Abstract
The antibiotic valanimycin is a naturally occurring azoxy compound isolated from Streptomyces viridifaciens. Detailed investigations have shown that valanimycin is derived from L-valine and L-serine via the intermediacy of O-(L-seryl)-isobutylhydroxylamine. Sequence analysis of the valanimycin biosynthetic genes provides relatively few clues to the nature of the later stages of the pathway. Two exceptions are provided by the vlmJ and vlmK genes. The translation product of vlmJ exhibits similarity to diacylglycerol kinases, while the translation product of vlmK exhibits low similarity to the MmgE/PrpD superfamily of proteins. This superfamily includes 2-methylcitrate dehydratase. This communication describes the isolation and structure elucidation of valanimycin hydrate from vlmJ and vlmK mutants of S. viridifaciens. Additional studies show that the conversion of valanimycin hydrate into valanimycin by S. viridifaciens requires both the vlmJ and vlmK genes, and that VlmJ catalyzes the ATP-dependent phosphorylation of the hydroxyl group of valanimycin hydrate prior to a VlmK-catalyzed dehydration.
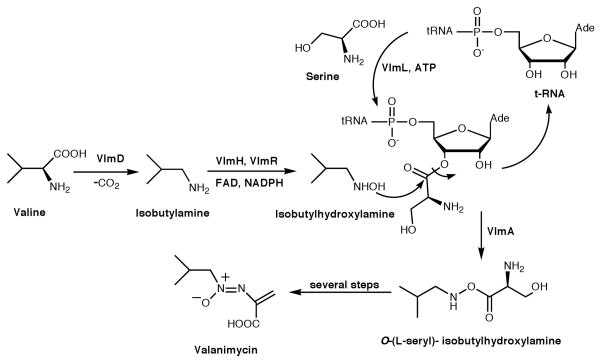
The antibiotic valanimycin is a naturally occurring azoxy compound isolated from the fermentation broth of Streptomyces viridifaciens MG456-hF10.1 Enzymatic and genetic investigations have led to the cloning of the valanimycin gene cluster, which was found to contain 14 genes (Figure S3, Supporting Information).2 The functions of seven of these genes have now been established.2,3 VlmF, which is a member of the major facilitator family of transport proteins, confers valanimycin resistance. VlmD, VlmH, and VlmR catalyze the conversion of L-valine into isobutylhydroxylamine, while VlmL catalyzes the formation of L-seryl-tRNA from L-serine. Recently, VlmA has been shown to catalyze the transfer of L-serine from L-seryl-tRNA to isobutylhydroxylamine to produce O-(L-seryl)-isobutylhydroxylamine. Finally, VlmI has been found to be a Streptomyces antibiotic regulatory protein (SARP) that is a positive regulator of valanimycin biosynthesis.2d These studies and the results from precursor incorporation4 experiments allow formulation of the biosynthetic pathway for valanimycin shown in Scheme 1. The remaining uncertainties in the valanimycin pathway involve the steps required to convert O-(L-seryl)-isobutylhydroxylamine into valanimycin. Sequence analysis of the valanimycin biosynthetic genes provides relatively few clues to the nature of the later stages of the pathway. Two exceptions are provided by the vlmJ and vlmK genes. The translation product of vlmJ exhibits similarity to diacylglycerol kinases, while the translation product of vlmK exhibits low similarity to the MmgE/PrpD superfamily of proteins. This superfamily includes 2-methylcitrate dehydratase, an enzyme required for propionate catabolism. In this communication, we provide evidence supporting the hypothesis that vlmJ and vlmK play a role in the final stages of valanimycin biosynthesis.
Scheme 1.
Previous investigations have shown that washed cells of S. viridifaciens efficiently incorporate labeled serine into valanimycin.4b Accordingly, [U-14C]-L-serine was administered to washed cells of vlmJ and vlmK mutants of S. viridifaciens along with L-valine to stimulate valanimycin production.5 After 24 h, the supernatants were acidified to pH 3, saturated with sodium chloride, and extracted with ethyl acetate. Tlc analysis of the concentrated ethyl acetate extracts revealed the presence of an unknown metabolite (1) that could be visualized by autoradiography (Figure 1A). When a similar experiment was carried out with a vlmH mutant,3c which cannot form isobutylhydroxylamine, the metabolite was absent.6 This suggested that the metabolite was related to the valanimycin pathway. Preliminary characterization of this metabolite was carried out by NMR analysis of the crude compound produced by administration of L-valine and L-serine labeled with combinations of carbon-13 and nitrogen-15 to washed cells (Supporting Information, Table S1, entries 1-3). In each of these experiments, a 13C resonance was observed at 64.7 ppm with multiplicities arising from coupling to 15N. A DEPT experiment confirmed that this resonance was due to a CH moiety. The 15N chemical shift data observed for the metabolite were consistent with the presence of an azoxy group.7 Additional support for the presence of an azoxy group in the unknown was provided by comparison with the NMR data for valanimycin biosynthesized from (15N)-L-valine and (2-13C, 15N)-L-serine (Table S1, entry 4).
Figure 1.
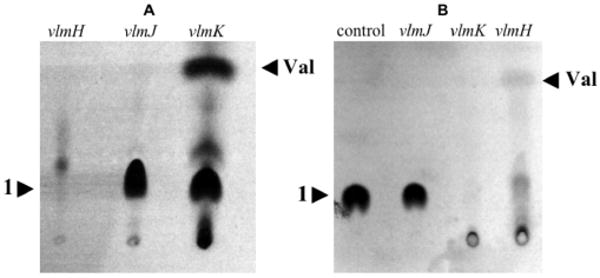
A. Tlc analysis of extractable metabolites produced by washed cells of S. viridifaciens vlmH, vlmJ, and vlmK mutants after administration of L-serine and L-valine. B. Tlc analysis of extractable metabolites produced by incubation of 1 with cell-free extracts of vlmJ, vlmK, and vlmH mutants. Val = valanimycin. See Supporting Information for details.
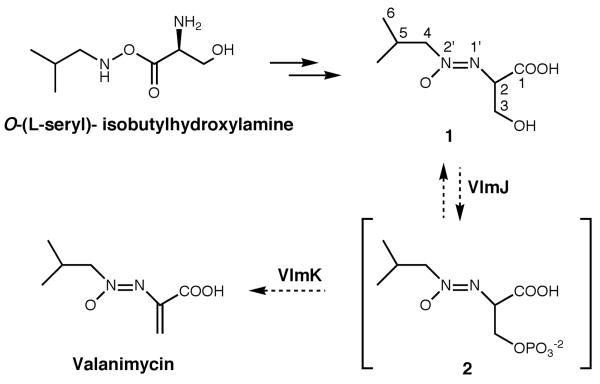
Since preliminary analysis suggested the unknown metabolite was probably on the valanimycin pathway, the compound was purified by reverse-phase, preparative HPLC. Detailed NMR analyses of the purified metabolite using 1H, 13C, 1H-1H COSY, 1H-13C HSQC, and 1H-13C HMBC experiments unequivocally demonstrated that the compound corresponds to valanimycin hydrate (1, Scheme 2) (Table 1). Additional support for the assigned structure was provided by high-resolution mass spectrometry, which showed an m/z at 191.1023 (M+H)+ (calculated for C7H15N2O4, 191.1032).
Scheme 2.
Table 1.
NMR Assignments (ppm) for Valanimycin Hydrate (1)a
| Position | 1H Resonance | 13C Resonance |
|---|---|---|
| 1 | N/A | 171.337 |
| 2 | 4.656 (1H, dd, 3JHH ≈ 4.9 Hz, 3JHH ≈ 4.9 Hz) | 64.580 |
| 3 | 4.114 (1H, dd, 2JHH = 11.5 Hz, 3JHH ≈ 5.0 Hz) 4.095 (1H, dd, 2JHH = 11.5 Hz, 3JHH ≈ 5.0 Hz) |
61.855 |
| 4 | 4.104 (1H, dd, 2JHH = 11.4 Hz, 3JHH =7.2 Hz) 4.134 (1H, dd, 2JHH = 11.4 Hz, 3JHH = 7.8 Hz |
76.875 |
| 5 | 2.479 (1H, nominal nonet, 3JHH ≈ 6.9 Hz) | 27.965 |
| 6 | 1.021 (3H, d, 3JHH = 6.72 Hz), 1.034 (3H, d, 3JHH = 6.72 Hz) | 19.638, 19.485 |
Measured in CDCl3 with shifts defined relative to TMS.
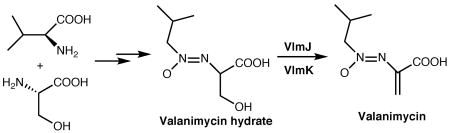
Once the structure of the metabolite produced by the S. viridifaciens vlmJ and vlmK mutants had been determined, experiments were conducted to determine if 1 is a viable intermediate in valanimycin biosynthesis. HPLC-purified, radiolabeled 1 was incubated with cell-free extracts prepared from an S. viridifaciens vlmH mutant. After 16 h, the incubation mixture was acidified, extracted with ethyl acetate, and the concentrated extract analyzed by tlc on silica gel for the presence of valanimycin. Visualization of the thin layer chromatogram by autoradiography showed the presence of a compound with the same Rf as authentic valanimycin (Figure 1B). A cell-free extract prepared from an S. viridifaciens vlmI mutant failed to catalyze the conversion of radiolabeled 1 into valanimycin. This observation shows that the transformation of 1 into valanimycin in the cell-free extracts requires the presence of valanimycin biosynthetic enzymes. The conversion of 1 into valanimycin by cell-free extracts of the vlmH mutant was confirmed by NMR and mass spectral analysis. Attempts to purify the valanimycin from the incubation mixture by standard methods failed due to the low concentration of valanimycin produced in the extract. However, proton NMR analysis of the unpurified valanimycin showed the presence of signals at 6.357 and 6.380 ppm that are assignable to the vinyl hydrogen atoms of valanimycin,8 and a 1H-13C HSQC experiment on the same sample showed that both of these vinyl hydrogens correlate to a 13C resonance at 122.0 ppm, a value that is close to the 13C resonance position for the vinyl CH2 group of purified valanimycin (120.0 ppm). LC-MS analysis of the crude valanimycin showed the presence of a compound with the same retention time and exact molecular mass as valanimycin: m/z 173.0932 (M+H)+, calculated for C7H13N2O3, 173.0926. We therefore conclude that 1 is an intermediate in valanimycin biosynthesis.
Additional insight into the nature of the dehydration reaction was obtained by experiments with cell-free extracts prepared from vlmJ and vlmK mutants of S. viridifaciens. The cell-free extract of the vlmJ mutant was unable to convert 1 into valanimycin, suggesting that vlmJ is required for the dehydration reaction (Figure 1B). On the other hand, the cell-free extract of a vlmK mutant, in which VlmJ is active, appeared to convert 1 into a water-soluble compound that is no longer extractable into ethyl acetate, This conversion was dependent upon the addition of ATP to the cell-free extract (Figure S2, Supporting Information). Furthermore, high-resolution LC-MS analysis of the crude product formed from 1 and ATP in cell-free extracts of the vlmK mutant revealed the presence of a compound whose exact mass corresponds to that of 2, thereby supporting the hypothesis that VlmJ catalyzes the conversion of 1 to 2 shown in Scheme 2 (see Supporting Information). Carbon-13 NMR analysis of the crude product formed from (2-13C)-1 and ATP in a vlmK cell-free extract also provided additional evidence for the formation of 2 (see Supporting Information). The excretion of 1 by the vlmK mutant can be explained by dephosphorylation of 2 or by feedback inhibition of VlmJ when 2 accumulates in vivo. Precedent for the type of elimination reaction shown in Scheme 2 is found in the mechanism for dehydration of serine residues during lantibiotic biosynthesis.9
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM053818) and the Robert A. Welch Foundation (C-0729) for financial support. We thank Dr. Tan Guo for mass spectral data.
Footnotes
Supporting Information Available: Experimental procedures for the purification of compound 1, NMR and mass spectral characterization of 1, the bioconversion of 1 in cell-free extracts; LC-MS and NMR evidence for the formation of 2 from 1. This material is available free of charge at http://www.pubs.acs.org.
References
- 1.Yamato M, Iinuma H, Naganawa H, Yamagishi Y, Hamada M, Masuda T, Umezawa H, Abe V, Hori M. J Antibiot (Tokyo) 1986;39:184–191. doi: 10.7164/antibiotics.39.184. [DOI] [PubMed] [Google Scholar]
- 2.(a) Parry RJ, Li W. J Biol Chem. 1997;272:23303–23311. doi: 10.1074/jbc.272.37.23303. [DOI] [PubMed] [Google Scholar]; (b) Parry RJ, Li W. Arch Biochem Biophys. 1997;339:47–54. doi: 10.1006/abbi.1996.9857. [DOI] [PubMed] [Google Scholar]; (c) Parry RJ, Li W, Cooper HN. J Bacteriol. 1997;179:409–416. doi: 10.1128/jb.179.2.409-416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Garg RP, Ma Y, Hoyt JC, Parry RJ. Mol Microbiol. 2002;46:505–517. doi: 10.1046/j.1365-2958.2002.03169.x. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ma Y, Parry RJ. Microbiology. 2000;146:345–352. doi: 10.1099/00221287-146-2-345. [DOI] [PubMed] [Google Scholar]; (b) Garg RP, Gonzalez JM, Parry RJ. J Biol Chem. 2006;281:26785–26791. doi: 10.1074/jbc.M603675200. [DOI] [PubMed] [Google Scholar]; (c) Garg RP, Qian XL, Alemany LB, Moran S, Parry RJ. Proc Natl Acad Sci U S A. 2008;105:6543–6547. doi: 10.1073/pnas.0708957105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Parry RJ, Li W. Chem Commun. 1994:995–996. [Google Scholar]; (b) Parry RJ, Li Y, Lii FW. J Am Chem Soc. 1992;114:10062–10064. [Google Scholar]
- 5.Yamato M, Takeuchi T, Umezawa H, Sakata N, Hayashi H, Hori M. J Antibiot (Tokyo) 1986;39:1263–1269. doi: 10.7164/antibiotics.39.1263. [DOI] [PubMed] [Google Scholar]
- 6.The presence of valanimycin in the extract of the vlmK mutant is due to partial reversion of this single crossover mutation to wild-type.2d
- 7.Witanowski M, Stefaniak L, Webb GA. Ann Rep NMR Spectrosc. 1986;18:171. [Google Scholar]; (b) See Supporting Information
- 8.The proton chemical shift positions for C-3 of valanimycin are concentration dependent. See Supporting Information for details.
- 9.You YO, van der Donk WA. Biochemistry. 2007;46:5991–6000. doi: 10.1021/bi602663x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.