Abstract
Tissue repair is a complex process that requires wound-edge cells to proliferate and migrate, which in turn necessitates induction of a large repair transcriptome. Epigenetic modifications have emerged as crucial regulators of gene expression. Here, we ask whether epigenetic reprogramming might contribute to the concerted induction of repair genes by wound-edge cells. Polycomb group proteins (PcGs) co-operatively silence genes by laying down repressive marks such as histone H3 lysine 27 trimethylation (H3K27me3), which can be removed by specific demethylases. We show that PcGs Eed, Ezh2 and Suz12 are significantly downregulated during murine skin repair, whereas the newly described demethylases Jmjd3 and Utx are markedly upregulated. Correspondingly, we find a striking reduction of repressive H3K27me3 in the wound epidermis. Quantitative chromatin immunoprecipitation studies have revealed that there is less Eed bound to the regulatory regions of two paradigm wound-induced genes, Myc and Egfr, suggesting that loss of polycomb-mediated silencing might contribute to the induction of repair genes.
Keywords: chromatin, epithelium, wound, polycomb
Introduction
Wound healing in adult tissues is a dramatic and complex tissue rebuilding process that requires a multitude of cell lineages at the site of tissue damage to proliferate, migrate and differentiate (Martin, 1997); these various cell behaviours are, in part, achieved by induction of a large repair transcriptome, including, among others, cell-cycle regulators, matrix molecules, integrins, proteases and antioxidant enzymes (Werner & Grose, 2003; Cooper et al, 2005; Roy et al, 2008). It is of fundamental and clinical importance to understand how these changes are regulated and coordinated. Epigenetic modifications, and in particular those controlled by the polycomb family of proteins, are known to be crucial regulators of gene transcription potential during development. On the basis of the striking commonalities between wound repair and many aspects of embryonic morphogenesis (Martin & Parkhurst, 2004), we investigated whether similar epigenetic reprogramming might also underpin the concerted induction of repair genes by cells at the wound site.
Polycomb group proteins (PcGs) are a family of epigenetic modifiers that direct cellular fates during embryogenesis by controlling the expression patterns of homeotic genes and other developmental regulators (Sparmann & van Lohuizen, 2006). They form chromatin-modifying complexes that are able to silence large numbers of target genes by post-translational modification of histones and methylation of the DNA template. Eed, Ezh2 and Suz12 form the Polycomb Repressive Complex 2 (PRC2), and they co-operatively silence target genes by laying repressive marks such as histone H3 lysine 27 trimethylation (H3K27me3; Cao et al, 2002). Historically, H3K27 methylation has been considered to be relatively stable, thus maintaining long-term transcriptional silencing; however, the recent discovery of lysine demethylases, Jmjd3 and Utx, which specifically demethylate H3K27, indicates that these modifications can be more transient than originally predicted (Agger et al, 2007).
Studies in Drosophila (Netter et al, 1998; Maurange & Paro, 2002), as well as more recent studies in mammalian stem cells and embryonic fibroblasts (Boyer et al, 2006; Bracken et al, 2006; Mikkelsen et al, 2007; Pan et al, 2007; Ku et al, 2008), have revealed many crucial patterning genes, as well as hundreds of other genes with numerous and diverse functions, to be potential PcG targets. We hypothesized that PcGs may also regulate the expression of developmental and other genes during the repair process. This study reports how polycomb-mediated gene silencing is downregulated during mammalian wound repair, which may lead to derepression, or ‘un-silencing', of target genes that regulate wound repair.
Results And Discussion
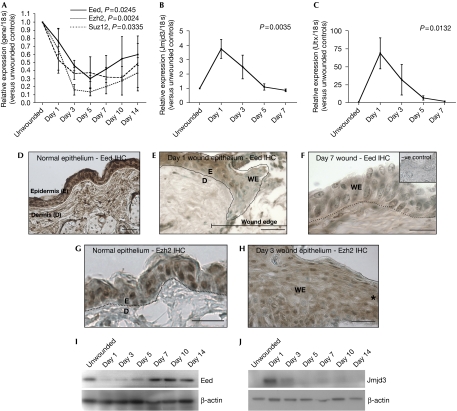
To determine whether PcGs and their associated demethylases contribute to regulating gene expression during in vivo mammalian tissue repair, we analysed full-thickness excisional wounds made to the shaved dorsal skin of adult male CD-1 mice (initial injury: 4 mm diameter biopsy punch; sample collection: 6 mm punch). Quantitative reverse transcription (RT)–PCR revealed that Eed, Ezh2 and Suz12, the three main components of PRC2, were significantly and transiently downregulated during the repair process (Fig 1A). Conversely, Jmjd3 (Fig 1B) and Utx (Fig 1C), the newly described lysine demethylases that specifically remove the PcG-mediated histone mark, were co-ordinately upregulated. Immunohistochemistry revealed that, spatially, Eed is expressed by the keratinocytes in unwounded skin (Fig 1D), but is downregulated in the leading-edge epithelium, which is an effect that extends approximately 10–20 cells back from the wound margin at day 1 post-wounding (Fig 1E), and persists within the continuous, newly formed immature epithelium of day 7 wounds (Fig 1F). Similarly, Ezh2 is abundant and predominantly nuclear in normal epidermis (Fig 1G), but in wound-edge epithelium its expression is reduced and diffuse throughout the cells (Fig 1H: day 3 wound). The changes in Eed and Jmjd3 expression were further shown by western blotting (Fig 1I,J). The timing of PcG re-expression is of interest as these proteins may have a role in re-silencing the repair machinery once the wound has healed, and there may be pathological consequences should this not occur properly. Our immunohistochemistry data show that Eed downregulation lasted beyond day 7 (Fig 1F) and remained low until maturation of the wound epidermis, between days 10 and 14. The western blot data (Fig 1I) indicate slightly earlier Eed re-expression, with levels seeming to be nearly restored by day 7; we attribute this apparent temporal discrepancy to wound contraction, which may result in an increased amount of normal unwounded epidermis in the biopsy samples at the later time-points.
Figure 1.
Polycomb group proteins are downregulated during skin wound healing, whereas their respective histone demethylases are upregulated. (A–C) Quantitative reverse transcription–PCR analysis of (A) Eed, Ezh2 and Suz12 (PcGs) and (B,C) Jmjd3 and Utx (demethylases) in excisional mouse skin wounds (initial injury: 4 mm biopsy punch; sample collection: 6 mm biopsy punch). The results, normalized to 18 s and presented relative to levels in unwounded skin, show mean±s.e.m. of at least three independent experiments (one-way ANOVA P-values indicated; n=3–5). (D–F) Eed immunohistochemistry (IHC; brown staining) in (D) normal unwounded skin indicates abundant expression in the epidermis (dotted white line indicates the epidermal–dermal boundary); which is downregulated in (E) the leading-edge epithelium 1 day post-wounding (black line demarks the epithelium); and (F) the newly formed epithelium overlying the wound 7 days post-wounding (inset: no primary antibody negative control). (G,H) Ezh2 immunohistochemistry in (G) normal unwounded skin indicates abundant expression in the nuclei of epidermal cells (dotted black line indicates the epidermal–dermal boundary), which is downregulated and predominantly cytoplasmic in (H) the leading-edge epithelium 3 days post-wounding (asterisk demarks the wound edge). (I,J) Western blot analysis of Eed and Jmjd3 during the time-course of wound repair, showing transient downregulation and upregulation, respectively. Scale bars: 50 μm (D,E,G); 30 μm (F,H). ANOVA, analysis of variance; D, dermis; E, epidermis; PcGs, polycomb group proteins; WE, wound epithelium.
Western blot analysis of wounded skin for H3K27me3—the repressive histone modification characteristic of PRC2 activity—showed a dramatic loss of this mark during the repair process (Fig 2A). Having observed changes in Eed and Ezh2 expression in the epidermis by immunohistochemistry, we predicted that the decrease in H3K27me3 would be occurring specifically in these cells. Indeed, although Eed and Ezh2 were also found to be expressed by cells in the dermis, the H3K27me3 mark was present only in isolated epidermis and not in the dermis of unwounded skin (Fig 2B). We wished to rule out that the decrease in H3K27me3 observed in whole wounds may in part be attributable to an under-representation of the epidermis in the samples owing to the gap created by the wound. However, this turns out to be incorrect because the loss of H3K27me3 persists through to 7 days post-wounding (Fig 2A), when re-epithelialization of the wound is complete (Fig 2D), and an equal or even greater number of epithelial cells is collected per biopsy than for unwounded skin. Furthermore, a comparison of disassociated epidermis from unwounded skin with that from day 3 wounded skin from three biological replicates also showed a reduction in the silencing modification (Fig 2C). Loss of the repressive H3K27me3 mark during repair is consistent with the downregulation of PcGs and the concomitant induction of demethylase expression. It supports the hypothesis that this may be a mechanism for ‘lifting the brakes' on a subset of the repair transcriptome, thus allowing for these genes to be expressed if the necessary transcription factors and machinery are present.
Figure 2.
The PRC2-mediated repressive histone mark is downregulated during skin wound healing. (A) Western blot analysis of H3K27me3 during the time-course of wound repair, showing transient downregulation. (B) Comparison of H3K27me3 levels in whole skin, epidermis and dermis by western blot, showing expression exclusively in the epidermal compartment. (C) Dispase-mediated isolation of epidermis from unwounded or wounded skin (3 days post-wounding) from three biological replicates, followed by western blot analysis, confirmed a wound-induced reduction in epidermal H3K27me3. (B,C) After blotting, membranes were silver stained, and representative bands are shown as loading controls. (D) A H&E-stained histological section of a day 7 wound, illustrating the complete re-epithelialization at this time point post-wounding (the dotted white line demarks the epidermis–dermis boundary over the central wound site). Scale bar, 130 μm. D, dermis; E, epidermis; GT, granulation tissue; H&E, haematoxylin and eosin; H3K27me3, histone H3 lysine 27 trimethylation; S, scab; WE, wound epithelium.
Downregulation of PcG expression has also been observed during imaginal disc regeneration in Drosophila (Lee et al, 2005), but this study provides the first evidence that PcG and reciprocal demethylase expression changes occur during mammalian wound healing.
Next, we asked whether this epigenetic reprogramming contributes directly to ‘repair genes' becoming un-silenced and induced at the wound edge. PcGs are thought to be recruited to specific sites in the genome, which are known as Polycomb Response Elements (PREs). During Drosophila and mouse development, these elements are clearly pivotal in spatially and temporally restricting the expression of Hox genes (Simon et al, 1992; Hanson et al, 1999); however, in vertebrates there is no clear consensus sequence defining these elements (Ringrose & Paro, 2007). To address this challenge of predicting mammalian PcG target genes, Boyer et al (2006), Bracken et al (2006) and others (Mikkelsen et al, 2007; Pan et al, 2007; Ku et al, 2008; Mohn et al, 2008) have used chromatin immunoprecipitation (ChIP) and microarray or sequencing strategies in murine embryonic stem cells and fibroblasts to identify potential developmental targets, and many of their hits are in fact genes that we know to also be upregulated and have functional roles in wound healing. Data mining and comparison of the Boyer et al (2006) and Bracken et al (2006) microarray experiments, which identified potential PcG target genes, with our own and others' work on wound-induced genes (Cooper et al, 2005; Roy et al, 2008) indicates that up to 20% of the known repair genes could potentially be regulated in this way.
We considered two such genes, Egfr and Myc, which are probable PcG targets based on the above studies and are well-established to be upregulated during, and important for, repair (Stoscheck et al, 1992; Zanet et al, 2005), and asked whether they might be regulated by PcGs in a wound setting.
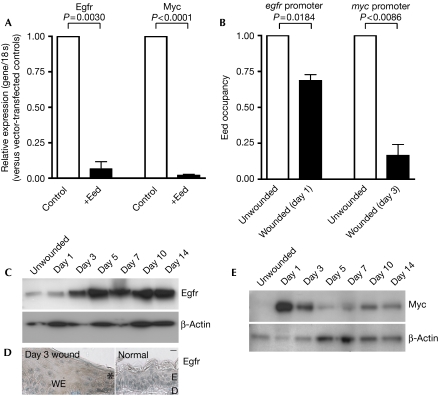
First, mIMCD3 epithelial cell cultures were transfected so that they overexpressed Eed and, after 24 h, were analysed by quantitative RT–PCR for target gene expression. Eed overexpression resulted in significant downregulation, or silencing, of both Egfr and Myc (Fig 3A). Next, in vivo wound samples were analysed by quantitative ChIP, which allowed for a comparison of the amount of PcG physically occupying the regulatory regions of specific genes. We compared the amount of Eed bound to the egfr and myc promoters in unwounded with wounded skin, and found that significantly less Eed bound to these promoter regions at 1 and 3 days post-wounding, respectively (Fig 3B). These findings provide correlative evidence that two paradigm wound-induced genes may be epigenetically regulated by PcGs during repair. We then sought to correlate this decrease in Eed binding with gene expression changes. Western blotting showed that Egfr and Myc expression are induced following loss of Eed binding (Fig 3C–E), albeit with a slight delay that we attribute to the time required for induction and/or recruitment of the required transcriptional machinery. For example, Egfr transcription during tissue repair may be positively regulated by serum exposure (Haley et al, 1987) and/or the transcription factor activator protein 1 (AP-1; Johnson et al, 2000), both of which are present in a wound setting.
Figure 3.
PRC2-mediated repression is decreased in the regulatory regions of important repair genes, myc and egfr, during wound healing. (A) Transfection of mIMCD3 cells with either empty vector (control) or Eed for 24 h, followed by quantitative reverse transcription–PCR analysis of Egfr and Myc, shows that they are both silenced by Eed. The results, normalized to 18 s and presented relative to levels in vector-transfected controls, show the mean±s.e.m. of at least three independent experiments (one-way ANOVA P-values indicated; n=3–5). (B) Quantitative chromatin immunoprecipitation experiments on unwounded compared with wounded skin, comparing the levels of Eed bound to egfr and myc promoter regions on days 1 and 3 post-wounding, respectively, show less Eed binding during repair. Quantitative PCR results are shown. Data are presented as mean±s.e.m. of three independent experiments (t-test P-values indicated; n=3). (C–E) Western blot analysis of Egfr and Myc during the time-course of wound repair and immunohistochemistry (D) showing increased Egfr staining (brown) in day 3 wound-edge epidermis (asterisk demarks the wound edge) compared with normal unwounded skin. Scale bar, 20 μm. ANOVA, analysis of variance; D, dermis; E, epidermis; WE, wound epithelium.
Although it is clear that several PcGs and demethylases show altered expression at the wound site, it is difficult to address precisely which are the rate-limiting components responsible for the induction of repair genes. Certainly, Eed, Ezh2 and Suz12 are known to function cooperatively (Hansen et al, 2008), and downregulation of all three in this context is likely to underpin the observed loss of PcG silencing. The role of the demethylases Jmjd3 and/or Utx in regulating repair genes will perhaps best be tested using various skin-specific knockout mice.
There are many other post-translational modifications of histone proteins and DNA itself that may also influence wound gene transcription. Our own data indicate that PcG regulation of histone methylation may be particularly relevant in un-silencing of genes in the wound epidermis, but this does not exclude a role for other epigenetic silencing and/or activating mechanisms. For example, H3K27me3 has been found to overlap with H3K4 methylation—an activating chromatin modification—at thousands of genetic loci, including many repair genes and specifically egfr and myc (Mikkelsen et al, 2007; Pan et al, 2007; Ku et al, 2008). Genes with these bivalent chromatin modifications are considered ‘poised' or ‘primed' for expression, and wound repair is precisely the sort of occasion when tissues might use such a mechanism to enable a coordinated and rapid induction of gene expression. DNA methylation is another epigenetic silencing mechanism that, acting independently or together with PcG-mediated silencing (Ku et al, 2008; Mohn et al, 2008), may contribute to regulating the repair transcriptome, including egfr and myc, which have both been shown to be regulated by DNA methylation in other settings (Meissner et al, 2008). Furthermore, PcGs and/or other epigenetic modifiers may act as regulators of equally dramatic changes in cell behaviours that occur in other wound tissues, including myofibroblast differentiation, inflammatory cell activation and angiogenesis. Indeed, Horswill et al (2008) have shown that, as fibroblasts differentiate during corneal repair, the maspin gene—a serine protease inhibitor that controls cell invasion—is epigenetically silenced by DNA methylation and histone H3 dimethylation at lysine 9.
This study has focused on the gene expression changes resulting from PcG downregulation, but the cell's behavioural consequences and how these changes contribute functionally to the marked transformation of dormant, unwounded epidermal cells into an activated, migrating and proliferative leading-edge remain largely unknown. Studies, primarily in embryonic stem cells and cancer cells, indicate that PcGs, in these contexts, can contribute to cell-cycle control, cell motility and differentiation switches (Sparmann & van Lohuizen, 2006), which are all components of the normal tissue repair process; however, future studies may unveil other unique ways in which cells of the wound epidermis respond to changes in PcG levels.
In summary, we have found that during in vivo mammalian skin repair there occurs a loss of PcG-mediated gene silencing, which is a mode of regulation that may have a powerful role during tissue repair, as it has the potential to coordinately regulate a considerable subset of known repair genes and thus function as a master regulator of wound re-epithelialization. In addition, our study highlights how mechanisms that underpin developmental growth and patterning of tissues during embryogenesis are re-used to rebuild tissues as part of a regenerative response.
Methods
Wound model. All experiments were conducted according to UK Home Office regulations. Mice (CD-1 age-matched males; 7–11 weeks) were halothane-anaesthetized and four full-thickness wounds (4 mm biopsy punch; Kai Industries, Oyana, Japan) were aseptically made to the shaved dorsal skin. Wound tissue was harvested with a 6 mm biopsy punch. The epidermis and dermis were separated by soaking in Dispase II (Roche Applied Science, Burgess Hill, UK; 0.145% in serum-free DMEM) overnight at 4°C.
Cell culture. MIMCD3 cells (mouse inner medullary collecting duct epithelial cells; gift from JA Davies, University of Edinburgh) were cultured in DMEM/F12 supplemented with 10% fetal calf serum (Invitrogen, Paisley, UK). Cells were transfected with either pCMV-Sport6 (empty vector, control) or pCMV-Sport6-Eed to overexpress mouse Eed (IMAGE ID: 3991086; Geneservice, Cambridge, UK) using Lipofectamine 2000 (Invitrogen). After transfection, cells were cultured for an additional 24 h, and harvested for RNA.
Histology. Tissue was fixed in 10% formalin for embedding in paraffin and 6 μm sections were subjected to Eed, Ezh2 or Egfr immunohistochemistry. Deparaffinized sections were incubated with primary antibody (Eed, Abcam, Cambridge, UK; Ezh2, Zymed, San Francisco, CA, USA; Egfr, Cell Signaling Technologies, Danvers, MA, USA) diluted 1:50 in antibody diluent (DAKO, Glostrup, Denmark) overnight at 4°C. After 3 × 5 min washes in Tris-buffered saline (TBS) containing 0.05% Tween-20, secondary swine anti-rabbit antibody (DAKO) diluted 1:500 in TBS was applied for 1 h at 37°C. Next, StreptABComplex/horseradish peroxidase (DAKO) was used to detect antibody binding and was visualized with 3,3′-diaminobenzidine; sections were counterstained with methyl green.
RNA isolation and RT–PCR analysis. Total RNA was extracted using the RNeasy kit (Qiagen, Crawley, UK). RNA (20 ng per reaction) was analysed for gene expression using the QuantiTect SYBR Green RT-PCR kit (Qiagen) and Quantitect Primer Assays.
Western blotting. Protein was extracted from homogenized tissue samples in either RIPA buffer (for Eed and Jmjd3 analysis) or acid extraction buffer (for H3K27me3 analysis) according to the manufacturer's instructions (Millipore, Watford, UK). Protein concentrations were determined using the Pierce (Cramlington, UK) BCA protein assay, and equal quantities were separated on 4–12% NuPAGE Novex Bis–Tris Gels (Invitrogen), transferred to polyvinylidene fluoride membrane, and blotted according to standard protocols using the following antibodies: Eed (Santa Cruz, Santa Cruz, CA, USA; 1:300), β-Actin (Sigma-Aldrich, Dorset, UK; 1:1,000), H3K27me3 (Millipore; 1:1,000), Jmjd3 (Abgent, San Diego, CA, USA; 1:300), Egfr (gift from PJ Cullen, University of Bristol; 1:1,000), Myc (Serotec, Oxford, UK; 1:500) and HRP-conjugated goat anti-rabbit or swine anti-mouse antibodies (GE Healthcare, Chalfont Saint Giles, UK; 1:3,000) or HRP-conjugated donkey anti-sheep antibody (Jackson Immunoresearch, Newmarket, UK; 1:10,000). Protein bands were visualized using the Amersham ECL Plus detection system (GE Healthcare). Colloidal silver stain, performed as described previously (Kovarik et al, 1987), served as a loading control for the H3K27me3 blots, and representative bands are shown.
Chromatin immunoprecipitation. Quantitative ChIP was carried out as described previously (Nelson et al, 2006). Briefly, tissue samples were diced with a scalpel in cell culture medium (DMEM, Invitrogen) containing 1.4% formaldehyde, and fixed by rotating at approximately 21°C for 15 min. Glycine was then added to a final concentration of 125 mM and samples were rotated for an additional 5 min, after which the tissue was washed twice with ice-cold PBS, homogenized in RIPA buffer, centrifuged at top speed for 10 min and the supernatant collected for ChIP analysis. Two micrograms of antibody were used (Eed (Abcam) compared with Rb IgG (Santa Cruz)), and the primers used were as follows: 5′MYC: TAGTTTAGAGAGACGTTTGGTCGT; 3′MYC: TTCCCTTTCTATACGATTATTCGAA; 5′EGFR: CCCCAGAGCCTTGTCTAGTG; and 3′EGFR: GGAGCGAAGAGGAGGAGAAT.
Acknowledgments
We thank R. Mori for advice and samples, and K. Malik for useful discussions. This work was supported by Cancer Research UK and Natural Sciences and Engineering Research Council of Canada (T.S., post-doctoral research fellowship).
Footnotes
The authors declare that they have no conflict of interest.
References
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K (2007) UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449: 731–734 [DOI] [PubMed] [Google Scholar]
- Boyer LA et al. (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441: 349–353 [DOI] [PubMed] [Google Scholar]
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K (2006) Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev 20: 1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y (2002) Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039–1043 [DOI] [PubMed] [Google Scholar]
- Cooper L, Johnson C, Burslem F, Martin P (2005) Wound healing and inflammation genes revealed by array analysis of ‘macrophageless' PU.1 null mice. Genome Biol 6: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J, Whittle N, Bennet P, Kinchington D, Ullrich A, Waterfield M (1987) The human EGF receptor gene: structure of the 110 kb locus and identification of sequences regulating its transcription. Oncogene Res 1: 375–396 [PubMed] [Google Scholar]
- Hansen KH, Bracken AP, Pasini D, Dietrich N, Gehani SS, Monrad A, Rappsilber J, Lerdrup M, Helin K (2008) A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol 10: 1291–1300 [DOI] [PubMed] [Google Scholar]
- Hanson RD et al. (1999) Mammalian Trithorax and polycomb-group homologues are antagonistic regulators of homeotic development. Proc Natl Acad Sci USA 96: 14372–14377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horswill MA, Narayan M, Warejcka DJ, Cirillo LA, Twining SS (2008) Epigenetic silencing of maspin expression occurs early in the conversion of keratocytes to fibroblasts. Exp Eye Res 86: 586–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Murphy BA, Matelis CM, Rubinstein Y, Piebenga EC, Akers LM, Neta G, Vinson C, Birrer M (2000) Activator protein-1 mediates induced but not basal epidermal growth factor receptor gene expression. Mol Med 6: 17–27 [PMC free article] [PubMed] [Google Scholar]
- Kovarik A, Hlubinova K, Vrbenska A, Prachar J (1987) An improved colloidal silver staining method of protein blots on nitrocellulose membranes. Folia Biol (Praha) 33: 253–257 [PubMed] [Google Scholar]
- Ku M et al. (2008) Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet 4: e1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Maurange C, Ringrose L, Paro R (2005) Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature 438: 234–237 [DOI] [PubMed] [Google Scholar]
- Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276: 75–81 [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM (2004) Parallels between tissue repair and embryo morphogenesis. Development 131: 3021–3034 [DOI] [PubMed] [Google Scholar]
- Maurange C, Paro R (2002) A cellular memory module conveys epigenetic inheritance of hedgehog expression during Drosophila wing imaginal disc development. Genes Dev 16: 2672–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A et al. (2008) Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454: 766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS et al. (2007) Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448: 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn F, Weber M, Rebhan M, Roloff TC, Richter J, Stadler MB, Bibel M, Schubeler D (2008) Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell 30: 755–766 [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Sova P, Bomsztyk K (2006) Fast chromatin immunoprecipitation assay. Nucleic Acids Res 34: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter S, Fauvarque MO, Diez del Corral R, Dura JM, Coen D (1998) white+ transgene insertions presenting a dorsal/ventral pattern define a single cluster of homeobox genes that is silenced by the polycomb-group proteins in Drosophila melanogaster. Genetics 149: 257–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA (2007) Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell 1: 299–312 [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R (2007) Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development 134: 223–232 [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Rink C, Biswas S, Sen CK (2008) Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics 34: 162–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Chiang A, Bender W (1992) Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development 114: 493–505 [DOI] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M (2006) Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6: 846–856 [DOI] [PubMed] [Google Scholar]
- Stoscheck CM, Nanney LB, King LE Jr (1992) Quantitative determination of EGF-R during epidermal wound healing. J Invest Dermatol 99: 645–649 [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83: 835–870 [DOI] [PubMed] [Google Scholar]
- Zanet J, Pibre S, Jacquet C, Ramirez A, de Alboran IM, Gandarillas A (2005) Endogenous Myc controls mammalian epidermal cell size, hyperproliferation, endoreplication and stem cell amplification. J Cell Sci 118: 1693–1704 [DOI] [PubMed] [Google Scholar]