Abstract
Leukocyte recruitment to tissues and organs is an essential component of host defense. The molecular mechanisms controlling this process are complex and remain under active investigation. The combination of biochemical techniques and live cell imaging using in vivo and in vitro flow model approaches have shed light on several aspects of neutrophil transmigration through the vascular endothelial lining of blood vessels. Here we focus on the role of adhesion molecule signaling in endothelial cells and their downstream targets during the process of transendothelial migration at cell-cell borders (paracellular transmigration). An emerging model involves leukocyte β2 integrin engagement of endothelial cell ICAM-1, which triggers integrin-ICAM-1 clustering (rings) and stabilizes leukocyte adhesion at cell-cell junctions. This step recruits nonreceptor tyrosine kinases that phosphorylate key tyrosine residues in the cytoplasmic tail of VE-cadherin, which destabilizes its linkage to catenins and the actin cytoskeleton, triggering the transient opening of VE-cadherin homodimers to form a gap in the cell junction, through which the neutrophil transmigrates. Interestingly, the signaling events that lead to neutrophil transmigration occur independent of shear flow in vitro.
Keywords: Inflammation, VE-cadherin, transmigration, src kinases, ICAM-1.
Introduction
Inflammation is a protective response in which blood leukocytes are recruited to vascularized tissues to remove injurious or infectious agents and then initiate the process of tissue repair. Acute inflammation is the earliest response, occurring in minutes or hours, and involves the influx of leukocytes, primarily polymorphonuclear leukocytes (neutrophils). If acute inflammation is self-limiting, then inflammation resolves and tissues are replaced by regeneration or scarring (or by a combination of both) through processes that are as yet incompletely understood (reviewed in (7,54)). On the other hand, chronic inflammation is characterized as inflammation extending over a sustained interval, with active inflammation and tissue destruction and repair occurring simultaneously. Chronic inflammatory leukocytes include blood monocytes, tissue macrophages, and lymphocytes (mononuclear leukocytes). In this review, we will address the recent advances in our understanding of blood neutrophil interactions with the vascular endothelium at sites of inflammation and ultimately transendothelial migration (also termed diapedesis) across the vessel lining.
During inflammation or injury, the peripheral vascular endothelium becomes “activated” and undergoes a dramatic alteration in phenotype, including conversion to a proadhesive surface to recruit circulating leukocytes. It is now well established that the vascular endothelium is an active participant in leukocyte recruitment and plays a critical role. Inflammatory cytokines, certain Gram-negative bacterial endotoxins, oxidized LDL, and many other stimuli initiate transcription-dependent induction and surface expression of adhesion molecules (Fig. 1) and release and/or presentation of chemokines by endothelial cells. This process involves activation of various combinations of transcription factors including NF-κB, Sp1, IFN regulatory factor-1, GATA motifs, and scaffold factors such as high-mobility group-I(Y) (HMG-1[Y]) (21). Further regulation of adhesion molecule expression has recently been shown to involve microRNAs (33).
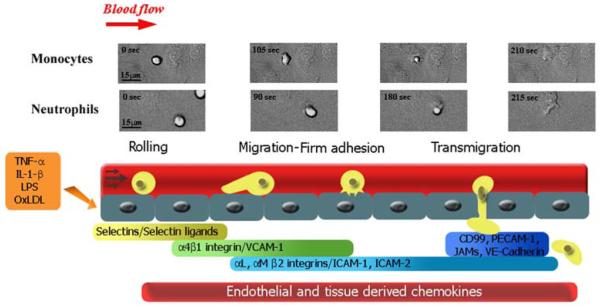
Fig legend 1. Sequence of steps during leukocyte recruitment culminating in transendothelial migration.
Circulating monocytes and neutrophils in the bloodstream initially attach to activated endothelium by selectin-mediated and α4β1 integrin-mediated interactions involved in the initial rolling step. The leukocytes locomote on the endothelial cell vessel wall by establishing Mac-1 integrin-mediated interactions with ICAM-1 on the endothelial cells until they firmly arrest near cell-cell junctions. This step is followed by stronger interactions mediated by several endothelial cell molecules besides ICAM-1, in which the leukocyte squeezes through the endothelial cell junction. Frames corresponding to live cell imaging digital microscopy show monocytes and neutrophils interacting with 4-hr TNF-α-activated HUVEC monolayers under flow conditions in vitro.
It is notable that neither monocytes nor neutrophils require laminar shear flow conditions to adhere to or transmigrate across the endothelium in vitro. One can observe robust levels of adhesion and transmigration under static conditions; however, leukocyte rolling, initial attachment, and the mechanisms that regulate these steps cannot be studied. In addition, there is a significant literature on the effects of shear on leukocytes (reviewed in (45)) and endothelial cells (22,27). The effects of fluid shear on leukocytes are interesting because physiological levels of fluid shear in the absence of stimuli diminish both neutrophil membrane adhesive pseudopod formation (49) and the level of cell surface β2 integrins adhesion molecules. The reduction in β2 integrin adhesion molecules is mediated in part by proteolytic cleavage through neutrophil-released cathepsin B (70). Both responses serve to decrease adhesion of circulating blood leukocytes. This regulatory function of shear stress may be most important during leukocyte passage through capillaries and small postcapillary venules. For a more complete discussion of the effects of shear stress on leukocytes, the reader is directed to the recent review by Makino and colleagues (45).
Current paradigm of circulating leukocyte capture by activated endothelium under shear flow conditions
Recruitment of leukocytes in laminar shear flow involves a sequential, multistep adhesion cascade (13). The initial step consists of leukocyte rolling on activated endothelium. This step is followed by leukocyte locomotion, then arrest, which precede transmigration under laminar fluid shear stress conditions (Fig. 1). In general, blood leukocytes follow this sequential cascade, although some differences are found among them in terms of the requirements for transendothelial migration in vivo and in vitro. In in vitro flow models, monocytes and neutrophils attach mostly (>70%) at cell-cell borders and therefore only migrate short distances on the apical surface before transmigrating at a junction (12,29,68,96). The propensity for neutrophils to adhere at or close to cell-cell junctions was also observed in inflamed mouse cremaster venules, where 75% of firmly adhered neutrophils overlapped an endothelial cell junction (92). More recent studies have refined this idea and identified a role for specific leukocyte adhesion molecules. The leukocyte β2 integrin Mac-1 was required for crawling of murine neutrophils to optimal emigration sites at the cell-cell borders in vivo (58). In addition Mac-1 mediated a similar process for human monocytes during transmigration in an in vitro model (66), although the endothelial cell ligand(s) were not identified in either report. In contrast, under flow conditions in vitro, human T lymphocytes do not behave the same. Importantly, T cells attach randomly on the apical endothelial cell surface in vitro, and their locomotion on the apical surface step is much more extensive than monocytes or neutrophils and lasts longer. Another significant difference between neutrophils and monocytes is that T cell transmigration requires shear flow and the presence of an apical chemokine (19). The reader is referred to the article in this issue by Alon and colleagues for discussion of T cell recruitment.
Leukocytes are also able to transmigrate at nonjunctional locations, as demonstrated in in vivo and in vitro models ((14,20,26,48,96); reviewed in (25)). The factors that influence the route, that is, junctional (paracellular) versus nonjunctional (transcellular), used for transmigration have yet to be firmly established but are probably influenced by the state of endothelial cell activation and endothelial cell shape (96), stability of endothelial junctions, local shear flow conditions, and location of vascular bed (41,51) or vessel wall matrix protein composition (86). The extent of transcellular transmigration was variable and depended on the leukocyte type and the anatomical site from which endothelial cells were isolated; microvascular cells showed a higher rate of T cell transcellular transmigration (48). This review will focus on leukocyte transmigration at cell-cell junctions.
Endothelial cell adhesion molecules that participate in junctional transendothelial migration of leukocytes
Several endothelial transmembrane proteins have been shown to be involved in leukocyte diapedesis and transmigration (Fig. 1). These proteins differ in their expression levels, and the ability of certain cytokines to affect their expression. Many are enriched at endothelial cell-cell contacts, which may explain, in part, why leukocytes transmigrate preferentially at cell-cell borders (i.e., specialized sites). Below we review some of the known adhesion molecules involved in neutrophil transmigration; we refer the reader to recent reviews and the references therein that cover more extensively the molecules involved in leukocyte recruitment (43,44,62,83).
PECAM-1 and membrane recycling in transmigration
PECAM-1 (CD31) has been demonstrated to play a role in leukocyte transmigration in vivo (65) and in vitro (53). The salient points are that PECAM-1 is expressed by endothelium, platelets, and most leukocytes (2) and is enriched at endothelial cell junctions. Homophilic adhesion between PECAM-1 on leukocytes and PECAM-1 on endothelium has been shown to function during transendothelial cell migration of most, but not all, leukocytes, as measured in various in vivo and in vitro models of inflammation (reviewed in (52)). A recent study has also suggested that neutrophil-expressed CD177 is a ligand for PECAM-1 and that the heterophilic interaction between CD177 and PECAM-1 functions specifically in neutrophil transmigration (64). Previous studies had shown that some endothelial PECAM-1 (∼30%) is localized to a subcellular compartment of small vesicular-like structures just beneath the apical surface. During leukocyte transmigration these PECAM-1-containing vesicles are targeted to the sites of monocyte transmigration (46). More recently the targeted recycling of this vesicular compartment (termed lateral border recycling compartment, LBRC) and efficient leukocyte transmigration were shown to depend on an intact endothelial cell microtubule system and require one or more members of the kinesin family of motor proteins (47). Moreover, the membrane recycling mediated by the LBRC was required for both PECAM-1-dependent and -independent transmigration. Future studies are necessary to fully characterize the contents of the LBRC and their contributions to leukocyte transmigration in vivo.
ICAM-1 and VE-Cadherin and their role in neutrophil transmigration
For the remainder of the review article, we will focus on newly described mechanisms that regulate junctional disruption during paracellular (aka, junctional) transmigration. These mechanisms involve outside-in signaling mediated by ICAM-1 clustering, subsequent recruitment and activation of kinases, and phosphorylation of VE-Cad that leads to junctional destabilization.
Several molecules that are not considered leukocyte adhesion molecules per se have been implicated in leukocyte transmigration. Certain of these may be important for transmigration due to their cellular localization near junctional proteins or because of the signals they transmit to them. We highlight the molecules that interact with ICAM-1 and VE-Cadherin and are thought to mediate junctional adhesion molecule disruption and thereby facilitate transmigration of leukocytes.
Catenins
This family of proteins link cadherins to the cytoskeleton (reviewed in (84)). α-catenin associates with the actin cytoskeleton; β-catenin and γ-catenin (or plakoglobin) associate directly with the cytoplasmic tail of VE-cadherin and with α-catenin, and hence couple VE-cadherin to the actin cytoskeleton in confluent endothelial cells (see Fig. 3a). While the α-catenin-mediated linkage of E-cadherin-catenin complex to the actin cytoskeleton in epithelium has been challenged (89,95), this paradigm has not been examined for the VE-cadherin-catenin complex in endothelium. p120-catenin was originally identified as a substrate of Src kinase (40) and was later found to bind to the juxtamembrane region in the VE-cadherin cytoplasmic tail. Thus, p120-catenin does not participate in linking VE-cadherin to the cytoskeleton (reviewed in (63,85)) and instead has been demonstrated to be essential for the maintenance of VE-cadherin surface expression in endothelium ((93). In addition, p120-catenin has been shown to regulate Rho family GTPases RhoA, Rac and Cdc42 (6), which may be relevant to leukocyte transmigration because of the key role that Rho GTPases play in dynamically regulating the actin cytoskeleton (18). There are other catenin family members that localize to cell junctions (e.g., ARVCF, p0071, δ-catenin) and associate with cadherins; the reader is directed to a review by Vincent and colleagues for in-depth discussions (85).
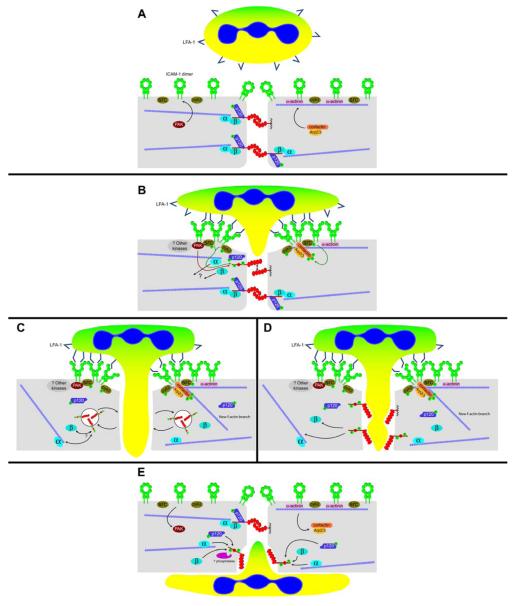
Figure 3. Outside-in signaling resulting in leukocyte transmigration at the junctions through activated endothelium: Interplay of endothelial cell molecules that participate actively in the dynamic reorganization of the adherens junctions.
A: Pretransmigratory state. Endothelial cells express ICAM-1 in the closed “O-form” dimer, homogeneously distributed in the cytoplasmic membrane (with slight but detectable enrichment at cell-cell junctions). In the cytoplasm, ICAM-1 is linked to actin filaments via α-actinin. VE-cadherin is shown as transdimers, forming the adherens junction. VE-cadherin is linked to the actin cytoskeleton by α-, β-, and γ-catenin (plakoglobin). For clarity, γ-catenin is not included in the figure, but it is anticipated to behave in a similar manner, although this has not been investigated in great detail (4,5). p120-catenin binds to the juxtamembrane region in the VE-cadherin tail and does not function in linkage to the cytoskeleton; rather it functions in VE-cadherin retention on the surface(93). Protein tyrosine kinases (src, pyk2) and serine kinases (PAK) participate in the induction of cytoskeletal remodeling after ICAM-1 engagement (3,98). Early events in leukocyte transmigration will include ICAM-1 clustering and consequent recruitment of cortactin-Arp2/3 and protein kinases to the clustered ICAM-1 tails. Others have shown that Rho G and its GEF, SEGF, also bind to the ICAM-1 cytoplasmic tail at this step in transmigration (82). A leukocyte is depicted with LFA-1 in the inactive state.
B: Initial stage of leukocyte transmigration. Engagement of ICAM-1 by leukocyte LFA-1 (in the active conformation) causes ICAM-1 clustering, and we suggest that a conformational switch occurs from the O-form to the W-form based on recent evidence from Yang and colleagues (99). Within the endothelial cell cytoplasm, protein kinases recruited to clustered ICAM-1 tails become phosphorylated and catalyze the phosphorylation of cortactin, VE-cadherin, and p120. Green arrows depict tyrosine phosphorylation, while PAK-catalyzed phosphorylation of VE-cadherin ser665 (80) is represented by a red arrow. The localization of PAK is not known, but the presence of a proline-rich SH3 binding region in PAK (11) suggests that it associates with Src kinase. As a result of VE-cadherin phosphorylation, the catenin complex bound to VE-cadherin begins to disassemble, uncoupling VE-cadherin from the actin cytoskeleton. The adherens junction itself begins to dissociate as well. Cortactin, once phosphorylated, can serve as a scaffold for new actin branches (depicted in panels C and D below).
C and D: Intermediate stage of leukocyte transmigration. Panel C illustrates the possibility that phosphorylated VE-cadherin is internalized within clathrin-coated vesicles (28,42,93,94) during leukocyte diapedesis, while panel D depicts VE-cadherin as remaining junctional but no longer participating in transdimerization with neighboring cells. The speed with which leukocyte diapedesis takes place suggests that the latter model is more likely than the former. This is pure speculation at this point.
E: Leukocyte transmigration nearly complete. The endothelial cell monolayer, having accommodated leukocyte transmigration, must reseal junctional gaps. This process is likely to involve protein tyrosine and serine phosphatases (whose identities have yet to be determined), which restore proteins to their resting states. VE-cadherin reassociates with the actin cytoskeleton, while actin branches formed by cortactin-Arp2/3 near the apical surface of the endothelial cell have dissolved.
The interactions between catenins and VE-cadherin are regulated by phosphorylation (reviewed in (84)). Indeed, Potter and colleagues showed that, in a CHO cell transfection system, phosphorylation of VE-cadherin in two tyrosine residues, Tyr 658 and Tyr 731, inhibited the binding of p120 and β-catenin, respectively, and reduced barrier function (59). More recently, these same tyrosine residues, as well as tyrosine residues 645 and 733, were found to be phosphorylated during THP-1 leukocyte adhesion and ICAM-1 engagement in human umbilical vein endothelial cells (HUVECs) (3) or during T lymphoblast adhesion in mouse and rat endothelial cell lines (80). An additional phosphorylation event at serine 665 was detected in the murine and rat models but has not been reported in human endothelium. Interestingly, phosphorylation of serine 665, catalyzed by the p21-activated kinase (PAK)(11), was shown to play a role in the internalization of VE-cadherin following stimulation by addition of vascular endothelial cell growth factor (VEGF) (28). Based on these reports, an attractive hypothesis is that tyrosine phosphorylation of VE-cadherin is involved in destabilization of junctional molecules to allow for VE-cadherin gap formation during leukocyte transmigration.
VE-Cadherin Protein Tyrosine Phosphatase (VE-PTP)
VE-PTP is a transmembrane receptor type tyrosine phosphatase that specifically and selectively associates with VE-cadherin (55). VE-PTP was shown to associate with the most membrane-proximal, extracellular domain of VE-cadherin. In a CHO cell transfection system, VE-PTP was shown to reverse the phosphorylation of VE-cadherin elicited by VEGFR-2, an effect that did not require VE-PTP to be catalytically active. The net affect of VE-PTP expression was to enhance barrier function. Further information from the Vestweber laboratory indicated that adhesion of neutrophils to endothelial cells triggers disassociation of VE-cadherin from VE-PTP (84).
Rho GTPases
The Rho family of GTPases belong to the Ras superfamily of small GTPases. Three members, RhoA, Rac, and Cdc42, have been studied in greatest detail. These proteins are widely recognized for mediating the cytoskeletal dynamics required for various cell adhesion processes including cell migration and cell-cell adhesion (39). Rho GTPases have been shown to regulate the expression, activity, and signaling of several adhesion molecules expressed by activated endothelium, including ICAM-1, VCAM-1, PECAM-1, and E-selectin (reviewed in (18)). In addition, there are multiple reports that p120-catenin in conjunction with p190RhoGAP regulates the activity of Rho GTPases (6,90). Rho activation has been implicated in leukocyte transmigration (18,61). Rho GTPases, under the control of p120, could regulate VE-cadherin stability at endothelial cell junctions and may also be of interest during the process of junctional transmigration.
Src kinases
This family of proteins includes important signaling molecules located in the cytosols of many different cell types, including endothelial cells, which respond to a wide range of stimuli including growth factors and adhesion proteins (37,77). Of the nine members, Fyn, Src, Yrk, and Yes are expressed in endothelium (35). In particular, Src has been shown to be activated by adhesion molecules, including ICAM-1, and to signal to the cytoskeleton (97,98) (see the next section). Src can also phosphorylate a variety of proteins present at the junctions, including VE-cadherin and p120 (63), and its inhibition leads to decreased transmigration of leukocytes (3,97,98). Src is also involved in regulating endothelial cell permeability (reviewed in (35)).
Cortactin
Cortactin is a multidomain scaffolding protein that binds F-actin, stimulates Arp2/3-dependent actin polymerization and dynamic actin rearrangement at the cell periphery, and is a substrate for Src kinases and Rho kinases (87,88). Cortactin is also implicated in the regulation of endothelial cell barrier function (24,38) and has been shown to associate with the cytoplasmic tail of ICAM-1 upon ligand engagement (78). More recently, cortactin has been shown to coordinate ICAM-1 clustering and cytoskeleton remodeling during neutrophil adhesion and transmigration (97,98).
α-Actinins
The nonmuscle isoforms of these proteins (isoforms 1 and 4) are binding partners of the cytoplasmic domain of ICAM-1 (17). There are multiple binding partners for alpha actinins (16), and these interactions are regulated by phosphoinositides PIP2 and PIP3 (67). The charged juxtamembrane domain of ICAM-1 is required for alpha-actinin interaction (16,17). □In particular, α-actinin 4 constitutively interacts with the ICAM-1 cytoplasmic tail and has been shown to play a role during neutrophil transmigration (17).
Ezrin/Radixin/Moesin (ERMs)
The ERMs are a class of proteins that, like the α-actinins, link the F-actin cytoskeleton to the plasma membrane by acting as adapter molecules for F-actin and membrane-associated proteins. Ezrin, radixin, and moesin associate with the cytoplasmic tails of CD43, CD44, PSGL-1, L-selectin, and ICAM-2 (reviewed in (79)), as well as to that of ICAM-1 (34). Unlike cortactin and the α-actinins, the ERM proteins appear to lack any F-actin cross-linking or branch forming abilities. Several studies have identified the membrane phospholipid PIP2 as a regulatory molecule for ERM proteins, affecting both actin binding and cellular distribution (10). It is, therefore, possible that ERMs and α-actinins respond to similar signals at the cytoplasmic membrane during leukocyte transmigration. The participation of ezrin and moesin in this process comes from the observations that both proteins co-localize with ICAM-1 in endothelial cells in docking structures (see next section below for further discussion and references (8,9).
In summary, many molecules contribute to the signaling pathways that regulate the recruitment of different leukocyte types. How these pathways integrate with one another and whether these connections are common to all leukocytes migrating in different vascular beds remains an active area of investigation.
Dynamics of endothelial cell ICAM-1 and VE-cadherin during leukocyte recruitment: A parable of rings and gaps
The combination of live-cell fluorescence-imaging microscopy and biochemical approaches has provided significant insight into the dynamic behavior of adhesion molecules during leukocyte adhesion, migration, and transendothelial cell migration in in vitro flow chamber models. The entire process of leukocyte transmigration takes only minutes both in vivo and in vitro. A number of investigators have addressed whether leukocyte transmigration leads to the alteration of endothelial cell junction proteins, specifically the adherens junction (AJ) components VE-cadherin and its cytosolic binding proteins α-, β-, and γ-catenins and p120-catenin or tight junction proteins. Several strategies were used to assess whether rapid alterations in AJ components, primarily VE-cadherin, occurred during neutrophil TEM. Initially, studies reported that neutrophil transmigration triggered loss of VE-cadherin and its associated cytosolic α-, β- γ- catenins, and plakoglobin under static assay conditions using immunofluorescence microscopy and biochemical approaches (4,23). Based on these data, a working model emerged wherein VE-cadherin acted as a gatekeeper in leukocyte transmigration: leukocyte adhesion triggered disassembly of the VE-cadherin complex via enzymatic degradation of the VE-cadherin complex, and leukocytes migrated through these gaps in VE-cadherin. This model was consistent with an in vivo model in which mAb inhibition of VE-cadherin in mice led to an exuberant inflammatory response in a murine peritonitis model (30). Subsequent experimental results concluded that the earlier reported adhesion-dependent loss of β-catenin and VE-cadherin was due to neutrophil proteases released during post assay manipulations (i.e., non-physiological proteolysis) (5,50). Nonetheless, our lab hypothesized that some form of disruption of this complex occurred and was a physiological step in transmigration. Therefore, we examined transmigration of monocytes and the differentiated monocytic cell line U937 because these leukocyte types lack neutrophil elastase, which can cleave VE-cadherin and overall express fewer proteases. Transmigrating monocytes and U937 cells induced localized and transient loss of VE-cadherin as assessed by confocal microscopy of fixed samples, and mAbs that inhibit leukocyte TEM also prevented the loss of VE-cadherin complex (5). Corroborating evidence for this idea arose by direct visualization of VE-cadherin during transmigration in live cell imaging, either by expressing a GFP-VE-cadherin fusion protein in endothelial cells, or by using an immunolabeled nonblocking anti-VE-cadherin antibody (68,76). These techniques allow direct visualization of the behavior of VE-cadherin during leukocyte transmigration and confirmed earlier observations that leukocyte transmigration indeed initiated transient alterations in VE-cadherin and subsequently reorganized to reseal the gap.
While leukocytes initiate de novo gaps or enlarge pre-existing gaps in VE-cadherin at sites of paracellular transmigration (68), the behavior of the endothelial adhesion molecules such as VCAM-1 and ICAM-1 had not been investigated. Although it was widely appreciated that LFA-1 — ICAM-1 interactions were critical for neutrophil transendothelial cell migration (71,72), until recently little was known about their physical location during TEM. Further insight has come from the work of Barreiro and colleagues, who reported interactions of members of the ERMs proteins with VCAM-1 and ICAM-1 during T cell adhesion and transmigration by live-cell time-lapse fluorescence microscopy (9). They observed that T cell attachment induced clustering of VCAM-1 with moesin on the endothelial apical surface but not at sites of transmigration. In contrast, ICAM-1 co-localized with moesin and ezrin during adhesion and transmigration under static assay conditions. These authors noted that both VCAM-1 and ICAM-1 associated with activated moesin and ezrin at sites of adhesion, and they termed such associations “docking structures.” Confocal analysis documented that these docking structures also contained actin, vinculin, α-actinin, and VASP, a member of the Wiscott-Aldrich Syndrome patient family of molecules. Pharmacological inhibition showed that maintenance of the docking structure was dependent, in part, on phosphoinositides and the Rho p160 ROCK and PI3K pathway. Given the complexity of the events in endothelial cells during leukocyte transmigration, it seemed likely that other signal pathways and other as yet unidentified actin-binding proteins participate and are not mutually exclusive.
Subsequent studies determined the distribution of neutrophil LFA-1 and endothelial ICAM-1 in leukocyte transmigration under shear flow conditions with a live-cell fluorescence-imaging system. This technique preserves the temporal sequence of events and the spatial organization and was the first demonstration of coordinated changes in leukocyte LFA-1 and endothelial cell ICAM-1 distribution during neutrophil transmigration (69). Neutrophil LFA-1 undergoes dramatic redistribution during transmigration. LFA-1 redistributes to the site of contact at endothelial cell junctions and forms a distinct ring-like cluster, while ICAM-1 simultaneously diffuses to the LFA-1 rings (69) . This LFA-1—ICAM-1 cluster remains around the neutrophil as it transmigrates through the endothelium. These data suggest that these changes in LFA-1 distribution reflect the molecular changes in LFA-1 affinity/avidity for ICAM-1 known to occur during integrin activation and adhesion to ligands such as ICAM-1, and probably represent their physiological correlates during transmigration. Other independent reports also describe dramatic changes in ICAM-1 localization in endothelium during leukocyte transmigration. Both ICAM-1 and VCAM-1 associated with microvilli-like projections that surrounded leukocytes in the process of transmigration; Carman and Springer coined the term “transmigratory cup” to describe this novel endothelial cell structure (15).
Inspection of the primary sequence of the ICAM-1 tail reveals a proline-rich region that conforms to a class I SH3 recognition site (74,75). The ICAM-1 tail is therefore a potential binding target for a wide variety of SH3 domain proteins, such as cortactin, as noted earlier. In a recent study, Rho G and an associated Rho G specific SH3-domain GEF (SGEF) were shown to be recruited to sites of ICAM-1 clustering, resulting in the activation of Rho G, formation of ICAM-1 clusters, and formation of a leukocyte transmigration cup (82). SGEF bound constitutively to the ICAM-1 tail, and its activity was increased after ICAM-1 engagement. These authors also suggested that Rho G activation was downstream Rho A activation. Earlier, endothelial cell Rho A activity was found to be critical for leukocyte adhesion and transmigration (1,91). Rho A activity can, in turn, be inhibited by p120-catenin (6,31,56). The variety of functions ascribed to p120-catenin suggests that this protein serves as a pathway for ICAM-1 and VE-cadherin to communicate with each other via signaling proteins such as Rho GTPases and/or src kinases, which can phosphorylate both VE-cadherin and p120-catenin, as well as ICAM-1 partners such as cortactin (3,63,97,98).
Numerous studies have established that the interaction between leukocyte LFA-1 and endothelial cell ICAM-1 results in the transmission of outside-in signals that facilitate leukocyte transmigration (81). By fluorescence recovery after photobleaching (FRAP) techniques in a HUVEC cell line transfected with ICAM-1-GFP, studies showed that ICAM-1 is a mobile molecule whose mobility is restricted due to the interaction of its cytoplasmic tail with the actin cytoskeleton (97). When a tailless version of ICAM-1-GFP was transduced into HUVECs, there was no cytoskeletal remodeling of actin upon ICAM-1 crosslinking, supporting the idea that ICAM-1 is anchored to the actin cytoskeleton via its cytoplasmic tail. More interesting, with an approach that involved siRNA knock down of cortactin, a known regulator of the endothelial actin cytoskeleton (36,78), endothelial cell cortactin was found to be critical for ICAM-1-induced cytoskeletal remodeling. This contribution is mediated by changes in phosphorylation induced by src kinases: transduction of HUVECs with retrovirus containing a mutated form of cortactin—GFP in three tyrosines known to be phosphorylated by src (36,78) resulted in impaired cytoskeletal remodeling induced by ICAM-1 crosslinking. When wild-type cortactin-GFP was expressed, it co-localized with ICAM-1 and formed a ring-like structure during neutrophil TEM; this structure was abolished when cortactin was knocked down by siRNA. Finally, inhibition of src kinases or knocking down of cortactin resulted in reduced transmigration of neutrophils. Thus, ICAM-1 clustering and ring formation during firm adhesion and transmigration of neutrophils results in src phosphorylation of cortactin and actin cytoskeleton rearrangement that facilitate the cytoskeletal changes that the endothelial cell undergoes during transmigration at junctions (97,98).
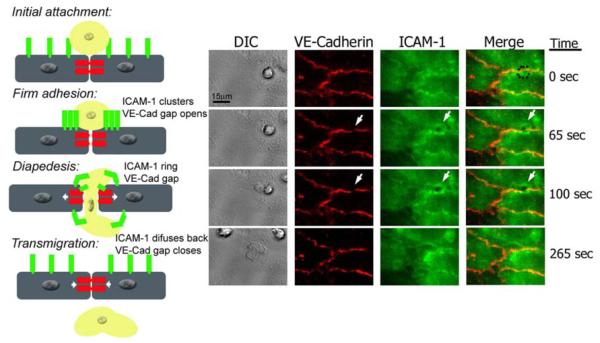
The use of live imaging techniques under flow conditions has contributed significantly to our understanding of the behavior and reorganization of ICAM-1 and VE-cadherin during transmigration at the junctions and has prompted our interest in commenting on these further. As mentioned earlier, it is important to note that other molecules besides VE-cadherin and ICAM-1 can participate in the process of leukocyte transmigration; however, ICAM-1 and VE-cadherin have been well characterized for their role in transmigration and therefore can serve as prototypic examples. Both VE-cadherin and ICAM-1 are mobile and dynamic during leukocyte transmigration under flow conditions. ICAM-1 forms a “ring” through which the leukocyte transmigrates and concomitantly relays outside-in signals that promote reorganization of the endothelial VE-cadherin to form a “gap” that opens a door at the endothelial cell contacts. These independent observations led us to carry out live-cell imaging experiments to monitor both molecules simultaneously during leukocyte transmigration (Fig 2 and supplemental movie 1). During initial attachment of the neutrophil, VE-cadherin is localized to cell-cell borders, and ICAM-1 is uniformly distributed at the apical surface of the endothelial cells. Upon firm adhesion, VE-cadherin begins to form a small gap at the transmigration site as ICAM-1 clusters around the neutrophil. Once diapedesis takes place, the VE-cadherin gap is at its maximum aperture, while ICAM-1 clusters into a ring-like structure through which the neutrophil transmigrates. When transmigration is complete, these molecules revert to their pretransmigration status: VE-cadherin and ICAM-1 are distributed uniformly at the cell-cell junction and the apical surface, respectively. The mechanisms that reset these molecules have not been examined in any detail. In some experiments, we have observed that a second leukocyte will transmigrate at the same site, sometimes even before the gap has completely resealed. This second neutrophil initiates an ICAM-1 ring and VE-cadherin gap, indicating the mechanism(s) for resetting the machinery can recycle quickly.
Figure legend 2. Live imaging of endothelial cell VE-Cadherin gaps and ICAM-1 rings dynamics during transmigration.
HUVECs were activated with TNF-α for 4 h and stained with nonblocking mAbs to VE-Cadherin and ICAM-1 directly conjugated in red (Alexa 568) and green (Alexa 488), respectively. Freshly isolated neutrophils were drawn through a parallel plate flow chamber apparatus at 1 dyne/cm2 of shear stress. Three-channel, live-cell digital microscopy of neutrophils and endothelium during the process of transmigration was carried out as described previously (69). At time= 0, the neutrophil approaches the brightly stained cell junction during the initial attachment to the 4-hr TNF-α activated HUVECs. The dynamic behavior of VE-Cadherin and ICAM-1 has not started. At time=65 sec, the neutrophil firmly adheres to the endothelial cells at the junctions, and as VE-Cadherin starts forming a de novo gap, ICAM-1 clusters at the cell-cell junction where the neutrophil is firmly adhered. At t= 100 sec, the neutrophil is undergoing diapedesis, and part of it is still on top of the monolayer, while part of it is underneath. At this point the VE-Cadherin gap reaches its maximum aperture, and ICAM-1 forms a ring-like structure surrounding the transmigrating neutrophil. Finally, at t= 265 sec, the neutrophil has completely transmigrated, and the endothelial cell junction returns to normal: the VE-Cadherin gap is closed, and ICAM-1 is again uniformly distributed on the apical surface of the endothelial cell.
In order to better understand the rapid and striking changes in endothelial molecules during transmigration, it is important to dissect the different intracellular signaling pathways that are triggered upon leukocyte firm adhesion and VE-cadherin and ICAM-1 redistribution at the junctions. These are mediated by several proteins cited earlier in this review, and the specific roles they play are discussed next.
Endothelial cell intracellular signaling during transmigration: the Yin - Yang of kinases
As discussed earlier, it is now well-documented that ICAM-1, VCAM-1, and PECAM-1 cluster at the interface between leukocyte and endothelial cell and can trigger localized redistribution and remodeling of cytoskeletal components in endothelium (15,46-48,69,96,97). Biochemical evidence suggests that new F-actin branching at the site of ICAM-1 clustering is mediated, at least in part, by cortactin and Arp2/3 recruitment to clustered ICAM-1 tail domains, and phosphorylation by kinases. The nonskeletal muscle α-actinins 1 and 4, which can serve as F-actin cross-linking proteins, may also regulate the mechanical properties of the underlying F-actin matrix during transmigration, as well as help anchor ICAM-1 to it, responding to localized enrichment of ICAM-1 tail domains, as discussed earlier (17). Cytoskeleton-associated proteins cooperate to transiently strengthen the attachment of the leukocyte to the endothelium by locally assembling a cytoskeletal network around the bound leukocyte to form a docking structure (9), a ring-like structure (69), or a transmigratory cup (15), as discussed earlier in this article.
Recent reports suggest that the influence of the cytoskeletal signaling pathway, both triggered by and affecting the distribution of ICAM-1, extends beyond ICAM-1 to the adherens junction (Fig 3). ICAM-1 clustering triggers the phosphorylation of the cytoplasmic domain of VE-cadherin, mediated in part by src and pyk2 tyrosine kinases (3) (Fig 3B). Phosphorylation of the cytoplasmic tail of VE-cadherin has been implicated in weakening of the homologous intracellular adhesive interactions of its extracellular domains, disrupting the adherens junction and increasing the permeability of the endothelial cell monolayer. There is no direct evidence, however, that VE-cadherin trans-dimers “come apart” as a result of phosphorylation, as opposed to being forced apart by the transmigrating leukocyte (see next section below). It is likely, however, that VE-cadherin phosphorylation triggers the release of bound catenins (59), which in turn, decouple VE-cadherin from the actin cytoskeleton (Figure 3 B and D). It is also possible that VE-cadherin becomes internalized into clathrin-coated vesicles (Fig 3 C) because this occurs constitutively (94) and after VEGF stimulation (28). Given the speed with which gaps in the endothelium form and reseal during leukocyte transmigration, a mechanism involving VE-cadherin internalization seems less likely to dominate during leukocyte transmigration.
Role of leukocyte force generation in transendothelial migration
In addition to the dynamic behavior of endothelial cell molecules and downstream signaling during leukocyte transmigration, the role played by the leukocyte itself in this process should be considered. Immune surveillance and rapid response to tissue inflammation or injury require leukocytes to not only travel considerable distances but also negotiate complex terrain. A few studies indicate that neutrophils generate significant levels of force when crawling randomly on glass surfaces and during chemotaxis to fMLP on polyacrylamide gel substrates (32,73). A recent report found that human neutrophils can exert forces on endothelial cell-cell contacts during transmigration, possibly contributing to VE-cadherin gap formation (60). Because of this finding, one can speculate that neutrophils physically force VE-cadherin trans-dimers (or additional proteins at junctions) apart during TEM. It has also been reported that leukocytes can modify their mechanical properties when confined or forced to enter microchannels (100). These findings suggest that mechanical forces exerted by the neutrophil also play a role in the disruption of intercellular junctions during diapedesis. Future studies are necessary to understand the role of force generation by neutrophils during transendothelial migration.
Summary and Future Directions
The engagement and clustering of endothelial cell ICAM-1 by LFA-1 on the leukocyte surface triggers activation of nonreceptor tyrosine kinases (src, Pyk-2, and likely others) that lead to two divergent responses within the endothelial cell cytoplasm: ICAM-1 becomes more firmly anchored to the cytoskeleton and VE-cadherin becomes less so. By contrast, the uncoupling of the endothelial cell cytoskeleton from VE-cadherin may serve to weaken locally the endothelial cell-cell junction at the area through which the leukocyte will eventually transmigrate.
As has been presented in this review, the many endothelial proteins that have been found to participate in leukocyte transmigration illustrate the complexity of this rapid and highly dynamic process. However, discerning how and when these proteins interplay, as well as whether there are other as yet undiscovered molecules or processes that also play a role, will be extremely relevant for the overall understanding of the process. The C-terminal tail of ICAM-1 has the capacity to bind either directly or indirectly to a number of proteins, including cortactin, ezrin and moesin, and nonskeletal muscle cell α-actinins as well as the Rho G-specific SH3-domain GEF (SGEF). ICAM-1 clustering indirectly leads to VE-cadherin phosphorylation and the disruption of VE-cadherin dimers, and p120-catenin regulates VE-cadherin stability (85,93,94) (Figure 3) and can also regulate VE-cadherin gap formation during transmigration (unpublished results, P Alcaide). The possible involvement of p120-catenin in the regulation of RhoA and RhoG activation may add additional routes of control and feedback for the entire process. These findings hint at a complex signaling pathway involving multiple regulatory proteins that link ICAM-1 clustering, VE-cadherin gap formation, and remodeling of the actin cytoskeleton and vesicular compartment. Two additional cytoskeleton-associated proteins, ezrin and moesin, also appear to associate with the charged juxtamembrane region of ICAM-1, as suggested by a number of studies (8,34,57). A picture of leukocyte transendothelial migration is now emerging, in which a variety of signaling molecules (Rho A, Rho G, protein tyrosine and serine kinases, vesicular recycling of adhesion molecule PECAM-1) as well as cytoskeletal proteins (cortactin, α-actinins, ezrin, moesin and perhaps others) mediate the response of the endothelial cell to leukocyte engagement and transmigration.
Although new information is constantly emerging and contributing to our understanding of the process of junctional disruption during transmigration, little is known about how the junctions come back together as the VE-cadherin gap closes and how this relates to endothelial cell and vessel wall barrier functions. Future studies are necessary to identify mechanisms that reverse the phosphorylation changes induced during leukocyte engagement once transmigration is complete. VE-PTP is another candidate that may participate by regulating VE-cadherin stability (55). Other phosphatases as well as other intracellular molecules could participate during the lateral diffusion and/or recycling of VE-cadherin, resulting in the resealing of the adherens junctions after transmigration. Lastly, more work is necessary to understand the effects of shear stress on neutrophil function, specifically what signaling mechanisms regulate pseudopod formation and retraction and β2 integrin expression. A more complete understanding of the whole process and possible insights into therapeutic approaches to this aspect of inflammation warrant additional study.
Supplementary Material
Acknowledgments
Supported by NIH grants HL36028 and HL53993 and a NIH NRSA training award F32 HL086217 (SA).
References
- 1.Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol. 1999;162:2964–2973. [PubMed] [Google Scholar]
- 2.Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allingham MJ, van Buul JD, Burridge K. ICAM-1-Mediated, Src- and Pyk2-Dependent Vascular Endothelial Cadherin Tyrosine Phosphorylation Is Required for Leukocyte Transendothelial Migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 4.Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: A process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med. 1997;186:517–527. doi: 10.1084/jem.186.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13:604–610. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 7.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends in Immunology. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Barreiro O, Vicente-Manzanares M, Urzainqui A, Yanez-Mo M, Sanchez-Madrid F. Interactive protrusive structures during leukocyte adhesion and transendothelial migration. Front Biosci. 2004;9:1849–1863. doi: 10.2741/1285. [DOI] [PubMed] [Google Scholar]
- 9.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol. 2000;151:1067–1080. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- 12.Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. J Leukoc Biol. 1999;65:299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- 13.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 14.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celli L, Ryckewaert JJ, Delachanal E, Duperray A. Evidence of a Functional Role for Interaction between ICAM-1 and Nonmuscle {alpha}-Actinins in Leukocyte Diapedesis. J Immunol. 2006;177:4113–4121. doi: 10.4049/jimmunol.177.6.4113. [DOI] [PubMed] [Google Scholar]
- 18.Cernuda-Morollon E, Ridley AJ. Rho GTPases and Leukocyte Adhesion Receptor Expression and Function in Endothelial Cells. Circ Res. 2006;98:757–767. doi: 10.1161/01.RES.0000210579.35304.d3. [DOI] [PubMed] [Google Scholar]
- 19.Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- 20.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant Signals and {beta}2 Integrin Occupancy at Apical Endothelial Contacts Combine with Shear Stress Signals to Promote Transendothelial Neutrophil Migration. J Immunol. 2004;173:7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 21.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kB and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 22.Davies PF, Zilberberg J, Helmke BP. Spatial microstimuli in endothelial mechanosignaling. Circ Res. 2003;92:359–370. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- 23.Del Maschio A, Zanetti A, Corada M, Rival Y, Ruco L, Lampugnani MG, Dejana E. Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J Cell Biol. 1996;135:497–510. doi: 10.1083/jcb.135.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 25.Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 26.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci U S A. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 29.Gopalan PK, Burns AR, Simon SI, Sparks S, Mcintire LV, Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J Leukoc Biol. 2000;68:47–57. [PubMed] [Google Scholar]
- 30.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 31.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 32.Guilford WH, Lantz RC, Gore RW. Locomotive forces produced by single leukocytes in vivo and in vitro. Am J Physiol. 1995;268:C1308–C1312. doi: 10.1152/ajpcell.1995.268.5.C1308. [DOI] [PubMed] [Google Scholar]
- 33.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. PNAS. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem. 1998;273:21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 35.Hu G, Place AT, Minshall RD. Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules. Chem Biol Interact. 2008;171:177–189. doi: 10.1016/j.cbi.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 37.Ingley E. Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol. 2006;291:L289–L295. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- 39.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeletal and cell adhesion by the rho family of GTPaese in mammalian cells. Annual Review of Biochemistry. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 40.Kanner SB, Reynolds AB, Parsons JT. Tyrosine phosphorylation of a 120-kilodalton pp60src substrate upon epidermal growth factor and platelet-derived growth factor stimulation and in polymovirus middle-T-antigen-transformed cells. Mol Cell Biol. 1991;11:713–720. doi: 10.1128/mcb.11.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kvietys PR, Sandig M. Neutrophil diapedesis: paracellular or transcellular? News Physiol Sci. 2001;16:15–19. doi: 10.1152/physiologyonline.2001.16.1.15. [DOI] [PubMed] [Google Scholar]
- 42.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 44.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 45.Makino A, Shin HY, Komai Y, Fukuda S, Coughlin MF, Sugihara-Seki M, Schmid-Schoenbein GW. Mechanotransduction in leukocyte activation: A review. Biorheology. 2007;44:221–240. [PubMed] [Google Scholar]
- 46.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 47.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 49.Moazzam F, DeLano FA, Zweifach BW, Schmid-Schonbein GW. The leukocyte response to fluid stress. Proc Natl Acad Sci U S A. 1997;94:5338–5343. doi: 10.1073/pnas.94.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moll T, Dejana E, Vestweber D. In vitro degradation of endothelial catenins by a neutrophil protease. J Cell Biol. 1998;140:403–407. doi: 10.1083/jcb.140.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller WA. Migration of leukocytes across endothelial junctions: some concepts and controversies. Microcirculation. 2001;8:181–193. doi: 10.1038/sj/mn/7800078. [DOI] [PubMed] [Google Scholar]
- 52.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 53.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nathan C. Points of control in inflammation. Nature. 2002;420:846. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 55.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 57.Oh HM, Lee S, Na BR, Wee H, Kim SH, Choi SC, Lee KM, Jun CD. RKIKK motif in the intracellular domain is critical for spatial and dynamic organization of ICAM-1: functional implication for the leukocyte adhesion and transmigration. Mol Biol Cell. 2007;18:2322–2335. doi: 10.1091/mbc.E06-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Potter MD, Barbero S, Cheresh DA. Tyrosine Phosphorylation of VE-cadherin Prevents Binding of p120- and {beta}-Catenin and Maintains the Cellular Mesenchymal State. J Biol Chem. 2005;280:31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 60.Rabodzey R, Alcaide P, Luscinskas FW, ladoux B. Mechanical forces induced by the transendothelial migration of human neutrophils. Biophys J. 2008 doi: 10.1529/biophysj.107.119156. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol. 2008;180:1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sachs UJH, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The Neutrophil-specific Antigen CD177 Is a Counter-receptor for Platelet Endothelial Cell Adhesion Molecule-1 (CD31) J Biol Chem. 2007;282:23603–23612. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- 65.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 66.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 67.Scott DL, Diez G, Goldmann WH. Protein-lipid interactions: correlation of a predictive algorithm for lipid-binding sites with three-dimensional structural data. Theor Biol Med Model. 2006;3:17. doi: 10.1186/1742-4682-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 69.Shaw SK, Ma S, Kim M, Rao RM, Hartman CU, Froio R, Liu Y, Yang L, Jones T, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompanies neutrophil transmigration. J Exp Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin HY, Simon SI, Schmid-Schonbein GW. Fluid shear-induced activation and cleavage of CD18 during pseudopod retraction by human neutrophils. J Cell Physiol. 2008;214:528–536. doi: 10.1002/jcp.21235. [DOI] [PubMed] [Google Scholar]
- 71.Smith CW, Kishimoto TK, Abbassi O, Hughes B, Rothlein R, Mcintire LV, Butcher EC, Anderson DC. Chemotactic factors regulate lectin adhesion molecule-1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothlial cells in vitro. J Clin Invest. 1991;87:609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith LA, Aranda-Espinoza H, Haun JB, Dembo M, Hammer DA. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys J. 2007;92:L58–L60. doi: 10.1529/biophysj.106.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sparks AB, Rider JE, Hoffman NG, Fowlkes DM, Quillam LA, Kay BK. Distinct ligand preferences of Src homology 3 domains from Src, Yes, Abl, Cortactin, p53bp2, PLCgamma, Crk, and Grb2. Proc Natl Acad Sci U S A. 1996;93:1540–1544. doi: 10.1073/pnas.93.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sparks AB, Rider JE, Kay BK. Mapping the specificity of SH3 domains with phage-displayed random-peptide libraries. Methods Mol Biol. 1998;84:87–103. doi: 10.1385/0-89603-488-7:87. [DOI] [PubMed] [Google Scholar]
- 76.Su WH, Chen H-I, Jen CJ. Differential movements of VE-cadherin and PECAM-1 during transmigration of polymorphonuclear leukocytes through human umbilical vein endothelium. Blood. 2002;100:3597–3603. doi: 10.1182/blood-2002-01-0303. [DOI] [PubMed] [Google Scholar]
- 77.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 78.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 2002;16:1257–1259. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 79.Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem. 1999;274:34507–34510. doi: 10.1074/jbc.274.49.34507. [DOI] [PubMed] [Google Scholar]
- 80.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turowski P, Adamson P, Greenwood J. Pharmacological Targeting of ICAM-1 Signaling in Brain Endothelial Cells: Potential for Treating Neuroinflammation. Cellular and Molecular Neurobiology. 2005;25:153–170. doi: 10.1007/s10571-004-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 84.Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 85.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm’s length. Am J Physiol Cell Physiol. 2004;286:C987–C997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- 86.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 89.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 91.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145:1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wojciechowski JC, Sarelius IH. Preferential binding of leukocytes to the endothelial cell junction region in venules in situ. Microcirculation. 2005;12:349–359. doi: 10.1080/10739680590934763. [DOI] [PubMed] [Google Scholar]
- 93.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin Regulates Clathrin-dependent Endocytosis of VE-Cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial Cell Cortactin Coordinates Intercellular Adhesion Molecule-1 Clustering and Actin Cytoskeleton Remodeling during Polymorphonuclear Leukocyte Adhesion and Transmigration. J Immunol. 2006;177:6440–6449. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- 98.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial Cell Cortactin Phosphorylation by Src Contributes to Polymorphonuclear Leukocyte Transmigration In Vitro. Circ Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 99.Yang Y, Jun CD, Liu JH, Zhang R, Joachimiak A, Springer TA, Wang JH. Structural basis for dimerization of ICAM-1 on the cell surface. Mol Cell. 2004;14:269–276. doi: 10.1016/s1097-2765(04)00204-7. [DOI] [PubMed] [Google Scholar]
- 100.Yap B, Kamm RD. Mechanical deformation of neutrophils into narrow channels induces pseudopod projection and changes in biomechanical properties. J Appl Physiol. 2005;98:1930–1939. doi: 10.1152/japplphysiol.01226.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.