Abstract
The central gustatory pathways are part of the brain circuits upon which rest the decision to ingest or reject a food. The quality of food stimuli, however, relies not only on their taste but also on properties such as odor, texture and temperature. We will review anatomical and functional evidence showing that the central gustatory system, in particular its cortical aspect, functions as an integrative circuit where taste-responsive neurons also display sensitivity to somatosensory and olfactory stimulation. In addition, gustatory pathways are modulated by the internal state of the body, with neuronal responses to tastes changing according to variations in physiological parameters such as gastrointestinal hormones and blood glucose levels. Therefore, rather than working as the receptive field of peripheral taste receptor cells, the central gustatory pathways seem to operate as a multisensory system dedicated to evaluate the biological significance of intra-oral stimuli.
Keywords: taste, gustatory cortex, multisensory integration, feeding
I. Introduction
When we are constantly and simultaneously bombarded with various types of sensory inputs, which brain mechanisms allow us to deal with the world in a meaningful manner? The problem of multisensory integration essentially refers to the set of brain processes involved in integrating incoming sensory inputs from several modalities, allowing for the formation of unified perceptual objects and consequently for appropriate behavioral responses to be generated1. In many cases, survival of an organism depends on appropriate responses to multisensory stimuli. Selecting foods for ingestion is a clear instance of a multisensory problem that must be solved, promptly and correctly, by any organism. In fact, the placing of food in the mouth simultaneously generates taste, olfactory and somatosensory (texture, temperature) inputs to the central nervous system. Once a stimulus is inside the oral cavity, the decision to ingest or reject it will depend on the evaluation of its multisensory aspects2,3 given that not only taste, but also other attributes such as odor and consistency, will function as cues to the nutritive value or potential toxicity of the stimulus.
Which brain regions control ingestive behaviors based on these multiple, simultaneous sensory inputs from the oral cavity and viscera? Much progress has been made recently on unveiling both the peripheral and central mechanisms of gustation3. One pattern emerging from these recent findings concerns the sensitivity of the central gustatory pathways to multiple sensory inputs arising from the oral cavity. In this review, we will focus on the apparent multisensory functions of the primary gustatory cortex (GC). In fact, both electrophysiological and functional neuroimaging studies make a strong case in favor of the hypothesis that the functions of the gustatory cortex are not restricted to reflecting taste receptor activity. In what follows, we will review evidence of multisensory responses in GC and propose that this primary sensory cortical region works as an integrative circuit, having the capability to encode multiple physical-chemical attributes of stimuli placed in the oral cavity.
II. Oral somatosensory olfactory, and gustatory inputs, contribute to the control of ingestive behaviors
We start by noting that not only gustatory, but also several other sensory attributes of intra-oral stimuli contribute to the organisms’ decision to ingest or reject foods. For example, a beloved sweet drink will evoke nociceptive responses and likely be rejected if served extremely hot. Usually acceptable and tasty foods, like fruits or meats, can eventually be rejected if associated with abnormal texture (e.g. sogginess) or unusual odors since both would indicate the potential presence of toxins. Therefore, it is not surprising that other oral senses modulate taste sensations and that a particular brain circuit should have evolved to assess the multisensory properties of intra-oral stimuli.
Let us take the exemplar case of temperature. In general, temperature has a strong influence on how we perceive the taste of foods4,5. For example, warming the anterior tongue from a cold temperature evokes the subjective sensation of sweetness6, whereas cooling can evoke sourness and/or saltiness7. Although many cellular processes are temperature dependent, recent attention has been given to the family of transient receptor potential (TRP) channels8. Trigeminal cold fibers express the channel TRPM8, which is activated by cooling and also by menthol, a compound that produces a cooling sensation. TRPV1 is found in trigeminal nociceptors and it is activated at temperatures above 40°C and also by capsaicin, the principle pungent compound in chili pepper that produces a burning taste sensation8. TRPM5 is found in type II taste cells9. This channel is sensitive to changes in both intracellular calcium levels and voltage, and is important for the transduction of sweet, bitter and umami tastes10. TRPM5 is also temperature dependent, in that its activation is triggered by increasing temperatures11. Because TRPM5 is expressed in the taste receptor cells of taste buds12, it has been associated with increased perceived sweetness with as a function of temperature11. For salt and sour tastants, temperature dependence can arise respectively from the temperature-dependent activation of epithelial sodium channels13 or proton-sensitive channels of the Polycystic Kidney Disease (PKD) family10,14, or from the intracellular pathway for weak acids15. More recently it has been shown that quinine inhibits TRPM5 channels and also reduces chorda tympani (CT) responses to sweet tastants16. The CT is a branch of the facial nerve innervating taste cells of the anterior 2/3 of the tongue. It does not affect salty or sour taste CT responses. These data may explain why bitter tastants inhibit human perception of sweet taste16.
More generally, most foods when masticated simultaneously activate the gustatory, somatosensory and olfactory systems – the latter via a retronasal route. One obvious example is fat, which after being degraded by lingual lipases to produce free-fatty acids activates receptors on taste cells17,18, whereas information about the texture and viscosity is conveyed by the somatosensory system19,20. Moreover, depending on the temperature and chain length of the fatty acid, low molecular weight fatty acids could, via the retronasal route, activate the olfactory system21. Other selected examples of chemicals that can activate multiple sensory systems3,9,22–25 include: NaCl (taste – the sensation of saltiness at relatively low concentrations; somatosensory – irritation at higher concentrations); acids (taste – sourness; somatosensory – nociception, olfactory – e.g., acetic acid), nicotine (taste – the sensation of bitterness at relatively low concentrations; somatosensory – burning at higher concentrations); and artificial sweeteners – the sensation of sweetness at relatively low concentrations; bitterness at higher concentrations as well as somatosensory – “metallic taste.”
In summary, a multitude of sensorial experiences can be evoked at once upon placing foods in the mouth. We will now review evidence that many of those modalities have their inputs centrally represented as neural activity in GC.
III. Anatomy of the central gustatory and oral somatosensory systems
Gustatory information from taste buds located on the anterior tongue and palate is transmitted to the brain via special sensory branches of the facial (VII) nerve. Somatosensory information from these same areas is transmitted via the trigeminal (V) nerve. Other regions of the oral cavity that contain taste buds such as the posterior tongue, the pharynx, larynx and epiglottis are innervated either by the glossopharyngeal (IX) or vagus (X) nerves26. Importantly, cranial nerves IX and X have both “special” (i.e. taste-specific) and “general” sensory neurons. In addition to containing mechano- and thermo-sensors, that transduce information on textural and thermal properties of intra-oral stimuli, the general sensory branches of cranial nerves IX and X also express polymodal nociceptors that are responsive to chemical stimuli (in the anterior tongue, the lingual branch of cranial nerve V has polymodal nociceptors).
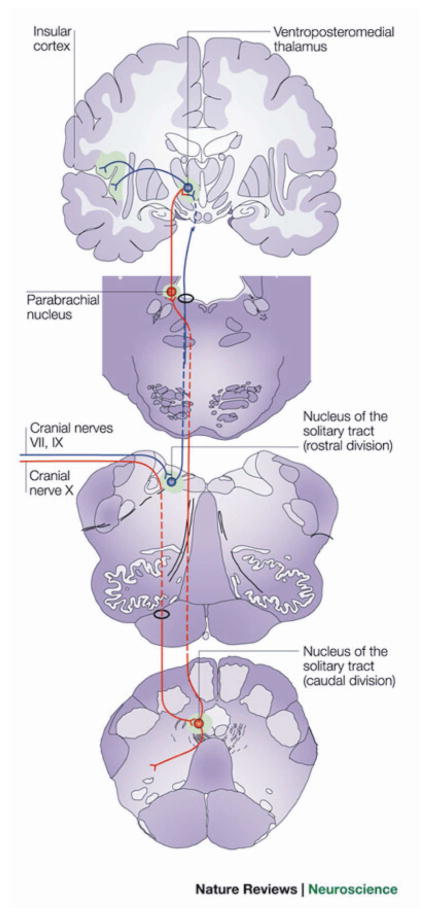
Figure 1 depicts a schematic view of the central taste pathways in mammals. Cranial nerves VII, IX and X transmit electrical signals that convey the chemical properties and quantity of tastants to the rostral division of the Nucleus of the Solitary Tract (NST) of the medulla, the principal visceral sensory nucleus of the brainstem. In rodents, second-order fibers (i.e. NST afferents) project ipsilaterally to the gustatory parabrachial nuclei (PBN) in the pons, proceeding then to the parvicellular part of the ventroposterior medial nucleus of the thalamus (VPMpc). In primates, however, the NST projection fibers bypass the PBN only to join the central tegmental tract and synapse directly into VPMpc. Thus, in primates, PBN circuits seem to be dedicated to convey general visceral information (e.g. from the vagus nerve) to specialized thalamic nuclei including the VPM27,28. The thalamic/cortical regions are depicted in Fig 1 based on the primate anatomy – the rodent case follows by analogy.
Figure 1. Anatomy of Gustatory –Reward Pathway.
Electrical signals from cranial nerves VII, IX and X that contain information on the chemical properties of tastants are conveyed to the rostral division of the nucleus tractus solitarius (rNTS) of the medulla, the principal visceral-sensory nucleus of the brainstem. In the rat, second-order fibers (that is, rNTS efferents) project ipsilaterally to gustatory centers in parabrachial nuclei (PBN) of the pons, from where a first (dorsal) pathway projects to the parvicellular part of the ventroposterior medial nucleus of the thalamus (VPMpc), the taste thalamic nucleus. The second (ventral) pathway includes direct projections from PBN to the central nucleus of the amygdala and lateral hypothalamus. In primates, however, the NTS projection fibers bypass the PBN only to join the central tegmental tract and synapse directly into the VPMpc, whereas the PBN seems to be dedicated to convey general visceral information (mainly through vagal afferents) to specialized thalamic nuclei. In either case, thalamic afferents then project to the primary gustatory cortex (GC), which is defined as the VPMpc cortical target. The VPMpc also sends projections to regions neighboring the primary somatosensory cortex, adjacent to the precentral gyrus, and that overlap with cortical somatotopic sites for the face and oral cavity. The primary taste cortex projects to the central nucleus of the amygdala, from where gustatory information reaches the lateral hypothalamus and midbrain dopaminergic regions. The primary taste cortex also projects anteriorly to the caudolateral orbitofrontal region, called the secondary taste cortex. Taste neurons in the caudolateral orbitofrontal cortex converge with cells receiving projections from the primary olfactory cortex, which might have implications for flavor perception. The orbitofrontal cortex is also targeted by projections from the lateral hypothalamus, allowing taste responses to be modulated by satiety states. Finally, cortical taste areas send afferents to the rNTS/PBN, allowing for top-down modulation of gustatory processing at the level of the brainstem. Blue, projections to rNTS; green, primary taste areas; red, projections to caudal NTS. (Used and modified with permission, originally published in Simon et al. 2006. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci 7:890–901.)
The primary taste cortex of mammals can be defined in terms of VPMpc afferents29. Pritchard et al. (1986) have studied the efferent projections of the VPMpc of the monkey, macaca fascicularis with tritiated amino acid autoradiography30. Two discrete cortical areas were characterized as a target of VPMpc projections. First, labeled cells were located in the ipsilateral insular-opercular cortex adjacent to the superior limiting sulcus and extending as far rostrally as the caudolateral orbitofrontal cortex. Moreover, further projections were located within the primary somatosensory cortex, in the precentral gyrus subjacent to the anterior subcentral nucleus (i.e. a precentral extension of the primary somatosensory cortex). This area is anterior to the VPM projection sites representing somatosensory information and is adjacent to or overlapping with the cortical somatotopic sites for the face and oral cavity 31. This area might be a target of somatosensory VPM and VPMpc projection fibers and thus implement the convergence in the cortex of the somatosensory and gustatory aspects of stimuli delivered in the mouth (see below).
Scott and Plata-Salaman (1999)29 defined the anterior limit of the primary taste cortex in the macaque as the junction of the orbitofrontal and opercular cortices, from which it extends 4.0 mm posteriorly. The mediolateral extension is defined ~16–19 mm lateral to the midline in an average adult macaque. The dorsal limit is defined as ~6 mm above the lateral fissure. The insular cortex, in the depth of the Sylvian fissure, has been divided into four rostrocaudal subdivisions: the most rostral portion has been designated the insular proisocortex; adjacent to it is the agranular subdivision of the insula, followed caudally by the dysgranular and the granular insular areas. In these terms, the VPMpc nucleus projects to the opercular and insular regions of the granular and dysgranular insula, and extends to adjacent agranular portions of the insula.
IV. The gustatory cortex (GC) in multisensory processing
Integration of gustatory, texture, temperature and olfactory inputs in GC
Electrophysiological recordings in monkeys29 provided an early indication that multisensory neuronal responses take place in primary taste cortex. In fact, a small proportion of cells in the primary taste cortex did actually respond exclusively and consistently to taste stimuli (~6.5%); a significantly higher proportion (~23%) responded during tongue or jaw movements, for example. This suggested in particular that the primate primary taste cortex might be simultaneously encoding both taste and oral somatosensory properties of intra-oral stimuli. However, we note that the number of cells in the gustatory cortex that are responsive to tastants is strongly dependent on several variables, including the method of analysis32,33.
More recently, human studies using functional neuroimaging methods such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) confirmed that homologous gustatory areas to those of primates are responsive to gustatory stimuli in humans, including the anterior insula/frontal operculum aspects of GC34–36. This includes responses to glucose, NaCl35, umami36, caffeine and citric acid37 (Fig 2A). More recently, intrinsic imaging studies revealed that rat gustatory areas are activated by salt, acid, bitter tastants and sweet tastants38 and, as seen below, many electrophysiological studies revealed, as expected, that GC is activated by a variety of tastants.
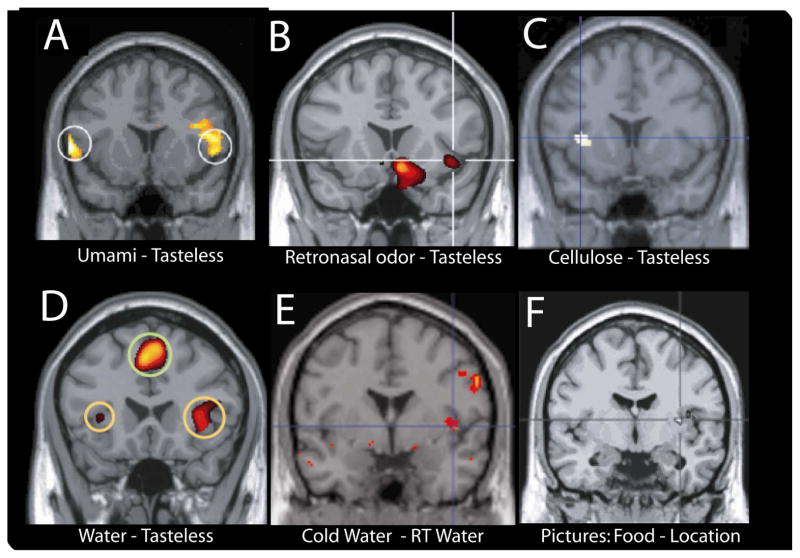
Figure 2. Multisensory inputs to the human GC.
A. Characterization of the human GC as defined by the responses to a prototypical taste stimulus (monosodium glutamate) subtracted from a control artificial saliva solution. Note that responses include and are restricted to the anterior insular and surrounding (frontal) opercular cortex (de Araujo et al. 2003a). B. A region of the human insular taste cortex, as defined by its response to a prototypical taste stimulus (sucrose), which is activated by olfactory stimuli, delivered by either retronasal or orthonasal routes (de Araujo et al. 2003c). C. A region of the human insular taste cortex, as defined by its response to a prototypical taste stimulus (glucose), which is activated by a purely somatosensory (viscous) stimulus, carboxymethylcellulose, and by fat oils (de Araujo and Rolls 2004). D. A region of the human insular/opercular taste cortex, as defined by its response to a prototypical taste stimulus (glucose), which is activated by water in the mouth subtracted from a control artificial saliva solution. (de Araujo et al. 2003b). E. A region of the human insular/taste cortex, as defined by its response to a prototypical taste stimulus (glucose), which is activated by water in the mouth at different temperatures subtracted from water at room temperature (Guest et al. 2007). F. A region of the human insular cortex which is activated by the viewing of food pictures subtracted from control location pictures. (Simmons et al. 2004). Noticeably, the exact anatomical locations of these activations are highly coincidental to the taste-related activations described above.
In addition, human studies also provide evidence that GC not only responds to the major perceptual categories of taste, but also support the encoding of the multisensory aspects of taste stimuli. In a study using taste and retronasal olfactory stimuli (and their combinations), de Araujo et al.39 have shown that taste and olfactory inputs to the human brain converge in the far anterior (putatively agranular) insular cortex. This region of the far anterior (agranular) insula is close to the part of the insular cortex where it adjoins the caudal orbitofrontal cortex. A homology between the rodent and primate cases with respect to the central anatomy of taste and olfactory integration has been previously suggested, and thus it is being proposed here that this homology would extend to humans to encompass at least three mammal species. See Fig 2B.
As mentioned, it has been found in monkeys that a representative number of neurons in the primary taste cortex respond to oral somatosensory/motor stimulation40,41. The same sensitivity to oral somatosensory inputs in GC seems to hold true for humans as well. In fact, it has been shown that activation of the human anterior insular (putative primary) taste cortex by tasteless viscous stimuli (carboxymethylcellulose, CMC) was proportional to the log of the viscosity, providing evidence of somatosensory/gustatory integration in primary taste cortex. Note that CMC, while allowing for manipulation of the degree of viscosity of a stimulus, is considered tasteless. It was also shown42 that GC is activated by the oral delivery of fatty vegetable oil, demonstrating that GC might use inputs from different sensory modalities to detect biologically (in this case nutritive) relevant stimuli in the oral cavity. See Fig 2C.
Another example of responses in the human primary taste cortex that are independent of the major perceptual categories of taste is activation to water in the mouth, when subtracted from activations produced by artificial saliva at the same level of viscosity43. This finding has later been confirmed in rats where neurons in GC were found to respond to water but not to some tastants, ruling out purely somatosensory effects44. Therefore, not only the stimulation of taste receptors, but substances generally relevant for behavior and survival seem to elicit responses in the mammal gustatory cortices. See Fig 2D.
Changes in intra-oral temperature levels also seem to modulate activity in GC. Indeed it has been shown45 that the same regions of the insular cortex activated by a prototypical taste stimulus (1M glucose) are also activated by thermal stimuli. For example, following delivery of intra-oral thermal stimuli (i.e. distilled water at different temperatures), activations in this anterior insula region were found in Guest et al. 45 for the contrasts “hot” (50°C –rinse) and “cold” (5°C –rinse), and “cold–hot” (5°C –50°C) (where “rinse” is defined as distilled water at room temperature). See Fig 2E.
Higher-order inputs to GC
In addition to the basic sensory modalities of taste, olfaction, oral somatosensation and temperature, higher-order, more cognitive influences seem to have the ability to influence GC activity.
From our experiences it is obvious that visual input can affect one’s response to the palatability of food. However, it is not so obvious that merely seeing food would affect responses in the GC. To this point during event-related fMRI experiments, Simmons et al.46 had subjects to view pictures of appetizing foods and, for comparison, pictures of locations. Compared to “location pictures” that also activate the visual pathway, food pictures specifically activate gustatory processing areas including the insula/operculum (Figure 2E). Therefore, the mere presentation of food pictures, independently of concomitant gustatory activation, is sufficient to evoke neural activity in GC. Importantly, the locations of the activations reported by Simmons et al. were highly coincidental with the purely taste-elicited activity36.
In another human fMRI study of taste, Nitschke et al.47 found that the taste responses in the insula and operculum (GC) are modulated by expectation of a tastant. In their experiment they gave subjects cues as to whether a particular tastant (quinine) was perceived to be more or less bitter and determined whether that perceptual change was reflected in the cortical responses. They found that when expectancies were manipulated to mislead subjects into believing that the taste would be less unpleasant than it actually was, the responses in the insular and opercular responses were reduced.
Electrophysiological recordings also found that GC encodes expectation of tastant delivery48. In these experiments, arrays of electrodes were implanted in the GC of rats while the animals were licking a sipper on a fixed-ratio 5 (FR5) protocol (see Figure 3). The FR5 protocol can be expressed as [DL1-DL2–DL3-DL4 –Ti ]8 – WR where, DL represents the four dry licks, Ti is tastant i, WR is water rinse between blocks, and [….]8 represents a block of eight trials. That is after eight deliveries of tastant i a new tastant is delivered. Stapleton et al. hypothesized that animals trained on the FR5 protocol would learn that, after a rinse, a new block of eight trials will be followed by a different tastant48. These researchers developed a model which enabled them to predict on each trial (lick for a tastant) the probability of correctly predicting the tastant and its concentration. They found that after the first trial in a block in the DLs there is above chance in the next seven DL trials of predicting the tastant and its concentration. Interestingly, no information is encoded during the first four dry licks that antecede the delivery of a new tastant. In other words, when an animal knows what tastant is coming it sets up an “engram” of the tastant. Changing the protocol such that tastants could be delivered anywhere in a block reduced the probability of predicting the tastants to chance.
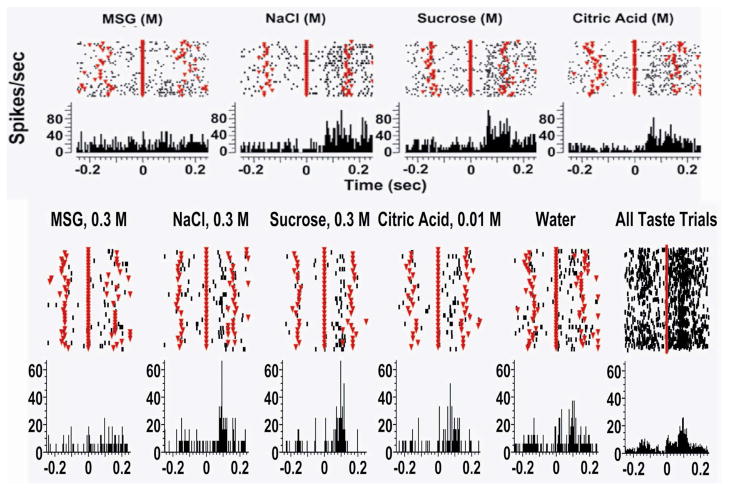
Figure 3. Electrophysiological properties of GC single units.
Single unit recordings from the primary gustatory cortex of rats licking on a FR5 (fixed ratio- see text for further details) schedule in which they licked a dry sipper four times and every fifth lick they received a tastant (at time 0 seconds as indicated by a solid red line). The tastants were delivered in blocks of eight. The dry licks (DL) before and after tastant deliveries are indicated by inverted triangles. The upper parts of each figure are raster plots and each dot indicates an action potential. Below are peri-stimulus time histograms (PSTHs). A. This trace is an example of a non-chemosensory response whose activity correlated with licking and preceded the licking of the sipper. It is important to note the temporal precision of the spikes and that the responses were the same for all tastants. B. An example of a chemosensitive neuron is presented. It is seen that the neuron is unresponsive to 0.3 M MSG but clearly responsive to the other taste stimuli including water. The panel on the right hand side, which represents the responses to all tastants, clearly shows that there is activity generated in the dry lick preceding the tastant delivery, indicating that this neuron also responds to somatosensory stimulation. (Permission requested, originally published in S. A. Simon, I E de Araujo, J. R. Stapleton, & M. A. L. Nicolelis (2008) Multisensory processing of gustatory stimuli. Chem. Percept. 1:95–102 23.)
Finally, a recent study reported significant responses in GC engaged when human subjects attempted to detect the presence of a tastant in a tasteless solution49, thus providing further evidence that cognitive processes, such as selective attention to taste, are sufficient to engage the GC circuitry even when no concomitant gustatory stimulation is provided. Overall, the sensitivity of GC neurons to multiple sensory modalities should therefore include modulatory activity by other cortical regions.
V. Electrophysiological properties of GC neurons responsive to intra-oral stimuli
Stapleton et al. have investigated somatosensory as well as gustatory responses in GC from rodents through neural ensemble recordings while animals licked nutritive solutions from a spout50. Like sniffing and whisking, licking produces stereotyped responses at theta frequencies51. Such behaviors (along with their analogues in other species) will engage the somatosensory and taste central pathways, often simultaneously. Figure 3 shows a single neuron in GC whose responses were recorded while rats licked on an FR5 schedule (described above)44,48. We investigated whether neural populations in the rat GC encoded sufficient information to allow the discrimination between taste and somatosensory features of tastants within single inter-lick intervals (~150 ms, an interval sufficient for trained rats to discriminate between tastants following a single lick, 52). We also investigated whether there is sufficient information in the evoked spike trains within a single lick to discriminate dry licks from wet licks as well as between different tastants.
Figure 3 depicts two distinct types of neuronal response that were active at some time within the lick cycle (about 150 ms). One type consisted of a temporally precise activation before the lick cycle (Figure 3A) and thus was obviously not chemosensitive. Neurons of this type could simply reflect oromotor responses such as opening or closing of the mouth or sticking out or retracting the tongue53,54. A second type of neuron response was activated both by licking a dry sipper and by the delivery of tastants (Figure 3B). As in the above example, GC neurons are broadly tuned and are usually activated by several tastants. Also seen is that licking the dry sipper elicits a small response (Figure 3B and especially right hand panel). In this figure it is seen that the response for licking plus the concurrent tastant delivery was much larger for such chemosensory neurons. This suggests that the somatosensory information elicited by licking might combine in a supralinear manner with chemosensory inputs. Such an effect is in fact expected to be observed during multimodal processing 55, and constitutes an important topic for future research.
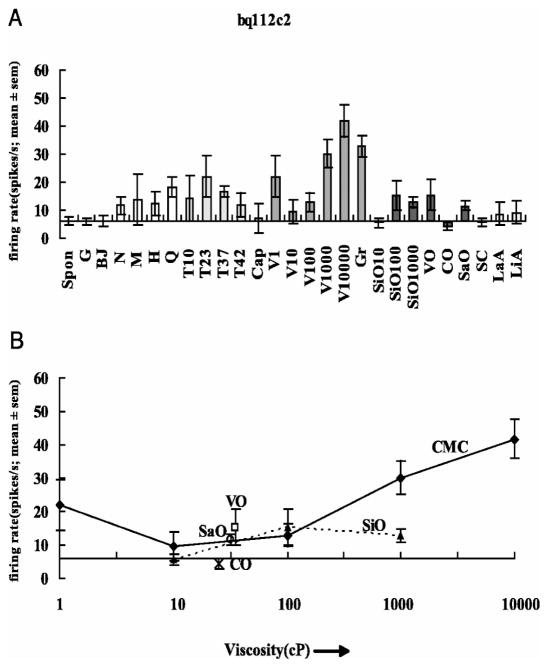
Neuronal responses in GC to somatosensory stimuli placed in the mouth are also observed during electrophysiological recordings in monkeys. Figure 4 provides such an example. Figure 4A shows a GC neuron recorded from a primate with differential responses primarily to viscosity as produced by CMC. (Note it is also responsive to changes in temperature (T) but not especially to tastants or various oils (O)). Figure 4B shows that the mean firing of a GC neuron to different viscosities of CMC increases from about 100 to 10,000 centipoise. Also shown is the relative absence of responses to oils of different viscosity. Other types of neurons, not shown, were responsive to oils and not to viscosity and still others were responsive only over specific temperature ranges. Taken together these data clearly demonstrate that single neurons in the primate GC respond to a variety of modalities including texture-related information, as indicated by the neuroimaging studies of the human GC shown in Fig 2.
Figure 4. Mean firing rate of a GC neuron to multiple stimuli.
A: Responses of neuron (bq112c2) with differential responses primarily to viscosity (V) as produced by the carboxymethylcellulose (CMC). The taste stimuli were 1 M glucose (G), 0.1 M NaCl (N), 0.1 M MSG (M), 0.01 M HCl (H), and 0.001 M quinine-HCl (Q); the temperature stimuli were T10, T23, T37, and T42 where the number indicates the temperature in degrees Celsius; the viscosity stimuli were V1, V10, V100, V1000, and V10000 where the numeral indicates the viscosity in centipoise at 23°C; fat texture stimul i were SiO10, SiO100, SiO1000 (silicone oil with the viscosity indicated), vegetable oil (VO), coconut oil (CO), and safflower oil (SaO). BJ. fruit juice; Cap, 10 μM capsaicin; LaA, 0.1 mM lauric acid; LiA, 0.1 mM linoleic acid; Gr, the gritty stimulus. B: The mean firing rate (±1 SE) to different viscosities of carboxymethylcellulose (CMC) shown as a graph, with the responses to the oils shown at their viscosity. The mean ± SE firing rate responses to each stimulus calculated in a 1-s period over 4–6 trials are shown. The spontaneous (Spon) firing rate is shown by the dashed horizontal line. (Used with permission, originally published in Verhagen JV, Kadohisa M, Rolls ET (2004) Primate Insular/Opercular Taste Cortex: Neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J Neurophysiol 92: 1685–1699 63.)
VI. Postingestive factors and visceral inputs influence GC activity
Postingestive effects, including visceral inputs that follow ingestion of foods, not only influence GC activity, but also seem to require the integrity of GC to exert their control on food intake. In fact, the GC is required for associations to be formed between sensory and postingestive aspects of foods. This is evident when an organism learns to use the taste of a novel food as a cue to the ensuing malaise (conditioned taste aversion), so that avoidance adaptive behaviors are acquired to protect from further contamination. Thus, conditioned taste aversion paradigms, pharmacological manipulations56, protein synthesis inhibition57 or irreversible lesions to GC58, disrupt the formation of a “memory trace” linking a conditioned taste cue to ensuing visceral malaise.
A recent intrinsic imaging study of the rat GC showed that the areas involved for different tastants changed when an animal received a visceral malaise (IP injection of LiCl) while being exposed to saccharin59. The imaging map of the saccharin response became closer to that evoked by the bitter tastant quinine. Upon extinguishing the conditioned taste aversion, the topography of the responses was partially reversible. These experiments showed that changes in hedonic perception are directly related to the maps’ plasticity in the GC. That is, an internal state of malaise induces plastic reshaping in the GC associated with the behavioral shift of the stimulus hedonic value.
The GC also seems to be important for the control of food intake by postingestive effects when such effects are rewarding. During appetitive conditioning, bilateral lesions to the gustatory cortex abolish the assignment of incentive value to food outcomes in instrumental tasks60. Furthermore, insular neuronal responses to palatable compounds are modulated by postingestive satiation in both rats61 and humans62.
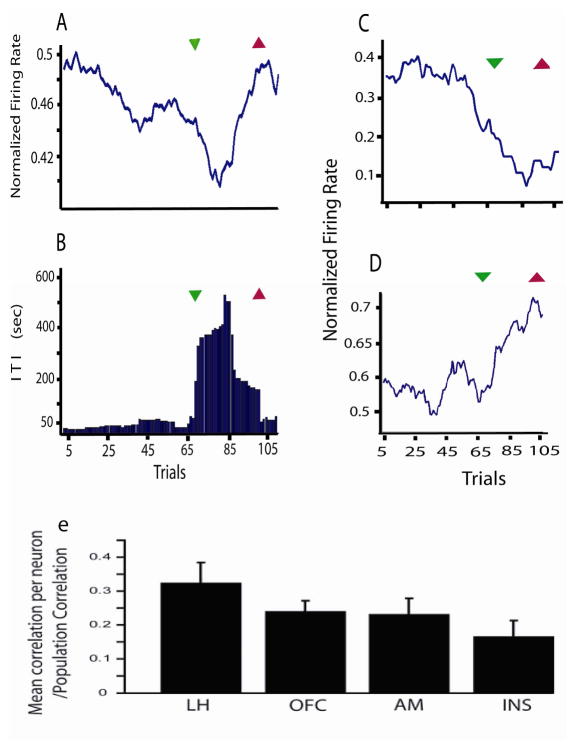
Figure 5 displays a representative example of the firing activity of ensembles of simultaneously recorded neural units in different areas of the rat forebrain while rats ingest a nutritive sucrose solution61. In a typical experimental session, an initially hungry rat will reduce the frequency with which it approaches and licks a sipping tube containing sucrose. The time interval measured between two consecutive licking bouts is called an inter-trial interval (ITI). These intervals can be used as behavioral indexes for the motivation of the animal to ingest sucrose, such that during ‘hunger’ periods they tend to be short (high sucrose consumption per unit of time) whereas during ‘satiation’ phases they tend to be longer. In this study, de Araujo et al61 reported that, when combined in a population mean, ensembles of simultaneously recorded neural units reflect more efficiently the hunger/satiation state of the animal compared to their constituent single units (See Fig 5A–D). It is important to note however that in this study neurons in GC contributed to approximately the same extent of the coding of the physiological state of the animal as other forebrain regions known to be involved in the homeostatic control of feeding. Thus, the ratio between the average performance of individual neurons in a given area and the performance of the entire corresponding ensemble (performance = correlation coefficient between firing rate across trials and ITIs during the corresponding session) was not significantly different between GC and lateral hypothalamus, amygdala or orbitofrontal cortex61. Therefore, it can be concluded that GC neurons contribute importantly, as other brain regions directly involved in the homeostatic control of food intake, to the neural encoding of the physiological state (hunger/satiety) of the organism.
Figure 5. Gustatory cortical neurons contribute significantly to the encoding of physiological states.
The firing activity of ensembles of simultaneously recorded neural units in different areas of the rat forebrain can represent the current motivation of the animal to ingest a nutritive sucrose solution more efficiently than its constituent single units. In a typical experimental session, an initially hungry rat will reduce the frequency with which it approaches and licks a sipping tube containing sucrose. The time interval measured between two consecutive licking bouts is called an inter-trial interval (ITI). These intervals can be used as behavioral indexes for the motivation of the animal to ingest sucrose, such that at ‘hunger’ periods they tend to be short (high sucrose consumption per unit of time) whereas at ‘satiation’ phases they tend to be longer. We found that when combined in a population mean, ensembles of simultaneously recorded neural units reflect more efficiently the hunger/satiation state of the animal compared to their constituent single units, with relatively higher population firing rates during hunger phases. A. Example of an experimental session in which the population mean firing rate correlated significantly with ITIs. Green and red arrows indicate start and end points respectively of a satiety phase. B. Corresponding ITIs for this session. Note the significant satiety phase (large ITI values) starting around trial number 65. However, in general, single units did not reflect the time course of the ITIs as precisely. C. Example of a cell from the original population monotonically decreasing its firing rate during the experiment. D. The same as in c, but depicting a monotonical increase in activity. The combination of these individual cell types in a population mean increases the accuracy of this distributed code to reflect feeding behavior. E. Neurons in GC contribute to approximately the same extent to the coding of the physiological state of the animal as other forebrain regions known to be involved in the homeostatic control of feeding. The graphs show that the ratio between the average performance of individual neurons in a given area and the performance of the entire ensemble (performance = correlation coefficient between firing rate across trials and ITIs during the corresponding session) was not significantly different between GC (INS) and lateral hypothalamus (LH), amygdala (AM) or orbitofrontal cortex (OFC). (Used with permission, originally published in Simon SA, de Araujo IE, Gutierrez R, Nicolelis MAL (2006) The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci 7:890–901.)
VI. Conclusion
We have reviewed evidence, from imaging, behavioral and electrophysiological studies, suggesting that GC functions as an integrative circuit by combining inputs from multiple sensory modalities arising from the oral cavity as well as visual and interoceptive inputs. These findings indicate that GC has a more general function beyond representing the gustatory aspects of intra-oral stimuli. In general, rather than working as the receptive field of peripheral taste receptor cells, the central gustatory pathways seem to operate as a multisensory system dedicated to evaluating the biological significance of intra-oral stimuli via activation of non-taste oro-gastro-intestinal receptors. Among these functions is the ability of GC to combine taste information with the postingestive consequences that follow the consumption of foods, a function that is reflected in the ability of GC neurons to represent faithfully the physiological, internal state of the organism.
Acknowledgments
The studies hereby reported in which both authors took part were supported in part by NIH grant DC-01065 and grants from Philip Morris USA Inc. and Philip Morris International.
References
- 1.Simon SA. The Merging of the Senses. Front Neurosci. 2008;2:13–14. doi: 10.3389/neuro.01.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breslin PA, Huang L. Human taste: peripheral anatomy, taste transduction, and coding. Adv Otorhinolaryngol. 2006;63:152–190. doi: 10.1159/000093760. [DOI] [PubMed] [Google Scholar]
- 3.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MAL. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 4.Breza JM, Curtis KS, Contreras RJ. Temperature Modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 2006;95:674–685. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz HR. Effects of solution temperature on taste Intensity in humans. Physiology & Behavior. 1973;10:289–292. doi: 10.1016/0031-9384(73)90312-0. [DOI] [PubMed] [Google Scholar]
- 6.Bartoshuk LM, Rennert K, Rodin J, Stevens JC. Effects of temperature on the perceived sweetness of sucrose. Physiology & Behavior. 1982;28:905–910. doi: 10.1016/0031-9384(82)90212-8. [DOI] [PubMed] [Google Scholar]
- 7.Cruz A, Green BG. Thermal stimulation of taste. Nature. 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey IS, Delling M, Clapham DE. An introduction to trp channels. Annual Review of Physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 9.Roper S. Signal transduction and information processing in mammalian taste buds. Pflugers Archiv. 2007;454:759–776. doi: 10.1007/s00424-007-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 11.Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 12.Perez CA, Margolskee RF, Kinnamon SC, Ogura T, et al. Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium. 2003;33:541–549. doi: 10.1016/s0143-4160(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Kurihara K. Temperature dependence of amiloride-sensitive and -insensitive components of rat taste nerve response to NaCl. Brain Research. 1988;444:159–164. doi: 10.1016/0006-8993(88)90923-7. [DOI] [PubMed] [Google Scholar]
- 14.Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. PNAS. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. The Journal of Physiology. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talavera K, Yasumatsu K, Yoshida R, Margolskee RF, Voets T, Ninomiya Y, Nilius B. The taste transduction channel TRPM5 is a locus for bittersweet taste interactions. FASEB J. 2008;22:1343–1355. doi: 10.1096/fj.07-9591com. [DOI] [PubMed] [Google Scholar]
- 17.Abumrad NA. CD36 may determine our desire for dietary fats. J Clin Invest. 2005;115:2965–2967. doi: 10.1172/JCI26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–R1832. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 19.Mattes RD. Fat taste and lipid metabolism in humans. Physiology & Behavior. 2005;86:691–697. doi: 10.1016/j.physbeh.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 20.Kadohisa M, Rolls ET, Verhagen JV. Neuronal representations of stimuli in the mouth: The Primate insular taste cortex, orbitofrontal cortex and amygdala. Chem Senses. 2005;30:401–419. doi: 10.1093/chemse/bji036. [DOI] [PubMed] [Google Scholar]
- 21.Small DM, Gerber JC, Mak E, Hummel T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47:593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Riera CE, Vogel H, Simon SA, le Coutre J. The capsaicin receptor participates in Artificial Sweetener Aversion. Bioche Biophys Res Comm. 2008;376:653–657. doi: 10.1016/j.bbrc.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Simon SA, de Araujo IE, Stapleton JA, Nicolelis MAL. Multisensory processing of gustatory stimuli. Chem Percept. 2008;1:95–102. doi: 10.1007/s12078-008-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halling DB, Aracena-Parks P, Hamilton SL. Regulation of Voltage-Gated Ca2+ Channels by Calmodulin. Sci STKE. 2005:re15. doi: 10.1126/stke.3152005re15. [DOI] [PubMed] [Google Scholar]
- 25.Roper S. Signaling in the chemosensory systems. Cellular and Molecular Life Sciences (CMLS) 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norgren R. Gustatory system. In: Paxinos G, editor. The rat nervous system. 2. Academic Press; San Diego: 1995. p. 1136pp. [Google Scholar]
- 27.Pritchard TC, Hamilton RB, Norgren R. Projections of the parabrachial nucleus in the old world monkey. Exp Neurol. 2000;165:101–117. doi: 10.1006/exnr.2000.7450. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard TC, Hamilton RB, Norgren R. Neural coding of gustatory information in the thalamus of Macaca mulatta. J Neurophysiol. 1989;61:1–14. doi: 10.1152/jn.1989.61.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Physiol & Behav. 1999;67:489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neur. 1986;244:213–288. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- 31.Jain N, Catania KC, Kass JH. Anatomic correlates of the face and oral cavity representations in the somatosensory cortical area 3b of monkeys. J Comp Neurol. 2001;429:455–468. doi: 10.1002/1096-9861(20010115)429:3<455::aid-cne7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Katz DB, Simon SA, Nicolelis MAL. Taste-Specific Neuronal Ensembles in the Gustatory Cortex of Awake Rats. J Neurosci. 2002;22:1850–1857. doi: 10.1523/JNEUROSCI.22-05-01850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz DB, Simon SA, Nicolelis MAL. Dynamic and multimodal response of gustatory cortical neurons. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- 35.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophys. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 36.de Araujo IET, Kringelbach ML, Rolls ET, Hobden P. Representation of Umami Taste in the Human Brain. J Neurophysiol. 2003;90:313–319. doi: 10.1152/jn.00669.2002. [DOI] [PubMed] [Google Scholar]
- 37.Schoenfeld MA, Neuer G, Tempelmann C, Ler K, Noesselt T, Hopf J-M, Heinze H-J. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127:347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Accolla R, Bathellier B, Petersen CH, Carleton A. Differential Spatial Representation of Taste Modalities in the Rat Gustatory Cortex. J Neurosci. 2007;27:1396–1404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Araujo IET, Rolls ET, Kringelbach ML, et al. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. European Journal of Neuroscience. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- 40.Scott TR, Yaxley S, Sienkiewicz ZJ, Rolls ET. Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol. 1986;56:876–890. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa H. Gustatory cortex of primates: anatomy and physiology. Neuroscience Research. 1994;20:1–13. doi: 10.1016/0168-0102(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 42.de Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Araujo IET, Kringelbach ML, Rolls ET, McGlone F. Human Cortical Responses to Water in the Mouth, and the Effects of Thirst. J Neurophysiol. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- 44.Stapleton JA, Lavine M, Wolpert R, Nicolelis MAL, Simon SA. Rapid taste response in the gustatory cortex during licking. J Neurosci. 2006;26:4126–4138. doi: 10.1523/JNEUROSCI.0092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guest S, Grabenhorst F, Essick G, et al. Human cortical representation of oral temperature. Physiology & Behavior. 2007;92:975–984. doi: 10.1016/j.physbeh.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Simmons WK, Martin A, Barsalou LW. Pictures of Appetizing Foods Activate Gustatory Cortices for Taste and Reward. Cereb Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 47.Nitschke JB, Dixon GE, Sarinopoulos I, Short SJ, Cohen JD, Smith EE, et al. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat Neurosci. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- 48.Stapleton JA, Lavine M, Nicolelis MAL, Simon SA. Ensembles of gustatory cortical neurons anticipate and discriminate between tastants in a single lick. Frontiers in Neuroscience. 2007;1:161–174. doi: 10.3389/neuro.01.1.1.012.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Sens. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira-Maia AJ, Simon SA, Nicolelis MAL. Neural Ensemble Recordings from Central Gustatory-Reward Pathways in Awake and Behaving Animals. In: Nicolelis MAL, editor. Methods for Neural Ensemble Recordings. Boca Raton: CRC Press Taylor and Francis Group; 2008. pp. 190–218. Chapter 10. [PubMed] [Google Scholar]
- 51.Gutierrez R, Carmena JM, Nicolelis MAL, Simon SA. Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards. J Neurophysiol. 2006;95:119–133. doi: 10.1152/jn.00467.2005. [DOI] [PubMed] [Google Scholar]
- 52.Halpern BP, Tapper DN. Taste stimuli: quality coding time. Science. 1971;171:1256–1258. doi: 10.1126/science.171.3977.1256. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Sensory inputs from the oral region to the cerebral cortex in behaving rats: An analysis of unit responses in cortical somatosensory and taste during ingestive behavior. J Neurophysiol. 1988;60:1303–1321. doi: 10.1152/jn.1988.60.4.1303. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol. 1989;61:1244–1258. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]
- 55.Laurienti PJ, Perrault TJ, Stanford TR, Wallace MT, Stein BE. On the use of superadditivity as a metric for characterizing multisensory integration in functional neuroimaging studies. Experimental Brain Research. 2005;166:289–297. doi: 10.1007/s00221-005-2370-2. [DOI] [PubMed] [Google Scholar]
- 56.Gutierrez R, Tellez LA, Bermudez-Rattoni F. Blockade of cortical muscarinic but not NMDA receptors prevents a novel taste from becoming familiar. European Journal of Neuroscience. 2003;17:1556–1562. doi: 10.1046/j.1460-9568.2003.02608.x. [DOI] [PubMed] [Google Scholar]
- 57.Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- 58.Bermudez-Rattoni F. Molecular mechanisms of taste -recognition memory. Nature Reviews Neuroscience. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 59.Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. PNAS. 2008;105:4010–4015. doi: 10.1073/pnas.0708927105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: Evidence for a role in incentive memory. J Neurosci. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MAL, Simon SA. Neural Ensemble Coding of Satiety States. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Small DM, Zatorre RJ. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 63.Verhagen JV, Kadohisa M, Rolls ET. Primate Insular/Opercular taste Cortex: Neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J Neurophysiol. 2004;92:1685–1699. doi: 10.1152/jn.00321.2004. [DOI] [PubMed] [Google Scholar]