Abstract
Objective
Deficits in sensory gating are a common feature of schizophrenia. Failure of inhibitory gating mechanisms, shown by poor suppression of evoked responses to repeated auditory stimuli, have been previously studied using EEG methods. These methods yield information about the temporal characteristics of sensory gating deficits, but do not identify brain regions involved in the process. Hence, the neuroanatomical substrates of poor sensory gating in schizophrenia remain largely unknown. This study used functional magnetic resonance imaging (fMRI) to investigate the functional neuroanatomy of sensory gating deficits in schizophrenia.
Methods
Twelve patients with schizophrenia and 12 healthy comparison subjects were scanned at 3 Tesla while performing a sensory gating task developed for fMRI. P50 EEG evoked potential recordings from a paired-stimulus conditioning-test paradigm were obtained from the same subjects.
Results
Compared to healthy comparison subjects, patients with schizophrenia exhibited greater activation in the hippocampus, thalamus, and dorsolateral prefrontal cortex (DLPFC) during the fMRI sensory gating task. No group difference was observed in the superior temporal gyrus. Schizophrenia subjects also showed decreased P50 suppression as measured with EEG. Hemodynamic response in the fMRI measure was positively correlated with test/conditioning ratios from the EEG sensory gating measure.
Conclusions
Poor sensory gating in schizophrenia is associated with dysfunction of an apparent network of brain regions, including the hippocampus, thalamus and DLPFC. Greater activation of these regions is consistent with evidence for diminished inhibitory function in schizophrenia.
Keywords: Schizophrenia, fMRI, sensory gating, hippocampus, thalamus, prefrontal cortex
1. Introduction
“During the last while back I have noticed that noises all seem to be louder to me than they were before…I notice it most with background noises—you know what I mean, noises that are always around but you don't notice them. Now they seem to be just as loud and sometimes louder than the main noises that are going on…It's a bit alarming at times because it makes it difficult to keep your mind on something when there's so much going on that you can't help listening to.”
This quotation from a schizophrenia patient presented by McGhie and Chapman in 1961 describes a problem commonly reported by patients with the disease – the inability to filter out irrelevant information from the environment (McGhie and Chapman 1961). Frequent reports of such problems, in the context of a disease with such varied presentation, have led investigators to suspect that this “sensory gating” deficit may be related to disease pathology. Venables (1967) suggested that the psychotic state might be characterized as a state of hypervigilance in that psychotic patients appear to be flooded by stimuli whose intensity they cannot regulate through sensory gating mechanisms (Venables 1967). Patients with schizophrenia might suffer from deficits in filtering sensory input, which leads to this flooding. Different types of psychopathology could then be conceptualized as attempts to deal with this flooding. Because understanding brain function during sensory gating deficits in schizophrenia may yield insights into disease pathophysiology, investigators have used these early descriptions to develop EEG methods to investigate sensory gating.
One measure of sensory gating is a conditioning-testing paradigm, in which P50 auditory evoked potentials are measured from repeated pairs of clicks, separated by 500 ms. In healthy controls, responses to the second click in the pair are suppressed, compared to the first. Schizophrenia patients have diminished inhibition of responses to the second stimulus (Adler et al. 1982). This measure has thus revealed inhibitory deficits in sensory gating in schizophrenia (Bramon et al. 2004). Sensory gating deficits have also been found in first-degree relatives of schizophrenia patients, suggesting these deficits represent heritable factors that increase vulnerability to schizophrenia (Freedman et al. 1997).
EEG studies lack the spatial resolution to determine which brain regions mediate this deficit in schizophrenia. Animal studies and a limited number of human intracranial recording and lesion studies point to the involvement of several brain regions, including the hippocampus, thalamus and prefrontal cortex (Miller and Freedman 1995;Erwin et al. 1991;Knight et al. 1989;Grunwald et al. 2003). The most often studied region is the hippocampus. In a rodent model of sensory gating, hippocampal activity is inhibited at the 500ms inter-stimulus interval, an inhibition which is dependent upon cholinergic activity from the medial septal fimbria-fornix input (Miller and Freedman 1993). Studies recording intracranial evoked potentials from patients undergoing invasive presurgical evaluation for epilepsy have also implicated the hippocampus in sensory gating (Freedman et al. 1991;Grunwald et al. 2003;Boutros et al. 2005). Involvement of the thalamus in sensory gating has also been proposed. Early studies of depth recordings of midlatency auditory potentials in cats suggested a generator system involving the thalamus (Hinman and Buchwald 1983). Correspondence of cat to human midlatency responses led investigators to propose the thalamus as a P50 source generator (Erwin and Buchwald 1987). Reports of thalamic dysfunction in schizophrenia led to the hypothesis that this region may be involved in sensory gating deficits in the disease (Erwin et al. 1991). Finally, both surface and intracranial recordings support the involvement of the dorsolateral prefrontal cortex in sensory gating (Knight et al. 1989;Grunwald et al. 2003).
Only one group has used magnetoencephalography (MEG), which has improved spatial resolution, compared to EEG, to study the neural correlates of auditory gating deficits in schizophrenia (Edgar et al. 2005;Thoma et al. 2005;Huang et al. 2003;Thoma et al. 2003). In a simultaneous MEG/EEG study, Thoma and colleagues (2003) found impaired gating in schizophrenia patients at the M50, the likely MEG analogue to the P50 (Thoma et al. 2003). M50 dipoles localized to the superior temporal gyrus (STG), consistent with prior MEG studies that did not evaluate gating (Reite et al. 1988). The relationship between M50 gating and established measures of sensory gating is unclear, however, as the M50 ratio did not correlate with the P50 ratio in patients and only weakly in the left hemisphere in controls. Additional limitations of MEG, including lack of uniform sensitivity throughout the brain and the difficulty of modeling multiple simultaneous sources, suggest that further studies are needed to determine the functional neuroanatomy of this deficit.
The present study is the first to use functional magnetic resonance imaging (fMRI) to investigate the neurobiology underlying auditory sensory gating deficits in schizophrenia. The superior spatial resolution of this technique complements the high temporal resolution of EEG and MEG, allowing an evaluation of the involvement of a network of multiple specific brain regions to the deficit. One reason for the lack of such studies to date likely stems from the loud scanning environment, which interferes with stimulus presentation. A related problem specific to auditory gating experiments is the requirement for a relatively long silent period prior to stimulus presentation for inhibitory circuitry to reset (Freedman et al. 1983). The present study addresses these issues with a paradigm that uses clustered volume acquisition. This scanning technique acquires all slices for each brain volume in 2 sec, interspersed with long periods of silence (6 sec), allowing silence during and prior to stimulus presentation, yet still providing sensitivity to the hemodynamic response.
Another issue hindering fMRI studies of sensory gating is the broad temporal resolution of the technique, which is limited to seconds, compared to the millisecond resolution obtainable in EEG/MEG. Thus, it is difficult to resolve responses from two stimuli, separated by only 500ms, as in the typical sensory gating paradigm. To address this, our paradigm compares the response of one click to the response of a brief click train (9 clicks, separated by 500s, over a total of 4000ms), rather than to two clicks, as in the typical paradigm (see Methods for paradigm details).
This study examines sensory gating deficits with fMRI and compares these results to evoked potentials from a typical paired-click paradigm using EEG. We test the hypotheses that 1) a network of brain regions, including the STG, hippocampus, thalamus and DLPFC, is involved in sensory gating deficits in schizophrenia, and 2) the newly developed fMRI sensory gating measure is correlated with typical EEG measures of this deficit.
2. Methods
2.1 Subjects
Twelve healthy controls and 12 subjects with DSM-IV schizophrenia participated in this study. Diagnoses were made by two raters following interviews with the Diagnostic Instrument for Genetic Studies (DIGS). Controls included 6 males, 6 females, mean age 36.4, SD 12.3 yrs, all Caucasian, with a mean education of 17, SD 1.8 yrs. Subjects with schizophrenia, all stable outpatients, also included 6 males, 6 females, with a mean age of 43.6, SD 12.3 yrs. Of the 12 schizophrenia subjects, 10 were Caucasian and 2 African American, with a mean education of 15.8, SD 3.0 yrs. No significant group difference was observed in age (t=1.46,df=22,p=0.15) or education (t=1.14,df=22,p=0.13). One additional schizophrenia subject was excluded due to excess head motion (> 1 mm) during scanning. Eight of 12 subjects with schizophrenia were treated with atypical neuroleptics, 2 were treated with typicals and 2 were treated with both typical and atypical neuroleptics. One subject was treated with clozapine. Subjects who smoked refrained from smoking for at least 30 minutes prior to the study. All volunteers provided written, informed consent approved by the University of Colorado IRB.
2.2 fMRI Methods
Studies were performed with at 3T GE MR system using a standard quadrature head coil. Following a hearing test (see below), a high-resolution, T1-weighted 3D anatomical scan was acquired for each subject (IR-SPGR, TR=9ms, TE=1.9ms, TI=500ms flip angle=10°, matrix = 256×256, FOV = 220mm2, 124 1.7 mm thick coronal slices) for coregistration to functional data. Functional images were acquired with a gradient-echo T2* Blood Oxygenation Level Dependant (BOLD) contrast technique, with TR=14000ms (as a clustered volume acquisition of 2000 ms, plus an additional 12000ms silent interval), TE=30ms, FOV=220mm2, 642 matrix, 31 slices, 4mm thick, no gap, angled parallel to the planum sphenoidale. Additionally, one IR-EPI (TI=505ms) volume was acquired to improve coregistration between EPIs and the IR-SPGR.
Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark). Auditory stimuli were presented via MR-compatible headphones (Resonance Technology, Inc., CA, USA). MR-compatible goggles (Resonance Technology, Inc, CA, USA) were used for visual stimuli. Motor responses for the hearing test were collected via a fiber optic response pad (Cedrus Corp, USA).
2.3 fMRI Paradigm
Prior to scanning, subjects were administered a hearing test in the scanner to set the task volume at 50dB above hearing threshold. Previous studies have shown this sound level to elicit maximal differences in sensory gating between controls and subjects with schizophrenia (Griffith et al. 1995). The same auditory stimuli were used in the hearing test and the gating task. Click stimuli consisted of “broadband chirps”, 10ms in duration, designed to elicit robust cortical responses (Dau et al. 2000).
After the hearing test and structural scan, subjects performed the sensory gating task while undergoing fMRI (Figure 1). Subjects watched a silent movie (“Wallace and Gromit”) during the scan while auditory stimuli were played in the background. The paradigm used a clustered volume acquisition approach to 1) allow a 6s silent period following scanner noise for neuronal inhibitory circuitry to reset (Freedman et al. 1983), and to 2) to allow auditory stimuli to be presented during silence. For each presentation during the 12s silent period between scanner noise, subjects heard either 1) silence 2) one click or 3) repeated clicks, separated by 0.5s, for 4s. Auditory stimuli were centered at 8s after scanner noise ended. The following volume was acquired 4-6s following auditory stimulation to capture peak hemodynamic response (Edmister et al. 1999). Two stimulus presentations comprised one block. Twenty-eight second blocks of “silence”, “one click” or “repeated clicks” were presented to the subjects over three runs, totaling 21 minutes. Five blocks of each stimulus type were randomly distributed throughout each run. Between runs, brief conversations were held with subjects to ensure that they were awake and responsive. A comparison of “repeated clicks” to “one click” was the measure of auditory sensory gating. This subtraction should remove some of the effects of overall auditory responsiveness, which was measured separately in the contrast “one click - silence.” Multiple clicks, rather than a click pair were used to maximize sensitivity to the difference in BOLD response between the two stimulus conditions. Inhibitory gating in a train of clicks separated by 500ms is nearly identical to gating in a paired click paradigm, in that responses to the second and subsequent clicks are diminished, compared to the first click (Rosburg et al. 2004). Patients with schizophrenia also show deficits in gating with click trains (Adler et al. 1985;Erwin et al. 1991).
Figure 1.
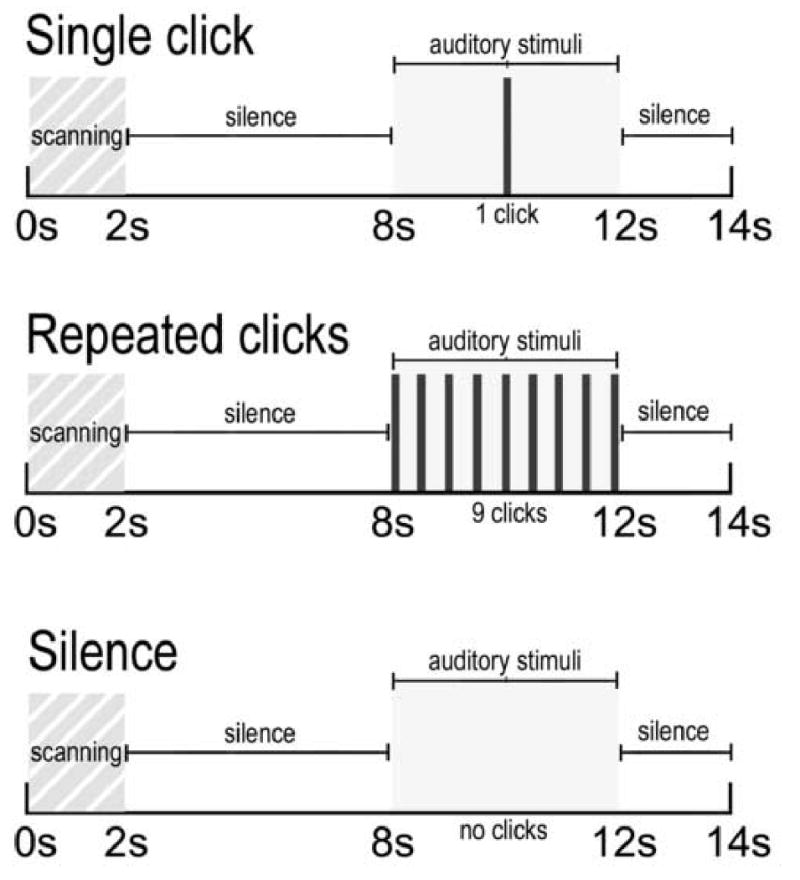
Schematic representation of experimental design. For all conditions, two seconds of scanning was followed by 6 s of silence for inhibitory circuitry to resent. Following the silent period, subjects were presented with a single click, repeated clicks each separated by 500ms, or silence.
2.4 fMRI Data Analysis
Data were analyzed using SPM2 (Wellcome Dept. of Imaging Neuroscience, London). Data from each subject were realigned to the first volume, normalized to the Montreal Neurological Institute template, using a gray-matter-segmented IR-EPI as an intermediate to improve registration between the EPI and IR-SPGR, and smoothed with an 8 mm FWHM Gaussian kernel. A 128s high pass filter was applied to remove low-frequency fluctuation in the BOLD signal.
To account for both within-group and within-subject variance, a random effects analysis was implemented in SPM2. The general linear model was used to estimate first-level repeated measures identifying the effects of condition for each subject. Data were modeled with a HRF-convolved boxcar function. The between subjects effect of diagnosis was evaluated by entering parameter estimates from each individual's first level analysis into a second level model. The result of this two-step process is mathematically analogous to evaluating a mixed-design ANOVA 2 × 2 interaction term. The measure of sensory gating was the contrast “repeated clicks – single click.” The contrasts “single click – silence” and “repeated clicks – silence” were also evaluated. T-contrasts, which in SPM2 are one-tailed, rather than F-contrasts (two-tailed in SPM2) were evaluated because we had apriori hypotheses about the directionality of group differences within the condition contrasts.
All analyses were performed on four regions of interest: the superior temporal gyrus (STG), hippocampus, thalamus and dorsolateral prefrontal cortex (DLPFC). A priori hypotheses about activation of these areas were evaluated using anatomically defined ROIs from the WFU Pickatlas (Maldjian et al. 2003). The conservative hippocampal and thalamic ROIs included the entire anatomical structures. The DLPFC ROI consisted of Brodmann Areas 9 and 46 combined, excluding the superior frontal gyrus. The mean response for all voxels in each ROI was determined using the Marsbar toolbox (Brett et al. 2002) in SPM2. To improve statistical power, results were masked with a gray-matter mask, consisting of the average gray-matter from all subjects obtained by their gray-segmented IR-EPIs. Functional results were overlaid onto the group average T1-weighted anatomical images and thresholded at a whole-brain p<0.01 for visualization.
2.5 EEG Paradigm and Methods
Details of the paired-click recording paradigm have been described previously (Adler et al. 2004). The P50 potential was identified and measured by using a previously described computer algorithm (Adler et al. 2004). The amplitude of the P50 test wave was divided by the amplitude of the P50 conditioning wave, expressed as a percentage: the P50 ratio. Subjects were given no special instructions concerning the clicks they were hearing. Recordings were obtained from 11 of 12 subjects in each group. One subject in each group was unavailable for EEG recording.
3. Results
3.1 fMRI Results
Relative to the comparison subjects, patients with schizophrenia exhibited greater activity during the sensory gating comparison (repeated clicks – single click) in three of the four regions of interest evaluated in this study, including the hippocampus (t=2.70, df=22, p=0.007, left; t=1.53, df=22, p=0.066, right), DLPFC (t=2.56, df=22, p=0.009, left; t=2.16, df=22, p=0.021, right) and thalamus (t=1.67, df=22, p=0.054, left; t=2.56, df=22, p=0.012, right) (Figure 2). Mean group differences in percent signal change and 95% confidence intervals were 0.46 [0.11 – 0.82] for left hippocampus, 0.15 [-0.05 – 0.35] for right hippocampus, 0.28 [0.04 – 0.53] for left thalamus, 0.31 [-0.07 – 0.70] for right thalamus, 0.35 [0.07 - .63] for left DLPFC, and 0.23 [0.01 – 0.46] for right DLPFC. No significant group difference in activity during sensory gating was observed in the primary auditory cortex (t=0.54, df=22, p=0.299, left; t=0.41, df=22, p=0.343, right).
Figure 2.
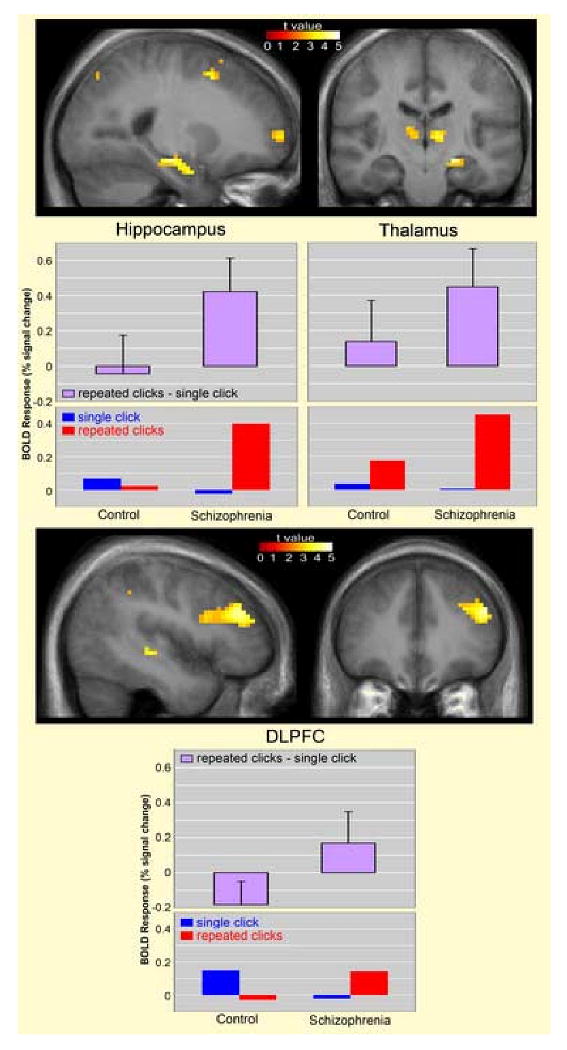
Increased hemodynamic response in subjects with schizophrenia (N=12) relative to healthy comparison subjects (N=12) during sensory gating. Statistical parametric maps thresholded at P < 0.01, overlaid onto the average T1-weighted anatomy of all subjects. Local maxima located at x = -24, y = -18, z = -15 (left hippocampus), x = -9, y = -12, z = 3 (left thalamus), x = 9, y = -21, z = 3 (right thalamus), x = -48, y = 36, z = 27 (left DLPFC). Graphs below show mean blood oxygenation level dependent (BOLD) responses in terms of % signal change, relative to the global mean, for the sensory gating measure, (repeated clicks – single click), and for each condition separately, compared to silence. Error bars indicate standard deviations.
Figure 2 also shows mean group responses for the single clicks (single click – silence) and repeated clicks (repeated clicks – silence) in the hippocampus, thalamus and DLPFC. The only significant group difference in response to the separate stimulus conditions was increased activation of the hippocampus during ‘repeated clicks’ in schizophrenia compared to control subjects (t=2.35, df=22, p=0.03).
Figure 3 shows the combined group response to the single click condition, demonstrating the sensitivity of this paradigm. A single click was associated with robust activity in both the left (t=3.25, df=23, p=0.002) and right (t=2.66, df=23, p=0.007) posterior Heschl's gyri. No significant group differences were observed in this region in response to the single click (t=0.89, df=22, p=0.383, left; t=0.85, df=22, p=0.403, right) or the repeated clicks (t=0.98, df=22, p=0.334, left; t=0.99, df=22, p=0.332, right). Mean BOLD responses in the primary auditory cortex to single clicks were 0.13 SD 0.32 (left), 0.11 SD 0.35 (right) for controls and 0.24 SD 0.23 (left), 0.21 SD 0.22 (right) for schizophrenia subjects. Primary auditory cortex responses to repeated clicks were 0.50 SD 0.55 (left), 0.46 SD 0.43 (right) in controls and 0.71 SD 0.46 (left) and 0.63 SD 0.41 (right) in schizophrenia subjects. All BOLD responses are expressed as the mean % signal change across all voxels within the ROI, relative to the global mean.
Figure 3.
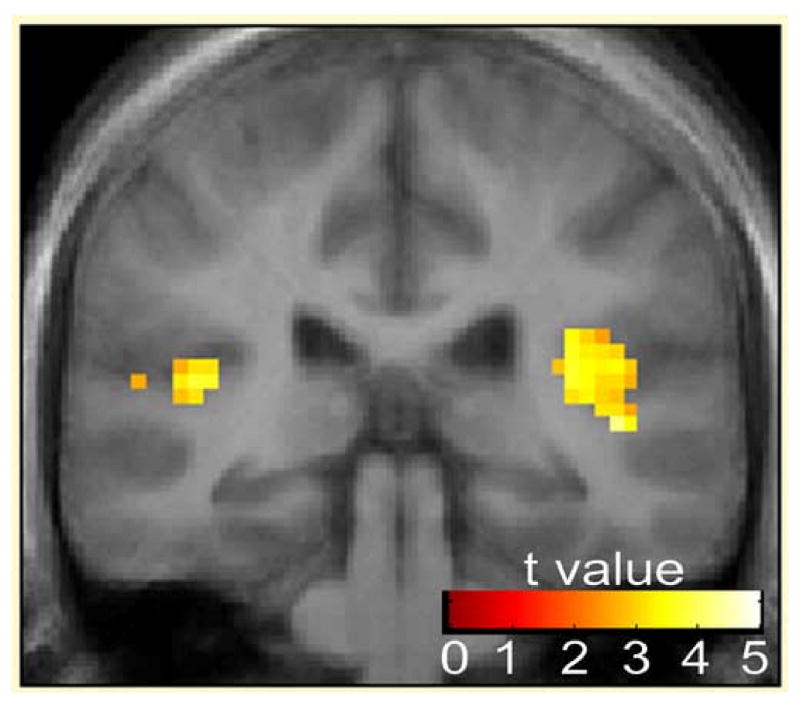
Activation across groups of bilateral primary auditory cortex in response to single click (N=24). Statistical parametric maps thresholded at P < 0.01, overlaid onto the average T1-weighted anatomy of all subjects. Local maxima located at x = -42, y = -33, z = -12 (left Heschl's gyrus), x = 42, y = -30, z = 12 (right Heschle's gryus)
In addition to the region of interest analysis, an exploratory, whole-brain analysis was conducted to examine responses in areas outside the hypothesized regions of interest. No areas of difference were observed in any region, after correcting for multiple comparisons using the false discovery rate technique (30). At an uncorrected threshold of p <0.001, differences were observed in the DLPFC (x = -48, y=36,z=27), hippocampus (x=-24,y=-18,z=-15), thalamus (x=-9,y=-21,z=3), precentral gyrus (x=-30,y=-24,z=60), parietal region (x=-57,y=-42,z=45), medial occipito-temporal gyrus (x=45,y=-45,z=-21) and middle temporal gyrus (x=-48,y=-27,z=-6). Inferences about activation of these areas are limited, due to the exploratory nature of this analysis.
3.2 EEG Results
Schizophrenia patients had significantly higher P50 test/conditioning ratios, relative to comparison subjects (t=5.28, df=20, p=0.001), Figure 4. The average evoked response to the conditioning click was 2.76μV, SD = 1.13 in schizophrenia patients and 2.71μV, SD = 1.61 in comparison subjects. The average response to the test clicks was 1.99μV, SD=0.86 in schizophrenia patients and 0.69μV, SD=0.63 in comparison subjects.
Figure 4.
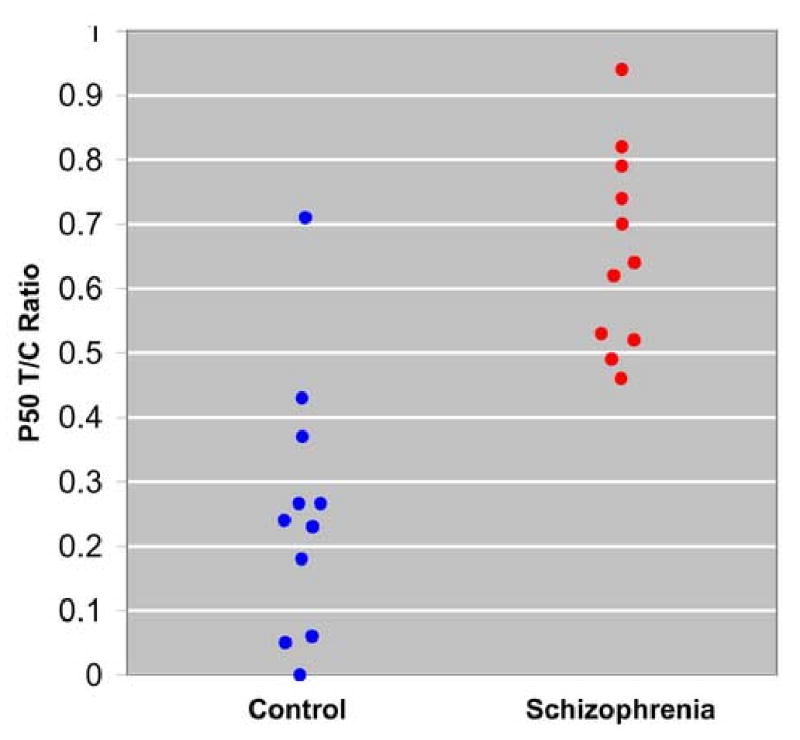
P50 sensory gating ratios in schizophrenia subjects (N=11) and healthy comparison subjects (N=11).
3.3 fMRI-EEG Correlations
Figure 5 shows significant positive correlations between the fMRI measure of sensory gating and P50 gating measured with EEG. The strongest correlation was observed in the hippocampus (R2 = 0.43, df=21,p=0.001). Significant correlations were also observed in the thalamus (R2 = 0.235, df=21,p=0.022) and dorsolateral prefrontal cortex (R2 = 0.40, df=21,p=0.002). Correlations were collapsed across group because no significant group × P50 T/C Ratio × BOLD response interaction was observed in any region. No significant relationships were observed between P50T/C Ratios and the “single click” condition in the fMRI task. Positive correlations were observed between P50 T/C ratios and fMRI task “repeated clicks” in the hippocampus (R2=0.304, df=21, p=0.008), thalamus (R2=0.196, df=21,p=0.04) and DLPFC (R2=0.211,df=21,p=0.031).
Figure 5.
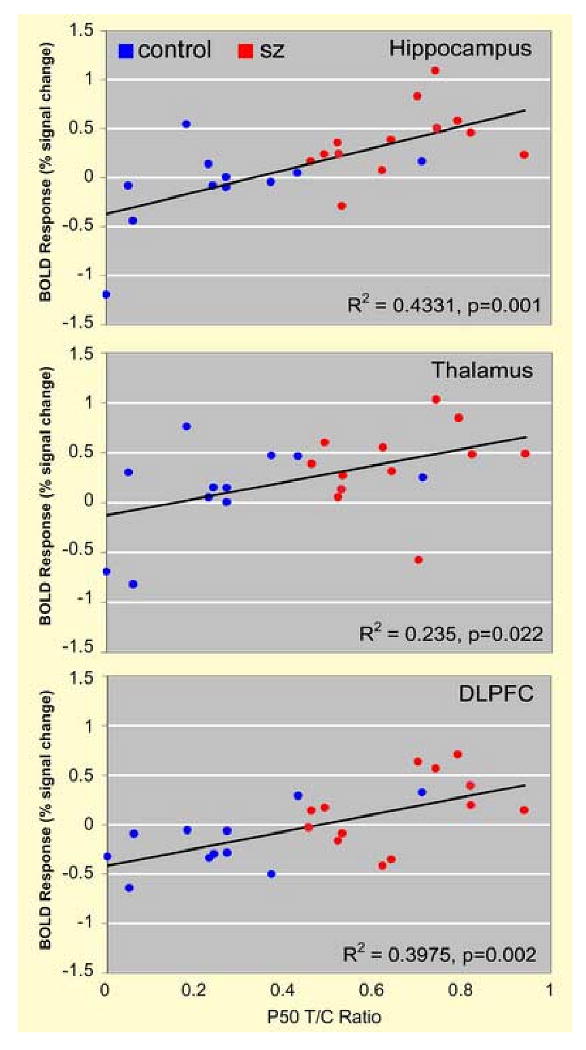
Correlations between P50 sensory gating ratios and hemodynamic response during fMRI sensory gating task in the thalamus, hippocampus and DLPFC.
4. Discussion
The primary finding of this study is greater activation of the hippocampus, DLPFC and thalamus during sensory gating in subjects with schizophrenia, relative to healthy comparison subjects. Furthermore, activation of these regions was positively correlated with EEG measures of a typical P50 conditioning-testing paradigm, suggesting this new fMRI measure of sensory gating may assess the same deficient gating mechanisms.
4.1 Hippocampus
Greater activation of the hippocampi, particularly the left hippocampus, during the fMRI sensory gating task was observed in schizophrenia subjects. This activation was strongly correlated with evoked responses during the EEG conditioning-testing sensory gating measure. These results are consistent with evidence from animal studies and human intracranial recording studies for the involvement of the hippocampus in sensory gating, and deficits in this process in schizophrenia.
In the rodent model of sensory gating, pyramidal neurons in the hippocampus respond to the initial stimulus of a typical dual-click conditioning-testing paradigm at about 20 ms post stimulus, on the rising phase of the P20 component of the P20-N40 complex, the putative rodent analogue of the P50 and N100, respectively. Interneurons fire continuously in bursts, with peak activity about 300 ms post stimulus, continuing through the next stimulus at 500 ms, suggesting that sensory gating is at least in part mediated by hippocampal interneurons (Miller and Freedman 1995).
A limited number of intracranial evoked potential studies have also implicated the hippocampus in sensory gating. Using subdural strip electrodes, we identified auditory evoked responses with a 50ms latency from the surfaces of the medial and lateral temporal lobe. The P50 wave showed polarity reversal at the hippocampus, suggesting this site may be involved in generation of the wave (Freedman et al. 1991). This finding was consistent with earlier reports showing auditory evoked responses in the hippocampus at a similar latency using depth electrodes (Goff et al. 1980). Using both hippocampal depth electrodes and subdural strip and grid electrodes, Grunwald, Boutros and colleagues recently identified gating in the hippocampus, temporo-parietal region and prefrontal cortex. These investigators observed hippocampal responses at a latency of about 250 ms, leading the authors to suggest that sensory gating may be a multi-step process, with an early phase subserved by temporo-parietal and prefrontal regions and a later phase mediated by the hippocampus.
While the above studies support the involvement of the hippocampus in sensory gating, the involvement of this structure in gating deficits in schizophrenia has until now been less direct, consisting of observations of pathology of both inhibitory interneurons and mediators of inhibitory function, and in animal models of gating deficits (Benes et al. 1991;Miller and Freedman 1995;Thoma et al. 2003) Data from the present study are the first to directly demonstrate the involvement of the hippocampus in sensory gating deficits in schizophrenia.
4.2 Thalamus
Schizophrenia subjects also exhibited greater activation during sensory gating in the medial thalamus. Thalamic involvement in sensory gating is consistent with prior studies of gating in animals (Hinman and Buchwald 1983;Erwin and Buchwald 1987). Filtering, or gating of sensory information occurs in the thalamus, likely mediated by the nucleus reticularis thalami, a thin lamina of neurons surrounding the thalamus composed entirely of GABAergic interneurons (Scheibel and Scheibel 1966). Thalamic involvement in sensory gating deficits has not been well studied recently, however, possibly due the increased reliance on the rodent model of sensory gating. Inhibitory circuitry of the rodent thalamus is markedly different from that of the primate thalamus, limiting inferences about thalamic inhibitory function between species (Bowery et al. 1999).
4.3 Dorsolateral Prefrontal Cortex
Subjects with schizophrenia also exhibited greater activation during sensory gating in the DLPFC. Activity in this region was also correlated with evoked responses from the EEG measure of sensory gating. Involvement the DLPFC in sensory gating has been previously suggested by human lesion studies and recent intracranial recording studies. A recent human intracranial recording study using a paired click paradigm found evidence of sensory gating in the prefrontal cortex, as well as the hippocampus (Grunwald et al. 2003).
Knight and colleagues (1989) recorded middle-latency auditory evoked potentials in controls and patients with focal lesions in the DLPFC and found that patients with unilateral DLPFC lesions showed increased amplitudes of evoked responses in the primary auditory cortex. The authors concluded that the DLPFC exerts early inhibitory modulation of input to the primary auditory cortex (Knight et al. 1989). The present data, which do not reveal auditory cortex differences, suggest an additional role for the DLPFC beyond its role in ‘top-down’ inhibitory modulation of the auditory cortex. It is possible that ‘bottom-up’ processes may also be at work, such that the hippocampus and thalamus may fail to inhibit afferent sensory information, leading to greater activation of the DLPFC. The notion of a different cause of greater activation in the DLPFC is also supported by evaluating the separate responses from the single and repeated click conditions. Unlike in the hippocampus and thalamus, where significant group differences in sensory gating were largely driven by robust responses to repeated clicks in schizophrenia subjects, greater DLPFC activation was driven equally by a more modest response to repeated clicks in schizophrenia and a larger response to single clicks in controls.
4.4 Superior Temporal Gyrus
No differences in activation during sensory gating were observed in the superior temporal gyrus. This result is consistent with previous physiological studies of sensory gating in this region. Gating of responses to auditory stimuli at the 500 ms interval or greater is not observed in the primary auditory cortex (Simpson and Knight 1993) nor in neurons in the entorhinal cortex, which receives projections from this region (Stafekhina and Vinogradova 1975). MEG studies suggest bilateral superior temporal gyrus sources for the M50, the putative analogue to the P50 (Reite et al. 1988). The left, but not right, hemisphere M50 gating ratio is moderately correlated with P50 gating in healthy controls (Thoma et al. 2003). In patients with schizophrenia, however, Thoma et al. (2003, 2005) have reported that P50 and M50 gating ratios do not correlate with each other significantly, which suggests that the EEG recordings at Cz are influenced by additional sources in schizophrenia patients not modeled by the bilateral STG dipoles.
4.5 Limitations
Results presented here should be considered in the context of the limitations of this study. Because evoked responses were acquired only for the typical paired-click P50 paradigm, and not for the click train task, inferences about the relationship between hemodynamic responses in the fMRI task and evoked responses in the EEG task are limited. Future studies should measure evoked and hemodynamic responses in the same paradigm. Another limitation stems from the broad temporal window of fMRI, which does not allow parsing of the discrete early and late components involved in response to auditory stimulation. While components from P50 and beyond will show suppression with multiple clicks, early components do not suppress. It is possible, therefore, that failure to detect group differences regions such as the STG may reflect the fact this structure is heavily involved in early processing, which may contribute less to the summed hemodynamic response. This temporal limitation underscores the importance of continued EEG and MEG studies of sensory gating.
4.6 Implications for the Pathophysiology of Schizophrenia
Taken together, greater activation in the hippocampus, thalamus and DLPFC during the fMRI sensory gating task suggests dysfunction of an apparent network of regions involved in this deficit in schizophrenia. While BOLD responses in all three regions were correlated with evoked responses during a typical conditioning-test paradigm, the strongest correlation was with the hippocampus (R2=0.43), followed closely by the DLPFC (R2=0.40), suggesting these structures may play more prominent roles in the gating deficit typically measured.
The increased hemodynamic response observed in the present study is interesting in light of previous imaging studies of these regions in schizophrenia. Many studies of processes thought to recruit specific brain regions, including the hippocampus (Heckers et al. 1998), DLPFC (Glahn et al. 2005) and thalamus (Andrews et al. 2006) have found decreased activation in schizophrenia. Greater activation of these regions in schizophrenia during a passive listening task in the present study suggests an interesting possibility. It is possible that, when patients are at rest, or performing simple cognitive tasks that do not normally engage these regions, these areas are inappropriately activated, as the patients are unable to filter out distracting stimuli from the environment. In this case, reduced activation during tasks in which these regions must be engaged may be the result of occlusion, i.e. these regions are already inappropriately activated due failure of sensory gating mechanisms, such that inadequate neuronal capacity remains for recruitment for task demands. While speculative, this interpretation is consistent with other data suggesting that these regions, particularly the hippocampus, may be overactive in schizophrenia during tasks where hippocampal recruitment was not expected, including eye movement tasks (Tregellas et al. 2004), passive face viewing (Holt et al. 2006) and auditory discrimination tasks, both during task and control conditions (Medoff et al. 2001).
Of particular clinical interest, emerging data has shown hippocampal activity to be inversely correlated with positive symptoms (Lahti et al. 2006b). Preliminary data from the same group also suggests that elevated baseline activity of the hippocampus in schizophrenia is reduced in patients who respond positively to neuroleptics (Lahti et al. 2006a). Results of the current study shed light on one possible mechanism for these observations, and point to new avenues for studying disease mechanisms and the evaluation of therapeutic interventions in schizophrenia.
Acknowledgments
The authors thank Jamey Ellis, Deb Singel and Laurie Woodward for help recruiting subjects and acquiring data, Jody Tanabe, M.D., for valuable methods contributions, and Lawrence Adler, M.D., Gary Zerbe, Ph.D. and David Arciniegas, M.D., for critical comments during manuscript preparation.
Role of Funding Sources: This work was supported by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award for Dr. Tregellas, NIH Silvio O. Conte Center grant 5 P50 MH068582, and VISN19/MIRECC. None of the funding sources played a role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Contributors: Jason Tregellas designed the study, analyzed and interpreted data and wrote the manuscript. Deana Davalos helped design the study and acquire data. Donald Rojas helped design the study, interpret data and prepare the manuscript. Merilyne Waldo helped acquire data and interpret results. Linzi Gibson helped acquire data and performed literature reviews. Korey Wylie assisted with literature reviews and data interpretation. Yiping Du assisted with study design and MR scanning. Robert Freedman helped with study design and data interpretation. All authors contributed to and have approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, Waldo MC, Hall MH, Bowles A, Woodward L, Ross RG, Freedman R. Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry. 2004;161:1822–1828. doi: 10.1176/ajp.161.10.1822. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Adler LE, Waldo MC, Freedman R. Neurophysiologic studies of sensory gating in schizophrenia: comparison of auditory and visual responses. Biol Psychiatry. 1985;20:1284–1296. doi: 10.1016/0006-3223(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry. 2006;163:463–469. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Trautner P, Rosburg T, Korzyukov O, Grunwald T, Schaller C, Elger CE, Kurthen M. Sensory gating in the human hippocampal and rhinal regions. Clin Neurophysiol. 2005;116:1967–1974. doi: 10.1016/j.clinph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Parry K, Goodrich G, Ilinsky I, Kultas-Ilinsky K. Distribution of GABA(B) binding sites in the thalamus and basal ganglia of the rhesus monkey (Macaca mulatta) Neuropharmacology. 1999;38:1675–1682. doi: 10.1016/s0028-3908(99)00130-6. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2-6, 2002; Sendai, Japan. 2002. Available on CD-ROM in NeuroImage. [Google Scholar]
- Dau T, Wegner O, Mellert V, Kollmeier B. Auditory brainstem responses with optimized chirp signals compensating basilar-membrane dispersion. J Acoust Soc Am. 2000;107:1530–1540. doi: 10.1121/1.428438. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Miller GA, Moses SN, Thoma RJ, Huang MX, Hanlon FM, Weisend MP, Sherwood A, Bustillo J, Adler LE, Canive JM. Cross-modal generality of the gating deficit. Psychophysiology. 2005;42:318–327. doi: 10.1111/j.1469-8986.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp. 1999;7:89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses in the human and the cat model. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:461–467. [PubMed] [Google Scholar]
- Erwin RJ, Mawhinney-Hee M, Gur RC, Gur RE. Midlatency auditory evoked responses in schizophrenia. Biol Psychiatry. 1991;30:430–442. doi: 10.1016/0006-3223(91)90304-5. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunctions in schizophrenia. Schizophr Res. 1991;4:233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff WR, Williamson RD, VanGilder JC, Allison T, Fisher TC. Neural Origins of Long Latency Potentials Recorded from the Depth and from the Cortical Surface of the Brain in Man. In: Desmedt JE, editor. Progress in Clinical Neurophysiology. S. Karger; Brussels: 1980. pp. 126–145. [Google Scholar]
- Griffith J, Hoffer LD, Adler LE, Zerbe GO, Freedman R. Effects of sound intensity on a midlatency evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Psychophysiology. 1995;32:460–466. doi: 10.1111/j.1469-8986.1995.tb02097.x. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, Elger CE. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hinman CL, Buchwald JS. Depth evoked potential and single unit correlates of vertex midlatency auditory evoked responses. Brain Res. 1983;264:57–67. doi: 10.1016/0006-8993(83)91120-4. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Rauch SL, Hootnick J, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, Paulson KM, Weisend MP, Irwin JG, Bustillo JR, Adler LE, Miller GA, Canive JM. Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin Neurophysiol. 2003;114:835–850. doi: 10.1016/s1388-2457(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL. Prefrontal cortex gating of auditory transmission in humans. Brain Res. 1989;504:338–342. doi: 10.1016/0006-8993(89)91381-4. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Tamminga CA, Weiler MA. Functional effects of haloperidol and olanzapine after 1 and 6 weeks of treatment in patients with schizophrenia. Neuroimage (Human Brain Mapping 2006 Abstract) 2006a;31:S1, S1800. [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006b;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. Medial septal neuron activity in relation to an auditory sensory gating paradigm. Neuroscience. 1993;55:373–380. doi: 10.1016/0306-4522(93)90506-b. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Zimmerman J, Davis K, Whalen J, Edrich J. Source origin of a 50-msec latency auditory evoked field component in young schizophrenic men. Biol Psychiatry. 1988;24:495–506. doi: 10.1016/0006-3223(88)90160-6. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Trautner P, Korzyukov OA, Boutros NN, Schaller C, Elger CE, Kurthen M. Short-term habituation of the intracranially recorded auditory evoked potentials P50 and N100. Neurosci Lett. 2004;372:245–249. doi: 10.1016/j.neulet.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. The organization of the nucleus reticularis thalami: a Golgi study. Brain Res. 1966;1:43–62. doi: 10.1016/0006-8993(66)90104-1. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Knight RT. Multiple brain systems generating the rat auditory evoked potential. II. Dissociation of auditory cortex and non-lemniscal generator systems. Brain Res. 1993;602:251–263. doi: 10.1016/0006-8993(93)90690-o. [DOI] [PubMed] [Google Scholar]
- Stafekhina VS, Vinogradova OS. Sensory characteristics of the cortical input to the hippocampus: the entorhinal cortex. Zh Vyssh Nerv Deiat Im I P Pavlova. 1975;25:119–127. [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang M, Weisend MP, Irwin J, Sherwood A, Paulson K, Bustillo J, Adler LE, Miller GA, Canive JM. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Ricker D, Huang M, Edgar C, Irwin J, Torres F, Weisend MP, Adler LE, Miller GA, Canive JM. M50 sensory gating predicts negative symptoms in schizophrenia. Schizophr Res. 2005;73:311–318. doi: 10.1016/j.schres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161:315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- Venables PH. Input Dysfunction in Schizophrenia. In: Maher BA, editor. Progress in Experimental Personality Research. Academic PRess; Orlando, FL: 1967. pp. 1–64. [PubMed] [Google Scholar]


