Abstract
We developed a photonic crystal sensing method for diol containing species such as carbohydrates based on a poly(vinyl alcohol) (PVA) hydrogel containing an embedded crystalline colloidal array (CCA). The polymerized CCA (PCCA) diffracts visible light. We show that in the presence of borax the diffraction wavelength shifts as the concentration of glucose changes. The diffraction shifts result from the competitive binding of glucose to borate, which reduces the concentration of borate bound to the PVA diols.
Introduction
There is great interest in the development of chemical sensors that can be used to visually determine the concentration of species in aqueous environments. We recently developed photonic crystal materials which can be used to construct such sensors.1-4 The photonic crystal sensors are fabricated from highly charged monodisperse polystyrene particles synthesized via emulsion polymerization. These particles self-assemble into a face-centered cubic crystalline colloidal array (CCA), which diffracts visible light.5 The CCA is embedded in an elastic hydrogel polymer matrix, forming a polymerized crystalline colloidal array (PCCA) which retains the diffraction property of the CCA.1 Molecular-recognition groups that bind analytes selectively are attached to the PCCA.6 Binding of the target analyte molecule causes the hydrogel volume to change which produces diffraction wavelength shifts that can be discernable to the eye.6
In the present study, we demonstrate a new competitive binding sensing mechanism where the analyte molecule competes for binding to a dissolved species (borate) which binds to the hydrogel backbone (poly(vinyl alcohol) (PVA) in this case). The PVA PCCA diols and added borate ions form 1 : 1 or 1 : 2 complexes with the hydrogel which alters the hydrogel volume by changing the free energy of mixing and the elastic free energy.7 Added glucose competes for binding to borate, which changes the free borate concentration in solution which changes the concentration of borate bound to the PVA PCCA; the diffraction of the PVA PCCA depends on the glucose concentration.
The sensor developed here can be used to determine the solution concentration of molecules with diols such as carbohydrates.8 Determination of carbohydrates is important in applications such as controlling glycemia and monitoring fermentation processes.9-11 Although enzyme-based assays offer the most selective detection methods for carbohydrate determination,12 only a few enzyme-based carbohydrate sensors exist.11,13 Numerous research groups are presently developing methods to determine carbohydrates.9,14
Experimental
Materials
Poly(vinyl alcohol) (PVA, 88% mol hydrolyzed, MW 25 kDa) was purchased from Polysciences, Inc. 2,2-Diethoxyacetophenone (DEAP) was purchased from Acros Organics. Ion exchange resin (AG 501-X8 (D) Resin, 20-50 mesh) was purchased from Bio-Rad Laboratories, Inc. Phosphate buffered saline (PBS) was purchased from Pierce Biotechnology. D-(+)-Glucose (99.5%) was purchased from Sigma-Aldrich. Borax, HCl, NaOH, and NaCl were purchased from J. T. Baker. All chemicals were used as received.
PCCA preparation
Highly-charged, monodisperse polystyrene particles were synthesized from styrene by emulsion polymerization.5 Synthesis of poly(vinyl alcohol) PCCA was reported previously.15 The PVA was functionalized with glycidyl methacrylate in order to introduce vinyl groups for the PCCA photopolymerization.16 In a typical PCCA fabrication, the modified PVA (0.25 g) was dissolved in nanopure water (1.22 g, 17.5 MΩ cm-1, Barnstead Nanopure Water Purification System) via heating and vortexing in a 2 dram vial. The PVA solution was then cleaned with ion exchange resin (0.1 g). One milliliter of the modified PVA solution was taken from the vial after centrifuging for 5 min, and combined with the CCA (2.06 g, ∼10 wt%, 118 nm diameter). Ion exchange resin (0.10 g) and photoinitiator (DEAP, 135 μL, 20% in DMSO) were added to this solution. The mixture was again vortexed and then centrifuged for another 5 min. The dispersion was injected into a cell consisting of two quartz plates, separated by a 125 μm thick Parafilm spacer. The cell was placed between two Black Ray mercury lamps (365 nm) and photopolymerized for 45 min. The cell was opened in nanopure water, and the resulting PCCA was rinsed with large quantities of nanopure water. To test the response reversibility, the PCCAs were put in 500 mL nanopure water for half an hour to wash out borate ions and glucose, and the procedure was repeated 5 times before being retested.
Diffraction measurement
Diffraction measurements were performed by using an Ocean Optics USB 2000-UV-VIS Spectrometer with an LS-1 Tungsten Halogen light source and an R-series fiber optic reflectance probe. A piece of the PCCA (1 cm × 1 cm) was placed in a Petri dish containing 50 mL 150 mM NaCl or 25 mM phosphate buffer solution. The PCCA was immobilized by a plastic washer which weighted it down to the bottom of the Petri dish.
Results and discussion
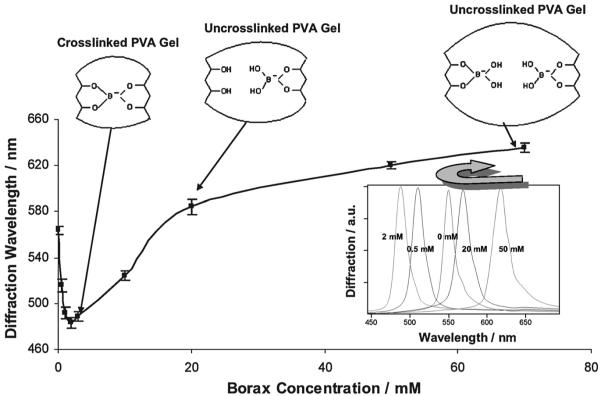
Fig. 1 shows that the addition of borax (sodium tetraborate) to a 150 mM NaCl solution bathing the PVA PCCA causes the diffraction wavelength to blueshift (0 to 2 mM) and then redshift as the concentration of borax increases (>2 mM), in a manner similar to that of our previous metal cation and glucose sensing PCCAs which rely on the formation of crosslinks.3,4 In dilute solution, borax forms boric acid and borate ions (≤25 mM, Scheme 1).17 The borate ions reversibly bind to cis-1,2- or -1,3-diols to generate five-or six-membered cyclic complexes.11,18 Scheme 2 shows that both 1 : 1 complexes and 1 : 2 crosslinking complexes can form between the borate and PVA 1,3-diols. At low borate ion concentrations where the PVA cis-1,3-diol groups are in excess, the 1 : 2 complexes will dominate (Scheme 2: reaction (1)). The resulting PVA crosslinks cause the diffraction to blueshift, in a manner analogous to that caused by the glucose crosslinks formed in our boronate PCCA that senses glucose4 and by the metal cation crosslinks formed in our 8-hydroxyquinoline PCCA.3
Fig. 1.
Dependence of PVA PCCA diffraction on borax concentration in 150 mM NaCl solutions.
Scheme 1.
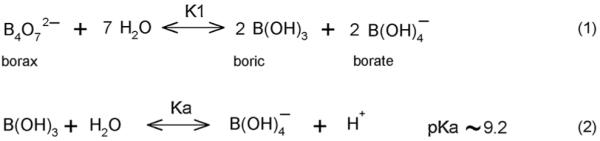
Borax solution equilibria.
Scheme 2.
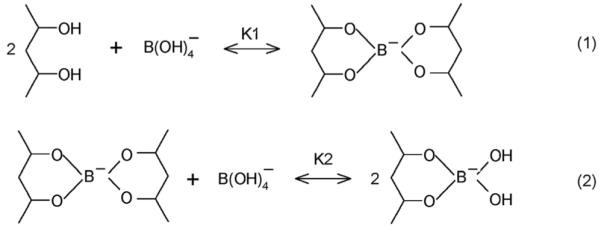
Reactions between cis-diols and borate.
As the borate concentration increases, borate forms 1 : 1 complexes with the PVA diols which break the crosslinks, resulting in diffraction redshifts (Scheme 2: reaction (2)). At borax concentrations $ 20 mM, the diffracted wavelength exceeds the initial diffracted wavelength in the absence of borax. Presumably, the PVA diol-borate ion complex has a more favorable free energy of mixing, which swells the hydrogel and redshifts the diffraction.
Boron analysis (atomic emission, R J Lee Group, Inc.) of the PVA PCCA hydrogels (3 cm × 3cm × 125 μm), immersed in 150 mM NaCl solutions as a function of borax concentration, shows that borate selectively partitions into the hydrogel (Table 1). The PCCA boron concentration is 30-fold larger than that in the 0.5 mM borax solution reservoir. The relative enrichment decreases to 6-fold for 50 mM borax concentrations due to the saturation of borate binding within the PCCA due to the estimated PVA hydrogel diol concentration of 0.59 M (see the Appendix).
Table 1.
Boron analysis of PVA PCCA (3 cm × 3 cm × 125 μm) immersed in 150 mM NaCl solutions as a function of borax concentration
| Borax concentration (mM) | 0.5 | 2 | 10 | 50 |
| Boron content (mM) | 15 | 22 | 91 | 307 |
We estimated the formation constants for forming 1 : 1 and 1 : 2 complexes between PCCA cis-diols and borate (see Appendix). As demonstrated in the Appendix, if we assume that 1 : 2 and 1 : 1 borate-diol complexes are dominantly formed in low and high borax concentration solutions, respectively, we estimate a formation constant for the 1 : 1 complex, K1 = 10.9 M-1, while for the 1 : 2 complex, K21 = 62.0 M-2. Our value of K1 is 5-fold and K21 is 13-fold larger than those reported by Lorand and Edwards19 for PVA dissolved in solution. In contrast, our K1 is only slightly larger than that (K1 ≈ 9 M-1) reported by Lin et al.20 It should be noted that the effective concentrations of hydroxyls in our PCCA hydrogel may be much greater than those in the solutions studied by Lorand et al. and Lin et al.
Diffraction dependence on glucose concentration
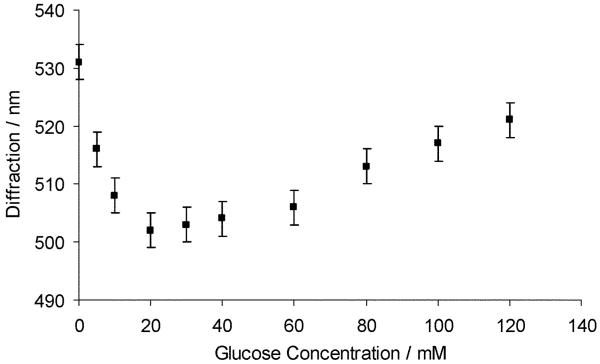
The response of the PVA PCCA to borate will depend on the presence of species such as carbohydrates in solution which can compete with the binding of PVA diols to borate. This is especially true for species like glucose with borate association constants larger than the PVA diol-borate association constant. The value of K1 for glucose binding is 44-fold and K21 is 179-fold larger than those for PVA binding.19,21 Fig. 2 shows that a bimodal PCCA diffraction response occurs due to the addition of glucose to the PVA PCCA in the presence of 10 mM borax; the PCCA diffraction wavelength blueshifts for glucose concentrations between 0 and 20 mM and then redshifts for glucose concentrations above 20 mM.
Fig. 2.
Dependence of PVA PCCA diffraction on glucose in 150 mM NaCl solution at a borax concentration of 10 mM.
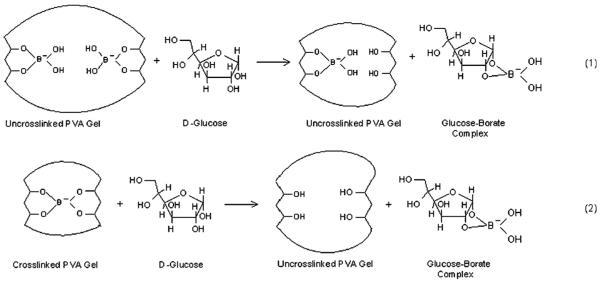
The mechanism for this bimodal response is that glucose-borate binding lowers the available uncomplexed borate ion concentration in solution. At the initial 10 mM borate concentration there is a mixture of the 1 : 2 and 1 : 1 PVA diol-borate complexes. Glucose decreases the free borate concentration, and shifts the equilibrium towards the 1 : 2 complex forming crosslinks (Scheme 3: reaction (1)). Glucose addition above 20 mM forms 1 : 1 PVA-boronate-glucose complexes which breaks the crosslinking and redshifts the diffraction (Scheme 3, reaction (2)).
Scheme 3.
Competitive glucose-borate-PVA-PCCA binding equilibria.
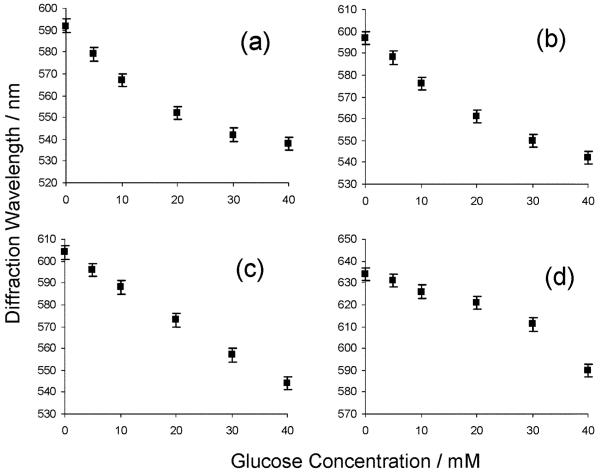
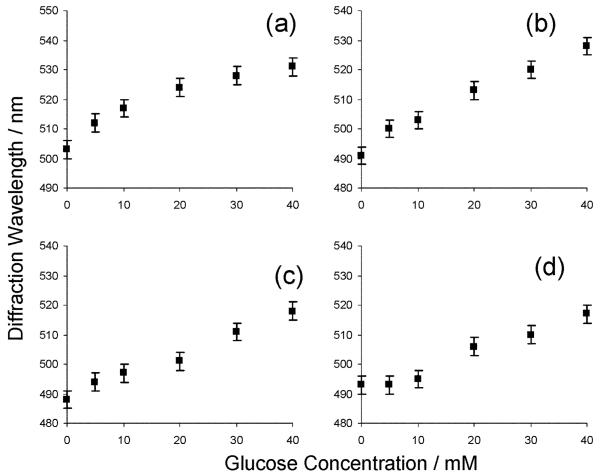
These PVA PCCA photonic crystal materials can act as glucose sensors if borax is present. At high borax concentrations (20-70 mM) where hydrogel 1 : 1 borate-PVA diol complexes dominate, glucose addition causes the diffraction to blueshift (Fig. 3). The error bars were calculated from replicate runs at borax concentrations of 50 mM. In this regime, glucose addition decreases the borate concentration which decreases the PVA diol-borate 1 : 1 complex concentration as shown by reaction (1) in Scheme 3. Boron analyses (atomic emission, R J Lee Group, Inc.) of a PVA PCCA immersed in 50 mM borax compared to a solution with 50 mM borax containing 40 mM glucose demonstrates a decrease in boron concentration from 307 to 231 mM.
Fig. 3.
Dependence of PVA PCCA diffraction on glucose in 150 mM NaCl solution at borax concentrations of (a) 20, (b) 30, (c) 50, (d) 70 mM.
In contrast, at low borax concentrations, where 1 : 2 borate-PVA diol crosslink complexes dominate, glucose addition decreases the free borate concentration resulting in a decrease in the number of crosslinks as shown by Fig. 4 which demonstrates that the diffraction redshifts upon the addition of glucose to solutions containing 0.5, 1, 2, and 3 mM of borax. The curvature in Fig. 3 and Fig. 4 occurs because the impact of glucose differs between different concentration regimes. For example, in Fig. 3a, the initial glucose addition significantly decreases the 1 : 1 borate-diol complex concentration within the PCCA. However, further glucose addition has little impact at low borate-diol complex concentrations.
Fig. 4.
Glucose dependence of PVA PCCA diffraction for 150 mM NaCl solutions at borax concentrations of (a) 0.5, (b) 1, (c) 2, (d) 3 mM.
The reproducibility in reading the diffraction appears to be the major error in the measurement. The magnitude of this error, 3σ ≈ 0.6 nm, allows us to estimate a glucose detection limit of 0.33 mM for 50 mM borax solution containing 150 mM NaCl (pH = 8.5).
pH dependence of the glucose measurement
We examined the pH dependence of the glucose response for 50 mM borax solutions (Fig. 5). The PCCA swelling signaled by the diffraction redshift with increasing pH results from the increased concentration of 1 : 1 borate-PVA complexes as the borate concentration increases. We originally expected that boric acid would show a pKa = 9.1-9.222 which would give rise to the pH dependence of boric acid and borate concentrations shown in the inset to Fig. 5. The line drawn through the Fig. 5 zero glucose pH dependent diffraction data shows a titration behavior suggestive of a process with an effective pKa for boric acid within the hydrogel decreased to ∼8.4. The origin of this shift in effective pKa results from the saturation of the binding of borate to the hydrogel. As the concentration of borate exceeds that of the PVA diols the binding of borate saturates and the slope of the diffraction redshift decreases.
Fig. 5.
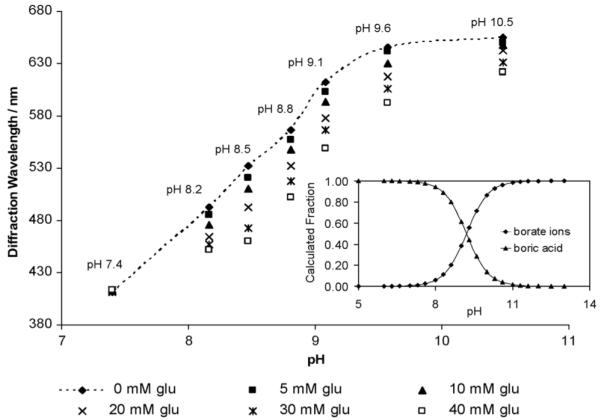
Dependence of the PVA PCCA glucose response for a solution containing 25 mM sodium phosphate and 150 mM NaCl containing 50 mM borax.
At pH 7.4 we expect and observe that there is little response of the PVA PCCA to glucose because the boric acid present binds glucose with a small affinity, much less than that of borate.23 As expected, the response increases with pH until pH = 8.5 where it starts to drop. The glucose dependence results from=competition of glucose for binding to the borate ions. Boron analysis of dried PVA hydrogels, immersed in 50 mM borax solutions, shows that the boron content increases with increasing pH from 7.4 to 9.2 where it saturates (Table 2).
Table 2.
Boron analysis for the PVA PCCAs (3 cm × 3 cm × 125 μm) immersed in 150 mM NaCl solutions with 50 mM borax as a function of pH values
| pH | 7.4 | 8.4 | 9.2 | 10.1 |
| Boron content (mM) | 155 | 231 | 307 | 292 |
As the borate concentration increases above that of the PVA diols at pH values above 8.5 the response to glucose decreases because we are already in a saturating borate-PVA binding regime (Fig. 5). A similar result is observed in Fig. 3d which occurs at the highest borax concentrations.
Reaction kinetics and response reversibility
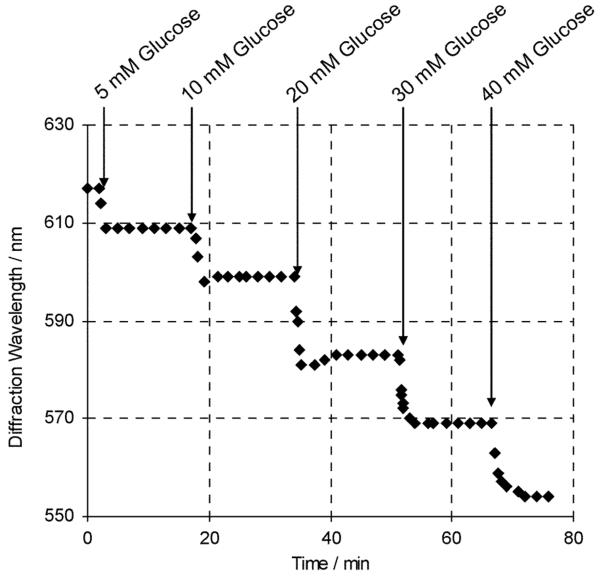
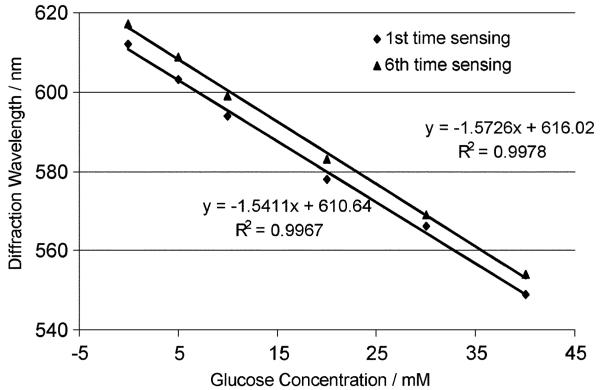
Fig. 6 shows that these PVA PCCA sensors respond in real time to glucose and that their diffraction wavelength shifts complete within 2-3 minutes after glucose addition. The PVA PCCA sensor response is both reversible and reproducible. Fig. 7 compares the initial sensing response of a PVA PCCA compared to that occurring after five replicate sensing runs where the sensor was washed between sensing runs with 150 mM NaCl solutions. The glucose sensitivity is unchanged but we see a ∼5 nm redshift in the sensor diffraction.
Fig. 6.
Response kinetics of the PVA PCCA glucose sensor upon exposure to multiple additions of a freshly prepared glucose stock solution.
Fig. 7.
Comparison of initial diffraction response of the PVA PCCA sensor containing 50 mM borax to glucose and after five glucose sensing measurements at pH 9.08, which were followed by washing with a pH 7.4 25 mM sodium phosphate and 150 mM NaCl solution. The sensitivity does not change, but there is a ∼5 nm diffraction redshift.
These PVA PCCAs have a major advantage over our classic acrylamide PCCAs in their ability to be reversibly dehydrated.15 When rehydrated they show similar sensitivity to that prior to dehydration. This enables storage of the sensors for later use.
Conclusions
We developed a competitive binding motif for use in photonic crystal PCCA sensing. We utilize a PVA PCCA and add borate to the solution which binds to the PVA hydrogel, crosslinking as well as increasing the hydrogel polymer solubility. We demonstrate sensing of glucose which results from the competitive binding between the glucose and the borate which decreases the borate binding to the PVA PCCA. The glucose sensing is reversible and the diffraction shifts occur in real time (3 min). The sensors can be dehydrated for long-term storage.
Acknowledgements
We gratefully acknowledge financial support from NIH Grant 2R01 EB004132.
Appendix: hydroxyl density and borate formation constants
The PVA for making the hydrogel is 88 mol% hydrolyzed, where 1.2% of the side chains are methacrylated according to NMR data. Thus the PVA molecular structure is: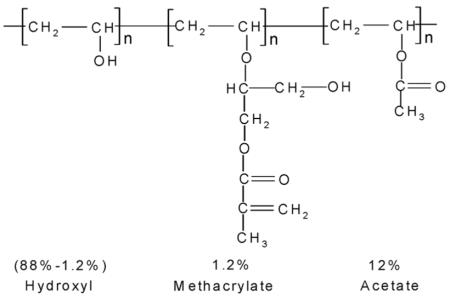
The weight percentage of hydroxyl groups is 29.2% in pure PVA. From the chemical composition used we calculate that the hydroxyl concentration of the PCCA hydrogel is 1.18 M. This would suggest a 0.59 M concentration of cis-1,3-diols. A PCCA sample (1cm × 1cm × 125 μm) would contain a total of 7.5 μmole cis-1,3-diols. The number of moles of PCCA cis-diol groups is, thus, small compared to the amount of borate ion in solution. This ensures that there will be no depletion of the borate concentration in the reservoir.
We can estimate the association constants for the borate with the PVA PCCA. Boric acid has the following acid-base equilibrium:
| (1) |
where BH is boric acid, B is the borate ion.
Within the hydrogel, borate ions form 1 : 1 and 1 : 2 complexes with the PVA cis-diol groups:
| (2) |
| (3) |
where L is a PVA diol group.
For a borax concentration ≤ 25 mM, where borax is completely dissociated into borate ions and boric acid, the borate ion concentration can be determined from the known association constant Ka and the solution pH.
At a 0.5 mM borax concentration, the 1 : 2 complexes appear to dominate as evidenced by the diffraction blue shift. The boron analysis (Table 1) shows that boron content is 15 mM within the hydrogel. Assuming that all borate ions form 1 : 2 complexes, [BL2] = 15 mM. Since [L] = [L]total - 2[BL2] and [B] can be calculated from eqn (1) with a pKa of 9.2. Thus,
At 10 mM borax concentrations, 1 : 1 complexes dominate and boron analysis shows that the boron concentration within the hydrogel is 91 mM. Assuming that all borate ions form 1 : 1 complexes, [BL] = 91 mM. Since [L] = [L]total - [BL] and [B] is known from the boric acid equilibrium (eqn (1)). Thus,
Footnotes
Dedicated to Professor Seiji Shinkai on the occasion of his 65th birthday
References
- 1.Holtz JH, Asher SA. Nature. 1997;389:829. doi: 10.1038/39834. [DOI] [PubMed] [Google Scholar]
- 2.Kimble KW, Walker JP, Finegold DN, Asher SA. Anal. Bioanal. Chem. 2006;385:678. doi: 10.1007/s00216-006-0453-y. [DOI] [PubMed] [Google Scholar]; Asher SA, Peteu SF, Reese CE, Lin MX, Finegold D. Anal. Bioanal. Chem. 2002;373:632. doi: 10.1007/s00216-002-1366-z. [DOI] [PubMed] [Google Scholar]; Alexeev VL, Das S, Finegold DN, Asher SA. Clin. Chem. 2004;50:2353. doi: 10.1373/clinchem.2004.039701. [DOI] [PubMed] [Google Scholar]; Ben-Moshe M, Alexeev VL, Asher SA. Anal. Chem. 2006;78:5149. doi: 10.1021/ac060643i. [DOI] [PubMed] [Google Scholar]
- 3.Asher SA, Sharma AC, Goponenko AV, Ward MM. Anal. Chem. 2003;75:1676. doi: 10.1021/ac026328n. [DOI] [PubMed] [Google Scholar]
- 4.Alexeev VL, Sharma AC, Goponenko AV, Das S, Lednev IK, Wilcox CS, Finegold DN, Asher SA. Anal. Chem. 2003;75:2316. doi: 10.1021/ac030021m. [DOI] [PubMed] [Google Scholar]
- 5.Reese CE, Guerrero CD, Weissman JM, Lee K, Asher SA. J. Colloid Interf. Sci. 2000;232:76. doi: 10.1006/jcis.2000.7190. [DOI] [PubMed] [Google Scholar]
- 6.Asher SA, Alexeev VL, Goponenko AV, Sharma AC, Lednev IK, Wilcox CS, Finegold DN. J. Am. Chem. Soc. 2003;125:3322. doi: 10.1021/ja021037h. [DOI] [PubMed] [Google Scholar]
- 7.Flory PJ. Principles of Polymer Chemistry. Cornell University Press; Ithaca, New York: 1953. [Google Scholar]
- 8.McNaught AD. Pure Appl. Chem. 1996;68:1919. [Google Scholar]
- 9.Aslan K, Zhang J, Lakowicz JR, Geddes CD. J. Fluoresc. 2004;14:391. doi: 10.1023/b:jofl.0000031820.17358.28. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cannizzo C, Amigoni-Gerbier S, Larpent C. Polymer. 2005;46:1269. [Google Scholar]; James TD, Shinkai S. Top. Curr. Chem. 2002;218:159. [Google Scholar]
- 10.Klonoff DC. Diabetes Care. 2005;28:1231. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]; de Menezes JC, da Silva Alves S, Lemos JM, de Azevedo SF. Biotechnol. Tech. 1992;6:1. [Google Scholar]; Cote GL, Cameron BD. J. Biomed. Opt. 1997;2:275. doi: 10.1117/12.275221. [DOI] [PubMed] [Google Scholar]; Myung J, Kim KB, Crews CM. Med. Res. Rev. 2001;21:245. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang W, Gao S, Gao X, Karnati VVR, Ni W, Wang B, Hooks WB, Carson J, Weston B. Bioorg. Med. Chem. Lett. 2002;12:2175. doi: 10.1016/s0960-894x(02)00339-6. [DOI] [PubMed] [Google Scholar]
- 11.James TD, Sandanayake Samankumara KRA, Shinkai S. Angew. Chem., Int. Ed. 1996;35:1911. [Google Scholar]
- 12.Jelinek R, Kolusheva S. Chem. Rev. 2004;104:5987. doi: 10.1021/cr0300284. [DOI] [PubMed] [Google Scholar]; Baldwin RP. J. Pharm. Biomed. Anal. 1999;19:69. doi: 10.1016/s0731-7085(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 13.James TD, Linnane P, Shinkai S. Chem. Commun. 1996:281. [Google Scholar]
- 14.Sadik OA, Yan F. Anal. Chim. Acta. 2007;588:292. doi: 10.1016/j.aca.2007.02.046. [DOI] [PubMed] [Google Scholar]; He F, Chen K, Fu J, Nie L, Yao S. Anal. Sci. 1995;11:1001. [Google Scholar]
- 15.Ward Muscatello MM, Asher SA. Adv. Funct. Mater. 2008;18:1186. doi: 10.1002/adfm.200701210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalieri F, Miano F, D’Antona P, Paradossi G. Biomacromolecules. 2004;5:2439. doi: 10.1021/bm049654g. [DOI] [PubMed] [Google Scholar]; Martens P, Anseth KS. Polymer. 2000;41:7715. [Google Scholar]
- 17.Ingri N, Lagerstrom G, Frydman M, Sillen LG. Acta Chem. Scand. 1957;11:1034. [Google Scholar]; Maeda M, Hirao T, Kotaka M, Kakihana H. J. Inorg. Nucl. Chem. 1979;41:1217. [Google Scholar]
- 18.Sugihara JM, Bowman CM. J. Am. Chem. Soc. 1958;80:2443. [Google Scholar]; Ma Y, Qian L, Huang H, Yang X. J. Colloid. Interface Sci. 2006;295:583. doi: 10.1016/j.jcis.2005.05.031. [DOI] [PubMed] [Google Scholar]; Smith HD, Jr., Wiersema RJ. Inorg. Chem. 1972;11:1152. [Google Scholar]; Chapelle S, Verchere JF. Tetrahedron. 1988;44:4469. [Google Scholar]
- 19.Lorand JP, Edwards JO. J. Org. Chem. 1959;24:769. [Google Scholar]
- 20.Lin H-L, Liu W-H, Liu Y-F, Cheng C-H. J. Polym. Res. 2002;9:233. [Google Scholar]
- 21.Roy GL, Laferriere AL, Edwards JO. J. Inorg. Nucl. Chem. 1957;4:106. [Google Scholar]
- 22.Davis HB, Mott CJB. J. Chem. Soc., Faraday Trans. 1980;76:1991. [Google Scholar]; Aruga R. J. Chem. Soc., Dalton Trans. 1988:2971. [Google Scholar]; Bishop M, Shahid N, Yang J, Barron AR. Dalton Trans. 2004:2621. doi: 10.1039/b406952h. [DOI] [PubMed] [Google Scholar]; Tossell JA. Geochim Cosmochim Acta. 2005;69:5647. [Google Scholar]
- 23.Shibayama M, Sato M, Kimura Y, Fujiwara H, Nomura S. Polymer. 1988;29:336. [Google Scholar]; Pezron E, Ricard A, Lafuma F, Audebert R. Macromolecules. 1988;21:1121. [Google Scholar]; Ivanov E, Larsson H, Galaev Y, Mattiasson B. Polymer. 2004;45:2495. [Google Scholar]; Ochiai H, Shimizu S, Tadokoro Y, Murakami I. Polymer. 1981;22:1456. [Google Scholar]