1)2 that are released from apoptotic cells. Consequently in vivo, wherever apoptosis is visible in standard histological sections, apoptotic cells and their remnants are invariably associated with macrophages. In keeping with the non-phlogistic nature of the apoptosis program, the other class of professional phagocytes, the granulocytes (or ‘microphages’ as they were originally termed) fail to seek out and engulf apoptotic cells. As we have recently demonstrated, this is due at least in part to the release from apoptotic cells of mediators, including lactoferrin, that inhibit granulocyte recruitment.3
Apoptotic cells impose effects on cells in their immediate vicinity. It is well known that they cause responses in phagocytes including engulfment (involving plasma membrane mobilisation) and anti-inflammatory events, perhaps the best known of which is TGF-β1 production.4 Under certain circumstances, apoptosis-in line with primary necrosis-leads to pro-inflammatory phagocyte responses, for example during progression to the post-apoptotic, necrotic (cytolysed) state that regularly occurs in vitro or when clearance is inhibited in vivo.5 Responses of phagocytes to apoptotic cells are not, of necessity, coupled only to phagocytosis since additional mechanisms dependent on mere contact between the dying cells and phagocytes can promote local anti-inflammatory effects, as well as suppression of pro-inflammatory signals. For example, switching of activated monocytes from pro-inflammatory to anti-inflammatory states follows contact with apoptotic neutrophils.6 Contact-dependent mechanisms that are independent of either soluble factors or phagocytic events have also been shown to inhibit pro-inflammatory signalling in macrophages downstream of TLR signalling pathways.7 Furthermore, contact with apoptotic cells can activate a “kiss and tell” response by phagocytes, a contactdependent licensing of macrophages to the anti-inflammatory effects of TGF-β1 that could play an important role in limiting the extent of the apoptosis-driven anti-inflammatory responses to the immediate neighborhood of the dying cell.8 Further intercellular signalling mechanisms between apoptotic cells and mononuclear phagocytes appear to be involved in creating a microenvironment that promotes apoptotic-cell clearance. Apoptotic cells can themselves produce anti-inflammatory mediators such as IL-109 and TGFβ-1,10 each of which is capable of up-regulating the capacity of macrophages to clear apoptotic cells.11,12 Intriguingly, the antiinflammatory signalling mechanisms that are activated in cells in contact with apoptotic cells occur in a wide range of different cell lineages in addition to professional phagocytes, including cells with no phagocytic capabilities.13 These observations provide strong evidence that dying cells have profound effects on the activities of cells in their neighborhood.
Local cellular responses to apoptotic cells are not limited to those that regulate inflammation and immunity. Although most studies to date have focused on the responses of macrophages that engulf apoptotic cells, evidence has been presented that exposure to apoptotic cells elicits repair responses, including growth factor and angiogenic factor production by neighbors.14–16 Apoptotic cells directly produce factors such as IL-10, TGFβ-1 and lactoferrin9,10,3 that can affect cell growth and differentiation. Furthermore, the presence of apoptotic cells has been found to initiate directional endothelial cell sprouting, an electrostatic mechanism resulting from the increased surface negative charge of apoptotic cells consequent to redistribution of phospholipids in their plasma membranes.17
In addition to releasing chemotactic mediators that allow phagocytes to sense them, apoptotic cells also undergo plasma membrane changes that are required for intercellular adhesion by phagocytes, as well as engulfment and anti-inflammatory responses. The best characterised architectural feature of the apoptotic cell surface is the loss of phospholipid asymmetry that results in the redistribution of the anionic phospholipid, phosphatidylserine (PS) from the inner plasma membrane leaflet to the outer.18–21 Exposed PS can engage with a number of glycoproteins such as MFG-E8 or Gas6 that act as bridging molecules to phagocyte receptors such as integrins αvβ3/5 or Mer22,23 or directly with integral PS receptors of the phagocyte surface such as TIM-1/4,24–26 BAI127 and Stabilin-2.28 Exposure or alteration at the surface of additional molecules of various biochemical classes such as sugars, proteins and nucleic acid may also be coupled to processes through which apoptotic cells condition their microenvironment and interact with neighbors and phagocytes.
Although cell culture systems are usually optimized to minimise loss in viability, cells growing in vitro are continuously exposed to apoptotic or other forms of non-viable cells and their remnants. Because it has proved difficult to remove dead cells by simple methods that do not themselves lead to loss in viability, little is yet known about the effects of cell death in culture on viable neighboring cells. Given the known effects of apoptotic cells upon neighboring cells in which they come into contact and the pathological consequences of dead cells that persist in vivo, it seemed likely to us at the onset of this work that the behaviour of viable cells in vitro could be modulated by the presence of non-viable cells. We reasoned that presence of dead cells could impose inhibitory effects on viable cells in culture and that depletion of non-viable cells—through emulation of the normal homeostatic process of dead-cell removal—might ‘release’ viable cells from the inhibitory effects of their non-viable counterparts. Here we demonstrate that monoclonal antibody-producing hybridoma cells can markedly improve in their individual capacity to produce antibody following a single round of depletion of dead neighbors, either by macrophages or by Dead-Cert™ Nanoparticles that can function as ‘substitute’ phagocytes that remove dead cells in a magnetic field. Remarkably, dead-cell removal at the start of cell culture significantly improved antibody production over protracted culture periods, suggesting that removal of non-viable hybridoma cells can cause programming of viable neighboring cells for improved antibody production. The mechanisms by which dead-cell removal releases/re-programmes hybridoma cell populations to produce higher quantities of antibody remain to be elucidated.
Results
Apoptotic cells inhibit antibody production by hybridomas.
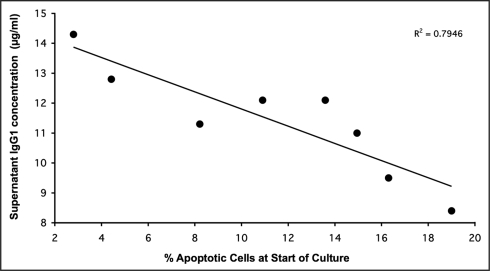
In order to establish whether apoptotic cells are capable of modulating antibody production in hybridoma cell populations, we first assessed antibody productivity in hybridoma cultures containing variable proportions of apoptotic cells at seeding of a constant number of viable cells. Variable proportions of apoptotic cells were achieved by spiking a constant number of viable cells with apoptotic cells from crashed cultures in which all cells had died. We sought a correlation between population viability at the onset of hybridoma culture and antibody productivity. As shown in Figure 1, a significant inverse correlation was found between antibody production and apoptotic cell number at the onset of short-term culture. Thus, in the experiment shown, for every percentage point increase in apoptosis at the start of the culture, antibody productivity by the equivalent number of viable cells (at seeding) fell by approximately two percentage points (Figure 1).
Figure 1.
Inverse relationship between proportion of non-viable (apoptotic) cells and antibody productivity in hybridoma cultures. 2 × 105 viable 4/C6 hybridoma cells/ml were seeded with different proportions of apoptotic cells (non-viable cells obtained from cultures at the end of plateau phase). Monoclonal antibody (IgG1) productivity was assessed in cell-free supernatants four days later. The inverse correlation was significant (p < 0.005)
These results suggested that non-viable hybridoma cells exert inhibitory effects on their viable neighbors.
Removal of apoptotic cells by macrophages promotes antibody production.
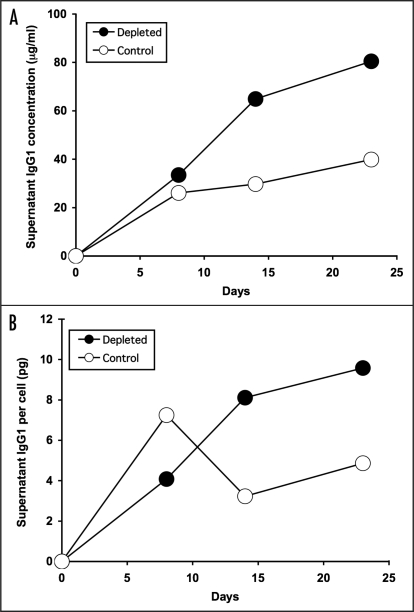
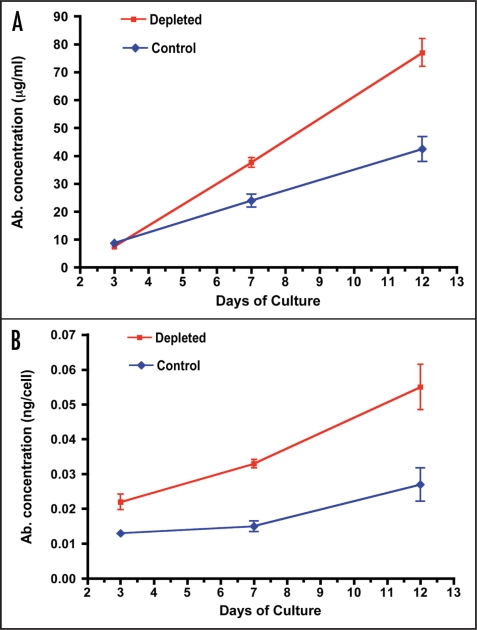
In order to obtain more direct evidence that non-viable hybridoma cells exert inhibitory effects on viable cells in the same cultures, we used macrophages as a physiological mode of depletion of non-viable cells and compared antibody productivity of depleted versus control cultures. As shown in Figure 2A, culture of 9e10 hybridoma cells in T flasks for a period of three weeks following a single depletion of non-viable cells using human monocyte-derived macrophages (improving viability in the experiment illustrated from 57 to 96% as assessed by trypan blue exclusion), resulted in substantially improved antibody productivity, particularly during the later stages of culture when antibody productivity rose to double that of the untreated cells. Similar results were obtained using 4/C6 hybridoma cells (data not shown).
Figure 2.
Antibody production by hybridoma cells after depletion of non-viable cells by macrophages. Equal numbers of viable 9e10/4 hybridoma cells (2 × 105 /ml) were cultured before (Control, open symbols) or after (Depleted, solid symbols) removal of non-viable cells on day 0 using monolayers of HMDM. In the experiment shown, viability was improved from 57 to 96% by HMDM-mediated depletion of non-viable cells. At the indicated times, cell-free supernatants were assayed for IgG1 content. (A) total IgG1 per ml; (B) IgG1 per cell.
In order to determine the extent to which the improved antibody productivity observed in depleted cultures reflected greater antibody production by individual cells, we assessed the antibody productivity per cell over the course of the culture period. As illustrated in Figure 2B, while the antibody productivity per cell (assessed by dividing the total antibody concentration measured at a particular time point by the total cell count at that time point) was greater in the untreated cultures during the first week, in depleted cultures the productivity per cell rose steadily to reach double that of the untreated cultures by the second week, and sustained that level for the remaining week. These results suggested that the removal of non-viable cells prior to culture set-up enhanced the antibody-producing capacity of individual cells during extended culture.
Dead-Cert™ Nanoparticles: new devices for effective removal of non-viable cells in vitro.
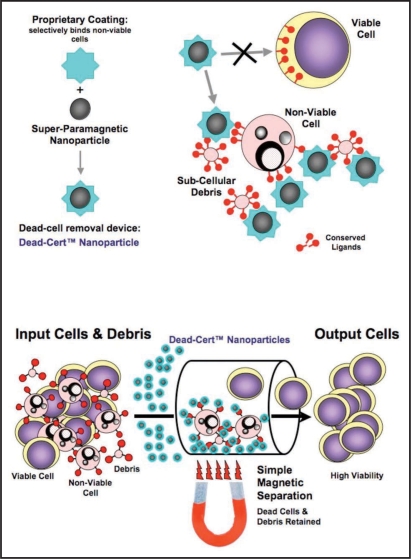
The results obtained above using macrophages for depletion of non-viable cells are consistent with the conclusion that dead and dying cells exert inhibitory effects on antibody production by hybridoma cells. An alternative explanation is that the observed effects were induced following ‘activation’ of hybridoma cells by macrophage—the latter cells providing signals that boost antibody productivity-rather than as a consequence of removal of non-viable cells per se. In order to differentiate between these two possibilities, we first investigated the utility of Dead-Cert Nanoparticles™ in depleting non-viable cells from hybridoma cell suspensions as an alternative to macrophages. We have developed the surface of these 250 nm super-paramagnetic particles to discriminate between viable and non-viable cells (Figure 3A) such that, following a short incubation step during which the particles bind to non-viable cells and sub-cellular debris, viable cells can be rescued by negative selection using a simple magnet (Figure 3B). We have found this approach to be non-traumatic and effective in depletion of non-viable cells of multiple lineages from many species (see www.immunosolv.com for further details).
Figure 3.
Dead-Cert™ Nanoparticles: simple devices for effective direct removal of non-viable cells in vitro. ImmunoSolv's antibody-based Dead-Cert™ technology (see www.immunosolv.com) has been developed to discriminate non-viable from viable cells in vitro. ImmunoSolv has used antibodies, together with additional discriminatory molecules coupled to the surface of 250 nm super-paramagnetic nanoparticles, to develop a simple, yet highly effective dead-cell removal device, the Dead-Cert™ Nanoparticle. Because of their ability to bind to membrane structures that are not accessible on viable cells-represented by the red symbols in the cartoons-the coated particles are able to bind selectively to apoptotic (dying) as well as necrotic (dead) cells and cell debris. Once bound to non-viable cells and debris, the Dead-Cert™ Nanoparticles can be readily removed with the aid of a simple magnet leaving the ‘untouched’ viable cells purified by negative selection.
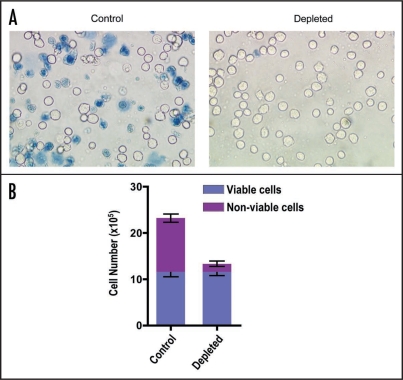
As shown in Figure 4, efficient depletion of non-viable hybridoma cells was achieved in a single separation using Dead-Cert™ Nanoparticles. In the examples shown, trypan blue positive cells were effectively removed using this method (Figure 4A and B) and the yield of viable cells was virtually 100% indicating full recovery of the negatively selected viable cells from the input population (Figure 4B).
Figure 4.
Depletion of non-viable hybridoma cells using Dead-Cert™ Nanoparticles. Hybridoma cells (9e10/18) taken from the end of plateau phase of culture were subjected to a single magnetic separation using Dead-Cert™ Nanoparticles. (A) Light micrographs of untreated (Control) and separated (Depleted) cells exposed to trypan blue to label dead cells. (B) Quantitative analyses of untreated and separated cell populations. Cells were counted in Neubauer haemocytometer chambers following exposure to trypan blue.
Removal of apoptotic cells by Dead-Cert™ Nanoparticles promotes monoclonal antibody production by hybridoma cells.
In order to determine whether depletion of non-viable hybridoma cells using Dead-Cert™ Nanoparticles could emulate the effects of macrophages on antibody productivity as described above, we set up hybridoma cultures using untreated (control) or depleted (separated using Dead-Cert™ Nanoparticles) hybridoma cells (illustrated here using 9e10 but observed also with other hybridomas- not shown) at culture seeding and compared antibody production levels 3, 7 and 12 days later. As shown in Figure 5 A, while similar levels of antibody were produced at 3 days in both types of culture, by 12 days, antibody production in the depleted cultures was running at almost double that of control cultures. When the productivity of antibody per cell was assessed (Figure 5B), differences in antibody production between control and depleted cultures were even more marked, with cells from depleted cultures producing more than double the amount of antibody of control cultures at 7 and 12 days. Since mere exposure to Dead-Cert™ Nanoparticles failed to affect cultures (data not shown) these results indicate that the enhanced and sustained productivity of the cultures seeded with depleted cells was due directly to the removal of inhibitory non-viable cells by the Dead-Cert™ Nanoparticles.
Figure 5.
Antibody production by hybridoma cells after depletion of non-viable cells by Dead-Cert™ Nanoparticles. Equal numbers of viable 9e10/18 hybridoma cells (2 × 105/ml) were cultured before (Control) or after (Depleted) removal of non-viable cells on day 0 using Dead-Cert™ Nanoparticles. In the experiment shown, viability was improved from 64% to 95% by Dead-Cert™ Nanoparticle-mediated magnetic depletion of non-viable cells. At the indicated times, cell-free supernatants were assayed for IgG1 content. (A) Total IgG1 per ml; (B) IgG1 per cell.
Discussion
Although relatively little is yet known of the underlying mechanisms, there is no doubt that non-viable cells condition their microenvironment, either directly or via responses of other cell types, notably phagocytes. Using two methods to remove non-viable cells, (1) macrophages and (2) super-paramagnetic particles, we have shown that non-viable hybridoma cells radically affect their viable neighbors, and inhibit the antibody-producing potential of individual cells. We were able to demonstrate this effect in the absence of phagocytes, which provides definitive evidence that non-viable hybridoma cells exert direct inhibitory effects on viable cells in the same cultures, and that efficient removal of non-viable cells can have surprisingly long-lasting effects on the remaining viable cells and their progeny. Following a single depletion of non-viable cells at culture seeding, we observed improvements in antibody productivity by hybridoma cells that were sustained—and indeed increased—with time of culture. These improvements were substantial, 100% or more, suggesting that this strategy has potential to boost monoclonal antibody manufacturing.
Cell death through apoptosis in cultures of antibody-producing hybridoma cells is well known,31,32 and strategies for improving viability of hybridoma cells via suppression of their apoptosis program (such as through expression of Bcl-2-family proteins) to improve antibody productivity have been widely studied.33,34 However, to our knowledge, the phenomenon described here of substantially improved antibody production by hybridoma cells following removal of non-viable cells prior to culture seeding has not been reported previously. The effect is particularly intriguing because it was not limited to the initial population of cells from which the non-viable cells were removed. Rather, these cells appeared, through removal of neighboring non-viable cells, to be in some way reprogrammed to produce more antibody, and that this reprogramming extended to the progeny of the cells that were used to seed the culture. Because persistent apoptotic cells have inhibitory effects on antibody production by hybridoma cells (as demonstrated in Figure 1), our interpretation of the apparent reprogramming mechanism is that it stems from release of viable cells from inhibitory properties of non-viable cells.
The nature of the inhibitory components of non-viable cells awaits definition, but two lines of evidence are noteworthy in this context. Firstly, activation of inhibitory signalling pathways that suppress pro-inflammatory responses by contact with apoptotic cells have been reported for multiple cell lineages, including non-phagocytic cells.13 Second, membranes of killed cells have been shown to harbor inhibitory lipid moieties capable of suppressing cell growth.35,36 Given the fundamental changes in lipid topology of plasma membranes that are induced during cell death, the most acclaimed of which being the loss of phospholipid asymmetry with externalisation of anionic phospholipids such as PS, it is tempting to speculate that such externalised components act as intercellular signalling structures to mediate the inhibitory effects described here. Although PS has been identified as a key component of anti-inflammatory signalling by phagocytes responding to apoptotic cells,4 such signalling to other cell types appears to occur independently of PS.13 Changes in plasma membrane topology are not limited to lipids: intracellular proteins and DNA also become exposed at the plasma membrane surface of dying cells, and localised alterations in molecular architecture also occur during apoptosis, including clustering of key proteins and lipids and alterations in surface carbohydrates.4,5 Furthermore, release of molecules such as immunomodulators and proteolytic enzymes from dying and dead cells provides ample scope for generating wide-ranging microenvironmental effects. Clearly, there are many candidate molecular mechanisms underlying the effects described here and substantial future work will be required to elucidate these definitively.
In conclusion, we report a novel negative mechanism controlling antibody production by hybridoma cells in vitro that is mediated by non-viable neighboring cells. Release from this inhibitory regulation can be readily achieved using an effective procedure of removal of non-viable cells using macrophages or, more simply, using Dead-Cert™ Nanoparticles at the initiation of culture. This procedure has obvious potential in improving antibody manufacture because the removal of non-viable cells can now be achieved simply with high yield and efficiency and without trauma to viable cells, and can substantially improve antibody productivity not only by the remaining viable cells but also by their progeny. Magnetic separation technology is amenable to scale-up and Dead-Cert™ Nanoparticles have significant potential to improve antibody manufacturing at industrial scales. Furthermore, because of the fundamental nature of cell death and the wide ranging responses of viable cells to non-viable neighbors, it is likely that the inhibitory effects of non-viable cells, as well as the release from such inhibitory activities, will prove to extend to many different cell systems. The ability to remove non-viable cells routinely in vitro provides the opportunity to enhance cell culture methods by emulating the dead-cell removal systems that operate in vivo and also provides a critical tool that allows the dissection of the mechanisms underlying the inhibitory effects of non-viable cells to begin.
Materials and Methods
Hybridoma culture.
In-house sub-clones of the murine hybridoma line, 9e10 (9e10/4) and (9e10/18) that produce IgG1 monoclonal antibody against c-Myc29 were used for the majority of these studies. Additional, and confirmatory experiments were performed using a different IgG1-producing hybridoma, 4/C6, generated in our laboratory. Cells were cultured in RPMI 1640 medium (InVitrogen) containing 10% (v/v) FCS (PAA Laboratories), 2 mM L-glutamine and antibiotics (PAA Laboratories). Viability was assessed routinely by microscopic analysis of trypan blue-stained (0.2%) cell populations. Cell death occurred through apoptosis, as diagnosed using DAPI or acridine orange staining as described30 and apoptotic hybridoma cells rapidly became permeable to trypan blue.
Assessment of antibody production.
IgG1 was measured in cell-free supernatants either by single radial immunodiffusion using a sheep anti-mouse IgG antiserum and purified mouse IgG1 as calibrator, or by sandwich ELISA using goat anti-mouse Ig (Sigma) as capture antibody and goat anti-mouse IgG-peroxidase conjugate (Sigma) followed by OPD for visualisation (Sigma). The reaction was stopped using H2SO4 and absorbances were read at 492 nm using an Anthos plate reader.
Removal of non-viable cells using macrophages.
Prior to culture seeding, 9e10/4 hybridoma cells from plateau-phase cultures were subjected to panning on monolayers of human monocyte-derived macrophages (HMDM) prepared as described.30 Monocytes were cultivated for seven days (approximately 107 monocytes per 25 cm2 tissue culture flask) in Iscove's modified Dulbecco's medium (IMDM) containing 2 mM L-glutamine and 10% autologous serum at 37°C in an atmosphere of 5% CO2 in air. Culture medium was replenished every three days. Immediately before panning, flasks containing mature HMDM were rinsed three times with pre-warmed (37°C) culture medium. Hybridoma cells (107 total cells) were subsequently added (approximately 20 non-viable cells per macrophage) in 2 ml culture medium and incubated for 2 hours at 37°C in 5% CO2 in air with gentle rocking every 30 minutes. Hybridoma cells which failed to attach to, or be engulfed by, HMDM were recovered by decanting culture medium and rinsing the monolayer gently with fresh culture medium (10 ml). Cells recovered from panning, together with controls, were resuspended at 2 × 105 viable cells/ml in 5 ml fresh hybridoma culture medium and cultured in 25 cm2 tissue culture T flasks. Viability of control and depleted cell preparations was determined using 0.2% trypan blue.
Removal of non-viable cells using Dead-Cert™ Nanoparticles.
9e10/18 hybridoma cell populations were depleted of non-viable cells using Dead-Cert™ Nanoparticles according to the manufacturer's instructions. Briefly, 5 × 106 hybridoma cells from plateau-phase cultures were resuspended in 200 µl culture medium containing Dead-Cert™ Nanoparticles (20 µl washed particles from stock). Care was taken to resuspend the stock suspension of particles by vortexing for at least 30 seconds and gentle pipetting. Cells were incubated with Dead-Cert™ Nanoparticles upright in a 1.5 ml Eppendorf tube at 4°C for 30 minutes. 0.8 ml culture medium was subsequently added to the mixture which was thoroughly resuspended by aspiration using a 1 ml pipette tip. The tube was then placed on a magnet for 3 minutes at ambient temperature and (viable) cells remaining in suspension were carefully removed, resuspended at 2 × 105 viable cells/ml in fresh hybridoma culture medium and cultured in 24-well tissue culture plates. Further details of the composition, specificity and application of Dead-Cert™ Nanoparticles including laboratory protocols and a video detailing the separation process can be obtained at www.immunosolv.com.
Abbreviations
- HMDM
human monocyte-derived macrophages
- PS
phosphatidylserine
Footnotes
Previously published online as a mAbs E-publication: www.landesbioscience.com/journals/mabs/article/9124
References
- 1.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3- mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 2.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 3.Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Gregory CD, Devitt A. The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically? Immunology. 2004;113:1–14. doi: 10.1111/j.1365-2567.2004.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 7.Cvetanovic M, Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. 2004;172:880–889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- 8.Lucas M, Stuart LM, Zhang A, Hodivala-Dilke K, Febbraio M, Silverstein R, et al. Requirements for apoptotic cell contact in regulation of macrophage responses. J Immunol. 2006;177:4047–4054. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- 9.Gao YK, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WJ, Frank ME, Jin WW, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 11.Szondy Z, Sarang Z, Molnar P, Nemeth T, Piacentini M, Mastroberardino PG, et al. Transglutaminase 2-/- mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci U S A. 2003;100:7812–7817. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CA, Pound JD, Batth BK, Owens S, Johannessen I, Wood K, et al. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt's lymphoma. J Immunol. 2005;174:3015–3023. doi: 10.4049/jimmunol.174.5.3015. [DOI] [PubMed] [Google Scholar]
- 13.Cvetanovic M, Mitchell JE, Patel V, Avner BS, Su Y, van der Saag PT, et al. Specific recognition of apoptotic cells reveals a ubiquitous and unconventional innate immunity. J Biol Chem. 2006;281:20055–20067. doi: 10.1074/jbc.M603920200. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, et al. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol. 2001;24:608–615. doi: 10.1165/ajrcmb.24.5.4292. [DOI] [PubMed] [Google Scholar]
- 15.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, et al. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. Faseb J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 16.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 17.Weihua Z, Tsan R, Schroit AJ, Fidler IJ. Apoptotic cells initiate endothelial cell sprouting via electrostatic signaling. Cancer Res. 2005;65:11529–11535. doi: 10.1158/0008-5472.CAN-05-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 19.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium- mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 21.Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J Leukoc Biol. 2006;79:896–903. doi: 10.1189/jlb.1005550. [DOI] [PubMed] [Google Scholar]
- 22.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 23.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 24.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 29.Chan S, Gabra H, Hill F, Evan G, Sikora K. A novel tumour marker related to the c-myc oncogene product. Mol Cell Probes. 1987;1:73–82. doi: 10.1016/0890-8508(87)90008-9. [DOI] [PubMed] [Google Scholar]
- 30.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 31.Franek F, Sramkova K. Protection of B lymphocyte hybridoma against starvationinduced apoptosis: survival-signal role of some amino acids. Immunol Lett. 1996;52:139–144. doi: 10.1016/0165-2478(96)02591-6. [DOI] [PubMed] [Google Scholar]
- 32.Singh RP, Al-Rubeai M, Gregory CD, Emery AN. Cell-death in bioreactors - a role for apoptosis. Biotechnol Bioeng. 1994;44:720–726. doi: 10.1002/bit.260440608. [DOI] [PubMed] [Google Scholar]
- 33.Perani A, Singh RP, Chauhan R, Al-Rubeai M. Variable functions of bcl-2 in mediating bioreactor stress- induced apoptosis in hybridoma cells. Cytotechnology. 1998;28:177–188. doi: 10.1023/A:1008002319400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terada S, Komatsu T, Fujita T, Terakawa A, Nagamune T, Takayama S, et al. Co-expression of bcl-2 and bag-1, apoptosis suppressing genes, prolonged viable culture period of hybridoma and enhanced antibody production. Cytotechnology. 1999;31:143–151. doi: 10.1023/A:1008080407581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stallcup KC, Dawson A, Mescher MF. Growth-inhibitory activity of lymphoid cell plasma membranes. I. Inhibition of lymphocyte and lymphoid tumor cell growth. J Cell Biol. 1984;99:1221–1226. doi: 10.1083/jcb.99.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stallcup KC, Burakoff SJ, Mescher MF. Growth-inhibitory activity of lymphoid cell plasma membranes. II. Partial characterization of the inhibitor. J Cell Biol. 1984;99:1227–1234. doi: 10.1083/jcb.99.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]