Abstract
Galanin expression markedly increases in the dorsal root ganglion (DRG) after sciatic nerve axotomy and modulates pain behavior and regeneration of sensory neurons. Here, we describe transgenic mice expressing constructs with varying amounts of sequence upstream of the murine galanin gene marked by LacZ. The 20 kb region upstream of the galanin gene recapitulates the endogenous expression pattern of galanin in the embryonic and adult intact DRG and after axotomy. In contrast, 1.9 kb failed to drive LacZ expression in the intact DRG or after axotomy. However, the addition of an additional 2.7 kb of 5′ flanking DNA (4.6 kb construct) restored the expression in the embryonic DRG and in the adult after axotomy. Sequence analysis of this 2.7 kb region revealed unique 18 and 23 bp regions containing overlapping putative Ets-, Stat-, and Smad-binding sites, and adjacent putative Stat- and Smad-binding sites, respectively. Deletion of the 18 and 23 bp regions from the 4.6 kb construct abolished the upregulation of LacZ expression in the DRG after axotomy but did not affect expression in the embryonic or intact adult DRG. Also, a bioinformatic analysis of the upstream regions of a number of other axotomy-responsive genes demonstrated that the close proximity of putative Ets-, Stat-, and Smad-binding sites appears to be a common motif in injury-induced upregulation in gene expression.
Keywords: galanin, DRG, Ets, Stat, Smad, axotomy
Introduction
Galanin is expressed at high levels in most cells of the developing DRG in late gestation and starts to downregulate in the early postnatal period (Xu et al., 1996). Low levels of the peptide are detected in <5% of adult DRG neurons, which are predominantly small-diameter C-fiber nociceptors (Hokfelt et al., 1987), with higher levels detected in the primary afferent terminals of the spinal cord (Skofitsch and Jacobowitz, 1985). After sciatic nerve section (axotomy), galanin levels in the DRG rise by up to 120-fold (Hokfelt et al., 1987; Wiesenfeld et al., 1992), and the peptide is abundantly expressed in ∼40% of sensory neurons (Hokfelt et al., 1994). The functional significance of these findings has been studied using various pharmacological tools (Liu et al., 2001) to modulate galanin signaling and by the phenotypic characterization of transgenic mice bearing loss-of-function (galanin knock-out, Gal-KO) (Holmes et al., 2000) or gain-of-function (galanin over-expressing) (Bacon et al., 2002; Hygge-Blakeman et al., 2004) mutations in the galanin gene. These studies have shown that the role played by galanin in pain signaling (nociception) is complex with facilitatory and inhibitory effects observed, sometimes in a modality-specific manner (for review, see Xu et al., 2000). More recently, we and others have shown that galanin overexpression in the DRG of transgenic mice reduces allodynia (the perception of pain from a normally innocuous stimulus) in a number of models of neuropathic pain (Holmes et al., 2003; Hygge-Blakeman et al., 2004).
In addition to its modulatory role in nociception, galanin also plays a trophic role in adult sensory neurons. We have previously shown that the rate of peripheral nerve regeneration after a crush injury to the sciatic nerve was reduced by 35% in adult Gal-KO animals (Holmes et al., 2000), associated with long-term sensorimotor functional deficits (Holmes et al., 2000). This compromised regenerative capacity in vivo was reflected by in vitro deficits in neuritogenesis. The addition of galanin to cultures from adult wild-type (WT) and Gal-KO animals significantly enhanced neurite outgrowth from WT sensory neurons and fully rescued the observed deficits in Gal-KO cultures (Mahoney et al., 2003). These results demonstrate that adult sensory neurons are dependent on galanin for optimal neurite extension.
In light of these findings, it is important to delineate the factors and signaling cascades that regulate galanin expression in the DRG. Previous studies have implicated nerve growth factor (NGF) (Verge et al., 1995; Kerekes et al., 1997; Shadiack et al., 2001), leukemia inhibitory factor (LIF), and interleukin-6 (IL-6) (Corness et al., 1996; Sun and Zigmond, 1996; Thompson et al., 1998; Murphy et al., 1999) in the regulation of galanin gene expression in the intact DRG and after axotomy. Most recently, a study has demonstrated that activation of the nitric oxide-cGMP pathway also induces galanin expression in cultured rat DRG neurons (Thippeswamy et al., 2007). The enhancer regions and transcription factors (TFs) that mediate the axotomy-induced upregulation of galanin in the DRG and responses to the above factors have yet to be fully elucidated.
In the present study, a transgenic approach was used to map and analyze the enhancer regions of the murine galanin gene that regulate basal expression of galanin gene transcription in the embryonic and adult DRG and the upregulation in expression in response to axotomy.
Materials and Methods
Transgene construction
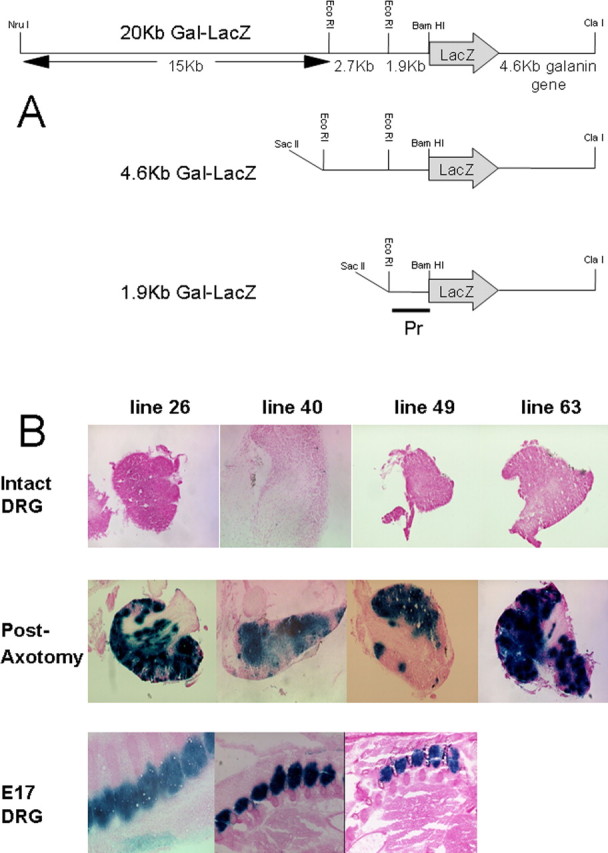
The 20 kb promoter Gal-LacZ transgene.
As described previously, a mouse 129Sv BamHI cosmid genomic library was screened using the full-length rat galanin cDNA as a probe and a ∼40 kb cosmid clone that contained the entire galanin gene, and ∼20 kb of upstream flanking sequence was isolated (Wynick et al., 1998). Figure 1A details the cloning strategy to construct the three transgenes that were microinjected. In brief, mapping of the cosmid clone identified two BspEI sites 13 kb apart. The 5′ BspEI site was ∼700 bp upstream of the transcriptional start site and the 3′ site was ∼7.5 kb downstream of the galanin gene. This 13 kb fragment was excised from the cosmid, leaving an insert of ∼26 kb. A 6.5 kb EcoRI fragment containing the galanin gene was subcloned into pBluescript KS+ (Stratagene, La Jolla, CA), and a 3.5 kb LacZ gene (kindly provided by Dr. D. Summerbell, National Institute for Medical Research, London, UK) was inserted into a unique BamHI site, in the 5′-UTR 13 bp downstream of the transcriptional start site [as described previously by Koefler et al. (1996)]. The unique SalI site in the vector polylinker was blunted, and a BspEI linker was inserted to replace it. The 8.8 kb BspEI fragment (containing the LacZ gene and the entire galanin coding region) was then excised and cloned into the now-unique BspEI site in the 26 kb cosmid vector. The entire transgene was then excised from the cosmid vector by digesting with NruI and ClaI, unique sites in the cosmid vector.
Figure 1.
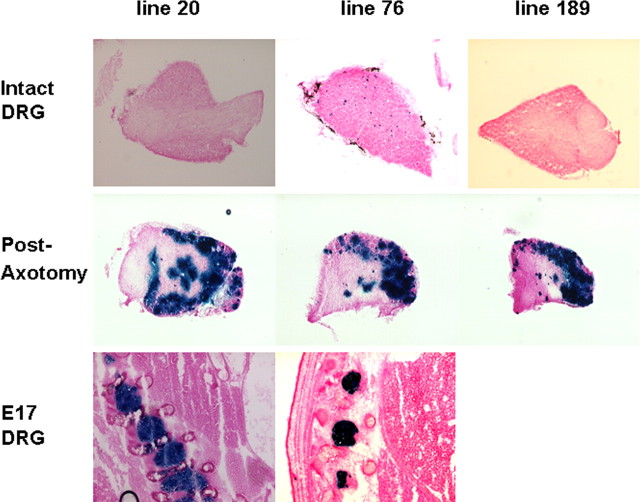
A, Construction of the 20, 4.6, and 1.9 kb Gal-LacZ transgenes. In each case, the LacZ gene (inserted into a BamH1 site in the 5′UTR) and the 4.6 kb galanin genomic locus are included with varying amounts of 5′ upstream sequence. The probe used to determine transmission and copy number of all of the transgenes is marked (Pr). B, Representative photomicrographs demonstrating expression in the four expressing 20 kb transgene lines. For each line, the expression pattern was determined for a minimum of five animals. Uninjured DRG (top panel) expression varied from no positive cells to 10–20 positive cells per DRG. Regardless of the level of expression in each line in the uninjured DRG, axotomy markedly increased the numbers of positive cells and the levels for β-galactosidase activity in the ipsilateral (axotomized) DRG (middle). High levels of staining were observed in the embryonic DRG harvested at day 17 of gestation for the three highest expressing 20 kb lines (bottom).
The 1.9 kb promoter Gal-LacZ transgene.
The 10 kb fragment containing the 1.9 kb of upstream sequence, the 3.5 kb LacZ gene, and the entire 4.6 kb galanin coding region was excised from the vector by digesting with SacII and SalI (each unique sites in the vector).
The 4.6 kb promoter Gal-LacZ transgene.
A further 2.7 kb of 5′ flanking DNA was subcloned into pBluescript KS+ as an EcoRI fragment. A NotI linker was inserted into the unique EcoRV site in the vector 3′ polylinker. The 2.7 kb insert was then isolated as a NotI fragment and cloned into the >1.9 kb transgene at the unique NotI site in the vector 5′ polylinker. The 12.7 kb insert was excised from the vector by digesting with SacII and SalI.
The 4.6Δ23,18 kb promoter Gal-LacZ transgene.
Site-directed mutagenesis was used to remove both the 23 bp 5′ Stat/Smad-binding site and the 18 bp 3′ Ets/Stat/Smad-binding site from the 4.6 kb LacZ construct (hereafter referred to as the 4.6Δ23,18 kb. Consecutive deletion of the 18 and 23 bp binding sites, within the 2.7 kb EcoRI fragment of the mouse galanin promoter, was achieved using a QuickChange XL Site-Directed Mutagenesis kit (Stratagene). HPLC-purified oligonucleotides (Invitrogen, San Diego, CA) flanking the putative 3′ Ets/Stat/Smad and 5′ Stat/Smad sites were, respectively, 5′-GGCAGCAGCAAGCAGGGC/TTACAGGTAGGAAATGACAGC-3′ (AY026768 nucleotides 2108–2125 plus nucleotides 2144–2164, where ′′/′′ represents the deletion site) and its reverse complement, and 5′-GAGCCTCGGGGCCCAGG/GCAAGAACCAGATCCCACT-3′ (AY026768 nucleotides 234–250 plus nucleotides 274–292) and its reverse complement. The reactions each used 25 ng of plasmid DNA (NotI-linkered pBluescript with 2.7 kb genomic DNA insert, see above), with annealing at 65°C for 1 min, extension at 68°C for 13 min, followed by DpnI digestion for 2 h. After transformation into XL10-Gold ultracompetent cells (Stratagene), colonies were screened by PCR with primers specific for either nondeletion (5′-CAGCAGCAAGCAGGGCTTCTGA-3′) or Ets/Stat/Smad deletion sequence (5′-CAGCAGCAAGCAGGGC/TTACAG-3′), together with reverse primer (5′-AGACTGAAGCCCATGCTTCAGTG-3′). A plasmid DNA clone with the specific deletion of the 18 bp putative Ets/Stat/Smad-binding site was then used for deletion of the putative 5′ Smad/Stat site, as above, with colony PCR screening using either nondeletion (5′-CCTCGGGGCCCAGGGTCTAG-3′) or 5′ Stat/Smad deletion primers (5′- CCTCGGGGCCCAGG/GCAAGA-3′), together with reverse primer (3kbAS2; 5′-GGGTTTGCTTATGTGTATAGGCATG-3′). Resulting efficiencies, including correct product size, were 13/18 (72%) for the 18 bp putative Ets/Stat/Smad-binding site and 44/48 (92%) for the 23 bp putative 5′ Stat/Smad-binding site. The 2.7 kb insert of the double deletion was excised with NotI and used to replace the corresponding fragment of the 4.6 kb promoter Gal-LacZ transgene (see above), resulting in the 4.6Δ23,18 kb Gal-LacZ transgene.
All transgenes were purified from LMP agarose gel (FMC Bioproducts, Rockland, ME) by the Geneclean II kit (BIO 101, La Jolla, CA) and subsequently purified with an Elutip column (Schleicher and Schuell, Keene, NH) before dilution in 1× TE to a final concentration of 5 ng/μl for microinjection.
Animals
All transgenic lines were generated and bred on the CBA/B6 F1 hybrid background. Age- and sex-matched wild-type animals were used as controls in all experiments. All animals were fed standard chow and water ad libitum, and animal care and procedures were performed within United Kingdom Home-Office protocols and guidelines. The generation of the transgenic lines was performed by microinjection of fertilized mouse oocytes with the above transgenes and subsequent transfer into pseudopregnant females, as per standard protocols (Hogan et al., 1994). Founders were identified by Southern blot analysis of genomic DNA digested with EcoRI. Blots were hybridized with a 32P-labeled 1.9 kb probe (Pr) (see Fig. 1A). Copy number was determined by comparing the intensities of the wild-type (6.5 kb) and transgenic bands (5 kb), quantified with a STORM 840 PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Surgery
Age-matched (10–12 weeks; 25–30 g) adult mice were used in all experiments (n = 5/genotype). Mice were anesthetized with Hypnorm (0.315 mg/ml fentanyl citrate plus 10 mg/ml fluanisone; Janssen Pharmaceuticals, High Wycombe, UK):Hypnovel (5 mg/ml midazolam; Roche Products, Welwyn Garden City, UK):water at a ratio of 1:1:2 at 4 μl/g. For axotomy, an incision was made in the lateral right hind leg at the level of the mid-thigh exposing the sciatic nerve. The common sciatic nerve was sectioned, and ∼2 mm of distal nerve stump was removed. The overlying muscle and skin was sutured and the animals allowed to recover. In sham-operated animals, the sciatic nerve was exposed but not lesioned.
Sequencing and analysis
DNA was sequenced by the Department of Biochemistry, University of Oxford with multiple synthetic oligonucleotide primers (Invitrogen) and analyzed with MatInspector software and a range of published TF-binding sites. The 4.6 kb galanin promoter sequence has been assigned GenBank accession number AY026768.
β-Galactosidase staining
Adult animals were killed and tissues dissected and frozen on solid CO2. Thirty micrometer sections were collected on polylysine slides (BDH Chemicals, Poole, UK). Sections were air-dried and then fixed for 30 s in 0.2% glutaraldehyde (Sigma, St. Louis, MO), 2 mm MgCl2 (BDH Chemicals), and 5 mm EGTA (BDH Chemicals) in 1×PBS (BDH Chemicals), then washed in 1× PBS three times for 5 min and incubated at 37°C overnight in staining solution [1× PBS containing 5 mm K4Fe(CN)6 3H2O (BDH Chemicals), 5 mm K3Fe(CN)6 (BDH Chemicals), 2 mm MgCl2, and 1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Sigma); this was dissolved in N-N-dimethylformamide (Sigma)]. Sections were then washed in 1× PBS for 10 min, rinsed in distilled water, and counterstained for 10 min in nuclear fast red (Vector Laboratories, Burlingame, CA) before being dehydrated in ethanol, cleared in xylene (BDH Chemicals), and mounted in distrene plasticizer xylene (BDH Chemicals).
For embryonic tissue, female mice carrying embryos at day 17 of gestation were killed and the embryos surgically removed, washed briefly in PBS, and frozen on solid CO2. Tissue was then prepared as for adult tissue (above) with the exception that whole embryos were sectioned transversely to allow visualization of the spinal cord and DRG.
In vitro dispersed DRG assay
Cultures were performed as described previously (Mahoney et al., 2003). In brief, DRG from the lumbar, cervical, and thoracic region of 8-week-old male mice were removed aseptically and collected in DMEM/F12 medium (Sigma). Ganglia were enzymatically treated with collagenase (Roche Products) for 1 h at 37°C, washed, and digested with trypsin EDTA (Sigma) for 10 min at 37°C. After washing, ganglia were mechanically dissociated in medium containing trypsin inhibitor (Sigma). After centrifugation, cells were resuspended in DMEM/F12 (Sigma) supplemented with 5% horse serum (Sigma), 1 mm glutamine (Sigma), and 10 ng/ml gentamycin (Sigma). To enhance the culture for neurons and eliminate much of the cellular debris, cells were plated on six-well plates coated with 0.5 mg/ml polyornithine (Sigma) and maintained overnight at 37°C in a humidified incubator with 95% air/5% CO2. Medium was removed and discarded. The neurons were removed from the surface by squirting with a jet of fresh medium. After centrifugation, cells were plated on 24-well plates treated with 0.5 mg/ml polyornithine and 5 μg/ml laminin (Sigma) and maintained for 4 h at 37°C in a humidified incubator with 95% air/5% CO2. Neurons were then stained for β-galactosidase as described above, and the number of β-galactosidase-positive neurons counted using NIH Image (Scion, Frederick, MD). Data are presented as mean ± SEM.
Results
The 20 kb Gal-LacZ construct
We first generated a construct that contained ∼20 kb of sequence upstream of the galanin gene, the 3.5 kb LacZ gene (inserted into the 5′ untranslated region of exon 1) and the entire 4.6 kb galanin coding region (Fig. 1A) (hereafter referred to as 20 kb Gal-LacZ construct). Six founder mice were generated by microinjection of the 20 kb Gal-LacZ transgene, and both copy number and transmission were assessed by Southern blot analysis. Five lines were bred and expression data are summarized in Table 1. DRG from naive, uninjured animals of each line displayed a low level of β-galactosidase staining, similar to the previously described endogenous galanin expression in the mouse and rat (Hokfelt et al., 1987; Holmes et al., 2003). This varied in different lines from no positive cells to 10–20 positive cells per DRG (Fig. 1B, top). Animals from each of these lines were then subjected to unilateral axotomy of the sciatic nerve. One week later, animals were killed; the bilateral lumbar L4 and L5 DRG were removed and stained for β-galactosidase activity. Identical staining patterns were observed in the contralateral (unaxotomized) DRG to those from naive, uninjured controls (data not shown). Regardless of the level of expression in each line, axotomy markedly increased the numbers of positive cells and the levels for β-galactosidase activity in the ipsilateral (axotomized) DRG (Fig. 1B, middle).
Table 1.
Characterization of the various transgenic lines
| Transgene | Line number | Transmission | Copy number | Expression in E17 DRG | Expression in adult intact DRG | >10-fold upregulation in adult DRG after axotomy |
|---|---|---|---|---|---|---|
| 20 kb | 26 | Yes | 11 | Yes | Yes | Yes |
| 20 kb | 40 | Yes | 2 | Yes | Yes | Yes |
| 20 kb | 49 | Yes | 11 | Yes | Yes | Yes |
| 20 kb | 63 | Yes | 2 | Yes | Yes | Yes |
| 20 kb | 148 | Yes | 4 | Yes | Yes | Yes |
| 4.6 kb | 20 | Yes | 7 | Yes | Yes | Yes |
| 4.6 kb | 76 | Yes | 17 | Yes | Yes | Yes |
| 4.6 kb | 189 | Yes | 3 | Yes | Yes | Yes |
| 4.6Δ23,18 kb | 125 | Yes | 5 | Yes | Yes | No |
| 4.6Δ23,18 kb | 200 | Yes | 12 | Yes | Yes | No |
| 4.6Δ23,18 kb | 234 | Yes | 7 | Yes | Yes | No |
| 1.9 kb | 64 | Yes | 4 | No | No | No |
| 1.9 kb | 66 | Yes | 14 | No | No | No |
| 1.9 kb | 73 | Yes | 7 | No | No | No |
| 1.9 kb | 77 | No | 1 | No | No | No |
| 1.9 kb | 128 | Yes | 4 | No | No | No |
Full recapitulation of the endogenous galanin expression pattern and responses to axotomy are noted in the 20 and 4.6 kb lines. In contrast, no expression was detected in any of the 1.9 kb lines. Removal of the 23 and 18 bp regions from the 4.6 kb transgene (4.6Δ23,18 kb) had no effect on embryonic or intact adult expression but completely abolished the axotomy-induced upregulation in the transgene.
Previous data from the rat had demonstrated high levels of galanin in the embryonic DRG at late gestation (Xu et al., 1996). We therefore harvested mouse embryos at day 17 of gestation (E17) and detected high levels of β-galactosidase expression in the DRG of all expressing lines (Fig. 1B, bottom, and data not shown).
The 20 kb transgene therefore appears to recapitulate the endogenous expression pattern of galanin in the embryonic and intact adult DRG and after axotomy.
The 1.9 kb Gal-LacZ construct
A second construct was generated that contained only 1.9 kb of sequence upstream of the galanin gene, the 3.5 kb LacZ gene, and the entire 4.6 kb galanin coding region (Fig. 1A) (hereafter referred to as 1.9 kb Gal-LacZ construct). Five founder mice were generated by microinjection of the 1.9 kb Gal-LacZ transgene, and both copy number and transmission were assessed by Southern blot analysis. As one of the lines did not transmit the transgene, β-galactosidase expression was measured in that founder animal. Expression data are summarized in Table 1, with none of the 1.9 kb lines showing any expression of the transgene in the adult DRG either before or after axotomy (data not shown). Similarly, no β-galactosidase expression was seen in the embryo at E17 in the four lines that transmitted the transgene (data not shown).
The lack of any β-galactosidase expression in either the embryo or adult in any of the 1.9 kb lines implies that neither the 1.9 kb of proximal promoter nor intronic enhancers are sufficient for either basal expression of galanin or the marked upregulation that occurs after axotomy.
The 4.6 kb Gal-LacZ construct
To define which part of the 18 kb region upstream of the proximal 1.9 kb galanin enhancer was required for the embryonic and adult expression and the response to axotomy (as observed with the 20 kb Gal-LacZ construct), a further transgene with 4.6 kb of upstream sequence was constructed (containing 4.6 kb of sequence upstream of the galanin gene, the 3.5 kb LacZ gene, and the entire 4.6 kb galanin coding region) (Fig. 1A) (hereafter referred to as 4.6 kb Gal-LacZ construct). Three founder mice were generated, and both copy number and transmission were assessed by Southern blot analysis. Lines were bred from all founders, and expression data are summarized in Table 1. Expression in the intact adult DRG (Fig. 2, top) was very similar to that observed in the 20 kb transgene (Fig. 1). Each line was axotomized and all demonstrated a marked increase both in the numbers of positive cells and the levels of β-galactosidase activity in the DRG (Fig. 2, middle). Also, E17 embryos from two of the 4.6 kb lines were harvested, and high levels of β-galactosidase expression were noted in the DRG of both lines (Fig. 2, bottom).
Figure 2.
Representative photomicrographs demonstrating expression in three expressing 4.6 kb lines. For each line, the expression pattern was determined for a minimum of five animals. Expression similar in distribution and intensity to the 20 kb line was noted in the intact DRG (top) after axotomy (middle) and in the embryonic DRG harvested at day 17 of gestation from the two highest expressing lines (bottom).
Therefore, we have demonstrated by transgenic analysis that a 2.7 kb region of DNA (nucleotides −4576 to −1850) 5′ to the galanin gene is necessary to direct expression to the embryonic and intact adult DRG and after axotomy.
Sequence analysis of the 4.6 kb region
The 4.6 kb of DNA [containing the adjacent 2.7 kb (nucleotides −4576 to −1850) and 1.9 kb (nucleotides −1849 to −1) regions 5′ to the galanin gene] were sequenced and checked for putative TF-binding sites using MatInspector (Quandt et al., 1995). The 3′ 620 bp is identical to that reported previously (Kofler et al., 1996). Within the upstream 2.7 kb region, an 18 bp region was identified that contained a single identity to the Smad3/Smad4 palindromic consensus binding site 5′-GTCTAGAC-3′ (Zawel et al., 1998) (−2441), adjacent to a putative Stat6-binding site 5′-TTC[N]4GAA-3′ (Leonard and O'Shea, 1998) (−2451). Overlapping these two binding sites is a putative Ets core binding site 5′-GGAA/T-3′ (Seth and Watson, 2005) (−2445) with high sequence similarity to a well characterized Ets-binding site 5′-TGAGGAAGTa-3′ (Bosc and Janknecht, 2002). One other region of 23 bp was also identified containing a Smad-binding sequence variant 5′-GTCTAGtC-3′ (−4326) with a putative Stat-binding site 13 bp downstream (TT[N]5/6AA, −4306), which did not contain and was not adjacent to a putative Ets-binding site. In contrast, the proximal 1.9 kb segment of DNA (which does not confer axotomy-inducibility), lacks a palindromic consensus Smad-binding site, although it does contain two Smad-binding sequence variants (20) 5′-GTCTgGct-3′ (−1607) and 5′-GTCTgGAt-3′ (−533), neither of these Smad-binding sequence variants have adjacent putative Stat- or Ets-binding sites.
Sequence analysis also identified a small number of other putative TF-binding sites that are present in the 2.7 kb region, but not in either the 1.9 kb of proximal promoter or the 4.6 kb of galanin gene: single p53 and Sry sites, three CDP (CCAAT displacement proteins), transcriptional repressor sites, and five sites to specific Fox (forkhead-box) proteins (data not shown).
Deletion of the 23 bp 5′ Stat/Smad and 18bp Stat/Smad/Ets putative binding sites from the 4.6 kb construct
We next used site-directed mutagenesis to remove both the 23 bp 5′ Stat/Smad and 18 bp 3′ Stat/Smad/Ets putative binding sites from the 4.6 kb-LacZ construct (hereafter referred to as the 4.6Δ23,18 kb construct).
Three founder mice were generated from the 4.6Δ23,18 kb construct and both copy number and transmission were assessed by Southern blot analysis. Lines were bred from all founders, and expression data are summarized in Table 1. Results demonstrate that expression in the intact adult DRG (Fig. 3A, top) and at E17 (Fig. 3A, bottom) was similar to that observed in the 20 kb and wild-type 4.6 kb constructs (Figs. 1 and 2, respectively). In contrast, the widespread upregulation in β-galactosidase staining in the DRG after axotomy is completely abolished, and the number of β-galactosidase-positive neurons in the DRG does not change after axotomy (Fig. 3A, middle).
Figure 3.
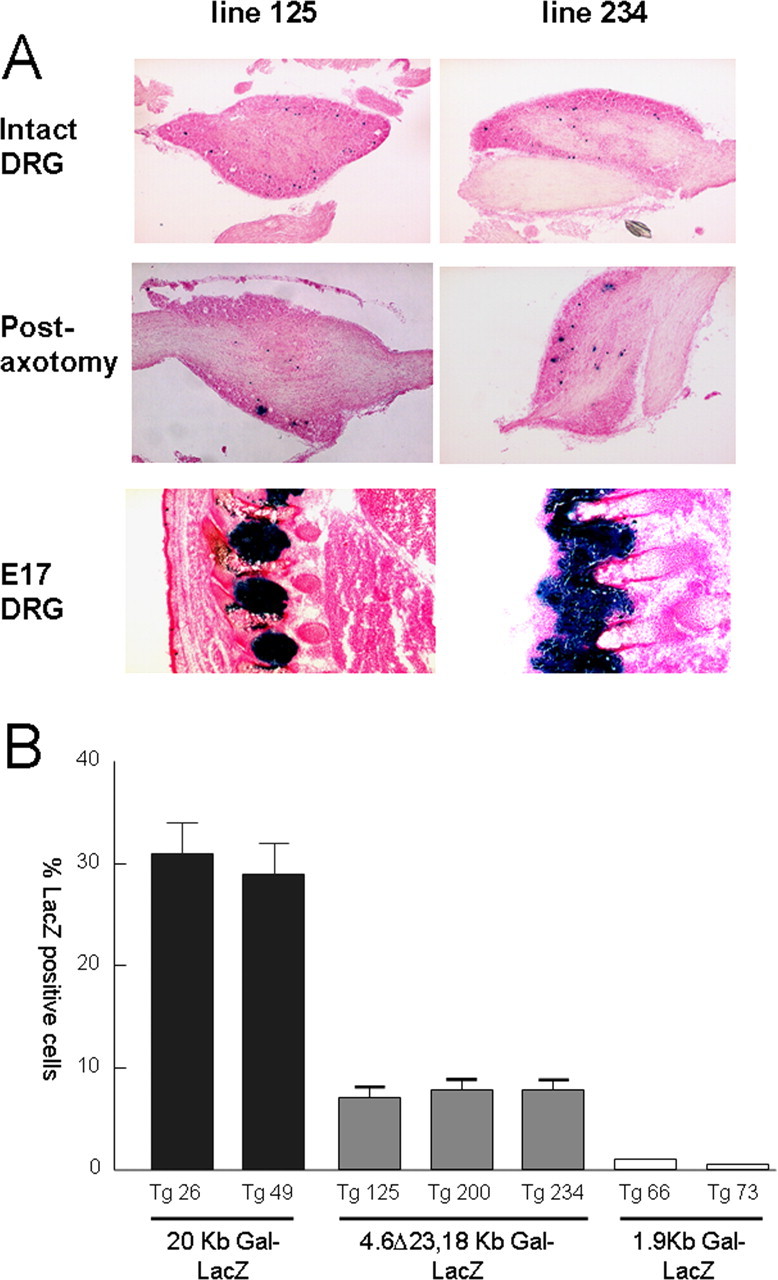
A, Representative photomicrographs demonstrating expression in two expressing 4.6Δ23, 18 kb lines. For each line, the expression pattern was determined for a minimum of five animals. Expression similar in distribution and intensity to the 20 kb line was noted in the intact DRG (top) and in the embryonic DRG harvested at day 17 of gestation (bottom). In contrast, expression in the adult DRG after axotomy was completely abolished. B, Quantitation of the number of β-galactosidase-positive cells in adult DRG neurons after 3 d in culture. From the adult DRG neurons, 30 ± 3% of the 20 kb transgenic animals were strongly positive for β-galactosidase staining. No β-galactosidase staining was noted in neurons harvested from any of the 1.9 kb lines. Only 8 ± 1% of DRG neurons exhibited β-galactosidase staining in the three 4.6Δ23,18 kb lines.
To further quantitate these findings, we used an in vitro dispersed DRG assay as a model of axotomy, as described previously (Mahoney et al., 2003), which allows us to accurately measure the number of β-galactosidase-positive cells after 3 d in culture. Of the adult DRG neurons obtained, 30 ± 3% from the 20 kb transgenic animals were strongly positive for β-galactosidase staining (Fig. 3B). This figure is identical to that obtained in a previous study that quantified the number of galanin-positive neurons (determined by immunocytochemistry) in the rat DRG after 3 d in culture (Kerekes et al., 1997). No β-galactosidase staining was noted in neurons harvested from any of the 1.9 kb lines (Fig. 3B). Only 8 ± 1% of DRG neurons exhibited β-galactosidase staining in the three 4.6Δ23,18 kb lines (Fig. 3B), similar to the percentage of galanin-positive neurons described previously in the intact adult mouse and rat DRG (Hokfelt et al., 1987; Holmes et al., 2003).
Sequence analysis of the enhancer regions of other axotomy-responsive genes
In light of the above findings demonstrating that Stat/Smad/Ets putative binding sites appear to be crucial to the axotomy-induced upregulation of galanin in the DRG, we used bioinformatics to analyze how frequently adjacent or overlapping putative Ets (Seth and Watson, 2005), Stat (Seidel et al., 1995), and Smad variant (Yingling et al., 1997; Dennler et al., 1998; Zawel et al., 1998; Zhang et al., 1998; Kusanagi et al., 2000) binding sites occurred in the proximal 10 kb enhancer regions of the vasoactive intestinal polypeptide (VIP), neuropeptide Y (NPY), and growth-associated polypeptide (GAP-43) genes, focusing on regions that were highly conserved between the mouse and rat. These three proteins were chosen because they are also known to be expressed in the DRG, upregulate their expression after axotomy, and have been shown previously to be important for regeneration of sensory neurons and/or modulation of nociception (for review, see Strittmatter et al., 1992; Schreyer and Skene, 1993; Klimaschewski, 1997; Hokfelt et al., 2007; Smith et al., 2007).
Table 2 demonstrates that in each of the three genes, at least two regions can be identified within 10 kb of the transcriptional start site that are conserved between the mouse and rat and contain adjacent or overlapping Stat, Smad, and Ets putative binding sites. Of note, no absolute conservation of the 23 or 18 bp regions found within the 2.7 kb galanin enhancer were identified in the proximal 10 kb upstream regions of the VIP, NPY, and GAP-43 genes.
Table 2.
Adjacent or overlapping putative Ets- Stat- or Smad-binding sequences (or reverse complement sequences) that were identical or highly similar in the mouse and rat VIP, NPY, and GAP-43 genes are shown
| Position | |
|---|---|
| Mouse VIP (gene sequence NW_000022) | |
| TTTGTGAA(N)12GTCTTGAC(N)21TTCC | nucleotide −9378 |
| TTTGCAGGAA(N)21TTTCTGCCT(N)15TTCCa | nucleotide −2374 |
| TTTATGGAAAGACAGACC(N)4GGAAb | nucleotide −2152 |
| TTTCCTGGAA(N)10GGAA (N)10AGTCAGACCc | nucleotide −1301 |
| Mouse GAP-43 (gene sequence NT_039624) | |
| TTCCTGAAA (N)13TCTGTCTGGCAc,d | nucleotide −9617 |
| AGCCAGAAGAATTGAATAAGGGAA | nucleotide −8462 |
| Mouse NPY (gene sequence NT_039343) | |
| GGACAGACT(N)5GGAA (N)13TTGGGAGTAA | nucleotide −8146 |
| TTCCTCTTAA(N)17GTCTAGTTb | nucleotide −7915 |
Ets core consensus sites (GGAA) are underlined, Stat consensus sites (TT[N]4–6AA) are in bold, and sequences similar to Smad-binding sites are italicized. Previously published specific consensus sites are referenced and denoted by superscripts. The location of the sequences that are shown are relative to exon 1 of the mouse VIP gene (Lamperti et al., 1991), the 5′ end of the mouse GAP-43 cDNA sequence (NM_008083) (Strausberg et al., 2002) that is equivalent to the rat P2 promoter transcription start site (Eggen et al., 1994), and to the 5′ end of the mouse NPY cDNA sequence (NM_023456) (Strausberg et al., 2002).
aSimilar to Stat-binding sites CTTC(N)3/4GAAG (Kraus et al., 2003).
bTTA (N)3GAA Stat-binding sites (Becker et al., 1998).
cPutative Stat-binding site with consensus sequence TTC(N)2–4GAA (Hebenstreit et al., 2006).
dSimilar to Stat-binding sites CTTC(N)3/4GAAG (Kraus et al., 2003).
Discussion
Despite the large body of data demonstrating that DRG galanin expression markedly increases after axotomy and modulates pain behavior and regeneration of sensory neurons, the trophic factors and down-stream TFs that mediate galanin gene transcription have yet to be fully elucidated. Previous work has shown that intrathecal infusion of NGF reduces axotomy-induced DRG galanin expression (Verge et al., 1995), whereas chronic peripheral injection of an NGF antiserum increases galanin synthesis and content in the uninjured DRG (Shadiack et al., 2001). Also, NGF reduces the expression of galanin in cultured rat DRG neurons (Kerekes et al., 1997). In contrast to the inhibitory effects of NGF, the cytokines LIF and IL-6 positively regulate galanin gene expression in the DRG, and injection of either protein into the sciatic nerve significantly increases galanin expression in the DRG (Thompson et al., 1998; Murphy et al., 1999). Conversely, axotomy-induced upregulation of galanin is markedly attenuated in LIF knock-out mice (Sun and Zigmond, 1996; Corness et al., 1996). Both LIF and IL-6 bind to the gp130 coreceptor and are thought to principally regulate target gene activation by the phosphorylation of Jak2 and Stat3 (Thompson et al., 1998). Interestingly, IL-6-mediated Stat activation is inhibited by NGF in superior cervical ganglion explant cultures, implying cross talk between the two signaling pathways after axotomy (Rajan et al., 1998).
Together, the above studies demonstrate that a number of neurotrophins and cytokines may mediate, at least in part, the upregulation of galanin that occurs after nerve injury, and implicates the Jak-Stat signaling pathway in this process. In the present study, we used transgenesis to map and analyze the enhancer regions of the murine galanin gene that regulate expression of galanin gene transcription in the embryonic DRG, the intact adult DRG, and the upregulation in response to axotomy. Unexpectedly, we found that deletion of both an 18 bp region (∼2.5 kb upstream of the transcriptional start site, with overlapping putative Stat-, Smad-, and Ets-binding sites) and a 23 bp region (∼4.3 kb upstream of the transcriptional start site, with adjacent Stat- and Smad-binding sites) abolishes the upregulation of galanin in the adult DRG after axotomy, but has no effect on galanin expression in the embryonic or intact adult DRG. At present, we cannot definitively state which of the two enhancer regions are more important to the axotomy response. These findings are of considerable interest in view of the continuing debate whether responses to peripheral nerve injury in the adult merely recapitulate development or can be independent of developmental expression. Our data demonstrates that whereas galanin is expressed during development and in the adult after injury, different regulatory elements appear to control these two transcriptional events, similar to that previously described for the GAP43 gene in zebrafish (Udvadia et al., 2001). In contrast, a small number of genes have been characterized (e.g., the small proline-rich repeat protein 1A) that are not expressed in the embryonic or intact adult DRG, and levels are detectable in the DRG solely after nerve injury (Bonilla et al., 2002).
The bioinformatic data implies that one or more members of the Ets, Stat, and/or Smad families of TFs may regulate galanin expression in the DRG after nerve injury. Furthermore, the finding that three other axotomy-responsive proteins also have putative binding sites for Ets, Stat, and Smad in close proximity in their upstream promoters implies that this may represent a common motif that underlies, at least in part, injury-induced upregulation in gene expression in the DRG. Many of the family members of each of these TFs are known to be expressed in the DRG. To date, 29 members of the Ets family of TFs have been described that share a highly conserved DNA-binding domain and an invariant DNA core-binding site (for review, see Gallant and Gilkeson, 2006). Members of the PEA-3 subfamily of Ets proteins have been identified in the embryonic DRG and spinal cord where they have been implicated in sensory and motor neuron survival (Arber et al., 2000; Ladle and Frank, 2002). Smad proteins are the target TFs of a family of serine-threonine kinase receptors that are activated by activins and bone morphogenic proteins (BMPs). Expression of Smad and many of the BMP family members have been detected in rat DRG (Farkas et al., 1999; Flanders et al., 2001; Cruise et al., 2004). Similarly, Stat3 is expressed in the DRG (Stromberg et al., 2000) and a sustained activation of Stat proteins occurs after axotomy (Schwaiger et al., 2000).
Interactions between Stat and Smad TFs have been described previously that mediate synergistic signaling between LIF and BMPs in cultures of fetal neuronal precursors (Nakashima et al., 1999) and embryonic astrocytes (Yanagisawa et al., 2001). Also, Smad/Ets (Lindemann et al., 2001; Jinnin et al., 2004) and Stat/Ets (Rameil et al., 2000) complexes have also been reported. Whether direct interactions between Stat, Smad, or Ets family members occurs and regulates galanin expression has yet to be established.
In summary, we have used transgenesis to map and analyze the enhancer regions of the galanin gene. A number of mouse lines with varying amounts of sequence upstream of the murine galanin gene (20, 4.6, and 1.9 kb each marked by the LacZ gene) have been generated and characterized. Here, we demonstrate that two regions upstream of the transcriptional start site of galanin appear to be critical to the upregulation of the peptide in the DRG after axotomy but not to expression in the embryonic or intact adult DRG. These data imply that Ets, Stat, and/or Smad proteins not only regulate galanin expression, but also control the transcription of other axotomy-responsive genes in the DRG. If these nuclear protein complexes and the genes they regulate can be further defined and dissected, then that may lead to the identification of new therapeutic targets for the treatment of neuropathy and associated neuropathic pain.
Footnotes
This work was supported by the Medical Research Council. We gratefully acknowledge the tuition and advice provided by Dr. P. Ashby (National Institute for Medical Research, London, UK) relating to the generation of transgenic mice.
References
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Bacon A, Holmes FE, Small CJ, Ghatei M, Mahoney S, Bloom SR, Wynick D. Transgenic over-expression of galanin in injured primary sensory neurons. NeuroReport. 2002;13:2129–2132. doi: 10.1097/00001756-200211150-00028. [DOI] [PubMed] [Google Scholar]
- Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394:145–151. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosc DG, Janknecht R. Regulation of Her2/neu promoter activity by the ETS transcription factor, ER81. J Cell Biochem. 2002;86:174–183. doi: 10.1002/jcb.10205. [DOI] [PubMed] [Google Scholar]
- Corness J, Shi TJ, Xu ZQ, Brulet P, Hokfelt T. Influence of leukemia inhibitory factor on galanin/GMAP and neuropeptide Y expression in mouse primary sensory neurons after axotomy. Exp Brain Res. 1996;112:79–88. doi: 10.1007/BF00227180. [DOI] [PubMed] [Google Scholar]
- Cruise BA, Xu P, Hall AK. Wounds increase activin in skin and a vasoactive neuropeptide in sensory ganglia. Dev Biol. 2004;271:1–10. doi: 10.1016/j.ydbio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen BJ, Nielander HB, Rensen-de Leeuw MG, Schotman P, Gispen WH, Schrama LH. Identification of two promoter regions in the rat B-50/GAP-43 gene. Brain Res Mol Brain Res. 1994;23:221–234. doi: 10.1016/0169-328x(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Farkas LM, Jaszai J, Unsicker K, Krieglstein K. Characterization of bone morphogenetic protein family members as neurotrophic factors for cultured sensory neurons. Neuroscience. 1999;92:227–235. doi: 10.1016/s0306-4522(98)00735-0. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1–6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-betas. Dev Dyn. 2001;220:141–154. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1096>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gallant S, Gilkeson G. ETS transcription factors and regulation of immunity. Arch Immunol Ther Exp (Warsz) 2006;54:149–163. doi: 10.1007/s00005-006-0017-z. [DOI] [PubMed] [Google Scholar]
- Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. New York: Cold Spring Harbor Laboratory; 1994. Manipulating the mouse embryo. [Google Scholar]
- Hokfelt T, Wiesenfeld HZ, Villar M, Melander T. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci Lett. 1987;83:217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Wiesenfeld HZ. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17:22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Brumovsky P, Shi T, Pedrazzini T, Villar M. NPY and pain as seen from the histochemical side. Peptides. 2007;28:365–372. doi: 10.1016/j.peptides.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Holmes FE, Mahoney S, King VR, Bacon A, Kerr NCH, Pachnis V, Curtis R, Priestley JV, Wynick D. Targeted disruption of the galanin gene reduces the number of sensory neurons and their regenerative capacity. Proc Natl Acad Sci USA. 2000;97:11563–11568. doi: 10.1073/pnas.210221897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes FE, Bacon A, Pope RJ, Vanderplank PA, Kerr NC, Sukumaran M, Pachnis V, Wynick D. Transgenic overexpression of galanin in the dorsal root ganglia modulates pain-related behavior. Proc Natl Acad Sci USA. 2003;100:6180–6185. doi: 10.1073/pnas.0937087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygge-Blakeman K, Brumovsky P, Hao JX, Xu XJ, Hokfelt T, Crawley JN, Wiesenfeld-Hallin Z. Galanin over-expression decreases the development of neuropathic pain-like behaviors in mice after partial sciatic nerve injury. Brain Res. 2004;1025:152–158. doi: 10.1016/j.brainres.2004.07.078. [DOI] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Tenascin-C upregulation by transforming growth factor-beta in human dermal fibroblasts involves Smad3, Sp1, and Ets1. Oncogene. 2004;23:1656–1667. doi: 10.1038/sj.onc.1207064. [DOI] [PubMed] [Google Scholar]
- Kerekes N, Landry M, Rydh RM, Hokfelt T. The effect of NGF, BDNF and bFGF on expression of galanin in cultured rat dorsal root ganglia. Brain Res. 1997;754:131–141. doi: 10.1016/s0006-8993(97)00056-5. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L. VIP – a ‘very important peptide' in the sympathetic nervous system? Anat Embryol (Berlin) 1997;196:269–277. doi: 10.1007/s004290050096. [DOI] [PubMed] [Google Scholar]
- Kofler B, Liu ML, Jacoby AS, Shine J, Iismaa TP. Molecular cloning and characterisation of the mouse preprogalanin gene. Gene. 1996;182:71–75. doi: 10.1016/s0378-1119(96)00477-5. [DOI] [PubMed] [Google Scholar]
- Kraus J, Borner C, Hollt V. Distinct palindromic extensions of the 5′-TTC.GAA-3′ motif allow STAT6 binding in vivo. FASEB J. 2003;17:304–306. doi: 10.1096/fj.02-0482fje. [DOI] [PubMed] [Google Scholar]
- Kusanagi K, Inoue H, Ishidou Y, Mishima HK, Kawabata M, Miyazono K. Characterization of a bone morphogenetic protein-responsive Smad-binding element. Mol Biol Cell. 2000;11:555–565. doi: 10.1091/mbc.11.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladle DR, Frank E. The role of the ETS gene PEA3 in the development of motor and sensory neurons. Physiol Behav. 2002;77:571–576. doi: 10.1016/s0031-9384(02)00907-1. [DOI] [PubMed] [Google Scholar]
- Lamperti ED, Rosen KM, Villa-Komaroff L. Characterization of the gene and messages for vasoactive intestinal polypeptide (VIP) in rat and mouse. Brain Res Mol Brain Res. 1991;9:217–231. doi: 10.1016/0169-328x(91)90005-i. [DOI] [PubMed] [Google Scholar]
- Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- Lindemann RK, Ballschmieter P, Nordheim A, Dittmer J. Transforming growth factor beta regulates parathyroid hormone-related protein expression in MDA-MB-231 breast cancer cells through a novel Smad/Ets synergism. J Biol Chem. 2001;276:46661–46670. doi: 10.1074/jbc.M105816200. [DOI] [PubMed] [Google Scholar]
- Liu HX, Brumovsky P, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hokfelt T. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: Selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney SA, Hosking R, Farrant S, Holmes FE, Jacoby AS, Shine J, Iismaa TP, Scott MK, Schmidt R, Wynick D. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J Neurosci. 2003;23:416–421. doi: 10.1523/JNEUROSCI.23-02-00416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PG, Ramer MS, Borthwick L, Gauldie J, Richardson PM, Bisby MA. Endogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. Eur J Neurosci. 1999;11:2243–2253. doi: 10.1046/j.1460-9568.1999.00641.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan P, Gearan T, Fink JS. Leukemia inhibitory factor and NGF regulate signal transducers and activators of transcription activation in sympathetic ganglia: convergence of cytokine- and neurotrophin-signaling pathways. Brain Res. 1998;802:198–204. doi: 10.1016/s0006-8993(98)00611-8. [DOI] [PubMed] [Google Scholar]
- Rameil P, Lecine P, Ghysdael J, Gouilleux F, Kahn-Perles B, Imbert J. IL-2 and long-term T cell activation induce physical and functional interaction between STAT5 and ETS transcription factors in human T cells. Oncogene. 2000;19:2086–2097. doi: 10.1038/sj.onc.1203542. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J Neurobiol. 1993;24:959–970. doi: 10.1002/neu.480240709. [DOI] [PubMed] [Google Scholar]
- Schwaiger FW, Hager G, Schmitt AB, Horvat A, Hager G, Streif R, Spitzer C, Gamal S, Breuer S, Brook GA, Nacimiento W, Kreutzberg GW. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT) Eur J Neurosci. 2000;12:1165–1176. doi: 10.1046/j.1460-9568.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- Seidel HM, Milocco LH, Lamb P, Darnell-JE J, Stein RB, Rosen J. Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc Natl Acad Sci USA. 1995;92:3041–3045. doi: 10.1073/pnas.92.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Shadiack A, Sun Y, Zigmond R. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci. 2001;21:363–371. doi: 10.1523/JNEUROSCI.21-02-00363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Galanin-like immunoreactivity in capsaicin sensitive sensory neurons and ganglia. Brain Res Bull. 1985;15:191–195. doi: 10.1016/0361-9230(85)90135-2. [DOI] [PubMed] [Google Scholar]
- Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;28:464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter SM, Vartanian T, Fishman MC. GAP-43 as a plasticity protein in neuronal form and repair. J Neurobiol. 1992;23:507–520. doi: 10.1002/neu.480230506. [DOI] [PubMed] [Google Scholar]
- Stromberg H, Svensson SP, Hermanson O. Distribution of the transcription factor signal transducer and activator of transcription 3 in the rat central nervous system and dorsal root ganglia. Brain Res. 2000;853:105–114. doi: 10.1016/s0006-8993(99)02260-x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur J Neurosci. 1996;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Thippeswamy T, Haddley K, Corness J, Howard MR, McKay JS, Beaucourt SM, Pope MD, Murphy D, Morris R, Hokfelt T, Quinn JP. NO-cGMP mediated galanin expression in NGF-deprived or axotomized sensory neurons. J Neurochem. 2007;100:790–801. doi: 10.1111/j.1471-4159.2006.04243.x. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Priestley JV, Southall A. gp130 cytokines, leukemia inhibitory factor and interleukin-6, induce neuropeptide expression in intact adult rat sensory neurons in vivo: time-course, specificity and comparison with sciatic nerve axotomy. Neuroscience. 1998;84:1247–1255. doi: 10.1016/s0306-4522(97)00553-8. [DOI] [PubMed] [Google Scholar]
- Udvadia AJ, Koster RW, Skene JH. GAP-43 promoter elements in transgenic zebrafish reveal a difference in signals for axon growth during CNS development and regeneration. Development. 2001;128:1175–1182. doi: 10.1242/dev.128.7.1175. [DOI] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Wiesenfeld HZ, Hokfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J Neurosci. 1995;15:2081–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld HZ, Bartfai T, Hokfelt T. Galanin in sensory neurons in the spinal cord. Front Neuroendocrinol. 1992;13:319–343. [PubMed] [Google Scholar]
- Wynick D, Small CJ, Bacon A, Holmes FE, Norman M, Ormandy CJ, Kilic E, Kerr NCH, Ghatei M, Talamantes F, Bloom SR, Pachnis V. Galanin regulates prolactin release and lactotroph proliferation. Proc Natl Acad Sci USA. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Hokfelt T, Bartfai T, Wiesenfeld-Hallin Z. Galanin and spinal nociceptive mechanisms: recent advances and therapeutic implications. Neuropeptides. 2000;34:137–147. doi: 10.1054/npep.2000.0820. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Hokfelt T. Expression of galanin and a galanin receptor in several sensory systems and bone anlage of rat embryos. Proc Natl Acad Sci USA. 1996;93:14901–14905. doi: 10.1073/pnas.93.25.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Nakashima K, Takizawa T, Ochiai W, Arakawa H, Taga T. Signaling crosstalk underlying synergistic induction of astrocyte differentiation by BMPs and IL-6 family of cytokines. FEBS Lett. 2001;489:139–143. doi: 10.1016/s0014-5793(01)02095-6. [DOI] [PubMed] [Google Scholar]
- Yingling JM, Datto MB, Wong C, Frederick JP, Liberati NT, Wang XF. Tumor suppressor Smad4 is a transforming growth factor beta-inducible DNA binding protein. Mol Cell Biol. 1997;17:7019–7028. doi: 10.1128/mcb.17.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]