Abstract
Aquaporin (AQP) facilitated water transport is common to virtually all cell membranes and is marked by almost perfect specificity and high flux rates. Simultaneously, protons and cations are strictly excluded to maintain ionic transmembrane gradients. Yet, the AQP cation filters have not been identified experimentally. We report that three point mutations turned the water-specific AQP1 into a proton/alkali cation channel with reduced water permeability and the permeability sequence: H+ ≫K+ >Rb+ >Na+ >Cs+ >Li+. Contrary to theoretical models, we found that electrostatic repulsion at the central asn-pro-ala (NPA) region does not suffice to exclude protons. Full proton exclusion is reached only in conjunction with the aromatic/arginine (ar/R) constriction at the pore mouth. In contrast, alkali cations are blocked by the NPA region but leak through the ar/R constriction. Expression of alkali-leaking AQPs depolarized membrane potentials and compromised cell survival. Our results hint at the alkali-tight but solute-unselective NPA region as a feature of primordial channels and the proton-tight and solute-selective ar/R constriction variants as later adaptations within the AQP superfamily.
Keywords: aquaporin, cation, proton, selectivity, water
Introduction
Water channels of the ubiquitous aquaporin (AQP) protein family selectively funnel water or small, uncharged solutes across cellular membranes at rates equal to those of free diffusion in bulk solution (Borgnia et al, 1999). A long-standing question in the field is how, at the same time, protons and other cations are strictly excluded to maintain vital transmembrane pH and ionic gradients (Murata et al, 2000). Numerous theoretical studies analysed the selectivity function of two highly conserved constrictions in the otherwise 4 Å wide AQP channel path (Figure 1A) (de Groot and Grubmüller, 2005; Hub et al, 2009). One constriction is located at the extracellular pore mouth and consists of an arginine residue (Arg195 in human or rat AQP1) in an aromatic environment, that is, the ar/R region (Sui et al, 2001). Its diameter defines whether the AQP is water specific (2.8 Å) or also conducts solutes, such as urea and glycerol (>3.4 Å) (Beitz et al, 2006). The second, somewhat wider constriction resides in the centre of the 20 Å long channel and is formed by the asparagine residues of two canonical NPA motifs that cap the positive ends of two short α-helices (Asn76 and Asn192 in human or rat AQP1; Figure 1A) (Sui et al, 2001). The current models predict congruently that the positive electrostatic field emanating from the helix dipoles poses a major energy barrier for proton passage in the form of hydroxonium ions, H3O+. However, in various studies, the contribution of positive charges and size limitations in the ar/R constriction is considered either to be small (de Groot and Grubmüller, 2005) or large (Sui et al, 2001; Chen et al, 2006, 2007). In earlier models, the NPA region was further thought to have the additional function of breaking hydrogen bonds between water molecules passing in single file to prevent protons from hopping along the water chain, that is, a proton wire (Murata et al, 2000). Despite great efforts to identify the location and mechanisms of AQP proton and cation filters, experimental data are missing.
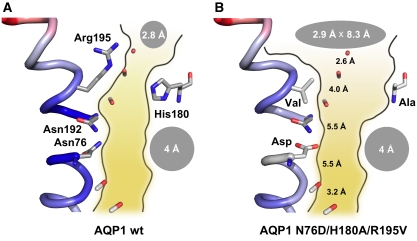
Figure 1.
Models of wild-type rat AQP1 (A) and AQP1-N76D/H180A/R195V (B). The channel path depictions are based on the crystal structure of bovine AQP1 (PDB #1j4n). Shape and diameters of the aromatic/arginine region (His180, Arg195) and the NPA region (Asn76, Asn192) are indicated by grey ellipses. Distances between the backbone carbonyl oxygens along the channel are given in Å. The figure was generated using Pymol.
We aimed to experimentally locate the cation filter in the prototypical water-specific AQP1 by point mutations of amino-acid residues in both constriction regions. Earlier, we had succeeded in generating a proton-leaking AQP1 mutant by replacing in the ar/R region Arg195 by valine and His180 by alanine (Figure 1B) (Beitz et al, 2006). The low proton conductance of the AQP1-H180A/R195V double mutant supported the model of the NPA region as a major, yet leaky, proton filter site. Calculations from molecular dynamics simulations using these mutants indicated that proton permeability was enabled because of a combination of (a) reduction of the electrostatic repulsion of Arg195, (b) an increase in the ar/R constriction diameter reducing the dehydration penalty, and (c) a lowering effect on the free energy barrier in the NPA region (Chen et al, 2006). In another theoretical study, free energy simulations in combination with continuum electrostatic calculations indicated that neutralizing the positive electrostatic field of the helix dipoles in the NPA region also could lead to proton conducting AQPs (Chakrabarti et al, 2004). Hence, in this study, we combined mutational changes in the ar/R constriction with changes in the NPA region. Specifically, we replaced Asn76 and Asn192 by negatively charged aspartate to compensate for the positive field in the NPA region and introduced the H180A/R195V double mutation to remove the positive charge and widen the diameter in the ar/R constriction (Figure 1B). We introduced the mutations individually and in combination and experimentally determined the resulting water/solute/cation permeability.
Results and discussion
Neutralization of the AQP helix dipoles does not generate proton permeability but a sodium leak
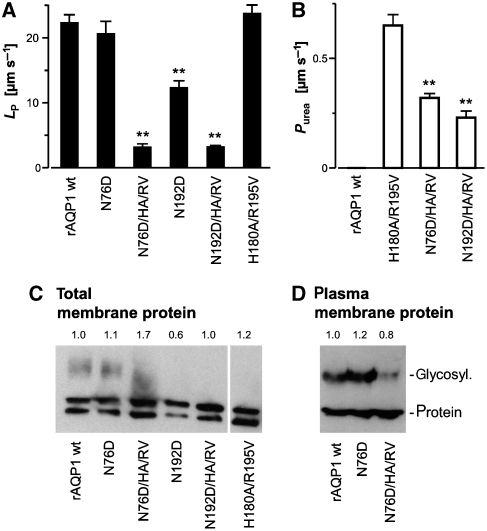
Both single mutations introduced in the NPA region, that is, AQP1-N76D and AQP1-N192D, produced functional and specific water channels when expressed in Xenopus laevis oocytes (Figure 2A). The water permeability was equal to wild-type AQP1 (N76D) or was reduced by 43% (N192D). These values correlate well with the expression levels of the mutants, which are similar (N76D) or at 60% (N192D) of wild-type AQP1 (Figure 2C). The exchange of asparagine with aspartate in terms of water–protein interactions cannot be regarded minor because a former hydrogen bond donor site was turned into an acceptor. Consequently, the orientation of a water molecule in the NPA region should be altered. Yet, this change apparently did not slow water passage. The situation is reminiscent of the AQP1-H180A/R195V double mutant in which all hydrogen bond donor sites in the ar/R constriction were removed and only a single carbonyl oxygen from the protein backbone, Gly190, remained as an interaction site with water. Still, water permeability was equal to wild-type AQP1 (Beitz et al, 2006).
Figure 2.
Water and solute permeability of AQP1 mutants. (A) Water permeability of rat wild-type AQP1 and the indicated AQP1 mutants was determined from the oocyte shrinkage obtained by adding 20 mosm mannitol to the bathing solution (Beitz et al, 2006). (B) Urea permeability was calculated from the oocyte swelling rate in a 130 mM isotonic inward gradient (Beitz et al, 2006). n=5–14, ±s.e.m, **P<0.01 versus wild-type AQP1 or AQP1-H180A/R195V. (C) Expression levels of wild-type AQP1 and AQP1 mutants were tested by western blotting with total oocyte membrane protein (protein from one oocyte per lane) and a specific anti-AQP1 antiserum. Non-glycosylated (lower band) as well as glycosylated forms of AQP1 (upper bands) were detected. (D) For detection of AQP1 in the plasma membrane, intact oocytes were treated with Ludox and polyacrylic acid to increase the specific weight of the plasma membrane (Leduc-Nadeau et al, 2007). After homogenization, the plasma membrane leaflets were collected by low-speed centrifugations and plasma membrane protein from 25 oocytes was loaded per lane. The numbers above the blots in C and D indicate expression levels relative to wild-type AQP1.
Having established integrity and functionality of the AQP1-N76D and AQP1-N192D mutants, we determined the permeability for H+ by two-electrode voltage-clamp measurements in AQP expressing Xenopus oocytes as described in Figure 3 and below in connection with other AQP1 mutants. Neither the AQP1-N76D mutant nor AQP1-N192D conducted protons as compared with wild-type AQP1 (Figure 3F). We confirmed, however, the low proton permeability P(H+) of 1.7 × 10–4 cm s−1 of the ar/R double mutant AQP1-H180A/R195V (Figure 3F) (Beitz et al, 2006). On the basis of the current theoretical models, this outcome was most unexpected. Apparently, the ar/R constriction controls proton exclusion more tightly than the NPA region.
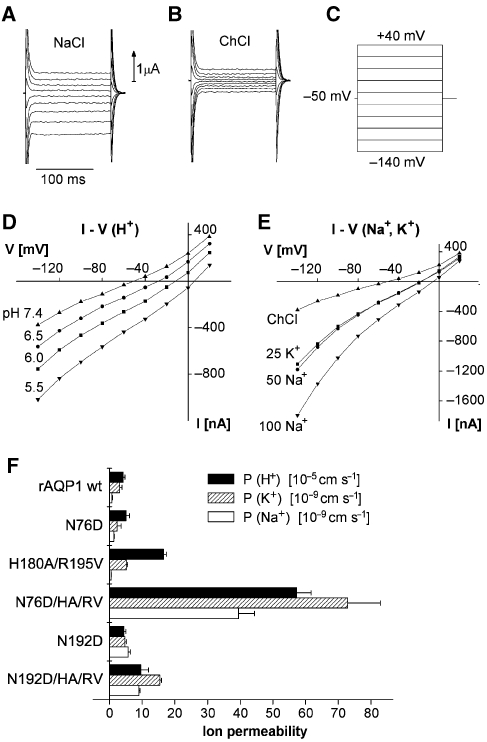
Figure 3.
Ion permeability of AQP1 mutants. Current–voltage relationships were obtained by the two-electrode voltage-clamp technique (Beitz et al, 2006). A series of voltage steps were applied and the resulting steady state currents recorded. The examples are from an oocyte expressing AQP1-N76D/H180A/R195V bathed in 100 mM NaCl (A) or in 100 mM ChCl (B), the latter also served as control bathing solution. All solutions contained an additional (mM) 2 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES or MES, and pH was adjusted using Tris base. (C) The voltage was changed from a holding potential of −50 mV to a value between +40 and −140 mV in steps of 20 mV and the current was read after 100 ms. The test solutions were applied for about 30 s, at which time the voltage steps were applied. (D) Steady state currents obtained in 100 mM ChCl at an external pH of 7.4, 6.5, 6.0, or 5.5. (E) Steady state currents plotted as a function of voltage in 100 mM choline chloride (ChCl), in 25 mM KCl + 75 mM ChCl (25 K+), in 50 mM NaCl + 50 mM ChCl (50 Na+), and in 100 mM NaCl (100 Na+). (F) Permeability (P) for H+, Na+, and K+, please note the different scales. The P-values were calculated from the clamp currents (IC) obtained at a clamp voltage V of −120 mV from the equation P=ICRT/(VF2C), an approximation to the Goldman–Hodgkin–Katz equation applicable at high negative potentials. C is the concentration, F is the Faraday's constant, and R is the gas constant. Values for the wild-type AQP1 (rAQP1 wt) and the mutants AQP1-N76D, AQP1-H180A/R195V, AQP1-N76D/H180A/R195V, AQP1-N192D, and AQP1-N192D/H180A/R195V are shown. Currents observed with uninjected oocytes were not significantly different from oocytes expressing wild-type AQP1. n=5–14, ±s.e.m.
We then tested AQP1-N76D and AQP1-N192D for Na+ and K+ permeability (Figure 3). AQP1-N76D prohibited passage of both cations. AQP1-N192D also excluded K+; we detected, however, some Na+ leakage at strongly negative potentials, that is, P(Na+) of 6 × 10–9 cm s–1 (Figure 3F). Together, low proton permeability of AQP1-H180A/R195V and Na+ leakage of AQP1-N192D hint at cation filter functions at both constriction sites with the NPA region being particularly geared to prevent Na+ from passing and the ar/R constriction posing a higher barrier for protons.
The proton permeability P(H+) of uninjected and water-injected oocytes is around 9 × 10–5 cm s−1 (Beitz et al, 2006; Søgaard et al, 2009). The Na+ permeability P(Na+) of the uninjected oocytes used in this study was not significantly different from zero (0.1±0.84 [10–9 cm s–1]), while the K+ permeability P(K+) was 3.6±2.0 [10–9 cm s–1] (data from three oocytes). Expression of wild-type AQP1 (rAQP1 wt in Figure 3F) did not significantly alter permeability for H+, Na+, and K+, and we chose to use the data for wild-type AQP1 as controls for the mutant AQPs. It is to be expected that any putative increase in membrane area or co-expression of other proteins is the same in oocytes expressing wild-type and mutant AQP1. It should be noted that in Figure 3F, we have not subtracted the background cation permeability of the oocyte membrane from the values obtained for the oocytes expressing AQP1 and its mutants. Thus, the bars shown for wild-type AQP1 represent the membrane leak currents.
Both AQP constrictions jointly prevent cation passage
We reasoned that if both constrictions in AQP1 individually have the capability to exclude cations then the combination of N76D or N192D with the H180A/R195V double mutation could turn AQP1 into a cation channel (see structure model in Figure 1B). First, however, we analysed the basic functionality of the AQP1-N76D/H180A/R195V and AQP1-N192D/H180A/R195V triple mutants. Osmotic oocyte swelling assays showed water permeability of only 14% that of wild-type AQP1 (Figure 2A). The reduced water permeability is intrinsic to the mutants and not because of reduced protein expression levels (Figure 2C and D). The amount of protein present in the Xenopus oocyte plasma membrane (Leduc-Nadeau et al, 2007) is within ±20% for wild-type AQP1, AQP1-N76D, and AQP1-N76D/H180A/R195V (Figure 2D), suggesting that the proteins fold correctly and are in a functional state. The ratio of glycosylated to unglycosylated AQP1 in the plasma membrane seemed to be slightly different among wild-type AQP1 (1.3:1), AQP1 N76D (1.6:1), and AQP1-N76D/H180A/R195V (1:1.9; Figure 2D). Yet, it was shown before that glycosylation does not affect AQP1 permeability (van Hoek et al, 1995). Expression of wild-type AQP1 and AQP1 N76D/H180A/R195V was further visualized by fluorescence microscopy using GFP-fusion constructs (Supplementary Figure S1). Passage of urea, at about half the permeability rate of AQP1-H180A/R195V provided additional evidence for correct folding of the triple mutants (Figure 2B; Beitz et al, 2006). The somewhat lower permeability observed can probably be explained by an unfavourable orientation of the urea molecule in an NPA region carrying an aspartate, because here interaction will be through hydrogens from one of urea's amide nitrogen atoms, whereas interaction with the original asparagine will be through urea's carbonyl oxygen moiety. It even seems possible that both interactions occur simultaneously because the mutants carry one aspartate and one asparagine, which would intensify binding and prolong the residing time of urea in the NPA region. The larger solute glycerol did not pass (not shown), indicating that the minimal pore diameter is below 3.4 Å (Beitz et al, 2006). In summary, combination of an N76D or N192D mutation in the NPA region with the H180A/R195V double mutation in the ar/R constriction results in intact AQP1 channel protein but with strongly reduced water permeability.
Electrophysiological measurements revealed high proton and even alkali cation permeability for AQP1-N76D/H180A/R195V (Figure 3D–F) and smaller, yet appreciable permeability for AQP1-N192D/H180A/R195V (Figure 3F). The H+ permeability of oocytes expressing AQP1-N76D/H180A/R195V was 5.8 × 10–4 cm s–1, that is, four times higher than with AQP1-H180A/R195V. With 1011 copies of AQP1 per cm2 (Beitz et al, 2006), this amounts to a unit H+ permeability of 0.6 × 10–15 cm3 s–1, about one twentieth of the unit water permeability of AQP1 of 10–14 cm3 s–1. Accordingly, the equivalent H+ conductance of the AQP1-N76D/H180A/R195V expressing oocytes at pH 5.5 is 7 × 10–6 S translating into a unit conductance of about 7 × 10–16 S. The unit conductance of canonical H+-specific channels is about 1.5 × 10–14 S at room temperature and physiological pH and increases to around 14 × 10–14 S at pH 5.5 (Cherny et al, 2003). Although this H+ conductance is about three orders of magnitude smaller than that of most other channels, it is still two orders of magnitude higher than that of the AQP1-N76D/H180A/R195V channel. In the light of the high abundance of AQP channels of up to 2 × 105 copies per cell (Denker et al, 1988), the necessity of efficient AQP proton filters becomes obvious. The permeability for K+ and Na+ was about four orders of magnitude smaller than that of H+. Yet, in a physiological setting, this is more than compensated by the high cation concentrations. At 100 mM Na+, for example, AQP1-N76D/H180A/R195V contributes a Na+ conductance of 3 × 10–5 S (Figure 3E), that is, a value four times larger than that for H+. The selectivity of canonical H+ channels is certainly higher than that of the AQP triple mutants. The P(H+)/P(Na+) ratio of H+ channels is typically in the range of 106–108 (DeCoursey, 2008), that is, 2–4 orders of magnitude higher than the ratio determined for AQP1-N76D/H180A/R195V and AQP1-N192D/H180A/R195V (Table I). AQP1-H180A/R195V with an intact NPA region, however, is highly selective for H+ further indicating different selectivity properties of both AQP pore constrictions.
Table 1.
Relative ion permeability (R) normalized to the water permeability of the AQP1 wild-type or the given mutants
| R(H+) [10−1] | R(Na+) [10−5] | R(K+) [10−5] | |
|---|---|---|---|
| rAQP1 wt | 0.18±0.024 | 0.031±0.0089 | 0.13±0.034 |
| N76D | 0.25±0.055 | 0.065±0.0059 | 0.12±0.056 |
| N192D | 0.35±0.047 | 0.45±0.055 | 0.37±0.040 |
| H180A/R195V | 0.70±0.035 | 0.020±0.0076 | 0.22±0.017 |
| N76D/H180A/R195V | 17.9±1.4 | 12.3±1.5 | 22.8±3.2 |
| N192D/H180A/R195V | 2.9±0.72 | 2.7±0.81 | 4.7±0.14 |
For the highly cation-permeable AQP1-N76D/H180A/R195V, a comparison of the permeability of five alkali metal cations in experiments analogous to those shown in Figure 3 gave a ratio P(K+):P(Rb+):P(Na+):P(Cs+):P(Li+) of 1:0.81:0.59:0.50:0.32, that is, an Eisenman sequence V, which is typical for K+ channels from nerve (Diamond and Wright, 1969). Yet, we expect that the mechanism of alkali metal transport in the AQP1 mutants is different from that of naturally occurring channels. K+ channels possess several high affinity binding sites, which ensure the presence of one or two K+ ions in the channel at all times (Zhou et al, 2001; Zhou and MacKinnon, 2003). In addition, the transport of ions and water is strictly coupled because of the restrictions imposed by single file transport. In a recent study using osmotic gradients on the HERG K+ channel, it was found that one K+ ion was co-transported for each 1.4 water molecules (Ando et al, 2008). In the AQP1 mutants, an osmotic gradient gave rise to comparatively very large fluxes of water (Figure 2A). As the occupation of the mutant AQP channel by a cation is a rare event, that is, about one cation per 4000 water molecules at unit osmotic and ion gradients (Table I), any sweeping away of cations would be quantitatively too small to be detected. The lack of electroosmotic coupling is also evident by the fact that the rate of osmotically induced water fluxes (i.e. the water permeability) was not a function of the ion flux through the channel: water permeability was not affected by applying or changing the clamp voltage or the type of cations present in the external solution (not shown).
The lower cation unit conductance of AQP1-N76D/H180A/R195V as compared with canonical cation channels probably results from lower affinity because of fewer carbonyl oxygens that are available for ion coordination as a water shell substitute (Figure 1B) (Zhou et al, 2001). Within the AQP1 channel, only four coordination sites of the passing cation can be satisfied, that is, two by carbonyl oxygens from the carbonyl ladder and two by water molecules above and below the cation. At the pore entry, because of the widened elliptic diameter by the H180A/R195V mutations (Figure 1B), two more water molecules may coordinate on the sides of the cation. The carbonyl oxygen introduced by the N76D mutation in the NPA region almost ideally bridges the gap in the carbonyl ladder forming a continuous path for cation coordination through the channel (Figure 1B). In the N192D triple mutant, somewhat less optimal orientation or spacing of the oxygen may account for the reduced cation conductance. Sub-angstrom deviations in the distance and angle of the coordination sites in a channel may have great influence on ion permeability as is proposed for the K+ over Na+ selectivity in potassium channels (Zhou et al, 2001). Further, the close location of the N192D mutation to Arg195 of the ar/R region (Figure 1) may readily affect the geometric and chemical properties of the constriction region.
The model shown in Figure 1 provides an intuitive visualization of why, in combination with electrostatic repulsion by the helix dipols, asparagine residues are the optimal building blocks for the centre of a cation excluding water channel. The amide moieties create a polar, hydrogen bond donating environment promoting the swift passage of water molecules; yet, proton-carrying hydroxonium, H3O+, as a hydrogen bond acceptor is also compatible with this environment and is not fully excluded by electrostatic repulsion of the helix dipoles (Beitz et al, 2006). For inorganic cations, however, the asparagines form an 11 Å wide barrier because of the lack of oxygen atoms for ion coordination.
The changes in the mechanism for water and ion transport induced by the mutations are linked to the nature of the given mutation and not to different expression levels. This appears from two facts. First, densitometric quantification of the western blot signals for wild-type AQP1 and the mutants suggests very strongly that the expression levels were similar (Figures 2C, D and 4A). The observed differences in expression levels of ±20–30% (in two cases 70%) are clearly below the changes in permeability. Second and more importantly, the ratio between water and cation permeability was found to be different between the various mutants, compare Figures 2A and 3F and see Table I. This ratio is independent from expression levels. For example, AQP1-N76D/H180A/R195V has a low water permeability and a high cation permeability, while the ratio is opposite for wild-type AQP1. Consequently, even if the cation permeability is normalized to the water permeability, the resulting relative cation permeability of the AQP1-N76D/H180A/R195V mutant is still much higher than that of the wild-type AQP1 (Table I).
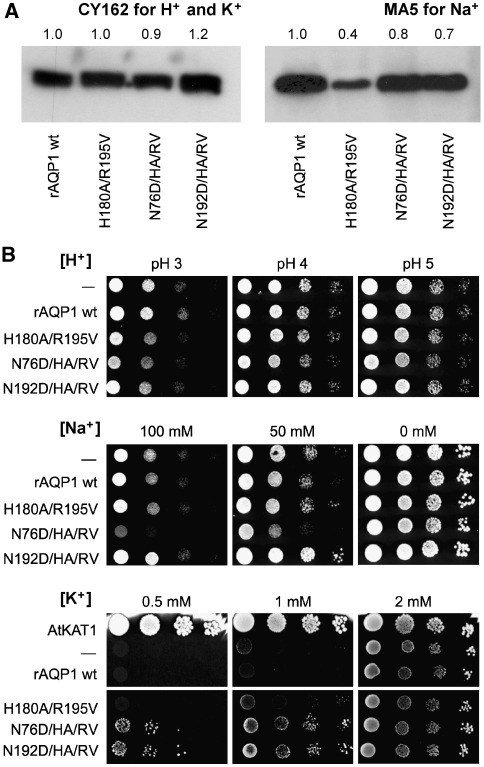
Figure 4.
Phenotypic yeast assays for ion permeability of AQP1 mutants. (A) Expression control of AQP1 mutants in the CY162 and MA5 yeast strains by western blot. (B) Yeast expressing rat wild-type AQP1 or the indicated mutants were spotted in serial 1:10 dilutions on low-salt arginine phosphate medium (Anderson et al, 1992) of different pH (H+) or supplemented with sodium (Na+) or potassium (K+) chloride at pH 5.5. Mock transformed yeast (−) or yeast transformed with an Arabidopsis potassium channel, AtKAT1, served as controls. For the H+ stress assay and the K+ growth complementation assay, the CY162 strain (Anderson et al, 1992) lacking both endogenous potassium channels Trk1 and 2 was used. The Na+ stress test was done with the sodium sensitive MA5 strain (Benito et al, 2004).
How does cation permeability interfere with water permeability? At least two options are thinkable and need to be addressed in later studies: (a) a changed electrostatic environment may affect the orientation of the molecules within the water file and may alter water–protein interactions (Chakrabarti et al, 2004); (b) the osmotic driving force may change from being hydraulic to being diffusive (Hill, 1982). According to this theory, osmotic pressure does not build up in a channel when entering osmolytes constantly attain equilibrium between the exterior and the interior of the channel. With the hydraulic driving force eliminated, water movement is controlled by diffusion alone and is lowered by a factor proportional to the number of water molecules in the channel. Considering the length of the water file in the AQP channel of 6–8 molecules, our water permeability measurements (Figure 2A) support this model exceedingly well.
Cation permeability of the AQP mutants has phenotypic effects
The physiological implication of the requirement of AQP cation filters becomes particularly clear when the behaviour of oocytes expressing the AQP1-N76D/H180A/R195V mutant is considered. Such oocytes were generally depolarized with membrane potentials of −10 to −15 mV when bathed in solutions containing 100 mM NaCl. When the major cation in the bath solution was non-permeating, that is, in 100 mM choline chloride (ChCl) the membrane potential was −50 mV. In addition, AQP1-N76D/H180A/R195V expressing oocytes only survived for 3 days probably due to the high Na+ leak. In contrast, oocytes expressing the other mutants or wild-type AQP1 survived for 1 week or more.
We further analysed the effects of cation-conducting AQP1-mutants by phenotypic yeast assays. Expression was controlled by western blot and appeared fairly even (Figure 4A). Protein insertion into the yeast plasma membrane was shown for wild-type AQP1 and AQP1-N76D/H180A/R195V by GFP-fusion and confocal fluorescence microscopy (Supplementary Figure S1). First, we applied pH and Na+ stress conditions and obtained similar results as in oocytes. Yeast growth of cells expressing H+ conducting AQP1-H180A/R195V or the triple mutants with N76D or N192D was only marginally reduced at acidic pH (Figure 4B, top panel), showing that a higher need for ATP-driven proton pumping leads to a rather mild physiological deficit. However, a sodium sensitive yeast strain, MA5 (Benito et al, 2004), expressing AQP1-N76D/H180A/R195V hardly survived on medium with 100 mM Na+ (Figure 4B, middle panel). We finally tested for K+ conductance using a growth complementation assay based on a yeast strain, CY162, that is devoid of its endogenous K+ channels, Trk1 and Trk2 (Anderson et al, 1992). At K+ concentrations above 2 mM uptake through unspecific yeast cation channels maintains cell growth. At lower K+ concentrations growth depends on the presence of more efficient channels, such as the Arabidopsis K+ channel AtKAT1 (Anderson et al, 1992), which we used as a positive control in the assay (Figure 4B, bottom panel). When tested under the same conditions, the K+ conductance of AQP1-N76D/H180A/R195V as well as of AQP1-N192D/H180A/R195V was sufficient to enable yeast growth even at the lowest K+ concentration of 0.5 mM (Figure 4B, bottom panel). The AQP triple mutants were also permeable for the ammonium ion, NH4+ (Supplementary Figure S2), which is similar to K+ in terms of ionic radius, hydration shell, and charge distribution (Zeuthen et al, 2006).
Evolutional implications and conclusion
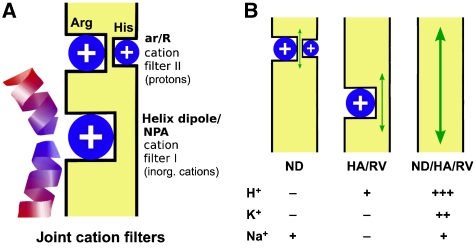
The fact that the NPA motifs in both AQP protein halves are almost perfectly conserved throughout the various phyla (archaea, plant, bacteria, animals) and among all types of AQPs while the composition of the ar/R region is variable can be understood in an evolutional context (Zardoya, 2005). Our findings suggest that AQPs have evolved in two stages. First, primitive primordial cells would have acquired a simple open channel by gene duplication permitting the uptake of nutrients and release of waste products. This channel already carried the optimal NPA motifs preventing passive movement of inorganic cations (Na+, K+, etc.; Figure 5) and enabling ionic gradients to be built up across the membrane by active transport. Yet, this channel may have leaked protons (Figure 5B, centre). Second, at a later stage of development, the ar/R region evolved to fully shut off proton permeability (Figure 5B, left) and to satisfy specific needs of the particular cell or organ. In this way, the spectrum of AQP types with water, urea, glycerol, and ammonia permeability arose carrying joint cation filters in the NPA and ar/R regions.
Figure 5.
Model of the AQP joint cation filters. (A) The primordial AQPs already selected against inorganic cations, such as Na+ and K+, because of a positive electrostatic field from the helix dipols and the lack of cation coordination sites in the NPA region (filter I); yet, protons leaked through. Later, a second cation filter evolved in the ar/R region, which fully excluded protons (filter II) and provided individual selectivity properties for water, glycerol, urea, and ammonia. (B) Disturbance of the filter regions by point mutations leads to Na+ leakage as was the case for the AQP1-N192D mutant (ND; exchange of asparagine for aspartate in the NPA region), enables low proton permeability (HA/RV; removal of positive charges in the ar/R region) or switches the AQP into a cation channel (ND/HA/RV; combination of the NPA and ar/R mutations).
Materials and methods
Mutation and expression of rat AQP1 mutants in X. laevis oocytes
Rat AQP1 and the AQP1-H180A/R195V double mutant in the pOG1 vector have been described (Beitz et al, 2006). Additional point mutations were introduced using the QuikChange protocol (Stratagene) and primers with respective nucleotide exchanges (list available from the authors upon request). cRNA synthesis was done from Not I linearized pOG1 plasmid with the mMessage mMachine T7 kit (Ambion). A measure of 5 ng of cRNA in 50 nl of water were injected into collagenase A (Roche) defolliculated stage V and VI X. laevis oocytes. The oocytes were incubated for 3 days at 15°C in ND96 buffer (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4). Control oocytes were water injected.
Oocyte water and solute permeability
Osmotic water permeability (LP) was measured by means of a high resolution method (Beitz et al, 2006). The volume of the individual oocyte was monitored continuously with a time resolution of 1 s and a volume resolution of 20 pL equivalent to 0.002% of the oocyte volume. To record the LP, 20 mM of the inert sugar alcohol mannitol (or raffinose) was added to the bathing solution and the rate of shrinkage of the oocyte was recorded. The bathing solution was changed in about 1 s, and the LP was obtained within 10 s. Oocyte swelling because of urea (Purea) or glycerol permeability was monitored for 60 s in a 130 mM inward solute gradient generated by isotonic replacement of 65 mM NaCl in the bath (Beitz et al, 2006).
Western blot analysis and densitometric quantification
For total membrane protein preparation, oocytes were lysed in cold hypotonic phosphate buffer (7.5 mM Na2HPO4/NaH2PO4, pH 7.5). Cell debris was removed by centrifugation at 500 × g for 5 min and the membranes were collected at 16 000 × g for 30 min. Membrane protein corresponding to one oocyte per lane was separated by SDS/PAGE and blotted on PVDF membranes (Amersham Biosciences) for later detection. Plasma membrane proteins were isolated according to Leduc-Nadeau et al (2007). Briefly, oocytes were rinsed in MES-buffered saline solution (MBSS; in mM: 80 NaCl, 20 MES, pH 6.0) and incubated with 0.003% subtilisin A in MBSS for 10 min at room temperature under mild agitation, followed by 60 min at 4°C in MBSS with 1% Ludox and 60 min in MBSS with 0.1% polyacrylic acid. After homogenization in cold HbA buffer (in mM: 5 MgCl2, 5 NaH2PO4, 1 EDTA, 80 sucrose, 20 Tris, pH 7.4) the plasma membrane leaflets were separated from internal membranes by low-speed centrifugation steps at 14 × g, 22 × g, 31 × g, and 42 × g and collected at 16 000 × g. Plasma membrane protein corresponding to 25 oocytes per lane was separated and blotted on PVDF. The membranes were probed with an anti-AQP1 monoclonal mouse antibody (1:1000, Santa Cruz Biotechnology) and detected with horseradish peroxidase-conjugated goat anti-mouse antiserum (Dianova) using the ECL Plus system (Amersham Biosciences). ImageJ software was used for quantification of western blot signals relative to AQP1 wild type. The evaluation procedure involved background substraction and subsequent determination of the band intensity.
Electrophysiology
The electrophysiological methods have been described earlier (Beitz et al, 2006; Zeuthen et al, 2006). To measure cation-induced currents in Xenopus oocytes expressing AQP, we used the two-electrode voltage-clamp technique, using a high performance oocyte clamp (DAGAN CA-1B, Minneapolis, MN, USA), DigiData 1322A, and MiniDigi 1B interfaces controlled by pCLAMP software, version 9.2 (Axon Instruments, Burlingame, CA, USA). Recording electrodes were filled with 1 M KCl and had resistances of about 1.5 MΩ. Oocytes were placed in a small circular recording chamber (diameter: 3 mm) and superfused with control or test solutions at 20 ml min−1. Reference electrodes in the bath were either Ag/AgCl or agar bridges filled with 2 M KCl. Solutions were exchanged using a custom-made mechanical valve; the time constant for solution change was <1 s. The control solution contained (in mM): 100 ChCl, 20 mannitol, 2 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES or MES; pH was adjusted to the required pH using MES or TRIS base. All experiments were carried out at room temperature. To measure the permeability of Na+, 50 or 100 mM Ch+ was replaced by Na+; to measure the permeability of K+, 25 mM of Ch+ was replaced by K+. To measure the H+ permeability, the pH of the bathing solution was changed to a value between 7.4 and 5.5. Before and after the solution change, the voltage of the voltage-clamped oocyte was jumped to potentials between +40 and −140 mV in steps of 20 mV lasting 150 ms. The corresponding steady state clamp currents were recorded after 100 ms.
Phenotypic yeast assays
The open reading frames coding for rat AQP1 wild type and mutants were ligated into the constitutive yeast expression vector pRS426MET. Yeast was grown on arginine phosphate medium (AP, in mM: 12 L-arginine, 1 MgCl2, 0.1 CaCl2, 2% glucose, vitamins and trace elements adjusted to pH 5.5 with phosphoric acid) (Anderson et al, 1992). For the H+ stress assay (AP medium plus 20% agar and 50 mM KCl adjusted to pH 3, 4, or 5) and the K+ growth complementation assay (AP medium plus 20% agarose and 0.5, 1, or 2 mM KCl) the CY162 strain was used (MAT α ura3-52 trk1Δ his3Δ200 his4-15 trk2Δ1∷pCK64) (Anderson et al, 1992). The Na+ stress test was done with the sodium sensitive MA5 strain (Mat a ade2 ura3 trp1 trkΔ∷LEU2 trk2Δ∷HIS3 ena1-4Δ∷HIS3 nha1Δ∷LEU2) (Benito et al, 2004) on AP medium plus 20% agar and supplemented with 0, 50, or 100 mM NaCl.
Supplementary Material
Supplementary Figures S1 and S2
Review Process File
Acknowledgments
We thank A Rodríguez-Navarro for providing the MA5 yeast strain. M Bleich and J von Bülow are acknowledged for their help with the Xenopus frogs, C Plieth for fluorescence microscopy, and B Henke and S Häuer for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft Be2253/3 (to EB), by the Nordic Centre of Excellence Program in Molecular Medicine, the Danish Research Council, the Lundbeck Foundation, and the NovoNordisk Foundation (to TZ and MA).
Footnotes
The authors declare that they have no conflict of interest.
References
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 89: 3736–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Kuno M, Shimizu H, Muramatsu I, Oiki S (2008) Coupled K+-water flux through the HERG potassium channel measured by an osmotic pulse method. J Gen Physiol 126: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T (2006) Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 103: 269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito B, Garciadeblás B, Schreier P, Rodríguez-Navarro A (2004) Novel p-type ATPases mediate high-affinity potassium or sodium uptake in fungi. Eucaryot Cell 3: 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgnia M, Nielsen S, Engel A, Agre P (1999) Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 68: 425–458 [DOI] [PubMed] [Google Scholar]
- Chakrabarti N, Roux B, Pomès R (2004) Structural determinants of proton blockage in aquaporins. J Mol Biol 343: 493–510 [DOI] [PubMed] [Google Scholar]
- Chen H, Ilan B, Wu Y, Zhu F, Schulten K, Voth GA (2007) Charge delocalization in proton channels, I: the aquaporin channels and proton blockage. Biophys J 92: 46–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wu Y, Voth GA (2006) Origins of proton transport behavior from selectivity domain mutations of the aquaporin-1 channel. Biophys J 90: L73–L75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny VV, Murphy R, Sokolov V, Levis RA, DeCoursey TE (2003) Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement. J Gen Physiol 121: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot BL, Grubmüller H (2005) The dynamics and energetics of water permeation and proton exclusion in aquaporins. Curr Opin Struct Biol 15: 176–183 [DOI] [PubMed] [Google Scholar]
- DeCoursey TE (2008) Voltage-gated proton channels: what's next? J Physiol 586: 5305–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker BM, Smith BL, Kuhajda FP, Agre P (1988) Identification, purification, and partial characterization of a novel Mr 28 000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem 263: 15634–15642 [PubMed] [Google Scholar]
- Diamond JM, Wright EM (1969) Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol 31: 581–646 [DOI] [PubMed] [Google Scholar]
- Hill AE (1982) Osmosis: a bimodal theory with implications for symmetry. Proc R Soc Lond 215: 155–174 [DOI] [PubMed] [Google Scholar]
- Hub J, Grubmüller H, de Groot BL (2009) Dynamics and energetics of permeation through aquaporins. What do we learn from molecular dynamics simulations? Handb Exp Pharmacol 190: 57–76 [DOI] [PubMed] [Google Scholar]
- Leduc-Nadeau A, Lahjouji K, Bissonnette P, Lapointe JY, Bichet DG (2007) Elaboration of a novel technique for purification of plasma membranes from Xenopus laevis oocytes. Am J Physiol 292: C1132–C1136 [DOI] [PubMed] [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Structural determinants of water permeation through aquaporin-1. Nature 407: 599–605 [DOI] [PubMed] [Google Scholar]
- Søgaard R, Alsterfjord M, Macaulay N, Zeuthen T (2009) Ammonium ion transport by the AMT/Rh Homolog TaAMT1;1 is stimula. Pflügers Archiv—Eur J Physiol (advance online publication 2 April 2009, doi:10.1007/s00424-009-0665-z) [DOI] [PubMed] [Google Scholar]
- Sui H, Han BG, Lee JK, Walian PB, Jap K (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414: 872–878 [DOI] [PubMed] [Google Scholar]
- van Hoek AN, Wiener MC, Verbavatz JM, Brown D, Lipniunas PH, Townsend RR, Verkman AS (1995) Purification and structure-function analysis of native, PNGase F-treated, and endo-beta-galactosidase-treated CHIP28 water channels. Biochemistry 34: 2212–2219 [DOI] [PubMed] [Google Scholar]
- Zardoya R (2005) Phylogeny and evolution of the major intrinsic protein family. Biol Cell 97: 397–414 [DOI] [PubMed] [Google Scholar]
- Zeuthen T, Belhage B, Zeuthen E (2006) Water transport by Na+-coupled cotransporters of glucose (SGLT1) and of iodide (NIS). The dependence of substrate size studied at high resolution. J Physiol 570: 485–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T, Wu B, Pavlovic-Djuranovic S, Holm LM, Uzcategui NL, Duszenko M, Kun JF, Schultz JE, Beitz E (2006) Ammonia permeability of the aquaglyceroporins from Plasmodium falciparum, Toxoplasma gondii and Trypansoma brucei. Mol Microbiol 61: 1598–1606 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R (2001) Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414: 43–48 [DOI] [PubMed] [Google Scholar]
- Zhou Y, MacKinnon R (2003) The occupancy of ions in the K+ selectivity filter: charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J Mol Biol 333: 965–975 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1 and S2
Review Process File