Abstract
Natural killer (NK) cells trigger cytotoxicity and interferon (IFN)-γ secretion on engagement of the natural-killer group (NKG)2D receptor or members of the natural cytotoxicity receptor (NCR) family, such as NKp46, by ligands expressed on tumour cells. However, it remains unknown whether T cells can regulate NK cell-mediated anti-tumour responses. Here, we investigated the early events occurring during T cell–tumour cell interactions, and their impact on NK cell functions. We observed that on co-culture with some melanomas, activated CD4+ T cells promoted degranulation, and NKG2D- and NKp46-dependent IFN-γ secretion by NK cells, probably owing to the capture of NKG2D and NKp46 ligands from the tumour-cell surface (trogocytosis). This effect was observed in CD4+, CD8+ and resting T cells, which showed substantial amounts of cell surface major histocompatibility complex class I chain-related protein A on co-culture with tumour cells. Our findings identify a new, so far, unrecognized mechanism by which effector T cells support NK cell function through the capture of specific tumour ligands with profound implications at the crossroad of innate and adaptive immunity.
Keywords: NK cells, T cells, tumour
Introduction
Tumour progression results from a balance between tumour escape and immune surveillance that is mediated by T cells and natural killer (NK) cells through cytotoxicity and interferon (IFN)-γ secretion (Rabinovich et al, 2007). However, it remains unknown whether T cells can regulate NK cell function, particularly during anti-tumour responses. NK cells trigger effector functions on engagement of receptors such as the natural-killer group (NKG)2D receptor, NKp30, NKp44 and NKp46 (Moretta et al, 2006), among others. Ligands recognized by NKp30, NKp44 and NKp46 (NKp46L) on tumour cells still remain elusive, but NKG2D recognizes inducible cell-surface molecules (NKG2D ligands (NKG2DLs)) such as MICA and MICB (major histocompatibility complex class I chain-related proteins A and B), and the glycosylphosphatidylinositol (GPI)-anchored molecules UL-16 binding protein (ULBP) 1–3 (Eagle & Trowsdale, 2007).
Intricate cell interactions of unknown consequences occur in a tumour microenvironment; some seem to involve trogocytosis or the transfer of cell membrane molecules from tumour cells to cells of the immune system (Hudrisier & Bongrand, 2002; Caumartin et al, 2007; Hudrisier et al, 2007), including MICA (McCann et al, 2007), but its physiological relevance awaits further elucidation. To characterize early events affecting the T cell-mediated response against tumours and their impact on NK cell function, we investigated whether a brief contact between activated human T cells and melanoma cells endows T cells with NK cell-stimulatory function. Here, we present evidence indicating that tumour-experienced T cells actively capture NKG2DLs and NKp46Ls from tumour cells, which, in turn, promote degranulation and IFN-γ secretion by NK cells, thus showing a new functional crosstalk between adaptive and innate immunity with crucial implications for the control of anti-tumour immunity.
Results And Discussion
Tumour-experienced T cells trigger NK cell effector functions
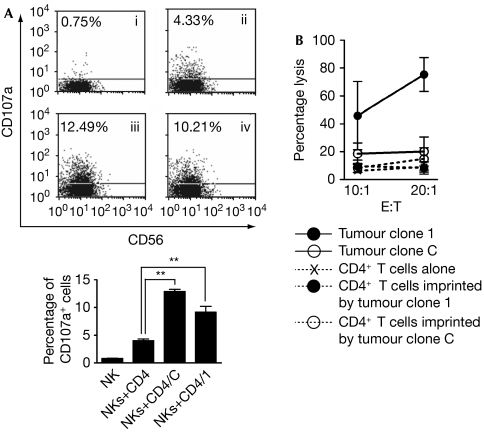
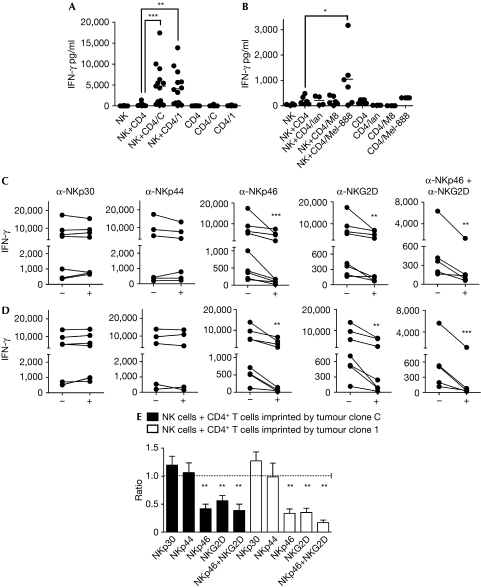
Despite considerable progress in elucidating the cellular mechanisms of anti-tumor responses, the capacity of T cells to regulate NK cell functions is still unknown. Here, we investigated whether tumours can endow CD4+ T cells with the capacity to modulate NK cell effector functions. By using the experimental approach illustrated in Supplementary Fig 1 online, we found that activated CD4+ T cells which had experienced a 1-h contact with an IIB-MEL-LES melanoma clone overexpressing MICA on the cell surface (tumour clone 1) or a mock-transfected clone (tumour clone C) acquired the ability to promote degranulation of NK cells, as shown by an increase in the percentage of CD3−CD56+CD107a+ cells from 4% to 10–12% (Fig 1A), but remained resistant to NK cell-mediated cytotoxicity. However, tumour clone 1, but not tumour clone C, was susceptible to this effect (Fig 1B). Such resistance of activated tumour-experienced CD4+ T cells could be associated with the expression of the serpin inhibitor of granzyme B PI-9, which prevents apoptosis of CD4+, CD8+ and NK cells (Hirst et al, 2003), or with the higher expression of human leukocyte antigen (HLA)-E compared with tumour cells (Supplementary Fig 2 online), which might engage the inhibitory receptor CD94/NKG2A. Tumour-experienced CD4+ T cells also promoted the secretion of substantially higher amounts of IFN-γ by NK cells from 14 different donors when compared with activated CD4+ T cells that had no contact with tumour cells (Fig 2A). IFN-γ secretion was also observed when we used Mel-888 cells but not with IIB-MEL-IAN or M8 cells indicating that this effect was limited to certain melanoma cells, but not restricted to transfected cells (Fig 2B). The purity of CD4+ T cells used for co-culture with NK cells ruled out the possibility of degranulation and IFN-γ secretion induced by contaminating tumour cells (Supplementary Fig 1 online). Similar results were obtained with syngeneic CD4+ T cells and NK cells (Supplementary Fig 3 online). The amount of IFN-γ detected in cell cultures was derived from NK cells as only CD3−CD56+IFN-γ+ cells were detected by flow cytometry (data not shown). Remarkably, no major differences were detected in the amount of IFN-γ secreted by NK cells co-cultured with activated T cells, which were imprinted by tumour clone C when compared with NK cells co-cultured with activated T cells imprinted by tumour clone 1. Blocking experiments showed that anti-NKp46 and anti-NKG2D monoclonal antibodies significantly prevented IFN-γ secretion (Fig 2C,D), indicating that NKG2DLs and NKp46Ls expressed on tumour-imprinted, activated CD4+ T cells are responsible for triggering IFN-γ secretion by NK cells in amounts comparable with that of NK cells stimulated by toll-like receptor (TLR) agonists and cytokines (Girart et al, 2007) or tumour clone 1 (Fuertes et al, 2008). Although the identity of NKp46Ls on tumour cells remains unknown, the NKG2DLs that could mediate this effect could be the transmembrane proteins MICA/B, ULBP4/retinoic acid early transcript (RAET)1E and/or RAET1G, or the GPI-anchored molecules ULBP1–3 (Eagle & Trowsdale, 2007). Although the involvement of ULBP4/RAET1E and RAET1G can be assessed only when blocking monoclonal antibodies become available, we can rule out a putative role for MICB in IIB-MEL-LES, as these cells do not express this NKG2DL (M.B.F., unpublished data). These observations prompted us to investigate the involvement of MICA in the observed effects.
Figure 1.
Cytotoxicity and degranulation of natural killer cells. The experimental approach is detailed in Supplementary Fig 1A online. (A) Degranulation of natural killer (NK) cells triggered by activated CD4+ T cells cultured in the absence (ii) or previously co-cultured for 1 h with tumour clone C (iii) or tumour clone 1 (iv); NK cells cultured alone are also shown (i). The graph shows results from three different donors. **P<0.01. (B) NK cells were used as effector cells of cytotoxicity against tumour clone 1, tumour clone C or activated CD4+ T cells cultured in the absence or in the presence of the tumour clones at two effector:target (E:T) ratios. Results correspond to one of four independent experiments carried out with cells from different donors (data are expressed as mean±s.e.m.).
Figure 2.
Interferon-γ secretion by natural killer cells. The experimental approach is detailed in Supplementary Fig 1B online. (A,B) Natural killer (NK), cells alone; NK+CD4, NK cells co-cultured with CD4 T cells that did not contact tumour cells; NK+CD4/C, NK+CD4/1, NK+CD4/Ian, NK+CD4/M8 and NK+CD4/Mel-888, NK cells co-cultured with CD4 T cells previously co-cultured with tumour clone C or tumour clone 1, or IIB-MEL-IAN, M8 or Mel-888 cells, respectively. CD4, CD4/C, CD4/1, CD4/Ian, CD4/M8 and CD4/Mel-888, CD4+ T cells cultured in the absence or in the presence of tumour clone C or 1, or IIB-MEL-IAN, M8 or Mel-888 cells, respectively. For blocking experiments, NK cells were co-cultured with CD4+ T cells imprinted by tumour clone C (C) or tumour clone 1 (D), in the absence (−) or in the presence (+) of the indicated blocking monoclonal antibodies. Each dot or line in (A–D) represents the result obtained with cells from a different donor. To circumvent the heterogeneity commonly observed in IFN-γ secretion among different donors, the ratio of IFN-γ secretion observed in the presence to that obtained in the absence of the blocking monoclonal antibodies is shown (E). The mean±s.e.m. obtained in independent experiments carried out with cells from five to eight different donors is shown. *P<0.05; **P<0.01; ***P<0.001. IFN-γ, interferon-γ.
NK cell stimulation is mediated by capture of MICA
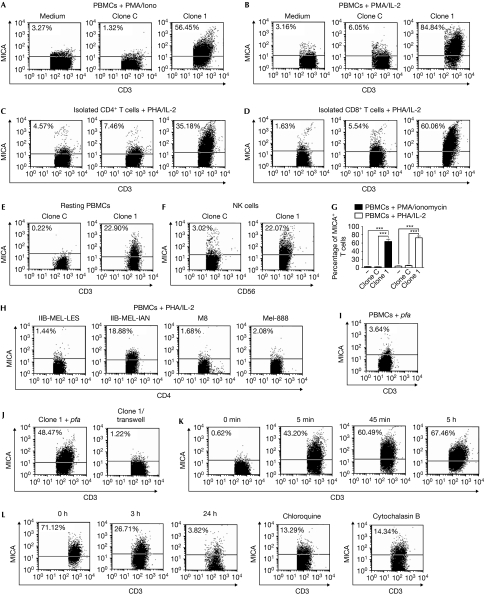
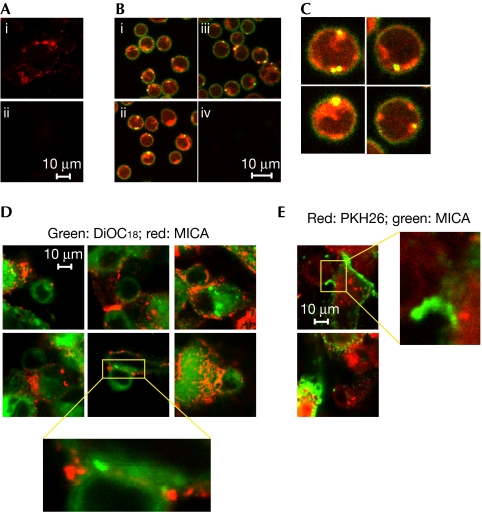
To investigate the mechanisms underlying NK-cell-mediated IFN-γ secretion induced by tumour-experienced, activated CD4+ T cells, we examined whether a brief contact of activated CD4+ T cells with the melanoma tumour clones C and 1 could promote the cell surface expression of MICA. Thus, cell-surface MICA was assessed on mitogen-activated T cells present in peripheral blood mononuclear cells (PBMCs; Fig 3A,B,G), purified CD4+ and CD8+ T cells stimulated with phytohaemagglutinin (PHA), interleukin (IL)-2 (Fig 3C,D), and in vitro-differentiated T helper (TH)1 and TH2 cells (Supplementary Fig 4 online) on co-culture for 1 h with tumour clone C or with tumour clone 1. In all cases, MICA was detected on T cells only on co-culture with tumour clone 1. Forward scatter and side scatter profiles, and gated CD3+ cells confirmed that MICA was localized mainly on T cells and not on T cell–tumour cell conjugates (Supplementary Fig 5 online). As even resting T cells and NK cells, which do not express MICA, exhibited cell surface MICA (Fig 3E,F), we speculated that this NKG2DL was acquired from tumour cells by cell-to-cell transfer of membrane molecules. Notably, transfer of MICA was also observed when we used IIB-MEL-IAN, but not using M8 or Mel-888 cells (Supplementary Fig 6 online), indicating that this effect was limited to certain melanoma cells, but not restricted to transfected cells (Fig 3H). Such selectivity might be due to differences in the amounts of MICA present on the surface of various melanoma cells and/or tumour-cell surface molecules that make them permissive to trogocytosis. As IIB-MEL-IAN cells express MICA and MICB, and the monoclonal antibody used in our experiments recognizes both NKG2DLs, it is possible that MICB might also become transferred. Several molecules have been described as mediators of trogocytosis (Hudrisier et al, 2007). In our study, the involvement of T-cell receptor/CD3, CD28, and major histocompatibility complex (MHC) class I and class II molecules was ruled out, as we used polyclonally activated T cells and the melanomas did not express MHC class II or CD80/CD86. In addition, blockade of inter-cellular adhesion molecule 1 (ICAM1), the ligand for lymphocyte function-associated antigen 1 (LFA1), did not abrogate the transfer of MICA (data not shown). Fixed tumour cells, but not fixed T cells, also promoted the transfer of MICA to T cells (Fig 3I,J) but trogocytosis was abrogated if cells were separated by transwells (Fig 3J), suggesting that transfer of MICA was dependent on T-cell viability and cell-to-cell contact. A contact of 5 min was sufficient for T cells to capture MICA (Fig 3K), and this NKG2DL was still detected on T cells after 72 h of co-culture (data not shown). However, the percentage of MICA+ T cells rapidly decreased from 71% to 26% after 3 h and to less than 4% after 24 h if melanoma tumour clone 1 and T cells were separated and cultured alone after co-culture (Fig 3L). Interestingly, both chloroquine and cytochalasin B delayed the clearance of MICA from the T cell surface—at 24 h, about 13–14% of T cells were still MICA+ in the presence of these inhibitors—suggesting that MICA was endocytosed. Thus, a fraction of MICA was cleared from the cell surface but, as long as T cells could establish physical contact with melanoma cells, they continuously captured and presented this molecule on their cell surface.
Figure 3.
Transfer of major histocompatibility complex class I chain-related protein A to T cells. (A) Peripheral blood mononuclear cells (PBMCs) stimulated with phorbol-12-myristate-13-acetate (PMA) and ionomycin (Iono) or (B) phytohaemagglutinin (PHA) and interleukin (IL)-2, (C) purified CD4+ T cells or (D) CD8+ T cells stimulated with PHA and IL-2, (E) resting PBMCs or (F) natural killer (NK) cells stimulated with IL-15 were cultured without (medium) or with tumour clone C or tumour clone 1 for 1 h and major histocompatibility complex class I chain-related protein A (MICA) was detected on non-adherent CD3+ T cells (A–E) or CD56+ cells (F). (H) Similar experiments were carried out with the indicated cell lines and MICA expression was assessed on CD4+ T cells. (I) Similar experiments as in (B) were carried out with fixed PBMCs (p-formaldehyde (pfa)). (J) PBMCs stimulated with PMA+Iono were co-cultured for 1 h with tumour clone 1 previously fixed with pfa, or with live cells of tumour clone 1 separated by transwells (clone 1/transwell), and MICA was assessed on CD3+ T cells. (K) Kinetic analysis of MICA on T cells stimulated with PMA+Iono on co-culture with tumour clone 1 for different periods of time (indicated at the top of each dot plot). 0 min: MICA on stimulated T cells not co-cultured with tumour clone 1. MICA expression was assessed as in (J). (L) PBMCs stimulated with PHA+IL-2 co-cultured for 1 h with tumour clone 1 were harvested, cultured alone for 3 or 24 h (in this case, in the absence or in the presence of chloroquine or cytochalasin B), and MICA was assessed as in (J). 0 h: MICA on activated T cells co-cultured with clone 1 for 1 h analysed immediately after co-culture. Numbers within dot-plots show the percentage of MICA+ cells. One of four representative experiments carried out with PBMCs from four different donors is shown. The mean±s.e.m. of these experiments is shown in (G). ***P<0.001. The increase in cell-surface MICA on T and NK cells co-cultured with tumour clone 1 was statistically significant, compared with equivalent cells cultured alone (P<0.05; data not shown).
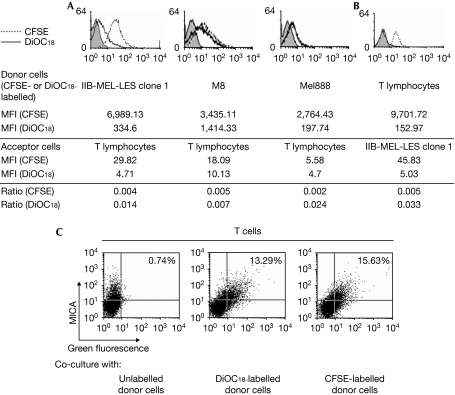
Transfer of surface molecules between melanoma cells and T cells was confirmed using 3,3′-dioctadecyloxacarbocyanine perchlorate (DiOC18)- or carboxyfluorescein succinimidyl ester (CFSE)-labelled cells, as green fluorescence was acquired by activated T cells co-cultured with labelled melanoma cells (Fig 4A), and by melanoma cells co-cultured with activated and labelled T cells (Fig 4B). Thus, transfer was bidirectional and involved cell-to-cell contact, as it was abrogated when cells were separated by transwells (data not shown). Fluorescence from donor cells was detected on acceptor cells but not on T cell–tumour cell conjugates, as shown by the analysis of forward scatter and side scatter profiles, and staining following specific gating (Supplementary Fig 5 online). Assessment of the extent of trogocytosis further showed that DiOC18 was more efficiently transferred than CFSE, probably because CFSE is a cytoplasmic probe that binds to the amino groups of cytoplasmic domains of transmembrane proteins.
Figure 4.
Transfer of molecules between tumour and T cells. Acceptor unlabelled cells (peripheral blood mononuclear cells (PBMCs) stimulated with phytohaemagglutinin (PHA) and interleukin (IL)-2 in (A) and cells of tumour clone 1 in (B)) were co-cultured for 1 h with 3,3′-dioctadecyloxacarbocyanine perchlorate (DiOC18)- or carboxyfluorescein succinimidyl ester (CFSE)-labelled donor cells (melanoma cells in (A) or PBMCs stimulated with PHA+ IL-2 in (B)), and fluorescence was assessed on (A) non-adherent CD3+ T cells or (B) tumour cells after removing PBMCs. Numbers represent the mean fluorescent intensity (MFI) of CFSE- or DiOC18-labelled donor cells or acceptor cells that captured CFSE or DiOC18. The extent of trogocytosis was calculated as the ratio of fluorescence between acceptor and donor cells for each fluorescent probe. In addition, tumour clone 1 (unlabelled or labelled with DiOC18 or CFSE) was co-cultured for 1 h with PBMCs stimulated with PHA and IL-2, and cell surface expression of major histocompatibility complex class I chain-related protein A (MICA) was assessed by flow cytometry on non-adherent CD3+ cells (C). The percentages in quadrant 2 represent T cells that captured the fluorescent probe and MICA (quadrants were set with acceptor cells that did not contact tumour cells). Results are representative of three experiments carried out with cells from three different donors. The increase in green fluorescence on acceptor cells after co-culture with CFSE- and DiOC18-labelled donor cells (A,B) or the increase in percentage of double-positive cells after co-culture with CFSE- and DiOC18-labelled donor cells (C) was statistically significant, compared with acceptor cells cultured alone (P<0.05).
Co-culture of DiOC18- or CFSE-labelled tumour clone 1 with unlabelled activated T cells promoted the appearance of MICA+ T cells that simultaneously showed DiOC18- or CFSE-derived fluorescence (Fig 4C). As above, the possibility of detecting T cell–tumour cell conjugates was ruled out by the analysis of forward-scatter profile, side-scatter profile and gating on CD3+ cells (Supplementary Fig 7 online). Thus, T cells acquire MICA and other tumour-cell surface molecules from certain melanoma cells by trogocytosis. Conversely, we did not observe the transfer of GPI-anchored ULBPs (Supplementary Fig 8 online), an effect that could be ascribed to differential segregation of these GPI-anchored molecules within microdomains of the tumour cell membrane.
Transfer of MICA was also analysed by confocal microscopy (Supplementary Fig 9 online), an experimental approach that avoids antibody-induced capping of MICA. As expected, we observed bright red fluorescence associated with MICA in tumour clone 1 (Fig 5A). Remarkably, CD4+ T cells isolated after co-culture showed yellow spots on their cell surface owing to the capture of MICA from the melanoma cell surface (Fig 5B,C). MICA localized in discrete areas of the T-cell membrane, which could indicate capping preceding endocytosis. In addition, red fluorescence observed inside CD4+ T cells was compatible with fast endocytosis of MICA after being captured. Co-transfer experiments carried out with DiOC18 (green)- or PKH26 (red)-labelled tumour clone 1 co-cultured for 1 h with unlabelled activated CD4+ T cells allowed visualization of MICA+CD4+ T cells that simultaneously showed DiOC18- or PKH26-derived fluorescence (Fig 5D,E). A closer examination revealed the existence of patches of fluorescence being conveyed from the tumour to the T cell surface at the tumour–T cell interface (insets in Fig 5D), confirming that MICA becomes co-transferred from the tumour cell surface to the T-cell surface by trogocytosis during their intimate contact in culture.
Figure 5.
Confocal microscopy. (A) The tumour clone 1 was stained with the MICA (major histocompatibility complex class I chain-related protein A) monoclonal antibody (mAb; i) or isotype-matched control mAb (IC mAb; ii) and Cy3-labelled donkey anti-mouse IgG. (B) Cells were cultured as detailed in Supplementary Fig 9 online. Fluorescein isothiocyanate (FITC)-labelled stimulated CD4+ T cells after co-culture with red-labelled MICA+ tumour clone 1 cells are shown in i–iii. CD4+ T cells stained with an IC mAb co-cultured with tumour clone 1 labelled with an IC control mAb are also shown (iv). (C) Higher magnification of some CD4+ T cells from (B). (D,E) Unlabelled CD4+ T cells stimulated with phytohaemagglutinin (PHA) and interleukin (IL)-2 were co-cultured for 1 h with cells of tumour clone 1 labelled with (D) 3,3′-dioctadecyloxacarbocyanine perchlorate (DiOC18; green) or (E) PKH26 (red), and with the anti-MICA mAb and Cy3-labelled anti-mouse IgG (for DiOC18-labelled tumour cells) or FITC-labelled anti-mouse IgG (for PKH26-labelled tumour cells). Thereafter, cells were analysed by confocal microscopy. Insets show the synapse between T cells and tumour cells. Results are representative of three independent experiments carried out with cells from three different donors.
Our findings provide an alternative explanation to previous results, indicating that tumour rejection requires T cells, NK cells and IFN-γ but that T cell-derived IFN-γ does not seem to be crucial for triggering anti-tumour immunity (Mumberg et al, 1999; Shankaran et al, 2001; Segal et al, 2002; Li et al, 2007). Instead, IFN-γ seems to be derived from NK cells, while T cells provide help for their priming. Hence, T-cell capture of NK-cell receptor ligands from tumours might operate as a new mechanism to stimulate IFN-γ secretion by NK cells in a T cell-dependent manner.
In summary, our results constitute the first demonstration that activated T cells are imprinted by tumour cells through the capture of NKG2DLs such as MICA and NKp46Ls from the tumour-cell surface, and that such tumour-experienced T cells are endowed with NK cell-stimulating function to promote increased degranulation, and NKG2D- and NKp46-dependent IFN-γ secretion to foster anti-tumour immunity. Thus, cells from adaptive immunity might provide T-cell receptor-independent help to innate immune cells through the capture of NK cell ligands from tumour cells, showing a new, so far, unrecognized role for activated T cells in NK cell-mediated effector functions.
Methods
Monoclonal antibodies. Fluorescein isothiocyanate-labelled anti-CD107a (BD, San José, CA, USA); anti-NKG2D (eBioscience, San Diego, CA, USA); anti-ULBPs 1–3 (R&D, Minneapolis, MN, USA); anti-MICA/B D7 (Fuertes et al, 2008); anti-HLA-E MEM-E08 (Santa Cruz Biotech, Santa Cruz, CA, USA); isotype-matched control (eBioscience); fluorescein isothiocyanate- or phycoerytherin-labelled anti-CD56, anti-CD4, anti-CD8 or anti-IFN-γ (eBioscience); spectral red-labelled anti-CD3 (Southern Biotech, Birmingham, AL, USA); blocking anti-NKp30 (F252), anti-NKp44 (KS38) and anti-NKp46 (KL247; kindly provided by Dr Alessandro Moretta (University of Genoa, Genoa, Italy)).
Peripheral blood mononuclear cells. PBMCs were isolated as described previously (Girart et al, 2007). CD4+, CD8+ T cells and NK cells were isolated using RosetteSep kits (StemCell, Vancouver, BC, Canada). Purity (>95%) was assessed by using flow cytometry. Cells were stimulated with phorbol-12-myristate-13-acetate (10 ng/ml) and ionomycin (0.5 μg/ml) for 72 h or with PHA (1 μg/ml) for 18 h and IL-2 (0.8 ng/ml) for 48 h. NK cells were used unstimulated for functional experiments or were stimulated with IL-15 (10 ng/ml) for 72 h for co-culture with melanomas. This study was approved by the institutional review committee. Mitogens, ionomycin and RPMI1640 were obtained from Sigma (St Louis, MO, USA), and cytokines from R&D or Peprotech (Rock Hill, NJ, USA).
Cell lines and co-cultures. The human melanoma cell lines IIB-MEL-LES, IIB-MEL-IAN, M8, A375 and MEL-888, and a clone of IIB-MEL-LES transfected with MICA (tumour clone 1) and a clone of transfected with an empty plasmid (tumour clone C) were cultured as described previously (Fuertes et al, 2008).
Equal numbers of melanoma cells and PBMCs, T cells or NK cells were co-cultured for up to 5 days. Non-adherent cells were harvested and used for flow cytometry, confocal microscopy or functional assays. In some cases, activated T cells were cultured further in the absence of melanoma cells for up to 24 h with PHA and IL-2 in the absence or presence of chloroquine (50 μM; Sigma) or cytochalasin B (20 μg/ml; Sigma). In some cases, melanoma cells and PBMCs were co-cultured for 1 h separated by 0.4 μm pore size transwells (NUNC, Rochester, NJ, USA). In addition, melanoma cells or PBMCs were fixed with 2% of p-formaldehyde before co-cultures. In other cases, melanomas or T cells were labelled with CFSE (10 μM; Invitrogen, Carlsbad, CA, USA), DiOC18 (30 μM; Invitrogen) or PKH26 (2 μM; Sigma), co-cultured with unlabelled T cells or melanoma cells, and fluorescence was assessed by flow cytometry or confocal microscopy. In addition, DiOC18- or PKH26-labelled melanoma cells were co-cultured for 1 h with activated CD4+ T cells, and MICA was thereafter assessed on non-adherent T cells with the anti-MICA mAb and PE-labelled anti-mouse IgG (for DiOC18-labelled cells) or FITC-labelled anti-mouse IgG (for PKH26-labelled cells). Cells were analysed by flow cytometry.
For functional assays, CD4+ T cells co-cultured for 1 h, with or without tumour clone C or 1, were harvested and further co-cultured for 4 or 15 h with an equal number of NK cells, followed by evaluation of cytotoxicity, degranulation or IFN-γ secretion by NK cells.
Flow cytometry. Expression of NKG2DLs on T cells was analysed by flow cytometry using specific monoclonal antibodies and phycoerytherin-labelled anti-mouse IgG (DAKO, Glostrup, Denmark). Viable lymphoid cells were gated according to their forward scatter and side scatter parameters, and stained for CD3 or CD4. NK cells were characterized as CD3−CD56+ cells. Cells were analysed in a FACSAria (BD) flow cytometer.
NK cell effector functions. For degranulation, activated CD4+ T cells cultured alone, with tumour clone C or with tumour clone 1 for 1 h were cultured overnight at 37°C with an equal number of NK cells. During the last 4 h, CD107a monoclonal antibody was added. Cells were harvested and stained with CD3 and CD56 monoclonal antibodies to gate NK cells (CD3−CD56+ cells) and to determine the percentage of CD107a+ cells. Cytotoxicity and IFN-γ secretion were assessed as described previously (Fuertes et al, 2008).
Confocal microscopy. Melanoma cells were fixed with 2% of p-formaldehyde and stained with anti-MICA monoclonal antibody and Cy3-labelled donkey anti-mouse IgG (Jackson, West Grove, PA, USA). Fixed and labelled tumour cells were also co-cultured for 1 h with activated CD4+ T cells pre-stained with a fluorescein isothiocyanate-labelled anti-CD4 monoclonal antibody (Supplementary Fig 7 online). T cells were harvested and seeded onto polylysine-coated coverslips. In addition, co-cultures of unlabelled activated CD4+ T cells with DiOC18- or PKH26-labelled melanoma cells and also labelled with the anti-MICA mAb and Cy3-labelled donkey anti-mouse IgG (Jackson) or FITC-labelled goat anti-mouse IgG (DAKO) were performed. Cells were observed using an Eclipse E800 Nikon C1 laser confocal microscope with Nikon Plan Apo × 60/1.40 oil objective (Nikon, Kawasaki, Kanagawa, Japan).
Statistical analysis. Paired test with Tukey comparison was used in Fig 1A. One-way analysis of variance with Dunnett's comparison test was used in Figs 2A,B and 3 and Supplementary Fig 3 online; Student's t-test was used in Fig 2C–E.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figs 1–9
Acknowledgments
We thank A. Moretta (University of Genoa, Italy) for providing the natural cytotoxicity receptor monoclonal antibodies, R. Alen (Centro de Hemoterapia ‘Dr Luis Agote', Buenos Aires, Argentina) and J. Mordoh (Instituto Leloir, Buenos Aires, Argentina) for providing monoclonal antibodies, buffy coats and cell lines. We also thank Fundación Sales for additional support. M.B.F., M.V.G., C.I.D. and D.E.A. are fellows of Consejo Nacional de Investigaciones Científicas y Técnicas. L.E.R. is a fellow of Agencia Nacional de Promoción Científica y Tecnológica. G.A.R. and N.W.Z. are members of the Researcher Career of Consejo Nacional de Investigaciones Científicas y Técnicas. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica, Consejo Nacional de Investigaciones Científicas y Técnicas and the University of Buenos Aires (to N.W.Z).
Footnotes
The authors declare that they have no conflict of interest.
References
- Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J (2007) Trogocytosis-based generation of suppressive NK cells. EMBO J 26: 1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle RA, Trowsdale J (2007) Promiscuity and the single receptor: NKG2D. Nat Rev Immunol 7: 737–744 [DOI] [PubMed] [Google Scholar]
- Fuertes MB, Girart MV, Molinero LL, Domaica CI, Rossi LE, Barrio MM, Mordoh J, Rabinovich GA, Zwirner NW (2008) Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell-mediated cytotoxicity. J Immunol 180: 4606–4614 [DOI] [PubMed] [Google Scholar]
- Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW (2007) Engagement of TLR3, TLR7, and NKG2D regulate IFN-γ secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J Immunol 179: 3472–3479 [DOI] [PubMed] [Google Scholar]
- Hirst CE, Buzza MS, Bird CH, Warren HS, Cameron PU, Zhang M, Ashton-Rickardt PG, Bird PI (2003) The intracellular granzyme B inhibitor, proteinase inhibitor 9, is up-regulated during accessory cell maturation and effector cell degranulation, and its overexpression enhances CTL potency. J Immunol 170: 805–815 [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Aucher A, Puaux AL, Bordier C, Joly E (2007) Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J Immunol 178: 3637–3647 [DOI] [PubMed] [Google Scholar]
- Hudrisier D, Bongrand P (2002) Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J 16: 477–486 [DOI] [PubMed] [Google Scholar]
- Li Z, Pradera F, Kammertoens T, Li B, Liu S, Qin Z (2007) Cross-talk between T cells and innate immune cells is crucial for IFN-{gamma}-dependent tumor rejection. J Immunol 179: 1568–1576 [DOI] [PubMed] [Google Scholar]
- McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM (2007) The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J Immunol 178: 3418–3426 [DOI] [PubMed] [Google Scholar]
- Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A (2006) Surface NK receptors and their ligands on tumor cells. Semin Immunol 18: 151–158 [DOI] [PubMed] [Google Scholar]
- Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD, Schreiber H (1999) CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-γ. Proc Natl Acad Sci USA 96: 8633–8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Sotomayor E, Gabrilovich D (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25: 267–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Glass DD, Shevach EM (2002) IL-10-producing CD4+ T cells mediate tumor rejection. J Immunol 168: 1–4 [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD (2001) IFN[gamma] and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410: 1107–1111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs 1–9