Abstract
Androgens have a variety of protective and therapeutic effects in both the central and peripheral nervous systems. Here we review these effects as they related specifically to spinal and cranial motoneurons. Early in development, androgens are critical for the formation of important neuromuscular sex differences, decreasing the magnitude of normally occurring cell death in select motoneuron populations. Throughout the lifespan, androgens also protect against motoneuron death caused by axonal injury. Surviving motoneurons also display regressive changes to their neurites as a result of both direct axonal injury and loss of neighboring motoneurons. Androgen treatment enhances the ability of motoneurons to recover from these regressive changes and regenerate both axons and dendrites, restoring normal neuromuscular function. Androgens exert these protective effects by acting through a variety of molecular pathways. Recent work has begun to examine how androgen treatment can interact with other treatment strategies in promoting recovery from motoneuron injury.
Keywords: motoneurons, androgens, axotomy, neuroprotection, cell death, sex differences, nerve regeneration, dendrites, testosterone, steroids
From cellular and molecular perspectives, the consequences of gonadal steroid action in the nervous system include profound trophic effects on target neurons. During development, gonadal steroids are involved in the establishment of sex differences in neural structure and organization that underlie sex differences in behavior [122], promoting the differentiation of a masculine pattern of neural organization [3,111]. For example, androgens have been shown to exert a potent influence on the determination of sex differences in neuron number [116,133,134]. Gonadal steroids also exhibit a wide array of neuroprotective and neurotherapeutic effects on a variety of neuronal populations [4,124]. In this case, the ability of androgens to impact the survival of neurons after injury or in disease, and to promote both neuronal plasticity and regeneration in the context of dendritic or axonal damage, has established androgens as neurotherapeutic agents with clinical potential in the treatment of both peripheral and central neural injuries [148].
The neuroprotective and neurotherapeutic effects of androgens have been the most thoroughly described and documented in motoneuron populations, including cranial motoneurons, sciatic motoneurons, and pudendal motoneurons. In this review, we summarize this work in motoneurons, describing androgenic action in a variety of phenomena, models, and species.
Protection from Cell Death: Sexual Differentiation
The death of neurons during normal development is a well documented phenomenon that occurs throughout the nervous system. Initially, neurons are overproduced and their numbers are subsequently reduced during a period of normally occurring cell death. The magnitude of death can range up to 80% of the initial neuron population in a given structure, and this death typically occurs over a brief period of time. Normally occurring neuron death contributes to the formation of a variety of structural specializations throughout the nervous system, creating local differences in neuron numbers that underlie functional specializations ranging from high acuity vision to sex differences [132]. In addition, neuron death is thought to establish appropriate ratios of cells in interconnecting populations, as well as to eliminate neurons that have established erroneous connections through diffuse growth of axons.
Abundant evidence exists in the neuroendocrine literature regarding the ability of gonadal steroids to positively impact neuronal viability during development. The first and most thoroughly studied example of the ability of androgens to protect neurons from normally occurring cell death during development comes from the sexual differentiation literature. The fifth and sixth lumbar segments of the spinal cord of the rat contain two sexually dimorphic motoneuron nuclei which innervate striated penile muscles. The smaller of these is the spinal nucleus of the bulbocavernosus (SNB), a medially located nucleus containing approximately 200 motoneurons in the adult male. This nucleus is also referred to as the dorsomedial nucleus (DM; [131]). In males, SNB motoneurons innervate the bulbocavernosus and levator ani muscles of the penis [10,131], as well as the external anal sphincter [103]. The SNB in mature females, who lack or have greatly reduced perineal musculature [60,19,151], is comprised of only approximately 60 motoneurons, innervating primarily the external anal sphincter [103,153]. Lateral to the SNB lies the sexually dimorphic dorsolateral nucleus (DLN), a much larger nucleus than the SNB, with approximately 600 motoneurons in the adult male but only half as many motoneurons in females [75]. The motoneurons of the DLN innervate the ischiocavernosus muscle of the penis as well as the urethral sphincter [131,75,106]. The ischiocavernosus muscle is also reduced or absent in adult females and thus, as in the SNB, females have a smaller number of DLN motoneurons. These muscles and the motoneurons innervating them control penile reflexes important for copulatory behavior [129,59,63].
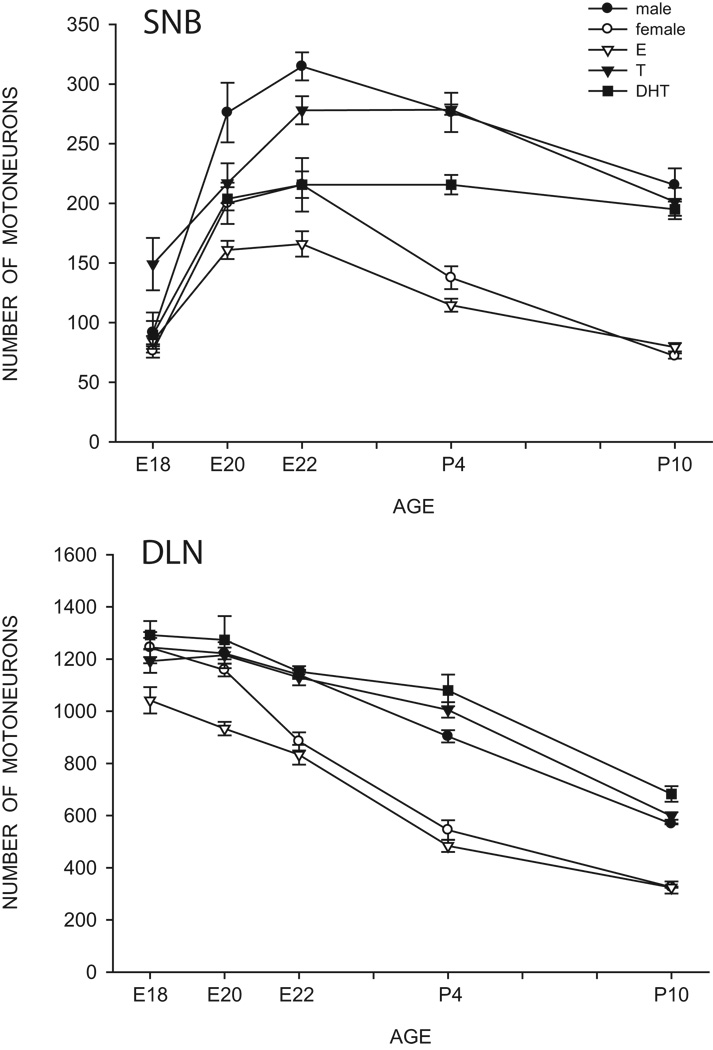
This sex difference in SNB and DLN motoneuron number develops perinatally. Before birth, the number of motoneurons in the SNB increases in both male and female rats, the result of a migration of developing SNB cells into their characteristic medial location [133]. SNB motoneuron numbers reach their maxima in both sexes just before birth, and this peak is followed by a decline through postnatal day (P) 10, when cell number reaches its adult range (Fig. 1). The decline is due to sexually dimorphic cell death, as revealed by counts of degenerating cells in the SNB [116]. Females typically lose up to 70% of their SNB motoneurons, whereas males lose about 25%. This sexually dimorphic motoneuron death is dependent on Bax, the pro-death member of the Bcl-2 gene family, and Bax depletion rescues SNB motoneurons [66]. In the DLN, large numbers of motoneurons are present in both males and females embryonically, and decline through P10 [134]. As in the SNB, this decline is due to the death of motoneurons; females typically lose over 70% of their DLN motoneurons, whereas males lose only about 50%.
Figure 1.
Counts of motoneurons in the SNB (top) and DLN (bottom) from embryonic (E) day 18 through postnatal (P) day 10 for normal males and females, as well as females treated with estradiol (E), testosterone (T), or dihydrotestosterone (DHT). Points represent means ± SEM. (Compiled from data originally published in [134,136], and Goldstein and Sengelaub, unpublished)
Gonadal hormones act in the establishment of sex differences in SNB and DLN motoneuron number by regulating this normally occurring motoneuron death. Females treated perinatally with testosterone propionate have reduced cell death during development and significantly more SNB and DLN motoneurons in adulthood than do normal females [13,75,116,133,134]. Conversely, male rats with testicular feminization mutation (Tfm, a loss-of-function mutation of the androgen receptor gene) experience a feminine pattern of cell degeneration in the developing SNB [135], and exhibit a feminine number of motoneuron in adulthood [11]. These genetic males (XY) develop functional testes that secrete normal levels of testosterone yet remain unresponsive to the steroid because of greatly reduced androgen binding relative to normal males [113]. Prenatal treatment with the antiandrogen flutamide, a drug known to block androgen receptor activation, also feminizes SNB motoneuron number in males [12].
This effect of androgens in the regulation motoneuron survival during early development has also been observed in the sexually dimorphic laryngeal motor nucleus in African clawed frogs [79]. These cranial motoneurons are part of a sexually dimorphic neuromuscular system involved in vocalization, and the greater number of motoneurons in males is thought to be due to an androgen-regulated developmental cell death. While treatment of male tadpoles with androgens has no effect, it increases the number of laryngeal motoneurons in females [79].
Androgens are thought to regulate the death of SNB motoneurons indirectly through their action at the target musculature. While treatment with androgens in the late prenatal or early postnatal period reduces SNB motoneuron death, SNB motoneurons do not express androgen receptors until the second postnatal week [44,76,77]. In addition, the normal androgen-induced masculinization of the SNB can be prevented by local application of the anti-androgen flutamide to the target muscles [43]. Studies implicating the SNB target muscles as the site of action have demonstrated that these muscles can be maintained by androgen treatment during development even if they have been denervated by removal of the lumbar spinal cord at birth [42], and moreover, the muscles possess androgen receptors at birth [44]. In a study by Freeman et al., [50], Tfm females who were genetically mosaic for androgen insensitivity were treated with testosterone during the period of SNB motoneuron death. These researchers capitalized on the fact that the androgen receptor gene resides on the X chromosome [160]. As a result of the random X-chromosome inactivation that occurs in every cell of XX embryos during development, female carriers of the Tfm mutation have some SNB cells that express the wild-type androgen receptors, and some that express the Tfm androgen receptors. Despite lacking functional androgen receptors, SNB motoneurons expressing the Tfm allele were spared by early testosterone treatment [50]. Because the SNB target musculature was also spared in these females, and these muscles contained functional androgen receptors, it seems plausible that androgen action in the neuromuscular periphery is responsible for the indirect support of SNB motoneuron survival.
Many of the actions of testosterone occur through its conversion to the non-aromatizable androgen dihydrotestosterone (DHT) or estrogenic metabolites [65]. For example, in rats, the sexually dimorphic nucleus (SDN) of the preoptic area of the hypothalamus is larger in adult males than in females. This sex difference is the result of estrogenic action during development. Females treated with estradiol perinatally develop a masculine SDN [27], while treatment of males with estrogen antagonists results in a feminine SDN [29]. Furthermore, DHT treatment is ineffective in masculinizing the SDN in female rats, and males given androgen antagonists perinatally develop an SDN that is no different from those of normal males [28]. Similarly, DHT treatment of female mice fails to completely masculinize the number of calbindin-immunoreactive neurons in the SDN [6].
Unlike SDN neurons, the protection from normally occurring motoneuron death during development appears to be strictly androgenic. Animals treated with DHT throughout the pre-and postnatal critical period have an SNB system that is completely masculine in terms of motoneuron number (Fig. 1) and presence of the target muscles [56]. Prenatal treatment with DHT is sufficient to completely masculinize DLN motoneuron number [136]. By contrast, in both the SNB and DLN, treatment of females with estradiol benzoate completely fails to alter cell number from the normal feminine pattern [15,55,9] (Fig. 1). Similarly, SNB motoneuron number is not affected in males treated postnatally with the aromatase inhibitor 4-OH-androstenedione [24], indicating that conversion of testosterone to an estrogen is not necessary for the development of a masculine number of motoneurons in this system. The findings in Tfm rats, and the failure of estrogens to prevent SNB motoneuron death, suggests that the sparing of both the SNB and its target musculature specifically requires activation of the androgen receptor.
Androgenic hormones may be required to stimulate the production of trophic factors from the neuromuscular periphery that then mediate the rescue of SNB motoneurons. Although these factors have not been identified, injection of ciliary neurotrophic factor into the perineum of newborn females spares SNB motoneurons [47], and the ability of testosterone to masculinize SNB motoneuron number is blocked by perineal injections of antagonists to trophic factors [156].
Protection from Cell Death: Injury
Gonadal steroids have been demonstrated to exert neuroprotective effects after nervous system trauma or disease, and there is substantial data in the literature that gonadal steroids can rescue neurons from cell death following a wide variety of neurological insults [155,4,124]. The molecular bases for gonadal steroid-mediated effects on neuron viability are quite intriguing; these effects can involve both androgenic and estrogenic mechanisms, and can be mediated by classical steroid receptors or by mechanisms independent of intracellular receptor populations.
In the 1990s, converging evidence from animal experiments and epidemiological studies indicated that estrogens can act as neuroprotectants in the central nervous system [52]. Less was known about the ability of androgens to act as neuroprotectants, however, until late in the decade, when the results of in vitro experiments demonstrated that cerebellar granule neurons can be protected from oxidative stress by androgens [1,2]. Testosterone was also shown to protect primary cultures of human neurons from cell death caused by serum deprivation [58] and cultured rat hippocampal neurons from beta-amyloid toxicity [123]. Androgens also protect cultured immortalized motoneurons from heat shock-induced cell death. Since these neuroprotective effects of androgens on cultured motoneurons occur in the absence of glial elements or synaptic input, the molecular mechanisms underlying rescue appear to be neuronally based [148].
Subsequent in vivo work has demonstrated that androgens are also capable of preventing neuronal cell death in the brain after various types of injury. For example, facial nerve transection kills facial motoneurons in neonatal hamsters, but androgen treatment prevents this [64]; and androgens can enhance neuronal viability after kainate lesions in rat hippocampus [126]. Similarly, treatment with DHT reduces laryngeal motoneuron loss after axotomy [120].
Axonal Injury
In mammals, axons in the injured central nervous system generally do not regenerate well. In contrast, axons in the peripheral nervous system are known for their remarkable regenerative capacity. As long as the connective tissues of the nerve sheath are left relatively intact, most peripheral axons readily regrow and reinnervate their original targets with a high degree of specificity. This is true of both cranial and spinal motoneurons. However, the regenerative capacity of peripheral motoneuron axons is not limitless. Both severity of the nerve injury and proximity of the injury to the cell body can lead to relatively poor nerve regeneration [142]. It is therefore important to uncover the factors that determine the regenerative ability of injured neurons, and begin to understand how to enhance these abilities. In fact, a significant amount of research has been done on these topics [95,109], and androgens have proven to be effective agents in enhancing nerve regeneration.
Androgen-Enhanced Nerve Regeneration
In the 1980s, several laboratories described the positive actions of androgens on neuronal regeneration in different motoneuron injury paradigms. Yu and collaborators used the rat hypoglossal motoneuron as one experimental paradigm [162,163], while others utilized spinal nerve injury models [154,161]. Early studies by Yu and colleagues demonstrated a sex difference in the rates of axon regeneration (males regenerating faster) following axotomy of the hypoglossal nerve in rats [162], and that androgen treatment enhances axon regeneration rates in both male [163,167] and female [166] rats.
One of the most useful in vivo models of axon regeneration is the rodent facial nerve axotomy model [68,109]. Injury to the facial nerve at its exit from the skull through the stylomastoid foramen leaves the blood-brain barrier intact and does not directly damage the cell bodies within the brainstem. Facial nerve injury results in a characteristic unilateral facial paralysis, associated with drooping of one corner of the mouth, flattened and paralyzed vibrissae, and loss of the eyeblink reflex. In rodents, complete recovery of gross motor movements is nearly always observed within three weeks.
Rodent facial motoneurons contain androgen receptor mRNA and protein [164,30] and, therefore, represent an ideal system in which to study the effects of androgens on peripheral nerve repair. The first indication that androgens might regulate axon regeneration in the facial nerve was in a series of experiments published in 1989 [85]. In this paper, the authors reported experiments in which adult male Syrian hamsters were subjected to unilateral facial nerve crush, then treated with various regimens of testosterone propionate and evaluated for the length of time until recovery of facial motor function. The results demonstrated that testosterone exerts a dramatic effect on the speed of functional recovery. Furthermore, the experiments revealed a dose-response relationship, with higher doses and more frequent treatments producing better results; the steady dose achieved by Silastic implants yielded the best results. Androgen treatment also accelerates functional recovery from facial nerve crush in mice [148].
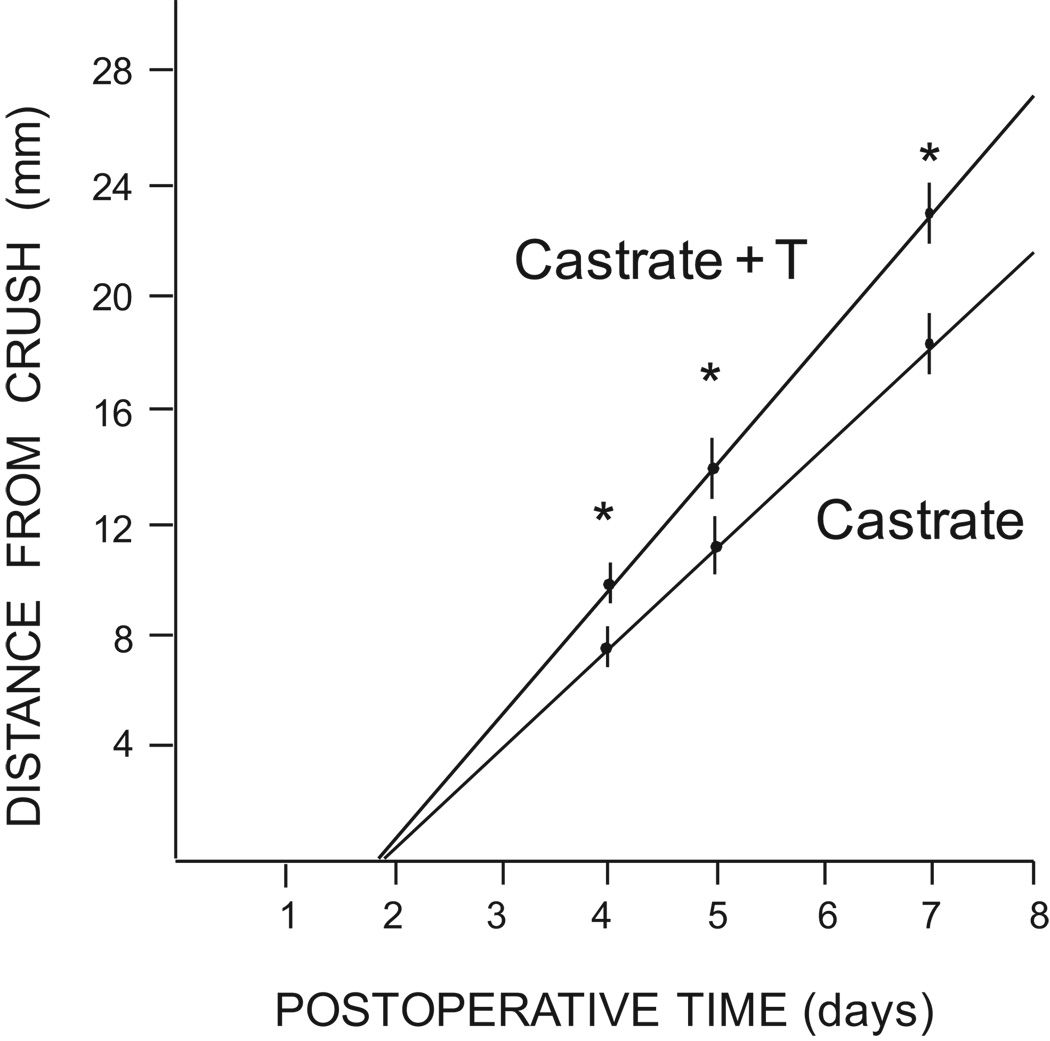
The initial finding that testosterone accelerates functional recovery following facial nerve injury led to the hypothesis that testosterone might exert its beneficial effect by increasing the temporal rate of regeneration of the injured axons. To test this hypothesis, Kujawa and colleagues [82] injected tritiated amino acids into the facial motor nuclei of male and female hamsters with facial nerve crush injuries. Because these radiolabeled amino acids were incorporated into protein and transported to the growing tips of the axons by fast axonal transport, it was then possible to measure the distance traveled by the fastest growing axons within the facial nerve. Testosterone administration led to a 26–30% increase in the rate of regeneration in males (Fig. 2). Interestingly, baseline axon regeneration rates were higher in females than in males. Testosterone also increased axon regeneration rates in females, but to a lesser degree than in males. There is a corresponding sex difference in speed of functional recovery following facial nerve crush, with females recovering before males [67]. Testosterone treatment speeds functional recovery in males, but has no significant effect on speed of recovery in females [67].
Figure 2.
Outgrowth distances from the point of nerve crush to the leading edge of the growing axons following facial nerve crush in castrated male hamsters, either treated with testosterone (T) or left untreated. Circle heights and bars represent means ± SEM. * indicates p < .05. Diagonal lines represent extrapolation of the data. (Data from [82])
This finding of a sex difference in testosterone-enhanced axon regeneration suggests that the facial nerve systems of male and female hamsters may be differentially responsive to androgens, perhaps due in part to an inherent sex difference in basal androgen receptor levels. This hypothesis is strengthened by the fact that administration of flutamide, a potent androgen receptor blocker, completely abolishes the ability of testosterone to enhance facial nerve regeneration rates, indicating that the effect of testosterone is indeed androgen receptor-dependent [86]. Additionally, DHT, which is often found to be a more potent ligand for androgen receptors than testosterone, produces a greater acceleration in regeneration than testosterone [145]. Drengler and colleagues [30] provided evidence for a sex difference in androgen receptor levels by demonstrating that female hamsters express androgen receptor mRNA in their facial motoneuron somata at only about half the levels that male hamsters do. It is unknown whether this difference in message is translated into a difference in protein, but if so, this could account for the reduced effect of testosterone on axon regeneration rates in females. There is a known sex difference in androgen receptor protein in rat facial motoneuron somata, with males having more [165]. Interestingly, while testosterone treatment of castrated males increases androgen receptor mRNA in facial motoneuron somata, this effect is completely blocked by axotomy [31,89]. Axotomy-induced downregulation of androgen receptor has also been found in spinal motoneurons [92,157]. These findings suggest that the regulation of androgen receptor levels in motoneuron somata depends at least in part on a peripherally-derived factor. More importantly, however, they suggest that the accelerative effects of testosterone on axon regeneration do not depend on increased transcription of androgen receptor message in the regenerating motoneuron.
Androgen enhancement of facial nerve regeneration has important temporal requirements. An early study of the effects of testosterone on the recovery of facial motor function following crush injury suggests that the first week following injury represents a “critical period” [84]. If testosterone treatment is delayed until 6 days after the injury, it is ineffective in accelerating recovery. However, if testosterone treatment is given immediately, it is beneficial even if withdrawn after 7 days, well before full behavioral recovery. In fact, the effective temporal window of testosterone is actually much shorter than this [146]. Administration of testosterone for only 6 hours post-injury has the same effectiveness on both functional recovery and axon regeneration rates as does continuous administration of testosterone throughout the full recovery phase. Moreover, delaying administration of testosterone until 6 hours after injury completely eliminates its effectiveness, even if it is administered continuously from that point forward.
Testosterone has also been shown to accelerate functional recovery from hind limb paralysis following sciatic nerve crush in rats [16], and to enhance sciatic axon regeneration rates [83]. In view of the importance of motoneuron viability to a successful outcome from spinal cord injury, this is a matter of critical therapeutic significance. Castrated male rats were subjected to a mid-thigh crush injury of the sciatic nerve (approximately 65 mm from the spinal cord), then treated with either testosterone or vehicle. Systemic exposure to androgens accelerated axon regeneration rates, but did not alter the delay before sprout formation occurred. This acceleration has important consequences for recovery, as rats given sustained-release testosterone implants recover from axotomy-induced hind limb paralysis more quickly than untreated rats [15]. The superior efficacy of implants over endogenous hormone in supporting androgen-dependent features has been observed previously (e.g., [25]), and may reflect the difference between a given (and fixed) circulating testosterone level from an implant and the same average (but fluctuating) level of hormone in intact males.
Interestingly, the sciatic nerve has been shown to express androgen receptor [94], apparently in the endoneurium [78], raising the possibility that androgens exert some of their beneficial effects on nerve regeneration by acting in the periphery.
Interestingly, androgen enhancement of functional recovery following peripheral axotomy appears to depend in part on route of administration. When testosterone is administered by subcutaneous injections rather than sustained-release implants, the steroid can still enhance axon regeneration in the sciatic nerve, but has no beneficial effects on functional recovery [144]. Similarly, sustained-release testosterone decreases functional recovery times following facial nerve axotomy, but injected testosterone does not [85].
Axotomy Effects on Dendrites
After peripheral axotomy motoneurons show a range of responses including structural, functional, and biochemical changes (e.g., [57,150,5]. For example, axotomy of sciatic motoneurons by nerve crush causes dendritic retraction after two months [117]. Axotomy also changes the electrophysiological properties of motoneuron dendrites, for example, giving rise to novel sodium-dependent partial spikes [137]. Permanent axotomy of gastrocnemius motoneurons reduces dendritic diameter within 3 weeks, and dramatically reduces dendritic membrane area and volume within 12 weeks [7]. Actual disconnection of motoneurons from their target musculature is not required to induce dendritic retraction; for example, chemical blockade of functional contact between hypoglossal motoneurons and the tongue results in dendritic retraction [141]. The dramatic regressions that occur in motoneuron dendritic arbors after axotomy can be reversed upon muscle reinnervation [141,6,8,117]. This association between dendritic arbor size and muscle contact suggests that target musculature provides some sort of trophic support for motoneurons.
Protective Effects on Dendrites after Axotomy
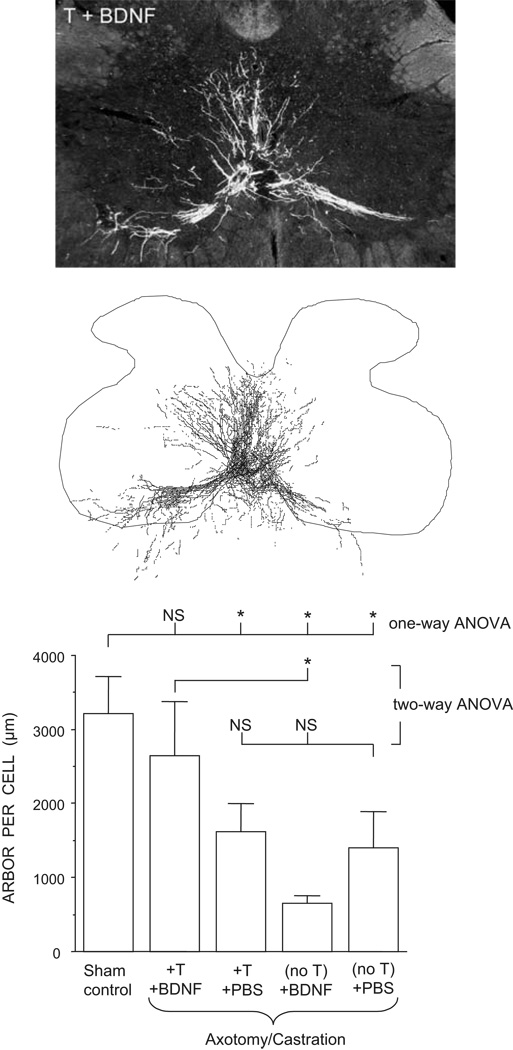
In addition to its effects on nerve regeneration, testosterone has been shown to have a variety of protective effects on other aspects of motoneuron structure and function. Treatment with either testosterone or brain derived neurotrophic factor (BDNF) alone can prevent axotomy- or castration-induced declines in soma size of SNB motoneurons [158]. Furthermore, treatment with testosterone and BDNF shows an interactive effect in the regulation of androgen receptor expression. Axotomy of adult SNB motoneurons causes a dramatic decline in the expression of androgen receptor immunoreactivity [157]. While application of BDNF alone to the cut SNB axons is ineffective in preventing this decline, combined treatment with both testosterone and BDNF is more effective than treatment with testosterone alone [157]. Androgen and BDNF show a similar interactive effect in the maintenance of dendritic morphology [159]. SNB dendritic lengths are dramatically reduced in axotomized castrated males, and treatment with either testosterone implants or BDNF alone applied to the cut axons is ineffective in preventing similar declines in dendritic length (Fig. 3). However, combined treatment with both testosterone and BDNF fully supports SNB dendritic morphology after axotomy. The requirement for testosterone may be at least partially due to the fact that the expression of the BDNF receptor, trkB, in spinal motoneurons is regulated by testosterone: motoneurons of castrated males deprived of testosterone show reduced expression of trkB receptors compared to motoneurons of intact animals or castrated males given testosterone replacement [118,119].
Figure 3.
Darkfield photomicrograph (top) and a computer-generated composite (middle) of BHRP-labeled SNB motoneurons in an axotomized castrated male treated with both testosterone and BDNF. (Bottom) SNB dendritic lengths were dramatically reduced in axotomized castrated males compared to those of intact males. Treatment with either testosterone (T) implants or BDNF applied to the cut axons alone was ineffective in preventing similar declines in dendritic length. However, axotomized castrate males given both testosterone and BDNF had SNB dendritic lengths that did not differ from those of intact males. Bar heights represent means ± SEM. * indicates p < .05. (Data from [159])
Androgen Maintenance of Motoneuron Morphology
Manipulating androgens in adulthood can have marked effects on motoneuron soma size. Castration of adult males leads to the shrinkage of SNB [11], and to a lesser degree DLN [87], motoneuron somata, which can be prevented by treating castrated males with testosterone. This effect is not universal in motoneurons and likely reflects differences in androgen sensitivity across motoneuron populations [91]. For example, castration with or without hormone replacement has no effect on soma size in quadriceps motoneurons [118].
DHT partially protects SNB cells from shrinkage after castration of adult male rats, but is not as effective as testosterone when given in the same dose [46]. Estrogenic metabolites of testosterone do not seem to explain the difference in efficacy between testosterone and DHT, however, as estradiol alone or in conjunction with DHT has no effect on soma size [46]. Similarly, blockade of estradiol synthesis in intact adult male rats with the aromatase inhibitor, fadrozole, does not affect SNB soma size [18].
A similar pattern of results is seen in gerbils, where long-term castration of adult males leads to a reduction in SNB soma size that can be prevented by treatment with testosterone, but not estradiol [49]. As in rats, DHT is partially effective in preventing the castration-induced reduction in SNB cell size in gerbils [49]. This suggests that testosterone increases SNB soma size of adult rodents by acting via androgen receptors, and may do so without conversion to either estradiol or DHT.
In adulthood, castration results in a substantial reduction in the length of dendrites in both SNB and DLN motoneurons which can be completely reversed with androgen replacement [88,87]. This kind of androgen-dependent, reversible plasticity had not been reported previously in an adult mammalian system, and was particularly exciting because changes in androgen levels occur normally and correlate with changes in the frequency of copulatory behaviors. For example, a naturally occurring analog of hormone depletion and replacement can be seen in seasonally breeding mammals such as white-footed mice. As in rats, castration results in decreased dendritic arbors in these mice [45]. Similarly, circulating testosterone levels decline with advanced aging, and these declines are accompanied by reductions in sexual behavior. Fargo et al., [35] found that the bulbocavernosus and levator ani muscles and their innervating SNB motoneurons underwent profound atrophy with advancing age, with muscle weight and motoneuron dendritic length declining to less than 50% of young adult levels. Treatment of aged animals with testosterone completely reverses the age-related declines in muscle weight and SNB motoneuron morphology, demonstrating that the SNB system retains its androgen-mediated plasticity throughout life.
Concomitant with changes in dendritic length, androgens control the number and size of gap junctions and synapses on SNB motoneurons [90,101,102]. Although the molecular bases for androgenic effects on dendrites, gap junctions or synapses are not fully understood, androgen manipulation directly or indirectly affects the expression of a number of candidate genes and proteins in SNB motoneurons, including those for the gap junction proteins [99], N-cadherin [107], calcitonin gene-related peptide [125], androgen receptor [98], the ciliary neurotrophic factor receptor α [48], and the major cytoskeletal elements, β-actin and β-tubulin [99,97].
Rand and Breedlove [127] showed that testosterone can regulate SNB dendrites by acting at the target musculature. In castrated males, SNB motoneurons projecting to testosterone-implanted bulbocavernosus muscles have significantly longer dendritic lengths than those projecting to muscles on the contralateral side treated with the anti-androgen flutamide. This result suggests that, similar to the effects described earlier for hormonal support of dendritic morphology after axotomy [159], androgens regulate a neurotrophic signal from the muscle that is critical in the maintenance of dendritic organization in adulthood.
Induced Atrophy after Motoneuron Depletion
Neurodegenerative disease or nerve injury often results in the loss of spinal motoneurons. For example, amyotrophic lateral sclerosis (ALS), the most common motoneuron disease in adult humans, is characterized by the selective death of upper or lower motoneurons (as well as small interneurons) in the brain and spinal cord. This cellular degeneration results in a progressive muscle weakness, atrophy and spasticity, and ultimately a nearly complete paralysis [20]. While ALS is the most prevalent, other diseases including the motor neuron diseases and spinal muscular atrophies (e.g., spinal and bulbar muscular atrophy [80]) are also characterized by progressive loss of motoneurons. Similarly, damage to spinal nerves resulting in laceration and avulsion of spinal roots (e.g., cauda equina injury with high impact motor vehicle accidents, [112]) can lead to the death of motoneurons and preganglionic autonomic neurons in the spinal cord, resulting in autonomic and motor dysfunction [62].
However, the death of motoneurons is not the only outcome, and importantly, remaining motoneurons after such insults show a variety of morphological and functional changes. Experimentally-induced partial depletion of motoneurons, produced by injections of the retrogradely transported neurotoxin saporin into the target musculature, results in substantial somal and dendritic atrophy in surviving SNB or quadriceps motoneurons [36,37,39,91]. In both cases, after the death of motoneurons by saporin injection, the cross-sectional area of surviving nearby SNB or quadriceps motoneurons is reduced [36,37,39,91] (Fig. 4). Similarly, induced motoneuron death results in a pronounced dendritic atrophy in surviving nearby motoneurons. Four weeks after partial motoneuron depletion, dendritic length in both SNB and quadriceps motoneurons is decreased by over 60% [36,37,39,91]. Evidence of recovery in dendritic lengths is present at 6 weeks post depletion, and by 10 weeks, dendritic lengths recover to those of normal, intact males [40].
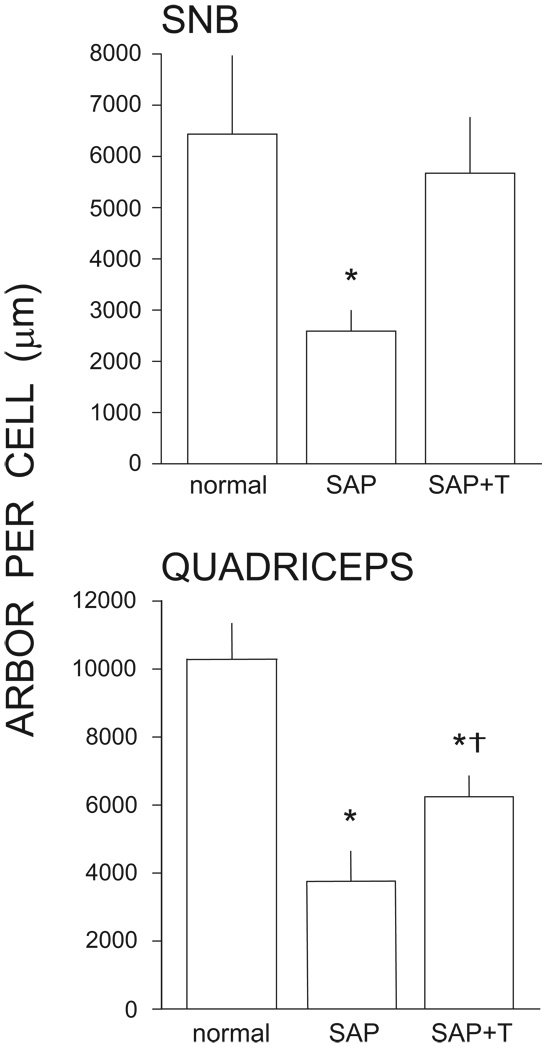
Figure 4.
Testosterone treatment is neuroprotective in SNB (top) and quadriceps (bottom) motoneurons, attenuating induced dendritic atrophy resulting from the death of nearby motoneurons. Motoneuron dendritic lengths are expressed as length of arbor per labeled motoneuron for normal males and saporin-treated males with (SAP+T) or without (SAP) supplemental testosterone. Bar heights represent means ± SEM. * indicates significantly different from normal males. † indicates significantly different from untreated saporin-injected animals. (Data from [37,91])
These regressive changes in motoneuron morphology are correlated with changes in motor activation: concomitant with atrophy, stimulation-evoked activation of motoneurons is attenuated. In saporin-injected animals, stimulation of the dorsal root afferents to the SNB or quadriceps motoneurons produces responses in the respective pudendal or femoral nerves whose amplitudes are dramatically reduced compared to those of normal males [38,91].
Treatment with testosterone is neuroprotective for remaining motoneurons following partial depletion. In the SNB, testosterone treatment attenuates dendritic atrophy, resulting in dendritic lengths that are reduced an average of only approximately 16% from normal lengths, and are greater than those of saporin-injected animals by an average of over 119% [36,37,39] (Fig. 4). While testosterone treatment is effective in attenuating dendritic atrophy in quadriceps motoneurons, it does not protect them to the same degree as observed in the highly steroid-sensitive SNB [91]. Quadriceps motoneurons in testosterone-treated saporin animals have dendritic lengths that are reduced almost 40% from normal lengths, and are greater than those of saporin-injected animals by only approximately 65% (Fig. 4). Changes in motor activation across the SNB and quadriceps motor populations reflect these differences in the magnitude of the neuroprotective effects of testosterone on dendritic morphology. Stimulation-evoked activation of SNB motoneurons is restored to intact levels with testosterone treatment, but in quadriceps motoneurons testosterone treatment is again only partially protective, resulting in intermediate levels of motor activation [38,91]. A likely explanation for this difference lies in the expression of androgen receptors in these two systems. SNB motoneurons abundantly express androgen receptors, and almost 70% of SNB motoneurons are labeled with tritiated testosterone at 5X background grain density; in contrast, less than 3% reach this criterion in other motoneurons [9,14]. Similarly, the principal SNB target muscles in males (the bulbocavernosus and levator ani muscles) are enriched for androgen binding sites and androgen receptor protein compared to other striated muscles [32,152,108]. Androgen receptor protein is present in substantially higher concentrations in the levator ani compared to other skeletal muscle [108], and the bulbocavernosus and levator ani muscles have over four times as many binding sites for testosterone than are present in the quadriceps muscles [32]. Thus, as differences in the density of androgen receptors are thought to underlie differences in androgen responsiveness across tissues [108], the lower density of androgen receptors in the quadriceps system might result in a smaller protective effect of testosterone. Regardless of the differences in degree, testosterone treatment is neurotherapeutic in both SNB and quadriceps motoneurons, protecting against the reductions in excitability seen after partial motoneuron depletion and providing a functional measure of recovery.
The time course of induced atrophy and recovery from partial motoneuron depletion with saporin is protracted, and treatment with testosterone appears to attenuate the atrophy rather than prevent it. While initial dendritic atrophy (2 weeks after motoneuron depletion) is similar to that of untreated saporin-injected males, testosterone treatment prevents further decrements in length [40]. Furthermore, in both SNB and quadriceps motoneurons this attenuation of dendritic atrophy by testosterone is dose-dependent, and four weeks after motoneuron depletion, dendritic lengths in males treated with physiological levels of testosterone are longer than those of untreated, saporin-injected males [23]. Treatment with higher dosages of testosterone does not produce any further attenuation in dendritic length [23].
As described earlier for the effects of androgen on facial nerve regeneration, protection from dendritic atrophy after partial motoneuron depletion with saporin also has important temporal requirements [22]. Four weeks of testosterone treatment (delivered immediately post-saporin), or two weeks of testosterone treatment (after a delay of two weeks post-saporin) are both effective in attenuating induced dendritic atrophy in quadriceps motoneurons. However, dendritic atrophy in animals with immediate testosterone treatment of shorter durations or longer delays in the start of treatment, is comparable to that of animals who received no supplemental testosterone. This result demonstrates that similar to facial motoneurons, the neuroprotective effect of testosterone on quadriceps motoneuron morphology may also have a “critical period”. While motoneuron depletion occurs within a few days after saporin injection, loss of dendrites in remaining quadriceps motoneurons occurs most dramatically between 2–4 weeks post-saporin (Coons and Sengelaub, unpublished), the period in which testosterone treatment is maximally effective.
Consistent with most other morphological effects in adult SNB motoneurons, the neuroprotective effects of steroid treatment appear to be strictly androgenic, as treatment with either testosterone or DHT, but not estradiol, attenuates induced somal and dendritic atrophy following saporin injections [39]. By contrast, dendritic atrophy following saporin injections in motoneurons innervating the quadriceps muscles is attenuated to a similar degree by treatment with testosterone or its metabolites (Muñoz et al., unpublished).
Cellular and Molecular Mediators
The mechanisms by which androgens act to impart neuroprotection on selective neuronal populations are currently under investigation by numerous laboratories. In view of substantial data demonstrating neuroprotective effects of estrogens [143] and the widespread distribution of aromatase in the brain [128], one likely molecular scenario includes the conversion of circulating androgens to estrogens via brain aromatase [53]. However, there is also recent evidence for direct androgenic activation of neuroprotective pathways via both classical androgen receptor and membrane-associated receptors [114,115,54]. These studies suggest that in some cases androgens are able to exert neuroprotective actions without conversion to estrogens.
Understanding the molecular mechanisms by which androgens act on injured neurons is likely to provide important insights for developing strategies for therapeutic interventions for nervous system trauma and neurodegenerative diseases. Several proteins thought to be involved in neuroprotection are regulated by androgens. For example, proteins with antioxidant functions (e.g., catalase [2]) or involved in the regulation of cytoplasmic calcium (e.g., calbindin [121] are androgen-mediated. As discussed above, the neurotrophin BDNF [119] and its receptor trkB [118] are both regulated by androgens. Androgens are also thought to be involved with the activation of signaling pathways involved in neuroprotection (e.g., MAPK/ERK [124]).
Direct axonal damage results in a retrograde cell body reaction as well as significant changes in surrounding glial cells. Androgen receptors are expressed in both neurons and glia [41,51, 130], so androgen treatment has the potential for both direct and indirect modulation of the central neuronal repair process. In the case of the facial motor nucleus, axotomy is known to result in a process known as synaptic stripping, in which the synaptic input to the injured motoneurons is dramatically reduced, and neuron-neuron contacts are replaced by glia-neuron contacts [71]. This is accompanied by an upregulation in the facial motor nucleus of the glial marker GFAP at both the mRNA [72] and protein [21] levels. However, androgen treatment attenuates the process of synaptic stripping, preserving central input to the motoneurons [71], as well as attenuating the axotomy-induced upregulation of GFAP [21,67,69]. Similar effects have also been observed in the rat [109].
Other cellular and molecular elements that might be involved in the neuroprotective effects of androgens include ribosomal RNA, neuronal and glial cytoskeletal proteins, and stress proteins. Intracellular protein synthesis events are mediated by the nucleolar response to injury, and androgen exposure enhances this response. Following facial nerve axotomy in the hamster, testosterone treatment causes ribosomal RNA levels to increase more rapidly and to a greater magnitude than injury alone [81].
GAP-43, a cytoskeletal protein associated with the membranes of axons, is upregulated by axotomy in the peripheral nervous system [147]. Injury of the facial motor nerve increases GAP-43 mRNA and protein in the facial nucleus, and androgen treatment augments this response [21,70]. Other cytoskeletal proteins are also involved in androgen-enhanced nerve regeneration. The primary cytoskeletal structures in axons are microtubules, which are composed of tubulin proteins. Tubulin mRNAs are upregulated in facial motoneurons as the axons regenerate following axotomy [73,74,147]; androgen treatment, which enhances axon regeneration rates, causes an additional upregulation of tubulin mRNAs above axotomy alone [73,74]. Importantly, tubulin mRNAs are upregulated by androgen treatment selectively. While αI-tubulin, βII-tubulin, and βIII-tubulin are all upregulated by axotomy, only βII-tubulin has been shown to be under the control of androgen in this model [73,74]. Tubulin genes are also regulated by androgen in axotomized sciatic motoneurons [17] and rubrospinal motoneurons [140,26], and in the SNB [97,100], raising the possibility that this may be a general phenomenon in motoneurons.
Androgenic enhancement of axon regeneration also appears to be under the control of neuritin. Neuritin mRNA levels are upregulated by androgen treatment in cultured motoneuron-neuroblastoma hybrid cells in an androgen receptor-dependent manner [34], and neuritin has been shown to be a critical downstream mediator of the ability of androgens to increase neurite outgrowth in cultured NSC34/mAR cells [96]. In the injured hamster facial motor nucleus, testosterone causes a ~300% increase in neuritin mRNA levels two days post-injury [34]. Because testosterone treatment increases the rate of axon regeneration in injured hamster facial motoneurons [82], these data demonstrate a relationship between neuritin expression and androgen-enhanced axon regeneration in vivo. This relationship is further supported by the fact that blocking androgen receptors with flutamide, which is known to block the effects of testosterone on axon regeneration rates [86], completely abolishes the effect of testosterone on neuritin mRNA expression in the injured hamster facial nucleus [34]. The time course of androgenic regulation of neuritin expression in the hamster facial motor nucleus, with the most pronounced effect of testosterone occurring at two days post-injury, is particularly interesting for two reasons. First, extrapolation of hamster facial motoneuron axon regeneration data indicates that axon sprouting occurs at about two days post-injury [82]. Second, androgen treatment enhances axotomy-induced upregulation of βII-tubulin expression between two days and seven days post-injury [73,74].
Facial nerve injury also results in a heat shock response, in the form of upregulation of HSP70, a protein known to be upregulated in response to a variety of cell stresses, including central nervous system injury [33]. It has been suggested that testosterone treatment exerts its neuroprotective effects in axotomized hamster facial motoneurons in part by modulating this response [148,149]. Unbound androgen receptors in the cytoplasm form protein complexes with various heat shock proteins, and upon androgen binding, these heat shock proteins are released. During the normal cell stress response, there is a temporary cessation of rRNA processing and of overall protein synthesis [110]. Heat shock proteins are usually induced in the cell during this time, in order to complex with intracellular proteins denatured by the stressor. However, exogenous androgens delay the stress response following hamster facial nerve axotomy, presumably because androgen receptor binding releases a pool of immediately available heat shock proteins [149]. This obviates the need to divert the cellular protein synthesis machinery from a regenerative response to a cell stress response. Thus, androgen treatment at the time of injury may allow an uninterrupted axonal repair process to proceed, with the result being marked increases in the rates of axon regeneration and overall functional recovery.
Finally, a significant body of work has emerged pointing to peripheral nerves as targets for steroid actions [93,78,104,105]. These studies suggest that neuroactive steroids can actually be synthesized in nerves, and can work in the nerves via both classical and nonclassical steroid receptors. Furthermore, these studies indicate that one molecular target of neuroactive steroids is peripheral myelin, specifically the myelin-related proteins Po and PMP22.
Androgens and Electrical Stimulation
Recently work has begun to explore the effectiveness of combinatorial treatment involving both androgen and electrical stimulation in promoting recovery from peripheral nerve injury in rats. In a typical experiment, rats are subjected to unilateral facial nerve crush injury, treated with testosterone or no steroid, and given electrical stimulation or sham stimulation. Stimulating electrodes are implanted just proximal to the site of crush injury, and short bursts of stimulus pulses are delivered at 1–4 mV while the animals are awake and unrestrained [61]. Using radiolabeled amino acids injected into the facial nucleus, it has been shown that testosterone treatment increases the rate of axon regeneration without decreasing the delay before sprout formation [138]; this result is identical to what has been found in hamsters [82]. In contrast, electrical stimulation decreases the delay before sprout formation, without increasing the rate of axon regeneration. Combinatorial treatment with both testosterone and electrical stimulation both decreases the delay to sprout formation and increases the rate of axon regeneration, resulting in a better outcome than either treatment alone [138]. This result is mirrored in measures of functional recovery from facial nerve injury, where combinatorial treatment with testosterone and electrical stimulation results in faster functional recovery than either treatment alone [61].
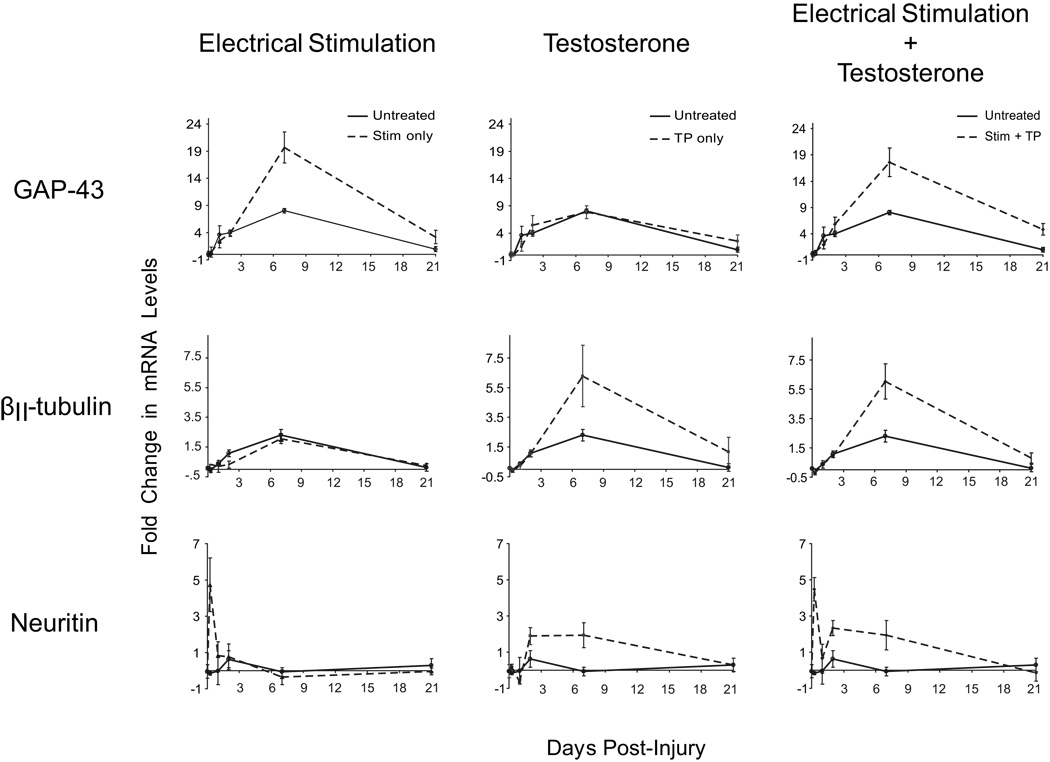
Electrical stimulation appears to have a very early effect on axon regeneration, reducing the delay to sprout formation from two days to one day post-injury, while testosterone has a more sustained effect, increasing the rate of axon regeneration through at least seven days post-injury [138]. This observation suggests that androgen treatment and electrical stimulation may act on different cellular and molecular processes in enhancing axon regeneration after injury. Expression of several regeneration-associated genes reveals that some of these genes are regulated only by electrical stimulation, some only by testosterone, and some by both [139] (Fig. 5). For example, GAP-43 mRNA levels are increased beyond nerve injury alone by electrical stimulation, but not by testosterone treatment. In contrast, βII-tubulin mRNA levels are increased by testosterone treatment, but not by electrical stimulation. Finally, some genes, such as neuritin, are regulated by both electrical stimulation and testosterone treatment.
Figure 5.
Testosterone and electrical stimulation differentially regulate regeneration-associated gene expression in the facial motor nucleus following crush axotomy. Unilateral crush injuries were performed in male rats, and each animal was treated with electrical stimulation only, testosterone propionate only, or a combination of both electrical stimulation and testosterone propionate. Tissue punches were taken from the facial motor nucleus on both the injured and uninjured sides of each animal, at 6 hours, 1 day, 2 days, 7 days, or 21 days after injury. mRNA levels were determined by reverse transcription and quantitative PCR, and are displayed as fold change with injury by comparing the injured and uninjured sides using the ΔΔCt method. Changes in mRNA levels are shown for GAP-43 (top row), βII-tubulin (middle row), and neuritin (bottom row), for untreated animals (solid lines, all panels), and animals treated with electrical stimulation only (left column), testosterone propionate only (center column), or a combination of both electrical stimulation and testosterone propionate (right column; all treated groups shown as dashed lines). GAP-43 was regulated by electrical stimulation only, βII-tubulin was regulated by testosterone propionate only, and neuritin was regulated by both electrical stimulation and testosterone propionate. Points represent means ± SEM. (Data from [139])
Conclusions
In summary, it is clear that androgens have robust neuroprotective effects on motoneurons. From the early developmental sparing from normally occurring neuron death through the enhancement of axonal and dendritic regeneration, androgens play an important role as protective and therapeutic agents in the nervous system. The accessibility of motor populations, the relative simplicity of their peripheral connectivity, and their clear behavioral role, combine to make them a powerful model in which to study processes of neuronal survival and regeneration. Androgens are profoundly effective in enhancing these processes, so exploring the neuroprotective effects of androgens on motoneurons has proven to be quite fruitful in recent decades. This approach has revealed a variety of mechanisms through which testosterone and its metabolites exert their protective effects on cellular morphology and function. Exploring these mechanisms and associated proteins and pathways will continue to provide valuable insights into regenerative processes, allowing us to identify specific treatment targets and establish new neurotherapeutic strategies.
Acknowledgements
This work was supported by NIH NINDS grant NS047264 (D.R.S.), NIH NINDS grant NS052997 (K.N.F.), NIH NIDCD grant DC000012 (K.N.F.), VA Rehab R&D grant B3756F (K.J.J.;K.N.F.), VA Rehab R&D grant B4952I (K.J.J.), VA Rehab R&D grant B6598W (K.N.F.), the Department of Otolaryngology--Head and Neck Surgery, Loyola University Chicago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur. J. Neurosci. 1999;11:1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 2.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 3.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 4.Bialek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Pol. J. Pharmacol. 2004;56:509–518. [PubMed] [Google Scholar]
- 5.Bisby MA, Tetzlaff W. Changes in cytoskeletal protein synthesis following axon injury and during regeneration. Mol. Neurobiol. 1992;6:107–123. doi: 10.1007/BF02780547. [DOI] [PubMed] [Google Scholar]
- 6.Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinol. 2008;149:4142–4150. doi: 10.1210/en.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brännström T, Havton L, Kellerth J-O. Changes in size and dendritic arborization patterns of adult cat spinal α-motoneurons following permanent axotomy. J. Comp. Neurol. 1992a;318:439–451. doi: 10.1002/cne.903180408. [DOI] [PubMed] [Google Scholar]
- 8.Brännström T, Havton L, Kellerth J-O. Restorative effects of reinnervation on the size and dendritic arborization patterns of axotomized cat spinal α-motoneurons. J. Comp. Neurol. 1992b;318:452–461. doi: 10.1002/cne.903180409. [DOI] [PubMed] [Google Scholar]
- 9.Breedlove SM. Neonatal androgen and estrogen treatments masculinize the size of motoneurons in the rat spinal nucleus of the bulbocavernosus. Cell. Mol. Neurobiol. 1997;17:687–697. doi: 10.1023/A:1022590104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 11.Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- 12.Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. I. Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J. Neurosci. 1983a;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J. Neurosci. 1983b;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breedlove SM, Arnold AP. Sex differences in the pattern of steroid accumulation by motoneurons of the rat lumbar spinal cord. J. Comp. Neurol. 1983c;215:211–216. doi: 10.1002/cne.902150208. [DOI] [PubMed] [Google Scholar]
- 15.Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237:173–181. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- 16.Brown TJ, Kahn T, Jones KJ. Androgen induced acceleration of functional recovery after rat sciatic nerve injury. Restor. Neurol. Neurosci. 1999;15:289–295. [PubMed] [Google Scholar]
- 17.Brown TJ, Storer P, Oblinger M, Jones KJ. Androgenic enhancement of βII-tubulin mRNA in spinal motoneurons following sciatic nerve injury. Restor. Neurol. Neurosci. 2001;18:191–198. [PubMed] [Google Scholar]
- 18.Burke KA, Kuwajima M, Sengelaub DR. Aromatase inhibition reduces dendritic growth in a sexually dimorphic rat spinal nucleus. J. Neurobiol. 1999;38:301–312. [PubMed] [Google Scholar]
- 19.Čihák R, Gutmann E, Hanzlikova V. Involution and hormone-induced persistence of the muscle sphincter (levator ani) in female rats. J. Anat. (Lond.) 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- 20.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 21.Coers S, Tanzer L, Jones KJ. Testosterone treatment attenuates the effects of facial nerve transection on glial fibrillary acidic protein (GFAP) levels in the hamster facial motor nucleus. Metab. Brain Dis. 2002;17:55–63. doi: 10.1023/a:1015415226799. [DOI] [PubMed] [Google Scholar]
- 22.Coons KD, Sengelaub DR. Protection from dendritic atrophy with testosterone following partial motoneuron depletion: Timing and duration of treatment, functional correlates in motor activation. Soc. Neurosci. Abstr. Viewer/Itinerary Planner, Program No. 556.23. 2008 [Google Scholar]
- 23.Coons KD, Wilson RE, Sengelaub DR. Protection from dendritic atrophy with testosterone following partial motoneuron depletion: dose-dependence in males and efficacy in females. Soc. Neurosci. Abstr. Viewer/Itinerary Planner, Program No. 56.11. 2007 [Google Scholar]
- 24.Currie JC, Houston PA, Henderson A, Payne AP. Neonatal hormone manipulations and the maintenance of perineal muscles and their motor neurones in Albino Swiss rats. J. Reprod. Fertil. 1990;89:597–603. doi: 10.1530/jrf.0.0890597. [DOI] [PubMed] [Google Scholar]
- 25.Damassa DA, Kobashigawa D, Smith ER, Davidson JM. Negative feedback control of LH by testosterone: A quantitative study in male rats. Endocrinol. 1976;99:736–742. doi: 10.1210/endo-99-3-736. [DOI] [PubMed] [Google Scholar]
- 26.DeLucia TA, Alexander T, Fargo KN, Jones KJ. Effects of single versus combinatorial treatment strategies on beta II-tubulin gene expression in axotomized hamster rubrospinal motoneurons. Restor. Neurol. Neurosci. 2007;25:573–584. [PubMed] [Google Scholar]
- 27.Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Pre- and postnatal influence of testosterone propionate and diethylstilbesterol on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res. 1984a;302:291–295. doi: 10.1016/0006-8993(84)90242-7. [DOI] [PubMed] [Google Scholar]
- 28.Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Sickmoller PM, Jarzab B, Gorski RA. Pre- and postnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Endocrinology. 1986;42:443–448. doi: 10.1159/000124484. [DOI] [PubMed] [Google Scholar]
- 29.Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen anatagonist. Neuroendocrinology. 1984b;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- 30.Drengler SM, Handa RJ, Jones KJ. Sex differences in androgen receptor mRNA levels and regulation in hamster facial motoneurons. Brain Res. Mol. Brain Res. 1996;35:131–138. doi: 10.1016/0169-328x(95)00197-z. [DOI] [PubMed] [Google Scholar]
- 31.Drengler SM, Handa RJ, Jones KJ. Effects of axotomy and testosterone on androgen receptor mRNA expression in hamster facial motoneurons. Exp. Neurol. 1997;14:374–379. doi: 10.1006/exnr.1997.6537. [DOI] [PubMed] [Google Scholar]
- 32.Dube JY, Lesage R, Tremblay RR. Androgen and estrogen binding in rat skeletal and perineal muscles. Can. J. Biochem. 1976;54:50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- 33.Dutcher SA, Underwood BD, Walker PD, Diaz FG, Michael DB. Patterns of heat-shock protein 70 biosynthesis following human traumatic brain injury. J. Neurotrauma. 1998;15:411–420. doi: 10.1089/neu.1998.15.411. [DOI] [PubMed] [Google Scholar]
- 34.Fargo KN, Alexander TD, Tanzer L, Poletti A, Jones KJ. Androgen regulates neuritin mRNA levels in an in vivo model of steroid-enhanced peripheral nerve regeneration. J. Neurotrauma. 2008;25:561–566. doi: 10.1089/neu.2007.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fargo KN, Iwema CL, Clark-Phelps MC, Sengelaub DR. Exogenous testosterone reverses age-related atrophy in a spinal neuromuscular system. Horm. Behav. 2007;51:20–30. doi: 10.1016/j.yhbeh.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Fargo KN, Sengelaub DR. Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J. Comp. Neurol. 2004a;469:96–106. doi: 10.1002/cne.10991. [DOI] [PubMed] [Google Scholar]
- 37.Fargo KN, Sengelaub DR. Exogenous testosterone prevents motoneuron atrophy induced by contralateral motoneuron depletion. J. Neurobiol. 2004b;60:348–359. doi: 10.1002/neu.20027. [DOI] [PubMed] [Google Scholar]
- 38.Fargo KN, Sengelaub DR. Testosterone treatment prevents deficits in motor activation caused by partial loss of motoneurons. Soc. Neurosci. Abstr. Viewer/Itinerary Planner, Program No. 672.8. 2005 [Google Scholar]
- 39.Fargo KN, Sengelaub R. Androgenic, but not estrogenic, protection of motoneurons from somal and dendritic atrophy induced by the death of neighboring motoneurons. Dev. Neurobiol. 2007;67:1094–1106. doi: 10.1002/dneu.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson AS, Sengelaub DR. Dendritic atrophy following partial motoneuron depletion: time course of recovery and protection with testosterone. Soc. Neurosci. Abstr. Viewer/Itinerary Planner, Program No. 56.24. 2007 [Google Scholar]
- 41.Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J. Neurobiol. 1999;40:446–457. [PubMed] [Google Scholar]
- 42.Fishman RB, Breedlove SM. Neonatal androgen maintains sexually dimorphic muscles in the absence of innervation. Muscle Nerve. 1988;11:553–560. doi: 10.1002/mus.880110606. [DOI] [PubMed] [Google Scholar]
- 43.Fishman RB, Breedlove SM. Local perineal implants of antiandrogen block masculinization of the SNB. Dev. Brain Res. 1992;70:283–286. doi: 10.1016/0165-3806(92)90208-e. [DOI] [PubMed] [Google Scholar]
- 44.Fishman RB, Chism L, Firestone GL, Breedlove SM. Evidence for androgen receptors in sexually dimorphic perineal muscles of neonatal male rats. Absence of androgen accumulation by the perineal motoneurons. J. Neurobiol. 1990;21:694–704. doi: 10.1002/neu.480210504. [DOI] [PubMed] [Google Scholar]
- 45.Forger NG, Breedlove SM. Seasonal variation in mammalian striated muscle mass and motoneuron morphology. J. Neurobiol. 1987;18:155–165. doi: 10.1002/neu.480180204. [DOI] [PubMed] [Google Scholar]
- 46.Forger NG, Fishman RB, Breedlove SM. Differential effects of testosterone metabolites upon the size of sexually dimorphic motoneurons in adulthood. Horm. Behav. 1992;26:204–213. doi: 10.1016/0018-506x(92)90042-t. [DOI] [PubMed] [Google Scholar]
- 47.Forger NG, Roberts LS, Wong V, Breedlove SM. Ciliary neurotrophic factor maintains motoneurons and their target muscles in developing rats. J. Neurosci. 1993;13:4720–4726. doi: 10.1523/JNEUROSCI.13-11-04720.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forger NG, Wagner CK, Contois M, Bengston L, MacLennan AJ. Ciliary neurotrophic factor receptor α in spinal motoneurons is regulated by gonadal hormones. J. Neurosci. 1998;18:8720–8729. doi: 10.1523/JNEUROSCI.18-21-08720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraley GS, Ulibarri CM. Long term castration affects motoneuron size but not number in the spinal nucleus of the bulbocavernosus in the adult male Mongolian gerbil. Brain Res. 2002;953:265–271. doi: 10.1016/s0006-8993(02)02949-9. [DOI] [PubMed] [Google Scholar]
- 50.Freeman LM, Watson NV, Breedlove SM. Androgen spares androgen-insensitive motoneurons from apoptosis in the spinal nucleus of the bulbocavernosus in rats. Horm. Behav. 1996;30:424–433. doi: 10.1006/hbeh.1996.0047. [DOI] [PubMed] [Google Scholar]
- 51.García-Ovejero D, Veiga S, García-Segura LM, DonCarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J. Comp. Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog. Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog. Neurobiol. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–2464. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein LA, Sengelaub DR. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. IV. Masculinization of the spinal nucleus of the bulbocavernosus with testosterone metabolites. J. Neurobiol. 1990;21:719–730. doi: 10.1002/neu.480210506. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein LA, Sengelaub DR. Timing and duration of dihydrotestosterone treatment affect the development of motoneuron number and morphology in a sexually dimorphic rat spinal nucleus. J. Comp. Neurol. 1992;326:147–157. doi: 10.1002/cne.903260113. [DOI] [PubMed] [Google Scholar]
- 57.Grafstein B, McQuarrie EI. Role of the nerve cell body in axonal regeneration. In: Cotman CW, editor. Neuronal Plasticity. New York: Raven Press; 1978. pp. 155–195. [Google Scholar]
- 58.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 59.Hart BL, Melese-D'Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol. Behav. 1983;31:807–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- 60.Hayes KJ. The so-called 'levator ani' of the rat. Acta Endocrinol. 1965;48:337–347. doi: 10.1530/acta.0.0480337. [DOI] [PubMed] [Google Scholar]
- 61.Hetzler LE, Sharma N, Tanzer L, Wurster RD, Leonetti J, Marzo SJ, Jones KJ, Foecking EM. Accelerating functional recovery after rat facial nerve injury: Effects of gonadal steroids and electrical stimulation. Otolaryngol. Head Neck Surg. 2008;139:62–67. doi: 10.1016/j.otohns.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Hoang TX, Nieto J, Tillakaratne NJK, Havton LA. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J. Comp. Neurol. 2003;467:477–486. doi: 10.1002/cne.10928. [DOI] [PubMed] [Google Scholar]
- 63.Holmes GM, Sachs BD. Erectile function and bulbospongiosus EMG activity in estrogen-maintained castrated rats vary with behavioral context. Horm. Behav. 1992;26:406–419. doi: 10.1016/0018-506x(92)90010-s. [DOI] [PubMed] [Google Scholar]
- 64.Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-β estradiol. J. Neurosci. 2005;25:4004–4013. doi: 10.1523/JNEUROSCI.5279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutchinson JB. Gender-specific steroid metabolism in neural differentiation. Cell. Molec. Neurobiol. 1997;17:603–626. doi: 10.1023/A:1022581902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacob DA, Bengston CL, Forger NG. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci. 2005;25:5638–5644. doi: 10.1523/JNEUROSCI.1200-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones KJ. Recovery from facial paralysis following crush injury of the facial nerve in hamsters: differential effects of gender and androgen exposure. Exp. Neurol. 1993;121:133–138. doi: 10.1006/exnr.1993.1079. [DOI] [PubMed] [Google Scholar]
- 68.Jones KJ, Brown TJ, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res. Brain Res. Rev. 2001;37:372–382. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- 69.Jones KJ, Coers S, Storer PD, Tanzer L, Kinderman NB. Androgenic regulation of the central glia response following nerve damage. J. Neurobiol. 1999a;40:560–573. doi: 10.1002/(sici)1097-4695(19990915)40:4<560::aid-neu11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 70.Jones KJ, Drengler SM, Oblinger MM. Gonadal steroid regulation of growth-associated protein GAP-43 mRNA expression in axotomized hamster facial motor neurons. Neurochem. Res. 1997a;22:1367–1374. doi: 10.1023/a:1022071123255. [DOI] [PubMed] [Google Scholar]
- 71.Jones KJ, Durica TE, Jacob SK. Gonadal steroid preservation of central synaptic input to hamster facial motoneurons following peripheral axotomy. J. Neurocytol. 1997b;26:257–266. doi: 10.1023/a:1018596316465. [DOI] [PubMed] [Google Scholar]
- 72.Jones KJ, Kinderman NB, Oblinger MM. Alterations in glial fibrillary acidic protein (GFAP) mRNA levels in the hamster facial motor nucleus: effects of axotomy and testosterone. Neurochem. Res. 1997c;22:1359–1366. doi: 10.1023/a:1022019106417. [DOI] [PubMed] [Google Scholar]
- 73.Jones KJ, Oblinger MM. Androgenic regulation of tubulin gene expression in axotomized hamster facial motoneurons. J. Neurosci. 1994;14:3620–3627. doi: 10.1523/JNEUROSCI.14-06-03620.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones KJ, Storer PD, Drengler SM, Oblinger MM. Differential regulation of cytoskeletal gene expression in hamster facial motoneurons: effects of axotomy and testosterone treatment. J. Neurosci. Res. 1999b;57:817–823. [PubMed] [Google Scholar]
- 75.Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982;249:309–314. doi: 10.1016/0006-8993(82)90065-8. [DOI] [PubMed] [Google Scholar]
- 76.Jordan CL, Breedlove SM, Arnold AP. Ontogeny of steroid accumulation in spinal lumbar motoneurons of the rat: implications for androgen's site of action during synapse elimination. J. Comp. Neurol. 1991;313:441–448. doi: 10.1002/cne.903130304. [DOI] [PubMed] [Google Scholar]
- 77.Jordan CL, Padgett B, Hershey J, Prins G, Arnold A. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J. Comp. Neurol. 1997;379:88–98. [PubMed] [Google Scholar]
- 78.Jordan CL, Price RH, Jr, Handa RJ. Androgen receptor messenger RNA and protein in adult rat sciatic nerve: implications for site of androgen action. J. Neurosci. Res. 2002;69:509–518. doi: 10.1002/jnr.10324. [DOI] [PubMed] [Google Scholar]
- 79.Kay JN, Hannigan P, Kelley DB. Trophic effects of androgen: Development and hormonal regulation of neuron number in a sexually dimorphic vocal motor nucleus. J. Neurobiol. 1999;40:375–385. [PubMed] [Google Scholar]
- 80.Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18:671–680. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- 81.Kinderman NB, Jones KJ. Testosterone enhancement of the nerve cell body response to injury: evidence using in situ hybridization and ribosomal DNA probes. J. Neurosci. 1993;13:1523–1532. doi: 10.1523/JNEUROSCI.13-04-01523.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kujawa KA, Emeric E, Jones KJ. Testosterone differentially regulates the regenerative properties of injured hamster facial motoneurons. J. Neurosci. 1991;11:3898–3906. doi: 10.1523/JNEUROSCI.11-12-03898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kujawa KA, Jacob JM, Jones KJ. Testosterone regulation of the regenerative properties of injured rat sciatic motor neurons. J. Neurosci. Res. 1993;35:268–273. doi: 10.1002/jnr.490350306. [DOI] [PubMed] [Google Scholar]
- 84.Kujawa KA, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis in male hamsters: temporal requirements of hormone exposure. Physiol. Behav. 1990;48:765–768. doi: 10.1016/0031-9384(90)90223-q. [DOI] [PubMed] [Google Scholar]
- 85.Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp. Neurol. 1989;105:80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- 86.Kujawa KA, Tanzer L, Jones KJ. Inhibition of the accelerative effects of testosterone on hamster facial nerve regeneration by the antiandrogen flutamide. Exp. Neurol. 1995;133:138–143. doi: 10.1006/exnr.1995.1016. [DOI] [PubMed] [Google Scholar]
- 87.Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J. Neurobiol. 1991;22:976–988. doi: 10.1002/neu.480220909. [DOI] [PubMed] [Google Scholar]
- 88.Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- 89.Larkowski TD, Drengler SM, Tanzer L, Jones KJ. Androgen receptor mRNA regulation in adult male and female hamster facial motoneurons: Effects of axotomy and exogenous androgens. J. Neurobiol. 2000;45:207–214. doi: 10.1002/1097-4695(200012)45:4<207::aid-neu2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 90.Leedy MG, Beattie MS, Bresnahan JC. Testosterone-induced plasticity of synaptic inputs to adult mammalian motoneurons. Brain Res. 1987;424:386–390. doi: 10.1016/0006-8993(87)91484-3. [DOI] [PubMed] [Google Scholar]
- 91.Little CM, Coons KD, Sengelaub DR. Neuroprotective effects of testosterone on the morphology and function of somatic motoneurons following the death of neighboring motoneurons. J. Comp. Neurol. 2009;512:359–372. doi: 10.1002/cne.21885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lubischer JL, Arnold AP. Axotomy transiently down-regulates androgen receptors in motoneurons of the spinal nucleus of the bulbocavernosus. Brain Res. 1995;694:61–68. doi: 10.1016/0006-8993(95)00766-j. [DOI] [PubMed] [Google Scholar]
- 93.Magnaghi V, Cavarretta I, Galbiati M, Martini L, Melcangi RC. Neuroactive steroids and peripheral myelin proteins. Brain Res. Brain Res. Rev. 2001;37:360–371. doi: 10.1016/s0165-0173(01)00140-0. [DOI] [PubMed] [Google Scholar]
- 94.Magnaghi V, Cavarretta I, Zucchi I, Susani L, Rupprecht R, Hermann B, Martini L, Melcangi RC. Po gene expression is modulated by androgens in the sciatic nerve of adult male rats. Brain Res. Mol. Brain Res. 1999;70:36–44. doi: 10.1016/s0169-328x(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 95.Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005;272:2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- 96.Marron TU, Guerini V, Rusmini P, Sau D, Brevini TA, Martini L, Poletti A. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J. Neurochem. 2005;92:10–20. doi: 10.1111/j.1471-4159.2004.02836.x. [DOI] [PubMed] [Google Scholar]
- 97.Matsumoto A, Arai Y, Hyodo S. Androgenic regulation of expression of β-tubulin messenger ribonucleic acid in motoneurons of the spinal nucleus of the bulbocavernosus. J. Neuroendocrinol. 1993;5:357–363. doi: 10.1111/j.1365-2826.1993.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 98.Matsumoto A, Arai Y, Prins GS. Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J. Neuroendocrinol. 1996;8:553–559. doi: 10.1046/j.1365-2826.1996.04899.x. [DOI] [PubMed] [Google Scholar]
- 99.Matsumoto A, Arai Y, Urano A, Hyodo S. Effect of androgen on the expression of gap junction and b-actin mRNAs in adult rat motoneurons. Neurosci. Res. 1992;14:133–144. doi: 10.1016/0168-0102(92)90089-u. [DOI] [PubMed] [Google Scholar]
- 100.Matsumoto A, Arai Y, Urano A, Hyodo S. Androgen regulates gene expression of cytoskeletal proteins in adult rat motoneurons. Horm. Behav. 1994;28:357–366. doi: 10.1006/hbeh.1994.1032. [DOI] [PubMed] [Google Scholar]
- 101.Matsumoto A, Arnold AP, Zampighi GA, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J. Neurosci. 1988a;8:4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsumoto A, Micevych PE, Arnold AP. Androgen regulates synaptic input to motoneurons of the adult rat spinal cord. J. Neurosci. 1988b;8:4168–4176. doi: 10.1523/JNEUROSCI.08-11-04168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J. Comp. Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 104.Melcangi RC, Ballabio M, Cavarretta I, Gonzalez LC, Leonelli E, Veiga S, Martini L, Magnaghi V. Effects of neuroactive steroids on myelin of peripheral nervous system. J. Steroid Biochem. Mol. Biol. 2003;85:323–327. doi: 10.1016/s0960-0760(03)00228-0. [DOI] [PubMed] [Google Scholar]
- 105.Melcangi RC, Cavarretta IT, Ballabio M, Leonelli E, Schenone A, Azcoitia I, Miguel Garcia-Segura L, Magnaghi V. Peripheral nerves: a target for the action of neuroactive steroids. Brain Res. Brain Res. Rev. 2005;48:328–338. doi: 10.1016/j.brainresrev.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 106.Micevych PE, Coquelin AW, Arnold AP. Immunohistochemical distribution of substance P, serotonin, and methionine enkephalin in sexually dimorphic nuclei in the rat spinal cord. J. Comp. Neurol. 1985;248:235–244. doi: 10.1002/cne.902480206. [DOI] [PubMed] [Google Scholar]
- 107.Monks DA, Getsios S, MacCalman CD, Watson NV. N-cadherin is regulated by gonadal steroids in adult sexually dimorphic spinal motoneurons. J. Neurobiol. 2001;47:255–264. doi: 10.1002/neu.1033. [DOI] [PubMed] [Google Scholar]
- 108.Monks DA, Kopachik W, Breedlove SM, Jordan CL. Anabolic responsiveness of skeletal muscles correlates with androgen receptor protein but not mRNA, Can. J. Physiol. Pharmacol. 2006;84:272–277. doi: 10.1139/y05-157. [DOI] [PubMed] [Google Scholar]
- 109.Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res. Brain Res. Rev. 2004;44:154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat. Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- 111.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 112.Moschilla G, Song S, Chakera T. Post-traumatic lumbar nerve root avulsion. Austral. Radiol. 2001;45:281–284. doi: 10.1046/j.1440-1673.2001.00921.x. [DOI] [PubMed] [Google Scholar]
- 113.Naess O, Haug E, Ahramadal A, Aakvvaag A, Hansson V, French F. Androgen receptors in the anterior pituitary and central nervous system of the androgen “insensitive” (Tfm) rat: Correlation between receptor binding and effects of androgens on gonadotropin secretion. Endocrinology. 1976;99:1295–1303. doi: 10.1210/endo-99-5-1295. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J. Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 115.Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148:2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- 116.Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- 117.O'Hanlon GM, Lowrie MB. Nerve injury in adult rats causes abnormalities in the motoneuron dendritic field that differ from those seen following neonatal nerve injury. Exp. Brain Res. 1995;103:243–250. doi: 10.1007/BF00231710. [DOI] [PubMed] [Google Scholar]
- 118.Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB expression in spinal motoneurons. J. Neurosci. Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- 119.Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;1458:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- 120.Perez J, Kelley DB. Trophic effects of androgen: Receptor expression and the survival of laryngeal motor neurons after axotomy. J. Neurosci. 1996;16:6625–6633. doi: 10.1523/JNEUROSCI.16-21-06625.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perez J, Kelley DB. Androgen mitigates axotomy-induced decreases in calbindin expression in motor neurons. J. Neurosci. 1997;17:7396–7403. doi: 10.1523/JNEUROSCI.17-19-07396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Phoenix C, Goy R, Gerall A, Young W. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 123.Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- 124.Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm. Behav. 2008;53:693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Popper P, Micevych PE. Steroid regulation of calcitonin gene-related peptide mRNA expression in motoneurons of the spinal nucleus of the bulbocavernosus. Mol. Brain Res. 1990;8:159–166. doi: 10.1016/0169-328x(90)90060-q. [DOI] [PubMed] [Google Scholar]
- 126.Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 127.Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J. Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roselli CF. Brain aromatase: roles in reproduction and neuroprotection. J. Steroid Biochem. Mol. Biol. 2007;106:143–150. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J. Reprod. Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- 130.Sarkey S, Azcoitia I, García-Segura LM, García-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Horm. Behav. 2008;53:753–764. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schrøder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J. Comp. Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- 132.Sengelaub DR. Neural development, in: Encyclopedia of Cognitive Science. Nature Publishing Group. 2002;Volume 3:253–261. [Google Scholar]
- 133.Sengelaub DR, Arnold AP. Development and loss of early projections in a sexually dimorphic rat spinal nucleus. J. Neurosci. 1986;6:1613–1620. doi: 10.1523/JNEUROSCI.06-06-01613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sengelaub DR, Arnold AP. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. I. Testosterone-regulated death in the dorsolateral nucleus (DLN) J. Comp. Neurol. 1989;280:622–629. doi: 10.1002/cne.902800411. [DOI] [PubMed] [Google Scholar]
- 135.Sengelaub DR, Jordan CL, Kurz EM, Arnold AP. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. II. Development of the spinal nucleus of the bulbocavernosus in androgen insensitive (Tfm) rats. J. Comp. Neurol. 1989a;280:630–636. doi: 10.1002/cne.902800412. [DOI] [PubMed] [Google Scholar]
- 136.Sengelaub DR, Nordeen EJ, Nordeen KW, Arnold AP. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. III. Differential effects of the androgen dihydrotestosterone. J. Comp. Neurol. 1989b;280:637–644. doi: 10.1002/cne.902800413. [DOI] [PubMed] [Google Scholar]
- 137.Sernagor E, Yarom Y, Werman R. Sodium-dependent regenerative responses in dendrites of axotomized motoneurons in the cat. Proc. Natl. Acad. Sci. USA. 1986;83:7966–7970. doi: 10.1073/pnas.83.20.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharma N, Tanzer L, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance rat facial nerve regeneration following injury. Experimental Biology meeting abstracts, Abstract #977.4. 2007 [Google Scholar]
- 139.Sharma N, Wurster RD, Marzo SJ, Foecking EM, Jones KJ. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Examining the effects of electrical stimulation and testosterone in a rat facial nerve injury model, Program No. 153.17. Online. [Google Scholar]
- 140.Storer PD, Houle JD, Oblinger M, Jones KJ. Combination of gonadal steroid treatment and peripheral nerve grafting results in a peripheral motoneuron-like pattern of BII-tubulin mRNA expression in axotomized hamster rubrospinal motoneurons. J. Comp. Neurol. 2002;449:364–373. doi: 10.1002/cne.10304. [DOI] [PubMed] [Google Scholar]
- 141.Sumner BEH, Watson WE. Retraction and expansion of the dendritic tree of motor neurons of adult rats induced in vivo. Nature. 1971;233:273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- 142.Sunderland S. Cranial nerve injury. Structural and pathophysiological considerations and a classification of nerve injury. In: Samii M, Jannetta PJ, editors. The Cranial Nerves. Berlin: Springer-Verlag; 1981. pp. 16–23. [Google Scholar]
- 143.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 144.Swallow RK, Leiser MR, Fink BE, Sauer C, Nicklous DM, Hoffman JR. Anatomical but not functional recovery from a sciatic nerve crush is enhanced by treatment with testosterone. Restor. Neurol. Neurosci. 1999;15:297–303. [PubMed] [Google Scholar]
- 145.Tanzer L, Jones KJ. Gonadal steroid regulation of hamster facial nerve regeneration: effects of dihydrotestosterone and estradiol. Exp. Neurol. 1997;146:258–264. doi: 10.1006/exnr.1997.6529. [DOI] [PubMed] [Google Scholar]
- 146.Tanzer L, Jones KJ. Neurotherapeutic action of testosterone on hamster facial nerve regeneration: temporal window of effects. Horm. Behav. 2004;45:339–344. doi: 10.1016/j.yhbeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 147.Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J. Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tetzlaff JE, Huppenbauer CB, Tanzer L, Alexander TD, Jones KJ. Motoneuron injury and repair: new perspectives on gonadal steroids as neurotherapeutics. J. Mol. Neurosci. 2006;28:53–64. doi: 10.1385/jmn:28:1:53. [DOI] [PubMed] [Google Scholar]
- 149.Tetzlaff J, Tanzer L, Jones KJ. Exogenous androgen treatment delays the stress response following hamster facial nerve injury. J. Neuroendocrinol. 2007;19:383–389. doi: 10.1111/j.1365-2826.2007.01538.x. [DOI] [PubMed] [Google Scholar]
- 150.Titmus MJ, Faber DS. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog. Neurobiol. 1990;35:1–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- 151.Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev. Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- 152.Tremblay RR, Dube JY, Y J, Ho-Kim MA, Lesage R. Determination of rat muscles androgen-receptor complexes with methyltrienolone. Steroids. 1977;29:185–195. doi: 10.1016/0039-128x(77)90038-1. [DOI] [PubMed] [Google Scholar]
- 153.Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat. Embryol. (Berl.) 1987;17:37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- 154.Vita G, Dattola R, Girlanda P, Oteri G, Lo Presti F, Messina C. Effects of steroid hormones on muscle reinnervation after nerve crush in rabbit. Exp. Neurol. 1983;80:279–287. doi: 10.1016/0014-4886(83)90282-0. [DOI] [PubMed] [Google Scholar]
- 155.Wise PM. Estrogens and neuroprotection. Trends Endocrinol. Metab. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- 156.Xu J, Gingras KM, Bengston L, Di Marco A, Forger NG. Blockade of endogenous neurotrophic factors prevents the androgenic rescue of rat spinal motoneurons. J. Neurosci. 2001;21:4366–4372. doi: 10.1523/JNEUROSCI.21-12-04366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang LY, Arnold AP. BDNF regulation of androgen receptor expression in axotomized SNB motoneurons of adult male rats. Brain Res. 2000a;852:127–139. doi: 10.1016/s0006-8993(99)02225-8. [DOI] [PubMed] [Google Scholar]
- 158.Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J. Neurobiol. 2000b;44:308–319. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 159.Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- 160.Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J. Biol. Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]
- 161.Yeagle SP, Mayer RF, Max SR. Contractile properties of rat fast-twitch skeletal muscle during reinnervation: effects of testosterone and castration. Exp. Neurol. 1983;82:344–357. doi: 10.1016/0014-4886(83)90407-7. [DOI] [PubMed] [Google Scholar]
- 162.Yu WH. Sex difference in the regeneration of the hypoglossal nerve in rats. Brain Res. 1982a;238:404–406. doi: 10.1016/0006-8993(82)90114-7. [DOI] [PubMed] [Google Scholar]
- 163.Yu WH. Effect of testosterone on the regeneration of the hypoglossal nerve in rats. Exp. Neurol. 1982b;77:129–141. doi: 10.1016/0014-4886(82)90149-2. [DOI] [PubMed] [Google Scholar]
- 164.Yu WH, McGinnis MY. Androgen receptor levels in cranial nerve nuclei and tongue muscles in rats. J. Neurosci. 1986;6:1302–1307. doi: 10.1523/JNEUROSCI.06-05-01302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu WH, McGinnis MY. Androgen receptors in cranial nerve motor nuclei of male and female rats. J. Neurobiol. 2001;46:1–10. doi: 10.1002/1097-4695(200101)46:1<1::aid-neu1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 166.Yu WH, Srinivasan R. Effect of testosterone and 5 alpha-dihydrotestosterone on regeneration of the hypoglossal nerve in rats. Exp. Neurol. 1981;71:431–435. doi: 10.1016/0014-4886(81)90101-1. [DOI] [PubMed] [Google Scholar]
- 167.Yu WH, Yu MC. Acceleration of the regeneration of the crushed hypoglossal nerve by testosterone. Exp. Neurol. 1983;80:349–360. doi: 10.1016/0014-4886(83)90288-1. [DOI] [PubMed] [Google Scholar]