Abstract
Objectives
To assess blood aldosterone-renin ratio (ARR) and its relationship to ambulatory blood pressure (ABP) and left ventricular mass (LVM) in children.
Study design
A cross-sectional clinical study was conducted in 102 children (71.6% African American and 62.7% male) aged 7-18 years (mean=13.6, median=14). ABP (24-hour monitoring) was expressed as blood pressure index (BPI = mean BP/95th percentile by sex and height). LVM was measured by echocardiography and expressed as an index (LVMI=grams/ht2.7). Regression analyses were used to estimate associations.
Results
African American children had significantly lower serum aldosterone concentration and plasma renin activity compared with European American children (aldosterone: 5.9 ng/dl vs. 11.4 ng/dl, P = <0.0001 and renin: 1.6 ng/ml/h vs. 2.8 ng/ml/h, P = 0.01 respectively). However, ARR was not significantly different by race. ARR was not associated with 24-hour ABP, but was significantly associated with LVMI (β = 0.4 g/m2.7, P = 0.02) after adjustment for the ratio of 24-hour urine Na to creatinine excretion, BMI-z score, and ABPI.
Conclusion
The study observed a significant association between ARR and LVMI but not ABP in children, which suggested early cardiac remodeling associated with a high ARR.
Keywords: renin, aldosterone, aldosterone-renin ratio, ambulatory blood pressure, left ventricular mass, pediatrics
The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of systemic blood pressure (BP) and sodium (Na) excretion. the aldosterone-renin ratio (ARR) has become recognized as a useful test to screen hypertensive individuals with primary hyperaldosteronism,1 predicts incident hypertension among adults,2 and has been proposed as a guide for targeting anti-hypertensive drug therapy. It appears to be more robust and relatively independent of time and position at sampling compared with the individual plasma levels of renin and aldosterone.1 The ARR was found to have significantly positive associations to measures of cardiovascular disease among hypertensive adults.3 There are limited data regarding ARR and its relationship to ambulatory blood pressure (ABP) and left ventricular mass (LVM) in children. The aims of this study were to examine, in children referred for primary hypertension, (1) serum aldosterone level, plasma renin activity, and ARR; and (2) the relationship of ARR with ABP and LVM.
METHODS
Children aged 7-18 years were recruited from primary care clinics and from referrals to the University of Tennessee Medical Group (UTMG) nephrology or cardiology clinics for evaluation of elevated BP. Individuals were excluded if they were on antihypertensive medications or oral contraceptives, or had a diagnosis of renal disease, cardiac diseases, Liddle’s syndrome, or secondary hypertension (HTN). A total of 124 subjects were enrolled. Eighteen subjects with insufficient readings to meet the criteria (≥ 30 total readings and ≥ 5 nocturnal readings in 24 hours) for adequate ABP monitoring (ABPM) and four individuals with ARR > 30 (potential primary hyperaldosteronism) were excluded. Thus, the final sample in this analysis included 102 participants (73 African Americans, AAs and 29 European Americans, EAs). Informed consent was obtained from the parent and assent obtained from the study subject, as appropriate. The research protocol was approved by the University of Tennessee Health Science Center Institutional Review Board and followed the guidelines for good clinical practice.
During the 2-day study period, the following procedures were performed: blood samples collection; 24-hour urine collection; ABPM; and echocardiographic measurement. Subject’s height and weight were measured using a balance beam scale and pediatric wall-mounted stadiometer. Ethnicity was categorized as that reported by the subjects or parents. The participants were not asked to alter their dietary sodium intake during the study period.
ABPM was performed using the AM5600 ambulatory BP monitor (Advanced Biosensor, Columbia, SC). The monitors were programmed to take the BP every 20 minutes for a 24-hour period using the auscultatory technique to detect Systolic BP (SBP) at Korotkoff phase I and diastolic BP (DBP) at Korotkoff phase V. After selection of the appropriate cuff size, the brachial artery of the non-dominant arm at the anticubital fossa was located by palpation. The microphone was taped to the subject’s arm over the strongest impulse followed by placement of the cuff and electrodes. During the AM5600 “office check period,” with the subject in a seated position, a minimum of 3 pairs of simultaneous readings were taken in the same arm via a 3-way stopcock, with a mercury sphygmomanometer and stethoscope, and the AM5600, using the recorder’s BP cuff. The calibration readings allowed for adjustments to be made in the recorder’s microphone amplification and to establish a baseline for ABPM versus manual SBP and DBP. The office check readings were not included in the 24-hour results; casual BP readings were derived from the mean of 3 readings by mercury sphygmomanometer. The parent/guardian was asked to record the subject’s bedtime and time of awakening; using the reported times, the asleep period was designated by the next reading after the time of sleep and the last reading prior to the time recorded for awakening. After 24 hours, the cuff and monitor were removed, and the data downloaded using the manufacturer’s software.
The mean SBP and DBP were calculated separately for the 24-hour period and for awake (designated as daytime) and asleep (designated as nighttime) periods. The blood pressure index (BPI) for each subject was calculated by expressing the mean SBP or DBP as a ratio to the appropriate 95th percentile as reported by Soergel et al.4 By definition, when the BPI is greater than 1.0, the mean BP is above the 95th percentile. According to ABPM standards, 70% of the participants had a 24 hour BP load greater than or equal to 25% which is abnormal. Forty percent had a BP index > 1, and 25% had white coat HTN. These details were published elsewhere.5
The Pearson correlation coefficients between the AM5600 monitor and the mercury sphygmomanometer were 0.992 and 0.979 for systolic and diastolic BP, respectively, with coefficients of variation of 1.5 and 3.5%. The AM5600 monitor has been validated for use in children and adolescents.6 ABPM waveform analysis to detect artifactual readings was performed by a blinded co-investigator. Artifactual readings were discarded based upon methods previously described.7 The accuracy of each reading was determined by inspection of the graphic display of the Korotkoff signal.
Echocardiography was performed according to the standard of the American Society of Echocardiography (ASE).8 Subjects were positioned in the partial left decubitus position. Measurements performed in M-mode were the dimensions of the interventricular septal thickness (IVS), posterior wall thickness (PWT), and left ventricular internal dimension (LVID), which were taken at or just below the mitral valve tips. Left ventricular mass (LVM) was calculated using the formula published by Devereux et al9 according to the ASE guidelines: LV mass (g) = 0.81[1.04(IVS + PWT + LVID)3 - (LVID)3] + 0.06. Echocardiography was performed by the same technician, and all measurements were performed in triplicate by the same cardiologist, who was blinded to the subject’s BP. LVM index (LVMI) was derived by dividing LVM in grams by the subject’s height in meters raised to the 2.7 power.10-12
Blood samples for the measurement of plasma renin activity (PRA) and serum aldosterone concentration were drawn in an upright-seated position after subjects were ambulatory and before placing the ABPM. The time at blood drawn for each subject was recorded. Blood samples were centrifuged, and the serum/plasma fraction was frozen and sent to American Esoteric Laboratories (AEL) in Memphis for analysis. PRA (ng/ml/hr) and serum aldosterone concentration (ng/dL) were measured by radioimmunassays. ARR was calculated as serum aldosterone concentration divided by PRA. For those whose PRA < 0.2 (n=3), PRA values were arbitrarily set to 0.2.13 A 24-hour urine sample was collected to measure urinary sodium and creatinine excretion. To correct for any missed samples in the timed urine collection, 24-hour urinary Na excretion (mmol/24-h) was standardized to urinary creatinine excretion (g/24-h), i.e., Na/Creatine ratio.14
SAS 9.1. (SAS, Inc., Cary, NC) statistical software was used for all analyses. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). BMI z scores, which reflect the standard deviation score for the age- and sex-appropriate BMI distribution, were calculated using the same methods as used in the 2000 CDC Growth Charts for the United States.15 Descriptive analyses were used to assess the mean values and standard errors of the means. The t-statistics test was applied to test significant differences in the distribution of continuous variables by sex or ethnic group. Pearson correlation coefficients were calculated for all continuous measurements. Scatter plot was applied to estimate the relationship between ARR and LVMI without adjustment for covariates. Effects of ARR on ABP and LVMI controlling for other covariates were assessed by linear regression. Collinearity was assessed to remove variables that were highly correlated with independent variables in regression models.16 Tiu et al reported that ARR was affected by the time at blood collection.1 The time at which the blood was drawn in this study ranged from 7:00 am to 4:15 pm. To control for time, as a possible confounder in the relationship between ARR and BP or LVMI, time was classified into morning (am) and afternoon (pm) with pm as the reference for comparisons.
RESULTS
There was a predominance of African-American children (73 AA/29 EA) as well as males (64 M/38 F) (Table I). Participants’ age ranged from 7 to 18 years with a mean ± standard deviation of 13.6 ± 2.5 years, and a median of 14 years. The age distribution was not significantly different by race. The distributions of other selected variables of interest were also similar between AAs and EAs, except for serum aldosterone concentration and PRA. Significantly lower PRA and serum aldosterone concentration were observed in AAs compared with their EA counterparts (PRA 1.61 vs. 2.84 ng/ml/hr, P = 0.01, and aldosterone 5.87 vs. 11.40 ng/dL, P < 0.0001). However, the ARR was not significantly different between AAs (6.31) and EAs (5.19, P = 0.41). Compared with EAs, AAs were likely to have lower daytime-average DBP (74.4 vs. 77.3 mmHg, P = 0.07) and daytime DBPI (0.88 vs. 0.92, P = 0.06). A sex difference in ABP was shown by significantly higher SBP in males compared with females (126.3 vs. 118.5 mm Hg, P = 0.0002 for mean 24-hour SBP, 132.1 vs. 123.5 mm Hg, P < 0.0001 for mean daytime SBP, 118.3 vs. 112.7 mm Hg, P = 0.02 for mean nighttime SBP respectively). These differences may be explained by a higher average age in males compared with females (13.97 vs. 13.05, P = 0.09) and the higher BMI, BMI percentile and BMI-z score in males than in females (Table I), although the differences between males and females were not statistically significant.
Table 1.
Participants’ characteristics
| AA (n=73) |
EA (n=29) |
Male (n=64) |
Female (n=38) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | se | Mean | se | p | Mean | se | Mean | se | p | |
| Age | 13.58 | 0.31 | 13.76 | 0.47 | 0.75 | 13.97 | 0.31 | 13.05 | 0.44 | 0.09 |
| BMI | 27.07 | 0.82 | 27.92 | 1.26 | 0.58 | 27.54 | 0.82 | 26.94 | 1.22 | 0.68 |
| BMI-PCT | 85.08 | 2.62 | 85.60 | 4.57 | 0.92 | 86.65 | 2.64 | 82.82 | 4.20 | 0.42 |
| BMI-Z | 1.47 | 0.12 | 1.58 | 0.20 | 0.63 | 1.58 | 0.12 | 1.38 | 0.18 | 0.34 |
| Aldo | 5.87 | 0.63 | 11.40 | 1.61 | <.0001 | 7.98 | 0.94 | 6.77 | 0.99 | 0.42 |
| PRA | 1.61 | 0.18 | 2.84 | 0.42 | 0.0102 | 2.10 | 0.27 | 1.79 | 0.19 | 0.50 |
| ARR | 6.31 | 0.82 | 5.19 | 0.83 | 0.41 | 6.55 | 0.84 | 4.94 | 0.87 | 0.22 |
| Na/CRT | 110.08 | 5.87 | 99.94 | 8.86 | 0.36 | 102.71 | 6.03 | 115.32 | 8.33 | 0.22 |
| SBP_24M | 122.80 | 1.17 | 125.01 | 2.23 | 0.34 | 126.34 | 1.11 | 118.50 | 1.89 | 0.0002 |
| DBP_24M | 70.57 | 0.79 | 72.37 | 1.34 | 0.23 | 72.00 | 0.77 | 69.55 | 1.27 | 0.08 |
| SBP_DM | 127.94 | 1.20 | 131.26 | 2.31 | 0.17 | 132.09 | 1.13 | 123.48 | 1.94 | <.0001 |
| DBP_DM | 74.39 | 0.87 | 77.34 | 1.38 | 0.07 | 76.05 | 0.89 | 73.87 | 1.30 | 0.16 |
| SBP_NM | 116.14 | 1.31 | 116.43 | 2.47 | 0.91 | 118.32 | 1.31 | 112.65 | 2.12 | 0.02 |
| DBP_NM | 65.74 | 0.95 | 65.28 | 1.48 | 0.79 | 66.43 | 0.93 | 64.20 | 1.45 | 0.18 |
| SBPI_24 | 0.98 | 0.01 | 0.99 | 0.02 | 0.38 | 0.99 | 0.01 | 0.97 | 0.02 | 0.14 |
| DBPI_24 | 0.92 | 0.01 | 0.94 | 0.02 | 0.20 | 0.93 | 0.01 | 0.92 | 0.02 | 0.40 |
| SBPI_D | 0.97 | 0.01 | 0.99 | 0.02 | 0.17 | 0.99 | 0.01 | 0.95 | 0.01 | 0.052 |
| DBPI_D | 0.88 | 0.01 | 0.92 | 0.02 | 0.06 | 0.89 | 0.01 | 0.88 | 0.02 | 0.46 |
| SBPI_N | 1.00 | 0.01 | 1.00 | 0.02 | 0.89 | 1.00 | 0.01 | 1.01 | 0.02 | 0.82 |
| DBPI_N | 0.99 | 0.01 | 0.98 | 0.02 | 0.77 | 0.99 | 0.01 | 0.97 | 0.02 | 0.47 |
| LVMI | 35.59 | 1.32 | 34.44 | 1.62 | 0.63 | 36.24 | 1.46 | 33.56 | 1.31 | 0.22 |
BMI: body mass index (kg/m2), BMI-PCT: BMI-Percentile, BMI-Z: BMI z score, Aldo: serum aldosterone concentration (ng/dL), PRA: plasma renin activity (ng/ml/hr), ARR: aldosterone to renin ratio, Na/CRT: 24-hour urine sodium to creatinine ratio, SBPI and DBPI: systolic and diastolic blood pressure index, BP_24M: mean of 24-hour blood pressure, BP_DM: mean of daytime blood pressure, BP_NM: mean of nighttime blood pressure, BPI_24: 24-hour blood pressure index, BPI_D: daytime blood pressure index, BPI_N: nighttime blood pressure index, LVMI: left ventricular mass index.
Blood samples were collected during 7am and 4:15pm: 10.1% of participants had blood drawn between 7:00 am and 9:59am; 24.7% between 10:00 am and 11:59am; 22.5% between 12:00 pm and 1:59pm; and 42.7% between 2:00 and 4:15 pm. The mean values of ARR at the 4 periods were 2.0, 1.3, 1.2 and 1.4. The mean values of ARR were not different by time at blood sample collections.
There was a significantly positive correlation between ARR and age (r = 0.26, P = 0.02) and an inverse correlation between urinary Na/creatine ratio and age (r = -0.33, P = 0.0007). Urinary Na/creatine ratio was significantly correlated with serum aldosterone (r = -0.21, P = 0.04) but with neither PRA (r = -0.04, P = 0.73) nor ARR (r = -0.14, P = 0.22). As previously reported, ABP was significantly correlated with LVMI.5
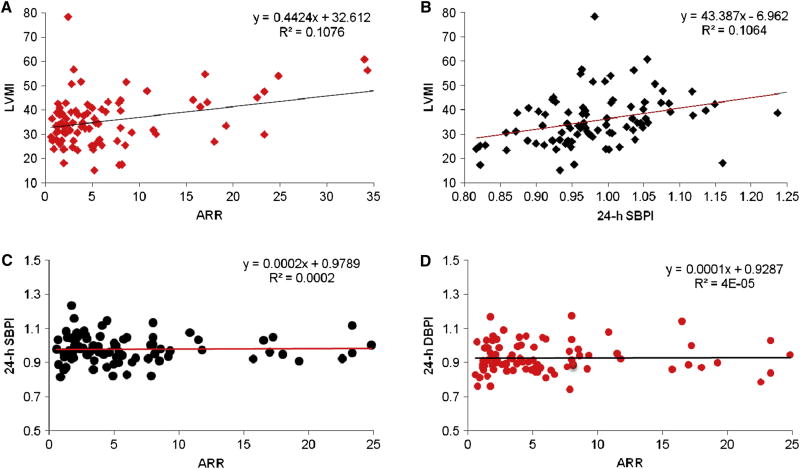
ARR was significantly associated with LVMI, but not ABP. The unadjusted relationship among ARR, ABPI and LVMI is shown in the Figure. Increased ARR was significantly associated with increased LVMI (Figure 1, A; β = 0.4, P = 0.04) but not ABPI (Figure 1, C and D). ABP was significantly associated with LVMI (Figure 1, B; P = 0.02 for 24-hour SBPI associated with LVMI). In multivariable regression analyses, ARR was also not associated with any ABP measurements. However, ARR was still significantly associated with LVMI (β = 0.367 g/m2.7, P = 0.047) after adjustment for time at blood collection, ratio of 24 hour-urine Na/Creatinine excretion, age, sex, ethnicity, and BMI-z score.
Figure 1.
Relationship among aldosterone/renin ratio (AAR), ambulatory blood pressure, and left ventricular mass index (LVMI). A: AAR and LVMI, β = 0.4, P = 0.04; B: 24-hour ambulatory systolic blood pressure index (24-h SBPI) and LVMI, β = 43.3, P = 0.02; C: ARR and 24-h SBPI, β = 0.0002, P = 0.91; and D: ARR and 24-h DBPI, β = 0.0001, P = 0.97
Table II presents the associations between ARR and LVMI independent from other covariates, including BP. Adding 24-hour SBP index (SBPI) and DBP index (DBPI), possible intermediate factors between ARR and left ventricular mass, into the multivariable model attenuated the ARR and LVMI association (before β = 0.41, P = 0.048, and after β = 0.38, P = 0.056). The regression coefficient of ARR associated with LVMI in the best fitting model was 0.40 with P = 0.022.
Table 2.
ARR associated with LVMI adjusted for other factors
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
Best Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| ARR | 0.41 | 0.048 | 0.38 | 0.056 | 0.37 | 0.047 | 0.35 | 0.054 | 0.36 | 0.046 | 0.36 | 0.052 | 0.40 | 0.022 |
| AM | -0.42 | 0.87 | -0.53 | 0.83 | 0.39 | 0.86 | 0.29 | 0.89 | 0.19 | 0.93 | 0.22 | 0.92 | ||
| Na/CRT (mmol/g) | 0.03 | 0.35 | 0.03 | 0.29 | 0.05 | 0.06 | 0.05 | 0.07 | 0.05 | 0.06 | 0.05 | 0.07 | 0.04 | 0.067 |
| Age (year) | -0.06 | 0.91 | 0.15 | 0.77 | 0.21 | 0.65 | 0.33 | 0.49 | 0.23 | 0.62 | 0.29 | 0.55 | ||
| Male | -1.92 | 0.45 | -1.37 | 0.58 | -1.36 | 0.55 | -1.05 | 0.65 | -1.30 | 0.57 | -1.16 | 0.62 | ||
| AA | -0.23 | 0.93 | 0.47 | 0.86 | 0.24 | 0.92 | 0.54 | 0.82 | 0.51 | 0.83 | 0.56 | 0.81 | ||
| BMI-Z (0.1) | 0.46 | <.0001 | 0.41 | 0.0004 | 0.42 | 0.0003 | 0.41 | 0.0004 | 0.41 | 0.0002 | ||||
| SBPI_24 (0.1) | 2.33 | 0.33 | 1.99 | 0.16 | 1.07 | 0.63 | 1.66 | 0.148 | ||||||
| DBPI_24 (0.1) | 1.48 | 0.46 | 1.67 | 0.16 | 0.99 | 0.59 | ||||||||
ARR: aldosterone to renin ratio, AM: blood drawn before 12 pm, Na/CRT: 24-hour urine sodium to creatinine ratio, AA: African American, BMI-Z: body mass index Z score, SBPI_24 and DBPI_24: 24-hour systolic and diastolic blood pressure index.
PRA was associated only with LVMI (β = 0.04, P = 0.04) after adjustment for BMI-z score and 24-hour urinary Na/creatinine excretion, but not associated with ABP. Aldosterone was neither associated with ABP nor with LVMI. However, association between 24-hour urinary Na/creatinine excretion and LVMI was significant (β = 0.04, P = 0.03) before adjustment for ARR but not significant at type I error less than 0.05 (Table II) after adjustment for ARR and BMI z-score.
DISCUSSION
This study observed a significant association between ARR and LVMI in children at risk for HTN. The association persisted after adjustment for significant covariates. Ethnic differences in the activity of specific components of the RAAS have been reported. Among healthy children participating in the Bogalusa Heart Study17 and in a study conducted among 20 schools in Indianapolis, Indiana,18 PRA was observed to be lower in AAs compared with EAs. The significantly lower renin in AAs in our study was consistent with that reported in previous studies of children and adults.17-20 The lower PRA in normotensive and hypertensive AA children and adults when compared with EAs may be due to expansion of the extracellular fluid volume, increased Na delivery to the macula densa, increased adrenal sensitivity to angiotensin II, higher dietary salt intake, lower dietary potassium intake, and/or increased activity of the distal tubular Na channel in AAs.18, 21-23
Studies of serum aldosterone concentration in African and European descendants have been inconclusive. Some studies have reported higher aldosterone concentrations in people of African origin,20 whereas other studies have observed higher aldosterone concentrations in people of European orgin,18, 24-27 or similar concentrations in both.28 In our study, serum aldosterone was significantly lower in AA children compared with their EA counterparts.
The relationship between PRA and BP has been examined in normotensive and hypertensive children, but the results have been inconsistent.17, 29-31 Of 159 normal children in the study conducted by Harshfield et al, subjects who had higher renin-sodium profiles (the relationship between PRA and 24-hour urinary Na excretion) had a blunted nocturnal decline in SBP and higher nocturnal DBP (assessed by ABPM).30 A recent study observed that PRA was significantly positively correlated with DBP (including DBP load by ABPM) in 70 untreated children (2.9% AA) with primary HTN.29 Our study did not find any significant PRA-BP association (data not shown) but a significant positive association between PRA and LVMI.
Aldosterone is an important determinant of BP control and has a major impact on cardiovascular health; its association with HTN is directly related to its known effects on renal salt excretion through its action on the distal tubular Na channel. In addition to its renal effects, plasma aldosterone has been associated with endothelial dysfunction,32 reduced vascular compliance,3, 33 increased LVM and BP-independent changes in myocardial structure and function.23 In a cohort study of the relationship between baseline aldosterone level and incident hypertension during follow-up, Vasan et al observed an increased risk for future development of HTN or worsening of HTN stage in adults whose plasma aldosterone levels were in the highest quartile, yet still within the normal range.34 Aldosterone appears to exert a BP- and epithelial sodium channel-independent effect on the left ventricle; aldosterone concentration has been associated with increased myocardial fibrosis and diastolic dysfunction.23 El-Gharbawy et al26 observed that aldosterone concentration was significantly positively correlated with nighttime SBP and DBP, but inversely correlated with nocturnal decline of both SBP and DBP in AA, but not in French Canadian, adults with essential HTN. Of note, plasma aldosterone also correlated with cardiac dimensions in AAs but not in French Canadians.26 Our study did not observe any association between serum aldosterone concentration and either ABP or LVMI.
Mahmud et al found that ARR was positively associated with increased aortic pulse pressure and arterial stiffness among untreated adults with essential HTN.3 A recent report of 1,773 normotensive adult participants from the Framingham Heart Study indicated that ARR was associated with the incidence of HTN.2 In our study of children, ARR was not significantly associated with ABP but was with LVMI without or with adjustment for ABP, although ABP was also significantly associated with LVMI without adjustment for ARR. Increased ARR has been found to correlate with left ventricular hypertrophy in adults.35 However, very little is known about ARR associated with cardiovascular target organ change in children. Our results suggested that ARR may be a marker to predict early cardiac remodeling in children.
Our study is not able to elucidate the mechanism by which increased ARR might promote increased left ventricular mass. However, other studies found that individuals with higher ARR may have increased adrenal sensitivity to angiotensin II.26 In addition, aldosterone not only enhances the pro-inflammatory actions of angiotensin II but is also known to promote vascular and cardiac remodeling independent of angiotensin II via activation of the mineralocorticoid receptor (MR).36 Our finding that the ARR is associated with increasing LVMI may be related to direct effects of the relative increase in circulating aldosterone on the vasculature and myocardium, or to a dual effect of both Ang II and aldosterone because there is synergism between the two agents.37
There are some limitations to this study. The majority of participants were AAs (72.4%) because of the population distribution in the catchment (Memphis metropolitan) area and the high prevalence of HTN in AAs; Tanner stage was not consistently documented in our participants, and therefore, could not be included in the analysis. However, we analyzed the association between ARR and LVMI adjusted for age and found that age was not a significant confounding factor in our study (Table II). Nutrient data, especially salt intake, were not collected in this study, but the study collected 24-hour urinary samples for sodium excretion estimate, which may indirectly reflect participants’ salt intake.38 The study did not measure 24-hour urinary aldosterone concentration, an important index of aldosterone status. The renin activity and aldosterone concentration are sensitive to the time at which blood is drawn.1 Even if the association was estimated with adjustment for the time at blood collection in this study, a one-time measure of these biomarkers may not capture their 24-hour variations. In addition, this cross-sectional study was unable to detect the longitudinal effects of ARR on ABP and LVM. Finally, there may have been inadequate 24-hour urine collections in a few participants. However, we applied the commonly used approach, 24-hour urinary Na to creatinine ratio, to justify the potential confounding due to the measurement error14.
Increased ARR was significantly associated with increased LVM after accounting for other risk. In addition, this relationship between ARR and LVM is independent from 24-hour ABP, which suggests ARR may predicate early organ damage in children.
Acknowledgments
The authors wish to acknowledge all participants in this study.
This study was supported by a grant from the Children’s Foundation Research Center at Le Bonheur Children’s Medical Center, Memphis, TN, and the University of Tennessee Health Science Center, MO1 USPHS Grant RR-00211 and NIH NHLBI #5K23HL83910-2.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rongling Li, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, TN.
Phyllis A. Richey, Departments of Preventive Medicine and Pediatrics, University of Tennessee Health Science Center, Memphis, TN.
Thomas G. DiSessa, Department of Pediatrics, University of Kentucky Medical School, Lexington, KY.
Bruce S. Alpert, Department of Pediatrics, University of Tennessee Health Science Center, General Clinical Research Center, Children’s Foundation Research Center at Le Bonheur Children’s Medical Center, Memphis, TN.
Deborah P. Jones, Department of Pediatrics, University of Tennessee Health Science Center, General Clinical Research Center, Children’s Foundation Research Center at Le Bonheur Children’s Medical Center, Memphis, TN.
References
- 1.Tiu SC, Choi CH, Shek CC, Ng YW, Chan FK, Ng CM, et al. The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005;90:72–8. doi: 10.1210/jc.2004-1149. [DOI] [PubMed] [Google Scholar]
- 2.Newton-Cheh C, Guo CY, Gona P, Larson MG, Benjamin EJ, Wang TJ, et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49:846–56. doi: 10.1161/01.HYP.0000258554.87444.91. [DOI] [PubMed] [Google Scholar]
- 3.Mahmud A, Feely J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18:50–5. doi: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, et al. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–84. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 5.Richey PA, Disessa TG, Hastings MC, Somes GW, Alpert BS, Jones DP. Ambulatory blood pressure and increased left ventricular mass in children at risk for hypertension. J Pediatr. 2008;152:343–8. doi: 10.1016/j.jpeds.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones DP, Richey PA, Alpert BS. Validation of the AM5600 ambulatory blood pressure monitor in children and adolescents. Blood Press Monit. 2008;13:349–51. doi: 10.1097/MBP.0b013e3283102cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richey PA, Jones CL, Harshfield GA, Somes GW, Johnson KC, Bailey JE, et al. The AM5600 ambulatory blood pressure recording system. Blood Press Monit. 1997;2:193–5. [PubMed] [Google Scholar]
- 8.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–30. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 10.Daniels SR. Hypertension-induced cardiac damage in children and adolescents. Blood Press Monit. 1999;4:165–70. [PubMed] [Google Scholar]
- 11.Daniels SR, Meyer RA, Liang YC, Bove KE. Echocardiographically determined left ventricular mass index in normal children, adolescents and young adults. J Am Coll Cardiol. 1988;12:703–8. doi: 10.1016/s0735-1097(88)80060-3. [DOI] [PubMed] [Google Scholar]
- 12.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 13.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 14.Flack JM, Grimm RH, Jr, Staffileno BA, Dnsc, Elmer P, Yunis C, et al. New salt-sensitivity metrics: variability-adjusted blood pressure change and the urinary sodium-to-creatinine ratio. Ethn Dis. 2002;12:10–9. [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 16.Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. Second. Boston: PWS-KENT Publishing Company; 1988. [Google Scholar]
- 17.Chen W, Srinivasan SR, Berenson GS. Plasma renin activity and insulin resistance in African American and white children: the Bogalusa Heart Study. Am J Hypertens. 2001;14:212–7. doi: 10.1016/s0895-7061(00)01274-7. [DOI] [PubMed] [Google Scholar]
- 18.Bloem LJ, Manatunga AK, Pratt JH. Racial difference in the relationship of an angiotensin I-converting enzyme gene polymorphism to serum angiotensin I-converting enzyme activity. Hypertension. 1996;27:62–6. doi: 10.1161/01.hyp.27.1.62. [DOI] [PubMed] [Google Scholar]
- 19.He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. The renin-angiotensin system and blood pressure: differences between blacks and whites. Am J Hypertens. 1999;12:555–62. doi: 10.1016/s0895-7061(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AD, Millasseau SC, Dawes M, Kyd PA, Chambers JB, Ritter JM, et al. Aldosterone and left ventricular hypertrophy in Afro-Caribbean subjects with low renin hypertension. Am J Hypertens. 2006;19:19–24. doi: 10.1016/j.amjhyper.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 21.Aviv A, Hollenberg NK, Weder AB. Sodium glomerulopathy: tubuloglomerular feedback and renal injury in African Americans. Kidney Int. 2004;65:361–8. doi: 10.1111/j.1523-1755.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 22.Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q, et al. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–7. doi: 10.1161/01.HYP.0000179582.42830.1d. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt BM, Schmieder RE. Aldosterone-induced cardiac damage: focus on blood pressure independent effects. Am J Hypertens. 2003;16:80–6. doi: 10.1016/s0895-7061(02)03199-0. [DOI] [PubMed] [Google Scholar]
- 24.Barbato A, Russo P, Siani A, Folkerd EJ, Miller MA, Venezia A, et al. Aldosterone synthase gene (CYP11B2) C-344T polymorphism, plasma aldosterone, renin activity and blood pressure in a multi-ethnic population. J Hypertens. 2004;22:1895–901. doi: 10.1097/00004872-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, Sagnella GA, Dong Y, Miller MA, Onipinla A, Markandu ND, et al. Molecular variants of the sodium/hydrogen exchanger type 3 gene and essential hypertension. J Hypertens. 2004;22:1269–75. doi: 10.1097/01.hjh.0000125428.28861.11. [DOI] [PubMed] [Google Scholar]
- 26.El-Gharbawy AH, Nadig VS, Kotchen JM, Grim CE, Sagar KB, Kaldunski M, et al. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension. 2001;37:845–50. doi: 10.1161/01.hyp.37.3.845. [DOI] [PubMed] [Google Scholar]
- 27.Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. N Engl J Med. 1989;321:1152–7. doi: 10.1056/NEJM198910263211703. [DOI] [PubMed] [Google Scholar]
- 28.Grim CE, Cowley AW, Jr, Hamet P, Gaudet D, Kaldunski ML, Kotchen JM, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005;45:766–72. doi: 10.1161/01.HYP.0000154364.00763.d5. [DOI] [PubMed] [Google Scholar]
- 29.Flynn JT, Alderman MH. Characteristics of children with primary hypertension seen at a referral center. Pediatr Nephrol. 2005;20:961–6. doi: 10.1007/s00467-005-1855-3. [DOI] [PubMed] [Google Scholar]
- 30.Harshfield GA, Pulliam DA, Alpert BS, Stapleton FB, Willey ES, Somes GW. Ambulatory blood pressure patterns in children and adolescents: influence of renin-sodium profiles. Pediatrics. 1991;87:94–100. [PubMed] [Google Scholar]
- 31.Weidmann P, Beretta-Piccoli C, Ziegler WH, Keusch G, Gluck Z, Reubi FC. Age versus urinary sodium for judging renin, aldosterone, and catecholamine levels: studies in normal subjects and patients with essential hypertension. Kidney Int. 1978;14:619–28. doi: 10.1038/ki.1978.171. [DOI] [PubMed] [Google Scholar]
- 32.Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann N Y Acad Sci. 2002;970:89–100. doi: 10.1111/j.1749-6632.2002.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 33.Blacher J, Amah G, Girerd X, Kheder A, Ben Mais H, London GM, et al. Association between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertension. Am J Hypertens. 1997;10:1326–34. doi: 10.1016/s0895-7061(97)00301-4. [DOI] [PubMed] [Google Scholar]
- 34.Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 35.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–62. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–7. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 37.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–74. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Engeli S, Boschmann M, Frings P, Beck L, Janke J, Titze J, et al. Influence of salt intake on renin-angiotensin and natriuretic peptide system genes in human adipose tissue. Hypertension. 2006;48:1103–8. doi: 10.1161/01.HYP.0000248837.88749.18. [DOI] [PubMed] [Google Scholar]