Abstract
Purpose of review
We review recent insights into the mechanisms and prevalence of accommodation. Accommodation refers to an acquired resistance of an organ graft to humoral injury and rejection.
Recent findings
Accommodation has been postulated to reflect changes in antibodies, control of complement and/or acquired resistance to injury by antibodies, complement or other factors. We discuss the importance of these mechanisms, highlighting new conclusions.
Summary
Accommodation may be a common, perhaps the most common outcome of organ transplantation, and in some systems a predictable outcome of organ xenotransplantation. Further understanding of how accommodation is induced and by what mechanisms it is manifest and maintained could have a profound impact on transplantation in general and perhaps on other fields.
Keywords: Xenotransplantation, allotransplantation, antibodies, complement, endothelial cells, accommodation
Introduction
In the 1980s, we [1] and a few others [2] studied kidneys transplanted across blood group A or blood group B barriers in humans. Although the prospect of ABO-incompatible transplants was then considered poor, the others and we found that if anti-donor antibodies were removed from the circulation at the time of transplantation the transplanted kidneys might survive and function for extended periods of time. These observations suggested that compatibility of blood groups need not pose a profound barrier to transplantation, and prompted us to question of how these kidneys survived. It had been shown that although some recipients had antibodies specific for donor blood groups, the transplanted kidneys continued to express donor antigen without incurring rejection [3]. Based on these findings, we reasoned that some condition heretofore unrecognized allowed the survival of the kidneys across a humoral barrier that otherwise should have been nearly insurmountable. We subsequently called this condition “accommodation” and reasoned that induction of accommodation, whatever it may be, might allow the clinical application of xenotransplantation [4].
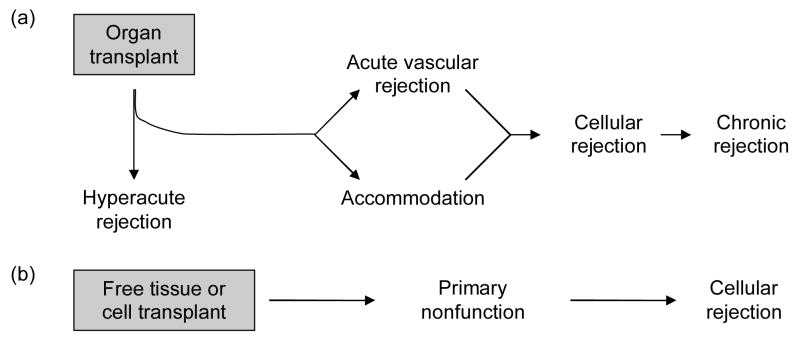
ABO-incompatible transplantation has been proposed as a model for xenotransplantation [5–8], and during the ensuing decade, the occurrence of accommodation was linked to one or more phenomena (Figure 1). These phenomena are still considered as potential mechanisms of accommodation, as a review of recent literature will show.
Figure 1. Occurrence of accommodation and sites of potential mechanisms.
(A) The outcome of organ transplantation. The outcomes of organ transplantation include hyperacute rejection (HAR), acute vascular (AVR) or antibody-mediated rejection, acute cellular rejection (ACR) and chronic rejection. These outcomes are often observed in the temporal sequence shown in the figure. HAR, AVR and chronic rejection can be mediated by antibodies. Accommodation (ACC) is defined as the survival and function of an organ transplant in the face of an antibody response that would otherwise cause injury and rejection. Accommodation can prevent HAR and AVR. As discussed in the text, accommodation might prevent chronic rejection or allow it to occur by avoiding more acute types of injury, or it might actually cause chronic rejection. (B) Mechanisms of accommodation. Transplantation of nearly every type engenders a B cell response. Anti-graft antibodies can ultimately injure organ grafts [10] through a series of events that include binding of antibody (Ab), activation of complement (C) and endothelial changes and injury induced by complement. Accommodation modifies the outcome of organ grafts at one or more of the steps in this sequence. These steps and the corresponding mechanisms are as follows: (i) change in antigen so that antibody does not bind; (ii) increased control of complement; and (iii) resistance to injury-mediated by complement. The original description of accommodation [4] postulated that accommodation might also be caused by a change in antibody, such that complement activation or other effector properties are mitigated. We now consider such changes to represent immunological “regulation” rather than accommodation, since they reflect changes in the immune response and not in the graft (discussed in the text). Manifestation of accommodation by change in antigen or by complement regulation may preclude detecting or evaluating resistance to injury caused by antibody and complement.
Potential Mechanisms of Accommodation
Because we consider accommodation a fundamental biological process [9] and because one may want to induce accommodation deliberately or in advance of transplanting an organ, we have emphasized the importance of understanding the mechanisms that bring about accommodation [10]. Below we discuss and summarize recent insights into mechanisms of accommodation.
Change in antibodies
Accommodation might result from a change in the effector properties of antibodies directed against the donor; such a change might limit the damage to the graft those antibodies cause [4,11]. Yu et al. [12] found that human IgG2 specific for Galα1-3Gal blocks activation of complement on target cells by blocking the binding of complement-fixing IgM and other IgG. Mohiuddin et al. [13] found that a transition from complement fixing to non-complement-fixing isotypes occur in the context of transplantation, and speculated that this change might explain accommodation. Conceivably, this transition could be sparked by immune deviation toward a Th2 phenotype after xenotransplantation [14].
Recent work suggests this mechanism might indeed mitigate graft injury. Galili [15] reviewed work in his laboratory that had suggested that stimulation of B cells in the absence of T cell help might generate antibodies which bind but do not injure grafts. This conclusion was reached using a model system in which mice deficient of α-1,3-galactosyl transferase (such mice, in contrast to wild type mice, do not make Gala1-3Gal and do make anti-Gala1-3Gal antibodies) [16] are transplanted with pig kidney membranes to stimulate immunity and then with hearts from wild type mice.
As interesting as the evolution of effector properties of immunoglobulin may be as a means by which tissue injury is limited, we no longer consider this phenomenon a primary mechanism of accommodation [17]. If a change in effector properties of an immune response occurs, we would prefer to think such a change reflects a type of immune regulation and is best understood in that context, allowing us to consider accommodation as a distinct entity better described as acquired resistance of a graft to injury [9]. Moreover, studies of clinical allografts, including ABO-incompatible transplants [18] and swine-to-primate xenografts by us [19,20] and by others [21], have not revealed a shift in the effector properties of antibodies directed against the graft in conjunction with accommodation. Indeed, careful analysis reveals these grafts contain C4d, indicating that antibody binding and initial steps in complement activation have occurred [18,22].
Change in antigen
Accommodation might reflect a change in the properties or expression of antigen targeted by anti-graft antibodies. Ulfvin et al. [23] reported that kidney allografts undergo a change in glycolipid metabolism over time. Yuzawa et al. [24] showed that glycoprotein and glycolipid antigens can undergo modulation following antibody binding; they speculated these changes might explain accommodation. We found that antibody binding to Galα1-3Gal depends on the positioning of that saccharide among the components of the cell membrane and glycocalix [25,26]. However, past [27] and recent studies in xenotransplantation suggest that accommodated organs can bind antibody to at least the same extent as normal organs [28]. Hence, modulation of antigen or change in antigen synthesis, while occurring in some settings, does not explain accommodation in clinically relevant models of xenotransplantation.
Modified control of complement
Accommodation might reflect modification of the complement cascade such that its effector functions cause little or no injury to graft endothelium. Incubation of cells with complement in sub-toxic amounts has long been known to modify cells in ways that cause the cells to resist subsequent exposure to higher concentrations of complement [29,30]. Resistance to complement is caused by some combination of: (i) shedding and internalization of terminal complement complexes [29]; modification of cellular metabolism such that cells intrinsically resist lysis [31]; and increased expression of complement regulatory proteins such as decay accelerating membrane co-factor protein or CD59 [32]. Dalmasso et al. [33,34] found that binding of antibodies or a lectin to Galα1-3Gal increases resistance of the cells to lysis, and this resistance is mediated at least in part by heightened expression of CD59 on endothelial cells [35]. Dorling et al. [36] found that binding of xenoreactive IgG to porcine cells increases resistance to lysis.
Consistent with these concepts, Ding et al. [28] recently reported that infusion of anti-Galα1-3Gal IgG1 into mice that are immunodeficient, lacking of α-1,3-galactosyl transferase (such mice, in contrast to wild type mice, do not make Gala1-3Gal and do make anti-Gala1-3Gal antibodies, as described above), and bearing rat cardiac xenografts (which express Galα1-3Gal) causes the grafts to increase expression of decay accelerating factor, Crry and CD59. These grafts resisted destruction when the recipients were administered a bolus of IgG1. Although other mechanisms were not excluded, the results suggested that heightened expression of complement regulatory proteins might have contributed to accommodation. Williams et al. [37] found that accommodated swine-to-baboon cardiac xenografts exhibit heightened control of complement at the level of C3, at least partly independent of the level of expression of known regulatory proteins. Wang et al. [38] reported that administration of anti-C5 antibodies in pre-sensitized allograft recipients in conjunction with immunosuppression prevented rejection and allowed accommodation to occur.
Although heightened control of complement activation provides a compelling explanation for accommodation, it cannot stand alone as a mechanism. Saadi et al. [39] showed that repair of endothelium following complement-mediated damage requires insertion of the membrane attack complex. McCurry et al. [40] and Cozzi et al. [41] among others showed that heightened expression of human complement regulatory proteins as the product of transgenes or administration of soluble forms of these receptors (which are potent inhibitors of complement) [42] does not by itself bring about accommodation; indeed, organs expressing those proteins undergo acute vascular rejection. Rather, manipulation of antibodies is still apparently needed to generate accommodation [43,44]. And, in human subjects and in some model systems, manipulation of antibodies suffices to allow accommodation to ensue [4,45]. We think that complement regulation is essential for survival of transplants over time and thus for accommodation to be manifest; however, regulation of complement per se does not appear to us to explain accommodation.
Acquired resistance to injury
Accommodation might reflect an acquired resistance of an organ or tissue to injury by complement or other noxious factors (independent of modifications in control of complement). Obviously, such protection could only be appreciated if some antibodies bind to a graft and some complement is activated. Acquired resistance to injury does not exclude other mechanisms or vice versa; however, if some other mechanism were fully manifest, an acquired resistance to injury would not be needed for accommodation, nor could it be proved.
Before considering evidence that organs acquire resistance to injury, we should offer a few further remarks on this subject. If the acquiring of resistance to injury depends on previous sub-toxic injury, then manipulations that prevent antibody binding or complement activation would preclude the development of resistance to injury and hence accommodation. Thus, we shall be very interested to know whether resistance, such as it may be, requires prior insult. If resistance to lethal injury requires sub-toxic injury, then we should want to know whether the trigger is homologous (i.e. antibody binding impairs further antibody binding or complement activation impairs further activation of complement) or whether it is heterologous (i.e. resistance to complement as one example might be induced by sub-toxic exposure to TNFα or endotoxin) [46]. The answer has obvious implications for therapeutics – if accommodation is homologous then full inhibition of complement might be detrimental. We should also mention that since resistance to injury is acquired and not constitutive, we would think that the condition of resistance must engender something that is dys-physiologic or toxic (otherwise resistance to injury would be a constitutive state). Following on that supposition, we might speculate that some chronic conditions, perhaps chronic rejection among them, might reflect “complications” of accommodation [47]. On the other hand, the existence of accommodation might also allow a graft to survive long enough for chronic rejection to occur as a consequence of factors, such as antibody binding and complement activation that would otherwise induce acute injury to an organ. Hence, a correlation of accommodation with chronic rejection should not be taken to infer one caused the other. Finally, one must be careful about inferring that a gene product, such as heme oxygenase-1, the absence of which is associated with organ injury and hence with the absence of accommodation, must be a critical mediator of accommodation. While heme oxygenase-1 or other protective genes might be central to accommodation, they might instead simply allow an organ graft to survive independent of whether accommodation has occurred. Thus, Matsuo et al. [48] found that inhibition of the function of decay accelerating factor in an organ causes the organ to undergo severe acute vascular disease, and hence DAF is essential for the integrity of an organ under stress or even under constitutive conditions. However, that is not to say that DAF is integral to accommodation, since as we mentioned heightened expression of that protein does not prevent acute vascular rejection of xenografts (rather it appears to be one of many proteins that help maintain integrity of organs or repair).
Several models systems have revealed evidence that organs can acquire resistance to injury. Nath et al. [49] showed that exposure of an organ to heme induces heme oxygenase, which can protect the organ against lethal injury by various toxins (consistent with a heterologous mechanism). Bach et al. [14] found that xenografts and Hancock et al. [50] that allograft with accommodation express a number of “protective genes”, that is to say genes that inhibit apoptosis and lack of heme oxygenase-1 precludes induction of accommodation. Delikouras et al. [51] found that exposure to xenoreactive antibodies induces expression of cytoprotective genes in endothelial cells. Jindra et al. [52] found that anti-HLA antibodies likewise induce such genes. On the other hand, Park et al. [53] found that cytoprotective genes may not be expressed at higher levels in ABO-incompatible allografts and Williams et al. [37] found these genes expressed at higher levels in xenografts with rejection than in xenografts with accommodation.
Other pathways may induce cytoprotection. Grehan et al. [54] and Black et al. [55] found that IL-7 and IL-13 confer resistance to lysis on endothelial cells that this resistance is owed at least in part to the PI-3 kinase/AKT pathways. Koch et al. [56] showed that the PI-3 kinase/AKT pathways makes hepatocytes naturally resist complement mediated lysis. Narayanan et al. [57] showed that anti-HLA antibodies activate the PI-3 kinase/AKT pathway and induce production of camp, which together protect endothelial cells from complement-mediated lysis at least in part by inducing cytoprotective genes.
The Prevalence of Accommodation
Based on our original definition of accommodation as a condition in which a graft recipient has antibodies directed against the graft but injury and/or dysfunction do not occur [4], one might conclude that accommodation is relatively infrequent. Thus, the proceedings of a recent Banff conference on organ allografts, accommodation was considered an unusual phenomenon, when it was considered at al [58]. Even more focused considerations of humoral immunity and graft outcome devote far more consideration to tolerance, which is only seen with regularity in ABO-incompatible transplants in infants [59] than to accommodation, which is seen both in infants and adults [60]. We think accommodation may be far more frequent than commonly though [17]. Healthy organs can absorb anti-donor antibodies in large amounts, removing those antibodies from the circulation [27]. Thus, we envision that organs may well be accommodated in recipients having no detectable anti-donor antibody, because the functioning graft serves as a ‘sink’ for that antibody. Consistent with that possibility, Adeyi et al. [61] found that the prevalence and level of anti-donor antibodies in the blood increase profoundly after an organ allograft is removed; we observe the same for organ xenografts [62]. As an extension of this idea, we might imagine that the higher levels of antibodies typically associated with vascular rejection may represent a consequence of rejection rather than a cause. The organ damaged by antibodies and complement is less able to absorb antibodies from the blood, and hence the level of those antibodies increases. We should add one further point – C4d and C3d are typically seen in accommodated allografts and xenografts [18,22,37]. Hence, deposition of C4d in an organ graft cannot be taken by itself as evidence of acute vascular, or humoral or antibody mediated rejection (whichever term may be preferred).
Concluding Remarks
We conclude that a fuller understanding of accommodation would profoundly benefit the field of transplantation. Not only might accommodation be crucial to successful xenotransplantation, but it might promote advances in allotransplantation, and perhaps to recovery from other disease states. Because severe vascular injury is all but inevitable in the xenograft, and injury occurs early and dramatically in the course, accommodation may be easiest to initially investigate in that setting.
Acknowledgments
This paper was supported by grants from the National Institutes of Health (HL79067 and HL52297).
References
- 1.Chopek MW, Simmons RL, Platt JL. ABO-incompatible renal transplantation: initial immunopathologic evaluation. Transplant Proc. 1987;19:4553–4557. [PubMed] [Google Scholar]
- 2.Alexandre GPJ, Squifflet JP, De Bruyere M, et al. Splenectomy as a prerequisite for successful human ABO-incompatible renal transplantation. Transplant Proc. 1985;17:138–143. [Google Scholar]
- 3.Bannett AD, McAlack RF, Morris M, et al. ABO incompatible renal transplantation: a qualitative analysis of native endothelial tissue ABO antigens after transplant. Transplant Proc. 1989;21:783–785. [PubMed] [Google Scholar]
- 4.Platt JL, Vercellotti GM, Dalmasso AP, et al. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990;11:450–456. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 5.Hammer C. Isohemagglutinins and preformed natural antibodies in xenogeneic organ transplantation. Transplant Proc. 1987;19:4443–4447. [PubMed] [Google Scholar]
- 6.Cooper DKC, Human PA, Rose AG, et al. The role of ABO blood group compatibility in heart transplantation between closely related animal species. J Thorac Cardiovasc Surg. 1989;97:447–455. [PubMed] [Google Scholar]
- 7.Parker W, Lundberg-Swanson K, Holzknecht ZE, et al. Isohemagglutinins and xenoreactive antibodies are members of a distinct family of natural antibodies. Hum Immunol. 1996;45:94–104. doi: 10.1016/0198-8859(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 8.Stussi G, West L, Cooper DK, et al. ABO-incompatible allotransplantation as a basis for clinical xenotransplantation. Xenotransplantation. 2006;13:390–399. doi: 10.1111/j.1399-3089.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 9.Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J Immunol. 2004;172:5143–5148. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 10.Platt JL. New directions for organ transplantation. Nature. 1998;392:11–17. doi: 10.1038/32023. [DOI] [PubMed] [Google Scholar]
- 11.Parker W, Saadi S, Lin SS, et al. Transplantation of discordant xenografts: a challenge revisited. Immunol Today. 1996;17:373–378. doi: 10.1016/0167-5699(96)10028-1. [DOI] [PubMed] [Google Scholar]
- 12.Yu PB, Holzknecht ZE, Bruno D, et al. Modulation of natural IgM binding and complement activation by natural IgG antibodies. J Immunol. 1996;157:5163–5168. [PubMed] [Google Scholar]
- 13.Mohiuddin MM, Ogawa H, Yin DP, et al. Antibody-mediated accommodation of heart grafts expressing an incompatible carbohydrate antigen. Transplantation. 2003;75:258–262. doi: 10.1097/01.TP.0000053616.61907.D5. [DOI] [PubMed] [Google Scholar]
- 14.Bach FH, Ferran C, Hechenleitner P, et al. Accommodation of vascularized xenografts: Expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 15.Galili U. Xenotransplantation and ABO incompatible transplantation: the similarities they share. Transfus Apher Sci. 2006;35:45–58. doi: 10.1016/j.transci.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Tanemura M, Yin D, Chong AS, et al. Differential immune responses to alpha-gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation. J Clin Invest. 2000;105:301–310. doi: 10.1172/JCI7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **17.Tang AH, Platt JL. Accommodation of grafts: implications for health and disease. Hum Immunol. 2007;68:645–651. doi: 10.1016/j.humimm.2007.04.003. This paper provides critical commentary on mechanisms of accommodation and proposes for the first time that accommodation is the most common outcome of organ transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **18.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. This manuscript reviews graft pathology, pointing out that C4d can mark accommodation as it does antibody-mediated rejection. [DOI] [PubMed] [Google Scholar]
- 19.Yu PB, Parker W, Everett M, et al. Immunochemical properties of anti-Galα1-3Gal after sensitization with xenogeneic tissues. J Clin Immunol. 1999;19:116–126. doi: 10.1023/a:1020506617105. [DOI] [PubMed] [Google Scholar]
- 20.Yu PB, Parker W, Nayak JV, et al. Sensitization with xenogeneic tissues alters the heavy chain repertoire of human anti-Galalpha1-3Gal antibodies. Transplantation. 2005;80:102–109. doi: 10.1097/01.tp.0000162976.07023.6d. [DOI] [PubMed] [Google Scholar]
- **21.Warner PR, Nester TA. ABO-incompatible solid-organ transplantation. Am J Clin Pathol. 2006;125 Suppl:S87–94. doi: 10.1309/8W4X9H6F8FTLCGYX. This paper reviews recent clinical observations on ABO-incompatible transplantation, considering accommodation as a clinical outcome. [DOI] [PubMed] [Google Scholar]
- 22.Platt JL. C4d and the fate of organ allografts. J Am Soc Nephrol. 2002;13:2417–2419. doi: 10.1097/01.asn.0000030140.74450.0b. [DOI] [PubMed] [Google Scholar]
- 23.Ulfvin A, Backer AE, Clausen H, et al. Expression of glycolipid blood group antigens in single human kidneys: change in antigen expression of rejected ABO incompatible kidney grafts. Kidney Int. 1993;44:1289–1297. doi: 10.1038/ki.1993.381. [DOI] [PubMed] [Google Scholar]
- 24.Yuzawa Y, Brett J, Fukatsu A, et al. Interaction of antibody with forssman antigen in guinea pigs. Am J Pathol. 1995;146:1260–1272. [PMC free article] [PubMed] [Google Scholar]
- 25.Parker W, Lateef J, Everett ML, et al. Specificity of xenoreactive anti-galα1-3gal IgM for α-galactosyl ligands. Glycobiology. 1996;6:499–506. doi: 10.1093/glycob/6.5.499. [DOI] [PubMed] [Google Scholar]
- 26.Parker W, Holzknecht ZE, Song A, et al. Fate of antigen in xenotransplantation: implications for acute vascular rejection and accommodation. Am J Pathol. 1998;152:829–839. [PMC free article] [PubMed] [Google Scholar]
- 27.Parker W, Lin SS, Platt JL. Antigen expression in xenotransplantation: how low must it go? Transplantation. 2001;71:313–319. doi: 10.1097/00007890-200101270-00025. [DOI] [PubMed] [Google Scholar]
- **28.Wen Ding J, Zhou T, Ma L, et al. Expression of complement regulatory proteins in accommodated xenografts induced by anti-alpha-Gal IgG1 in a rat-to-mouse model. Am J Transplant. 2007;7:1–9. doi: 10.1111/j.1600-6143.2007.02016.x. This paper provides compelling evidence that accommodation correlates with heightened expression of complement-regulating proteins. [DOI] [PubMed] [Google Scholar]
- 29.Carney DF, Koski CL, Shin ML. Elimination of terminal complement intermediates from the plasma membrane of nucleated cells: the rate of disappearance differs for cells carrying C5b-7 or C5b-8 or a mixture of C5b-8 with a limited number of C5b-9. J Immunol. 1985;134:1804–1809. [PubMed] [Google Scholar]
- 30.Carney DF, Hammer CH, Shin ML. Elimination of terminal complement complexes in the plasma membrane of nucleated cells: influence of extracellular Ca2+ and association with cellular Ca2+ J Immunol. 1986;137:263–270. [PubMed] [Google Scholar]
- 31.Shin ML, Hansch G, Mayer MM. Effect of agents that produce membrane disorder on lysis of erythrocytes by complement. Proc Natl Acad Sci USA. 1981;78:2522–2525. doi: 10.1073/pnas.78.4.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryant RW, Granzow CA, Siegel MI, et al. Phorbol esters increase synthesis of decay-accelerating factor, a phosphatidylinositol-anchored surface protein, in human endothelial cells. J Immunol. 1990;144:593–598. [PubMed] [Google Scholar]
- 33.Dalmasso AP, He T, Benson BA. Human IgM xenoreactive antibodies can induce resistance of porcine endothelial cells to complement-mediated injury. Xenotransplantation. 1996;3:54–62. [Google Scholar]
- 34.Dalmasso AP, Benson BA, Johnson JS, et al. Resistance against the membrane attack complex of complement induced in porcine endothelial cells with a Gal alpha(1–3)Gal binding lectin: up-regulation of CD59 expression. J Immunol. 2000;164:3764–3773. doi: 10.4049/jimmunol.164.7.3764. [DOI] [PubMed] [Google Scholar]
- 35.Grubbs BC, Benson BA, Dalmasso AP. Characteristics of CD59 up-regulation induced in porcine endothelial cells by alphaGal ligation and its association with protection from complement. Xenotransplantation. 2003;10:387–397. doi: 10.1034/j.1399-3089.2003.02088.x. [DOI] [PubMed] [Google Scholar]
- 36.Dorling A, Stocker C, Tsao T, et al. In vitro accommodation of immortalized porcine endothelial cells: resistance to complement mediated lysis and down-regulation of VCAM expression induced by low concentrations of polyclonal human IgG antipig antibodies. Transplantation. 1996;62:1127–1136. doi: 10.1097/00007890-199610270-00018. [DOI] [PubMed] [Google Scholar]
- 37.Williams JM, Holzknecht ZE, Plummer TB, et al. Acute vascular rejection and accommodation: divergent outcomes of the humoral response to organ transplantation. Transplantation. 2004;78:1471–1478. doi: 10.1097/01.tp.0000140770.81537.64. [DOI] [PubMed] [Google Scholar]
- **38.Wang H, Arp J, Liu W, et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179:4451–4463. doi: 10.4049/jimmunol.179.7.4451. This paper reports that inhibition of terminal complement complex formation prevents humoral rejection and allows accommodation to occur. [DOI] [PubMed] [Google Scholar]
- 39.Saadi S, Platt JL. Transient perturbation of endothelial integrity induced by natural antibodies and complement. J Exp Med. 1995;181:21–31. doi: 10.1084/jem.181.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCurry KR, Kooyman DL, Alvarado CG, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995;1:423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 41.Cozzi E, Yannoutsos N, Langford GA, et al. Effect of transgenic expression of human decay-accelerating factor on the inhibition of hyperacute rejection of pig organs. In: Cooper DKC, Kemp E, Platt JL, White DJG, editors. Xenotransplantation: the transplantation of organs and tissues between species. 2. Springer; 1997. pp. 665–682. [Google Scholar]
- 42.Pruitt SK, Kirk AD, Bollinger RR, et al. The effect of soluble complement receptor type 1 on hyperacute rejection of porcine xenografts. Transplantation. 1994;57:363–370. doi: 10.1097/00007890-199402150-00009. [DOI] [PubMed] [Google Scholar]
- 43.Lin SS, Weidner BC, Byrne GW, et al. The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest. 1998;101:1745–1756. doi: 10.1172/JCI2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin SS, Hanaway MJ, Gonzalez-Stawinski GV, et al. The role of anti-Galα1-3Gal antibodies in acute vascular rejection and accommodation of xenografts. Transplantation (Rapid Communication) 2000;70:1667–1674. doi: 10.1097/00007890-200012270-00002. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y, Vandeputte M, Waer M. Accommodation and T-independent B cell tolerance in rats with long term surviving hamster heart xenografts. J Immunol. 1998;160:369–375. [PubMed] [Google Scholar]
- 46.Sibley DR, Lefkowitz RJ. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985;317:124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- 47.Platt JL, Nath KA. Heme oxygenase: protective gene or Trojan horse. Nat Med. 1998;4:1364–1365. doi: 10.1038/3947. [DOI] [PubMed] [Google Scholar]
- 48.Matsuo S, Ichida S, Takizawa H, et al. In vivo effects of monoclonal antibodies that functionally inhibit complement regulatory proteins in rats. J Exp Med. 1994;180:1619–1627. doi: 10.1084/jem.180.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hancock WW, Buelow R, Sayegh MH, et al. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 51.Delikouras A, Fairbanks LD, Simmonds AH, et al. Endothelial cell cytoprotection induced in vitro by allo- or xenoreactive antibodies is mediated by signaling through adenosine A2 receptors. Eur J Immunol. 2003;33:3127–3135. doi: 10.1002/eji.200323566. [DOI] [PubMed] [Google Scholar]
- 52.Jindra PT, Zhang X, Mulder A, et al. Anti-HLA antibodies can induce endothelial cell survival or proliferation depending on their concentration. Transplantation. 2006;82:S33–35. doi: 10.1097/01.tp.0000231447.34240.3c. [DOI] [PubMed] [Google Scholar]
- 53.Park W, Grande JP, Ninova D, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3:952–960. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 54.Grehan JF, Levay-Young BK, Fogelson JL, et al. IL-4 and IL-13 induce protection of porcine endothelial cells from killing by human complement and from apoptosis through activation of a phosphatidylinositide 3-kinase/Akt pathway. J Immunol. 2005;175:1903–1910. doi: 10.4049/jimmunol.175.3.1903. [DOI] [PubMed] [Google Scholar]
- **55.Black SM, Grehan JF, Rivard AL, et al. Porcine endothelial cells and iliac arteries transduced with AdenoIL-4 are intrinsically protected, through Akt activation, against immediate injury caused by human complement. J Immunol. 2006;177:7355–7363. doi: 10.4049/jimmunol.177.10.7355. This paper relates accommodation to Akt activation. [DOI] [PubMed] [Google Scholar]
- 56.Koch CA, Kanazawa A, Nishitai R, et al. Intrinsic resistance of hepatocytes to complement-mediated injury. J Immunol. 2005;174:7302–7309. doi: 10.4049/jimmunol.174.11.7302. [DOI] [PubMed] [Google Scholar]
- **57.Narayanan K, Jendrisak MD, Phelan DL, et al. HLA class I antibody mediated accommodation of endothelial cells via the activation of PI3K/cAMP dependent PKA pathway. Transpl Immunol. 2006;15:187–197. doi: 10.1016/j.trim.2005.09.005. This paper reports that anti-HLA antibodies can induce cytoprotection by activation of PI-3 kinase and Akt. [DOI] [PubMed] [Google Scholar]
- 58.Solez K, Colvin RB, Racusen LC, et al. Banff ′05 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 59.Fan X, Ang A, Pollock-BarZiv SM, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10:1227–1233. doi: 10.1038/nm1126. [DOI] [PubMed] [Google Scholar]
- 60.Kirk AD, Baldwin WM, Cascalho MI, et al. American society of transplantation symposium on B cells in transplantation: harnessing humoral immunity from rodent models to clinical practice. Am J Transplant. 2007;7:1464–1470. doi: 10.1111/j.1600-6143.2007.01815.x. [DOI] [PubMed] [Google Scholar]
- 61.Adeyi OA, Girnita AL, Howe J, et al. Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol. 2005;14:53–62. doi: 10.1016/j.trim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 62.McCurry KR, Parker W, Cotterell AH, et al. Humoral responses in pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol. 1997;58:91–105. doi: 10.1016/s0198-8859(97)00229-2. [DOI] [PubMed] [Google Scholar]