Abstract
Background
Little is known about the timing of changes in glucose metabolism prior to type 2 diabetes development. We aimed to characterize trajectories of fasting and postload glucose, insulin sensitivity and insulin secretion in individuals developing type 2 diabetes.
Methods
Prospective occupational cohort study of 6538 (70.7% male, 91.3% Caucasian) British civil servants free of diabetes mellitus at baseline. During a median follow-up of 8.2 years, 505 incident diabetes cases were diagnosed (49.1% based on oral glucose tolerance test). Trajectories of fasting and 2-hour postload glucose, homeostasis model assessment (HOMA) insulin sensitivity, and β-cell function until diabetes diagnosis (the diabetic group) or the end of follow-up (the non-diabetic controls) were determined.
Findings
Multilevel models adjusted for age, sex and ethnicity confirmed that all metabolic measures followed linear trends in the controls except for insulin secretion that did not change during the follow-up. In the diabetic group a linear increase in fasting glucose was followed by a steep quadratic increase starting 3 years prior to the diagnosis of diabetes. For 2-hour postload glucose levels a fast elevation started 3 years prior to the diagnosis and for HOMA insulin sensitivity a steeper decrease was seen during the last 5 years before the diagnosis. HOMA β-cell function showed an increase between years 4 and 3 prior to the diagnosis and then a decrease until the diagnosis.
Interpretation
In this large cohort of British adults changes in glucose levels, insulin sensitivity, and insulin secretion were evident already 3–6 years before the diabetes diagnosis.
INTRODUCTION
The current global focus on the prevention of type 2 diabetes has highlighted the need to understand the pathophysiological changes leading to diabetes at their earliest possible stage.1–5 While definitions of pre-diabetic conditions such as Impaired Fasting Glycemia (IFG) or Impaired Glucose Tolerance (IGT) are predictive of the risk of future diabetes, they only reflect an individual’s glycemic state at a single point in time.6–9 It is suspected that the risk of diabetes and macrovascular complications begin to develop at glucose levels below the current cut-off levels for pre-diabetes.10,11
The multistage model of diabetes development describes an unstable period prior to diabetes onset.12 Although this model is widely accepted and supported by several studies13–25, important questions remain unanswered. An abrupt increase is suspected in fasting glucose 1.5–3 years before diagnosis, but the exact shape of this increase is unknown.14,19,22 Only one study in Pima Indians describes postload glucose trajectories before diabetes diagnosis based on yearly measurements21, whereas other studies on changes in postload glucose are based on a few repeats at least 3 years apart.14 Some prospective studies have measured or estimated insulin sensitivity and insulin secretion employing sophisticated methods, but generally describe changes as a function of pre-diabetes stage rather than time.13,15–18,20,23–25
As the data from previous studies provide a poorly defined picture of the natural history of diabetes development, we set out to observe the multistage model of diabetes development in a large population. To improve the timing of screening and prevention it is important to obtain high resolution data that could describe the timing of early changes in glucose metabolism prior to the incidence of type 2 diabetes. In this study from the 5 longitudinal Whitehall II cohort of British civil servants we characterize population trajectories of fasting glucose, 2-hour post load glucose, insulin sensitivity and insulin secretion during 13 years of follow up and compare such trajectories between those who did and did not develop type 2 diabetes.
METHODS
Participants and Design
All nonindustrial civil servants 35 to 55 years of age working in the London offices of 20 departments were invited to participate in this study; 10,308 (6895 men) were recruited between 1985 and 1988 (Phase 1).26 At Phase 3 of the study in 1991–1993, all participants known to be alive and in the country were invited to the screening clinic including an oral glucose tolerance test (OGTT); 6058 men and 2758 women (85.5% of the original sample) attended. Screening was repeated at Phase 5 (1997–1999, 5444 men and 2385 women participated) and Phase 7 (2003–2004, 4894 men, 2074 women). Additional questionnaire-only phases assessed diabetes status at Phase 4 (1995–1996, 5928 men, 2700 women), Phase 6 (2001, 5151 men, 2204 women) and Phase 8 (2006, 5017 men, 2156 women). The University College London ethics committee reviewed and approved the study, and written informed consent was obtained from each participant.
For the current analysis, Phase 3 when OGTT was performed for the first time served as the baseline. After the exclusion of non-participants (n=1492), individuals with prevalent diabetes at Phase 3 (n=42), those with missing follow-up (n=552), missing ethnicity data (n=27), or serum values not suitable for homeostasis model assessment (HOMA) analysis at any screening visit (n=1657) the final sample consisted of 6538 participants (74.2% of the baseline sample, Table 1). Participants included in the analyses, compared to those excluded, were more likely Caucasian (91.3% vs 88.8%) and men (70.7% vs 63.0%) (P < 0.05). They were 1.8 (95% CI 1.5 to 2.1) years younger, had 0.12 (95% CI 0.06 to 0.18) mmol/L higher fasting glucose and 8 (95% CI 4 to 11) pmol/L higher fasting insulin than excluded participants. There was no difference in body mass index (−0.16, 95% CI −0.37 to 0.05 kg/m2) between the groups.
Table 1.
Baseline characteristics of incident diabetes cases and controls included in the trajectory analysis.
| Variable | Control | Incident diabetes | P |
|---|---|---|---|
| N | 6033 | 505 | |
| Age (yrs) | 52.6 ± 7.1 | 53.1 ± 6.6 | 0.12 |
| Male (%) | 71.1 | 66.3 | 0.029 |
| Caucasian (%) | 92.3 | 79.8 | <0.0001 |
| Body mass index (kg/m2) | 25.60 ± 3.63 | 28.18 ± 4.99 | <0.0001 |
| Fasting glucose (mmol/L) | 5.21 ± 0.47 | 5.71 ± 0.91 | <0.0001 |
| 2h postload glucose (mmol/L) | 5.38 ± 1.42 | 7.06 ± 2.48 | <0.0001 |
| Fasting insulin (pmol/L) | 47 ± 30 | 73 ± 30 | <0.0001 |
| 2h postload insulin (pmol/L) | 259 ± 222 | 473 ± 351 | <0.0001 |
| HOMA2-%S (%)* | 145.1 ± 63.2 | 103.4 ± 58.8 | <0.0001 |
| HOMA2-%B (%) # | 78.4 ± 30.3 | 88.5 ± 39.0 | <0.0001 |
Data are presented as Mean ± SD or %. Comparisons were done using 2-sample t-tests, or Fisher’s exact tests as appropriate. HOMA2-%S and HOMA2-%B were calculated using HOMA2 calculator v2.2 (Diabetes Trials Unit, University of Oxford, Oxford, UK).27,28
HOMA2-%S – homeostasis model assessment insulin sensitivity
HOMA2-%B - homeostasis model assessment beta cell function
Of the potential 19614 person-examinations that would have been generated had every participant completed all three screenings, 398 person-examinations related to screenings after diabetes diagnosis and were excluded. We excluded 5188 person-examinations because the participant was not fasting according to WHO criteria (<8 hours fasting, or afternoon sampling), 108 because fasting plasma glucose values were extreme (≤3 or ≥25 mmol/L), and 2130 because fasting insulin levels were extreme (≤20 or ≥400 pmol/L), exceeding the published validity ranges for HOMA calculations.27,28 Thus, the dataset for analysis included a total of 11790 person-examinations.
Measurements
The samples at all phases of the study were handled according to similar standard protocols. Venous blood samples were taken in the fasting state (≥8 hours of fasting) before undergoing a standard 2-hour OGTT. Glucose samples were drawn into fluoride monovette tubes and insulin samples into native tubes which were centrifuged on site within an hour. Plasma/serum was immediately removed from the monovette tubes into microtubes and stored at −70 °C. Blood glucose was measured using glucose oxidase method29 on YSI Model 23A Glucose Analyzer (Phase 3, mean coefficient of variation (CV): 2.9–3.3%)30 and YSI MODEL 2300 STAT PLUS Analyzer (Phase 5 and 7, mean CV: 1.4–3.1%)31 (YSI Corporation, Yellow Springs, OH, USA). Serum insulin was measured using an in-house human insulin radioimmunoassay at Phase 3 (mean CV 7%)32 and a DAKO Insulin ELISA kit (DakoCytomation Ltd, Ely, UK) at Phases 5 and 7 (mean CV 4.2–9.3%).33 HOMA insulin sensitivity (HOMA2-%S) and HOMA β cell function (HOMA2-%B) were calculated based on model-derived estimates (rather than linear approximations) using the HOMA2 calculator v2.2 (http://www.dtu.ox.ac.uk/index.php?maindoc=/homa/index.php, Diabetes Trials Unit, University of Oxford, Oxford, UK).27,28
Diabetes was defined by a fasting glucose ≥7.0mmol/L or a 2-hour postload glucose ≥11.1 mmol/L.34,35 During the median 8.2 (interquartile range [IQR] – 8.7) year of follow-up 505 incident diabetes cases were diagnosed mostly on the basis of 75g OGTT (248 cases, 49.1%) except for those reporting doctor diagnosed diabetes (179 cases, 35.4%) or use of diabetes medication (78 cases, 15.4%) at screening or additional questionnaire phases. As shown in the number of measurements per year in Figure 1 and Figure 2, the date of diabetes diagnosis fell anywhere within the time window of the study (i.e. at screening dates or between the screenings) and therefore incident diabetes cases with only 1 screening, for example, may have had their OGTT years before the diagnosis.
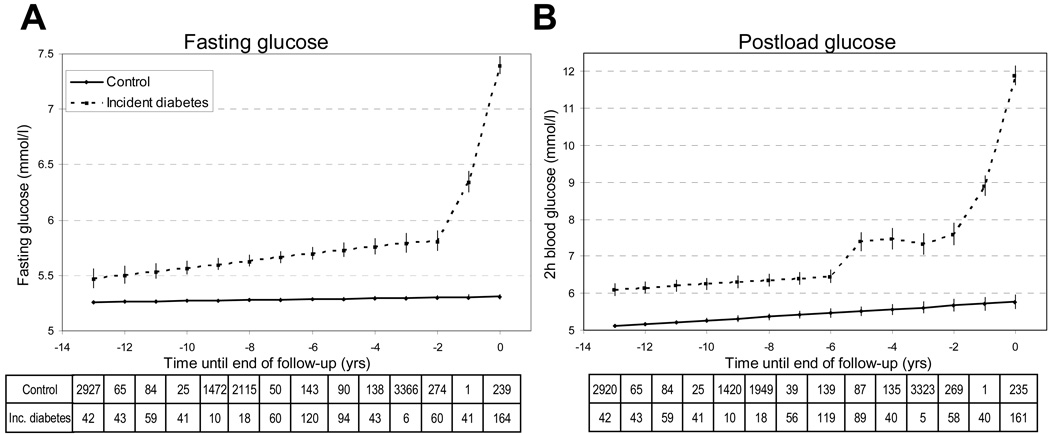
Figure 1.
Fasting and 2-hour postload glucose trajectories (panels A and B) before the diagnosis of diabetes mellitus or the end of follow-up in 505 incident diabetes cases compared to 6033 non-diabetic controls.
Multilevel longitudinal modeling using either linear growth model for non-diabetic and piecewise approach including cubic terms for time for incident diabetic subjects with OGTT fasting glucose (A) and 2-hour glucose (B) as outcomes. Adjusted for age, sex, ethnicity and study phase. Estimated for a hypothetical population of 72% male, 91% Caucasian aged 63 years at time 0 yrs. Error bars show 95% confidence intervals for the fixed effects.
Tables show the number of measurements for each year at and before diabetes diagnosis / end of follow-up.
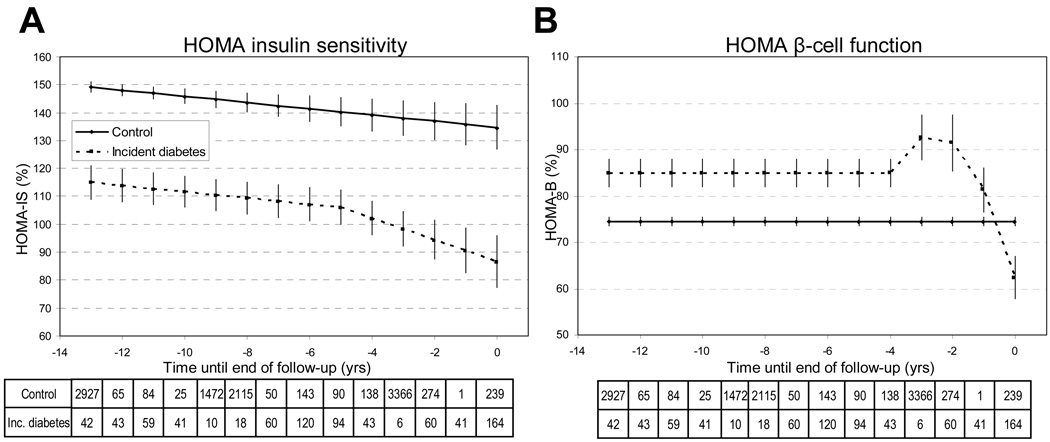
Figure 2.
Homeostasis model assessment insulin sensitivity (HOMA2-%S) and HOMA β-cell function (HOMA2-%B) trajectories (panels A and B) before the diagnosis of diabetes mellitus or the end of follow-up in 505 incident diabetes cases compared to 6033 non-diabetic controls.
Multilevel longitudinal modeling using either linear growth model for non-diabetic and non-piecewise or piecewise approach including linear or quadratic terms for time for incident diabetic subjects with HOMA2-%S (A) and HOMA2-%B (B) as outcomes. Adjusted for age, sex, ethnicity and study phase. Estimated for a hypothetical population of 72% male, 91% Caucasian aged 63 years at time 0. Error bars show 95% confidence intervals for the fixed effects.
Tables show the number of measurements for each year at and before diabetes diagnosis / end of follow-up.
Statistical Analysis
Statistical analyses were undertaken using SPSS 14.0 statistical software (SPSS Inc., Chicago, IL, USA). We divided participants into two groups: those who developed and those who did not develop diabetes during the follow-up. The observation period started (year 0) at the date of diagnosis for those who became diabetic (i.e., cases) and at the last screening or questionnaire phase for controls. Participants were then traced backwards to their first participation in clinical screening. Data at each phase during this retrospective observation period were collated to build trajectories for each outcome (fasting glucose, 2-hour glucose, HOMA2-%S and HOMA2-%B). In a preliminary analysis we plotted these trajectories for the cases and controls as a function of time and fitted nonparametric curves using locally weighted scatterplot smoother for graphical representation. We then used multilevel longitudinal modeling to estimate trajectories of fasting glucose, 2-hour glucose, HOMA2-%S and HOMA2-%B in cases prior to the diagnosis and controls prior to the last screening.36 Data were structured so that the repeated measurements of the three screening phases (i.e., person-examinations) were nested within participants and the non-independence of the person-examinations (the same individuals contributed more than one person-examination in the dataset) was taken into account in estimating the standard errors. Differences in trajectories between cases and controls were modeled using either a linear or non-linear growth model. Observation time was treated either as one period (a non-piecewise approach) or split into two distinct periods (a piecewise approach).37 In the latter approach, we created two time variables, one a continuous variable centered at the start of the second period (time=0) and the other a dummy variable indicating the period (0=1st period; 1=2nd period). We first determined the most parsimonious model for each centering point (from -9 to -0) and then chose the centering point which had the lowest information criteria for the final model. All analyses were adjusted for age, sex, ethnicity and study phase. To provide figures adjusted for baseline characteristics, trajectories were fitted for a hypothetical population of 72% male, 91% Caucasian at age 63 years at the end of follow-up. Finally, we checked how the models matched with the nonparametric scatterplot curves obtained from the preliminary analysis (online Appendix Figure 1) and ran a series of sensitivity analyses to test whether our findings were robust (online Appendix Tables 1 and 2). Statistical significance was inferred at a 2-tailed P<0.05.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
The 505 incident diabetic participants contributed a total of 801 fasting measurements. The corresponding number for the 6033 controls was 10,989. The mean age did not differ between the groups. As expected, the incident cases were more likely to be men and non-Caucasian, and they had higher body mass index than the controls (Table 1). At baseline, they also had higher fasting and postload plasma glucose, fasting and postload insulin, and HOMA2-%B, as well as a lower HOMA2-%S (all P <0.05).
Fasting plasma glucose (Table 2, Figure 1A)
Table 2.
Fixed effects for the multilevel models of change for fasting glucose, 2-hour postload glucose, homeostasis model assessment insulin sensitivity (HOMA2-%S) and HOMA β-cell function (HOMA2-%B) before the diagnosis of diabetes mellitus or the end of follow-up in 505 incident diabetes cases compared to the trajectories in 6033 non-diabetic controls.
|
Fasting (mmol/L) |
glucose |
Postload (mmol/L) |
glucose |
HOMA2-%S (%) | HOMA2-%B (%) | |||
|---|---|---|---|---|---|---|---|---|
| Regression coefficient |
SE |
Regression coefficient |
SE |
Regression coefficient |
SE |
Regression coefficient |
SE |
|
| Time (per year) | 0.004* | 0.001 | 0.051* | 0.007 | −1.11# | 0.30 | - | - |
| Case | 0.50* | 0.04 | 0.99* | 0.09 | −34.21* | 3.15 | 10.45* | 1.55 |
| Case * time | 0.028# | 0.007 | - | - | - | - | - | - |
| Case * time * 2nd period | −0.27† | 0.09 | 1.54* | 0.22 | −2.76† | 0.85 | 12.13# | 3.21 |
| Case * time2 * 2nd period | 0.26* | 0.027 | −0.75* | 0.10 | - | - | −4.44* | 0.84 |
| Case * time3 * 2nd period | - | - | 0.11* | 0.01 | - | - | - | - |
Multilevel longitudinal modelling using either a linear growth model for non-diabetic and a piecewise approach including cubic terms for time for incident diabetic subjects with OGTT fasting glucose, 2-hour glucose, HOMA2-%S and HOMA2-%B as outcomes. Adjusted for age, sex, ethnicity and study phase. Only models with the lowest information criteria are shown for each outcome. Time = a continuous variable centered (time = 0) at 3 years prior to diagnosis / end of follow-up for fasting glucose, 6 years for postload glucose, 5 years for HOMA2-%S, and 4 years for HOMA2-%B; 2nd period = a dummy variable, 1 for positive values in the time variable and 0 for non-positive values; Case = incident diabetes case.
P < 0.0001
P < 0.001
P < 0.01
P < 0.05
Among the controls there was a slight increase of fasting plasma glucose over time (mean ± S.E. 0.004±0.001 mmol/L/year) with levels of 5.26±0.008 mmol/L 13 years before and 5.31±0.010 mmol/L at the end of follow-up. For incident diabetes cases a linear trend from 13 years to 3 years before the diagnosis was apparent but with a steeper slope (slope difference between cases and controls 0.028±0.007 mmol/L/year) – the corresponding levels were 5.47±0.04 and 5.79±0.04 mmol/L. In the last 3 years the trajectory followed a quadratic curve reaching 7.40±0.04 mmol/L at the time of the diagnosis.
2-hour postload glucose (Table 2, Figure 1B)
Among the controls, 2-hour postload glucose values increased from 5.11±0.024 to 5.77±0.098 mmol/L during the 13 years of follow-up with a slope of 0.051±0.007 mmol/L/year. The slopes were not significantly different (case * time term) between the incident diabetes group and the controls during 13 to 6 years before the end of follow up, but incident cases had a 0.99±0.09 mmol/L higher glucose value throughout this period.
From the last 6 years before diagnosis postload glucose levels of incident diabetes cases followed a cubic trajectory with a flatter part between 5 to 3 years before the diagnosis. There was an approximately 1.5 times larger difference of postload glucose values between incident cases and controls during this period than in the preceding period of 13 to 6 years before the diagnosis.
Among the incident diabetes cases, a fast elevation of glucose levels was evident from 2 years before to the diagnosis onward (from 7.60±0.15 to 11.90±0.13 mmol/L).
HOMA insulin sensitivity (Table 2, Figure 2A)
During 13 to 5 years before the end of follow-up, HOMA2-%S decreased linearly with the same slope of 1.11±0.30 % per year among the incident diabetes cases and controls. Those with incident diabetes had a 34.2±3.1% lower insulin sensitivity value during this period. In the last 5 years before the diagnosis, HOMA2-%S decreased with a steeper slope in the incident cases compared with the controls (difference in slopes per year 2.76±0.85%), reaching the level of 86.7±4.7% at the end of follow-up.
HOMA β-cell function (Table 2, Figure 2B)
The calculated insulin secretion (HOMA2-%B) was flat for both groups between 13 and 4 years before the end of follow up. However, the HOMA2-%B value of 85.0% (SE 1.5) among the incident diabetes cases was on the average 10.4±1.5% higher than that in the controls. During the last 4 years before diagnosis, HOMA2-%B values of the incident diabetes cases followed a negative quadratic trajectory with a steep increase to 92.6±2.5% between years 4 to 3 before diagnosis followed by a steep decrease to a value of 62.4±2.3%.
Sensitivity analyses
The final models for trajectories of fasting and postload glucose, HOMA2-%S and HOMA2-%B were largely supported in a number of sensitivity analyses: in an extended study population including also those with 5 to 8 hours of fasting (total n = 7148, sensitivity analysis 1); in a sub-cohort of participants with no missing data prior to diagnosis (cases) or phase 8 (controls)(n = 1332, sensitivity analysis 2); and when the timing of diabetes was set to the midpoint between date of the diagnosis and the preceding examination to approximate the onset of the disease (n = 6290, sensitivity analysis 3) (online Appendix Table 1). Adjustment for time-varying BMI attenuated the difference in insulin sensitivity between cases and controls, but it had little effect on the differences in trajectories between these groups (online Appendix Table 2).
DISCUSSION
This is the first study to describe the 13-year trajectories of fasting and postload blood glucose, insulin sensitivity and insulin secretion until diabetes diagnosis in a large middle-aged, metabolically healthy population at baseline. All changes in metabolic measures among individuals who did not develop diabetes were well described by linear trajectories (modest rises for fasting and postload glucose, steady values for insulin secretion and slight falls for insulin sensitivity). Among individuals who developed diabetes, the levels of fasting and postload glucose and insulin secretion were higher and insulin sensitivity was lower than those among the controls already 13 years before the diagnosis. In the incident diabetes cases, linear increases in fasting and postload glucose were followed by a fast elevation in the last 6 to 3 years prior to diabetes diagnosis. For HOMA insulin sensitivity, there was a steeper decrease during the last 5 years prior to the diagnosis and HOMA β-cell function showed an increase between years 4 and 3 prior to the diagnosis and then a decrease until the diagnosis.
Comparison with other studies
Our findings provide support for multistage models of diabetes aetiology12: a long “compensatory” period, when insulin secretion increases to compensate insulin resistance without any major changes in glucose values; a “stable adaptation” when the β-cell mass is decreasing in spite of the β-cell adaptation; and a “transient unstable period” with a rapid rise of glucose values to overt diabetes. Our results suggest a stable adaptation when fasting blood glucose values increase linearly with a steeper slope in later diabetes cases compared to healthy participants, while the post-load glucose increases parallel with that of the controls. The observed accelerated rises fit with the unstable period leading to frank diabetes.
Our study is in agreement with a number of previous studies that found an abrupt increase in fasting glucose values 1.5 to 3 years before the diagnosis of diabetes.14,19,22 We observed yearly increases of 0.02–0.8 mmol/L/year in glucose near diagnosis which are of the same magnitude as those reported previously (range 0.4–1.2 mmol/L)14,19,22 and confirm previous observations regarding the accelerated increases in fasting glucose in IGT or when β-cell dysfunction is present.25,38 However, none of the previous studies performed a continuous prediction of fasting glucose similar to our study and most of them were based on fewer number of cases (<200) and a shorter follow-up period.
Our findings on postload glucose trajectories are in line with smaller-scale studies. A study including 3 data collections, found an approximately 5–6 mmol/L increase of postload glucose in the last 7 years preceding diabetes.14 In younger Pima Indians a linear increase of postload glucose until 4–8 years before diabetes diagnosis with a similar slope was found as in the present investigation.21 Our results confirm the finding of the abrupt increase of postload glucose and extend it to a broader, mostly Caucasian population.21
It seems likely that low insulin sensitivity is a prerequisite for incident diabetes.13,16–18,20,24,25 There is scarce and much less convincing evidence regarding the association between insulin sensitivity and development of IGT.16,17,20,24 It has been suggested that decrease in insulin sensitivity among those who develop IGT does not differ from that observed in people who remain normoglycemic, but is much smaller than that in those who develop incident diabetes.16 This is in agreement with our findings: we found that the decrease in insulin sensitivity before the 4 preceding years of diabetes diagnosis corresponded to that in normoglycemic controls, but a steeper decrease in insulin sensitivity was apparent during the last few years before the diagnosis.
The role of insulin secretion and β-cell function as predictors of type 2 diabetes has been unclear. Most studies report no association between insulin secretion and diabetes13,16,19,24,39, while the association of low disposition index with diabetes is well-established.13,16,17,20,24 Furthermore there is evidence of a higher insulin secretion in IGT compared to normal glucose tolerance, and a lower value in diabetes compared to IGT at least in non-Asian populations.15,23
Our findings suggest that part of the explanation for inconsistencies in the above described results might arise from differences in time frames between the studies. Higher values of insulin secretion may be associated with increased risk of type 2 diabetes if measured years or decades before the diagnosis, but lower values predict the short term diabetes risk. This is consistent with longitudinal studies reporting modest changes in acute insulin response or even in disposition index during the development from normal glucose tolerance to IGT, but marked decreases nearer the diabetes diagnosis.16,17,24 Further research is needed to examine whether the timing of the changes in insulin secretion, insulin sensitivity and fasting and post-load glucose point to causal relationships between these indexes.
Study strengths and limitations
The current study benefits from a well-phenotyped, well-described occupational cohort of people and diagnosis of incident diabetes based on OGTT using the current definition of the disease.26,34 We applied a sophisticated approach for data analysis taking into account the interrelationship between repeated measurements from the same individual at different time points. The long follow-up time provided a unique opportunity to describe different periods of “prediabetes” according to trajectories based on piecewise modeling.
The inclusion of the diagnostic glucose value in the analysis might be criticized since any diagnostic threshold would produce a rapid rise in the mean value among those who exceed this threshold.21 To overcome this problem we refitted the multilevel models excluding the diagnostic value from analysis. These models produced largely similar trajectories as those presented here. In addition, several other reports suggest a rapid rise in fasting and 2-hour glucose values14,19,21,22 and the modeling of the individual growth curves of post-load glucose also support the presence of a rapid true rise at the time of diagnosis.21 These findings suggest that we detected a genuine pre-diagnosis trajectory in glucose levels.
We used measures of HOMA insulin sensitivity/insulin resistance which are well-accepted in the literature. HOMA insulin sensitivity is extensively validated against the gold standard clamp and minimal model methods.28,40 In contrast, the calculated insulin secretion is less widely used.28,40,41 Since the HOMA uses fasting values for estimation, it mostly describes hepatic insulin resistance and steady state insulin secretion. While hepatic insulin resistance is strongly correlated with muscle and fat insulin resistance, the steady state insulin secretion is a late marker of β-cell dysfunction and shows only a moderate relationship with the most sensitive measures of the 1st phase insulin secretion.28,42 Considering this, our findings may provide an underestimate for the timing of early β-cell decompensation.
We did not use disposition index to describe changes in glucose metabolism with a single parameter because its calculation based on HOMA values has several theoretical problems and the compensation described by it could be incomplete even in normal glucose tolerant subjects.42–44
The data from several thousand blood glucose values provided an excellent power to examine general trajectories in a piecewise model based on between- and within-subject comparisons. The proposed trajectories give a good description of the events leading to diabetes. However, the limited number of repeated observations for each participant (max 3) means that individual differences in trajectories could not be examined in these data and should be studied in the future.
Of the baseline population, 25.8% was excluded because of missing data, extreme glucose or insulin values, or prevalent diabetes. Selection bias is an unlikely explanation for our results as comparisons of participants included and excluded from the analyses revealed modest differences, although statistical significance was often reached due to large numbers. In the main analysis we excluded individuals who had fasted less than 8 hours, but the sensitivity analyses showed that including those who had fasted 5 to 8 hours (the Whitehall II protocol) had little effect on the findings. Furthermore, the main findings were replicated in a sub-cohort with no missing data, suggesting that missingness is an unlikely source of bias in this study.
Implications
The description of biomarker trajectories leading to diabetes diagnosis may contribute to future attempts of building more accurate risk prediction models that make use of the wealth of repeated measures available for patients through regular check-ups. These models may be able to give an estimation which trajectory describes best the individual’s results. We anticipate that these models will have a better prediction than models using only the most recent glucose measurements.
Our findings show clearly that there are different windows of opportunity for screening and prevention. While most of the prevention studies were targeted on prediabetic people, our findings suggest that people with prediabetes are already on the accelerating part of the glucose trajectory. We hypothesize that prevention would be more effective prior to this instable period, but more research is needed to identify people at that stage of disease development. If a person could be kept on the shallower (linear) part of the fasting glucose (or postload glucose21) trajectory, the onset of diabetes might be substantially delayed. Further research is needed to confirm or refute these hypotheses.
Acknowledgements
We thank for Dr. Jane E. Ferrie for her comments on an earlier version of this paper. The presented work is funded by the Medical Research Council New Investigator Award (G0501184). The Whitehall II study is supported by the following bodies in the UK: Medical Research Council; Economic and Social Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; and in the US: National Heart Lung and Blood Institute (HL36310), NIH: National Institute on Aging (AG13196), NIH; Agency for Health Care Policy Research (HS06516); The John D and Catherine T. MacArthur Foundation. MK is supported by the Academy of Finland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
We declare that we have no conflict of interest.
REFERENCES
- 1.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 4.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 6.Meigs JB, Muller DC, Nathan DM, et al. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 7.Shaw JE, Zimmet PZ, de Court, et al. Impaired fasting glucose or impaired glucose tolerance. What best predicts future diabetes in Mauritius? Diabetes Care. 1999;22:399–402. doi: 10.2337/diacare.22.3.399. [DOI] [PubMed] [Google Scholar]
- 8.Gabir MM, Hanson RL, Dabelea D, et al. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23:1108–1112. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- 9.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med. 2002;136:575–581. doi: 10.7326/0003-4819-136-8-200204160-00006. [DOI] [PubMed] [Google Scholar]
- 10.DECODE Study Group EDEG. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26:688–696. doi: 10.2337/diacare.26.3.688. [DOI] [PubMed] [Google Scholar]
- 11.Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) European Heart Journal. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 12.Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53 Suppl 3:S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- 13.Cnop M, Vidal J, Hull RL, et al. Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care. 2007;30:677–682. doi: 10.2337/dc06-1834. [DOI] [PubMed] [Google Scholar]
- 14.Ferrannini E, Nannipieri M, Williams K, et al. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes. 2004;53:160–165. doi: 10.2337/diabetes.53.1.160. [DOI] [PubMed] [Google Scholar]
- 15.Ferrannini E, Gastaldelli A, Miyazaki Y, et al. beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: A new analysis. Journal of Clinical Endocrinology and Metabolism. 2005;90:493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 16.Festa A, Williams K, D'Agostino R, et al. The natural course of beta-cell function in nondiabetic and diabetic individuals - The insulin resistance atherosclerosis study. Diabetes. 2006;55:1114–1120. doi: 10.2337/diabetes.55.04.06.db05-1100. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero-Romero F, Rodriguez-Moran M. Assessing progression to impaired glucose tolerance and type 2 diabetes mellitus. European Journal of Clinical Investigation. 2006;36:796–802. doi: 10.1111/j.1365-2362.2006.01728.x. [DOI] [PubMed] [Google Scholar]
- 18.Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laspa E, Christen A, Efstathiadou Z, et al. Long-term changes and variability in diabetes risk factors prior to the development of impaired glucose homeostasis. Diabet Med. 2007;24:1269–1278. doi: 10.1111/j.1464-5491.2007.02225.x. [DOI] [PubMed] [Google Scholar]
- 20.Lyssenko V, Almgren P, Anevski D, et al. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005;54:166–174. doi: 10.2337/diabetes.54.1.166. [DOI] [PubMed] [Google Scholar]
- 21.Mason CC, Hanson RL, Knowler WC. Progression to type 2 diabetes characterized by moderate then rapid glucose increases. Diabetes. 2007;56:2054–2061. doi: 10.2337/db07-0053. [DOI] [PubMed] [Google Scholar]
- 22.Sattar N, McConnachie A, Ford I, et al. Serial metabolic measurements and conversion to type 2 diabetes in the west of Scotland coronary prevention study: specific elevations in alanine aminotransferase and triglycerides suggest hepatic fat accumulation as a potential contributing factor. Diabetes. 2007;56:984–991. doi: 10.2337/db06-1256. [DOI] [PubMed] [Google Scholar]
- 23.Tripathy D, Carlsson M, Almgren P, et al. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49:975–980. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]
- 24.Weyer C, Bogardus C, Mott DM, et al. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang AH, Wang C, Peters RK, et al. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006;55:1074–1079. doi: 10.2337/diabetes.55.04.06.db05-1109. [DOI] [PubMed] [Google Scholar]
- 26.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 29.Cooper GR. Methods for determining the amount of glucose in blood. CRC Critical Reviews in Clinical Laboratory Sciences. 1973;4:101–145. doi: 10.3109/10408367309151554. [DOI] [PubMed] [Google Scholar]
- 30.Alpert L. Instrument series: Model 23A Glucose Analyzer. Lab World. 1976;27:8–13. [Google Scholar]
- 31.Astles JR, Sedor FA, Toffaletti JG. Evaluation of the YSI 2300 glucose analyzer: algorithm-corrected results are accurate and specific. Clinical Biochemistry. 1996;29:27–31. doi: 10.1016/0009-9120(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 32.Hanning I, Home PD, Alberti KG. Measurement of free insulin concentrations: the influence of the timing of extraction of insulin antibodies. Diabetologia. 1985;28:831–835. doi: 10.1007/BF00291073. [DOI] [PubMed] [Google Scholar]
- 33.Andersen L, Dinesen B, Jorgensen PN, et al. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem. 1993;39:578–582. [PubMed] [Google Scholar]
- 34.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Brunner EJ, Marmot MG, Nanchahal K, et al. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 1997;40:1341–1349. doi: 10.1007/s001250050830. [DOI] [PubMed] [Google Scholar]
- 36.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 37.Naumova EN, Must A, Laird NM. Tutorial in Biostatistics: Evaluating the impact of 'critical periods' in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–1341. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]
- 38.Hong J, Gu WQ, Zhang YF, et al. The interplay of insulin resistance and beta-cell dysfunction involves the development of type 2 diabetes in Chinese obeses. Endocrine. 2007;31:93–99. doi: 10.1007/s12020-007-0002-2. [DOI] [PubMed] [Google Scholar]
- 39.Weyer C, Tataranni PA, Bogardus C, et al. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 40.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 41.Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia. 2004;47:943–956. doi: 10.1007/s00125-004-1381-z. [DOI] [PubMed] [Google Scholar]
- 42.Mari A, Ahren B, Pacini G. Assessment of insulin secretion in relation to insulin resistance. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8:529–533. doi: 10.1097/01.mco.0000171130.23441.59. [DOI] [PubMed] [Google Scholar]
- 43.Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol. 2004;150:97–104. doi: 10.1530/eje.0.1500097. [DOI] [PubMed] [Google Scholar]
- 44.Mari A, Pacini G, Brazzale AR, et al. Comparative evaluation of simple insulin sensitivity methods based on the oral glucose tolerance test. Diabetologia. 2005;48:748–751. doi: 10.1007/s00125-005-1683-9. [DOI] [PubMed] [Google Scholar]