Abstract
Calpain is a ubiquitous calcium-sensitive protease that is essential for normal physiologic neuronal function. However, alterations in calcium homeostasis lead to persistent, pathologic activation of calpain in a number of neurodegenerative diseases. Pathologic activation of calpain results in the cleavage of a number of neuronal substrates that negatively affect neuronal structure and function, leading to inhibition of essential neuronal survival mechanisms. In this review, we examine the mechanistic underpinnings of calcium dysregulation resulting in calpain activation in the acute neurodegenerative diseases such as cerebral ischemia and in the chronic neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, Huntington's disease, multiple sclerosis, prion-related encephalopathy, and amylotrophic lateral sclerosis. The premise of this paper is that analysis of the signaling and transcriptional consequences of calpain-mediated cleavage of its various substrates for any neurodegenerative disease can be extrapolated to all of the neurodegenerative diseases vulnerable to calcium dysregulation.
Keywords: Neurodegenerative disease, Calpain, Cerebral ischemia, Alzheimer's, Parkinson's, Huntington's, Amylotrophic lateral sclerosis, Multiple sclerosis, Prion-related encephalopathy, Excitotoxicity, Calcium
Introduction
Neurodegenerative disorders are becoming increasingly prevalent. Disorders such as cerebral ischemia, Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) manifest in various stages of adulthood and involve the dysfunction and ultimate death of neurons in the central nervous system (CNS). While the etiology underlying each disorder varies, the pathologic mechanisms, at least in part, converge on impaired intracellular calcium homeostasis, leading to activation of the cytoplasmic cysteine protease—calpain. Accordingly, calpain inhibition is neuroprotective in most models of neurodegeneration [1-3].
This review examines the role of calpain in the pathogenesis of neurodegenerative diseases. The mechanisms leading to calcium homeostasis dysregulation identified thus far will be discussed (Fig. 1), as will the impact of calpain-mediated proteolysis on the function of calpain substrates and their role in eventual cell death (Fig. 2). The primary tenant of this paper is that delineating the role of calpain in one neurodegenerative disease will lead to greater understanding of the core mechanisms of neuronal death in other neurodegenerative disorders and potentially to the development of viable therapeutic interventions.
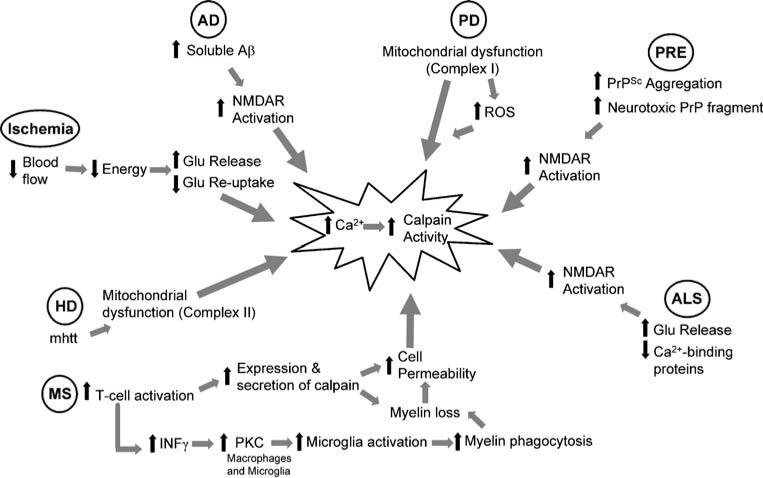
Fig. 1.
Mechanism of calpain activation in various neurodegenerative diseases. Outline of the specific mechanisms that lead to increased intracellular calcium and calpain activation. Ischemia, traumatic brain injury, and epilepsy all cause an acute increase in glutamate release resulting in increased intracellular calcium. The chronic neurodegenerative diseases AD, ALS, and PRE all result in increased NMDA receptor activation, while calcium dysregulation in HD and PD are thought to be due to mitochondrial dysfunction. MS is unique because pathologic calpain activation is initiated by T-cells and propagated by other immune cells such as macrophages and microglia. Examination of calpain activation in MS provides insight into another avenue for calpain activation in neurodegenerative diseases. Aβ amyloid β, AD Alzheimer's disease, ALS amyotrophic lateral sclerosis, Glu glutamate, HD Huntington's disease, INFγ interferon gamma, mhtt mutant huntingtin, MS multiple sclerosis, NMDAR N-methyl-d-aspartate receptor, ROS reactive oxygen species, PD Parkinson's disease, PKC protein kinase C, PRE prion-related encephalopathy, PrP prion-related peptide, PrPSc prion-related peptide, scrapie form
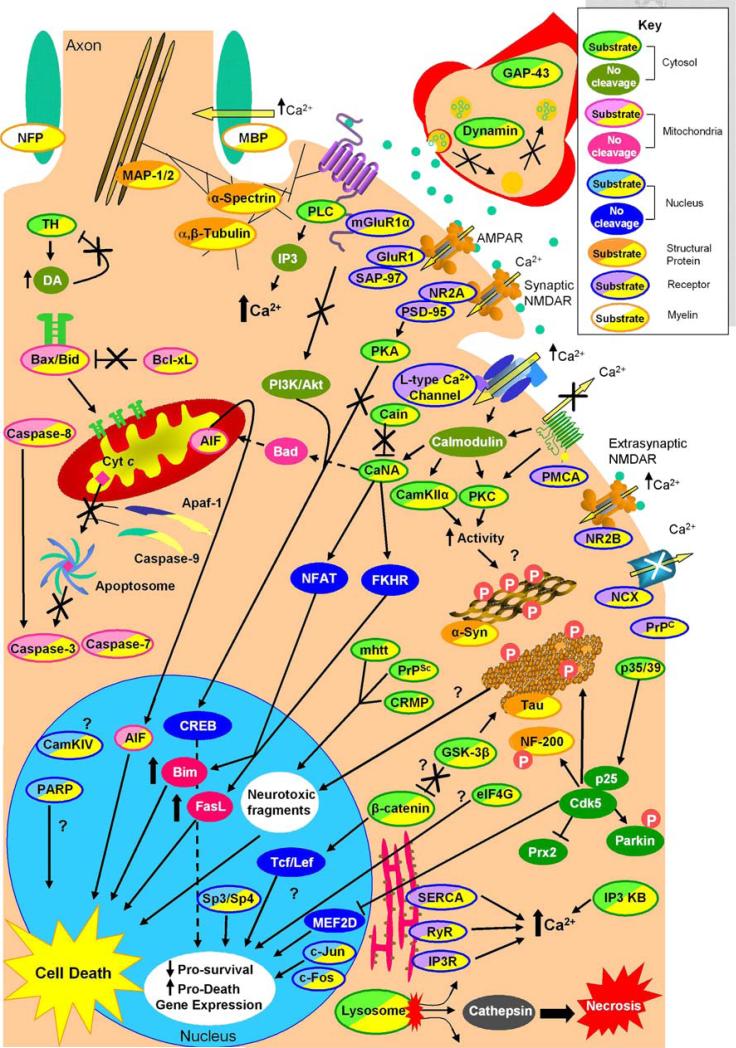
Fig. 2.
Intracellular signaling consequences of calpain-mediated substrate cleavage. Examination of the effects of calpain cleavage on its numerous substrates can be categorized by subcellular localization or protein class. Here, calpain substrates are divided into receptor, cytosolic, mitochondrial, nuclear, structural, and myelin proteins (see key). Intracellular calcium concentration is tightly regulated by membrane and endoplasmic reticulum associated receptors. Calpain propagates its own activation by blocking the calcium purging actions of PMCA and NCX, increasing calcium influx via l-type calcium channel cleavage and causing ER release of calcium via cleavage of SERCA, RyR, and IP3R. Cleavage of mGluR1α and the NMDAR NR2A subunit the C-termini uncouples both receptors from prosurvival CREB activation and potentiates increased calcium levels via increased PLC activity. Cleavage of calcium-regulated proteins such as PKC and CamKIIα also increase their activity to potentially hyperphosphorylate and cause aggregation of α-synuclein and tau. CaN A cleavage increases its activity causing dephosphorylation and translocation of transcription factors NFAT and FKHR leading to increased Bim and FasL levels. Cleavage of p35 to p25 alters Cdk5 activity, which is also involved with hyperphosphorylation of α-synuclein and tau, and also translocates to the nucleus and turns off prosurvival gene expression via MEF2D and increases ROS by inhibition of Prx2. The substrate β-catenin may also be involved with calpain induced cell death as cleavage mediates gene transcription. Finally, in the cytosol, calpain cleavage products from proteins such as mhtt, CRMP, PrPSc, and potentially tau translocate to the nucleus and are neurotoxic. Calpain also has substrates involved with mitochondria-associated programmed cell death. Calpain directly cleaves most of the caspases, Bax and Bcl-xL, and AIF, and it indirectly activates Bad via increased CaN A activation to initiate programmed neuronal death. However, calpain can also inactivate this pathway by cleaving Apaf-1 and caspase-9 in a model-dependent manner. Calpain is also active in the nucleus where it cleaves CamKIV and PARP, the transcription factors Sp3, Sp4, and the immediate early genes c-Jun and c-Fos. The effect of CamKIV and PARP cleavage has not been delineated. Sp3, Sp4, c-Fos, and c-Jun cleavage is thought to decrease housekeeping gene transcription leading to cell death; however, c-Jun-mediated transcription has also bee implicated in cell death. Lastly, structural proteins such as α-spectrin, MAP-1/2, and α- and β-tubulin are calpain substrates. Cleavage of these proteins is thought to impair microtubule transport and cell body and synaptic neuronal structures, potentially leading to neuronal death. α-Syn α-Synuclein, AIF apoptosis-inducing factor, AMPAR alpha-amino-3hydroxy-5-methyl-4-isoxazolepropionic acid receptor, Apaf-1 apoptosis-activating factor 1, CAMKIIα Ca2+/calmodulin-dependent protein kinase IIα, CAMKIV Ca2+/calmodulin-dependent protein kinase IV, CaN A calcineurin A, Cdk5 cyclin-dependent kinase 5, CREB cyclic AMP response element binding protein, CRMP collapsing response mediator protein, Cyt c cytochrome c, DA dopamine, eIF4G eukaryotic initiation factor 4G, ER endoplasmic reticulum, FasL Fas ligand, FKHR forkhead in rhabdomyosarcoma, GAP-43 growth-associated protein-43, GluR1 AMPA receptor 1, GSK-3β glycogen synthase kinase 3β, IP3 inositol triphosphate, IP3 KB inositol 1,4,5 triphosphate kinase B, IP3R inositol 1,4,5 triphosphate receptor, MAP1,2 microtubule-associated protein 1, 2, MBP myelin basic protein, mhtt mutant huntingtin, mGluR1α metabotropic glutamate receptor, NFP axonal neurofilament protein, NCX sodium–calcium exchanger, NF 200 neurofilament protein 200, NFAT nuclear factor activating T-cells, NMDA NR2A N-methyl-d-aspartate receptor NR2A subunit, NMDA NR2B N-methyl-d-aspartate receptor NR2B subunit, PI3K/Akt phosphatidyl inositol 3 kinase/protein kinase B, PLC phospholipase C, PMCA plasma membrane Ca2+ ATPase, PARP poly (ADP-ribose) polymerase, PKA protein kinase A, PKC protein kinase C, PrPC prion-related peptide, PrPSc scrapie form of prion-related protein, PSD-95 postsynaptic density-95 protein, RyR ryanodine receptor, SAP-97 synapse-associated protein-97, SERCA sarcoplasmic/endoplasmic reticulum calcium ATPase, TH tyrosine hydroxylase
Structure and Function of Calpain
Calcium-dependent protease with papain-like activity, or calpain, is a cytoplasmic cysteine protease that is activated by calcium. It is a highly evolutionarily conserved protease with homologues present in invertebrates, plants, fungi, and mammals, and it consists of 15 ubiquitous and tissue-specific isoforms in humans [4]. The catalytic subunits found in the CNS include isoforms 1, 2, 3, 5, and 10 [5]. The typical calpain isoform consists of an 80 kDa catalytic subunit and a 30-kDa regulatory subunit. The regulatory subunit possesses a hydrophobic, glycine-rich domain for membrane association. Each subunit contains an EF-hand domain, characteristic of most calcium-binding proteins [6]. For extensive reviews on the structure, tissue, and subcellular localization of calpain, see [4, 5, 7].
There are two prototypical calpains, μ-calpain, and mcalpain. Mu-calpain, or calpain I, is located in the cytosol or near the membrane and is activated by μM concentrations of calcium in vitro. In contrast, m-calpain, or calpain II, is located at the membrane and requires mM concentrations of calcium for activation. Calcium sensitivity is functionally important as physiologic concentrations of calcium range from 100−1,000 nM [8] and rise to 5−10 μM during excitotoxic conditions [9]; therefore, it is expected that μ-calpain would be affected by small changes in calcium concentrations, while m-calpain is likely activated by intracellular signaling via phosphorylation by protein kinase A (PKA) [5] and other yet undefined kinases. Following calcium stimulation, the 80-kDa subunit is autocatalytically processed to a 76-kDa fragment, and the 30-kDa regulatory subunit is processed to 18 kDa [10]. The regulatory subunit is critical for calpain activity; for example, it is necessary for embryonic development as genetic deletion of the subunit is embryonic lethal at E11.5 [11]. The requirement of m-calpain for development has also been demonstrated as it is necessary for embryo implantation [12].
Calpain activity is modulated in vivo by one known endogenous inhibitor—calpastatin. This cytosolic protein contains four calpain inhibitor domains and a consensus phosphorylation site for PKA, which increases calpastatin's specificity for m-calpain [13]. Both calpain and caspase-3 can cleave calpastatin. The calpain cleavage products retain their calpain-inhibitory activity [14], while caspase cleavage abrogates the calpain inhibition of calpastatin.
The prototypical calpain substrate is the cytoskeletal protein α-spectrin (for review, see [15]). Calpain cleaves this protein into characteristic 150 and 145 kDa fragments that are detectable by an antibody directed against α-spectrin for Western blot and immunohistochemistry [16, 17] and is stimulated by calmodulin binding of α-spectrin [18]. Thus, α-spectrin cleavage is a straightforward method to detect calpain cleavage in vitro and in vivo and has been used extensively as a quantitative measure of calpain activity. Despite numerous attempts to predict a preferential sequence of calpain cleavage [19, 20], it has been determined that calpain likely cleaves via recognition of protein secondary or tertiary structure making substrate identification unpredictable.
As calpain activity is ubiquitous, it is not surprising that it is involved in the pathogenesis of many neurodegenerative diseases. This review will now concentrate on the effect of calpain cleavage on its various substrates in specific neurodegenerative diseases. The review is organized by neurodegenerative disease and further by location of the calpain substrate. While it is acknowledged that some calpain substrates are found at multiple locations within the cell (i.e., some signaling pathways start in the cytosol and end with transcription in the nucleus), the substrates are categorized by the location of the preponderate activity of the protein once it is cleaved by calpain.
Cerebral Ischemia
Pathophysiology of Activation
Cerebral ischemia is caused by the sudden loss of cerebral blood flow to either select brain regions (focal ischemia or stroke) [21] or to the entire brain (cardiac arrest) [22]. Clinically, patients present with focal neurologic deficits such as dysphagia, hemianopia, weakness, ataxia, sensory loss, and neglect for stroke or coma, seizures, or delirium for global ischemia [21, 22]. The initial pathology is caused by energy depletion to affected brain regions resulting in diminished sodium driving force, disrupting the resting membrane potential and causing unregulated depolarization [23]. Uncontrolled release of glutamate [24], exacerbated by impaired reuptake, ultimately results in increased calcium influx through the hyperactivation of glutamate receptors and excitotoxicity [25]. Calcium dysregulation then causes the pathologic activation of calpain [26] and was shown to occur in models of acute neurodegeneration such as cerebral ischemia [27], traumatic brain injury (TBI), and epilepsy [28] (Fig. 1). Indeed, many proteins have been found to be calpain substrates following an excitotoxic insult both in vitro and in vivo (Table 1).
Table 1.
Calpain substrates: localization, functional alterations and neurodegeneration
| Substrate | Category | Location | Cleavage consequence | Disease | References |
|---|---|---|---|---|---|
| AIF | Cell death | Mitochondria/nucleus | Released from the mitochondria—translocates to the nucleus | Ischemia | [146, 267] |
| α-synuclein | Cytoskelatal protein | Cytosol | Promotes aggregation | Ischemia, PD | [147, 148, 150] |
| Apaf-1 | Cell death | Cytosol | No longer able to activate caspase cascade | Ischemia | [268] |
| APP | Membrane protein | Membrane/cytosol | Putative alpha secretase necessary for normal processing of APP | AD | [84-86] |
| Bax | Cell death | Cytosol/mitochondria | Promotes insertion into mitochondria and subsequent cytochrome c release | PD | [269, 270] |
| Bcl-xL | Cell death | Cytosol | Becomes proapoptotic | Ischemia, AD | [271] |
| Bid | Cell death | Cytosol/mitochondria | Promotes mitochondria permeability transition and release of proapoptotic factors | Ischemia | [272] |
| Cain/cabin1 | Calcium signaling | Cytosol | Calcineurin inhibitor—cleavage increases calcineurin activity | Ischemia | [273] |
| Cadherin | Cytoskelatal protein | Extracellular/cytosol | Disrupts extracellular ligand binding and intracellular signaling consequences | [274] | |
| Calpastatin | Calcium signaling | Cytosol | Retains calpain inhibitory activity | [14] | |
| CaMKIIα | Calcium signaling | Cytosol (synapse) | Removes calmodulin regulation; increases kinase activity | Ischemia | [100, 275] |
| CaMKIV | Calcium signaling | Nucleus | Reduces kinase activity decreasing CREB phosphorylation and activation | Ischemia | [30, 56] |
| CaN A | Calcium signaling | Cytosol | Removes calmodulin regulation—constitutively active phosphatase | Ischemia, AD | [60, 276] |
| Caspase-3 | Cell death | Cytosol | Cleaves into an inactive fragment | Ischemia | [30, 277] |
| Caspase-7 | Cell death | Cytosol | Cleaves into an inactive fragment | Ischemia | [278, 279] |
| Caspase-8 | Cell death | Cytosol | Truncated form unable to activate caspase-3 | Ischemia | [278] |
| Caspase-9 | Cell death | Cytosol | Truncated form unable to activate caspase-3 | Ischemia | [278, 280] |
| Caspase-12 | Cell death | Cytosol | Converts proform to active form | Ischemia, AD | [271, 281] |
| Caspase-14 | Cell death | Cytosol | Converts proform to active form | Ischemia | [282] |
| β-Catenin | Transcription factor | Cytosol/nucleus | Translocates to the nucleus; induces gene transcription | Ischemia | [105] |
| Catenin (p-120) | Cytoskeletal protein | Cytosol/membrane | Decreased cell aggregation | Ischemia | [283] |
| c-Fos | Immediate early gene | Nucleus | Decreased transcription | Ischemia, PD | [163] |
| c-Jun | Immediate early gene | Nucleus | Decreased transcription | Ischemia, PD | [163] |
| CRMP 1−5 | Cytosolic protein | Cytosol/nucleus | Truncated N terminus translocates to nucleus and is neurotoxic | Ischemia | [67, 68] |
| Dynamin 1 | Synaptic protein | Cytosol | Inhibits synaptic vesicle recycling leading to LTP/memory impairments | AD | [87] |
| eIF4G | Translation | Cytosol | Inhibits translation | Ischemia | [61, 62] |
| GAP-43 | Synaptic protein | Cytosol | Inhibits/regulates m-calpain activity | Ischemia, TBI, AD | [284, 285] |
| GFAP | Cytoskeletal protein | Cytosol | Unknown | PRE | [245] |
| GluR1 | Receptor | Membrane | C-terminal cleavage—suppression of AMPA current | Ischemia | [53, 286] |
| GSK-3β | Cytosolic enzyme | Cytosol | Increases kinase activity phosphorylation of tau | Ischemia, AD | [103] |
| htt, mhtt | Cytosolic protein | Cytosol | N-terminal fragments aggregate and translocate to nucleus | HD | [189, 190] |
| IP3 Kinase B | Cytosolic enzyme | Membrane/cytosol | Separates membrane-anchoring domain from kinase domain | Ischemia | [46, 287] |
| IP3R | Calcium signaling | ER | Reduces binding of the IP3 ligand | Ischemia | [45] |
| Kyotorphin | Neuropeptide | Cytosol | Produced by calpain cleavage of calpastatin; involved with analgesia | [288, 289] | |
| l-type calcium channel | Calcium signaling | Membrane | Removes phosphorylation site increasing calcium-influx | Ischemia | [40, 41] |
| Lysosomes | Protein degradation | Cytosol | Cleaves membrane releasing proteases leading to necrosis | Ischemia | [32, 33] |
| MAP-1 | Cytoskeletal protein | Cytosol | Likely alters microtubule transport and dendritic and synaptic structure | Ischemia | [290] |
| MAP-2 | Cytoskeletal protein | Cytosol | Likely alters microtubule transport and dendritic and synaptic structure | Ischemia | [26, 291] |
| mGluR1α | Receptor | Membrane | C-terminal cleavage—retains calcium transmission; uncouples from prosurvival Akt signaling | Ischemia | [55] |
| Myelin-associated glycoproteina | Myelin protein | Extracellular | Demyelination, axonal degeneration | MS | [214] |
| Myelin basic proteina | Myelin protein | Extracellular | Demyelination, axonal degeneration | MS | [213, 214] |
| NCX | Membrane protein | Membrane | Inhibits calcium-clearing from cytosol | Ischemia | [39] |
| NF200, NF160, and NF68 | Cytoskeletal protein | Cytosol | Cleavage potentially causes NF inclusions; may be phosphorylation “sink” for Cdk5/p25 | ALS, TBI, Ischemia | [255, 256, 260, 292] |
| NFPa | Myelin protein | Extracellular | Demyelination, axonal degeneration | MS | [213, 214] |
| nNOS | Cytosolic enzyme | Cytosol | Decreased activity | Ischemia | [275] |
| NMDA NR2A subunit | Receptor | Membrane | Uncouples synaptic NMDAR from prosurvival cascade | Ischemia | [47, 49] |
| NMDAR NR2B subunit | Receptor | Membrane | Uncouples extrasynaptic NMDAR from cell death pathway | Ischemia | [49, 50, 293] |
| p35/p39 | Cytosolic enzyme | Cytosol/nucleus | Produces stable p25/p29 fragments, which bind CDK5 and translocate to the nucleus | Ischemia, AD, PD, HD, ALS | [111, 112] |
| PLCβ | Cytosolic enzyme | Cytosol | Enhanced activation | Ischemia | [294-296] |
| PMCA | Membrane protein | Membrane | Inhibits calcium-clearing from cytosol | Ischemia | [38] |
| PARP | DNA repair | Nucleus | Inhibits activity leading to increased DNA fragmentation | Ischemia, AD | [30, 297] |
| PKA | Cytosolic enzyme | Cytosol | Decreased CREB activation; blocks inhibition of PKA of m-calpain | AD | [91, 92] |
| PKC | Calcium signaling | Cytosol | Produces a catalytically active fragment | Ischemia, AD, MS | [99, 298-300] |
| PrPC | Membrane protein | Membrane | Physiologic processing | PRE | [239] |
| PrPSc | Prion protein | Cytosol | Produces neurotoxic C2 cleavage product | PRE | [237] |
| PSD-95 | Structural protein | Cytosol (synapse) | Alters anchoring and signaling of NMDA receptors | Ischemia | [47, 237, 301] |
| Ryanodine receptor | Receptor | ER | Increase in release of ER calcium stores into cytosol | Ischemia | [42] |
| SAP-97 | Structural protein | Cytosol (synapse) | Decreases AMPA receptor levels | Ischemia | [302] |
| SERCA | Calcium signaling | ER | Degrades/ATPase activity uncoupled from calcium uptake | Ischemia | [43, 44] |
| Sp3 and Sp4 | Transcription factor | Nucleus | Decreased survival-dependent transcription | Ischemia | [65] |
| Spectrin | Structural protein | Membrane | Hallmark of calpain activity; cleavage likely alters membrane structure and function | Ischemia, AD, PD, HD, ALS, MS, PRE | [16, 17] |
| Tau | Cytoskeletal protein | Cytosol | Cleavage inhibited by PKA; formation of 17 kDA neurotoxic fragment | Ischemia, AD | [121, 303, 304] |
| Tubulin (α, β) | Cytoskeletal protein | Cytosol | Likely alters microtubule transport and dendritic and synaptic structure | Ischemia | [305] |
| Tyrosine Hydroxylase | Cytosolic enzymes | Cytosol | Proteolysis of N-term increases activity by eliminating feedback inhibition | PD | [130] |
AIF Apoptosis-inducing factor, AMPA alpha-amino-3hydroxy-5-methyl-4-isoxazolepropionic acid, Apaf-1 apoptosis-activating factor 1, APP amyloid precursor protein, CAMKIIα Ca2+ /calmodulin-dependent protein kinase IIα, CAMKIV Ca2+ /calmodulin-dependent protein kinase IV, CaN A calcineurin A, Cdk5 cyclin-dependent kinase 5, CREB cyclic AMP response element binding protein, CRMP 1−5 collapsing response mediator protein 1−5, eIF4G eukaryotic initiation factor 4G, ER endoplasmic reticulum, GAP-43 growth-associated protein-43, GFAP glial fibrillary acidic protein, GluR1 AMPA receptor 1, GSK-3β glycogen synthase kinase 3β, htt/mhtt huntingtin/mutant huntingtin, IP3 Kinase B inositol 1,4,5 triphosphate kinase B, IP3R inositol 1,4,5 triphosphate receptor, LTP long-term potentiation, MAP1,2 microtubule-associated protein 1, 2, mGluR1α metabotropic glutamate receptor, NFP axonal neurofilament protein, NCX sodium–calcium exchanger, NF 200, 160, 68 neurofilament protein 200, 160, 68, nNOS neuronal nitric oxide synthase, NMDA NR2A N-methyl-d-aspartate receptor NR2A subunit, NMDA NR2B N-methyl-d-aspartate receptor NR2B subunit, PLCβ phospholipase Cβ, PMCA plasma membrane Ca2+ ATPase, PARP poly (ADP-ribose) polymerase, PKA protein kinase A, PKC protein kinase C, PrPC prion-related peptide, PrPSc scrapie form of prion-related protein, PSD-95 postsynaptic density-95 protein, SAP-97 synapse-associated protein-97, SERCA sarcoplasmic/endoplasmic reticulum calcium ATPase, AD Alzheimer's disease, ALS amyotrophic lateral sclerosis, HD Huntington's disease, MS multiple sclerosis, PD Parkinson's disease, PRE prion-related encephalopathy, TBI traumatic brain injury
All major myelin proteins are calpain substrates [213]
Initially, calpain activation was thought to cause only necrotic cell death, while activation of caspases led to programmed cell death. However, calpain does play a role in apoptotic cell death in mixed glial and primary cortical neuronal cultures following oxygen–glucose deprivation (OGD) that is just as important as caspases [29]. Moreover, calpain has been shown to cleave and activate caspase-3 following maitotoxin treatment [30] and OGD [31]. The role of calpain in necrotic versus apoptotic cell death is still not without controversy. The calpain–cathepsin hypothesis posits calpain activation due to excitotoxic stimulus disrupts lysosomal membranes. This is ensued by the release of the lysosomal proteases, including cathepsins, the breakdown of cellular proteins, and, ultimately, necrosis [32, 33]. In support of this model, the sequential activation of calpain, cathepsins, and caspases occurs in a model of focal ischemia [34], while the administration of E64d, a combined μ-calpain–cathepsin B inhibitor, decreases infarct volume, edema, and neurologic deficit following focal ischemia in rats [35, 36].
A large number of proteins are cleaved by calpain following an ischemic or excitotoxic insult (for reviews, see [4, 8, 28, 37]). A comprehensive list of calpain substrates found in neurons thus far is shown in Table 1. Here, we examine recently identified, novel substrates, and new signaling consequences of calpain stimulation.
Membrane and Receptor
Briefly, calpain activation following excitotoxic insult positively regulates its own activity via cleavage of a number of proteins involved the homeostatic control of intracellular calcium. Calpain cleavage of the plasma membrane calcium ATPase (PMCA) [38], sodium–calcium exchanger (NCX) [39], l-type calcium channel [40, 41], ryanodine receptor (RyR) [42], sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) [43, 44], and inositol (1, 4, 5) triphosphate receptor [45] and inositol triphosphate kinase B [46] all result in the maintenance of elevated intracellular calcium levels. Cleavage of the C-termini of NMDA [47-50] and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) [51-53] receptor subunits results in decreased glutamatergic transmission through these channels by disabling downstream signaling mechanisms [54]. The effect of cleavage on calcium transmission, if any, remains unknown, as the proteins forming the calcium channel are not altered by calpain. Similar to calpain mediated C-terminal truncation of the metabotropic glutamate receptor mGluR1α disrupting coupling to the prosurvival PI3K–Akt pathway [55], cleavage of the C terminus of the NMDA NR2A subunit, and postsynaptic density (PSD) 95 uncouples synaptic NMDA receptors from CREB-mediated prosurvival expression [47].
Cytosol
Calpain also cleaves key cytosolic enzymes involved with calcium homeostasis such as Ca2+/calmodulin-dependent protein kinase (CaMK) type IV [56] and the protein phosphatase calcineurin (CaN) A [5]. Although the effect of CaMKIV cleavage on neuronal death has not been determined, regulation of CaN A has been examined in ischemia due to the neuroprotective effects of the CaN inhibitor FK506 [57]. This may be due to blocking the dephosphorylation and activation of the proapoptotic protein Bad by CaN A [58]. Calpain cleavage of CaN A also results in increased phosphatase activity leading to the translocation of the transcription factors nuclear factor of activated T-cells (NFAT) and forkhead in rhabdomyosarcoma (FKHR) and increased expression of proapoptotic Bim and Fas-ligand [59, 60]. Further experiments are necessary to determine if inhibition of the downstream effectors of CaN A can provide neuroprotection.
Calpain may also cleave eukaryotic initiation factor 4G (eIF4G), a scaffolding protein critical for the delivery of mRNA cap-binding protein to the ribosome for translation. Decreases in eIF4G levels were demonstrated to be calpain-mediated following cardiac arrest, as the pharmacological calpain inhibitor, MDL-28,170, abrogated diminished protein levels [61]. Calpain-mediated decreases in eIF4G were also shown following NMDA toxicity in primary neuronal culture as the calpain inhibitor calpeptin also blocked reductions in eIF4G protein levels [62]. Reduced eIF4G levels were correlated with inhibition of protein synthesis; however, in this model, although calpain inhibition was neuroprotective, it did not restore protein synthesis, leaving the significance of eIF4G reduction for neuronal survival in excitotoxicity unknown. Further studies are necessary to definitively show that eIF4G is a direct substrate of calpain as decreased protein levels could be indirectly linked to calpain via release of cathepsins or due to proteasomal degradation.
Nuclear
Calpain can alter the availability of a number of transcription factors, including those of the Sp-family proteins. These transcription factors regulate the expression of “housekeeping genes” and are critical for development and survival [63]. Following glutamate exposure, a decrease in Sp transcription occurs in cortical neurons [64] that is due to calpain cleavage of Sp3 and Sp4 [65]. The direct effects of diminished Sp-mediated transcription are unknown, but it has been suggested that they may mediate decreased expression of the NR1 subunit following NMDA-mediated excitotoxicity [66].
Pathologic proteolytic activation of calpain also occurs with the N-terminal cleavage of the collapsing response mediator proteins 1−5 (CRMP 1−5) [67, 68]. Nuclear translocation of CRMP N-terminal cleavage products has been implicated in neuronal death [67] and with the alteration of NMDA NR2B subunit expression at the neuronal surface [69]. Calpain-mediated increased activity of cyclin-dependent kinase 5 (Cdk5; the mechanism is discussed in greater detail below) following excitoxicity leads to phosphorylation of the NMDA NR2A subunit and increased neuronal death [70]. Overactivation of Cdk5 has also been implicated with the accumulation of hyperphosphorylated tau in ischemic areas [71].
Thus, the acute insult of cerebral ischemia rapidly overactivates glutamate receptors, sharply increases intracellular calcium concentrations, and results in pathological calpain activation. Calpain potentiates its own activation via cleavage of calcium regulatory proteins at the membrane (l-type calcium channels, PMCA, and NCX) and ER (RyR, SERCA, and IP3R) resulting in further increases in intracellular calcium. Calpain-mediated cleavage uncouples NMDAR from prosurvival cascades (PI3K/Akt and PKA) and propagates mitochondrial dysfunction (Fig. 2). Another consequence of ischemia-induced calpain activation is alteration of transcription through direct cleavage of transcription factors (Sp3 and Sp4) or through modification of phosphatase (CaN A) and kinase (Cdk5) activity. Finally, calpain-mediated CRMP N-terminal cleavage product translocation to the nucleus results in neuronal death—a phenomenon shared by many of the neurodegenerative diseases discussed below. Calpain activation due to cerebral ischemia is the most extensively studied and thus provide a foundation for the examination of discovered calpain substrates in other neurodegenerative diseases.
Alzheimer's Disease
Pathophysiology of Activation
Alzheimer's disease is clinically manifested by progressive dementia accompanied by neuronal death. The underlying neuronal histopathology consists of neurofibrillary tangles (NFTs) comprised of hyperphosphorylated collections of the microtubule-associated protein tau [72] and senile plaques made up of aggregated amyloid β-protein (Aβ) fibrils [73]. Amyloid β is produced by the cleavage of the integral membrane protein amyloid precursor protein (APP) by the sequential action of β- and γ-secretases [74]. Under physiologic conditions, APP is processed by a yet undefined α-secretase into a product of unknown function. Mounting evidence has demonstrated that increased levels of soluble Aβ is the primary cause of neuronal pathology in AD [75, 76]. Higher concentrations of soluble Aβ result in higher intracellular calcium levels via NMDA receptor activation [77-79]. Calcium dysregulation, in turn, leads to increased calpain activation which has been shown in post mortem human AD patients’ brains [80-82] (Fig. 1).
Membrane and Receptor (Physiologic Activation)
Calpain has been hypothesized as a candidate α-secretase responsible for the normal processing of APP [83-85]. Using siRNA directed against μ-calpain decreased overall phorbol ester-induced APP release with concomitant increase in Aβ production [86]. It would seem then that calpain is critical for normal APP processing. It is thus essential to understand the full temporal dynamics underlying the role of calpain in AD pathology. Based on this data, there could be an initial, subclinical decrease in calpain activity that alters APP processing toward the production of Aβ. The subsequent rise in Aβ concentration could then result in NMDA-receptor activation, leading to hyperactivation of calpain and the negative consequences unmasked by persistent calpain activity. Therefore, it is not sufficient to simply block calpain activity to provide therapeutic benefit, as this could lead to greater Aβ production.
Cytosol
The critical facet in mediating neurotoxicity in AD is how increased intracellular calcium-induced activation of calpain results in AD pathology. Examination of calpain substrates has identified a role for calpain in both the pathology and in the memory and cognitive impairment associated with the disease. Recently, it was discovered that Aβ application to primary hippocampal cultures resulted in the depletion of a synaptic vesicle recycling protein, dynamin 1, in an NMDA receptor- [79] and calpain-dependent manner [87]. Calpain-mediated dynamin depletion in the absence of overt neuronal or synaptic loss was corroborated in vivo using the AD mouse model Tg2576 [87]. These studies concluded that impairments in synaptic vesicle recycling due to dynamin 1 depletion could impair long-term potentiation and may play a role in the cognitive decline seen in AD patients [87]. This conclusion is controversial, however, because a triple transgenic mouse model of AD [88] failed to show a decrease in dynamin 1 levels or decreases in synaptic vesicle recycling despite AD-like pathology and abnormal synaptic plasticity [89].
An additional mechanism whereby pathologic calpain activation may produce cognitive and memory impairment is via decreases in cAMP-dependent protein kinase (PKA). PKA plays a prominent role in the activation of cAMP response element binding protein (CREB), an important transcription factor involved in the conversion of short-term to long-term memory [90]. Expression of both PKA regulatory subunits RIIα and RIIβ and the catalytic subunit Cβ are diminished in AD [91]. These lower levels of PKA should attenuate CREB activation and result in impaired memory. It must also be noted that PKA negatively regulates m-calpain via phosphorylation [92]; thus, calpain-mediated decreases in PKA activity will further enhance calpain activity. This positive feedback favoring further calpain activation may produce persistent calpain activity.
Calpain overactivation due to Aβ-induced calcium dysregulation may compliment the decrease in CREB activation via cleavage of protein phosphatase (PP) 2B or calcineurin A (CaN A) in the cytosol [5]. Calpain cleaves subunit A, the autoinhibitory domain of CaN A in vitro [93, 94], and in AD brain leading to a twofold increase in CaN A activity [95]. The activation of CaN A is also correlated with the number of NFTs [95], further suggesting a role of CaN A in the underlying pathogenesis of AD. CaN A inactivates inhibitor of protein phosphatase 1 (PP1) by dephosphorylation [96], which leads to increased PP1 activity and subsequent dephosphorylation and further inactivation of CREB.
Hyperphosphorylation and subsequent aggregation of tau protein is another hallmark of AD pathology [97, 98]. Calpain may mediate altered phosphorylation through activation of specific kinases. Tau hyperphosphorylation occurs concurrent with activation of the mitogen-activated protein kinases Erk 1/2 and calpain in AD but not normal brains [82]. Treatment of primary neurons with the calcium ionophore ionomycin recapitulated this phenomenon in vitro, demonstrating that Erk 1/2 activation and subsequent tau hyperphosphorylation was calpain dependent [82]. Calpain can also cleave protein kinase C (PKC) and calcium/calmodulin kinase (CamK) IIα, resulting in increased kinase activity [99, 100] that may further contribute to tau hyperphosphorylation via downstream PKC-substrate activation [101].
Glycogen synthase kinase (GSK) 3 is another kinase that mediates tau hyperphosphorylation [102] and has been shown to be a substrate of calpain [103]. Calpain cleaves the inhibitory domain of GSK-3β, thus increasing its kinase activity [103]. Overactivation of GSK-3β may contribute directly to AD pathology via tau hyperphosphorylation or since it plays a role in many key cell regulatory processes, it may contribute to neuronal death through the β-catenin pathway [104].
Nuclear
Modulation of GSK-3β substrates by calpain also occurs. Calpain can cleave β-catenin at its N terminus, removing its GSK-3β phosphorylation site, and lead to increased nuclear translocation and Tcf/Lef transcription [105]. While dysregulated β-catenin/Tcf pathway has been implicated in AD [106] and carcinogenesis [107], calpain cleavage of β-catenin and Tcf pathway activation is involved in the exploratory behavior in mice [105]. Further research is required to delineate the physiologic and pathologic roles of β-catenin and GSK-3β cleavage by calpain.
Pathological calpain activity in AD and AD models also results in the activation of other kinases involved in the hyperphosphorylation of tau. One kinase targeting the nucleus that has been extensively studied in AD is Cdk5 (for review, see [108, 109]). Cdk5 is physiologically activated by p35 [110]; however, under conditions of calcium dysregulation and calpain activation, p35 is cleaved to form p25 [111, 112]. This truncated form of p35 does not contain the membrane localization sequence of p35 and has a longer half-life, leading to increased activity often in abnormal subcellular locations, including the nucleus [112]. Increased levels of p25 have been found in AD post mortem brain tissue [112], suggesting that Cdk5 plays a role in the pathogenesis of AD. Furthermore, using in vitro models of AD applying Aβ to primary neurons activates calpain, p25 production, and hyperphosphorylation of AD-specific epitopes of tau [111, 113-115].
In a model of endoplasmic reticulum stress, calpain-dependent cleavage of p35 to p25 results in the translocation of Cdk5/p25 complexes to the nucleus, increasing histone H1 kinase activity [116]. Aberrant Cdk5 activation within the nucleus was hypothesized to activate cell cycle promoting factors, a phenomenon thought to cause cell death in terminally differentiated neurons [117]. The use of a dominant negative form of Cdk5 that inhibits the Cdk5/p25 but not the Cdk5/p35 complex protected cells from ER stress [116]. Alternatively, Cdk5 inhibitors, but not other cell cycle inhibitors, were demonstrated to diminish neuronal death by blocking mitochondrial dysfunction [118]. The pathological activity of Cdk5 is complex as hyperactivation has consequences within both the cytosol and nucleus. Thus, the exact mechanism of Cdk5-induced neuronal death remains controversial, albeit aberrant activation due to p35 cleavage to p25 by calpain is generally accepted.
Phosphorylation state of tau may not be linked to tau-induced cell death [119, 120]. In support of this, it was discovered that Aβ exposure to primary hippocampal neurons resulted in the calpain-mediated cleavage of tau into a toxic 17 kDa fragment—an occurrence that preceded Aβ-induced tau phosphorylation [121]. Overexpression of tau fragments (1−441) or (1−44) in primary neurons also resulted NMDAR-mediated, calpain-dependent activation of Erk 1/2 and neuronal death in primary neuronal culture [122]. Indeed, the tau fragments that induced cell death do not have AD-specific phosphoepitopes. Together, these results indicate that calpain not only contributes to AD pathogenesis indirectly via activation of a number of important kinases but it is also directly involved in AD neuronal death by creating a neurotoxic tau fragment. The subcellular localization of the neurotoxic tau fragment has not been determined, but the nuclear translocation of other calpain-induced fragments is associated with neuronal toxicity. The tau fragment produced by calpain may be functioning in a similar manner. Further research is necessary to delineate the full role of calpain in the pathogenesis of AD and the downstream effects of the kinase substrates of calpain on eventual neuronal demise.
Parkinson's Disease
Pathophysiology of Activation
Parkinson's disease is a neurodegenerative disease afflicting greater than 1% of the population aged 65 years and older. The clinical signs and symptoms are comprised of progressive bradykinesia, rigidity, resting tremor, and dementia [123]. The hallmark pathology associated with PD is the formation of fibrillar α-synuclein-containing inclusions called Lewy bodies along with the selective death of dopaminergic (DA) neurons in the substantia nigra (SN), the locus coeruleus, and the ventral tegmental area, which is thought to produce the aforementioned clinical motor symptoms, and eventual loss of cortical matter ending with dementia [124]. The etiology of PD remains largely unknown as most of the cases presented are sporadic, with only 5−10% of patients with PD possessing known PD-associated genetic mutations [125]. Currently, there are 13 known genetic loci that have been implicated in inherited PD [125], and these gene products have provided researchers insight into the complex pathophysiology fundamental to PD. As will be discussed in this section, calpain activation is implicated with two of these loci, Parkin and α-synuclein, along with other potential mediators of dopaminergic cell death.
The pathophysiology underlying the selective susceptibility of DA neurons to degeneration in PD is at least in part due to mitochondrial dysfunction, leading to increased free radical production and higher intracellular calcium concentrations [126]. Catecholaminergic neurons are more vulnerable to excitotoxic stress due to a paucity of the calcium binding proteins calbindin and calretinin [127, 128]. The lack of these proteins would allow for activation of calpain at lower calcium levels. Indeed, increased mcalpain expression was found in the fibers of mesencephalic neurons of patients with PD, with the highest concentration in the SN. Moreover, m-calpain colocalized with neurons positive for tyrosine hydroxylase, the rate limiting step in catecholaminergic synthesis, and was present within Lewy bodies [129].
Interestingly, tyrosine hydroxylase is a substrate of calpain. Calpain proteolysis of the N terminus of this key enzyme in DA synthesis increases its activity by eliminating feedback inhibition [130]. Therefore, cleavage of tyrosine hydroxylase leads to increased DA production under normal physiologic conditions; however, persistent, pathologic activation of calpain might cause production of dopamine to toxic levels [131, 132]. Furthermore, it was determined that overexpression of mutant α-synuclein sensitized cells to dopamine toxicity [132], and α-synuclein itself can reduce the activity of tyrosine hydroxylase [133]. Thus, α-synuclein aggregation may allow calpain activation of tyrosine hydroxylase to dominate and result in dopamine overproduction.
The endogenous inhibitor of calpain, calpastatin, was also examined in PD patient brains to determine if there was a loss of calpastatin expression which might account for the increase in calpain activation. This was found not to be the case, as there was no correlation of calpastatin immunoreactivity and neuronal vulnerability to PD [134].
Using these findings as a starting point, examination of calpain activation continued in PD models. Administration of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to nonhuman primates and humans resulted in the selective lesion of the nigrostriatial pathway mimicking the signs and symptoms of severe PD [135, 136]. The blood brain barrier (BBB) permeable MPTP is oxidized to its active metabolite 1-methyl-4-phenylpyridinium ion (MPP+) by monoamine oxidase B [137]. Once in the cell, MPP+ was found to irreversibly inhibit mitochondrial respiratory chain complex 1 resulting in decreased ATP levels [138], increased reactive oxygen species [139], and sustained increases in cytoplasmic calcium [140]. Determining the mechanism of MPTP-induced toxicity has provided great insight into the pathophysiology underlying PD (Fig. 1).
Based on increased calcium concentrations induced by MPTP, it was hypothesized that calpain may be activated and involved in the neuronal death observed following MPTP administration. The loss of TH-immunoreactive cells in the mouse striatum following MPTP exposure was abrogated by administration of a pharmacological calpain inhibitor MDL-28170 or using an adenoviral vector expressing calpastatin, suggesting calpain-mediated DA neuron death [141]. Moreover, calpain inhibition resulted in increased motor activity compared to MPTP-only treated mice. The authors also examined the effect of MPTP and calpain inhibition following MPTP administration on neurotensin expression. Neurotensin potentially blocks DA neurotransmission [142] and has been demonstrated to increase following MPTP administration in mice [143] and in human postmortem PD brain [144]. Calpain inhibition following MPTP treatment abrogated the increase in neurotensin expression caused by MPTP alone [141]. Finally, the authors observed an increase in calpain cleaved α-spectrin levels in post mortem PD brains. Taken together, it seems that calpain is likely to be activated following MPTP administration in mice and paralleled in the PD brain.
Cytosol
Using neuronally differentiated PC-12 cells to examine the mechanism behind MPTP-induced calpain activity has shown that activation of c-Jun N-terminal kinase and c-Jun increases BimEL expression [145]. BimEL interacts with other Bcl-2 family members to facilitate the proapoptotic proteins Bax and Bak in disrupting mitochondrial membrane permeability. In turn, disrupted mitochondria release cytochrome c and alter calcium homeostasis leading to caspase and calpain activation, respectively. Increased mitochondrial calpain activity has been shown to cleave apoptosis inducing factor (AIF) near its N terminus, releasing it from the mitochondria and inducing cell death [146].
One specific calpain substrate implicated in PD pathology is α-synuclein. Calpain has been demonstrated to cleave fibrillated α-synuclein in the C-terminal region in vitro [147, 148], generating fragments that promote aggregation into putative Lewy bodies. These fragments are implicated in DA neuron death via induction of oxidative stress shown in vitro [149], in vivo [150, 151], and in postmortem PD brain [149, 150]. There is some controversy as to the role α-synuclein cleavage plays, since calpain-mediated cleavage of the N-terminal region removes the nonamyloid component and prevents fibrillation, and this would be thought to be protective [147, 148]. Upon examination of the fragments present in α-synuclein aggregates in vivo [150, 151] and in postmortem PD brain [149, 150], there is a prevalence of C-terminal truncated α-synuclein, suggesting this is the neurotoxic and predominate form of α-synuclein found in Lewy bodies in PD brain.
A potential physiologic mechanism of α-synuclein cleavage by calpain was shown in an embryonic hippo-campal cell line where overexpression of Parkin, a ubiquitin-protein ligase E3 [152, 153], diminished α-synuclein-induced cell death via calpain activation [154]. However, Parkin is one of the PD-related genetic mutations [125], and decreased activity of this enzyme would suggest decreased physiologic calpain activation leading to α-synuclein aggregation, increasing oxidative stress in neurons leading to excitotoxicity and pathologic calpain activation.
Calpain activation is also activated in both the rotenone and the 6-hydroxy dopamine (6-OHDA) models of PD. Rotenone, a specific and irreversible inhibitor of mitochondrial complex 1, activates calpain prior to caspase activation and increases phosphorylation of the tumor suppressor gene p53 in mouse cortical neurons [155]. Increased phospho-p53 did not increase expression of cell cycle proteins indicating that calpain did not induce cell death via cell cycle activation [156]; however, it has been shown that following DNA damage calpain inhibition blocked activation of p53 and subsequent cytochrome c release and caspase activation [157]. Thus, calpain acts upstream of p53- and caspase-mediated neuronal death. It should be mentioned that p53 has been shown to be a substrate of calpain in MCF-7 human breast carcinoma cell line [158], but this has not yet been demonstrated in neurons.
Rotenone exposure to rats in vivo also activates calpain and induces greater than 90% of motor neuron death in spinal cord [159, 160]. Exposure to hydrogen peroxide, known to be induced by 6-OHDA [161], activated caspase-3 in a calpain-dependent manner [162]. This demonstrates that calpain activation occurs along not just one but several mechanisms in various models of PD.
Nuclear
Calpain in the nucleus can also cleave the transcription factors c-Jun and c-Fos [163], and blocking c-Jun activation has been shown to be protective in MPTP model of PD [164]. Thus, while calpain is involved in the pathology of PD, under physiologic conditions, it may also function to mitigate the transcription of messages detrimental to the cell.
In the MPTP PD model, as was found for AD, calpain cleaves p35 to p25, resulting in the abnormal activation of Cdk5 [165, 166]. Decreased MPTP-induced DA cell death and diminished behavioral deficits were seen using p35 deficient mice [166]. Furthermore, the authors demonstrated that the transcription factor involved with transcription of survival signals, myocyte enhancing factor 2D (MEF2D), was phosphorylated and inactivated by Cdk5; blocking MEF2D phosphorylation by overexpressing a nonphosphorylatable mutant also blocked MPTP-induced TH-positive neuron loss [166]. It has also been shown that Cdk5/p25 can phosphorylate Parkin decreasing autoubiquitilation and promoting α-synuclein inclusions [167]. Calpain cleavage of p25 with subsequent Cdk5 overactivation also results in phosphorylation of peroxiredoxin 2 (Prx2), an antioxidant with peroxidase activity [165]. Identified via p35 binding and mass spectrometry, Prx2 was found to be phosphorylated by Cdk5, an event that diminishes its activity. Accordingly, downregulation of Prx2 activity resulted in increased oxidative stress and cell death due to MPTP exposure, and p35 knockout mice showed decreased oxidative stress [165]. Substantiating this finding, phosphorylated Prx2 is increased in the SN of PD brains [165], and complexes of Cdk5/p25 have also been found in post mortem PD brains in the cingulate gyrus [168]. Further confirming the pathological role of calpain/Cdk5/p25, it was shown that blocking Cdk5 activity using melatonin [169] or the broad spectrum Cdk inhibitor, flavopiridol, blocked MPTP-induced cell death in rat cerebellar granule neuron culture.
The equilibrium underlying normal, regulated calpain activation and its persistent pathologic activation is evident in the mechanisms affecting nuclear factors. When combined with the cytosolic effects, calpain is a potent mediator of pathologic events in PD brain. Furthermore, modulation of the membrane or receptors by calpain in PD has not yet been examined, thus providing an additional topic to investigate in PD.
Huntington's Disease
Pathophysiology of Activation
The underlying etiology of Huntington's disease, an autosomal dominant neurodegenerative disorder, is a mutation resulting in the expansion of the polyglutamine (polyQ) region in the N terminus of huntingtin (htt) protein [170]. Despite having the mutation in their entire lives, patients tend to manifest signs and symptoms beginning in the fourth decade of life consisting of involuntary movement, emotional disturbances, and dementia [171]. Currently, the exact mechanism whereby mutated htt causes neuronal death has yet to be determined. The fundamental pathology of HD is the selective loss of medium spiny projection neurons in the striatum [172] and cortical projection neurons in layers V and VI [173].
One hypothesis concerning the mechanism of mutant htt (mhtt) causing neuronal death is that mhtt induces mitochondrial defects through the selective inhibition of mitochondrial complex II—succinate dehydrogenase [174-176] leading to aberrant calcium homeostasis [177, 178] (Fig. 1). Indeed, transgenic mice that overexpressed full length htt had a twofold increase in intracellular calcium [179], decreased inactivation of glutamate via vesicle uptake [180], and increased NMDA receptor currents [181-183]. Furthermore, mitochondria in a transgenic htt mouse model overexpressing a 128 polyQ repeat domain were sensitized to NMDAR activation, leading to increased intracellular calcium and the loss of mitochondrial membrane potential [184]. The HD specific striatal degeneration has also been linked to increased calcium sensitivity of striatal mitochondria [185, 186]. Using the mitochondrial complex II inhibitor 3-nitroproprionic acid (3NP), striatal neurons were shown to be more susceptible to alterations in calcium equilibrium than cortical neurons in vitro [187] and in vivo [188].
Cytosol
One of the consequences of mitochondrial calcium dysfunction is calpain activation. The presence of N-terminal truncated mhtt in the nucleus and cytoplasm in the human brain, a pathologic hallmark of HD [189, 190], is neurotoxic both in vitro and in vivo [191, 192]. Initially, caspase-3 was shown to cleave both wild-type and mhtt into N-terminal fragments [193, 194]. However, the caspase-dependent fragments were found in both control and HD brain, suggesting that caspase-mediated cleavage of htt was not producing the pathology of HD [195]. Further processing of the caspase-3 N-terminal fragments is also performed by calpain [195]. Subsequent exploration of calpain-mediated proteolysis of htt demonstrated active calpain in the caudate of HD but not control brains [196]. Calpain was able to cleave htt in a polyglutamine repeat-dependent manner [196, 197] and was induced by calcium excitotoxicity [198]. Furthermore, the fragment produced by calpain was smaller (∼45 kDa) than the caspase-mediated fragment [154, 195, 196], thus enabling it to translocate to the nucleus and potentially result in increased toxicity [199].
Further evidence suggests that calpain alone, and not caspases, is involved in producing the pathologic proteolysis of htt. Using the 3NP HD model in vivo, it was demonstrated that caspase was activated only in acute administration of 3NP whereas calpain was activated in both the acute and chronic 3NP treatment models. Calpain inhibition blocked htt cleavage, decreased striatal degeneration, and completely prevented DNA fragmentation [200]. Inhibition of calpain proteolysis of htt has also been shown to be neuroprotective in vitro via mutation of calpain cleavage sites of htt [201]. Additional evidence implicating calpain in HD neurodegeneration has shown that 3NP induces mitochondrial dysfunction leading to cytochrome c release and calpain activation in striatal cultures. While cytochrome c release did result in activation of caspase-9 and subsequent activation of caspase-3, calpain was shown to cleave both caspases-3 and 9 rendering this pathway inactive [202]. Interestingly, the pan-caspase inhibitor zVAD has also been shown to inhibit calpain, decreasing 3NP toxicity [203], and casting ambiguity on the results of previous experiments examining the role of caspases in HD pathology.
Nucleus
Analyses of calpain substrates other than htt in the pathogenesis of HD have been limited; however, the role of Cdk5 has been investigated. As with the previous neurodegenerative diseases discussed, calpain activation following 3NP administration in vivo resulted in the processing of p35 to p25, leading to increased Cdk5 activity and decreased phosphorylation of MEF2D [204]. Direct Cdk5 phosphorylation of htt at specific serine residues resulted in decreased htt cleavage and increased fragment aggregates [205], while constitutive phosphorylation of htt decreased toxicity from polyglutamine containing htt [206]. The role of htt phosphorylation is interpreted as a response to DNA damage that is dependent on p53. Increased DNA damage and a decrease in total Cdk5 and p35 levels are found in the striatum of HD brains [206]; however, calpain activation, p25 levels, or Cdk5 activation have not been investigated. Clearly, more studies are necessary to elucidate the function of Cdk5 in HD pathology.
In summary, mitochondrial sensitivity, likely due to complex II inhibition, mediates intracellular calcium dysregulation. Activation of calpain then cleaves htt and mhtt, resulting in the formation htt N-terminal fragment aggregates pathognomonic of HD. It remains to be determined, however, which occurs first: mitochondrial dysfunction or mhtt fragmentation. Further experiments need to explore this question as well as what other calpain substrates are important in HD pathogenesis in order to fully understand the disease and to develop therapeutics for HD. Unlike the previous disorders discussed, a physiologic role of calpain in HD has not been discerned.
Multiple Sclerosis
Pathophysiology of Activation
Multiple sclerosis is a progressive T-cell-mediated autoimmune disorder characterized by plaques of demyelination occurring in the CNS. The demyelination results in loss of saltatory nerve conduction with eventual axonal degeneration [207]. Clinically, MS is more prevalent in females (2:1, female to male) and patients present with visual disturbances, loss of motor coordination, and limb weakness [208]. The pathogenesis underlying MS is thought to be due to T-lymphocyte activation and inflammation directed against myelin proteins [207]. The histological features of MS are infiltration of T cells, macrophages, B cells, and reactive astrocytes and microglia in the proximity of demyelination plaques [207]. T-cells reactive to myelin proteins are thought to be activated in the periphery [209]. They then cross the blood brain barrier and are presented with and process the antigen [210]. This results in a secondary influx of lymphocytes and macrophages leading to the destruction of myelin and axonal damage [211, 212] (Fig. 1).
Membrane/Receptor
Loss of myelin proteins occurs by protease-mediated breakdown. The role of calpain was examined since all major myelin proteins, including myelin basic protein (MBP) and axonal neurofilament protein (NFP), are substrates of calpain [213, 214]. Activated T-cells also have increased intracellular calcium [215] and express and secrete calpain [216-218]. Using an animal model of MS, experimental allergic encephalitis (EAE), calpain expression was increased twofold over control with decreased levels of myelin associated glycoprotein and NFP [219]. Increased calpain expression was also seen in activated microglia, macrophages, reactive astrocytes, and activated T-cells in EAE [220], and calpain activity was correlated with myelin loss [221], optic neuritis [222], increased calcium, and neuronal cell death [223]. Higher calpain-induced α-spectrin cleavage products in MS-plaques have also been shown in human MS brain [224, 225]. As in the EAE model, increased expression was seen in reactive astrocytes and monocytes, activated T-cells [225], and in relation to axon damaged areas [224].
Cytosol
In addition to secretion of calpain [218], activated T-cells release the cytokine interferon γ (INFγ), which stimulates the protein kinase C signaling cascade in effector cells such as glia and macrophages [226]. Accordingly, stimulation of the PKC pathway in cultured macrophages and microglia using phorbol myristate acetate led to macrophage apoptosis including release of calpain into media and activation of microglia leading to increased myelin phagocytosis [227]. Release of calpain into the extracellular milieu would be expected to activate the protease due to the high calcium concentration (∼2 mM); the proteolysis of myelin proteins and membrane proteins on oligodendrocytes could also compromise the membrane and lead to increased calcium influx into the cells. Thus, calpain could play a pathophysiologic role at both extracellular and intracellular sites.
Oligodendrocytes treated with pharmacologic agents that lead to alterations in calcium homeostasis such as kainate and thapsigargin were shown to undergo apoptosis in a calpain-dependent manner [228], and TNFα-induced cell death was shown to be AIF-dependent [229], probably due to calpain-mediated cleavage [146]. Using another cell type known to be affected in MS, retinal ganglion cells, it was determined that exposure to a calcium ionophore or INFγ initiated calpain-dependent cell death [230]. To further the argument that calpain is involved in the pathogenesis of MS, oral administration of a BBB-permeable calpain inhibitor cysteic–leucyl–argininal 7 days postinduction of EAE in mice prevented demyelination, inflammation, and improved the behavioral signs of EAE [231]. In addition, peripheral blood mononuclear cells isolated from MS patients showed a correlation between increased calpain activation and T-helper 1 and 2 (Th1/Th2) cell dysregulation during relapse and remission [232]. Inhibition of calpain resulted in an abrogation of activated T-cell cytokines and an overall decrease in MBP degradation [232]. These studies suggest that calpain inhibition in vivo may abrogate both the direct extracellular degradation of myelin and the indirect action of INFγ-mediated cell death of oligodendrocytes and retinal ganglion cells in MS.
The pathologic mechanisms underlying and consequences of calpain activation in MS are distinct from the other neurodegenerative diseases discussed thus far. The mechanisms discussed such as alterations in PKC signaling and the potential for calpain release into the extracellular milieu to damage surrounding neurons can lead to further avenues of research into the pathology of these diseases.
Prion-Related Encephalopathy
Pathophysiology of Activation
A second neurodegenerative disease characterized by the abnormal aggregation of protein is prion disease or transmissible spongiform encephalopathies, including scrapie in sheep, bovine spongiform encephalopathy in cattle (or mad cow disease), and Creutzfeldt–Jakob disease in humans [233]. In the vast majority of cases, prion-related encephalopathies (PRE) are sporadic with an insidious onset consisting of a triad of symptoms: dementia, pseudosporadic electroencephalograph changes, and myoclonus [234]. The pathogenic protein implicated in the “protein-only” model of PRE is formation of a misfolded form of the ubiquitous mammalian protein, PrPC [235, 236], into an abnormal, protease-resistant, scrapie form, PrPSc [233]. The PrPSc form has a different conformation, increased stability and hydrophobicity, is insoluble, and forms aggregates similar to Aβ [234]. The normal PrPC is processed by a disintegrin and metalloprotease/TNFα-converting enzyme metalloproteases to form the C-terminal fragment C1 [237]; however, the neurotoxic PrPSc form is processed into the alternate C2 cleavage product [237].
Membrane/Receptor (Physiologic Activation)
Calpain is also implicated in the normal physiological processing of PrPC. PrPC is normally a cell surface N-linked glycoprotein that is neurotoxic when present in the cytosol [238]. However, retrotranslational located PrPC (unglycosylated PrPC from the ER that is shuttled to the cytosol for degradation) was increased following proteasome inhibition and increased further with concomitant proteasome and calpain inhibition [239]. Thus, calpain may play a role in reducing toxicity of endogenous, untransformed PrPC in normal neurons. However, this remains controversial as reactive oxygen species (ROS)-induced cleavage of PrPC, which also induces the formation of the C2 fragment, was not mediated by calpain in SHSY-5Y cells [240].
Cytosol
Calpain was thought to mediate the generation of C2 from PrPSc because of the known abnormalities in calcium homeostasis caused by scrapie infection [241]. Furthermore, the known inhibitor of PrPSc aggregation, quinacrine, also abrogates calcium influx [242], and the treatment of SHSY-5Y cells with the neurotoxic fragment, PrP (106−126), increased intracellular calcium inducing calpain activation [243] (Fig. 1). Indeed, using scrapie-infected mouse brain cells, there was an increase in the presence of the C2 fragment compared to cells that were cleared of infection by chronic pentosan sulfate treatment. The formation of C2 was inhibited by calpain inhibitors, including calpastatin overexpression, but not by cathepsin, caspase, or proteasome inhibitors [244]. A different model using intracerebral injection of the ME7 strain of prion protein into mouse brain, caused PRE-like gliosis, increased GFAP expression and GFAP fragments in hippocampus of infected mouse brain [245]. The increased expression of GFAP correlated with increased expression of μ-calpain in these cells [245], indicating increased calpain activation, as increased calpain activity occurs in other models inducing reactive gliosis [246, 247].
Exposure of rat primary neurons to cytotoxic PrP (106−126) also induced calpain and Cdk5 activation in a manner similar to Aβ-induced toxicity [115]. PrP (106−126) treatment resulted in the calpain-mediated processing of p35 to p25, leading to increased Cdk5 overactivation as measured by tau hyperphosphorylation. Neuronal death due to PrP (106−126) was reduced following pharmacologic calpain inhibition, and tau hyperphosphorylation was reduced by both calpain and Cdk5 inhibition [115]. Further validation of tau hyperphosphorylation as a measure of PrP toxicity has been demonstrated as increased levels of phosphorylated tau were found in the CSF of patients with PRE [248]. Clearly, more research is needed to characterize the role of calpain and its substrates on neuronal toxicity induced by PrP.
Amyotrophic Lateral Sclerosis
Pathophysiology of Activation
Loss of motor neurons in brain and spinal cord leading to progressive paralysis and ultimately death are the hallmarks of amyotrophic lateral sclerosis [249]. The pathogenesis of this motor neuron disease is thought to be due to excess extracellular glutamate [250, 251], and associated excitotoxicity as motor neurons vulnerable in ALS are deficient in calcium-binding proteins (as seen in PD; Fig. 1). These neurons are unable to efficiently buffer calcium changes [252, 253], and overexpression of the calcium binding protein, parvalbumin, delayed the onset of ALS in mutant superoxide dismutase (mSOD) transgenic mice [254]. Together, these phenomena would suggest that excitotoxicity coupled with impaired calcium buffering capability would lead to the persistent activation of calpain.
Cytosol
One of the histological findings consistent with ALS pathology is the increase in neurofilament (NF) inclusions. Calpain has been linked to NF processing [255], and NF processing is modulated by the NF phosphorylation state [256]. Phospho-NFH (a high molecular weight NF) protects against proteolysis [256], and calmodulin interactions with NFH also decrease calpain-mediated cleavage [257]. Despite these in vitro findings, there were no differences in the physiochemical processing of NFH in ALS patients or normal controls [258].
While calpain may not be involved in the pathogenesis of ALS by processing NF inclusions, it has been implicated in Cdk5 hyperactivation. Abnormal subcellular localization of Cdk5 occurs in ALS tissue and is implicated in hyperphosphorylation of NFH [259]. Furthermore, mSOD transgenic mice were found to have an increased p25/p35 ratio, increased Cdk5 activity, and hyperphosphorylation of tau and NF [260]. However, mSOD transgenic mice that also had increased expression of NFH and had an increased life span compared to mSOD mice [261] showed diminished tau hyperphosphorylation compared to mSOD-only mice [260]. Thus, it was concluded that NFH acts as a type of competitive antagonist for the pathogenic action of Cdk5-mediated tau hyperphosphorylation. The role of Cdk5 in the pathogenesis of ALS is controversial, however, since mSOD mice with p35 knocked out did not affect disease onset or progression when compared to p35 wild-type mice, despite reduced Cdk5 activity in the p35-null mice [262].
Calpain activity needs to be assessed further in ALS to determine the substrates that may be involved with motor neuron death. Recently, it was shown that double transgenic mSOD and X-linked inhibitor of apoptosis had decreased caspase-12 cleavage and calpain activity that was associated with increased life span compared to mSOD-only mice [263]. Thus, decreased calpain activity is linked to prolonged life in an ALS model, but the mechanism is not clearly defined.
Conclusions
There is a complex interplay between normal physiologic and persistent, pathologic calpain activity that underlies the pathophysiology of several neurodegenerative diseases. Alterations in calcium homeostasis due to energy depletion in acute neurodegenerative disorders, such as ischemia, TBI, and epilepsy, result in the overwhelming activation of calpain in vitro, in vivo, and in postmortem brain. Chronic neurodegenerative diseases also show calcium dysregulation with ensuing calpain activation via NMDA receptor overactivation in AD, ALS, and PRE, mitochondrial dysfunction, in PD and HD, and by immune cell-mediated release of calpain and cytokines in MS.
In addition to the shared mechanism of calpain activation in neurodegenerative diseases, there is also a commonality in the substrates cleaved by calpain. The prototypical calpain substrate, α-spectrin, is cleaved in all of the diseases mentioned, and it has been used as a biomarker of injury severity [264-266]. Alterations in the function and substrates of Cdk5 have been revealed in all of the neurodegenerative diseases discussed in this review except MS; however, there are no studies examining Cdk5 activation in MS, so it cannot be ruled out. The inactivation of the prosurvival factors MEF2D and Prx2 by Cdk5 as seen in PD could very well turn out to be components of neuronal death in the other neurodegenerative diseases. Similarly, mGluR1α and NMDA subunit NR2A C terminus cleavage in ischemia, PKA subunit cleavage in AD, and CaN A overactivation seen in both, all decrease prosurvival CREB activation. It remains to be determined if similar mechanisms exist in cerebral ischemia, PD, HD, PRE, MS, and ALS. Production of neurotoxic fragments of proteins is also a commonality among some neurodegenerative diseases. Calpain-mediated cleavage of CRMPs in ischemia, tau in AD, α-synuclein in PD, htt and mhtt in HD, and PrPSc all result in neuronal death. Further examination of calpain activation in MS and ALS may also uncover similar mechanisms underlying the pathology of these diseases.
Finally, delineating the time course of pathology in the chronic neurodegenerative diseases in relation to calpain activation would be extremely helpful in determining the point where calpain inhibition would prove most useful therapeutically. This is especially important as calpain serves many physiologic roles where its inhibition will be detrimental to neuronal viability [4]. It is probable, as with AD, that abnormal calpain activation is present in PD, HD, PRE, and ALS patients prior to the onset of symptoms and definitely before any pathognomonic indications. Clearly, there are similarities between calpain activation and pathology in both acute and chronic neurodegenerative diseases, and a thorough understanding of the alterations in function of calpain substrates would prove useful in designing therapeutics to ameliorate neuronal death and eventually patient morbidity and mortality to these devastating diseases.
Acknowledgments
This work was supported by NIH/NINDS grants (NS43802, NS45048, NS44178, NS 56118, and NS36736) and VA Merit Review to J.C. Additional support was provided by the American Heart Association to P.V. (0715254U). We thank Armando P. Signore for editorial assistance and Pat Strickler for secretarial support.
Contributor Information
P. S. Vosler, Department of Neurology, University of Pittsburgh School of Medicine, S-507, Biomedical Science Tower, Pittsburgh, PA 15213, USA Center for Neuroscience University of Pittsburgh, Pittsburgh, PA, USA.
C. S. Brennan, Department of Neurology, University of Pittsburgh School of Medicine, S-507, Biomedical Science Tower, Pittsburgh, PA 15213, USA
J. Chen, Department of Neurology, University of Pittsburgh School of Medicine, S-507, Biomedical Science Tower, Pittsburgh, PA 15213, USA Department of Pharmacology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Geriatric Research, Educational and Clinical Center, Veterans Affairs Pittsburgh Health Care System, Pittsburgh, PA, USA; Center for Neuroscience University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.Camins A. Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev. 2006;12:135–148. doi: 10.1111/j.1527-3458.2006.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray SK. Currently evaluated calpain and caspase inhibitors for neuroprotection in experimental brain ischemia. Curr Med Chem. 2006;13:3425–3440. doi: 10.2174/092986706779010342. [DOI] [PubMed] [Google Scholar]
- 3.Saez ME. The therapeutic potential of the calpain family: new aspects. Drug Discov Today. 2006;11:917–923. doi: 10.1016/j.drudis.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Goll DE. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 5.Wu HY. Calpain–calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama. 2007;61:123–137. doi: 10.18926/AMO/32905. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard H. Structure of a calpain Ca(2+)-binding domain reveals a novel EF-hand and Ca(2+)-induced conformational changes. Nat Struct Biol. 1997;4:532–538. doi: 10.1038/nsb0797-532. [DOI] [PubMed] [Google Scholar]
- 7.Bevers MB. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 2008;28:655–673. doi: 10.1038/sj.jcbfm.9600595. [DOI] [PubMed] [Google Scholar]
- 8.Chan SL. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- 9.Hyrc K. Ionized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicators. J Neurosci. 1997;17:6669–6677. doi: 10.1523/JNEUROSCI.17-17-06669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goll DE. Is calpain activity regulated by membranes and autolysis or by calcium and calpastatin? Bioessays. 1992;14:549–556. doi: 10.1002/bies.950140810. [DOI] [PubMed] [Google Scholar]
- 11.Arthur JS. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutt P. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:3. doi: 10.1186/1471-213X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontremoli S. Modulation of inhibitory efficiency of rat skeletal muscle calpastatin by phosphorylation. Biochem Biophys Res Commun. 1992;187:751–759. doi: 10.1016/0006-291x(92)91259-s. [DOI] [PubMed] [Google Scholar]
- 14.DeMartino GN. Proteolysis of the protein inhibitor of calcium-dependent proteases produces lower molecular weight fragments that retain inhibitory activity. Arch Biochem Biophys. 1988;262:189–198. doi: 10.1016/0003-9861(88)90181-6. [DOI] [PubMed] [Google Scholar]
- 15.Czogalla A. Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci. 2005;62:1913–1924. doi: 10.1007/s00018-005-5097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nixon RA. Fodrin degradation by calcium-activated neutral proteinase (CANP) in retinal ganglion cell neurons and optic glia: preferential localization of CANP activities in neurons. J Neurosci. 1986;6:1264–1271. doi: 10.1523/JNEUROSCI.06-05-01264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siman R. Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci U S A. 1984;81:3572–3576. doi: 10.1073/pnas.81.11.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seubert P. Calmodulin stimulates the degradation of brain spectrin by calpain. Synapse. 1987;1:20–24. doi: 10.1002/syn.890010105. [DOI] [PubMed] [Google Scholar]
- 19.Cuerrier D. Determination of peptide substrate specificity for mu-calpain by a peptide library-based approach: the importance of primed side interactions. J Biol Chem. 2005;280:40632–40641. doi: 10.1074/jbc.M506870200. [DOI] [PubMed] [Google Scholar]
- 20.Tompa P. On the sequential determinants of calpain cleavage. J Biol Chem. 2004;279:20775–20785. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- 21.van der Worp HB. Clinical practice. Acute ischemic stroke. N Engl J Med. 2007;357:572–579. doi: 10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]
- 22.Harukuni I. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Hara MR. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol. 2007;47:117–41. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- 24.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 25.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 26.Siman R. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- 27.Hong SC. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke. 1994;25:663–669. doi: 10.1161/01.str.25.3.663. [DOI] [PubMed] [Google Scholar]
- 28.Bevers MB. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 2007;28(4):655–673. doi: 10.1038/sj.jcbfm.9600595. [DOI] [PubMed] [Google Scholar]
- 29.Newcomb-Fernandez JK. Concurrent assessment of calpain and caspase-3 activation after oxygen–glucose deprivation in primary septo-hippocampal cultures. J Cereb Blood Flow Metab. 2001;21:1281–1294. doi: 10.1097/00004647-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 30.McGinnis KM. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- 31.Malagelada C. Contribution of caspase-mediated apoptosis to the cell death caused by oxygen–glucose deprivation in cortical cell cultures. Neurobiol Dis. 2005;20:27–37. doi: 10.1016/j.nbd.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Yamashima T. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on ‘calpain–cathepsin hypothesis’. Eur J Neurosci. 1998;10:1723–1733. doi: 10.1046/j.1460-9568.1998.00184.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamashima T. Sustained calpain activation associated with lysosomal rupture executes necrosis of the postischemic CA1 neurons in primates. Hippocampus. 2003;13:791–800. doi: 10.1002/hipo.10127. [DOI] [PubMed] [Google Scholar]
- 34.Chaitanya GV. Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res. 2008 doi: 10.1007/s11064-007-9567-7. doi:10.1007/s11064−007−9567−7. [DOI] [PubMed] [Google Scholar]
- 35.Tsubokawa T. Cathepsin and calpain inhibitor E64d attenuates matrix metvalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- 36.Tsubokawa T. Neurovascular and neuronal protection by E64d after focal cerebral ischemia in rats. J Neurosci Res. 2006;84:832–840. doi: 10.1002/jnr.20977. [DOI] [PubMed] [Google Scholar]
- 37.Verkhratsky A. Calcium and cell death. Subcell Biochem. 2007;45:465–480. doi: 10.1007/978-1-4020-6191-2_17. [DOI] [PubMed] [Google Scholar]
- 38.Pottorf WJ., 2nd Glutamate-induced protease-mediated loss of plasma membrane Ca2+ pump activity in rat hippocampal neurons. J Neurochem. 2006;98:1646–1656. doi: 10.1111/j.1471-4159.2006.04063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bano D. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 40.De Jongh KS. Differential proteolysis of the full-length form of the L-type calcium channel alpha 1 subunit by calpain. J Neurochem. 1994;63:1558–1564. doi: 10.1046/j.1471-4159.1994.63041558.x. [DOI] [PubMed] [Google Scholar]
- 41.Hell JW. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci U S A. 1996;93:3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rardon DP. Digestion of cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles with calpain II. Effects on the Ca2+ release channel. Circ Res. 1990;67:84–96. doi: 10.1161/01.res.67.1.84. [DOI] [PubMed] [Google Scholar]
- 43.French JP. Ischemia–reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H128–H136. doi: 10.1152/ajpheart.00739.2005. [DOI] [PubMed] [Google Scholar]
- 44.Parsons JT. Global ischemia-induced inhibition of the coupling ratio of calcium uptake and ATP hydrolysis by rat whole brain microsomal Mg(2+)/Ca(2+) ATPase. Brain Res. 1999;834:32–41. doi: 10.1016/s0006-8993(99)01504-8. [DOI] [PubMed] [Google Scholar]
- 45.Magnusson A. Calcium-induced degradation of the inositol (1,4,5)-trisphosphate receptor/Ca(2+)-channel. FEBS Lett. 1993;323:229–232. doi: 10.1016/0014-5793(93)81345-z. [DOI] [PubMed] [Google Scholar]
- 46.Pattni K. Calpain cleavage of the B isoform of Ins(1,4,5) P3 3-kinase separates the catalytic domain from the membrane anchoring domain. Biochem J. 2003;375:643–651. doi: 10.1042/BJ20030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gascon S. Excitotoxicity and focal cerebral ischemia induce truncation of the NR2A and NR2B subunits of the NMDA receptor and cleavage of the scaffolding protein PSD-95. Mol Psychiatry. 2008;13:99–114. doi: 10.1038/sj.mp.4002017. [DOI] [PubMed] [Google Scholar]
- 48.Guttmann RP. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- 49.Guttmann RP. Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J Pharmacol Exp Ther. 2002;302:1023–1030. doi: 10.1124/jpet.102.036962. [DOI] [PubMed] [Google Scholar]
- 50.Simpkins KL. Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci. 2003;23:11322–11331. doi: 10.1523/JNEUROSCI.23-36-11322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bi R. Phosphorylation regulates calpain-mediated truncation of glutamate ionotropic receptors. Brain Res. 1998;797:154–158. doi: 10.1016/s0006-8993(98)00433-8. [DOI] [PubMed] [Google Scholar]
- 52.Bi X. Calpain-mediated truncation of glutamate ionotropic receptors. Methods for studying the effects of calpain activation in brain tissue. Methods Mol Biol. 2000;144:203–217. doi: 10.1385/1-59259-050-0:203. [DOI] [PubMed] [Google Scholar]
- 53.Bi X. Calpain-mediated proteolysis of GluR1 subunits in organotypic hippocampal cultures following kainic acid treatment. Brain Res. 1998;781:355–357. doi: 10.1016/s0006-8993(97)01365-6. [DOI] [PubMed] [Google Scholar]
- 54.Wu HY. Regulation of N-methyl-D-aspartate receptors by calpain in cortical neurons. J Biol Chem. 2005;280:21588–21593. doi: 10.1074/jbc.M501603200. [DOI] [PubMed] [Google Scholar]
- 55.Xu W. Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 2007;53:399–412. doi: 10.1016/j.neuron.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 56.Tremper-Wells B. Nuclear calpain regulates Ca2+-dependent signaling via proteolysis of nuclear Ca2+/calmodulin-dependent protein kinase type IV in cultured neurons. J Biol Chem. 2005;280:2165–2175. doi: 10.1074/jbc.M410591200. [DOI] [PubMed] [Google Scholar]
- 57.Sharkey J. Immunophilins mediate the neuroprotective effects of FK506 in focal cerebral ischaemia. Nature. 1994;371:336–339. doi: 10.1038/371336a0. [DOI] [PubMed] [Google Scholar]
- 58.Uchino H. Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol Dis. 2002;10:219–233. doi: 10.1006/nbdi.2002.0514. [DOI] [PubMed] [Google Scholar]
- 59.Shioda N. Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J Neurochem. 2007;102:1506–1517. doi: 10.1111/j.1471-4159.2007.04600.x. [DOI] [PubMed] [Google Scholar]
- 60.Shioda N. Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem. 2006;98:310–320. doi: 10.1111/j.1471-4159.2006.03874.x. [DOI] [PubMed] [Google Scholar]
- 61.Neumar RW. Calpain mediates eukaryotic initiation factor 4G degradation during global brain ischemia. J Cereb Blood Flow Metab. 1998;18:876–881. doi: 10.1097/00004647-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Petegnief V. Nitric oxide mediates NMDA-induced persistent inhibition of protein synthesis through dephosphorylation of eukaryotic initiation factor 4E-binding protein 1 and eukaryotic initiation factor 4G proteolysis. Biochem J. 2008;411(3):667–677. doi: 10.1042/BJ20071060. [DOI] [PubMed] [Google Scholar]
- 63.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 64.Mao X. Neuronal kappa B-binding factors consist of Sp1-related proteins. Functional implications for autoregulation of N-methyl-D-aspartate receptor-1 expression. J Biol Chem. 2002;277:44911–44919. doi: 10.1074/jbc.M204292200. [DOI] [PubMed] [Google Scholar]
- 65.Mao X. Glutamate receptor activation evokes calpain-mediated degradation of Sp3 and Sp4, the prominent Sp-family transcription factors in neurons. J Neurochem. 2007;100:1300–1314. doi: 10.1111/j.1471-4159.2006.04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gascon S. Transcription of the NR1 subunit of the N-methyl-D-aspartate receptor is down-regulated by excitotoxic stimulation and cerebral ischemia. J Biol Chem. 2005;280:35018–35027. doi: 10.1074/jbc.M504108200. [DOI] [PubMed] [Google Scholar]
- 67.Hou ST. Calpain-cleaved collapsin response mediator protein-3 induces neuronal death after glutamate toxicity and cerebral ischemia. J Neurosci. 2006;26:2241–2249. doi: 10.1523/JNEUROSCI.4485-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang SX. Calpain cleavage of collapsin response mediator proteins in ischemic mouse brain. Eur J Neurosci. 2007;26:801–809. doi: 10.1111/j.1460-9568.2007.05715.x. [DOI] [PubMed] [Google Scholar]
- 69.Bretin S. Calpain product of WT-CRMP2 reduces the amount of surface NR2B NMDA receptor subunit. J Neurochem. 2006;98:1252–1265. doi: 10.1111/j.1471-4159.2006.03969.x. [DOI] [PubMed] [Google Scholar]
- 70.Rashidian J. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102:14080–14085. doi: 10.1073/pnas.0500099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wen Y. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta. 2007;1772:473–483. doi: 10.1016/j.bbadis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Grundke-Iqbal I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haass C. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 74.Vincent B. Regulation of betaAPP and PrPc cleavage by alpha-secretase: mechanistic and therapeutic perspectives. Curr Alzheimer Res. 2008;5:202–211. doi: 10.2174/156720508783954749. [DOI] [PubMed] [Google Scholar]
- 75.Lue LF. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naslund J. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 77.De Felice FG. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 78.Demuro A. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 79.Kelly BL. Beta-amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- 80.Nixon RA. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer's disease. Ann N Y Acad Sci. 1994;747:77–91. doi: 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- 81.Saito K. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veeranna Calpain mediates calcium-induced activation of the Erk1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer's disease. Am J Pathol. 2004;165:795–805. doi: 10.1016/S0002-9440(10)63342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen M. Stimulation of beta-amyloid precursor protein alpha-processing by phorbol ester involves calcium and calpain activation. Biochem Biophys Res Commun. 2004;316:332–340. doi: 10.1016/j.bbrc.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 84.Li QX. Proteolytic processing of Alzheimer's disease beta A4 amyloid precursor protein in human platelets. J Biol Chem. 1995;270:14140–14147. doi: 10.1074/jbc.270.23.14140. [DOI] [PubMed] [Google Scholar]
- 85.Siman R. Proteolytic processing of beta-amyloid precursor by calpain I. J Neurosci. 1990;10:2400–2411. doi: 10.1523/JNEUROSCI.10-07-02400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen M. Mu-calpain is functionally required for alpha-processing of Alzheimer's beta-amyloid precursor protein. Biochem Biophys Res Commun. 2005;330:714–721. doi: 10.1016/j.bbrc.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 87.Kelly BL. Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. J Biol Chem. 2005;280:31746–31753. doi: 10.1074/jbc.M503259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oddo S. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 89.Yao PJ. Preserved synaptic vesicle recycling in hippo-campal neurons in a mouse Alzheimer's disease model. Biochem Biophys Res Commun. 2005;330:34–38. doi: 10.1016/j.bbrc.2005.02.121. [DOI] [PubMed] [Google Scholar]
- 90.Barco A. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 91.Liang Z. Down-regulation of cAMP-dependent protein kinase by over-activated calpain in Alzheimer disease brain. J Neurochem. 2007;103:2462–2470. doi: 10.1111/j.1471-4159.2007.04942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shiraha H. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lakshmikuttyamma A. In vitro proteolytic degradation of bovine brain calcineurin by m-calpain. Neurochem Res. 2004;29:1913–1921. doi: 10.1023/b:nere.0000042218.27842.79. [DOI] [PubMed] [Google Scholar]
- 94.Wang KK. Characterization of the fragmented forms of calcineurin produced by calpain I. Biochem Cell Biol. 1989;67:703–711. doi: 10.1139/o89-105. [DOI] [PubMed] [Google Scholar]
- 95.Liu F. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- 96.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 97.Delobel P. Stable-tau overexpression in human neuroblastoma cells: an open door for explaining neuronal death in tauopathies. Ann N Y Acad Sci. 2003;1010:623–634. doi: 10.1196/annals.1299.115. [DOI] [PubMed] [Google Scholar]
- 98.Zheng YL. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. Embo J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kishimoto A. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- 100.Yoshimura Y. Purification and characterization of active fragment of Ca2+/calmodulin-dependent protein kinase II from the post-synaptic density in the rat forebrain. J Biochem. 1996;119:268–273. doi: 10.1093/oxfordjournals.jbchem.a021234. [DOI] [PubMed] [Google Scholar]
- 101.Shea TB. Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: involvement of calpain-mediated hydrolysis of protein kinase C. J Neurochem. 1996;66:1539–1549. doi: 10.1046/j.1471-4159.1996.66041539.x. [DOI] [PubMed] [Google Scholar]
- 102.Lucas JJ. Decreased nuclear beta-catenin, tau hyper-phosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. Embo J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goni-Oliver P. N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. J Biol Chem. 2007;282:22406–22413. doi: 10.1074/jbc.M702793200. [DOI] [PubMed] [Google Scholar]
- 104.Forde JE. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abe K. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 106.Chong ZZ. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease. Brain Res Brain Res Rev. 2005;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Cheung ZH. Cdk5: mediator of neuronal death and survival. Neurosci Lett. 2004;361:47–51. doi: 10.1016/j.neulet.2003.12.117. [DOI] [PubMed] [Google Scholar]
- 109.Monaco EA., 3rd Recent evidence regarding a role for Cdk5 dysregulation in Alzheimer's disease. Curr Alzheimer Res. 2004;1:33–38. doi: 10.2174/1567205043480519. [DOI] [PubMed] [Google Scholar]
- 110.Tsai LH. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 111.Jamsa A. Glutamate treatment and p25 transfection increase Cdk5 mediated tau phosphorylation in SH-SY5Y cells. Biochem Biophys Res Commun. 2006;345:324–331. doi: 10.1016/j.bbrc.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 112.Patrick GN. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 113.Alvarez A. Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett. 1999;459:421–426. doi: 10.1016/s0014-5793(99)01279-x. [DOI] [PubMed] [Google Scholar]
- 114.Lee MS. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 115.Lopes JP. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-beta and prion peptides: implications for Alzheimer's disease and prion-related encephalopathies. Cell Mol Neurobiol. 2007;27:943–957. doi: 10.1007/s10571-007-9224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saito T. p25/cyclin-dependent kinase 5 promotes the progression of cell death in nucleus of endoplasmic reticulum-stressed neurons. J Neurochem. 2007;102:133–140. doi: 10.1111/j.1471-4159.2007.04540.x. [DOI] [PubMed] [Google Scholar]
- 117.Becker EB. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol. 2004;72:1–25. doi: 10.1016/j.pneurobio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 118.Weishaupt JH. Inhibition of CDK5 is protective in necrotic and apoptotic paradigms of neuronal cell death and prevents mitochondrial dysfunction. Mol Cell Neurosci. 2003;24:489–502. doi: 10.1016/s1044-7431(03)00221-5. [DOI] [PubMed] [Google Scholar]
- 119.Wittmann CW. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 120.Yoshiyama Y. Reduction of detyrosinated microtubules and Golgi fragmentation are linked to tau-induced degeneration in astrocytes. J Neurosci. 2003;23:10662–10671. doi: 10.1523/JNEUROSCI.23-33-10662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park SY. The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J Neurosci. 2005;25:5365–5375. doi: 10.1523/JNEUROSCI.1125-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amadoro G. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 124.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 125.Belin AC. Parkinson's disease: a genetic perspective. Febs J. 2008;275(7):1377–1383. doi: 10.1111/j.1742-4658.2008.06301.x. [DOI] [PubMed] [Google Scholar]
- 126.Hirsch EC. Why are nigral catecholaminergic neurons more vulnerable than other cells in Parkinson's disease? Ann Neurol. 1992;32(Suppl):S88–S93. doi: 10.1002/ana.410320715. [DOI] [PubMed] [Google Scholar]
- 127.Iacopino AM. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc Natl Acad Sci U S A. 1990;87:4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mouatt-Prigent A. Does the calcium binding protein calretinin protect dopaminergic neurons against degeneration in Parkinson's disease? Brain Res. 1994;668:62–70. doi: 10.1016/0006-8993(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 129.Mouatt-Prigent A. Increased m-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience. 1996;73:979–987. doi: 10.1016/0306-4522(96)00100-5. [DOI] [PubMed] [Google Scholar]
- 130.Kiuchi K. Limited proteolysis of tyrosine hydroxylase by Ca(2+)-activated neutral protease (calpain). Biochemistry. 1991;30:10416–10419. doi: 10.1021/bi00107a008. [DOI] [PubMed] [Google Scholar]
- 131.Barzilai A. The molecular mechanism of dopamine-induced apoptosis: identification and characterization of genes that mediate dopamine toxicity. J Neural Transm Suppl. 2000:59–76. doi: 10.1007/978-3-7091-6301-6_4. [DOI] [PubMed] [Google Scholar]
- 132.Tabrizi SJ. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum Mol Genet. 2000;9:2683–2689. doi: 10.1093/hmg/9.18.2683. [DOI] [PubMed] [Google Scholar]
- 133.Tehranian R. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem. 2006;99:1188–1196. doi: 10.1111/j.1471-4159.2006.04146.x. [DOI] [PubMed] [Google Scholar]
- 134.Mouatt-Prigent A. Calpastatin immunoreactivity in the monkey and human brain of control subjects and patients with Parkinson's disease. J Comp Neurol. 2000;419:175–192. doi: 10.1002/(sici)1096-9861(20000403)419:2<175::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 135.Kolata G. Monkey model of Parkinson's disease. Science. 1983;220:705. doi: 10.1126/science.6403987. [DOI] [PubMed] [Google Scholar]
- 136.Langston JW. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 137.Chiba K. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984;120:574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- 138.Greenamyre JT. Mitochondrial dysfunction in Parkinson's disease. Biochem Soc Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- 139.Wu DC. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100:6145–650. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen TS. MPP+ selectively affects calcium homeostasis in mesencephalic cell cultures from embryonal C57/Bl6 mice. J Neural Transm Gen Sect. 1995;100:153–163. doi: 10.1007/BF01271538. [DOI] [PubMed] [Google Scholar]
- 141.Crocker SJ. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Radke JM. Atypical antipsychotic drugs selectively increase neurotensin efflux in dopamine terminal regions. Proc Natl Acad Sci U S A. 1998;95:11462–11464. doi: 10.1073/pnas.95.19.11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martorana A. Dopamine denervation induces neurotensin immunoreactivity in GABA-parvalbumin striatal neurons. Synapse. 2001;41:360–362. doi: 10.1002/syn.1093. [DOI] [PubMed] [Google Scholar]
- 144.Schimpff RM. Increased plasma neurotensin concentrations in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2001;70:784–786. doi: 10.1136/jnnp.70.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liou AK. BimEL up-regulation potentiates AIF translocation and cell death in response to MPTP. Faseb J. 2005;19:1350–1352. doi: 10.1096/fj.04-3258fje. [DOI] [PubMed] [Google Scholar]
- 146.Cao G. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mishizen-Eberz AJ. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J Neurochem. 2003;86:836–47. doi: 10.1046/j.1471-4159.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- 148.Mishizen-Eberz AJ. Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry. 2005;44:7818–7829. doi: 10.1021/bi047846q. [DOI] [PubMed] [Google Scholar]
- 149.Li W. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dufty BM. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–1738. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tofaris GK. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1−120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shimura H. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim SJ. Parkin cleaves intracellular alpha-synuclein inclusions via the activation of calpain. J Biol Chem. 2003;278:41890–41899. doi: 10.1074/jbc.M306017200. [DOI] [PubMed] [Google Scholar]
- 155.Chen MJ. Early induction of calpains in rotenone-mediated neuronal apoptosis. Neurosci Lett. 2006;397:69–73. doi: 10.1016/j.neulet.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 156.Kruman II. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 157.Sedarous M. Calpains mediate p53 activation and neuronal death evoked by DNA damage. J Biol Chem. 2003;278:26031–26038. doi: 10.1074/jbc.M302833200. [DOI] [PubMed] [Google Scholar]
- 158.Kubbutat MH. Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol. 1997;17:460–468. doi: 10.1128/mcb.17.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Samantaray S. The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats. Neuroscience. 2007;146:741–755. doi: 10.1016/j.neuroscience.2007.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Samantaray S. Calpain activation in apoptosis of motoneurons in cell culture models of experimental parkinsonism. Ann N Y Acad Sci. 2006;1074:349–356. doi: 10.1196/annals.1369.034. [DOI] [PubMed] [Google Scholar]
- 161.Cohen G. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974;249:2447–2452. [PubMed] [Google Scholar]
- 162.Saito Y. Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and -independent action. Free Radic Biol Med. 2007;42:675–685. doi: 10.1016/j.freeradbiomed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 163.Hirai S. Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett. 1991;287:57–61. doi: 10.1016/0014-5793(91)80015-u. [DOI] [PubMed] [Google Scholar]
- 164.Xia XG. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qu D. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 166.Smith PD. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Avraham E. Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitinligase activity and aggregation. J Biol Chem. 2007;282:12842–12850. doi: 10.1074/jbc.M608243200. [DOI] [PubMed] [Google Scholar]
- 168.Alvira D. Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson's disease. Parkinsonism Relat Disord. 2007;14(4):309–313. doi: 10.1016/j.parkreldis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 169.Alvira D. Inhibition of the cdk5/p25 fragment formation may explain the antiapoptotic effects of melatonin in an experimental model of Parkinson's disease. J Pineal Res. 2006;40:251–258. doi: 10.1111/j.1600-079X.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 170.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 171.Young AB. Huntingtin in health and disease. J Clin Invest. 2003;111:299–302. doi: 10.1172/JCI17742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferrante RJ. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985;230:561–563. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- 173.Cudkowicz M. Degeneration of pyramidal projection neurons in Huntington's disease cortex. Ann Neurol. 1990;27:200–204. doi: 10.1002/ana.410270217. [DOI] [PubMed] [Google Scholar]
- 174.Brouillet E. Partial inhibition of brain succinate dehydrogenase by 3-nitropropionic acid is sufficient to initiate striatal degeneration in rat. J Neurochem. 1998;70:794–805. doi: 10.1046/j.1471-4159.1998.70020794.x. [DOI] [PubMed] [Google Scholar]
- 175.Brouillet E. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci U S A. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gu M. Mitochondrial defect in Huntington's disease caudate nucleus. Ann Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 177.Panov AV. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 178.Panov AV. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington's disease individuals. Mol Cell Biochem. 2005;269:143–152. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- 179.Hodgson JG. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- 180.Li H. Amino-terminal fragments of mutant huntingtin show selective accumulation in striatal neurons and synaptic toxicity. Nat Genet. 2000;25:385–389. doi: 10.1038/78054. [DOI] [PubMed] [Google Scholar]
- 181.Chen N. Subtype-specific enhancement of NMDA receptor currents by mutant huntingtin. J Neurochem. 1999;72:1890–1898. doi: 10.1046/j.1471-4159.1999.0721890.x. [DOI] [PubMed] [Google Scholar]
- 182.Zeron MM. Mutant huntingtin enhances excitotoxic cell death. Mol Cell Neurosci. 2001;17:41–53. doi: 10.1006/mcne.2000.0909. [DOI] [PubMed] [Google Scholar]
- 183.Zeron MM. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 184.Fernandes HB. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington's disease. J Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brustovetsky N. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J Neurosci. 2003;23:4858–4867. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brustovetsky N. Age-dependent changes in the calcium sensitivity of striatal mitochondria in mouse models of Huntington's disease. J Neurochem. 2005;93:1361–1370. doi: 10.1111/j.1471-4159.2005.03036.x. [DOI] [PubMed] [Google Scholar]
- 187.Galas MC. Death of cortical and striatal neurons induced by mitochondrial defect involves differential molecular mechanisms. Neurobiol Dis. 2004;15:152–159. doi: 10.1016/j.nbd.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 188.Jacquard C. Brain mitochondrial defects amplify intracellular [Ca2+] rise and neurodegeneration but not Ca2+ entry during NMDA receptor activation. Faseb J. 2006;20:1021–1023. doi: 10.1096/fj.05-5085fje. [DOI] [PubMed] [Google Scholar]
- 189.DiFiglia M. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 190.Sapp E. Huntingtin localization in brains of normal and Huntington's disease patients. Ann Neurol. 1997;42:604–612. doi: 10.1002/ana.410420411. [DOI] [PubMed] [Google Scholar]
- 191.Davies SW. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 192.Scherzinger E. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–58. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- 193.Goldberg YP. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet. 1996;13:442–449. doi: 10.1038/ng0896-442. [DOI] [PubMed] [Google Scholar]
- 194.Wellington CL. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J Biol Chem. 1998;273:9158–9167. doi: 10.1074/jbc.273.15.9158. [DOI] [PubMed] [Google Scholar]
- 195.Kim YJ. Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington's disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc Natl Acad Sci U S A. 2001;98:12784–12789. doi: 10.1073/pnas.221451398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gafni J. Calpain activation in Huntington's disease. J Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sun B. Polyglutamine repeat length-dependent proteolysis of huntingtin. Neurobiol Dis. 2002;11:111–122. doi: 10.1006/nbdi.2002.0539. [DOI] [PubMed] [Google Scholar]
- 198.Goffredo D. Calcium-dependent cleavage of endogenous wild-type huntingtin in primary cortical neurons. J Biol Chem. 2002;277:39594–39598. doi: 10.1074/jbc.C200353200. [DOI] [PubMed] [Google Scholar]
- 199.Hackam AS. The influence of huntingtin protein size on nuclear localization and cellular toxicity. J Cell Biol. 1998;141:1097–1105. doi: 10.1083/jcb.141.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bizat N. Calpain is a major cell death effector in selective striatal degeneration induced in vivo by 3-nitropropionate: implications for Huntington's disease. J Neurosci. 2003;23:5020–5030. doi: 10.1523/JNEUROSCI.23-12-05020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gafni J. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J Biol Chem. 2004;279:20211–20220. doi: 10.1074/jbc.M401267200. [DOI] [PubMed] [Google Scholar]
- 202.Bizat N. In vivo calpain/caspase cross-talk during 3-nitropropionic acid-induced striatal degeneration: implication of a calpain-mediated cleavage of active caspase-3. J Biol Chem. 2003;278:43245–43253. doi: 10.1074/jbc.M305057200. [DOI] [PubMed] [Google Scholar]
- 203.Bizat N. Neuroprotective effect of zVAD against the neurotoxin 3-nitropropionic acid involves inhibition of calpain. Neuropharmacology. 2005;49:695–702. doi: 10.1016/j.neuropharm.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 204.Crespo-Biel N. 3-Nitropropionic acid activates calpain/cdk5 pathway in rat striatum. Neurosci Lett. 2007;421:77–81. doi: 10.1016/j.neulet.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 205.Luo S. Cdk5 phosphorylation of huntingtin reduces its cleavage by caspases: implications for mutant huntingtin toxicity. J Cell Biol. 2005;169:647–656. doi: 10.1083/jcb.200412071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Anne SL. Phosphorylation of huntingtin by cyclin-dependent kinase 5 is induced by DNA damage and regulates wild-type and mutant huntingtin toxicity in neurons. J Neurosci. 2007;27:7318–7328. doi: 10.1523/JNEUROSCI.1831-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Frohman EM. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 208.Noseworthy JH. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 209.Ofosu-Appiah W. Characterization of in vivo-activated T cell clones from peripheral blood of multiple sclerosis patients. Clin Immunol Immunopathol. 1991;58:46–55. doi: 10.1016/0090-1229(91)90147-3. [DOI] [PubMed] [Google Scholar]
- 210.Sospedra M. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 211.Compston A. The pathogenesis and basis for treatment in multiple sclerosis. Clin Neurol Neurosurg. 2004;106:246–248. doi: 10.1016/j.clineuro.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 212.Hohlfeld R. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Inuzuka T. Neutral protease in cerebrospinal fluid from patients with multiple sclerosis and other neurological diseases. Acta Neurol Scand. 1987;76:18–23. doi: 10.1111/j.1600-0404.1987.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 214.Shields DC. Pathophysiological role of calpain in experimental demyelination. J Neurosci Res. 1999;55:533–541. doi: 10.1002/(SICI)1097-4547(19990301)55:5<533::AID-JNR1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 215.Clementi E. Intracellular Ca2+ stores of T lymphocytes: changes induced by in vitro and in vivo activation. Eur J Immunol. 1994;24:1365–1371. doi: 10.1002/eji.1830240619. [DOI] [PubMed] [Google Scholar]
- 216.Deshpande RV. Differential distribution of calpain in human lymphoid cells. Neurochem Res. 1993;18:767–773. doi: 10.1007/BF00966771. [DOI] [PubMed] [Google Scholar]
- 217.Deshpande RV. Calpain expression in lymphoid cells. Increased mRNA and protein levels after cell activation. J Biol Chem. 1995;270:2497–2505. doi: 10.1074/jbc.270.6.2497. [DOI] [PubMed] [Google Scholar]
- 218.Deshpande RV. Calpain secreted by activated human lymphoid cells degrades myelin. J Neurosci Res. 1995;42:259–265. doi: 10.1002/jnr.490420214. [DOI] [PubMed] [Google Scholar]
- 219.Shields DC. Upregulation of calpain activity and expression in experimental allergic encephalomyelitis: a putative role for calpain in demyelination. Brain Res. 1998;794:68–74. doi: 10.1016/s0006-8993(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 220.Shields DC. Increased calpain expression in experimental demyelinating optic neuritis: an immunocytochemical study. Brain Res. 1998;784:299–304. doi: 10.1016/s0006-8993(97)01381-4. [DOI] [PubMed] [Google Scholar]
- 221.Schaecher K. Calpain expression and infiltration of activated T cells in experimental allergic encephalomyelitis over time: increased calpain activity begins with onset of disease. J Neuroimmunol. 2002;129:1–9. doi: 10.1016/s0165-5728(02)00142-x. [DOI] [PubMed] [Google Scholar]
- 222.Guyton MK. A role for calpain in optic neuritis. Ann N Y Acad Sci. 2005;1053:48–54. doi: 10.1196/annals.1344.005. [DOI] [PubMed] [Google Scholar]
- 223.Guyton MK. Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J Neurosci Res. 2005;81:53–61. doi: 10.1002/jnr.20470. [DOI] [PubMed] [Google Scholar]
- 224.Diaz-Sanchez M. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 2006;111:289–299. doi: 10.1007/s00401-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 225.Shields DC. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Benveniste EN. TNF-alpha- and IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. Faseb J. 1995;9:1577–1584. doi: 10.1096/fasebj.9.15.8529837. [DOI] [PubMed] [Google Scholar]
- 227.Smith ME. Effects of phorbol myristate acetate (PMA) on functions of macrophages and microglia in vitro. Neurochem Res. 1998;23:427–434. doi: 10.1023/a:1022478005243. [DOI] [PubMed] [Google Scholar]
- 228.Benjamins JA. Protection of mature oligodendrocytes by inhibitors of caspases and calpains. Neurochem Res. 2003;28:143–152. doi: 10.1023/a:1021612615554. [DOI] [PubMed] [Google Scholar]
- 229.Jurewicz A. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain. 2005;128:2675–2688. doi: 10.1093/brain/awh627. [DOI] [PubMed] [Google Scholar]
- 230.Das A. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res. 2006;1084:146–157. doi: 10.1016/j.brainres.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 231.Hassen GW. A novel calpain inhibitor for the treatment of acute experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;180:135–146. doi: 10.1016/j.jneuroim.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 232.Imam SA. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol. 2007;190:139–145. doi: 10.1016/j.jneuroim.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Eggenberger E. Prion disease. Neurol Clin. 2007;25:833–842. viii. doi: 10.1016/j.ncl.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 235.Wopfner F. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J Mol Biol. 1999;289:1163–1178. doi: 10.1006/jmbi.1999.2831. [DOI] [PubMed] [Google Scholar]
- 236.Zanusso G. Prion protein expression in different species: analysis with a panel of new mAbs. Proc Natl Acad Sci U S A. 1998;95:8812–8816. doi: 10.1073/pnas.95.15.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chen SG. Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem. 1995;270:19173–19180. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- 238.Ma J. Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science. 2002;298:1781–1785. doi: 10.1126/science.1073725. [DOI] [PubMed] [Google Scholar]
- 239.Wang X. Calpain and other cytosolic proteases can contribute to the degradation of retro-translocated prion protein in the cytosol. J Biol Chem. 2005;280:317–325. doi: 10.1074/jbc.M410649200. [DOI] [PubMed] [Google Scholar]
- 240.Watt NT. Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. J Biol Chem. 2005;280:35914–35921. doi: 10.1074/jbc.M507327200. [DOI] [PubMed] [Google Scholar]
- 241.Kristensson K. Scrapie prions alter receptor-mediated calcium responses in cultured cells. Neurology. 1993;43:2335–2341. doi: 10.1212/wnl.43.11.2335. [DOI] [PubMed] [Google Scholar]
- 242.Xiao YF. Mechanism of suppression of cardiac L-type Ca(2+) currents by the phospholipase A(2) inhibitor mepacrine. Eur J Pharmacol. 2000;399:107–116. doi: 10.1016/s0014-2999(00)00366-6. [DOI] [PubMed] [Google Scholar]
- 243.O'Donovan CN. Prion protein fragment PrP-(106−126) induces apoptosis via mitochondrial disruption in human neuronal SH-SY5Y cells. J Biol Chem. 2001;276:43516–43523. doi: 10.1074/jbc.M103894200. [DOI] [PubMed] [Google Scholar]
- 244.Yadavalli R. Calpain-dependent endoproteolytic cleavage of PrPSc modulates scrapie prion propagation. J Biol Chem. 2004;279:21948–21956. doi: 10.1074/jbc.M400793200. [DOI] [PubMed] [Google Scholar]
- 245.Gray BC. Increased expression of glial fibrillary acidic protein fragments and mu-calpain activation within the hippocampus of prion-infected mice. Biochem Soc Trans. 2006;34:51–54. doi: 10.1042/BST0340051. [DOI] [PubMed] [Google Scholar]
- 246.Du S. Calcium influx and activation of calpain I mediate acute reactive gliosis in injured spinal cord. Exp Neurol. 1999;157:96–105. doi: 10.1006/exnr.1999.7041. [DOI] [PubMed] [Google Scholar]
- 247.Lee YB. Rapid increase in immunoreactivity to GFAP in astrocytes in vitro induced by acidic pH is mediated by calcium influx and calpain I. Brain Res. 2000;864:220–229. doi: 10.1016/s0006-8993(00)02180-6. [DOI] [PubMed] [Google Scholar]
- 248.Riemenschneider M. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt–Jakob disease from other dementias. Mol Psychiatry. 2003;8:343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 249.Cleveland DW. From Charcot to SOD1: mechanisms of selective motor neuron death in ALS. Neuron. 1999;24:515–520. doi: 10.1016/s0896-6273(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 250.Ludolph AC. The role of excitotoxicity in ALS—what is the evidence? J Neurol. 2000;247(Suppl 1):17–16. doi: 10.1007/s004150050552. [DOI] [PubMed] [Google Scholar]
- 251.Ludolph AC. Amyotrophic lateral sclerosis and glutamate. Restor Neurol Neurosci. 1998;13:59–67. [PubMed] [Google Scholar]
- 252.Alexianu ME. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- 253.Siklos L. Intracellular calcium parallels motoneuron degeneration in SOD-1 mutant mice. J Neuropathol Exp Neurol. 1998;57:571–587. doi: 10.1097/00005072-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 254.Beers DR. Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis. J Neurochem. 2001;79:499–509. doi: 10.1046/j.1471-4159.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- 255.Malik MN. Purification and degradation of purified neurofilament proteins by the brain calcium-activated neutral proteases. Life Sci. 1986;39:1335–1343. doi: 10.1016/0024-3205(86)90331-0. [DOI] [PubMed] [Google Scholar]
- 256.Pant HC. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J. 1988;256:665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Johnson GV. The regulatory role of calmodulin in the proteolysis of individual neurofilament proteins by calpain. Neurochem Res. 1991;16:869–873. doi: 10.1007/BF00965535. [DOI] [PubMed] [Google Scholar]
- 258.Strong MJ. Phosphorylation state of the native high-molecular-weight neurofilament subunit protein from cervical spinal cord in sporadic amyotrophic lateral sclerosis. J Neurochem. 2001;76:1315–1325. doi: 10.1046/j.1471-4159.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 259.Bajaj NP. Cyclin dependent kinase-5 (CDK-5) phosphor-ylates neurofilament heavy (NF-H) chain to generate epitopes for antibodies that label neurofilament accumulations in amyotrophic lateral sclerosis (ALS) and is present in affected motor neurones in ALS. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:833–850. doi: 10.1016/s0278-5846(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 260.Nguyen MD. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- 261.Couillard-Despres S. Protective effect of neurofilament heavy gene overexpression in motor neuron disease induced by mutant superoxide dismutase. Proc Natl Acad Sci U S A. 1998;95:9626–9630. doi: 10.1073/pnas.95.16.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Takahashi S. Mutant superoxide dismutase 1 causes motor neuron degeneration independent of cyclin-dependent kinase 5 activation by p35 or p25. J Neurochem. 2004;88:1295–1304. doi: 10.1046/j.1471-4159.2003.02256.x. [DOI] [PubMed] [Google Scholar]
- 263.Wootz H. XIAP decreases caspase-12 cleavage and calpain activity in spinal cord of ALS transgenic mice. Exp Cell Res. 2006;312:1890–1898. doi: 10.1016/j.yexcr.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 264.Cardali S. Detection of alphaII-spectrin and breakdown products in humans after severe traumatic brain injury. J Neurosurg Sci. 2006;50:25–31. [PubMed] [Google Scholar]
- 265.Lewis SB. Alpha-II spectrin breakdown products in aneurysmal subarachnoid hemorrhage: a novel biomarker of proteolytic injury. J Neurosurg. 2007;107:792–796. doi: 10.3171/JNS-07/10/0792. [DOI] [PubMed] [Google Scholar]
- 266.Siman R. Novel surrogate markers for acute brain damage: cerebrospinal fluid levels corrrelate with severity of ischemic neurodegeneration in the rat. J Cereb Blood Flow Metab. 2005;25:1433–1444. doi: 10.1038/sj.jcbfm.9600138. [DOI] [PubMed] [Google Scholar]
- 267.Polster BM. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- 268.Reimertz C. Ca(2+)-induced inhibition of apoptosis in human SH-SY5Y neuroblastoma cells: degradation of apoptotic protease activating factor-1 (APAF-1). J Neurochem. 2001;78:1256–1266. doi: 10.1046/j.1471-4159.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- 269.Choi WS. Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2. J Neurochem. 2001;77:1531–1541. doi: 10.1046/j.1471-4159.2001.00368.x. [DOI] [PubMed] [Google Scholar]
- 270.Wood DE. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 271.Nakagawa T. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Takano J. Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways in adult brains: evidence from calpastatin mutant mice. J Biol Chem. 2005;280:16175–16184. doi: 10.1074/jbc.M414552200. [DOI] [PubMed] [Google Scholar]
- 273.Kim MJ. Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc Natl Acad Sci U S A. 2002;99:9870–9875. doi: 10.1073/pnas.152336999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Covault J. Calcium-activated proteolysis of intracellular domains in the cell adhesion molecules NCAM and N-cadherin. Brain Res Mol Brain Res. 1991;11:11–16. doi: 10.1016/0169-328x(91)90015-p. [DOI] [PubMed] [Google Scholar]
- 275.Hajimohammadreza I. Neuronal nitric oxide synthase and calmodulin-dependent protein kinase IIalpha undergo neurotoxin-induced proteolysis. J Neurochem. 1997;69:1006–1013. doi: 10.1046/j.1471-4159.1997.69031006.x. [DOI] [PubMed] [Google Scholar]
- 276.Tallant EA. Activation of a calmodulin-dependent phosphatase by a Ca2+-dependent protease. Biochemistry. 1988;27:2205–2211. doi: 10.1021/bi00406a059. [DOI] [PubMed] [Google Scholar]
- 277.Blomgren K. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- 278.Chua BT. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- 279.Ruiz-Vela A. Implication of calpain in caspase activation during B cell clonal deletion. Embo J. 1999;18:4988–4998. doi: 10.1093/emboj/18.18.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Volbracht C. The critical role of calpain versus caspase activation in excitotoxic injury induced by nitric oxide. J Neurochem. 2005;93:1280–1292. doi: 10.1111/j.1471-4159.2005.03122.x. [DOI] [PubMed] [Google Scholar]
- 281.Nakagawa T. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 282.Krajewska M. Early processing of Bid and caspase-6, -8, -10, -14 in the canine brain during cardiac arrest and resuscitation. Exp Neurol. 2004;189:261–279. doi: 10.1016/j.expneurol.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 283.Ohno H. Ischemia promotes calpain-mediated degradation of p120-catenin in SH-SY5Y cells. Biochem Biophys Res Commun. 2007;353:547–552. doi: 10.1016/j.bbrc.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 284.Zakharov VV. Site-specific calcium-dependent proteolysis of neuronal protein GAP-43. Neurosci Res. 2001;39:447–453. doi: 10.1016/s0168-0102(01)00201-2. [DOI] [PubMed] [Google Scholar]
- 285.Zakharov VV. M-calpain-mediated cleavage of GAP-43 near Ser41 is negatively regulated by protein kinase C, calmodulin and calpain-inhibiting fragment GAP-43−3. J Neurochem. 2007;101:1539–1551. doi: 10.1111/j.1471-4159.2007.04452.x. [DOI] [PubMed] [Google Scholar]
- 286.Yuen EY. Calpain regulation of AMPA receptor channels in cortical pyramidal neurons. J Physiol. 2007;580:241–254. doi: 10.1113/jphysiol.2006.122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lee SY. Purification and properties of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. Susceptibility to calpain. J Biol Chem. 1990;265:9434–9440. [PubMed] [Google Scholar]
- 288.Yoshihara Y. Purification of a novel type of calcium-activated neutral protease from rat brain. Possible involvement in production of the neuropeptide kyotorphin from calpastatin fragments. J Biol Chem. 1990;265:5809–5815. [PubMed] [Google Scholar]
- 289.Yoshihara Y. Calcium-activated neutral protease (CANP), a putative processing enzyme of the neuropeptide, kyotorphin, in the brain. Biochem Biophys Res Commun. 1988;155:546–553. doi: 10.1016/s0006-291x(88)80529-1. [DOI] [PubMed] [Google Scholar]
- 290.Sato C. Rapid proteolysis of brain MAP-1 related cytoskeleton-associated 350 kd protein by purified calpain. Cell Struct Funct. 1986;11:253–257. doi: 10.1247/csf.11.253. [DOI] [PubMed] [Google Scholar]
- 291.Inuzuka T. Changes in the concentrations of cerebral proteins following occlusion of the middle cerebral artery in rats. Stroke. 1990;21:917–922. doi: 10.1161/01.str.21.6.917. [DOI] [PubMed] [Google Scholar]
- 292.Posmantur RM. Cytoskeletal derangements of cortical neuronal processes three hours after traumatic brain injury in rats: an immunofluorescence study. J Neuropathol Exp Neurol. 1996;55:68–80. doi: 10.1097/00005072-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 293.Dong YN. Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J Neurosci. 2004;24:11035–11045. doi: 10.1523/JNEUROSCI.3722-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Banno Y. Endogenous cleavage of phospholipase C-beta 3 by agonist-induced activation of calpain in human platelets. J Biol Chem. 1995;270:4318–4324. doi: 10.1074/jbc.270.9.4318. [DOI] [PubMed] [Google Scholar]
- 295.Low MG. Multiple forms of phosphoinositide-specific phospholipase C of different relative molecular masses in animal tissues. Evidence for modification of the platelet enzyme by Ca2+-dependent proteinase. Biochem J. 1984;221:813–820. doi: 10.1042/bj2210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Park D. Removal of the carboxyl-terminal region of phospholipase C-beta 1 by calpain abolishes activation by G alpha q. J Biol Chem. 1993;268:3710–3714. [PubMed] [Google Scholar]
- 297.Boland B. beta-Amyloid (1−40)-induced apoptosis of cultured cortical neurones involves calpain-mediated cleavage of poly-ADP-ribose polymerase. Neurobiol Aging. 2003;24:179–186. doi: 10.1016/s0197-4580(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 298.Mikawa K. Studies on proteolysis of protein kinase C with calpain I and II. Kobe J Med Sci. 1990;36:55–69. [PubMed] [Google Scholar]
- 299.Sessoms JS. Ca(2+)-induced persistent protein kinase C activation in rat hippocampal homogenates. Second Messengers Phosphoprot. 1992;14:109–126. [PubMed] [Google Scholar]
- 300.Ziemka-Nalecz M. Decrease of PKC precedes other cellular signs of calpain activation in area CA1 of the hippocampus after transient cerebral ischemia. Neurochem Int. 2003;42:205–214. doi: 10.1016/s0197-0186(02)00096-7. [DOI] [PubMed] [Google Scholar]
- 301.Lu X. Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neurosci Lett. 2000;286:149–153. doi: 10.1016/s0304-3940(00)01101-0. [DOI] [PubMed] [Google Scholar]
- 302.Jourdi H. Effects of positive AMPA receptor modulators on calpain-mediated spectrin degradation in cultured hippocampal slices. Neurochem Int. 2005;46:31–40. doi: 10.1016/j.neuint.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 303.Johnson GV. Proteolysis of tau by calpain. Biochem Biophys Res Commun. 1989;163:1505–1511. doi: 10.1016/0006-291x(89)91150-9. [DOI] [PubMed] [Google Scholar]
- 304.Xie HQ. Calcineurin inhibition prevents calpain-mediated proteolysis of tau in differentiated PC12 cells. J Neurosci Res. 1998;53:153–164. doi: 10.1002/(SICI)1097-4547(19980715)53:2<153::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 305.Billger M. Proteolysis of tubulin and microtubule-associated proteins 1 and 2 by calpain I and II. Difference in sensitivity of assembled and disassembled microtubules. Cell Calcium. 1988;9:33–44. doi: 10.1016/0143-4160(88)90036-x. [DOI] [PubMed] [Google Scholar]