Abstract
Under the canopy, far-red (FR) light represses seed germination by inactivating phytochrome photoreceptors. This elicits a decrease in gibberellins (GA) levels and an increase in abscisic acid (ABA) levels. GA promotes germination by enhancing the proteasome-mediated destruction of DELLA repressors. ABA prevents germination by stimulating the expression of ABI repressors. How phytochromes elicit changes in hormone levels or how GA- and ABA-dependent signals are coordinated to repress germination remains poorly understood. We show that repression of germination by FR light involves stabilized DELLA factors GAI, RGA and RGL2 that stimulate endogenous ABA synthesis. In turn, ABA blocks germination through the transcription factor ABI3. The role of PIL5, a basic helix-loop-helix transcription factor stimulating GAI and RGA expression, is significant, provided GA synthesis is high enough; otherwise, high GAI and RGA protein levels persist to block germination. Under white light, GAI and RGA driven by the RGL2 promoter can substitute for RGL2 to promote ABA synthesis and repress germination, consistent with the recent findings with RGL2. The three DELLA factors inhibit testa rupture whereas ABI3 blocks endosperm rupture.
Keywords: abscisic acid, DELLA, gibberellins, phytochrome, seed germination
Introduction
In Arabidopsis, the mature seed consists of a protective outer layer of dead tissue, the testa, under which the endosperm, a single layer of cells, surrounds the embryo (Debeaujon et al, 2000). Arabidopsis seed germination chronologically involves testa rupture followed by concomitant endosperm rupture and embryonic axis (i.e. radicle) protrusion, the latter being the usual definition of germination (Kucera et al, 2005; Muller et al, 2006). When seeds are non-dormant, as in this study, imbibition by water is sufficient to trigger germination. Under normal conditions, the process can be completed within 48–72 h after seed imbibition (Piskurewicz et al, 2008).
Germination is under tight control by the environment, being affected by light quality, temperature and water potential. Environmental factors eventually determine the relative levels of the phytohormones gibberellins (GA) and abscisic acid (ABA) that have an antagonistic and important function in the control of seed germination (Olszewski et al, 2002; Nambara and Marion-Poll, 2005). Conditions favourable for germination lead shortly after seed imbibition to an increase of GA levels (Ogawa et al, 2003), which is essential for germination to occur. In parallel, the levels of ABA drop rapidly after dry seed imbibition, and thereafter the role of ABA becomes facultative: a sudden osmotic stress or direct ABA application effectively blocks endosperm rupture and its effect on testa rupture is significantly lesser (Muller et al, 2006; Piskurewicz et al, 2008). Importantly, exogenous GA does not stimulate endosperm rupture in ABA-treated seeds, suggesting that ABA acts downstream of GA for the control of endosperm rupture (Muller et al, 2006). The ABA-dependent growth arrest occurs only within a limited time window of about 48 h after seed imbibition (Lopez-Molina et al, 2001). ABA or osmotic stress stimulates the de novo accumulation of embryonic transcription factors ABI3 and ABI5, which are both necessary to repress germination (Lopez-Molina et al, 2001, 2002). Similarly, as during seed maturation, ABI5 expression is positively regulated by ABI3 and both transcription factors ensure the de novo induction of LEA gene expression, thus maintaining the embryonic nature of the arrested embryo (Parcy et al, 1994; Finkelstein and Lynch, 2000; Lopez-Molina and Chua, 2000).
GA promotes seed germination by enhancing the destruction of the DELLA repressor proteins through the 26S-proteasome machinery. DELLA genes are predicted to encode GRAS-family transcription factors, although no DNA-binding activity has been characterized so far. Rather, it has been proposed that they influence transcription by interacting with other DNA-binding transcription factors (Zentella et al, 2007). In the proposed model, GA binds to Arabidopsis GID1-like receptors, so as to enhance DELLA protein interaction with the F-box protein SLY1, thus facilitating DELLA ubiquitination and subsequent degradation (Sun and Gubler, 2004; Feng et al, 2008). There are five DELLA genes in the Arabidopsis genome: RGA, GAI, RGL1, RGL2 and RGL3. Even though all DELLA genes are expressed during seed germination, only RGL2, GAI and RGA have been shown to have a function to repress germination (Lee et al, 2002; Tyler et al, 2004; Penfield et al, 2006).
Light quality affects seed germination by invoking changes in GA and ABA levels (Seo et al, 2006). This notably involves phytochrome B (phyB) and PIL5, a basic helix-loop-helix (bHLH) transcription factor indirectly modulating GA and ABA levels and directly stimulating RGA and GAI gene transcription (Bae and Choi, 2008; Seo et al, 2009). phyB is a protein photoreceptor with a covalently attached light-sensitive chromophore, whose activity is mainly set by the intensity ratio of red light to far-red (FR) light. An elevated ratio of red light to FR light (R conditions) leads to the active state of phyB (Pfr state). When active, phyB triggers the proteasome-mediated destruction of PIL5 through a process in which phyB and PIL5 interact (Oh et al, 2004, 2006). As a result, GA synthesis occurs normally and GAI and RGA transcription is low (Oh et al, 2007).
In contrast, under the canopy, a low ratio of red to FR light (FR conditions) will inactivate phyB (Pr state) (Chen et al, 2004). When inactive, phyB no longer interacts with PIL5 and this prevents PIL5 destruction by the 26S-proteasome machinery. As a result, PIL5 accumulates and represses seed germination. Under this model, PIL5-dependent repression involves (1) GAI and RGA, (2) SOMNUS (SOM), a CCCH-type zinc-finger protein also repressing germination and (3) regulation of ABA and GA metabolic genes (Kim et al, 2008; Seo et al, 2009). PIL5 activates the transcription of GAI, RGA and SOM genes by directly binding to their promoter sequences. In parallel, both PIL5 and SOMNUS are proposed to indirectly lower GA levels and elevate ABA levels by modulating the expression of GA and ABA metabolic genes in an unknown manner. In turn, lower GA levels further increase DELLA protein stability, thus enhancing RGA-, GAI- and RGL2-dependent repression, whereas higher ABA levels repress seed germination by stimulating ABI3 and ABI5 expression.
Some key aspects of the model described above have not been addressed. First, ga1/pil5 (and ga1/som) double mutants cannot germinate under FR conditions, suggesting that additional unidentified repressive activities occur when GA synthesis is severely prevented (by the ga1 mutation). Second, the role of ABA and ABA-response factors to repress germination has not been directly investigated. Third, the nature of the PIL5- and SOM-dependent regulation of the expression of genes involved in hormone metabolism is unclear (Oh et al, 2007; Kim et al, 2008). Indeed, the observed changes in the expression of genes involved in GA and ABA metabolism in pil5 or som mutants may be interpreted as secondary effects resulting from pil5 and som seed germination under FR conditions. Finally, the notion that the balance of GA and ABA levels determines the germination potential of the Arabidopsis seed lacks precision in terms of their effects on development. Thus, even though the observation that GA and ABA levels are inversely correlated strongly points to a regulatory crosstalk between their metabolic pathways, this does not necessarily imply that each hormone regulates germination through symmetrically inversed mechanisms. Indeed, in a recent report, we showed that RGL2 and ABI5 have distinct developmental functions under white light that are consistent with how GA and ABA differently influence testa and endosperm rupture. In response to low GA levels, a stabilized RGL2 efficiently blocks testa rupture and increases endogenous ABA levels. ABA in turn stimulates ABI5 expression and product activity to repress endosperm rupture (Piskurewicz et al, 2008). This points to the general notion that GA promotes testa rupture and limits ABA levels, thus ensuring low expression and activity of the ABA-response factors repressing endosperm rupture.
Here, we report that this view can be extended to the phyB-dependent control of seed germination. We show that under FR conditions, low GA levels allow an overaccumulation of GAI, RGA and RGL2, which block testa rupture and promote an increase in the levels of ABA, ultimately responsible to prevent endosperm rupture. This may involve DELLA-dependent stimulation of the expression of XERICO, encoding an RING-H2 factor promoting ABA synthesis in an unknown manner. ABA-dependent repression under FR conditions is orchestrated by ABI3. We show that the role of PIL5 to influence GAI and RGA protein levels is limited to FR conditions in which GA levels are not drastically reduced. As a result, in pil5 seeds under low GA conditions, GAI and RGA levels increase sufficiently to block testa rupture and promote higher endogenous ABA levels. Finally, we show that GAI and RGA can complement rgl2 mutants when both are under the control of RGL2 promoter sequences, further confirming their role to repress testa rupture and to stimulate endogenous ABA synthesis during germination.
Results
RGL2, GAI and RGA are the main GA-response repressors of testa and endosperm rupture under FR light
Under phyB-dependent repression of seed germination, a pulse of FR light (FR conditions) ensures a complete blockade of seed germination in darkness unless followed by a pulse of red light (R conditions, phyB is active). FR conditions (i.e. phyB is inactive) are associated with lower GA levels and higher ABA levels, whereas the converse (high GA, low ABA) occurs under R conditions (Seo et al, 2006). The relative role of each hormone as well as each GA- or ABA-response factor to repress seed germination remains unclear.
Earlier reports have suggested that in conditions other than white light illumination, RGL2 is no longer the main DELLA factor repressing seed germination in response to low GA levels (Cao et al, 2005). Indeed, contrarily to what is observed under white light conditions, ga1/rgl2 double mutants can no longer germinate in darkness. In contrast, ga1/rgl2/gai/rga mutants can germinate in the dark, which shows that RGL2, GAI and RGA redundantly repress germination in the absence of light. However, darkness is a rather ill-defined treatment from the perspective of phytochrome-dependent repression of seed germination. Indeed, varied seed germination responses may occur in darkness depending on the seed batch, including germination of the entire seed population (Cao et al, 2005; Supplementary Figure 1). This is most likely because of the presence of different pools of active phyB in imbibed seeds that could be inherited from embryogenesis or from the experimental manipulations performed in the presence of light before seed imbibition. Therefore, seed germination in ga1/rgl2/gai/rga could still involve active phyB, so that the relative contribution of phyB and DELLA factors remains unclear.
Here, we explore the role of RGL2, GAI and RGA together with ABA to repress seed germination under conditions in which phyB is inactive (i.e. FR conditions). A 5-min FR irradiation treatment is sufficient to inactivate phyB and ensure repression of germination in darkness (Supplementary Figure 1). We first examined testa and endosperm rupture events in ga1, ga1/rgl2 and ga1/rgl2/gai/rga seeds under FR conditions, a situation that had not been yet explored.
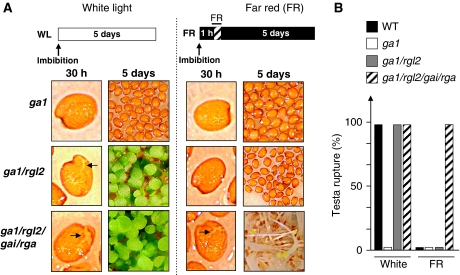
Under white light conditions, testa and endosperm rupture could be observed in ga1/rgl2 and ga1/rgl2/gai/rga seeds, but not in ga1 seeds, consistent with the earlier results (Figure 1A and B). In contrast, under FR conditions, testa rupture and endosperm rupture could only be observed in ga1/rgl2/gai/rga seeds (Figure 1A and B). FR conditions were associated with higher GAI and RGA protein contents in ga1 and ga1/rgl2, consistent with the earlier reports (Supplementary Figure 2). This further supports the notion that GAI and RGA, together with RGL2, become key GA-response repressors of testa and endosperm rupture when the phytochrome is inactive.
Figure 1.
RGL2, GAI and RGA redundantly repress testa rupture under FR conditions. (A) Representative pictures showing ga1-3, ga1-3/rgl2-13 and ga1-3/rga-t2/gai-t6/rgl2-1 seeds 30 h and 5 days after imbibition under white light conditions or far-red (FR) light conditions. Picture showing testa rupture in ga1-3/rgl2-13 seed is taken 72 h after imbibition. Arrows indicate testa rupture events. (B) Histogram shows percentage of testa rupture events 5 days after imbibition of WT (Col), ga1-3, ga1-3/rgl2-13 and ga1-3/rgat2/gait6/rgl2-1 seeds under white (white) or FR light conditions (in three independent seed batches (n=150–300) testa rupture percentage is always either 0 or 100% depending on the genotype and the light conditions as shown in the histogram).
GAI, RGA and RGL2 are necessary to increase endogenous ABA levels under FR conditions
Earlier reports have suggested that GAI and RGA may stimulate endogenous ABA synthesis in seedlings (Zentella et al, 2007). In addition, we showed earlier that RGL2-dependent repression of seed germination in white light involves stimulation of endogenous ABA synthesis (Piskurewicz et al, 2008). We thus hypothesized that under FR conditions, GAI and RGA, together with RGL2, are necessary to repress germination also by stimulating ABA synthesis.
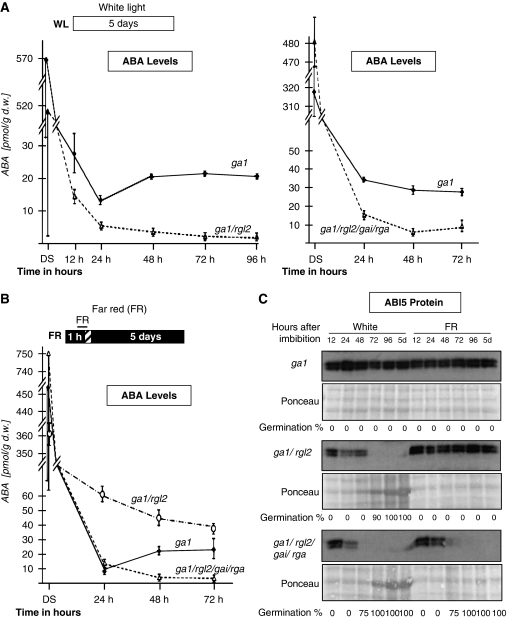
To address this possibility, we monitored endogenous ABA levels as well as ABI5 accumulation in protein blots, as it is a convenient marker for changes in endogenous ABA levels on seed imbibition and before seedling establishment (Piskurewicz et al, 2008). ABA levels, high in dry seeds, dropped markedly after imbibition under white light conditions in all ga1, ga1/rgl2 and ga1/rgl2/gai/rga seeds, consistent with the earlier results (Figure 2A) (Piskurewicz et al, 2008). Thereafter, ABA levels remained 5–10-fold higher in ga1 relative to ga1/rgl2 or ga1/rgl2/gai/rga seeds, consistent with the earlier results (Figure 2A) (Piskurewicz et al, 2008). As expected, ABI5 protein levels were high in ga1 seeds, but rapidly decayed in ga1/rgl2 or ga1/rgl2/gai/rga seeds under white light conditions (Figure 2C). In contrast, endogenous ABA and ABI5 protein levels remained elevated in ga1 and ga1/rgl2 seeds under FR conditions (Figure 2B and C). In contrast, ga1/rgl2/gai/rga seeds maintained 5–10-fold lower endogenous ABA levels over time relative to ga1 and ga1/rgl2 and failed to maintain ABI5 protein levels (Figure 2B and C). Higher endogenous ABA levels were consistently associated with higher mRNA levels of NCED6, encoding a 9-cis-epoxycarotenoid dioxygenase involved in the first-step specific to ABA biosynthesis (Supplementary Figure 3) (Lefebvre et al, 2006). This is consistent with their ability to germinate and indicates that GAI and RGA, together with RGL2, are necessary to maintain high endogenous ABA levels under FR conditions.
Figure 2.
RGL2, GAI and RGA are necessary to promote endogenous ABA and ABI5 levels under FR conditions. (A) Time course of endogenous ABA levels in ga1-3, ga1-3/rgl2-13 and ga1-3/rga-t2/gai-t6/rgl2-1 seeds under white light conditions (normal MS medium). Units are pmol per gram of fresh weight. Error bars indicate s.d. (n=3). (B) Same as in (A) under far-red light conditions. (C) Protein gel blot analysis of a time course of ABI5 protein levels upon ga1-3, ga1-3/rgl2-13 and ga1-3/rga-t2/gai-t6/rgl2-1 seed imbibition under far-red (FR) or white (white) light conditions. Signals can be directly compared between different genetic backgrounds. Each lane contains proteins extracted from 5 mg of seeds stained with Ponceau S (Ponceau) before incubation with antibodies against ABI5. At each time point, the percentage of germination (i.e. endosperm rupture events) in the seed population material used in the protein blot is indicated. We measured endosperm rupture in three different seed batches (n=150–300): at 5 days endosperm rupture percentage is either 0 or 100% depending on the genotype and the light condition as shown in Figure 1.
XERICO encodes a putative RING-H2 factor promoting ABA synthesis in an unknown manner (Ko et al, 2006; Zentella et al, 2007). We showed earlier that under white light conditions, RGL2 is necessary to elevate XERICO mRNA expression under low GA conditions (Piskurewicz et al, 2008). This suggested a possible mechanism accounting for the elevation of endogenous ABA levels after seed imbibition under low GA conditions. A similar mechanism, involving GAI and RGA, had been proposed earlier in seedlings, in which GAI and RGA bind XERICO promoter sequences and positively regulate its mRNA accumulation (Ko et al, 2006; Zentella et al, 2007). We, therefore, wished to explore whether GAI and RGA are necessary to promote XERICO mRNA accumulation under white and FR light conditions during seed germination and under low GA conditions. Supplementary Figure 4 shows that under white light conditions, XERICO mRNA expression was highest in ga1 seeds and markedly lower in ga1/rgl2 seeds, consistent with the earlier results using paclobutrazol (PAC)-treated wild type (WT) and rgl2 seeds, and reached its lowest levels in ga1/rgl2/gai/rga seeds (Piskurewicz et al, 2008). However, XERICO mRNA accumulation was comparable in ga1 and ga1/rgl2 seeds under FR light, but not in ga1/rgl2/gai/rga seeds, in which it was markedly lower (Supplementary Figure 4). Taken together, these data are further consistent with the notion that XERICO is a target gene of the DELLA factors RGL2, GAI and RGA to promote an elevation in endogenous ABA levels during seed germination.
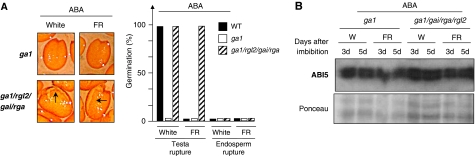
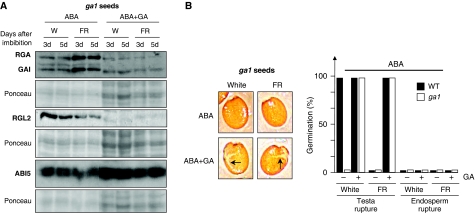
To discriminate between the role of DELLA factors to prevent testa rupture and their role to repress endosperm rupture by stimulating endogenous ABA levels, we treated ga1/rgl2/gai/rga with ABA under FR conditions. ABA-treated ga1/rgl2/gai/rga seeds ruptured their testa, but failed to rupture their endosperm (Figure 3A). As expected, this also correlated with higher ABI5 protein levels (Figure 3B, compare with Figure 2C). Similarly, treating ga1 seeds with both GA and ABA led to lower DELLA factor accumulation but maintained high ABI5 protein levels (Figure 4A), consistent with the earlier results (Zentella et al, 2007). Under these conditions, testa rupture was visible, but no endosperm rupture took place (Figure 4B).
Figure 3.
ABA blocks rupture of endosperm in ga1/rga/gai/rgl2 seeds, but not that of testa. (A) Representative pictures show testa rupture events in ga1-3 and ga1-3/rga-t2/gai-t6/rgl2-1 seeds 5 days after imbibition under white (white) or far-red (FR) conditions in the presence of 3 μM ABA. Histogram shows percentage of testa and endosperm rupture events 5 days after seed imbibition. Arrows indicate testa rupture event (in three independent seed batches (n=150–300) testa rupture percentage is always either 0 or 100% depending on the genotype and the light conditions as shown in the histogram). (B) Protein gel blot analysis of ABI5 protein levels in ga1-3 and ga1-3/rga-t2/gai-t6sol;rgl2-1 seeds at the indicated times upon imbibition under far-red (FR) or white (W) light conditions in the presence of 3 μM ABA. Protein gel blot conditions as in Figure 2C.
Figure 4.
Exogenous GA promotes testa rupture by downregulating DELLA protein levels without overcoming ABA-dependent blockade of endosperm rupture. (A) Protein gel blot analysis of GAI, RGA, RGL2 and ABI5 protein levels in ga1-3 seeds at the indicated times upon imbibition under white (W) or far-red (FR) light conditions in the presence of 3 μM ABA without (ABA) or with 50 μM GA (ABA+GA). Each lane contains proteins extracted from 5 mg of seeds. (B) Representative pictures showing ga1-3 seeds after 5 days under white (white) or far-red (FR) light conditions in the presence of 3 μM ABA without (ABA) or with 50 μM GA (ABA+GA). Arrows indicate testa rupture event. Histogram shows percentage of testa and endosperm rupture events at this time point (in three independent seed batches (n=150–300) testa rupture percentage is always either 0 or 100% depending on the genotype and the light conditions as shown in the histogram).
GAI and RGA can restore normal sensitivity to low GA in rgl2 mutants
We suggested earlier that under white light conditions, RGL2 is the main factor repressing seed germination because it achieves the highest protein accumulation relative to other DELLA factors when GA levels are low. This may be due to the fact that ABA positively and strongly regulates RGL2 mRNA levels, which is not the case for GAI and RGA (Piskurewicz et al, 2008). Here, we provided genetic evidence that GAI and RGA are also necessary to elevate endogenous ABA levels under FR conditions. Thus, we reasoned that both GAI and RGA should be able to substitute RGL2's function under white light conditions, provided their expression is under the control of RGL2 promoter.
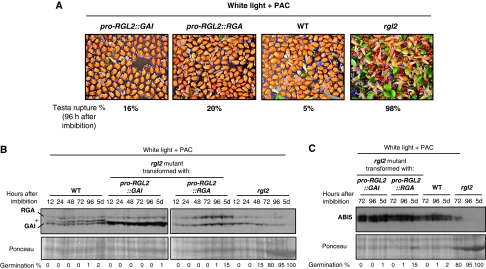
To explore this possibility, we generated rgl2 mutant transgenic lines carrying a transgene containing either GAI or RGA coding sequences under the control of RGL2 promoter sequences (rgl2/pro-RGL2∷GAI and rgl2/pro-RGL2∷RGA). We examined seed germination responses to low GA levels in independent lines using PAC, which inhibits ent-kaurene oxidase, a key enzyme of the GA synthesis pathway. As expected, repression of testa and endosperm rupture could be observed in PAC-treated rgl2/pro-RGL2∷GAI and rgl2/pro-RGL2∷RGA transgenic lines (Figure 5A; Supplementary Figure 5B). RNA and protein blot analysis confirmed that this was associated with higher GAI or RGA mRNA and protein product levels relative to PAC-treated WT and rgl2 seeds (Figure 5B; Supplementary Figure 5A). In addition, rgl2/pro-RGL2∷GAI and rgl2/pro-RGL2∷RGA arrested seeds accumulated high ABI5 protein levels, unlike rgl2 mutant seeds (Figure 5C). This is consistent with the notion that sufficient GAI or RGA accumulation can increase endogenous ABA levels during seed germination even under white light conditions.
Figure 5.
When expressed under RGL2 promoter sequences, GAI and RGA can inhibit rgl2 mutant seed germination in response to low GA levels. (A) Pictures show rgl2-13 (Col) mutants transformed with pro-RGL2∷GAI and pro-RGL2∷RGA DNA constructs as well as rgl2-13 and WT (Col) plants 96 h upon imbibition under white light conditions in the presence of 15 μM PAC (concerning the use of PAC, see Supplementary Figure 10). Average percentage of testa rupture events measured 96 h on imbibition is indicated (see also Supplementary Figure 5B). In the absence of PAC, all genotypes germinated similarly (not shown). (B) Protein gel blot analysis of GAI and RGA protein levels in WT (Col), rgl2-13/pro-RGL2∷GAI, rgl2-13/pro-RGL2∷RGA and rgl2-13 seeds at the indicated time points upon seed imbibition under same conditions as in A; 10 μg of total protein is loaded per lane. The band indicated by a plus sign (+) is RGL2, which may be detected with anti-GAI antibody. At each time point, the percentage of germination (i.e. endosperm rupture events) in the seed population material used in the protein blot is indicated. (C) Same as in B, but ABI5 protein levels are monitored.
Taken together, these data support the hypothesis that the DELLA factors RGL2, GAI and RGA collectively repress seed germination under FR conditions by (1) repressing testa rupture and (2) stimulating endogenous ABA synthesis. In turn, higher ABA levels prevent endosperm rupture by stimulating the expression and activity of ABA-response factors, such as ABI5.
Absence of PIL5 does not prevent high GAI and RGA accumulation under low GA conditions
PIL5 is a bHLH transcription factor preferentially interacting with the active Pfr form of the phytochrome, which facilitates its degradation by the 26S proteasome (Oh et al, 2004, 2006). Under FR conditions, PIL5 is stable and stimulates the transcription of GAI and RGA (Oh et al, 2007). pil5 mutants can germinate under FR conditions and this is associated with lower endogenous ABA levels. However, pil5 mutants cannot germinate in the absence of GA synthesis (as in a ga1 background), which is associated with increased endogenous ABA levels (Oh et al, 2006). This shows that PIL5 is no longer essential to repress seed germination under low GA levels. We wished to further clarify the role of PIL5 in the context of the repressive function of RGL2, GAI and RGA outlined above.
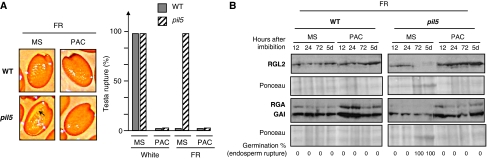
Under FR conditions, pil5 mutants ruptured their testa before endosperm rupture (i.e. germination), unlike WT seeds (Figure 6A). Treating seeds with PAC prevented testa and endosperm rupture in both WT and pil5 seeds (Figure 6A). These observations are consistent with the earlier results (Oh et al, 2006).
Figure 6.
Absence of PIL5 does not prevent high GAI and RGA accumulation under low GA conditions. (A) Representative pictures show WT (Col) and pil5 seeds 24 h upon imbibition under far-red (FR) conditions in the absence (MS) or the presence of 5 μM PAC (PAC) (concerning the use of PAC, see Supplementary Figure 10). Arrows indicate testa rupture event. Histogram shows percentage of testa rupture events 5 days upon imbibition under white (white) or far-red (FR) conditions in the absence (MS) or the presence of 5 μM PAC (PAC) (in three independent seed batches (n=150–300) testa rupture percentage is always either 0 or 100% depending on the genotype and the light conditions as shown in the histogram). (B) Protein gel blot analysis of a time course of GAI, RGA and RGL2 protein levels in WT (Col) and pil5 upon imbibition under far-red (FR) conditions in the absence (MS) or the presence of 5 μM PAC (PAC); 10 μg of total protein is loaded per lane. At each time point, the percentage of germination (i.e. endosperm rupture events) in the seed population material used in the protein blot is indicated.
Direct measurement of endogenous GAI and RGA protein levels in pil5 seeds under FR conditions was not reported earlier. Under FR conditions, we observed, as expected, that pil5 seeds had lower endogenous RGA and GAI protein levels relative to WT, although they could still be detected (Figure 6B). In contrast, PAC treatment led to higher RGA and GAI protein levels in both WT and pil5 seeds (Figure 6B). This may result from higher RGA and GAI protein stability as a result of lower GA levels. Critically, RGA and GAI protein levels in PAC-treated pil5 seeds were similar to those observed in WT seeds under FR conditions in the absence of PAC treatment (Figure 6B). This observation readily provides an explanation as to why preventing GA synthesis in pil5 mutants leads to blockade of testa rupture and higher endogenous ABA levels: sufficient GAI and RGA protein levels accumulate to stimulate endogenous ABA synthesis given that they are similar to those in WT seeds under FR conditions. In turn, higher ABA levels would ensure high RGL2 protein levels by increasing RGL2 mRNA levels (Piskurewicz et al, 2008). Consistent with this view, RGL2 protein levels increased in PAC-treated pil5 seeds (Figure 6B). In the absence of PAC treatment, RGL2 protein levels were roughly similar between WT and pil5 seeds at early time points after imbibition (12, 24 h), but rapidly decayed thereafter in pil5 (Figure 6B). Thus, failure to maintain RGL2 protein levels is consistent with the fact that pil5 have low endogenous ABA levels rather than the result of the absence of PIL5. Indeed, PIL5 does not seem to directly regulate RGL2 transcription (Oh et al, 2007), whereas ABA strongly upregulates RGL2 mRNA levels (Piskurewicz et al, 2008).
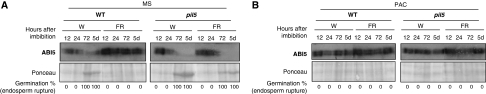
Furthermore, WT seeds under FR conditions accumulated high ABI5 protein levels relative to white light conditions and could not germinate (Figure 7A). In contrast, pil5 mutants, which accumulate low GAI, RGA and RGL2 levels (Figure 6B), failed to maintain high ABI5 protein levels and germinated (Figure 7A). As expected, PAC-treated pil5 seeds, which accumulate high levels of GAI, RGA and RGL2 (Figure 6B), maintained high ABI5 protein levels and did not germinate (Figure 7B). These results are consistent with the notion that GAI and RGA factors stimulate endogenous ABA levels under FR conditions.
Figure 7.
Absence of PIL5 does not prevent ABI5 accumulation under low GA conditions. (A) Protein gel blot analysis of a time course of ABI5 protein levels in WT (Col) and pil5 seeds upon imbibition under far-red (FR) or white (W) light conditions; 10 μg of total protein is loaded per lane. At each time point, the percentage of germination (i.e. endosperm rupture events) in the seed population material used in the protein blot is indicated. (B) Same as in (A), but WT (Col) and pil5 seeds were imbibed in the presence of 5 μM PAC (PAC) (concerning the use of PAC, see Supplementary Figure 10).
ABI3 is the main factor repressing germination under FR conditions
We further evaluated the role of ABA to repress seed germination under FR conditions when GA synthesis is prevented. If RGL2, GAI and RGA prevent endosperm rupture by promoting an increase in endogenous ABA levels, then preventing ABA synthesis should lead to seed germination, as reported earlier under white light (Leon-Kloosterziel et al, 1996; Piskurewicz et al, 2008).
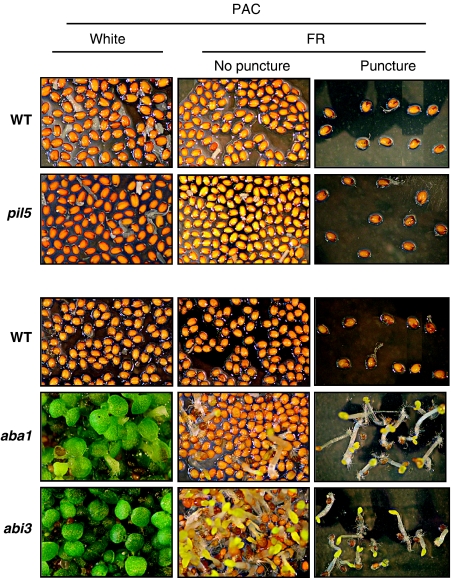
Unexpectedly, PAC-treated aba1 mutant seeds, unable to synthesize ABA, germinated poorly (20% after 5 days) under FR conditions (Figure 8; Supplementary Figure 7) even though they accumulated lower RGL2 and ABI5 protein levels relative to WT seeds, consistent with earlier results (Piskurewicz et al, 2008) (Supplementary Figure 8). However, we found that aba1 seeds did not rupture their testa, consistent with their high RGA and GAI protein accumulation, which was similar to that of WT seeds (Figure 9A). Thus, we reasoned that failure of aba1 seeds to rupture their testa might mechanically block radicle elongation, therefore, preventing the visualization of endosperm rupture (i.e. defined here as visible radicle protusion out of the seed coat). To address this possibility, we punctured aba1 seeds with a needle before FR irradiation (Material and methods). This surgical procedure triggered germination in each PAC-treated aba1 mutant seed (Figure 8; Supplementary Figures 6 and 7). Seed germination could not be observed in punctured PAC-treated WT or pil5 mutant seeds (Figure 8; Supplementary Figures 6 and 7). Control experiments performed in parallel showed that the puncture assay does not kill embryos (Supplementary Figures 6 and 7).
Figure 8.
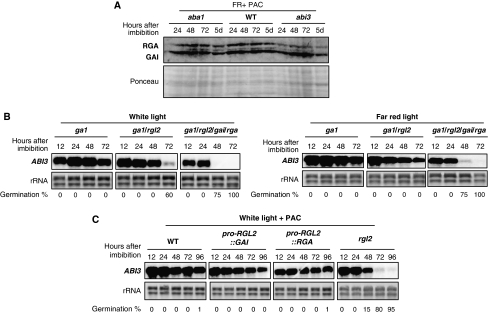
ABA acts through ABI3 to inhibit endosperm rupture in FR conditions. Pictures show WT (Col), pil5, WT (Ler), aba1-1 and abi3-1 seeds 7 days upon imbibition under white (white) or far-red (FR) conditions in the presence of 5 μM PAC (PAC) (concerning the use of PAC, see Supplementary Figure 10). Seeds were used for puncture experiments (Materials and methods). Punctured seeds were transferred to normal medium (MS) under white light conditions to assess seed viability after surgical procedure (Supplementary Figure 6). The procedure did not prevent seed germination (Supplementary Figure 6 shows WT (Col and Ler) and pil5 seeds after puncture).
Figure 9.
aba1 and abi3 seeds accumulate WT-like levels of GAI and RGA consistent with their inability to rupture the testa. DELLA-dependent increase in endogenous ABA levels correlates with high ABI3 mRNA expression. (A) Protein gel blot analysis of RGA and GAI protein levels in WT (Ler) and aba1-1 and abi3-1 seeds at the indicated times upon seed imbibition under far-red conditions in the presence of 5 μM PAC (FR+PAC) (concerning the use of PAC, see Supplementary Figure 10); 10 μg of total protein is loaded per lane. (B) RNA gel blot analysis of the time course of ABI3 mRNA accumulation in ga1-3, ga1-3/rgl2-13 and ga1-3/rga-t2/gai-t6/rgl2-1 seeds under white or far-red light conditions (normal MS medium). Two micrograms of total RNA was used per lane. rRNA is shown as a loading control. At each time point, the percentage of germination (i.e. endosperm rupture events) in the seed population material used in the RNA blot is indicated. (C) RNA gel blot analysis of the time course of ABI3 mRNA accumulation in PAC-treated (15 μM) WT (Col), pro-RGL2∷GAI, pro-RGL2∷RGA transgenic lines and rgl2-13 seeds under white light conditions. Two micrograms of total RNA was used per lane. rRNA is shown as a loading control. At each time point, the percentage of germination (i.e. endosperm rupture events) in the seed population material used in the RNA blot is indicated.
Finally, we wished to identify key ABA-response factors repressing endosperm rupture under FR conditions. An obvious candidate is ABI5, which indeed is necessary to repress endosperm rupture under white light conditions (Piskurewicz et al, 2008). However, PAC-treated abi5 mutants germinated poorly under FR conditions, even after mechanical puncture (Supplementary Figures 6 and 7). This could be due to higher endogenous ABA levels under FR conditions, resulting from the concerted activity of GAI, RGA and RGL2. In turn, higher ABA levels may increase the activity of additional ABA-response factors. Given that ABI3 is known to act upstream of ABI5 during seed germination in response to ABA (Lopez-Molina et al, 2002), we reasoned that it may also control the expression of additional ABA-response repressors of seed germination. Indeed, we observed 20–80% seed germination in PAC-treated abi3-1 (Ler ecotype) seeds depending on the seed batch (Figure 8). We also examined a weak abi3 mutant allele (abi3-9, Col ecotype) seed population, in which 0% of seed germination took place under FR conditions (Supplementary Figure 7). We noticed that abi3-1 or abi3-9 seed germination took place without prior visible testa rupture, similar to what is observed in PAC-treated aba1 mutant seeds under FR conditions (Figure 8). Consistently, abi3-1 accumulated high GAI and RGA protein levels under FR conditions (Figure 9A). Thus, we reasoned that failure to rupture testa may also mask endosperm rupture events in abi3-1 and abi3-9 seeds. Consistent with this hypothesis, puncturing abi3-1 and abi3-9 seeds strongly promoted germination (i.e. radicle elongation) in each case (Figure 8; Supplementary Figure 7).
We also monitored ABI3 expression in ga1, ga1/rgl2 and ga1/rgl2/gai/rga seeds under white and FR light conditions. Figure 9B shows that under white light conditions ABI3 mRNA expression was maintained over time in ga1 seeds, but not in ga1/rgl2 and ga1/rgl2/gai/rga seeds. Under FR light conditions, ABI3 mRNA expression was maintained in ga1 and ga1/rgl2 seeds, but not in ga1/rgl2/gai/rga seeds (Figure 9B). Similarly, high ABI3 mRNA expression was maintained in PAC-treated WT, rgl2/pro-RGL2∷GAI and rgl2/pro-RGL2∷RGA transgenic lines, but not in PAC-treated rgl2 mutants under white light conditions (Figure 9C). Collectively, these data are consistent with the notion that DELLA-dependent accumulation of endogenous ABA levels stimulates ABI3 expression.
In conclusion, our observations strongly support the notion that under FR conditions, only ABA levels determine the potential of a seed to rupture its endosperm (i.e. defined here as visible radicle protusion out of the seed coat), irrespective of GA levels. In contrast, DELLA factors have an important function to repress testa rupture and to promote higher endogenous ABA levels. This conclusion strengthens and extends our earlier studies of seed germination under white light conditions.
Discussion
In the present work, we assessed the role of the GA- and ABA-response factors under FR light conditions ensuring phyB inactivity. The salient findings of this study are (1) in the absence of GA synthesis, RGA, GAI and RGL2 act to (a) repress testa rupture and (b) repress endosperm rupture by increasing endogenous ABA levels, which in turn inhibit endosperm rupture through ABI3 and (2) to put the role of PIL5 in a new perspective: PIL5 ensures sufficient GAI and RGA protein accumulation under FR conditions to block testa rupture and promote high endogenous ABA levels. However, the role of PIL5 is restricted to FR conditions and can be overshadowed under conditions in which GA synthesis is severely prevented (as in PAC-treated pil5 seeds or in pil5/ga1 seeds). In this case, sufficient GAI and RGA accumulation occurs, so that testa and endosperm rupture is prevented despite the absence of PIL5. Thus, this work extends and confirms our recent studies on the control of Arabidopsis seed germination under white light conditions. Below, we put these findings in the perspective of the earlier studies.
The antagonistic effects that ABA and GA have on Arabidopsis seed germination have long been established. After seed imbibition, ABA levels, high in the mature seed, drop rapidly, a process most likely involving CYP707 hydroxylases (Okamoto et al, 2006). In parallel, GA levels start to rise as soon as 12–16 h on imbibition (Ogawa et al, 2003). Preventing GA synthesis in seeds or exposing them to ABA will block their germination. Similarly, environmental conditions that stimulate seed germination will tend to be associated with high GA levels and low ABA levels, whereas conditions unfavourable with seed germination will be associated with low GA levels and high ABA levels (Olszewski et al, 2002; Nambara and Marion-Poll, 2005).
Taken together, these observations have led to the view that it is the balance of ABA and GA levels that determines the germination potential of the seed (Seo et al, 2009). In this view, GA and ABA control the expression and activity of their respective response factors that then independently act to control germination. However, closer inspection indicates that this representation can be misleading. Indeed, this is because it may suggest that (1) GA and ABA levels exert their influence on the same developmental steps during germination and (2) it is only the ratio of GA to ABA that determines the germination potential of a seed. Moreover, another consideration is often overlooked: conclusions about the role of GA and ABA in controlling seed germination may be flawed depending on the developmental state of the seed, that is commitment to germinate or not. We further discuss these issues below.
GA and ABA exert striking different influences on Arabidopsis early germination processes (Muller et al, 2006; Piskurewicz et al, 2008). Seed germination involves a succession of developmental processes, notably, at an early stage, testa rupture, which is followed after a few hours by the rupture of the endosperm (defined here as the earliest visible protrusion of the radicle out of the testa) (Muller et al, 2006; Piskurewicz et al, 2008). The latter step is usually chosen to assess whether seed germination has taken place, but this completely overlooks testa rupture. Indeed, depending on the germination conditions and the genetic background, visible testa rupture may not take place before endosperm rupture (e.g. as in PAC-treated abi5 mutants) (Piskurewicz et al, 2008). A seed arrested because of low GA levels does not have the same appearance as a seed arrested because of high ABA levels. Indeed, in ga1-3 seeds, both testa and endosperm rupture is completely blocked. In contrast, in ABA-treated WT seeds, testa rupture is only delayed, which contrasts with the robust blockade of endosperm rupture (Muller et al, 2006; Piskurewicz et al, 2008). As a result, all ABA-arrested seeds eventually have a ruptured testa, although the embryo remains surrounded by the endosperm. This indicates that ABA blocks endosperm weakening and radicle elongation. Moreover, even high GA concentrations (e.g. 50 μM) cannot overcome relative low ABA concentrations that are sufficient to prevent endosperm rupture (e.g. 3 μM) (Supplementary Figure 9).
These observations strongly suggest that GA synthesis favours germination mainly because it promotes testa rupture. In contrast, ABA mainly represses endosperm rupture irrespective of GA levels. These considerations are not easy to reconcile with the view that the ratio of GA to ABA levels defines the germination capacity. The observation that low GA levels in seeds can lead to germination when ABA levels are low (as in an PAC-treated aba1 seeds) (Leon-Kloosterziel et al, 1996; Piskurewicz et al, 2008) can be interpreted as follows: lack of testa rupture when GA levels are low is associated with high endogenous ABA, which prevents endosperm rupture (Piskurewicz et al, 2008). When ABA synthesis is prevented (as in aba1), endosperm rupture may take place, provided the mechanical resistance of the un-ruptured testa is overcome by the embryo (Piskurewicz et al, 2008).
The evidence that PIL5 and SOMNUS repress germination by altering, in an unknown manner, the expression of GA and ABA metabolic genes is rather circumstantial. Indeed, it is based on the observation that pil5 and som seed germination under FR conditions is associated with high GA and low ABA levels and concomitant changes in GA and ABA metabolic gene expression. In this context, it is significant to note that no direct regulation of metabolic gene expression by PIL5 or SOM has been evidenced so far (such as PIL5 or SOM binding to promoter elements of metabolic genes) (Oh et al, 2007; Kim et al, 2008). Without further investigation, a given state of metabolic gene expression or hormone levels may be interpreted as the consequence rather than the cause of seed germination. This underlies the difficulty to assess the relative role of GA and ABA in controlling seed germination. In particular, there is also little direct evidence available to support the proposed function of ABA and ABA-responses factors to repress seed germination under FR conditions (Seo et al, 2009). To better understand the role of ABA, we chose to keep GA levels always low, such as in a ga1 seeds or PAC-treated seeds. In this case, testa and endosperm rupture is prevented. We could then examine the role of DELLA factors, ABA and ABA-response factors in controlling testa and endosperm rupture, thus allowing to establish causal effects.
In Piskurewicz et al, we argued that under white light conditions GA controls the levels of ABA through RGL2 (Piskurewicz et al, 2008). Indeed, when GA levels are low, a stabilized RGL2 stimulates endogenous ABA levels, a process that may involve direct stimulation of XERICO transcription by RGL2 (see below) (Supplementary Figure 4) (Zentella et al, 2007; Piskurewicz et al, 2008). XERICO encodes a putative RING-H2 zinc-finger factor increasing endogenous ABA levels in an unknown manner (Ko et al, 2006; Zentella et al, 2007). It is highly relevant to mention here that XERICO was first proposed to be a target gene of GAI and RGA in seedlings, in which both were shown to bind XERICO promoter sequences (Zentella et al, 2007). In turn, in seeds, higher endogenous ABA, through its positive control of ABI5 protein levels and activity, is the main, if not only variable, determining the potential of the seed to rupture endosperm. Equally important is the fact that endogenous ABA is also essential to stimulate high RGL2 mRNA levels, thus ensuring a positive feedback loop to maintain high RGL2 and, therefore, high ABI5 protein levels to ensure repression of endosperm rupture (Piskurewicz et al, 2008). On the other hand, GA levels are essential, through RGL2, to determine the potential for testa rupture. Indeed, ABA has a moderate influence on testa rupture. These conclusions are mainly based on the observation that rgl2 mutants have low ABA and low ABI5 protein levels when GA levels are low. Moreover, the converse is not true: when GA levels are low, abi5 mutants have high RGL2 protein levels and cannot break the testa. This suggests that ABI5 acts downstream of RGL2 and that both factors do not repress germination independently and in parallel as proposed earlier (Oh et al, 2007; Kim et al, 2008; Seo et al, 2009).
Here, we show that this view can be generalized under FR conditions, the main difference being that RGA and GAI participate together with RGL2 to repress testa rupture and to elevate endogenous ABA levels. Thus, only ga1/rgl2/gai/rga can rupture testa and accumulate low levels of ABA and ABA-response factors under FR conditions. In contrast, aba1 and abi3 mutants maintain high DELLA factors and do not rupture testa (Figure 9A), consistent with the notion that they act downstream of DELLA factors. We showed that in this case, it is necessary to surgically puncture seeds to unveil the capacity of PAC-treated aba1 and abi3 seeds to germinate (i.e. in this case, to elongate their radicle as puncture experiments rupture both testa and endosperm).
Our work provides a new perspective for PIL5 and its role to regulate GAI and RGA expression. Under white light or red light, the role of PIL5 is irrelevant, as the Pfr form of phyB triggers its degradation. In contrast, PIL5 accumulates under FR conditions and was proposed to positively regulate GAI and RGA transcription (Oh et al, 2007). Consistently, we confirm that endogenous GAI and RGA protein levels are lower in pil5 under FR conditions. However, we show that in the absence of GA synthesis (as in PAC-treated pil5 seeds), the role of PIL5 becomes marginal as GAI and RGA accumulate at levels similar to that of WT seeds under FR conditions. This offers a simple explanation as to why ga1/pil5 seeds do not rupture testa and endosperm: they produce sufficient GAI, RGA and RGL2 protein accumulation to block testa rupture and to produce sufficient ABA accumulation. Consistent with this view, surgical rupture assays in PAC-treated pil5 seeds do not lead to germination (i.e. radical elongation).
This raises the possibility that pil5 seed germination under FR conditions is only because of low GAI and RGA levels. This is unlikely, as PIL5 also positively regulates the transcription of SOM, which is indirectly involved in promoting ABA synthesis (Kim et al, 2008). SOM regulates GA and ABA metabolic genes indirectly, but it is not expected to do so through GAI and RGA, as it does not regulate DELLA transcript accumulation (Kim et al, 2008). Thus, it is plausible that GAI, RGA, RGL2 and SOM collectively contribute to raise endogenous ABA levels under FR conditions. Moreover, GAI and RGA promotion of higher endogenous ABA levels does not seem to be restricted to seed germination under FR conditions. This is substantiated by the observation that GAI and RGA can substitute for RGL2's function under white light conditions by restoring high ABI5 protein levels and hence blocking germination (Figure 5). Thus, the positive regulation of GAI and RGA mRNA levels by FR light through PIL5 can be viewed similarly to ABA's positive regulation of RGL2 mRNA levels: it is essential to ensure sufficient GAI and RGA protein levels, so that they promote high endogenous ABA levels under FR conditions (see model Figure 10). This underlines the fact that the role of DELLA genes in plant growth and development cannot be solely described or understood from the perspective of DELLA protein stability, but necessitates an understanding of how mRNA levels are regulated.
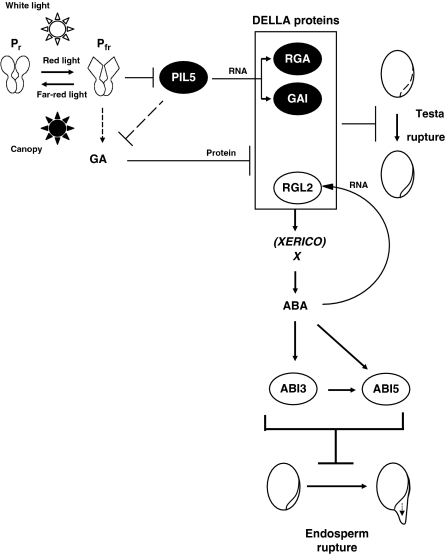
Figure 10.
A model for the control of seed germination under canopy conditions. Upon seed imbibition, canopy conditions (FR conditions) inactivate phytochromes, thus repressing GA synthesis in an unknown manner while stabilizing PIL5, which also represses GA synthesis indirectly. In turn, PIL5 stimulates GAI and RGA transcription, which ensures that sufficient GAI and RGA protein will accumulate to elevate, together with RGL2, endogenous ABA levels. PIL5, GAI and RGA are represented by black ovals to emphasize that they are relevant only under FR conditions. DELLA proteins repress seed germination by (1) blocking testa rupture and (2) stimulating ABA levels, which may involve stimulation of XERICO expression as well as other unidentified factors (X). In turn, ABA blocks endosperm rupture by stimulating ABI3 and ABI5 expression and product activity. RGL2 (white oval) has a central role to repress germination because RGL2 mRNA levels are positively regulated by ABA. This ensures high RGL2 protein levels irrespective of light conditions (i.e. FR or white light conditions).
Finally, it remains to be understood which are the DELLA-dependent mechanisms that lead to higher endogenous ABA levels when GA levels are low. This necessitates the understanding of the molecular function of DELLA factors, which is still uncertain. Recent reports have suggested that DELLA factors inhibit hypocotyl elongation by preventing PIL/PIF factors to bind target genes involved in cellular elongation, such as LTP3 and Expansin (Feng et al, 2008; de Lucas et al, 2008). In the case of XERICO, Zentella et al showed that XERICO promoter DNA sequences were enriched in ChIP experiments looking for RGA target genes in seedlings (Zentella et al, 2007). Our data are consistent with the proposition that DELLA factors may stimulate the transcription of XERICO to promote ABA synthesis to block germination (Zentella et al, 2007). Whether this involves the interaction with a bHLH transcription factor is speculative at the present time. Here, we have considered the case of the bHLH transcription factor PIF1/PIL5, whose proposed function is to activate the transcription of RGA and GAI. This is supported by genetic evidence and ChIP data (Oh et al, 2007). However, in the case of PIL5, there is no biochemical or genetic evidence that PIL5 activity is dependent on one or several DELLA factors (e.g. no interaction between PIL5 and a DELLA factor has been evidenced so far). Thus, although no direct DNA-binding activity of a DELLA factor has been shown, the evidence so far suggests that DELLA factors stimulate or repress gene transcription to exert their developmental role. However, it should be noted that seed germination is a rather peculiar process during which ABA has a central and temporally restricted regulatory function (Lopez-Molina et al, 2001; Piskurewicz et al, 2008). The regulation of hypocotyl elongation occurs well after the developmental window, in which the plant growth is highly sensitive to ABA. We have performed medium shift experiments in darkness that indicate that ABA poorly inhibits hypocotyl elongation in the dark (UP and LLM, unpublished observations). This could suggest that DELLA-dependent inhibition of hypocotyl elongation does not involve an increase in endogenous ABA levels. Although it seems highly likely that DELLA-dependent stimulation of XERICO expression is involved in promoting endogenous ABA synthesis, this does not exclude additional DELLA-dependent processes that do not involve changes in gene transcription. Thus, DELLA factors may regulate ABA metabolism by interacting with proteins other than transcription factors, by perhaps interfering with the activity of proteins regulating ABA metabolism.
Materials and methods
Plant material
Throughout this study, we use non-dormant seeds in the absence of seed stratification procedure. We refer to seeds sown under ‘normal conditions' when seeds are imbibed in a standard germination medium (MS).
The Arabidopsis mutants aba1-1 (‘aba1' in the text) and ga1-3 (Ler, ga1) were obtained by Marteen Koornneef. rgl2-13 is in the Columbia (Col-0) background (Tyler et al, 2004) and ga1-3/rgl2-13 (ga1/rgl2) seeds were obtained from TP Sun. ga1-3/rgl2-1/gai-t6/rga-t2 (Ler, ga1/rgl2/gai/rga) were obtained by NP Harberd. The abi5-3 (Col-0 ecotype) mutant was obtained from RR Finkelstein (Finkelstein and Lynch, 2000). abi3-1 (Ler) was obtained by F Parcy and abi3-9 (Col) by E Nambara (Nambara et al, 2002). pil5 (pif1-2) was obtained from C Fankhauser and first described in Huq et al (2004). Supplementary Figure 11 lists seed ecotypes and donors.
Germination assays
All seed batches compared in this study were harvested on the same day from plants grown side by side (i.e. identical environmental conditions). Dry siliques were obtained about 8 weeks after planting and left for a further 4 weeks at room temperature before seed harvesting. Seeds were then permanently stored at 4°C. Seeds obtained in this manner lacked dormancy. A minimum of three independently grown seed batches were used for measuring the per cent of testa and endosperm rupture events. For each seed batch, a minimum of two replication experiments was performed.
For germination assays, seeds were surface sterilized as described (Lopez-Molina and Chua, 2000) and sown in plates with MS medium containing 0.8% (wt/vol) Agar (Applichem). Medium was supplemented with GA3 (G7645, Sigma), ABA (A1049, Sigma) or PAC (46046, Riedel-de Haen) according to the germination condition examined. Plates were incubated in a climate-controlled room (20–25°C, 16 h light/day, light intensity 80μE /m−2 s−1, humidity 70%). Between 150 and 300 seeds were examined with a Stemi 2000 (Zeiss) stereomicroscope and photographed with a high-resolution digital camera (Canon Power G6, 7.1 Megapixels) at different times of seed imbibition. Photographs were enlarged electronically for measurement of testa and endosperm rupture events.
Light treatment and surgical procedure
After sterilization, seeds were incubated in the dark for 1 h before irradiation for 5 min with FR light (2 μmol m−2 s−1). Irradiation was performed in a growth chamber (CLF Plant Climatics, Percival CU-36L5) with 740 nm LEDs (L735-03AU, Epitex Inc, Japan). Seeds were kept in darkness by wrapping plates in several layers of aluminium foil.
The puncture procedure (using a needle) was performed 20 min after sterilization before 1 h incubation in the dark followed by FR light treatment. In a typical experiment, 15–19 seeds are punctured under white light illumination under the stereomicroscope. The procedure takes between 5 and 10 min. Thereafter, 10–14 seeds are subject to FR light irradiation, whereas five seeds are grown under normal white light illumination (positive control for seed viability). Figure 8 shows a typical example of 10 punctured seeds. These experiments were performed twice for each genotype. The seed viability test is always positive (5/5) (Supplementary Figures 6 and 7). PAC-treated WT and pil5 seeds never elongate the radicle after the puncture procedure, whereas an average of more than 90% of aba1 and abi3 seeds do elongate (>90%). The results are compiled in a table in Supplementary Figure 7.
Plasmid constructs and plant transformation
DNA manipulations were performed according to standard methods (Sambrook et al, 1989). prom-RGL2∷GAI and prom-RGL2∷RGA transgene construction: GAI coding sequences were amplified with 5′-ATAGGCGCGCCATGTATCCATATGACGTGCCGGACTACGCCTCC CTCATGAAGAGAGATCATCATCATCATC and 5′-GCTTAATTAACTAATTGGTGGAGAGTTTCCAAGCCGAGG and RGA coding sequences with 5′-ATAGGCGCGCCATGTATCCATATGACGTGCCGGACTA CGCCTCCCTCATGAAGAGAGATCATCACCAATTCC and 5′-GCTTAATTAATCAGTACGCCGCCGTCGAGAGTTTCC. In each case, the first primer contains an Asc I site and the HA sequence (MYPYDVPDYASL), whereas the second primer contains a Pac site. Both restriction sites were used for cloning into pBA002a, a promoterless version of pBA002 (Kost et al, 1998) lacking the 35S promoter of Cauliflower mosaic virus, containing approximately 2 kbp of upstream genomic promoter sequences of RGL2 amplified with 5′-ATAGGCGCGCCCTTTCTTGTTCTTGTGATGGTGAGTTAAG and 5′-ATAGGCGCGCCGTCTCAACAGTCTCATGCCGAGATGATGG. Transgenic Arabidopsis lines were generated using the Agrobacterium tumefaciens vacuum-infiltration method (Bechtold and Pelletier, 1998). Seeds (T1) from infiltrated plants were plated in selection medium as described (Lopez-Molina et al, 2001).
Antibody production, protein and RNA blot analysis
The antibodies and their use for protein gel blot analysis were as described in Piskurewicz et al (2008). RNA blot analysis and probes are as described earlier (Piskurewicz et al, 2008). A NCED6 DNA probe was generated using the following primers: 5′-CTTCTTCCGACGAAGACTTCTCC and 5′-CCGTCCGATCGTCTCAAGATCTCC.
ABA analysis
The seeds (10–100 mg of each sample f.w.) were ground using 3-mm tungsten carbide beads (Retsch GmbH & Co. KG, Haan, Germany) with an MM 301 vibration mill at a frequency of 27.5 Hz for 3 min (Retsch GmbH & Co.). Internal standard (50 pmol of (+)-3′,5′,5′,7′,7′,7′-2H6-ABA) and 1 ml cold methanol/water/acetic acid (80/19/1, v/v) were added to the samples. After 24 h of shaking in the dark at 4°C, the homogenates were centrifuged (20 000 r.p.m., 5 min, 4°C) and the pellets were then re-extracted in 0.5 ml extraction solvent for 60 min. The supernatants were transferred to a fresh glass tube and dried under vacuum. Extracts were dissolved in 100 μl 99% methanol:1% acetic acid (v/v) topped up to 1 ml with 99% water:1% acetic acid (v/v) and purified by solid-phase extraction on an Oasis HLB cartridges (60 mg, 3 ml, Waters, Milford, MA, USA). The fraction containing ABA was eluted with 3 ml methanol/water/acetic acid (80/19/1, v/v) and evaporated to dryness in a Speed-Vac (UniEquip). Subsequently, the evaporated samples were methylated, purified by ABA-specific immunoaffinity extraction (Hradecka et al, 2007) and analysed by UPLC-ESI(+)-MS/MS (Turečková et al, unpublished).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Supplementary Figure 9
Supplementary Figure 10
Supplementary Figure 11
Supplementary Figure Legends
Review Process File
Acknowledgments
We are especially grateful to Pierre Vassalli for help and numerous suggestions to write the manuscript. We are greatly indebted to the following colleagues for their generous gifts: Tai-Ping Sun for ga1-3/rgl2-13 mutants, Ruth Finkelstein for abi5-3 mutant, Nicholas Harberd for ga1-3/rgl2-1/gai-t6/rga-t2 mutants, Marteen Koornneef for aba1-1 and ga1-3 mutants, Eiji Nambara for abi3-9 mutant, François Parçy for abi3-1 and Christian Fankhauser for pil5 (pif1-2). We thank our colleague Jean-David Rochaix for letting us use his growth chamber and LED equipment (expertly assembled by the members of the workshop of the Molecular Biology Department, University of Geneva). We thank Christophe Belin, Jean-David Rochaix and Michel Goldschmidt-Clermont for critical comments on the manuscript. This work has been supported by Swiss National Science Foundation grants to LLM, by the State of Geneva, by the Société Académique de Genève. VT is supported by Czech Ministry of Education (MSM 6198959216).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bae G, Choi G (2008) Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol 59: 281–311 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Cao D, Hussain A, Cheng H, Peng J (2005) Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta 223: 105–113 [DOI] [PubMed] [Google Scholar]
- Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Genet 38: 87–117 [DOI] [PubMed] [Google Scholar]
- de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Leon-Kloosterziel KM, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122: 403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, Schafer E, Fu X, Fan LM, Deng XW (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradecka V, Novak O, Havlicek L, Strnad M (2007) Immunoaffinity chromatography of abscisic acid combined with electrospray liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 847: 162–173 [DOI] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH (2004) Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Kim DH, Yamaguchi S, Lim S, Oh E, Park J, Hanada A, Kamiya Y, Choi G (2008) SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 20: 1260–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Yang SH, Han KH (2006) Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J 47: 343–355 [DOI] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J 16: 393–401 [DOI] [PubMed] [Google Scholar]
- Kucera B, Alan Cohn M, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15: 281–307 [Google Scholar]
- Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion-Poll A (2006) Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45: 309–319 [DOI] [PubMed] [Google Scholar]
- Leon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Chua NH (2000) A null mutation in a bZIP factor confers ABA-insensitivity in Arabidopsis thaliana. Plant Cell Physiol 41: 541–547 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32: 317–328 [DOI] [PubMed] [Google Scholar]
- Muller K, Tintelnot S, Leubner-Metzger G (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47: 864–877 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Nambara E, Suzuki M, Abrams S, McCarty DR, Kamiya Y, McCourt P (2002) A screen for genes that function in abscisic acid signaling in Arabidopsis thaliana. Genetics 161: 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15: 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Kim J, Park E, Kim J-I, Kang C, Choi G (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in arabidopsis thaliana. Plant Cell 16: 3045–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19: 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G (2006) Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J 47: 124–139 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E (2006) CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol 141: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14 (Suppl): S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J (1994) Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6: 1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Gilday AD, Halliday KJ, Graham IA (2006) DELLA-mediated cotyledon expansion breaks coat-imposed seed dormancy. Curr Biol 16: 2366–2370 [DOI] [PubMed] [Google Scholar]
- Piskurewicz U, Jikumaru Y, Kinoshita N, Nambara E, Kamiya Y, Lopez-Molina L (2008) The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20: 2729–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun TP, Koshiba T, Kamiya Y, Yamaguchi S, Nambara E (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48: 354–366 [DOI] [PubMed] [Google Scholar]
- Seo M, Nambara E, Choi G, Yamaguchi S (2009) Interaction of light and hormone signals in germinating seeds. Plant Mol Biol 69: 463–472 [DOI] [PubMed] [Google Scholar]
- Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55: 197–223 [DOI] [PubMed] [Google Scholar]
- Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP (2004) Della proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol 135: 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentella R, Zhang ZL, Park M, Thomas SG, Endo A, Murase K, Fleet CM, Jikumaru Y, Nambara E, Kamiya Y, Sun TP (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19: 3037–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Supplementary Figure 9
Supplementary Figure 10
Supplementary Figure 11
Supplementary Figure Legends
Review Process File