Abstract
In mammals, primordial follicles are generated early in life and remain dormant for prolonged intervals. Their growth resumes via a process known as primordial follicle activation. Recent genetic studies have demonstrated that phosphoinositide 3-kinase (PI3K) is the essential signaling pathway controlling this process throughout life, acting via Akt to regulate nucleocytoplasmic shuttling of Foxo3, which functions as a downstream molecular switch. The receptor tyrosine kinase Kit has been implicated by numerous studies as the critical upstream regulator of primordial follicle activation via PI3K/Akt. Here we present a genetic analysis of the contribution of Kit in regulating primordial follicle activation and early follicle growth, employing a knock-in mutation (KitY719F) that completely abrogates signaling via PI3K. Surprisingly, homozygous KitY719F female mice undergo primordial follicle activation and are fertile, demonstrating that Kit signaling via PI3K is dispensable for this process. However, other abnormalities were identified in KitY719F ovaries, including accelerated primordial follicle depletion and accumulation of morphologically abnormal primary/secondary follicles with persistent nuclear Foxo3 localization. These findings reveal specific roles of Kit in the maintenance of the primordial follicle reserve and in the primary to secondary follicle transition, but argue that Kit is dispensable in primordial follicle activation.
Keywords: Forkhead transcription factor, Foxo3, ovary, oocyte, primordial follicle, PI3K, Kit, imatinib
Introduction
Primordial follicle activation (PFA) is the metered process by which primordial follicles are selected from the reserve pool into the growing follicle pool (McLaughlin and McIver, 2009). PFA and the ensuing follicle growth are irreversible; follicles that have initiated growth undergo atresia if not selected for subsequent stages of maturation (Peters et al., 1975). Morphologically, PFA is characterized by oocyte growth and transition of flat to cuboidal granulosa cells, accompanied by granulosa cell proliferation (Lintern-Moore and Moore, 1979). Primordial follicle numbers decrease as a function of aging, and their depletion culminates in the cessation of ovulation and reproductive senescence (menopause). Thus, the study of pathways regulating primordial follicle maintenance is necessary to shed light on the biological basis of menopause and several forms of female infertility characterized by accelerated primordial follicle depletion, such as primary ovarian insufficiency (Gosden et al., 1983; Nelson, 2009; Richardson et al., 1987).
PFA, unlike later stages of follicle maturation, is regulated largely within the ovary itself, and is independent of pituitary gonadotropins (Eppig and O’Brien, 1996; Mason et al., 1986; Matzuk et al., 2002; Peters et al., 1973). This and other findings have suggested that the oocyte communicates with and receives inputs from surrounding somatic cells (granulosa cells) and that these signals are integrated within the oocyte itself—i.e., the growth trigger mechanism functions within the oocyte itself. This general model was supported by the discovery of the forkhead transcription factor Foxo3 as a key regulator that functions within oocytes to regulate PFA (John et al., 2008). Foxo3-deficient mice complete primordial follicle development normally (John et al., 2007) but then undergo spontaneous global activation of all their primordial follicles immediately after their assembly is complete, leading to premature loss of all follicles and ensuing ovarian failure (Castrillon et al., 2003; Hosaka et al., 2004).
Consistent with their participation in diverse metabolic processes, the Foxo transcription factors are regulated by numerous post-translational modifications including phosphorylation, acetylation, and ubiquitination through a number of distinct biochemical pathways (van der Horst and Burgering, 2007). Thus, it was not initially clear which signaling pathway(s) might be relevant in the control of PFA via Foxo3. However, recent studies have provided strong evidence that the PI3K pathway is the critical signaling pathway regulating PFA via Foxo3. Foxo3 becomes phosphorylated at an Akt consensus phosphorylation sequence and this phosphorylation appears to mediate nuclear vs. cytoplasmic localization (John et al., 2008). Akt is regulated by PI3K, implicating the PI3K pathway as the critical signaling pathway regulating PFA via Foxo3. Genetic evidence supporting a role for PI3K in control of Foxo3 was obtained through oocyte-specific knockout of Pten (a potent inhibitor of PI3K signaling), which resulted in a global PFA phenotype closely resembling that observed in Foxo3 mice (John et al., 2008; Reddy et al., 2008). Importantly, Pten inactivation within the oocyte resulted in Akt and Foxo3 hyperphosphorylation, resulting in the nuclear export and inactivation of Foxo3. Thus, the PI3K pathway controls PFA by regulating Foxo3 phosphorylation and hence its subcellular localization (John et al., 2008).
In most physiologic contexts, PI3K activity is regulated by transmembrane receptor tyrosine kinases (RTK), raising the question of the identity of the relevant upstream receptor (and ligand) in the context of PFA. It seems likely that an RTK expressed on the oocyte surface, or perhaps multiple cooperating RTKs, would serve to integrate currently unidentified external signals produced by granulosa cells or other ovarian cellular compartments to regulate PFA throuth the PI3K/Akt/Foxo3 pathway.
Although several RTKs have been proposed to serve this function (reviewed in McLaughlin and McIver, 2009) a wide range of observations have made the Kit receptor a particularly attractive and favored candidate in this regard (Thomas and Vanderhyden, 2006). Kit and Kit ligand (Kitl) have well-documented functions in the germ cell lineage, being required for multiple stages of primordial germ cell survival, proliferation, migration, and differentiation (Bedell and Mahakali Zama, 2004). Kit signals through multiple pathways, including PI3K (Linnekin, 1999), which is especially important for Kit functions within the germline (De Miguel et al., 2002; Kissel et al., 2000; Serve et al., 1994). Kit is expressed on the primordial oocyte membrane, while KL is produced by granulosa cells, further making functional roles of this receptor/ligand pair in PFA plausible. In addition, certain alleles of the murine Kitl (a.k.a. Steel (Sl)) locus result in severe ovarian defects. Female mice homozygous for the hypomorphic Kitl allele Steelpanda (KitlSl-pan), which results in decreased ovarian expression of Kitl due to a translocation breakpoint involving the Kitl upstream regulatory region, form ovaries with follicles that become arrested at an early stage of maturation (Bedell et al., 1995; Besmer et al., 1993; Huang et al., 1993). Other combinations of Kitl alleles have similar phenotypic consequences (Kuroda et al., 1988). Although these phenotypes have in the past been interpreted as evidence for a role of Kitl in PFA, the severe and pleiotropic ovarian phenotypes associated with these alleles has obscured their interpetation (Bedell et al., 1995; Huang et al., 1993).
In addition to these genetic studies, antibody-mediated blockade of Kit reportedly disrupts primordial follicle growth in vivo (Yoshida et al., 1997) and in vitro (Packer et al., 1994), and recombinant Kitl induces PFA in cultured ovaries (Parrott and Skinner, 1999; Thomas et al., 2008). On the other hand, the observed effects have been small in magnitude or carried out with in vitro systems that can be maintained for only short intervals and thus provide only an extremely limited timescale for the study of PFA. In addition, some of these studies have not distinguished between oocyte growth in general and PFA per se.
Kit activates PI3K through a direct interaction with an SH2 domain on the p85 regulatory subunit of PI3K. This interaction is entirely dependent on Kit tyrosine residue 719, which undergoes autophosphoryolation following ligand binding. Mutation of this tyrosine residue prevents binding of Kit to p85 and thus completely abrogates Kit signaling via PI3K (Serve et al., 1994). “Knock-in” mice harboring this mutation (KitY719F) have been engineered by homologous recombination, permitting a formal genetic dissection of the contribution of PI3K signaling to diverse Kit-dependent biological processes (Blume-Jensen et al., 2000; Kissel et al., 2000). Homozygous KitY719F males are sterile with severe defects in spermatogenesis, demonstrating an important role of PI3K-dependent Kit signaling in the context of germ cell function. However, the impact of this mutation on PFA has not been formally evaluated. In light of the recent demonstration that PI3K is the prime regulator of Foxo3 and PFA we were prompted to perform detailed analyses of KitY719F ovaries in vivo. These analyses were complemented by studies of the impact of pharmacologic inhibition of Kit through the use of imatinib in vitro. The results also prompted us to re-evaluate the ovarian phenotype associated with the KitlSl-pan mutation. Surprisingly, our findings suggest that Kit plays a relatively minor and dispensable role in PFA, arguing that other receptors contribute to this process. However, we uncovered other essential roles of Kit-dependent PI3K signaling in the ovary, namely, in the maintenance of primordial follicles and in the transition of primary to secondary follicles. The latter phenotype was associated with abnormal stabilization of the Foxo3 protein in the oocyte nucleus.
Materials and methods
Mouse Strains, Breeding, and Analysis
This study was approved by an Institutional Animal Care and Use Committee. Generation and characterization of the KitlSl-pan and KitY719F alleles was described previously (Bedell et al., 1995; Huang et al., 1993; Kissel et al., 2000). Ovaries for organ culture were obtained from newborn mice (Jackson Laboratories). Ovaries from at least n = 3 experimental and n = 3 control animals were evaluated for each timepoint in all analyses.
Tissue Processing, Immunohistochemistry and Immunofluorescence
Tissue sections from experimental and control samples were placed on the same slide to ensure identical processing; at least n = 3 slides were evaluated for each antibody. For immunohistochemistry, tissues were fixed in 10% formalin 1–12 hours, then processed and embedded in paraffin. 5 μ sections were deparaffinized in xylene, and hydrated in an ethanol series. Slides were subjected to antigen retrieval by boiling in 10mM NaCitrate and cooled at RTx20 min. Antibodies and titers for immunohistochemistry were: Foxo3 1:200 (Santa Cruz # sc-11351), p-Foxo3 (Thr32) 1:200 (Upstate # 07-695); Ki67 1:50 (Lab Vision) Inhibin 1:200 (Lab Vision); p-Akt (Ser473) 1:50 (Cell Signaling # 9271) and t-Akt 1:1000 (Cell Signaling # 9272). The detection system was Immpress (Vector, Burlingame, CA) or Envision Plus (Dako, Carpinteria, CA). TUNEL was performed with the Apoptag kit from Chemicon International (Temecula, California) per the manufacturer’s instructions.
Organ Culture and Histomorphometry, and Follicle Counts
Ovaries were photographed after explantation and at 4 and 8 days and the relative ovarian volume was approximated by the equation V = (long diameter × short diameter2). Average oocyte diameter was determined on H&E tissue sections per the long diameter of oocytes with nuclei in the plane of section (at least n>100 such oocytes were analyzed per ovary). Follicle counts were performed on H&E stained tissue sections of entirely serially-sectioned ovaries. Only follicles where the oocyte nucleus was in the plane of section were counted. Every other section was used for counts of primordial and primary follicles, every 6th section for secondary, antral and atretic follicles and every 10th section for corpora lutea. A total of n=3 mice for each genotype and age were counted (total of 6 ovaries). For ovarian culture experiments, explanted ovaries were placed (37C, 5% CO2) on Transwell Permeable supports (Costar, catalog # 3413) in Waymouth’s medium (Invitrogen catalog # 11220-035) supplemented with 10% fetal bovine serum, 0.23 mM Pyruvic acid, 3 mg/ml BSA, 1x ITS supplement (Invitrogen, catalog # 51300-044) and 1x Antibiotic-Antimycotic (Invitrogen catalog # 15240-062). Kit inhibition was performed by addition of 3 μM of Imatinib (Gleevec, Novartis) to the media.
Results
KitlY719F females are born with a normal complement of primordial follicles that undergo PFA
To understand the role of Kit signaling in PFA, a PI3K-dependent process, females homozygous for the KitY719F “knock-in” allele were generated through breeding of heterozygotes, which are phenotypically normal (Kissel et al., 2000). Homozygous mice were born at expected Mendelian ratios and were externally normal and in good overall health. At postnatal day (PD) 3, KitY719F/KitY719F ovaries were of normal size relative to wildtype ovaries (n=3) (Fig. 1A and data not shown). Primordial follicle assembly is normally completed by PD3; at this timepoint, primordial follicle numbers and morphology were indistinguishable in wt vs. mutant ovaries, indicating that germ cell function and oogenesis were normal up to this stage (Fig. 1A–B, E).
Figure 1.
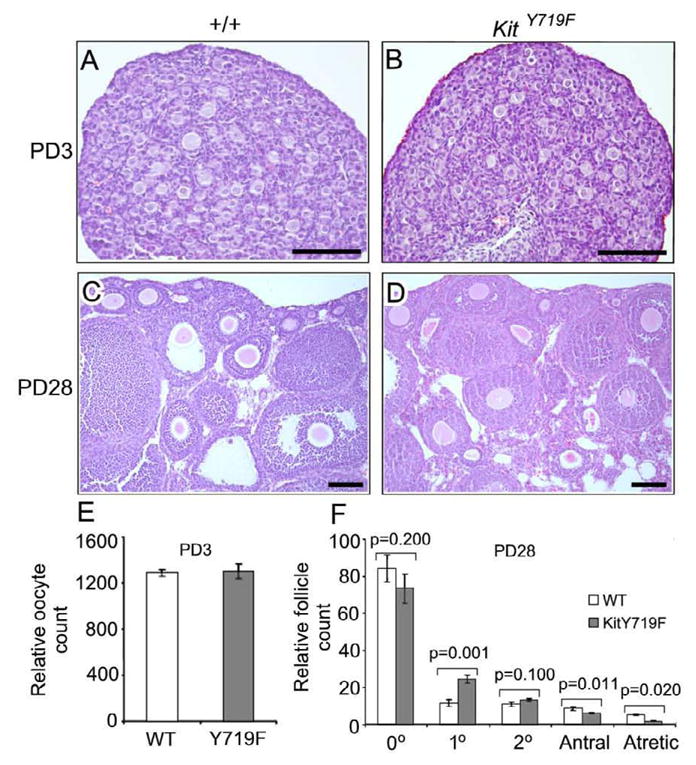
Analysis of KitY719F/KitY719F and sibling control (+/+) ovaries at postnatal day 3 reveals a normal endowment of primordial follicles and a lag in the transition of primary to secondary follicles but no evidence of decreased PFA. A–D) H&E stained tissue sections, bars = 100 microns. E) Relative oocyte counts in serially-sectioned ovaries (n=6 ovaries per genotype; error bars=s.e.m.). F) Relative follicle counts in serially sectioned ovaries (n=6 ovaries per genotype; error bars=s.e.m.). Note: 0°=primordial, 1°=primary, 2°=secondary.
By PD28 (prior to sexual maturity) wild-type ovaries contained significant numbers of growing follicles at various stages of maturation, consistent with the fact that PFA begins at around PD3. Detailed histologic analyses failed to reveal any defects in PFA at PD28 in mutant ovaries, which contained large numbers of primary, secondary, and more advanced follicles (Fig. 1C, D). Follicle counts in serially-sectioned ovaries did reveal some significant abnormalities in mutant ovaries, however. Primordial follicle numbers were slightly decreased, although this was not statistically significant (p=0.200, Fig. 1F). However, there was a significant increase (>2x) in the number of morphologically normal primary follicles (p=0.001).
Abnormal primordial follicle maintenance and early follicle growth due to deficient PI3K signaling downstream of Kit
To identify more subtle or cumulative defects in follicle maturation that might become evident only in older females, ovaries at 16 weeks of age were analyzed. KitY719F/KitY719F ovaries contained some normal follicles of all stages of maturation, but distinct abnormalities were identified. There was an accumulation of abnormal follicles arrested at an early stage of development, as previously reported (Kissel et al., 2000). These abnormal follicles accumulated throughout the ovarian stroma (Fig. 2A,B). Morphologically, these abnormal, apparently growth-arrested follicles were morphologically intermediate between primary (single layer of granulosa cells) and secondary follicles (two layers of granulosa cells). The oocytes showed minimal enlargement, but all of the associated granulosa cells had adopted a cuboidal morphology (Fig. 2C,D). This demonstrates that the follicles had undergone PFA, but were arrested at the transition of the primary to the secondary stage. Some of these follicles exhibited “pseudoantral” spaces, suggesting that the granulosa cells continued to differentiate to some extent despite their apparent growth arrest (antrum formation normally takes place in much more advanced larger follicles). The accumulation of these morphologically abnormal follicles indicates that they persist as stable, long-lived structures and don’t undergo atresia despite their growth arrest. These findings are consistent with prior studies, e.g. (Dong et al., 1996; Trombly et al., 2009; Yang and Fortune, 2006; Yang and Fortune, 2007), that argue for the existence of a distinct primary to secondary follicle checkpoint. Despite the decreased numbers of primordial follicles and the observed lag in follicle maturation, KitY719F/KitY719F females were fertile at 16 weeks of age. In single pair mating assays performed at 16 weeks, mutant females had an average of 7.0 viable progeny vs. 6.8 for +/+ siblings (n=5 females per genotype). This result further underscores the lack of an overt PFA phenotype in KitY719F ovaries.
Figure 2.
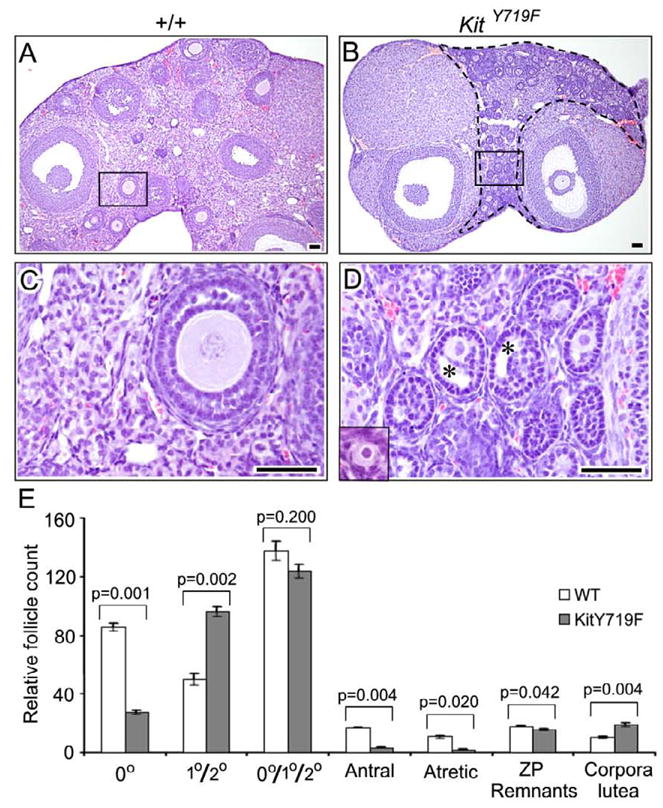
Analysis of KitY719F/KitY719F and sibling control (+/+) ovaries at 16 weeks reveals defects in primordial follicle survival/maintenance and in early follicle maturation (primary to secondary follicle transition). A–D) H&E stained tissue sections, bars = 50 microns. B) Abundance of morphologically abnormal follicles arrested at the primary/secondary follicle stage within the area enclosed by a dashed line. C–D) Higher magnification in boxed areas shown in A and B; insets show morphologically normal primordial follicles from elsewhere in each section. E) Relative follicle counts in serially sectioned ovaries (n=6 ovaries per genotype; error bars=s.e.m.). Due to the difficulty of reliably distinguishing primary from secondary follicles (because of abnormal maturation phenotype), primary + secondary follicles were counted together. P values were calculated per the student t test.
Follicle counts of serially-sectioned ovaries (n=3) showed a significant (approximately 3-fold) reduction in primordial follicle numbers (p=0.001), and also an increase in abnomal primary/secondary follicles (p=0.002), as discussed above. The increased numbers of primary/secondary follicles matched the decrease in primordial follicles, such that the sum of primordial plus primary/secondary follicles was similar in wt and mutant ovaries (Fig. 2E). There were also significant decreases in antral and atretic follicles, consistent with the arrest phenotype and limited progression to these more advanced stages in KitY719F/KitY719F ovaries. Somewhat unexpectedly given these results, mutant ovaries also harbored an increased number of corpora lutea (p=0.004). The basis of this abnormality has not been further explored, but raises the possibility that Kit also regulates the growth or persistence of corpora lutea (Brankin et al., 2004).
Growth arrest of primary/secondary follicles in KitY719F/KitY719F ovaries is associated with abnormal retention and stabilization of Foxo3 protein within oocyte nucleus
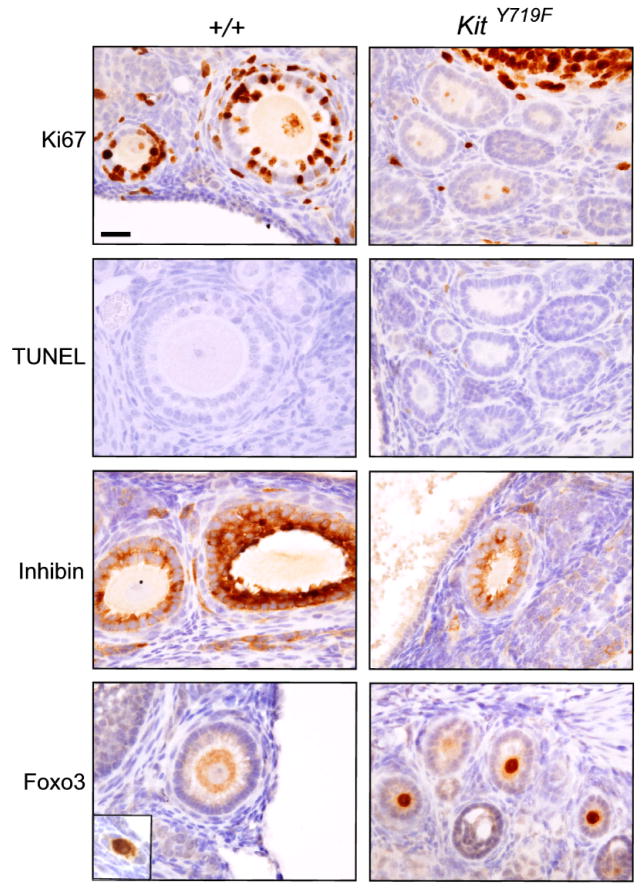
To gain further insights into the observed defects in follicle maturation, the expression of several markers was analyzed. Granulosa cells in normal follicles are actively proliferating, as evidenced by a high rate of positivity for the proliferation marker Ki67. In contrast, Ki67 immunostaining of mutant ovaries showed that granulosa cells in the growth arrested follicles had ceased proliferating (Fig. 3). Consistent with their apparent long-term stability (Fig. 3), there was no increase in apoptosis in either the granulosa cells or oocytes of the growth-arrested follicles by TUNEL. Markers such as inhibin that are induced following follicle activation and are detectable at the primary follicle stage were unaltered in mutant follicles of comparable stage (Fig. 3).
Figure 3.
Characterization of early follicle maturation defect in ovaries from adult (16 week KitY719F/KitY719F and sibling (+/+) controls. Tissue sections were stained for various markers as indicated. For Ki67, positivity of scattered cells serves as internal positive control; for TUNEL stains, positive cells were present elsewhere in the slide (not shown). Inhibin is expressed at similar levels in mutant and control follicles of comparable size and stage (a more advanced secondary follicle on the right side of the +/+ panel shows higher expression). Foxo3 localizes to the nucleus in primordial oocytes (inset) and shifts to the oocyte cytoplasm in primary follicles (+/+) but is abnormally retained within the nucleus of lagging primary/secondary follicles in KitY719F/KitY719F ovaries.
During normal ovarian development, Foxo3 protein is initially cytoplasmic but becomes translocated into the nucleus during primordial follicle assembly. The Foxo3 protein remains nuclear in primordial follicles throughout life, where it serves to suppress their growth. Following PFA, the Foxo3 protein shuttles from the nucleus to the cytoplasm (Fig. 3), and then becomes degraded during the secondary follicle stage (John et al., 2008). In KitY719F/KitY719F ovaries, Foxo3 export and degradation did not occur; instead Foxo3 remained distinctly nuclear. This suggests that Foxo3 exit from the nucleus is required for the primary to secondary follicle transition. Strongly supporting this idea, transgenic mice with enforced expression of Foxo3 are subfertile and exhibit early follicular maturation defects that resemble those of KitY719F/KitY719F mice (Liu et al., 2007). Our interpretation of these findings is that nuclear export of Foxo3 is required for the primary to secondary follicle transition, and that Kit plays an important function in regulating this PI3K-dependent process. It is suprising, however, given previous studies, that Kit signaling through PI3K is dispensable for PFA itself.
Reevaluation of the Steelpanda allele of Kit ligand (KitlSl-pan) reveals a severe defect in the primary to secondary follicle transition with abnormal nuclear Foxo3 retention, but not a deficiency of PFA per se
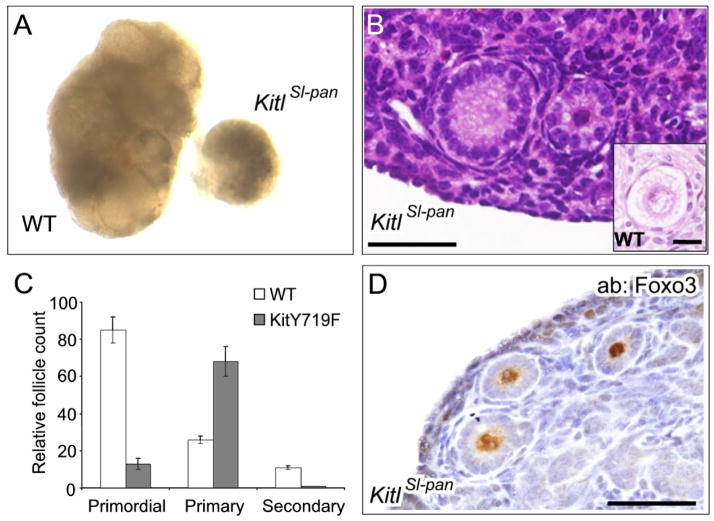
The unexpected finding that the KitY719F mutation did not prevent PFA prompted a reevaluation of the phenotype associated with the Steelpanda allele of Kit ligand (KitlSl-pan) particularly as its phenotype has been interpreted by some investigators as evidence supporting a role for Kit in PFA. As previously described, KitlSl-pan/KitlSl-pan ovaries at 3 weeks of age were much smaller than sibiling controls (Bedell et al., 1995; Huang et al., 1993), reflecting a significantly diminished initial endowment of primordial follicles (Fig. 4A). Histologic examination of these ovaries revealed a sparsity of primordial follicles, as previously reported. However, morphologic analysis of the remaining follicles revealed a growth arrest not at the primordial to primary follicle transition (which would represent evidence for a defect in PFA) but rather at the primary to secondary follicle transition, akin to what was observed in KitY719F/KitY719F mice. The vast majority of the follicles in KitlSl-pan/KitlSl-pan ovaries were associated with a distinct layer of cuboidal granulosa cells that were increased in number relative to a normal (unactivated) primordial follicle (Fig. 4B, see also inset). Follicle counts of serially-sectioned ovaries (n=3) confirmed that the great majority of follicles in KitlSl-pan/KitlSl-pan ovaries were arrested at the primary, not the primordial stage (Fig. 4C). Only rare secondary follicles were observed, and none beyond the secondary follicle stage. These results are consistent with our analysis of KitY719F/KitY719F mice in that both mutations resulted in an arrest at the primary to secondary follicle stage, pointing to a specific role of Kit-PI3K signaling in promoting the primary to secondary follicle transition. Immunolocalization of the Foxo3 protein in KitlSl-pan/KitlSl-pan ovaries again showed retention and stabilization of nuclear Foxo3, further supporting the notion that Kit regulates the primary to secondary follicle transition through the nuclear retention/stabilization of Foxo3. Thus, although the pleiotropic and severe nature of the KitlSl-pan/KitlSl-pan phenotype does obscure its interpretation, rigorous morphologic analysis does not provide compelling evidence for a defect in PFA itself but rather further support roles in primordial follicle survival/maintenance and in the primary to secondary follicle transition.
Figure 4.
Evaluation of KitlSl-pan/KitlSl-pan ovaries reveals defect in primary to secondary follicle transition associated with abnormal Foxo3 nuclear retention and stabilization. A) KitlSl-pan ovaries are highly atrophic due to pleitropic defects in primordial follicle numbers and follicle maturation. B) H&E of tissue section showing predominant follicular morphology. Most follicles in KitlSl-pan/KitlSl-pan ovaries are arrested at the primary, not the primordial follicle stage, as evidenced by the cuboidal morphology and numbers of granulosa cells associated with each follicle (inset: normal primordial follicle from control ovary). C) Relative follicle counts in serially-sectioned ovaries (n=6 ovaries per genotype; error bars=s.e.m.). D) Foxo3 immunohistochemistry reveals nuclear retention of Foxo3 in KitlSl-pan ovaries. Bars for B and D = 50 microns.
In vitro pharmacologic inhibition of Kit results in decreased PFA
To further explore roles of Kit in the regulation of PFA and to attempt to reconcile these in vivo observations with prior in vitro studies, we performed studies with the Kit small molecule inhibitor imatinib mesylate (trade name, Gleevec). Treatment of ovaries explanted from newborn wild-type pups resulted in a small but statistically significant decrease in ovarian size after 8 days in culture (Fig. 5A,B). Furthermore, this decrease in ovarian size correlated with a decreased average oocyte diameter (Fig. 5C,D). Western analysis showed decreased Foxo3 phosphorylation (Fig. 5E). One significant technical limitation is that in situ (immunohistochemical) and Western approaches proved of insufficient sensitivity to detect Kit autophosphorylation and thus document Kit inhibition, whereas we were readily able to document Kit inhibition in Mo7e cells (data not shown). With the additional caveat that this high dose may inhibit a variety of other related RTKs or possibly unrelated kinases, and may also produce other non-specific toxic effects at the level of the oocyte or other ovarian compartments, these results are consistent with previous in vivo studies (discussed below).
Figure 5.
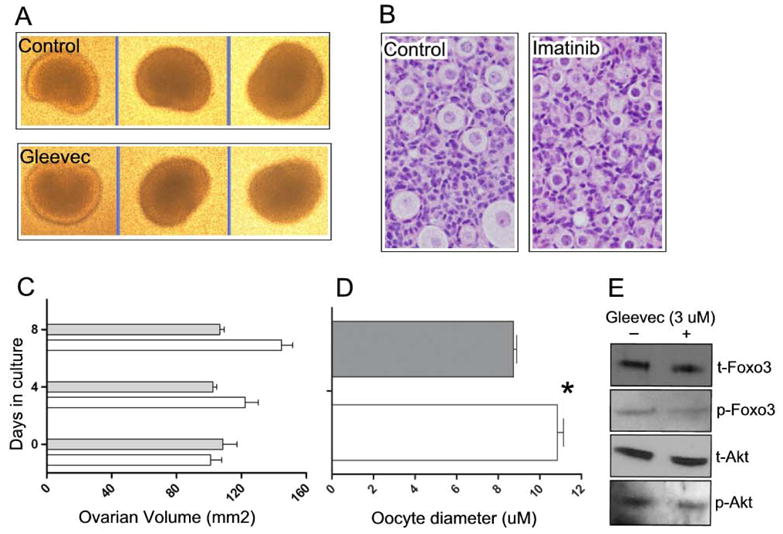
Effects of imatinib treatment on ovary and follicle size in vitro. Ovaries were explanted on the day of birth and placed in culture media +/− 3 μM imatinib. A) Representative photographs of ovaries after 8 days of culture. B) H&E stained sections of treated and untreated ovaries. Imatinib treatment results in decreased average oocyte size. C) Ovarian volume in untreated vs. treated ovaries. Imatinib treatment results in decreased average ovarian volume, (n=3 ovaries; error bars = s.e.m.). D) Average oocyte diameters (n=3 ovaries; error bars = s.e.m.). Asterisk indicates P value <0.05 per student’s t test. For C and D, grey bars = imatinib treated; white bars = untreated controls. E) Western analyses of imatinib-treated and untreated controls. t=total protein; p=phosphorylated protein.
Discussion
This study demonstrates that inactivation of Kit signaling via the PI3K pathway results in decreased primordial follicle survival and the abnormal accumulation of ovarian follicles arrested at an early stage of maturation. Surprisingly, however, the KitY719F mutation previously shown to disrupt binding of Kit to PI3K (and hence Kit-induced activation of PI3K (Blume-Jensen et al., 2000; Kissel et al., 2000)) did not result in a significant failure of PFA, as would be expected if Kit were the primary upstream receptor controlling this biological process. To the contrary, KitY719F/KitY719F female mice appear to undergo PFA normally, and adult females contain large numbers of growing follicles. Consistent with these results, we also found that a Kitl mutation that results in decreased ovarian levels of Kitl (Bedell et al., 1995; Huang et al., 1993) similarly affects primordial follicle survival and the primary to secondary follicle transition, but not PFA per se. These findings suggest that other upstream receptors may serve this function. Previous studies showing that increased PI3K signaling triggered by an oocyte-specific Pten gene knockout promotes global PFA (John et al., 2008; Reddy et al., 2008) argues against the alternative hypothesis that Kit is the overriding upstream receptor regulating PFA but that it acts through other (i.e. non-PI3K) signaling pathways.
There are two possible interpretations of the accelerated depletion of primordial follicles observed in the KitY719F mutant. First, the observed depletion may be due to decreased survival of primordial oocytes. This is an attractive hypothesis because Kit and Kitl are well-established survival factors for germ cells at various stages of development, particularly primordial germ cells (Bedell and Mahakali Zama, 2004). TUNEL analyses at 12 weeks and earlier timepoints failed to identify increased apoptosis in primordial oocytes of KitY719F mutant mice (data not shown). However, an increase in oocyte apoptosis in KitY719F ovaries would be cumulative and very gradual (at 16 weeks one-third of primordial follicles still remain). Thus the increased rate of apoptosis would likely be below limits of detection, particularly as the rate of primordial oocyte apoptosis in normal adult ovaries is low (Tilly, 2001). It is also formally possible that the observed depletion is due to increased PFA (i.e. that Kit signaling, counter to expectations, normally suppresses PFA), because the decrease in primordial follicles numbers roughly approximates the observed increase in primary+secondary follicles (Fig. 2E). However, this is more likely a coincidence due to the block in the transition of primary to secondary follicles. Consistent with this idea, imatinib treatment of cultured ovaries was not associated with an increased oocyte diameter (Fig. 5). Thus, we favor the idea that Kit serves an important physiologic function as a pro-survival factor for primordial oocytes.
We also observed that the Foxo3 protein is abnormally stabilized within the oocyte nucleus in both the KitY719F and KitlSl-pan mutants, arguing that Kit signaling through PI3K is important for regulating the primary to secondary follicle transition, and furthermore, that Foxo3 is a negative regulator of the primary to secondary follicle transition in addition to PFA. This suggests that Foxo3 degradation promotes the primary to secondary follicle transition, a finding that is entirely consistent with an earlier study showing that enforced Foxo3 expression in oocytes results in an arrest at the primary follicle stage (Liu et al., 2007). Thus, while PFA appears to be Kit-independent, the primary to secondary follicle transition clearly is not.
The results of the imatinib studies were consistent with previous in vitro studies showing a growth inhibition with various Kit inhibitory treatments (Driancourt et al., 2000; Huang et al., 1993; Packer et al., 1994; Parrott and Skinner, 1999; Thomas et al., 2008; Thomas and Vanderhyden, 2006; Yoshida et al., 1997). The results could be reconciled with the in vivo genetic analyses by hypothesizing that Kit is essential for PFA but that it signals through multiple downstream pathways, such that the PI3K pathway is nonessential. This however appears to be at odds with the Pten knockout studies discussed above. Despite the high concentrations of imatinib employed, it is possible that effective concentrations were not achieved within oocytes. For example, Mdr1, the ATP-dependent transporter that exports small-molecule drugs from chemoresistant tumor cells) is normally expressed at very high levels within oocytes and functions to limit the accumulation of various drugs within oocytes (Elbling et al., 1993; Gallardo et al., 2007). It would thus be highly desirable for these studies to be able to document effective inibition of Kit within the oocyte. In principle, this could be performed through in situ immunodetection of phosphorylated Kit protein, but currently available phospho-specific antibodies were not sufficiently specific and/or sensitive (data not shown). We also cannot exclude the possibility that the relatively high concentrations of imatinib inhibited other kinases, and thus, that the observed results are non-specific.
In summary, our analyses of two distinct mutants provide evidence that Kit functions via PI3K to promote the survival of primordial oocytes and the transition of primary to secondary follicles. Although in vitro studies provide some support for a role of Kit in promoting PFA, in vivo genetic analyses argue that Kit is nonessential in engaging the PI3K pathway to regulate PFA. The identification of upstream components and presumptive extracellullar signals regulating PFA remains an outstanding challenge in ovarian biology that will benefit from refinements in genetic and other experimental approaches.
Acknowledgments
We thank Teresa Gallardo, Lane Shirley, and Marshall Haynie for technical support and helpful discussions, and Mary Bedell for comments on the manuscript. The project described was supported by Award Number R01HD048690 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health. This work was also supported through the National Center for Research Resources (K26RR024196) and grants from the Lance Armstrong Foundation and the Flight Attendant Medical Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedell MA, Brannan CI, Evans EP, Copeland NG, Jenkins NA, Donovan PJ. DNA rearrangements located over 100 kb 5′ of the Steel (Sl)-coding region in Steel-panda and Steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development. Genes Dev. 1995;9:455–70. doi: 10.1101/gad.9.4.455. [DOI] [PubMed] [Google Scholar]
- Bedell MA, Mahakali Zama A. Genetic analysis of Kit ligand functions during mouse spermatogenesis. J Androl. 2004;25:188–99. doi: 10.1002/j.1939-4640.2004.tb02779.x. [DOI] [PubMed] [Google Scholar]
- Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl. 1993:125–37. [PubMed] [Google Scholar]
- Blume-Jensen P, Jiang G, Hyman R, Lee KF, O’Gorman S, Hunter T. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat Genet. 2000;24:157–62. doi: 10.1038/72814. [DOI] [PubMed] [Google Scholar]
- Brankin V, Hunter MG, Horan TL, Armstrong DG, Webb R. The expression patterns of mRNA-encoding stem cell factor, internal stem cell factor and c-kit in the prepubertal and adult porcine ovary. J Anat. 2004;205:393–403. doi: 10.1111/j.0021-8782.2004.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- De Miguel MP, Cheng L, Holland EC, Federspiel MJ, Donovan PJ. Dissection of the c-Kit signaling pathway in mouse primordial germ cells by retroviral-mediated gene transfer. Proc Natl Acad Sci U S A. 2002;99:10458–63. doi: 10.1073/pnas.122249399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–5. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod. 2000;5:143–52. doi: 10.1530/ror.0.0050143. [DOI] [PubMed] [Google Scholar]
- Elbling L, Berger W, Rehberger A, Waldhor T, Micksche M. P-glycoprotein regulates chemosensitivity in early developmental stages of the mouse. FASEB J. 1993;7:1499–506. doi: 10.1096/fasebj.7.15.7903262. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biology of Reproduction. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Gallardo TD, John GB, Shirley L, Contreras CM, Akbay EA, Haynie JM, Ward SE, Shidler MJ, Castrillon DH. Genomewide discovery and classification of candidate ovarian fertility genes in the mouse. Genetics. 2007;177:179–94. doi: 10.1534/genetics.107.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28:255–60. doi: 10.1095/biolreprod28.2.255. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–80. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157:100–9. doi: 10.1006/dbio.1993.1115. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133:855–63. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow SR, Manova K, Besmer P. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. Embo J. 2000;19:1312–26. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda H, Terada N, Nakayama H, Matsumoto K, Kitamura Y. Infertility due to growth arrest of ovarian follicles in Sl/Slt mice. Dev Biol. 1988;126:71–9. doi: 10.1016/0012-1606(88)90240-0. [DOI] [PubMed] [Google Scholar]
- Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–74. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Lintern-Moore S, Moore GP. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod. 1979;20:773–8. doi: 10.1095/biolreprod20.4.773. [DOI] [PubMed] [Google Scholar]
- Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134:199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–71. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–80. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11. doi: 10.1530/REP-08-0118. [DOI] [PubMed] [Google Scholar]
- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c-kit receptor promotes oocyte growth. Dev Biol. 1994;161:194–205. doi: 10.1006/dbio.1994.1020. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–71. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Himelstein-Braw R, Faber M. Follicular growth: the basic event in the mouse and human ovary. J Reprod Fertil. 1975;45:559–66. doi: 10.1530/jrf.0.0450559. [DOI] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Lintern-Moore S, Faber M, Andersen M. The effect of gonadotrophin on follicle growth initiation in the neonatal mouse ovary. J Reprod Fertil. 1973;35:139–41. doi: 10.1530/jrf.0.0350139. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–3. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–7. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Serve H, Hsu YC, Besmer P. Tyrosine residue 719 of the c-kit receptor is essential for binding of the P85 subunit of phosphatidylinositol (PI) 3-kinase and for c-kit-associated PI 3-kinase activity in COS-1 cells. J Biol Chem. 1994;269:6026–30. [PubMed] [Google Scholar]
- Thomas FH, Ismail RS, Jiang JY, Vanderhyden BC. Kit ligand 2 promotes murine oocyte growth in vitro. Biol Reprod. 2008;78:167–75. doi: 10.1095/biolreprod.106.058529. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Vanderhyden BC. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol. 2006;4:19. doi: 10.1186/1477-7827-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–48. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod. 2006;75:924–32. doi: 10.1095/biolreprod.106.051813. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol Reprod Dev. 2007;74:1095–104. doi: 10.1002/mrd.20633. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol. 1997;184:122–37. doi: 10.1006/dbio.1997.8503. [DOI] [PubMed] [Google Scholar]