Abstract
Objective
We investigated the effect of short viremic episodes on soluble markers associated with endothelial stress and cardiovascular disease risk in chronically HIV-1-infected patients followed during continuous antiretroviral therapy, antiretroviral therapy interruption and antiretroviral therapy resumption.
Design and methods
We assessed changes in plasma levels of von Willebrand factor, soluble vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 by enzyme-linked immunosorbent assay, as well as T-cell activation (CD8+/CD38+, CD8+/HLA-DR+ and CD3+/CD95+) by flow cytometry, in 36 chronically HIV-1-infected patients participating in a randomized study. Patients were divided into the following three groups: a, on continuous antiretroviral therapy; b, on a 6-week antiretroviral therapy interruption; or c, on antiretroviral therapy interruption extended to the achievement of viral set point.
Results
Although all measurements remained stable over a 40-week follow-up on antiretroviral therapy, plasma levels of soluble vascular cell adhesion molecule-1 (P < 0.0001) and soluble intercellular adhesion molecule-1 (P = 0.003) increased during treatment interruption in correlation with viral rebound and T-cell activation. No significant changes in von Willebrand factor were observed in any of the groups. After resuming antiretroviral therapy, soluble vascular cell adhesion molecule-1 levels remained elevated even after achievement of viral suppression to less than 50 copies/ml.
Conclusion
The prompt rise in plasma soluble vascular cell adhesion molecule-1 and soluble intercellular adhesion molecule-1 upon viral rebound suggests an acute increase in endothelial stress upon treatment interruption, which may persists after viral resuppression of virus. Thus, viral replication during short-term treatment interruption may increase the overall cardiovascular risk during and beyond treatment interruption.
Keywords: cardiovascular risk, endothelial stress, HIV-1, treatment interruption
Introduction
Patient-initiated treatment interruptions are common as a consequence of antiretroviral therapy (ART)-related adverse events. In addition, supervised treatment interruption (STI) strategies are commonly used in clinical care to manage specific clinical situations (e.g. toxicity, immune reconstitution syndrome, etc.) [1–3]. Although in the past STIs have been proposed as a means to minimize ART-related adverse events, results from a recent large randomized study of CD4-monitored open-ended treatment interruptions [Strategies for Management of Antiretroviral Therapy (SMART)] [4] indicated that the risk for disease progression was almost double in individuals interrupting therapy as compared with individuals on continuous therapy. Unexpectedly, a small but significantly higher rate of cardiovascular events was observed in the treatment interruption arm, raising the hypothesis that the resurging viremia itself may increase the risk of cardiovascular adverse events. Although the effects of long-term ART and chronic viral replication on cardiovascular risk have been described [5–8], it remains unclear whether short-term viremic episodes in immune-reconstituted individuals carry a similar risk.
A role for chronic viral infection in the etiology of endothelial stress, atherosclerosis, thrombosis, and thickening of the arterial tunica intima has been suggested [5,8,9] and could be mediated by a number of mechanisms such as either viral replication in endothelial cells or increased expression of inflammatory cytokines or both [10–14], which result in the activation of both immune effectors and endothelial cells. Increased immune activation and imbalance in cytokine microenvironment [5,15] associated with chronic HIV replication are thought to result either in endothelial dysfunction or damage or both [16,17] that could elevate the risk of atherosclerosis and related cardiovascular events [18]. Importantly, toxicity related to a number of antiretroviral medications (in particular, protease inhibitors) may itself result in dyslipidemia, insulin resistance and body fat redistribution, and thus, independently impact overall cardiovascular risk [5,7,18–21]. The SMART study finding of higher cardiovascular clinical event rate in ART-interrupting individuals raised the hypothesis that the inflammatory cascade associated with viral rebound may compound the preexisting ART-related endothelial damage, resulting in increased risk of atherosclerosis [5,22] and cardiovascular disease [6–8].
Various biological markers associated with endothelial stress, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), von Willebrand factor (vWF) and thrombomodulin, have been directly associated with chronic endothelial activation, atherosclerosis and risk of cardiovascular events [8,23]. A significant increase in circulating levels of these molecules was observed in patients with hyperlipidemia, hypertension, diabetes mellitus, oxidative stress, changes in hormone metabolism and a history of cigarette smoking [8,24]. An acute change in the plasma level of these molecules has been reported to reflect a vascular stress response associated with the development of cardiovascular disease [8,24]. In addition, a number of studies have shown increased plasma levels of endothelial cell markers in chronically viremic HIV-1-infected individuals who suffer clinical cardiovascular adverse events [8,25–29], suggesting that the normal endothelium function may be impaired in HIV infection. On the basis of these observations, the association between cardiovascular adverse events and treatment interruptions, and data showing that viral-mediated inflammation can increase endothelial expression of vWF and ‘shedding’ of cellular adhesion molecules (CAMs) from endothelial cells and leukocytes [10,30], we hypothesized that short-term treatment interruption-induced viral replication and associated T-cell activation could acutely increase endothelial stress resulting in levels of soluble (s)VCAM-1, sICAM-1 and vWF above those observed in the course of continuous ART.
Extending prior findings in chronic viremia, we show that sVCAM-1 and sICAM-1, but not vWF, rise in response to acute viremic episodes. Importantly, we present novel evidence indicating that sVCAM-1 levels remain elevated after ART resumption and successful viral resuppression, suggesting persistent endothelial damage.
Study participants, materials and methods
Participants
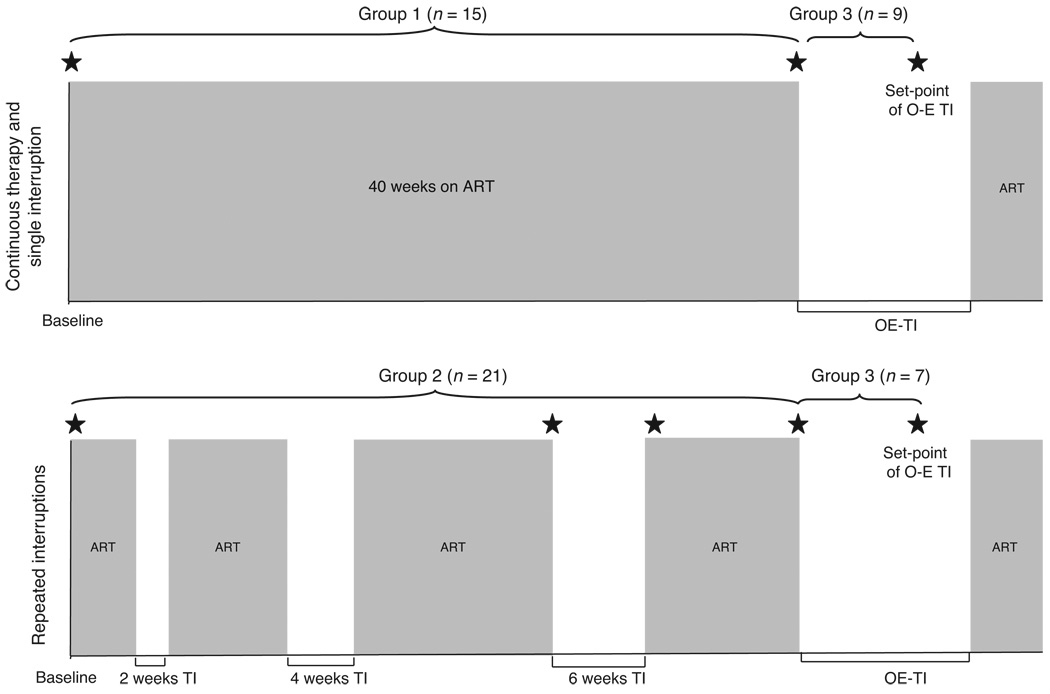
Cryopreserved plasma samples were derived from peripheral blood collected from 36 chronically suppressed HIV-1 infected patients participating in a parent treatment interruption study (parent cohort of 42 patients) based in Philadelphia (USA). Patients in the parent study were randomized to either a continuous therapy group followed for 40 weeks on ART and during a single ART interruption or to a repeated interruptions group undergoing four subsequent ART interruptions (Fig. 1). Recruitment characteristics of the patients were an age of at least 18 years, presence of ART (≥three drugs), CD4 cell count of more than 400 cells/µl with a history of nadir CD4 cell count of at least 100 cells/µl and plasma HIV-1 RNA (viral load) of less than 50 copies/ml with more than 6 months history of less than 500 copies/ml. Baseline and demographic characteristics of the patients participating in the parent study are shown in Table 1. The main findings of this study have been described in PLoS Medicine [3]. Cryopreserved plasma samples from 36 patients of the parent study were available for the current study and were divided in three analysis groups (Fig. 1): group 1: samples from 15 patients of the continuous therapy group collected at study baseline (viral load <50 copies/ml) and at the end of a 40-week follow-up on continuous ART; group 2: samples from 21 patients from the repeated interruptions group collected at study baseline, start (viral load <50 copies/ml) and end of a 6-week treatment interruption (plasma levels were measured during 6-week treatment interruption in this study because viral rebound was observed in all participating patients during this time). Samples from 18 of these patients were also available for analysis after ART resumption and viral resuppression to less than 50 copies/ml; group 3: samples from 16 patients from group 1 (n = 9) and group 2 (n = 7), analyzed at the start of an open-ended treatment interruption (viral load <50 copies/ml) and viral set point (average viral load of the first three consecutive measurements of viral load with <0.5 log variation) of the open-ended treatment interruption.
Fig. 1. Study design.
Parent study groups (continuous therapy/single interruption group, repeated interruptions group) as well as current analysis study groups (groups 1, 2 and 3) are shown. Grey and white shade areas represent periods on and off ART, respectively. Asterisks indicate time points studied in the current study. ART, antiretroviral therapy; O-E TI, open-ended treatment interruption; TI, treatment interruption.
Table 1.
Baseline demographic and clinical characteristics per study arm.
| Characteristics | Continuous therapy/single interruptions group (n = 21) |
Repeated interruptions group (n = 21) |
|---|---|---|
| Age (years), median, (25th and 75th percentiles) | 46 (41.5, 53) | 42 (38.5, 42) |
| Men (%) | 81 | 100 |
| Ethnicity (%) | 48 C, 42 AA, 10 H | 81 C, 14 AA, 5 H |
| Route of infection (%) | 90 S, 10 IV | 95 S, 5 IV |
| Years since diagnosis, median (25th and 75th percentiles) | 7 (5, 11.5) | 11 (6.5, 15.5) |
| Years on ART, median (25th and 75th percentiles) | 4.5 (4, 6.7) | 7 (6, 11) |
| CD4+ T-cell count at recruitment (cells/µl), median (25th and 75th percentiles) |
637 (481, 793) | 658 (506.5, 815.8) |
| Follow-up period (weeks), median (25th and 75th percentiles) | 79 (59, 88.5) | 81 (67, 88.5) |
| Drug classes used at entrya | ||
| PI | 7/21 | 10/21 |
| NNRTI | 15/21 | 13/21 |
AA, African American; ART, antiretroviral therapy; C, Caucasian; H, Hispanic; IV, intravenous drug usage; NNRTI, nonnucleoside reverse-transcriptase inhibitors; PI, protease inhibitors; S, sexual transmission.
Numbers include cases of PI/NNRTI combined use at study entry.
Flow cytometry analysis
Assessment of T-cell activation (CD8+/CD38+, CD8+/HLA-DR+ and CD3+/CD95+) was performed as part of the parent study, using whole blood flow cytometry at the time of blood collection as previously described [31].
Enzyme-linked immunosorbent assay
Plasma levels of markers of endothelial stress (sVCAM-1, sICAM-1 and vWF) were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's specifications (sVCAM-1 and ICAM-1 by R&D, Minneapolis, Minnesota, USA; vWF by American Diagnostica, Stamford, Connecticut, USA) on a kinetic absorbance reader at 450 nm (Rainbow Reader, SLT-Labinstruments, Grodig/Salzburg, Austria). All measurements were based on the average of duplicate samples. Lower limits of sVCAM-1, sICAM-1 and vWF detection were 6.25 ng/ml, 2.25 ng/ml and 0.5 mU/ml, respectively.
Statistical analysis
The data are described as medians, 25th and 75th percentiles for all variables. Variable distributions were analyzed for normality using the Shapiro–Wilk W test (P > 0.05); between-time points comparisons were performed using nonparametric Wilcoxon Signed–Rank test or paired t-tests depending on the data distribution. Correlations between variables were assessed using Spearman's or pairwise correlation tests. All statistical tests were performed using JMP 4.0 (SAS Institute, Cary North Carolina, USA).
Results
Increased plasma levels of soluble vascular cell adhesion molecule-1 and soluble intercellular adhesion molecule-1, but not von Willebrand factor, during acute rebounding viremia
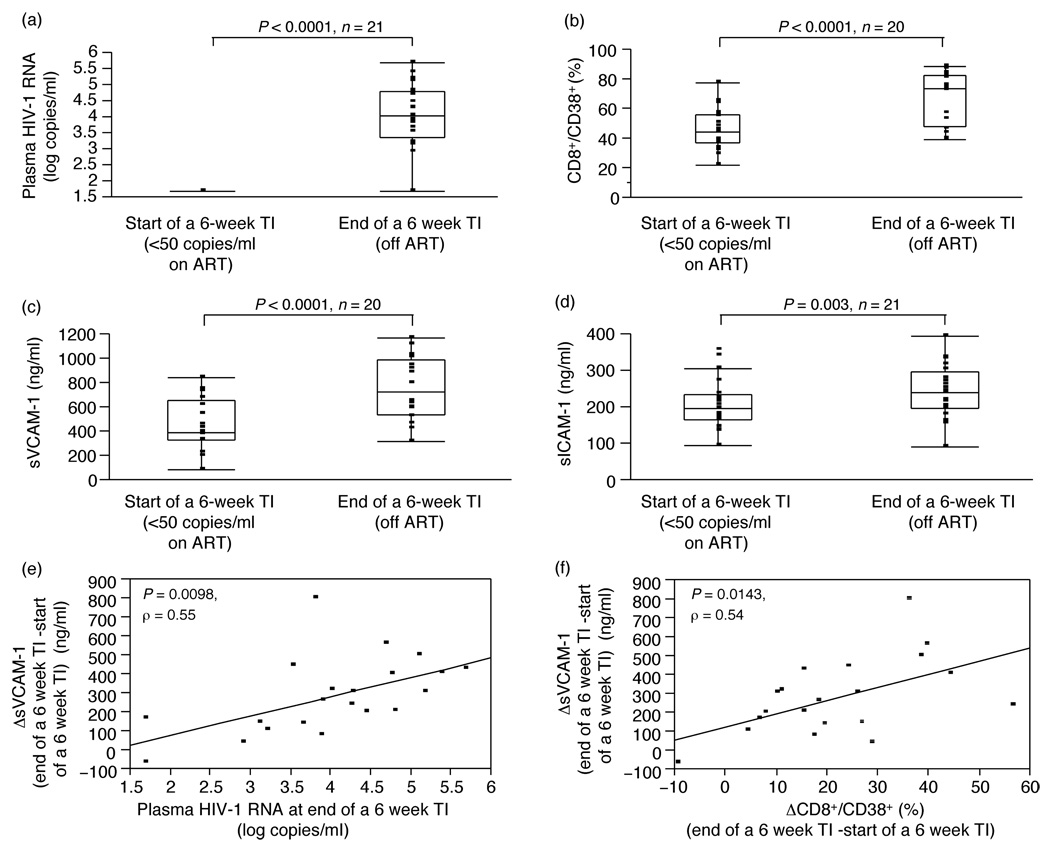
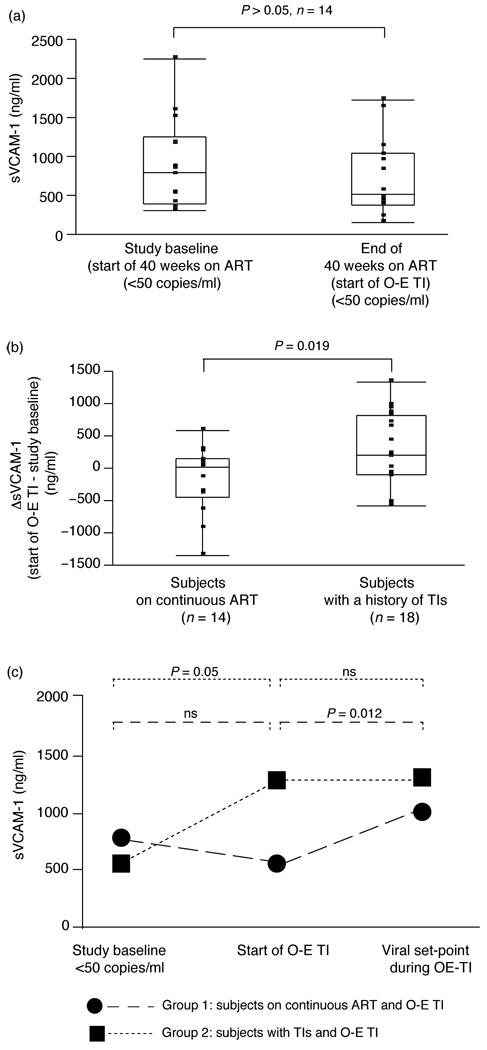
As described in the parent study results [3], a 6-week treatment interruption resulted in viral rebound (Fig. 2a). Concurrent with the rise in viral load and related increase in immune activation, reflected by a significant rise in the frequency of CD3+/CD95+ (P = 0.0022, n = 21), CD8+/CD38+ (P < 0.0001, n = 20, Fig. 2b) or CD8+/HLA-DR+ (P = 0.001, n = 20) T-cells, we observed an increase in plasma levels of sVCAM-1 (P < 0.0001, n = 20, Fig. 2c) and sICAM-1 (P = 0.003, n = 21, Fig. 2d), but not vWF, from start (viral load <50 copies/ml) of the 6-week treatment interruption. No change in plasma levels of any of the molecules (vWF, sICAM-1 and sVCAM-1) was observed in patients followed for 40 weeks on continuous ART during which the viral load was repeatedly tested to confirm suppression at less than 50 copies/ml (group 1, data for sVCAM-1;Fig. 3a, n = 14). A subsequent open-ended treatment interruption in these patients confirmed a rise in sVCAM-1 from suppression levels similar to that observed in group 2 patients during the 6-week treatment interruption (Fig. 3c).
Fig. 2. Plasma HIV-1 RNA, T-cell activation and markers of endothelial dysfunction: effect of treatment interruption and interrelationship of these variables.
(a) Plasma HIV-1 RNA at start (VL <50 copies/ml) and end of a 6-week treatment interruption is shown in HIV-1-positive patients interrupting therapy. (b) Percentages (%) of CD8+/CD38+ T-cells are shown at start (VL <50 copies/ml) and end of a 6-week treatment interruption in HIV-1-positive patients interrupting therapy. (c, d) Levels of sVCAM-1 (panel c) and sICAM-1 (panel d) are shown at start (VL <50 copies/ml) and end of a 6-week treatment interruption in HIV-1-positive patients interrupting therapy. Patient S49 although included in the analysis is not shown in the graph for graphic purposes. (e) Correlation between the increase in sVCAM-1 levels (ng/ml) during a 6-week therapy interruption (ΔsVCAM-1: sVCAM-1end of 6 week TI – sVCAM-1start of 6 week TI) and plasma HIV-1 RNA (log copies/ml) at week 6. (f) Correlation between the increase in sVCAM-1 levels (ng/ml) during a 6-week therapy interruption (ΔsVCAM-1: sVCAM-1end of 6 week TI – sVCAM-1start of 6 week TI) and the increase in the % of CD8+/CD38+ for the same period (ΔCD8+/CD38+: CD8+/CD38+end of 6 week TI – CD8+/CD38+start of 6 week TI). Data in panels a–d are shown as inter-quartile box plot (median and outliers), with significant P values on the top of each graph, whereas data in panels e and f is shown as regression lines, with correlation and P values. ART, antiretroviral therapy; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; TI, treatment interruption.
Fig. 3. Differential effect of continuous ART and multiple TIs on sVCAM-1 levels.
(a) Levels of sVCAM-1 are shown at study baseline and end of a 40-week follow-up on continuous ART (group 1), (b) change in sVCAM-1 levels is shown in patients followed for 40 weeks on ART prior to an O-E TI (group 1, sVCAM-1start of O-E TI – sVCAM-1study baseline) and in patients undergoing three TIs prior to an O-E TI (group 2, ΔsVCAM-1: sVCAM-1start of O-E TI – sVCAM-1study baseline), (c) levels of sVCAM-1 are shown at study baseline, start of O-E TI and when viral set-point was reached during O-E TI, in groups 1 and 2. Note that for group 1, the end of the 40-week follow-up is also the start of the O-E TI. Data in panels (a) and (b) are shown as inter-quartile box plot (median and outliers) and in panel (c) as medians. Significant P values corresponding in paired (panels a and c) and nonpaired (panel b) analysis are shown on the top of each graph. ART, antiretroviral therapy; O-E TI, open-ended treatment interruption; sVCAM-1, soluble vascular cell adhesion molecule-1; TI, treatment interruption.
Correlation between soluble vascular cell adhesion molecule-1 and soluble intercellular adhesion molecule-1 levels, viral load and T-cell activation
The increase m sVCAM-1 levels (ΔsVCAM-1: sVCAM-1end of 6-week treatment interruption – sVCAM-1start of 6-week treatment interruption) was positively associated with peak HIV-1 RNA during treatment interruption (P = 0.0098, ρ = 0.55, Fig. 2e). We also detected a positive association between the change in T-cell activation markers (CD3+/CD95+: P = 0.0181, ρ = 0.51; CD8+/CD38+: P = 0.0143, ρ = 0.54; CD8+/HLA-DR+: P = 0.0116, ρ = 0.55) and the change in sVCAM-1 levels during a 6-week treatment interruption (correlation of ΔsVCAM-1: sVCAM-1end of 6-week treatment interruption – sVCAM-1start of 6-week treatment interruption with ΔCD8+/CD38+: CD8+/CD38+end of 6-week treatment interruption – CD8+/CD38+start of 6-week treatment interruption, Fig. 2f). Accordingly the change in sICAM-1 levels during the same 6-week treatment interruption period was positively associated with the change in CD3+/CD95+ (P = 0.0315, ρ = 0.47, data not shown). No other correlations were found among the variables analyzed. Taken together, data indicate both viral replication and T-cell activation to be associated with endothelial stress change during treatment interruption.
Persistence of soluble vascular cell adhesion molecule-1 elevation upon antiretroviral therapy reinitiation and despite viral suppression
In patients with repeated treatment interruptions (group 2), ART resumption at the end of a 6-week treatment interruption achieving viral suppression to less than 50 copies/ml by the start of the open-ended treatment interruption resulted, as expected, in a significant decrease in T-cell immune activation reflected by a decrease in the frequency of CD3+/CD95+ (P = 0.0002, n = 17), CD8+/CD38+ (P = 0.018, n = 16) or CD8+/HLA-DR+ (P < 0.0001, n = 16) T-cells. In contrast to the decrease in T-cell activation, sVCAM-1 continued to increase (although this increase did not reach statistical significance, P = 0.06, n = 18) (Table 2). Interestingly, no additional increase of sVCAM-1 from elevated levels after ART resumption was observed in group 2 during the subsequent open-ended treatment interruption when the viral load was again documented (Table 2, Fig. 3c).
Table 2.
Endothelial markers levels during follow-up.a
| Variable | Repeated interruptions group | Continuous therapy/ single interruption group |
|||||
|---|---|---|---|---|---|---|---|
| Study baseline |
End of 6-week TI |
Start of O-E TI |
Set point of O-E TI |
Study baseline |
Start of OE-TI | Set point of O-E TI |
|
| sVCAM-1 (ng/ml) | 563.8 (396.4, 1505.9) |
794.8 (554.4, 1013.5) |
1301.8 (372.7, 1572.4) |
1287.1 (801.1, 1400) |
769.6 (406.9, 1260.4) |
558.8 (384.5, 1130.3) |
1028.3 (687.4, 1626.9) |
| sICAM-1 (ng/ml) | 253.8 (111.5, 289.3) |
242.4 (198.8, 310.1) |
209.7 (83.5, 302.6) |
292.7 (58.5, 307.1) |
276.4 (111.2, 371.2) |
151.3 (98.1, 295.5) |
179.5 (106.5, 370.2) |
| vWF (mU/ml) | 93.4 (88.4, 103.1) |
95.7 (87.2, 108.7) |
89.7 (78, 95.3) |
89.3 (81.5, 99.3) |
91.9 (75.1, 98.9) |
80.1 (75.4, 95.7) |
83.8 (72.2, 92.9) |
O-E TI, open-ended treatment interruption; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; TI treatment interruption; vWF, von Willebrand factor.
Medians are listed together with 25th and 75th percentiles in parenthesis.
In contrast to patients on continuous therapy (group 1, Fig. 3a), patients with repeated treatment interruptions (group 2) showed higher sVCAM-1 levels at the start of the open-ended treatment interruption (viral load <50 copies/ml on ART) when compared with their respective baselines before any monitored treatment interruption (study baseline, viral load <50 copies/ml on ART, P = 0.05, n = 18; Table 2, Fig. 3c), whereas no increase was observed for the same period for sICAM-1 or vWF (Table 2). This finding, together with a significantly higher change in sVCAM-1 levels (ΔsVCAM-1: sVCAM-1baseline of open-ended treatment interruption – sCAM-1study baseline) in group 2 patients as compared with group 1 patients (sVCAM-140-week follow-up – sVCAM-1study baseline) (P = 0.019, Fig. 3b), further support the interpretation that treatment interruptions result in a persistent elevation of sVCAM-1 despite ART-mediated viral resuppression.
Discussion
We report that short viremic episodes result in increased plasma levels of soluble VCAM-1 and ICAM-1, but not vWF, indicative of endothelial stress changes associated with rebounding viral load. These markers also show a positive association with T-cell activation and with viral load, supporting a direct pathogenetic relationship between viral replication, immune activation and changes in endothelial stress markers. Therapy resumption with viral resuppression did not reverse sVCAM-1 elevation, suggesting a persistent vascular stress, outlasting viral replication.
Complementing prior data obtained in steady-state viremia settings, in which vWF, sVCAM-1 and sICAM-1 were all shown to decrease with ART [8], our data clearly identifies sVCAM-1 as an early pronounced change upon viral rebound. The evidence of retained elevation of sVCAM-1 after a brief (6 weeks) treatment interruption suggests that endothelial stress may not be rapidly reversed to preinterruption levels following viral resuppression. Imbalance of the cytokine environment as a result of HIV-1 infection has been associated with an impairment of the endothelium function [5]. On the basis of our findings, we interpret that even a short viremic episode can trigger a cascade of events that cannot be immediately reversed by ART-associated viral suppression and an acute decrease in the frequency of activated T-cells. This highlights that even the treatment interruption associated with management of ART toxicities may result in a persistent increase in endothelial activation, which may in turn contribute to overall cardiovascular risk, compounding the described adverse effects of antiretroviral medications. Future studies will be needed to determine whether a sustained elevation of sVCAM-1 can segregate patients with a higher risk for cardiovascular adverse events upon treatment interruption. Our data indicate a clear correlation between viral replication, immune activation and rise in sVCAM-1/sICAM-1. However, their reciprocal pathogenic role in cardiovascular disease remains controversial based on several studies showing significant associations between atherosclerosis and either sICAM-1 [32–36] or sVCAM-1 [22,37,38] or both [22,39,40]. Although we cannot directly extrapolate the changes observed in the present study to an actual increase in incidence of clinical cardiovascular events, the levels we document for sVCAM-I and sICAM-1 as a result of viral rebound are comparable to those independently associated with underlying atherosclerosis and cardiovascular diseases such as myocardial infarction, stroke and peripheral arterial disease [41–44].
By suggesting a rise in endothelial stress, our findings are in keeping with reports from the SMART study [4], in which cardiac events were more frequent in patients interrupting ART. The majority of SMART patients interrupting ART, however, did not present with cardiovascular events, even though we would posit that all experienced an endothelial stress response. It remains possible that underlying risk factors, low CD4 nadirs and opportunistic infection among a relatively small subset of SMART patients, might have compounded the endothelial stress associated with the rebounding of viral replication.
Although our data identifies the increase in endothelial stress as a response to short-term viral replication, our study does not address a cumulative risk of cardiovascular risk. Apart from randomized assignment to study groups, we did not independently control for the contribution of ART regimen [protease inhibitors, nonnucleoside reverse transcriptase inhibitors (NNRTIs), etc.], ethnicity, obesity, lipids, smoking history and/or alcohol consumption. Future studies are underway to address the interrelationship between these factors, short viremic episodes and cardiovascular risk. However, we interpret that viral replication and T-cell activation remain a strong correlate of endothelial stress because patients in our study had an equal recruitment criteria, were randomized into study groups, and changes reported in the study were observed both within (thus accounting for baseline levels) and between the study groups indicating that although preexisting conditions may contribute to endothelial stress, these alone would not solely explain our results.
Of the variables analyzed in our study, sVCAM-1 was the most sensitive to changes in viral replication, when compared with sICAM-1 or vWF being the only variable positively associated with viral load (Fig. 2); the only variable confirmed to change in two separate patient groups studied after short-term therapy interruption (Fig. 3) and the only marker with sustained increase over time in patients with a history of treatment interruptions (Fig. 3). In contrast to both sCAMs, we did not detect changes in vWF levels in response to viral rebound in our study groups. Other investigators have shown significantly higher levels of vWF in chronically HIV-1-infected patients in comparison with normal controls [5], together with a correlation between increased circulating vWF, disease progression and plasma viral load [5,25,45]. Kinetic differences in sICAM-1, sVCAM-1 and vWF response to viremia could account for the lack of change in vWF levels observed during short-term viremia, as our data does not address long-term follow-up of chronic viremia outcomes. It also remains to be determined how additional cardiovascular risk markers such as C reactive protein (CRP) levels, tissue type plasminogen activator (tPA) and plasminogen activator inhibitor 1 (PAI-1) change following treatment interruption and the acute rise in sVCAM-1 in helping to determine what may be early versus delayed changes of cardiovascular stress.
A limitation to the assessment of soluble CAMs is that sICAM-1 and sVCAM-1 can be derived from other sources in addition to the endothelium (i.e. smooth muscle cells, leukocytes and tumor cells) [27,46]. However, an association between endothelial stress response and immune activation has been documented widely, and our data are in keeping with prior observations associating treatment interruption with increased frequency of cardiovascular events [5,18].
Taken together, our data shows the potential for a rapid and persistent endothelial stress response during short-term acute viremia that could be of relevance to a rise in total cardiovascular disease risk.
Acknowledgements
We would like to thank the HIV-1+ patients who participated in the study, their providers and the Board and Staff of Philadelphia FIGHT.
This work was primarily supported by a grant to L.J.M. by the National Institute of Allergy and Infectious Disease NIH AI48398. Additional support was provided by The Philadelphia Foundation (Robert I. Jacobs Fund), The Stengel-Miller family, AIDS funds from the Commonwealth of Pennsylvania and from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
No commercial affiliations were present.
References
- 1.Lori F, Lisziewicz J. Structured treatment interruptions for the management of HIV infection. JAMA. 2001;286:2981–2987. doi: 10.1001/jama.286.23.2981. [DOI] [PubMed] [Google Scholar]
- 2.Montaner LJ. Structured treatment interruptions to control HIV-1 and limit drug exposure. Trends Immunol. 2001;22:92–96. doi: 10.1016/s1471-4906(00)01809-3. [DOI] [PubMed] [Google Scholar]
- 3.Papasavvas E, Kostman JR, Mounzer K, Grant RM, Gross R, Gallo C, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 2004;1:e64. doi: 10.1371/journal.pmed.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 5.de Larranaga GF, Petroni A, Deluchi G, Alonso BS, Benetucci JA. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul Fibrinolysis. 2003;14:15–18. doi: 10.1097/00001721-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d'Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients: association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 7.Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 8.Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185:456–462. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos JA, Hennekens CH, Ridker PM. Plasma concentration of soluble vascular cell adhesion molecule-1 and subsequent cardiovascular risk. J Am Coll Cardiol. 2000;36:423–426. doi: 10.1016/s0735-1097(00)00742-7. [DOI] [PubMed] [Google Scholar]
- 11.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 12.Pober JS, Gimbrone MA, Jr, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- 13.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002;106:820–825. doi: 10.1161/01.cir.0000025636.03561.ee. [DOI] [PubMed] [Google Scholar]
- 14.Steffan AM, Lafon ME, Gendrault JL, Schweitzer C, Royer C, Jaeck D, et al. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:1582–1586. doi: 10.1073/pnas.89.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zietz C, Hotz B, Sturzl M, Rauch E, Penning R, Lohrs U. Aortic endothelium in HIV-1 infection: chronic injury, activation, and increased leukocyte adherence. Am J Pathol. 1996;149:1887–1898. [PMC free article] [PubMed] [Google Scholar]
- 16.Passalaris JD, Sepkowitz KA, Glesby MJ. Coronary artery disease and human immunodeficiency virus infection. Clin Infect Dis. 2000;31:787–797. doi: 10.1086/313995. [DOI] [PubMed] [Google Scholar]
- 17.Paton P, Tabib A, Loire R, Tete R. Coronary artery lesions and human immunodeficiency virus infection. Res Virol. 1993;144:225–231. doi: 10.1016/s0923-2516(06)80033-6. [DOI] [PubMed] [Google Scholar]
- 18.Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis. 2006;185:1–11. doi: 10.1016/j.atherosclerosis.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Al-Attar I, Orav EJ, Exil V, Vlach SA, Lipshultz SE. Predictors of cardiac morbidity and related mortality in children with acquired immunodeficiency syndrome. J Am Coll Cardiol. 2003;41:1598–1605. doi: 10.1016/s0735-1097(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 21.Safrin S, Grunfeld C. Fat distribution and metabolic changes in patients with HIV infection. AIDS. 1999;13:2493–2505. doi: 10.1097/00002030-199912240-00002. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer IM, de Maat MP, Bots ML, Breteler MM, Meijer J, Kiliaan AJ, et al. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2002;22:838–842. doi: 10.1161/01.atv.0000016249.96529.b8. [DOI] [PubMed] [Google Scholar]
- 23.Muller MM, Griesmacher A. Markers of endothelial dysfunction. Clin Chem Lab Med. 2000;38:77–85. doi: 10.1515/CCLM.2000.013. [DOI] [PubMed] [Google Scholar]
- 24.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 25.Aukrust P, Bjornsen S, Lunden B, Otterdal K, Ng EC, Ameln W, et al. Persistently elevated levels of von Willebrand factor antigen in HIV infection. Downregulation during highly active antiretroviral therapy. Thromb Haemost. 2000;84:183–187. [PubMed] [Google Scholar]
- 26.Blann AD. Endothelial cell damage and the development or progression of atherosclerosis. Clin Sci (Lond) 1999;97:119–121. [PubMed] [Google Scholar]
- 27.Blann AD, Lip GY. Cell adhesion molecules in cardiovascular disease and its risk factors: what can soluble levels tell us? J Clin Endocrinol Metab. 2000;85:1745–1747. doi: 10.1210/jcem.85.5.6594. [DOI] [PubMed] [Google Scholar]
- 28.Galea P, Vermot-Desroches C, Le Contel C, Wijdenes J, Chermann JC. Circulating cell adhesion molecules in HIV1-infected patients as indicator markers for AIDS progression. Res Immunol. 1997;148:109–117. doi: 10.1016/s0923-2494(97)82482-0. [DOI] [PubMed] [Google Scholar]
- 29.Nordoy I, Aukrust P, Muller F, Froland SS. Abnormal levels of circulating adhesion molecules in HIV-1 infection with characteristic alterations in opportunistic infections. Clin Immunol Immunopathol. 1996;81:16–21. doi: 10.1006/clin.1996.0151. [DOI] [PubMed] [Google Scholar]
- 30.Gearing AJ, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann N Y Acad Sci. 1992;667:324–331. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 31.Papasavvas E, Ortiz GM, Gross R, Sun J, Moore EC, Heymann JJ, et al. Enhancement of human immunodeficiency virus type 1 -specific CD4 and CD8 T cell responses in chronically infected persons after temporary treatment interruption. J Infect Dis. 2000;182:766–775. doi: 10.1086/315748. [DOI] [PubMed] [Google Scholar]
- 32.Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, et al. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol. 1999;19:2355–2363. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- 33.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 34.Luc G, Arveiler D, Evans A, Amouyel P, Ferrieres J, Bard JM, et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis. 2003;170:169–176. doi: 10.1016/s0021-9150(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 37.De Caterina R, Basta G, Lazzerini G, Dell'Omo G, Petrucci R, Morale M, et al. Soluble vascular cell adhesion molecule-1 as a biohumoral correlate of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2646–2654. doi: 10.1161/01.atv.17.11.2646. [DOI] [PubMed] [Google Scholar]
- 38.Peter K, Nawroth P, Conradt C, Nordt T, Weiss T, Boehme M, et al. Circulating vascular cell adhesion molecule-1 correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule-1, E-selectin, P-selectin, and thrombomodulin. Arterioscler Thromb Vase Biol. 1997;17:505–512. doi: 10.1161/01.atv.17.3.505. [DOI] [PubMed] [Google Scholar]
- 39.Rohde LE, Lee RT, Rivera J, Jamacochian M, Arroyo LH, Briggs W, et al. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:1765–1770. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 40.Shai I, Pischon T, Hu FB, Ascherio A, Rifai N, Rimm EB. Soluble intercellular adhesion molecules, soluble vascular cell adhesion molecules, and risk of coronary heart disease. Obesity (Silver Spring) 2006;14:2099–2106. doi: 10.1038/oby.2006.245. [DOI] [PubMed] [Google Scholar]
- 41.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–1342. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 42.Blann AD, McCollum CN. Circulating endothelial cell/leukocyte adhesion molecules in atherosclerosis. Thromb Haemost. 1994;72:151–154. [PubMed] [Google Scholar]
- 43.Morisaki N, Saito I, Tamura K, Tashiro J, Masuda M, Kanzaki T, et al. New indices of ischemic heart disease and aging: studies on the serum levels of soluble intercellular adhesion molecule-1 (ICAM-1) and soluble vascular cell adhesion molecule-1 (VCAM-1) in patients with hypercholesterolemia and ischemic heart disease. Atherosclerosis. 1997;131:43–48. doi: 10.1016/s0021-9150(97)06083-8. [DOI] [PubMed] [Google Scholar]
- 44.O'Malley T, Ludlam CA, Riemermsa RA, Fox KA. Early increase in levels of soluble inter-cellular adhesion molecule-1 (sICAM-1); potential risk factor for the acute coronary syndromes. Eur Heart J. 2001;22:1226–1234. doi: 10.1053/euhj.2000.2480. [DOI] [PubMed] [Google Scholar]
- 45.Lafeuillade A, Alessi MC, Poizot-Martin I, Boyer-Neumann C, Zandotti C, Quilichini R, et al. Endothelial cell dysfunction in HIV infection. J Acquir Immune Defic Syndr. 1992;5:127–131. [PubMed] [Google Scholar]
- 46.Braun M, Pietsch P, Schror K, Baumann G, Felix SB. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res. 1999;41:395–401. doi: 10.1016/s0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]