Abstract
G protein-coupled receptors (GPCRs) have critical functions in intercellular communication. Although a wide range of different receptors have been identified in the same cells, the mechanism by which signals are integrated remains elusive. The ability of GPCRs to form dimers or larger hetero-oligomers is thought to generate such signal integration. We examined the molecular mechanisms responsible for the GABAB receptor-mediated potentiation of the mGlu receptor signalling reported in Purkinje neurons. We showed that this effect does not require a physical interaction between both receptors. Instead, it is the result of a more general mechanism in which the βγ subunits produced by the Gi-coupled GABAB receptor enhance the mGlu-mediated Gq response. Most importantly, this mechanism could be generally applied to other pairs of Gi- and Gq-coupled receptors and the signal integration varied depending on the time delay between activation of each receptor. Such a mechanism helps explain specific properties of cells expressing two different Gi- and Gq-coupled receptors activated by a single transmitter, or properties of GPCRs naturally coupled to both types of the G protein.
Keywords: calcium signalling, G protein-coupled receptor, homogenous time-resolved FRET, oligomerization, resonance energy transfer
Introduction
G protein-coupled receptors (GPCRs) are encoded by the largest mammalian gene family; there are about 900 receptors in humans. Owing to the critical functions they have in intercellular communication and their involvement in all major physiological functions, these receptors and their associated signalling complexes represent major targets for drug development (Overington et al, 2006). Each cell usually expresses several subtypes of these GPCRs, integrating their numerous signals both spatially and temporally to produce the cellular response (Selbie and Hill, 1998). However, the molecular mechanisms allowing such signal integrations are still not clearly understood and may occur at the level of receptor signaling pathways or even at the level of receptors themselves.
A recent proposal suggests that such signal integration is facilitated through the ability of GPCRs to form dimers or larger oligomers. Indeed, two different GPCRs can physically associate together in complexes, either as heterodimers or hetero-oligomers, which could in turn show specific pharmacological and signaling behaviour, have an impact on cellular physiology, or be involved in pathologies (Bouvier, 2001; Angers et al, 2002; Milligan, 2004; Franco et al, 2007), making them interesting new targets for more selective drugs (Ferre et al, 2007; Pin et al, 2007; Gurevich and Gurevich, 2008). For example, the pharmacologically well-defined κ, μ and δ opioid receptor subtypes have been proposed to assemble into hetero-oligomers showing different ligand binding profiles and G protein-coupling properties (Jordan and Devi, 1999; George et al, 2000), as well as be involved in specific functional responses in animals (Waldhoer et al, 2005). Furthermore, in rat, the oligomerization of the metabotropic glutamate receptor type 2 (mGlu2) and the serotonin receptor 5-HT2A has recently been shown to control the action of hallucinogens in the prefrontal cortex (Gonzalez-Maeso et al, 2008). Another interesting example is the orphan receptor, GPR50, which interacts with the melatonin MT1 receptor to abolish both high-affinity agonist binding and G protein coupling, thus modifying the melatonin-induced cellular response (Levoye et al, 2006).
In cerebellar Purkinje cells, activation of the GABAB receptor increases calcium responses generated by the mGlu1a receptor (Hirono et al, 2001; Tabata et al, 2004). Such a phenomenon would have an important function in generation of long-term depression at the parallel fibre—Purkinje cell synapses (Kamikubo et al, 2007). Both the receptors are indeed co-expressed in these neurons, and both were described in dendritic spines (Ige et al, 2000). Moreover, it was reported that both receptors could be co-immunoprecipitated (Tabata et al, 2004), suggesting that the observed crosstalk could result from the existence of possible mGlu1a–GABAB heteromers. Such a proposal is supported by the absence of potentiation of the mGlu1a response by other Gi-coupled receptors like the adenosine A1 receptors also expressed in Purkinje cells (Hirono et al, 2001). Consequently, it would be of interest to determine whether such a GABAB–mGlu1a oligomer could be selectively targeted pharmacologically, thus enabling a specific effect on this cross-regulation. However, it may well also result from a functional crosstalk, as previously reported for other Gq- and Gi-coupled receptor pairs (Carroll et al, 1995).
The aim of this study was to analyse the molecular basis for such physiological crosstalk between GABAB and mGlu1a receptors. Our data show that the functional crosstalk can be observed with other pairs of Gi- and Gq-coupled receptors and reproduced in primary cells, again without a direct physical interaction occuring between these receptors in transfected cells. This general phenomenon may improve our understanding of the mechanisms involved in the spatial and temporal integration of GPCR signals in any given cell.
Results
mGlu1a and GABAB are co-localized in dendritic spines of Purkinje cells
Previous data have suggested that the functional crosstalk between GABAB and mGlu1a receptors is the result of their physical association in the dendritic spines of Purkinje cells facing the parallel fibre terminals (Hirono et al, 2001; Tabata et al, 2004; Kamikubo et al, 2007). Such a proposal was primarily based on the co-immunoprecipitation of both receptors from cerebellar extracts. Using an SDS freeze-fracture replica labelling technique coupled to electronic microscopy, we visualized the precise co-localization of both receptors in Purkinje-cell dendritic spines on a nanometric scale (Figure 1). We observed mGlu1a labelling distributed in a circular pattern within the spine plasma membrane and surrounding the postsynaptic membrane specialization in individual spines. The distribution of GB1 immunoreactivity was similar but was also found in other areas, such as within the specialization (Figure 1). Specificity of the replica labelling with the primary antibodies against GB1 and mGlu1a was previously confirmed using respective knockout mice (Kulik et al, 2006; Kaufmann et al, 2009). Although not definitive, these data are consistent with a possible physical association between both receptors, leading us to examine whether the assembly of mGlu1a and GABAB receptors is necessary for their functional crosstalk.
Figure 1.
Co-localization of GB1 and mGlu1a receptors in Purkinje-cell spines as shown by SDS freeze-fracture replica labelling. Freeze-fracture replicas prepared from mouse cerebellum were labelled with 5-nm (small black dots pointed by arrows in panel B) and 10-nm (bold black dots in panels A and B) immunoparticles to detect GB1 and mGlu1a receptors, respectively. (A) Low magnification view of a Purkinje-cell dendrite from which two dendritic spines emerge. Post-synaptic membrane specialization in individual spines was indicated by a dotted line. (B) High magnification view of a detailed Purkinje-cell dendrite spine from A. Scale bars=500 nm in A; 100 nm in B.
GABAB enhanced mGlu1a-mediated responses in cortical neurons and transfected cells
We first examined whether it was possible to observe the GABAB–mGlu1a crosstalk in other neuronal cell types. Indeed, as reported in Purkinje cells, GABAB activation enhanced the mGlu1a-mediated increase in intracellular calcium level (Ca2+i), both in cultured cortical neurons (Figure 2A) and in HEK293 cells co-expressing both GABAB and mGlu1a receptors (Figure 2B–E). Indeed, in HEK293 cells, the GABAB-induced potentiation of the mGlu1a-induced calcium response also led to an increased efficacy and potency of glutamate (2.41-±0.96-fold increase in the EC50 by 50 μM GABA). Notably, activation of the Gi/o-coupled GABAB receptor did not lead to a significant change in intracellular Ca2+ concentration. Conversely, the maximal calcium response induced by 100 μM glutamate was increased in a dose-dependent manner by GABA with an EC50 of 0.37±0.10 μM, in agreement with the known potency of GABA at this receptor (0.31±0.07 μM; (Binet et al, 2007)). Surprisingly, the strength of the potentiation seemed to depend on the initial amplitude of the mGlu1a-induced calcium response; a low mGlu1a-induced calcium response was generally more potentiated by GABAB activation than a stronger response, suggesting the involvement of complex signal integration pathways (Figure 2C). These results were not because of a ceiling effect, as the experiments were carried out in conditions in which the mGlu1a calcium responses were not saturating (Supplementary data 1C).
Figure 2.
Functional crosstalk between GABAB and mGlu1a receptors in cortical neurons and in transfected HEK293 cells. (A) Activation of GABAB receptor alone by Baclofen 100 μM (filled squares) and mGlu1a by DHPG in the presence (open circles) or in the absence (filled circles) of baclofen (100 μM), in cortical neurons. (B, C) Potentiation of the glutamate response by co-activation of the GABAB receptors in HEK293 cells. In (B), Ca2+ responses mediated by various concentrations of glutamate (circles) and GABA (triangles) in cells expressing both mGlu1a and GABAB receptors. Glutamate responses were measured in the absence (filled circles) and in the presence (open circles) of 50 μM GABA. The GABA-mediated responses were measured in the absence (filled triangles) and in the presence (open triangles) of 100 μM glutamate. (C) Glutamate responses, as in (B), in cells expressing either a low (triangles) or a high (circles) density of mGlu1a. (D, E) The GABAB effect needs Gi/o protein activation. (D) Same as in (C) in cells co-expressing the WT (circles) or a G protein activation-deficient (L686P represented by triangles) GABAB receptor and mGlu1a receptor. (E) Same as in (C), in cells expressing GABAB and mGlu1a receptors, treated OVN with PTX (squares) or not (circles). The Gαo protein was co-expressed in all of these experiments. Data are means±s.e.m. of triplicate determinations from a representative experiment reproduced at least three times.
When the G protein-activating GABAB receptor GB2 subunit bore the L686P mutation that suppresses coupling to G proteins (Duthey et al, 2002), potentiation was not observed, showing the necessity for functional coupling of the GABAB receptor to G proteins (Figure 2D). Similarly, inhibiting the Gi/o proteins with pertussis toxin (PTX) also prevented GABAB-mediated potentiation of the mGlu1a response (Figure 2E). Moreover, although this potentiation occurred without co-transfecting the G protein αo or i1 subunits (1.25-±0.05-fold increase of the maximal response, Supplementary data 1A), co-expression of the Gαo or i1 subunits further increased the GABAB potentiating effect, consistent with the necessity for Gi/o activation (Figure 2 and Supplementary data 1). Taken together, these observations led us to question the requirement for mGlu1a–GABAB receptor oligomerization for such crosstalk to occur, rather than a functional signal integration mechanism.
GABAB and mGlu1a receptors did not form hetero-oligomers in transfected cells
Using various approaches, we observed no oligomerization of GABAB and mGlu1a receptors in HEK293 cells (Figures 3, 4 and 5). First, we used antibody-based time-resolved (TR)-FRET technology, allowing the detection of energy transfer between anti-HA antibodies bearing the donor fluorophore europium-cryptate (HA–K) and anti-Flag antibodies bearing the acceptor fluorophore d2 (Flag–d2) (Maurel et al, 2004). Under these conditions, we observed a highly significant FRET signal in cells expressing HA-tagged GB1 (HA–GB1) and Flag-tagged GB2 (Flag–GB2) subunits of the heterodimeric GABAB receptor (Figure 3A and B). We obtained a similar FRET signal within the mGlu1a homodimer (between the HA-tagged mGlu1a and the Flag-tagged mGlu1a), the β2-adrenergic receptor dimer (between the HA-tagged β2AR and the Flag-tagged β2AR), as well as a significant FRET signal between two different receptors known to form hetero-oligomeric complexes, 5-HT2a and mGlu2 (Gonzalez-Maeso et al (2008); Figure 3A). In contrast, in a similar range of receptor expression levels (Supplementary data 2A), we detected no significant FRET signal between HA–mGlu1a and GABAB receptors, in which either GB1 or GB2 was tagged with the Flag epitope (Figure 3A and B). Similarly, we observed no significant TR-FRET signal in cells expressing either mGlu3 and GABAB (Figure 3A and B) or mGlu4 and GABAB (Figure 3A, data not shown).
Figure 3.
Absence of FRET between GABAB and mGlu1a receptors co-expressed in HEK293 cells. The FRET experiments were carried out using antibody- and SNAP-tag-based HTRF technologies. (A) Antibody-based HTRF experiments. The receptors were labelled with anti-HA and anti-Flag antibodies coupled to cryptate (HA–Ab–K) or D2 (Flag–Ab–D2) dyes. (B) FRET signal in function of the expression of the Flag-tagged receptors, in antibody-based HTRF experiments. Open squares: HA–GB1+Flag–GB2. Filled squares: HA–mGlu1a, GB1 and Flag–GB2. Open circles: HA–mGlu3+GB1+Flag–GB2. Cells were transfected with a constant amount (30 ng) of GB1, mGlu1a, mGlu3 or mGlu4 plasmids, and increasing amounts of the Flag–GB2 plasmid (0–30 ng per well) Expression of the receptors was determined with an ELISA assay against the Flag epitopes. (C) Combined antibody- and SNAP-tag-based TR-FRET experiment. The FRET experiment was carried out after labelling of the SNAP-tag with BG–K2 and then incubation of the anti-Flag antibodies coupled to D2. FRET signal is shown as a function of the cell-surface expression of the receptors, determined by an ELISA assay against the Flag epitope. Filled circles: ST–GB1 and Flag–GB2. Open circles: ST–5HT2a and Flag–mGlu2. Open squares: ST–GB1+GB2 and Flag–mGlu1a. Data are means±s.e.m. of triplicate determinations from a representative experiment reproduced at least three times.
Figure 4.
No significant BRET signal detected between mGlu1a and GABAB in HEK293 cells. (A, B) BRET signal was monitored in cells expressing combinations of receptors fused to YFP or RLuc proteins. (A) Cells were transfected with a constant amount (30 ng) of GB2-RLuc plasmid with increasing amounts of GB1–YFP plasmid (0–50 ng per well) (open circles), or with constant amount of GB2–RLuc and GB1 plasmids (30 ng each per well) and increasing amounts (0–50 ng per well) of PAR1–YFP plasmid (filled squares), or mGlu1a–YFP (open squares). (B) Cells were transfected with a constant amount (30 ng) of mGlu1a-RLuc plasmid and increasing amounts (0–50 ng per well) of mGlu1a–YFP (open squares), YFP(venus)–Homer3 (filled squares), GB1–YFP and GB2 (open circles), or GB2–YFP and GB1 (filled circles) plasmids. Data are means±s.e.m. of triplicate determinations from a representative experiment reproduced at least three times.
Figure 5.
No cell-surface co-immunoprecipitation between mGlu1a and GABAB in HEK293 cells. Upper panel: Cell-surface expression of the tagged receptors, determined by an ELISA assay. Lower panel: Cell-surface co-immunoprecipitation of the Flag-tagged receptors and western Blot carried out using anti-HA and anti-Flag antibodies from cells transfected with indicated plasmids. Data are representative of several experiments.
To exclude the possibility that the absence of FRET was because of steric hindrance imposed by the large size of the antibodies, we replaced one epitope tag by a SNAP-tag, allowing specific labelling with either the europium cryptate or d2 fluorophores as previously reported (Maurel et al, 2008). The SNAP-tag is a 20 KDa modified alkyl guanine transferase that can be covalently labelled with fluorophore-coupled benzyl guanines (BG): europium cryptate-coupled BG (BG-K) or d2-coupled BG (BG-d2). A high FRET signal was detected in cells expressing SNAP-tagged GB1 (ST–GB1) and Flag–GB2, whereas a lower but significant FRET signal was also detected within the 5HT2a–mGlu2 receptor complex (Figure 3C). However, no (or a very weak) FRET signal was measured between Flag–mGlu1a receptor and the ST–GB1-containing GABAB receptor over a range of receptor expressions (Figure 3C). Moreover, a combination of ST–GB2-containing GABAB receptor and ST–mGlu1a (in which only one protomer was labelled) did not show any significant FRET signal (Supplementary data 2D). The overexpression of the Gαo protein, found to increase the functional crosstalk, did not modify the TR-FRET signal between GABAB and mGlu1a receptors (Supplementary data 2C).
Consistent with our FRET results, a low linear BRET signal was observed in cells expressing mGlu1a–YFP and GB2–Rluc-containing GABAB receptors. This signal was in the same range as the one in negative control cells expressing PAR1–YFP and GB2–Rluc-containing GABAB receptors (Figure 4A). In contrast, BRET signals in positive control cells expressing GB1–YFP and GB2–Luc, fit a parabolic curve with a high maximum value (max=340.9 milliBRET). Similar results were obtained on inverting the fluorescent tags on the receptors (Figure 4B). A low linear BRET signal was observed in cells expressing mGlu1a–RLuc and either GB1–YFP- or GB2–YFP-containing GABAB receptors (Figure 4B). We validated the ability of mGlu1a constructs to generate BRET signals by measuring a saturating BRET signal in cells expressing either mGlu1a–RLuc and mGlu1a–YFP (Figure 4B); mGlu1a–RLuc and Homer3–YFP(Venus) or mGlu1a–YFP and Homer3–RLuc (Figure 4B and data not shown).
GABAB and mGlu1a receptors did not co-immunoprecipitate from transfected HEK293 cell-surface extract
Although GABAB and mGlu1a receptors have been found to co-immunoprecipitate from brain extract (Tabata et al, 2004), no co-immunoprecipitation of HA–GB1-containing GABAB and Flag–mGlu1a receptors was obtained from HEK293 cell-surface extracts (Figure 5). In contrast, HA–GB1 co-immunoprecipitated with Flag–GB2 in cells expressing both subunits, as well as with Flag–GB1 in cells expressing HA–GB1, Flag–GB1 and GB2, as GABAB receptors form tetramers through interaction between the GB1 subunits (Maurel et al, 2008). As expected, we also succeeded in detecting a high level of cell-surface interaction between the Flag-tagged mGlu2 and the HA-tagged 5HT2a receptors, but no co-immunoprecipitation between GABAB and mGlu4 receptors used as a negative control.
Although the C-terminal tail of the mGlu1a receptor known to interact with intracellular partners (Fagni et al, 2004) has been reported to be involved in mGlu1a assembly with other receptors (Ciruela et al, 2001), functional crosstalk could still be observed between the GABAB receptor and the mGlu1 short splice variant mGlu1b receptor possessing a short C-terminal tail (Pin and Duvoisin (1995); data not shown). Taken together, the data above provide evidence against oligomerization between GABAB and mGlu1a receptors in HEK293 cells, however, we did observe a functional crosstalk between them, such as that reported in neurons.
Mechanism of the functional interaction: involvement of the βγ subunits of the Gi/o protein
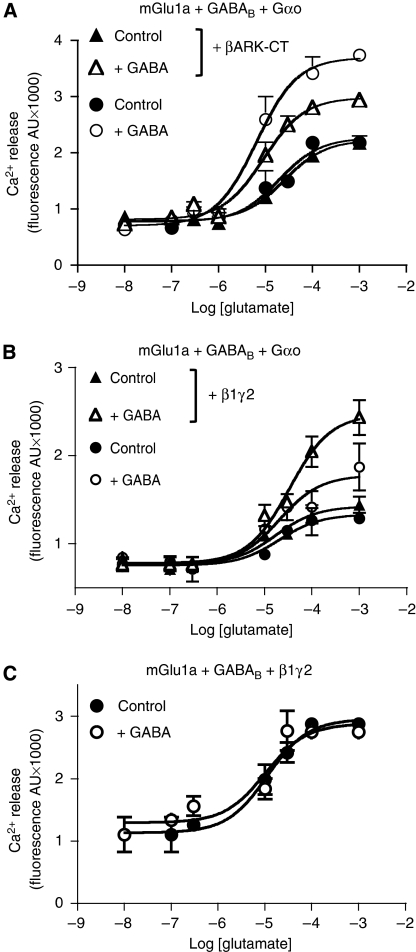
Evidence for the involvement of the βγ subunits of Gi/o G proteins was obtained from different sets of results from cells expressing GABAB, mGlu1a and αo G protein subunit. First, the GABAB-induced potentiation of the mGlu1a-induced calcium response (Figure 6A) was decreased by trapping the endogenous βγ subunits, using the co-expressed C-terminus of the β-adrenergic receptor kinase (βARK-CT), which is known to interact with βγ subunits. Second, the co-expression of β1γ2 in the presence of αo increased the potentiation (Figure 6B). We then overexpressed β1γ2 alone (not αo), with the hope that, due to a limitation in the amount of α subunits, free β1γ2 will be constantly available (Supplementary data 3). As such, activating the GABAB receptor was not expected to have an effect, as it would have already been generated by the free β1γ2 overexpressed in the cells. Consistent with this idea, GABAB receptor activation did not enhance mGlu1a-mediated response in cells overexpressing β1γ2 (Figure 6C). Such an effect of β1γ2 probably results from their action on PLC activity (Quitterer and Lohse, 1999) that has been clearly shown for PLCβ type 3 (Park et al, 1993), which is expressed in the HEK293 cells (Supplementary data 6).
Figure 6.
The crosstalk was dependent on the βγ subunits. (A) Ca2+ responses induced by various concentrations of glutamate was monitored in HEK293 cells expressing both mGlu1a and GABAB (GB1+GB2) receptors and the αo G protein subunit, in the absence (circles) or in the presence (triangles) of βARK C-terminal domain (βARK-CT), a chelator of the βγ subunits. Glutamate responses were measured in the absence (filled shapes) and in the presence (open shapes) of 50 μM GABA. (B) Ca2+ responses mediated by various concentrations of glutamate in cells expressing both mGlu1a and GABAB (GB1+GB2) receptors and the αo G protein subunit, in the absence (circles) or in the presence (triangles) of co-expressed β1 and γ2 subunits. Glutamate responses were measured in the absence (filled shapes) and in the presence (open shapes) of 50 μM GABA. (C) Ca2+ responses mediated by various concentrations of glutamate in cells expressing both mGlu1a and GABAB (GB1+GB2) receptors and βγ subunits, but not the Gαo subunit. Glutamate responses were measured in the absence (filled circles) and in the presence (open circles) of 50 μM GABA. Data are means±s.e.m. of triplicate determinations from a representative experiment reproduced at least three times.
Kinetics of the GABAB receptor-mediated potentiation
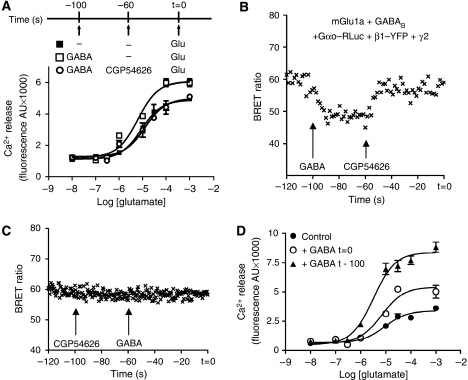
If βγ production is responsible for the GABAB receptor-mediated potentiation of the mGlu1a-induced calcium response, one might expect that the activation of the GABAB receptor initiated before the activation of mGlu1a could still induce a potentiation of the mGlu1a Ca2+ signal. As shown in Figure 7D, this was clearly the case, as a stronger potentiation of the mGlu1a-induced calcium response was obtained when activating the GABAB receptor 100 s before the mGlu1a receptor. Such a kinetic profile probably reflects the kinetics of GABAB-induced activation of the G protein. Indeed, when Go-protein activation was recorded in living cells using a BRET approach (based on the use of αo—RLuc and β1 (Figure 7B and C) or γ2 (Supplementary data 7) fused to YFP (YFP–β1 and YFP–γ2, respectively)), G-protein activation was observed on GABAB receptor activation by GABA, as seen by the decrease in BRET signal (Figure 7B; Gales et al (2006)). Moreover, when the GABAB receptor was first activated and then antagonized with CGP54626, a full inhibition of G-protein activation was observed and correlated with an inhibition of the potentiation of the mGlu1a-mediated response (Figure 7A and B). These data highlight the importance of the temporal integration of receptor-induced signals.
Figure 7.
Correlation between Ca2+-response potentiation and kinetics of association of the αo–βγ G protein subunits. (A) Ca2+ responses mediated by various concentrations of glutamate in HEK293 cells expressing both mGlu1a and GABAB receptors and Gαo subunit. Glutamate responses were measured in the absence of GABA (filled squares), after incubation with 10 μM of GABA for 100 s (open squares), or after incubation with 10 μM of GABA for 40 s followed by a second incubation with 10 μM of GABA+50 μM of the GABAB antagonist CGP54626 for 60 s (open circles). (B, C) Kinetics of the BRET signal generated by Gαo–RLuc and β1–YFP on GABAB receptor activation, in cells transfected with mGlu1a and GABAB receptors. (B) BRET signal detected after activation of the GABAB receptor with GABA (10 μM), followed 40 s later by the application of the antagonist CGP54626 (50 μM). Data are collected every 0.5 s, and each point corresponds to the average of three consecutive measurements. (C) BRET signal detected after application of CGP54626 (50 μM) followed 40 s later by application of GABA (10 μM). (D) In cells transfected with mGlu1a and GABAB and Gαo subunit, the mGlu1a calcium responses (filled circles) were measured on simultaneous co-activation of the GABAB receptor (open circles) or incubation with GABA for 100 s before glutamate application (filled triangles). Data are means±s.e.m. of triplicate determinations from a representative experiment reproduced at least three times.
Temporal signal integration
The mGlu1a and GABAB receptor signal is therefore temporally integrated, generating different calcium responses depending on simultaneous or delayed GABA and glutamate stimulations. We next explored this integration further by analysing the effect of GABA applied after glutamate receptor activation. By delaying the stimulation of GABAB for 180 s after mGlu1a stimulation, we observed that the GABAB receptor generated its own calcium signal (Figure 8A). This signal occurred after the completion of the mGlu1a-induced calcium response. Importantly, the GABAB receptor activation alone did not induce any calcium signal (Figure 8A). The acquired GABAB calcium response involved a Gi/o, rather than Gq, protein-dependent pathway, as it was blocked by PTX (Figure 8C). A dose-response curve generated by plotting the GABA-induced calcium signal values obtained by analysis of the fluorescence traces gave an EC50 of 0.3±0.1 μM for GABA, similar to that obtained for other GABAB signalling (see Figures 2 and 8C; Duthey et al (2002)). These data suggest that mGlu1a activation, in addition to a transient calcium response, produces a long-lasting signal that allows the GABAB receptor to couple to a calcium signal in a priming process. The primed response of the GABAB receptor was blocked by BAY367620, an allosteric antagonist of the mGlu1a receptor (Figure 8B and C), showing the necessity of the constant mGlu1a activity. We hypothesized that the long-lasting event necessary for this priming is the production of IP second messengers by mGlu1a activation. Indeed, as expected, the IP production by mGlu1a was increased by GABAB stimulation (Figure 8D).
Figure 8.
Temporal integration of the calcium response. (A, B) Fluorescence signal generated by the calcium sensor Fluo4 in cells transfected with GABAB, mGlu1a and Gαo. (A) Fluorescence signal was measured on GABA application or on glutamate (1 mM) application followed by GABA (10 μM) application, 180 s later. (B) Fluorescence signal was measured on application of glutamate, followed by application of the mGlu1a inverse agonist BAY367620 before application of GABA, 180 s later. (C) The mGlu1a pre-stimulation-induced GABAB-mediated calcium response (filled circles) in the presence of PTX (filled triangles) or BAY367220 (open circles). (D) IP second messenger production mediated by various concentrations of glutamate in HEK293 cells expressing both mGlu1a and GABAB receptors and Gαo in the absence (filled circles) and in the presence (open circles) of 50 μM GABA. (E) IP production mediated by various concentrations of GABA in cells expressing either mGlu1a and GABAB receptors (filled shapes) or the GABAB receptor alone (open circles). GABA-induced IP production was measured in the absence (circles) and in the presence (filled triangles) of the mGlu1a inverse agonist BAY367220. Data are means±s.e.m. of triplicate determinations from a representative experiment reproduced at least three times.
Constitutive activity of mGlu1a makes GABAB coupled to the PLC pathway
Although expression of the GABAB receptor alone did not lead to PLC activation, co-expression with mGlu1a led to the accumulation of IP (Figure 8D and E) even in the absence of agonist activation of the mGlu1a receptor. Under the same conditions, no Ca2+ signals could be recorded on GABAB receptor activation, probably because the kinetics of the PLC activation were too slow to generate a synchronous Ca2+ signal in all transfected cells (Lechleiter et al, 1990). As the GABAB-mediated IP production was observed in the absence of glutamate, we hypothesized this to be the result of the amplification of IP production induced by constitutive mGlu1a receptor activity (Prezeau et al, 1996; Ango et al, 2001). We confirmed this using the mGlu1a inverse agonist BAY367620 that prevented the GABAB-induced IP production (Figure 8E). Production of IP by the GABAB receptor was also blocked by PTX (Supplementary data 4). These data suggest that GABAB Gi/o coupling potentiates the Gq-induced PLC activation brought about by the constitutive activity of the mGlu1a receptors.
Generalization of signal integration to other GPCRs in transfected and primary cells
We hypothesized that the expression of different pairs of Gq- and Gi/o-coupled receptors allows such an oligomerization-independent functional crosstalk. This was found to be the case with the Gq-coupled mGlu1a and the Gi/o-coupled mGlu2 receptors (Figure 9A) or the Gq-coupled 5-HT2C and the Gi/o-coupled GABAB receptors (Figure 9B). The effect was not due to the overexpression of the receptors, as it was also observed when activating two endogenous receptors in a native model. For example, such a crosstalk is found in cortical primary astrocytes expressing Gi/o-coupled mGlu3 and Gq-coupled mGlu5 receptors (Ciccarelli et al, 1997; Biber et al, 1999) (Figure 9E and F). The calcium response generated by the mGlu5-receptor agonist DHPG is potentiated by the mGlu3-receptor agonist LY354740 in a PTX-sensitive way. In this study, we detected no significant FRET signal in HEK293 cells co-transfected with the Flag–mGlu2 and the ST–mGlu1a or with the ST–5HT2c and the Flag–tagged GB1-containing GABAB receptors, even for high receptor expression levels (Figure 9C and D). These data reinforce the idea that the crosstalk observed in recombinant systems or between native receptors depends on receptor compartmentalization rather than oligomerization. Thus, the specificity of temporally defined crosstalk could be achieved by the spatial pattern of expression or association of GPCRs into signalling platforms, both coupled to Gq or Gi/o and activated by either the same or two different ligands (Figure 10).
Figure 9.
Functional crosstalk between Gq- and Gi/o-coupled GPCRs. (A) mGlu2 agonist LY354740-induced mGlu1a potentiation in HEK293 cells co-expressing mGlu2 and mGlu1a receptors. (B) Functional crosstalk between GABAB receptor and the Gq-coupled 5-HT2C receptors in transfected cells. (C, D) FRET signal detected between receptor pairs co-expressed in HEK293 cells (C) HA–ST-mGlu1a labelled with a BG-DY647 dye and Flag–mGlu2 labelled with an antibody anti-Flag–K, and (D) between HA–ST-5HT2c labelled with a BG-DY647 dye and Flag–GABAB (Flag–GB1+GB2) labelled with an antibody anti-Flag–K. (E) Potentiation of mGlu5 calcium response by co-activation of mGlu3 receptors in cortical astrocytes. Calcium dose-response generated by activation of mGlu3 by LY354740 (filled triangles), of mGlu5 (DHPG, filled circles) or by co-activation of mGlu5 (DHPG) and mGlu3 (LY354740, 10 μM, open circles). (F) mGlu5 calcium responses induced by DHPG (filled circles) in the presence of LY354740 (10 μM, open circles) in PTX-treated cells.
Figure 10.
Schematic representation of three different situations in which potentiation of the calcium signalling can be measured.
Discussion
In parallel fiber–Purkinje cell synapses, a functional crosstalk between mGlu1a and GABAB receptors has been described and reported to likely influence the induction of long-term depression (LTD) (Hirono et al, 2001; Kamikubo et al, 2007). This crosstalk was suggested to be the result of a direct association of these receptors into oligomers (Tabata et al, 2004; Kamikubo et al, 2007). Here, we have shown that such crosstalk between mGlu1a and GABAB does not require their physical interaction but rather relates to a functional crosstalk between their signalling pathways. We analysed the molecular mechanism behind this crosstalk and found that the observed potentiation of the mGlu1a Gq-mediated calcium response occurs through GABAB receptor Gi/o activation. We therefore showed the dependence of the mechanism on respective Gq- and Gi/o-signalling pathways, and highlighted its likely convergence at the level of IP production by PLC.
Importantly, this mechanism allows the temporal and spatial integration of signals generated by these receptors. First, we extended this observation to other GPCR pairs, such as mGlu1a and mGlu2 or 5-HT2c and GABAB receptors, thereby showing its occurrence between diverse Gq- and Gi/o-coupled receptors; and second, to native models, such as cortical neurons (GABAB and mGlu1a receptors) and cortical astrocytes (mGlu3 and mGlu5 receptors). Understanding this mechanism is essential for the analysis of pharmacological and physiological actions of drugs and also in GPCR signalling analysis.
Oligomerization-independent crosstalk
Crosstalk involves either receptor transactivation because of allosteric coupling resulting from their oligomerization or integration of their respective signalling pathways. Here we present several arguments in favour of an oligomerization-independent crosstalk between mGlu1a and GABAB receptors. First, the absence of TR-FRET and BRET signals and receptor co-immunoprecipitation in HEK293 cells pointed to the absence of their spontaneous physical association in these cells. Second, the active conformation of the GABAB receptor, per se, was insufficient and required its coupling to Gi/o proteins, as shown by the lack of potentiation following PTX-induced inhibition of Gi/o proteins or when using a GABAB receptor unable to couple to G proteins. Third, the GABA effect was mimicked by the allosteric positive modulator CGP7930 that binds directly in the transmembrane domain of GABAB2 (Supplementary data 5; Binet et al, 2004). Lastly, the crosstalk was reproduced by pairs of Gq- and Gi/o-coupled GPCRs that were shown not to physically interact with each other, indicating that this crosstalk is not specific to the GABAB–mGlu1a receptor pair.
Owing to a number of studies reporting Gq/Gi-coupled receptor hetero-oligomers, it was proposed that this crosstalk was a consequence of such receptor oligomerization (for example, mGlu1a-A1 adenosine receptor association (Ciruela et al, 2001)), and could then be used as an argument to validate the existence of GPCR oligomers in native tissues (Franco et al, 2007). Our data indicate that the physical association between Gq- and Gi/o-coupled receptors is not needed for such crosstalk, indicating that other functional consequences of receptor association have to be identified (Pin et al, 2007).
Specificity of the crosstalk: co-localization versus oligomerization
One argument used in favour of crosstalk between GABAB and mGlu1a receptors requiring their physical association was its apparent specificity, as no such crosstalk was observed between the Gi/o-coupled A1 adenosine receptor and mGlu1a (Hirono et al, 2001), despite both being present in Purkinje neurons (Ciruela et al, 2001). However, activation of the A1 adenosine receptor has been shown to increase the mGlu1a calcium response in co-transfected HEK293 cells (Ciruela et al, 2001). Moreover, an adenosine A1 agonist-induced potentiation of the mGlu1a receptor calcium response has been observed in cultured astrocytes, although no clear mechanism was proposed (Toms and Roberts, 1999). Surprisingly, A1 adenosine and mGlu1a receptors co-immunoprecipitated from cerebellar synaptosome extracts (Ciruela et al, 2001).
These data indicate that a similar crosstalk can occur between A1 adenosine and mGlu1a, or GABAB and mGlu1a receptors. This suggests that the absence of crosstalk between A1 and mGlu1a receptors in Purkinje neurons probably results from a different subcellular compartmentalization of these receptors explaining why, in Purkinje cells, mGlu1a receptor calcium response is potentiated by GABAB, but not by A1 adenosine receptor activation (Hirono et al, 2001).
Indeed, both mGlu1a and GABAB receptors are compartmentalized in Purkinje cells at the annuli of their dendritic spines (Lujan et al, 1997; Kulik et al, 2002) and are found co-localized in the dendritic spines of cultured Purkinje cells by light microscopy (Kamikubo et al, 2007), as well as at the electronic microscopy level as shown here (Figure 1). Thus, the GABAB activation that leads to an increased mGlu1a response and facilitates the induction of LTD, would be dependent on the co-compartmentalization of both receptors in these spines. The likely explanation for this compartmentalized crosstalk in native cells is that both receptors are co-localized thanks to targeting and stabilization in protein platforms (Bockaert et al, 2004). Indeed, mGlu1a and GABAB receptors interact with proteins involved in such platforms, such as MUPP1, Homer or Shank (Fagni et al, 2004; Kornau, 2006). It will be necessary to determine the proteins involved in co-compartmentalization of both receptors. In HEK293 cells, the C-terminal of mGlu1a is important for its precise neuronal localization but not for the crosstalk itself, as the mGlu1b variant was still potentiated by GABAB receptor activation (data not shown) despite possessing a short C-terminal domain (66 residues instead of 361 for mGlu1a).
Functional relevance of such a GABAB–mGlu1a crosstalk
In glutamatergic parallel fiber–Purkinje cell synapses, activation of post-synaptic mGlu1a allows the induction of LTD and it has been described that activation of the GABAB receptor facilitates this mGlu1a-mediated LTD induction (Kamikubo et al, 2007). Consistent with our observations, this phenomenon probably involves ambient GABA or GABA spillover from surrounding synapses. Spillover has an important function in neuronal communication (Ventura and Harris, 1999), as can be observed by following the repetitive synaptic release of glutamate or GABA (Scanziani, 2000; Brasnjo and Otis, 2001). These molecules then diffuse out of the synaptic cleft and reach targets either on adjacent synapses (Scanziani, 2002) or at extrasynaptic sites, such as mGlu1a or GABAB receptors (Baude et al, 1993). In line with our observations of a differing pattern of the calcium response depending on which receptors were activated first, one might imagine therefore that either glutamate or GABA reaches its target first thus leading to a signal that is not only integrated spatially but also temporally. Indeed, the priming action of the Gq-coupled mGlu1a receptor activation drives the calcium response pattern.
Interestingly, the calcium response obtained by a delayed GABAB receptor activation occurred even when the calcium signalling of the mGlu1a receptor was over and the calcium level had returned to basal level. We concluded that the Gq pathway was activated as long as glutamate was present and that it lasted even after the disappearance of the mGlu1a-induced calcium response (Lechleiter et al, 1990), thus allowing a GABAB activation-induced calcium response.
Indeed, IP production does not always induce a calcium response, as shown by mGlu1a receptor constitutive activity leading to low IP production and no calcium response (Prezeau et al, 1996). In addition, GABAB receptor activation alone was unable to produce either a calcium response or IP production in our model, however, when co-expressed with mGlu1a, the GABAB receptor became coupled to detectable IP production (Figure 8E). Our data indicate that IP production induced by GABAB activation occurs as an amplification of that induced by the constitutive activity of mGlu1a, as it was blocked by an mGlu1a inverse agonist. Thus, the small activation of PLCs by the constitutive activity of mGlu1a, acting as a priming event, was potentiated by GABAB stimulation.
However, the GABAB-induced IP production in the presence of the mGlu1a receptor was not high enough to induce a calcium response until mGlu1a became activated. We previously showed that in cerebellar granule cells, the constitutive activity of mGlu1a is regulated by the interaction with Homer proteins (Ango et al, 2001). Indeed, the Homer1a-inducible form displaced the Homer3 protein interacting with mGlu1a and enabled a constitutive activity that not only led to the production of IP3 but also to a highly activated BigK channel (Ango et al, 2001). Thus, depending on whether or not mGlu1a shows constitutive activity, which itself depends on the expression of Homer1a, it is expected that GABAB receptor activation will or will not, respectively, generate an IP response. However, this has to be explored further.
Signal integration through crosstalk between Gi- and Gq-coupled GPCRs
According to our results, this crosstalk can occur in several different situations (Figure 10). The first is the integration of a signal from two different receptors, coupled differently and activated by two different ligands, such as the GABAB and mGlu1a receptors. The second is in which the receptors are coupled differently but activated by the same ligand, as shown with mGlu1 and mGlu2 in HEK293 cells, or mGlu3 and mGlu5 in astrocytes. However, one can imagine a third situation in which one receptor is coupled to both pathways, leading to an autopotentiation of its Gq pathway by its own Gi/o pathway, generating a calcium signal sensitive to PTX. Preliminary data show that in our model, 5-HT4 receptor is coupled to both Gq and Gi/o and generates a high calcium response almost completely blocked by PTX (Supplementary data 8). This suggests a weak Gq coupling of the receptor but a strong Gi/o coupling, resulting in an autopotentiation process that occludes any possible potentiation by the activation of another Gi/o-coupled receptor, such as GABAB (Figure 10 and Supplementary data 8). Depending on the receptors expressed, this may enable the cells to control the calcium response and increase the complexity of the signal transduction analysis. This may be of interest when considering new pharmacological applications, as the idea of autopotentiation of calcium signalling could have consequences in the field of functional selectivity (Mailman, 2007). Depending on the action of the ligand used, one could generate a solely Gq calcium response, a solely Gi/o response or an autopotentiated response by activating both pathways. Thus, crosstalk with another GPCR would depend on the pathway mobilized by the ligand and would be important in the therapeutic action of drugs with functional selectivity.
An example of physiopathological functional selectivity is given by the activation of the 5-HT2A receptor by hallucinogens (Gonzalez-Maeso et al, 2008). Non-hallucinogenic compounds lead to a Gq coupling of the receptor, whereas hallucinogenic compounds induce both Gq- and Gi-protein activation. It would be interesting to analyse calcium signalling under both conditions, as a calcium response could come from either a high Gq coupling or an autopotentiation mechanism.
Materials and methods
SDS-digested freeze-fracture replica labelling (SDS-FRL)
Three-month-old C57/BL6 mice was perfused with PB (0.1 M, pH 7.4) containing 2% formaldehyde and 15% of a saturated solution of picric acid. The cerebellum was cut into 150-μm-thick parasagittal sections using a vibrating microslicer (DTK-1000; Dosaka EM, Kyoto, Japan) and cryoprotected using 30% glycerol in 0.1 M PB overnight at 4°C. The sections were then frozen using a high-pressure freezing machine (HPM 010; Bal-Tec, Balzers, Liechtenstein) and fractured at −150°C using a double replica method in a freeze etching system (BAF060; Bal-Tec). Fractured faces were replicated by deposition of carbon (5 nm) from a 90° angle above the horizontal level, shadowed unidirectionally by platinum–carbon (2 nm) with the gun positioned at a 60° angle, followed by the application of carbon (15 nm) from a 90° angle. Tissue was dissolved in solution containing 2.5% SDS and 20% sucrose made up in 15 mM Tris buffer, pH 8.3, at 80°C on a shaking platform for 18 h. Replicas were washed in 25 mM TBS containing 0.05% bovine serum albumin (BSA) and incubated in a blocking solution containing 5% BSA in 25 mM TBS for 1 h. Subsequently, the replicas were incubated with mixtures of primary antibodies for GB1 (guinea pig, (Kulik et al, 2006)) and mGlu1a (rabbit, (Shigemoto et al, 1997)) in a solution containing 1% BSA in 25 mM TBS OVN at RT. After several washes, the replicas were reacted with a mixture of 10 nm gold-coupled goat anti-rabbit and 5 nm gold-coupled goat anti-guinea pig secondary antibodies (1:30; BioCell Research Laboratories, Cardiff, UK) made up in 25 mM TBS containing 5% BSA at RT for 2 h. They were then washed, picked up on 100-line grids and examined in a transmission electron microscope (Tecnai 10; FEI, Eindhoven, The Netherlands) equipped with a digital camera (MegaView III; Olympus Soft Imaging Solution GmbH, Munster, Germany). Specificity of the replica labelling with the primary antibodies was confirmed previously using respective knockout mice (Kulik et al, 2006; Kaufmann et al, 2009).
Plasmids and site-directed mutagenesis
Plasmids encoding GB1, GB2 and mGlu1 receptors, epitope-tagged at their N-terminus with HA and Flag, have previously been described by Kniazeff et al (2004). SNAP-tag sequence (Covalys, Geneva, Switzerland) was added as described by Maurel et al (2008), to generate the HA–SNAP-tagged 5HT2c and 5HT2a receptor sequences. mGlu1a–YFP and GB2–Rluc receptors (YFP or RLuc tags at their C-terminus) were generated as previously described for the PAR1–YFP construct (Ayoub et al, 2007) and the same strategy was used to generate Go–RLuc fusion protein. The Venus-tagged γ subunit was generously provided by Dr C Galès (INSERM U858, Toulouse, France; Gales et al, 2006) and the YFP-tagged β1 subunit was generated by inserting the YFP at the N-terminus of the coding sequence obtained from the cDNA Resource Center (Missouri, USA).
Cell culture and transfection
As previously described by Maurel et al (2008), HEK293 cells were cultured in DMEM—10% FCS and transfected using LipofectamineTM2000 in 96-well plates. For each well, the DNA (μg):LipofectamineTM2000 (μl) mix ratio was 0.4. A total of 0.2 μg of DNA was incubated with 0.5 μl of LipofectamineTM 2000 in 50 μl Opti-MEM (1 ×) (Invitrogen Cat. No. 31985-062) for 20 min at RT. 100 000 cultured cells were added in each well on the mix and incubated at 37°C in a CO2 incubator for 6 h. The medium was replaced by DMEM-GlutamaxI (Invitrogen/GIBCO Cat. No. 61965-026) for at least 20 h. Treatment with PTX (200 ng/ml) (Calbiochem, Cat. No. 516560) was carried out OVN in Glutamax.
ELISA assay for quantification of cell-surface expression
As previously described by Binet et al (2007), the cells were fixed with 4% paraformaldehyde and blocked with PBS+1% FCS. Rat anti-HA (clone 3F10, Roche Bioscience, Cat. No. 1 867 423) or mouse monoclonal anti-Flag M2 antibodies (Sigma-Aldrich, F3165) were applied for 30 min at 0.5 mg/l. After washes with blocking buffer, the horseradish peroxidase-conjugated goat anti-rat IgG (Jackson ImmunoResearch Laboratories, Code 112-036-006, 0.5 mg/l) or the anti-mouse HRP (Amersham Biosciences, NXA931, 0.5 mg/l) secondary antibodies were applied for 30 min. Chemoluminescence was detected using SuperSignal substrate (Pierce, Rockford, IL, USA) and a Mithras reader (Berthold Biotechnologies, Bad Wildbad, Germany). Data were collected using the MicroWin2000 software.
Intracellular calcium measurements
As previously described by Maurel et al (2008), transfected cells were washed with HBSS buffer (20 mM Hepes, 1 mM MgSO4, 3.3 mM Na2CO3, 1.3 mM CaCl2, 0.1% BSA, and 2.5 mM probenecid) and loaded with 1 μM fluorescent dye Fluo-4 AM (Molecular Probes, Eugene, OR, USA) for 1 h at 37°C and incubated with the indicated drugs. Fluorescence signals (excitation 485 nm, emission 525 nm) were measured using the reader Flexstation (Molecular Devices, Sunnyvale, CA, USA) at intervals of 1.5 s for 60 s. Data were analysed using the program Soft Max Pro (Molecular Devices) and the dose-response curves were generated by the max–min analysis of the signals and fitted using Prism GraphPad software (San Diego, CA, USA).
TR-FRET between antibodies or between antibodies and SNAP-tag fluorophores
As previously described by Maurel et al (2008), transfected cells were washed with DMEM 10% FCS. For SNAP-tag/antibodies HTR-FRET, cells were labelled for 1 h at 37°C, 5% CO2, washed twice with Tris-KREBS buffer (20 mM Tris (pH 7.4), 118 mM NaCl, 5.6 mM glucose, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.7 mM KCl and 1.8 mM CaCl2), and incubated with 2 nM of anti-Flag antibodies conjugated with K or DY647 OVN at 4°C. For antibodies HTR-FRET, cells were incubated in Tris-KREBS buffer with 2 nM of anti-Flag and anti-HA antibodies conjugated with K or DY647 OVN at 4°C. Homogenous HTR-FRET signal was measured at 665 nm between 50 and 450 μs after laser excitation at 337 nm. Signals were expressed as delta 665=(total signal at 665 nm)−(background at 665 nm). The background signal corresponds to SNAP-tag cells labelled with K only.
BRET experiments
As previously described by Ayoub et al (2007), for saturation curves, a constant quantity of the donor (Luc) was co-transfected with a range of expression of the acceptor (YFP). After washing the cells, Coelenterazine h (Invitrogen Cat. No. C-6780) was added (5 μM in 50 μl per well). Readings were recorded using the Mithras LB 940 reader (Berthold Biotechnologies) (Rluc filter: 485±20 nm and YFP filter: 530±25 nm), data collected using the MicroWin2000 software (Berthold Biotechnologies) and BRET signal expressed in milliBRET units of BRET ratio. Dose-response curves were fitted with a linear regression or sigmoid dose-response equation using Prism GraphPad software.
Inositol phosphate measurements
Experiments were carried out as previously described by Binet et al (2007). Briefly, after OVN incubation with 3H-myo inositol, cells were washed with Krebs buffer and incubated for 1 h with 2 mM pyruvate and 0.2 U GPT in Krebs buffer at 37°C. The cells were incubated with 20 mM of LiCl in 75 μl of Krebs buffer for 5 min at 37°C, the drugs added and the stimulation stopped after 30 min using formic acid. The lysate was filtered on a DOWEX resin in a 96-well Millipore Multiscreen 0.45-μm plate, washed with water and after 5 min incubation with elution buffer, the IP collected in a 96-well Wallac 1450-401 plate containing 100 μl of scintillant. Signal was normalized on the radioactivity in cell membranes per well.
Cell-surface co-immunoprecipitation
At 24 h after transfection in 100 mm plates, cells were washed with ice-cold PBS and incubated with blocking buffer PBS+0.2% BSA at 4°C for 1 h with mild shaking. The cells were incubated in 3 ml PBS+0.2% BSA containing mouse anti-Flag M2 monoclonal antibody (1/500) for 3 h at 4°C. After washes with blocking buffer, cells were treated with NEM for 20 min, washed with PBS and lysed with RIPA Lysis buffer (50 mM TrisHCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS+PI) for 45 min at 4°C with mild shaking. The soluble fraction was centrifuged for 30 min at 12 000 g and 4°C to collect the solubilized receptors in the supernatant. The supernatant was added on Prot A/G beads (ref 20421, Pierce) washed with 50 mM Tris–HCl (pH 7.4), 150 mM NaCl+protease inhibitor. After OVN rotation at 4°C, beads were washed with PBS and loading buffer was added. The samples were heated at 95°C for 5 min and centrifuged 2 min at 3000 g, the supernatant was loaded on a 8% SDS–PAGE. The primary rabbit anti-HA (ZYMED, 71-5500) antibody was used at 0.6 mg/l and the mouse anti-Flag antibody (Sigma, F3165) at 2 mg/l. The secondary antibodies conjugated to the DYLight 488 fluorophore (Rockland, ref. 610-141-003 and 611-141-003) were used at 0.1 mg/l and the fluorescence was read on the Odyssey (LI-COR Biosciences).
Primary cortical neurons and astrocytes
Primary cortical cultures were prepared by dissecting the cortex of Swiss mice at embryonic day 16. The tissue was dissociated in Ham's F12 with 1% antibiotic solution containing 10% Nu serum (BD Biosciences, 355100) for cortical astrocytes or containing 10% of a mix of hormones (transferin 100 μg/ml, insulin 25 μg/ml, putrescine 60 μM, progesterone 20 nM, and sodium selenite 30 nM) for cortical neurons. The cells were plated at a density of 300 000 cells per well for astrocyte culture and 200 000 cells per well for neuron culture in poly-ornithine, then for neurons in Ham's F12+10% SVF, pre-coated 96-well plates and maintained at 37°C in 95% air/5% CO2.
Supplementary Material
Supplementary data 1
Supplementary data 2
Supplementary data 3
Supplementary data 4
Supplementary data 5
Supplementary data 6
Supplementary data 7
Supplementary data 8
Supplementary Material
Acknowledgments
We thank Laetitia Comps-Agrar, Damien Maurel, Siluo Huang and Philippe Rondard for their technical and scientific assistance; Drs Philippe Marin and Aline Dumuis (IGF, Montpellier France) for providing the 5-HT2C and 5-HT4 cDNA respectively; Celine Gales (INSERM U858, Toulouse, France) for the G protein subunits fused to YFP and Rluc; and the screening facilities (Pharmacology Screening and Interaction Platform) of the Institut Federatif de Recherche 3. MLR was supported by a BDI fellowship (Bourse Docteur-Ingenieur) from the CNRS. This study was supported by CNRS, INSERM, Cisbio and by grants from the French Ministry of Research, the Agence Nationale de la Recherche (ANR-BLAN06-3_135092, ANR-05-NEUR-035, and ANR-BLANC09-InnovGABAB) and by an unrestricted grant from Senomyx.
Author contributions: MLR executed most of the experiments and participated in the writing of the paper; CV carried out some initial experiments on calcium signalling; NT and ET generated the tools for HTRF experiments; MAA generated and developed BRET tools; YF and RS carried out electronic microscopy experiments; JPP contributed to the supervision of the work and to the writing; LP supervised the project and contributed to the writing of the paper.
References
- Angers S, Salahpour A, Bouvier M (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435 [DOI] [PubMed] [Google Scholar]
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L (2001) Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962–965 [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Maurel D, Binet V, Fink M, Prezeau L, Ansanay H, Pin JP (2007) Real-time analysis of agonist-induced activation of protease-activated receptor 1/Galphai1 protein complex measured by bioluminescence resonance energy transfer in living cells. Mol Pharmacol 71: 1329–1340 [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P (1993) The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 11: 771–787 [DOI] [PubMed] [Google Scholar]
- Biber K, Laurie DJ, Berthele A, Sommer B, Tolle TR, Gebicke-Harter PJ, van Calker D, Boddeke HW (1999) Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem 72: 1671–1680 [DOI] [PubMed] [Google Scholar]
- Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prezeau L (2004) The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J Biol Chem 279: 29085–29091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet V, Duthey B, Lecaillon J, Vol C, Quoyer J, Labesse G, Pin JP, Prezeau L (2007) Common structural requirements for heptahelical domain function in class A and class C G protein-coupled receptors. J Biol Chem 282: 12154–12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P (2004) GPCR interacting proteins (GIP). Pharmacol Ther 103: 203–221 [DOI] [PubMed] [Google Scholar]
- Bouvier M (2001) Oligomerization of G-protein-coupled transmitter receptors. Nature Rev 2: 274–286 [DOI] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS (2001) Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron 31: 607–616 [DOI] [PubMed] [Google Scholar]
- Carroll RC, Morielli AD, Peralta EG (1995) Coincidence detection at the level of phospholipase C activation mediated by the m4 muscarinic acetylcholine receptor. Curr Biol 5: 536–544 [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, Sureda FX, Casabona G, Di Iorio P, Caruso A, Spinella F, Condorelli DF, Nicoletti F, Caciagli F (1997) Opposite influence of the metabotropic glutamate receptor subtypes mGlu3 and -5 on astrocyte proliferation in culture. Glia 21: 390–398 [DOI] [PubMed] [Google Scholar]
- Ciruela F, Escriche M, Burgueno J, Angulo E, Casado V, Soloviev MM, Canela EI, Mallol J, Chan WY, Lluis C, McIlhinney RA, Franco R (2001) Metabotropic glutamate 1alpha and adenosine A1 receptors assemble into functionally interacting complexes. J Biol Chem 276: 18345–18351 [DOI] [PubMed] [Google Scholar]
- Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin JP, Prezeau L (2002) A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J Biol Chem 277: 3236–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Ango F, Perroy J, Bockaert J (2004) Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Semin Cell Dev Biol 15: 289–298 [DOI] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Woods AS, Lluis C, Franco R (2007) Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci 30: 440–446 [DOI] [PubMed] [Google Scholar]
- Franco R, Casado V, Cortes A, Ferrada C, Mallol J, Woods A, Lluis C, Canela EI, Ferre S (2007) Basic concepts in G-protein-coupled receptor homo- and heterodimerization. Scientific World Journal 7: 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol 13: 778–786 [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF (2000) Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem 275: 26128–26135 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452: 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2008) How and why do GPCRs dimerize? Trends Pharmacol Sci 29: 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Yoshioka T, Konishi S (2001) GABA(B) receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat Neurosci 4: 1207–1216 [DOI] [PubMed] [Google Scholar]
- Ige AO, Bolam JP, Billinton A, White JH, Marshall FH, Emson PC (2000) Cellular and sub-cellular localisation of GABA(B1) and GABA(B2) receptor proteins in the rat cerebellum. Brain Res Mol Brain Res 83: 72–80 [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399: 697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikubo Y, Tabata T, Kakizawa S, Kawakami D, Watanabe M, Ogura A, Iino M, Kano M (2007) Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells. J Physiol 585: 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WA, Ferraguti F, Fukazawa Y, Kasugai Y, Shigemoto R, Laake P, Sexton JA, Ruth P, Wietzorrek G, Knaus HG, Storm JF, Ottersen OP (2009) Large-conductance calcium-activated potassium channels in Purkinje cell plasma membranes are clustered at sites of hypolemmal microdomains. J Comp Neurol 515: 215–230 [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Saintot P-P, Goudet C, Liu J, Charnet A, Guillon G, Pin J-P (2004) Locking the dimeric GABAB G-protein coupled receptor in its active state. J Neuroscience 24: 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC (2006) GABA(B) receptors and synaptic modulation. Cell Tissue Res 326: 517–533 [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R (2002) Distinct localization of GABA(B) receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Neurosci 15: 291–307 [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R (2006) Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci 26: 4289–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J, Hellmiss R, Duerson K, Ennulat D, David N, Clapham D, Peralta E (1990) Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J 9: 4381–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R (2006) The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J 25: 3012–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P (1997) Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat 13: 219–241 [DOI] [PubMed] [Google Scholar]
- Mailman RB (2007) GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28: 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prezeau L, Trinquet E, Pin JP (2008) Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods 5: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel D, Kniazeff J, Mathis G, Trinquet E, Pin JP, Ansanay H (2004) Cell surface detection of membrane protein interaction with homogeneous time-resolved fluorescence resonance energy transfer technology. Anal Biochem 329: 253–262 [DOI] [PubMed] [Google Scholar]
- Milligan G (2004) G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol 66: 1–7 [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL (2006) How many drug targets are there? Nat Rev Drug Discov 5: 993–996 [DOI] [PubMed] [Google Scholar]
- Park D, Jhon DY, Lee CW, Lee KH, Rhee SG (1993) Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem 268: 4573–4576 [PubMed] [Google Scholar]
- Pin JP, Duvoisin R (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34: 1–26 [DOI] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M, Spedding M (2007) International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev 59: 5–13 [DOI] [PubMed] [Google Scholar]
- Prezeau L, Gomeza J, Ahern S, Mary S, Galvez T, Bockaert J, Pin J-P (1996) Changes of the C-terminal domain of mGluR1 by alternative splicing generate receptors with different agonist independent activity. Mol Pharmacol 49: 422–429 [PubMed] [Google Scholar]
- Quitterer U, Lohse MJ (1999) Crosstalk between Galpha(i)- and Galpha(q)-coupled receptors is mediated by Gbetagamma exchange. Proc Natl Acad Sci USA 96: 10626–10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M (2000) GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron 25: 673–681 [DOI] [PubMed] [Google Scholar]
- Scanziani M (2002) Competing on the edge. Trends Neurosci 25: 282–283 [DOI] [PubMed] [Google Scholar]
- Selbie LA, Hill SJ (1998) G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci 19: 87–93 [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17: 7503–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, Kano M (2004) Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc Natl Acad Sci USA 101: 16952–16957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms NJ, Roberts PJ (1999) Group 1 mGlu receptors elevate [Ca2+]i in rat cultured cortical type 2 astrocytes: [Ca2+]i synergy with adenosine A1 receptors. Neuropharmacology 38: 1511–1517 [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19: 6897–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL (2005) A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA 102: 9050–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data 1
Supplementary data 2
Supplementary data 3
Supplementary data 4
Supplementary data 5
Supplementary data 6
Supplementary data 7
Supplementary data 8
Supplementary Material