Abstract
The zinc transporter ZIP8 is highly expressed in T cells derived from human subjects. T cell ZIP8 expression was markedly up-regulated upon in vitro activation. T cells collected from human subjects who had received oral zinc supplementation (15 mg/day) had higher expression of the activation marker IFN-γ upon in vitro activation, indicating a potentiating effect of zinc on T cell activation. Similarly, in vitro zinc treatment of T cells along with activation resulted in increased IFN-γ expression with a maximum effect at 3.1 μM. Knockdown of ZIP8 in T cells by siRNA decreased ZIP8 levels in nonactivated and activated cells and concomitantly reduced secretion of IFN-γ and perforin, both signatures of activation. Overexpression of ZIP8 by transient transfection caused T cells to exhibit enhanced activation. Confocal microscopy established that ZIP8 is localized to the lysosome where ZIP8 abundance is increased upon activation. Loss of lysosomal labile zinc in response to activation was measured by flow cytometry using a zinc fluorophore. Zinc between 0.8 and 3.1 μM reduced CN phosphatase activity. CN was also inhibited by the CN inhibitor FK506 and ZIP8 overexpression. The results suggest that zinc at low concentrations, through inhibition of CN, sustains phosphorylation of the transcription factor CREB, yielding greater IFN-γ expression in T cells. ZIP8, through control of zinc transport from the lysosome, may provide a secondary level of IFN-γ regulation in T cells.
Keywords: signal transduction, adaptive immunity, zinc nutrition, nutritional immunity
Introduction
Zinc homeostasis in cells, including those of the immune system, is maintained through tight regulation of zinc influx, efflux, and distribution to intracellular organelles. Zinc transporter proteins are essential for these metabolic and functional adjustments. Zinc transporters are found within two protein families: ZIP (SLC39) and ZnT (SLC30) [1, 2]. ZIP transporters function in zinc influx into the cytosol, and ZnT transporters function in zinc efflux from the cytosol.
Zinc has a variety of effects on the immune system in vivo and in vitro. These effects are mainly concentration-dependent [3]. For example, zinc increases proinflammatory cytokine mRNA levels in monocytes in vitro and also exhibits a biphasic effect on cytokine expression as zinc levels are increased [4]. IFN-γ production by PHA-stimulated T cells was decreased in experimental human zinc deficiency [5]. Similarly, reduced IFN-γ production in response to presentation of the Heligmosomoides polygyrus antigen was identified in zinc-deficient mice [6]. These findings suggest that immune cells require a finite amount of zinc to function optimally.
T cells play one of the central roles in adaptive immunity. Upon activation through the TCR, T cells mediate the immune response by secreting cytokines or a granule exocytosis-mediated cytotoxic mechanism [7]. The cytokine, IFN-γ, is a hallmark of T cells, and it is crucial for immunity against intracellular pathogens and for tumor suppression. IFN-γ expression is controlled by specific transcription factors in activated T cells. Among them, CREB regulates IFN-γ production positively in human T cells through binding to the IFN-γ promoter [8], which is enhanced by CREB phosphorylation [9]. It has been shown that CREB is dephophorylated by CN in many cell types including pancreatic islet cells and neurons [10], suggesting that CREB might be dephosphorylated by CN in T cells as well. CN phosphatase activity is inhibited by zinc in vitro in a concentration-dependent manner [11, 12]. Therefore, zinc may have a role in increased IFN-γ expression through inhibition of CN during T cell activation.
The granule exocytosis-mediated cytotoxicty pathway is used by CD4 and CD8 T cells [7]. Upon TCR activation, T cells gain the ability to execute granule exocytosis. Degranulation releases the pore-forming protein perforin and several proteases or granzymes. Perforin is secreted from lysosomes to form pores, allowing proteases and granzymes to enter target cells and induce apoptosis. Pore formation is a calcium-dependent process, and it is inhibited by zinc [13], suggesting that the concentration of lysosomal zinc is an important component for effective perforin function during T cell activation.
Relative to the involvement of zinc in immune cells, the transporter protein ZIP8 has been shown by Begum et al. [14] to be highly expressed in human immune-stimulated monocytes and differentiated macrophages. In transfected cells, it was localized to the lysosome and was inducible by immune stimuli such as LPS. Those findings support the 5.6- to 9.8-fold up-regulation of ZIP8, which we identified within a dataset derived from a microarray analysis of RNA from PBMCs of humans that were infused with LPS [15]. Furthermore, we have shown previously that ZIP8 is highly expressed in purified human T cells compared with monocytes or granulocytes [16]. Based on the high ZIP8 expression in human T cells and that zinc supplementation of human subjects produces enhanced IFN-γ expression upon activation in vitro [16], we have examined the relationship of ZIP8 expression further and related it to a process that appears to potentiate activation of human T cells.
Here, we used CD3+ circulating primary human T cells to conduct our experiments. Our data demonstrate that ZIP8 is up-regulated during T cell activation. ZIP8 protein was localized in the lysosome, and ZIP8 transports zinc from the lysosome to cytoplasm of T cells during TCR-mediated activation. The increased cytoplasmic zinc caused inhibition of CN activity, resulting in mild increases in phosphorylated CREB and production of IFN-γ, and the decreased lysosomal zinc caused an increase in perforin secretion. These experiments relate ZIP8 expression/function directly to the TCR-mediated T cell activation process.
MATERIALS AND METHODS
Human subjects
Healthy male subjects (21–31 years old) were used for peripheral blood collection after 4 days of zinc supplementation for the assessment of the effect of zinc on T cell activation. During this study, each subject consumed a zinc supplement (15 mg zinc as ZnSO4) or a placebo. For the mechanistic experiments, other healthy male subjects, who were not zinc-supplemented, provided samples of peripheral blood. Procedures used informed consent and had the approval of the University of Florida Institutional Review Board (Gainesville, FL, USA).
Isolation and TCR-mediated activation of T cells
Whole blood was mixed with PBS and was layered on Histopaque 1.077 (Sigma Chemical Co., St. Louis, MO, USA) and centrifuged at 600 g at room temperature for 30 min. The PBMCs at the interface were removed and washed two times with PBS using 250 g for 10 min. The cells were suspended in MACS buffer (PBS, pH 7.2, with 0.5% BSA and 2 μM EDTA) and mixed with magnetically labeled microbeads (Miltenyi Biotec, Germany) conjugated to a cocktail of antibodies against nonlymphocytic cells for isolation of T cells through negative selection. Flow cytometry-demonstrated purity of CD3+ T cells was >95%. Isolated T cells were plated at a density of 2 × 106 cells/ml in X-VIVO15® medium (Cambrex, Charles City, IA, USA) containing 5% human AB serum in 48-well plates. This formulation produced a medium with a zinc concentration of 1.2 μM. In some experiments, zinc was added (as ZnSO4) to increase the zinc concentration of the medium incrementally by between 1.6 and 25 μM. For TCR activation, the T cells were incubated for 48 h at 37°C in 5% CO2 with microbeads conjugated to antibodies against human CD2, CD3, and CD28 (Miltenyi Biotec). These methods, which mimic antigen presentation, have been described previously [16]. For the assessment of the effect of zinc in vitro, cells were pretreated with indicated concentrations of zinc sulfate for 2 h.
Overexpression and siRNA-mediated suppression of hZIP8 expression
The T cells were plated at a density of 2 × 106 cells/ml with medium (Cambrex) as above. For hZIP8 overexpression, the cells were transiently transfected with pCMV-Sport6 containing full-length hZIP8 cDNA (OriGene, Rockville, MD, USA) or empty pCMV-Sport6 using Effectene reagent (Qiagen, Valencia, CA, USA). Activation was initiated 2 h after transfection. Cells were harvested 48 h after activation was initiated. For ZIP8 knockdown, the cells were plated at a density of 1 × 106 cells/ml and were transfected using Hyperfect reagent (Qiagen) with 750 ng hZIP8 SMARTpool siRNA (Dharmacon, Boulder, CO, USA) or nontargeting random siRNA (Dharmacon). After 16 h, fresh medium was added, and cells were activated as above. Cells were harvested after a 48-h activation period. Cell viability, as measured by trypan blue exclusion, was not influenced by these treatments.
RNA isolation and qPCR analysis
Total RNA was isolated from the purified T cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To protect against residual DNA contamination, all RNA samples were treated with Turbo DNA-free reagents (Ambion, Austin, TX, USA) as described by the manufacturer. Primers and TaqMan probe sequences used for qPCR have been reported [16, 17]. Relative quantitation for all assays used 18S rRNA as the normalizer.
Antibody production
A polyclonal rabbit antibody against hZIP8 was raised to a peptide (FGNDNFGPQEKT) selected from the full-length sequence [14]. A cysteine residue was added to the N terminus for coupling to the carrier protein and for conjugation to Sulfolink (Pierce, Rockford, IL, USA) for affinity purification. This antibody was prepared in rabbits using methods we have described previously [18].
Confocal laser-scanning microscopy and flow cytometry
After 48 h in culture, the cells were fixed in 2% paraformaldehyde in PBS and incubated with 0.1% Triton X-100 for permeabilization. Immunolabeling was with the anti-hZIP8 polyclonal antibody or goat anti-LAMP1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Secondary labeling was performed with Alexa Flour 594, conjugated to anti-rabbit IgG and Alexa Flour 488 (Invitrogen), conjugated to anti-goat IgG, respectively. Nonactivated cells were also incubated with LysoTracker (Invitrogen) and the zinc-specific fluorophore, FluoZin-3 (Invitrogen), to examine labile zinc localization. Imaging was with a Leica TCS SP5 laser-scanning confocal microscope with LAS-AF imaging software, using a 40× oil objective. For detection of labile zinc by flow cytometry, cells were incubated with FluoZin-3 for 30 min. After an additional 30-min incubation in serum-free media without fluorophore, cells were washed, and fluorescence was measured. This was followed by addition of 100 μM ZnCl2 for 90 s, and fluoresence was measured again.
SDS-PAGE and Western analysis
Membrane proteins were isolated using a specialized protein extraction method (BioVision, Mountain View, CA, USA) prior to PAGE. To confirm equivalent loading, protein bands were visualized by Ponceau staining after transfer to nitrocellulose membranes. Alternatively, total cell lysates were analyzed. For these preparations, cells were placed in a buffer containing 1% Triton X-100, 50 μM Tris HCl (pH 7.4), and protease inhibitor (Sigma Chemical Co.) and sonicated for 10 s. Actin (Sigma Chemical Co.) was used as the loading control. Western blots were produced as described previously [18] with the affinity-purified rabbit anti-hZIP8 antibody above. Immunoreactivity was visualized by chemiluminescence (Pierce) and X- ray film. Band intensities for upper and lower bands of ZIP8 and loading controls were quantified by using GenTools (SynGene, UK) software.
Perforin and cytokine assays and TCR signaling analysis
T cells were removed by centrifugation, and the perforin concentration of the medium was measured using a sandwich ELISA (Diaclone, Bensancon, France). IFN-γ secretion into the medium was measured with Beadlyte human 26-plex multicytokine detection reagents (Millipore, Bedford, MA, USA). Fluorescence measurements using a Luminex 200 system were normalized for total input protein in the medium. Cells were lysed with Beadlyte universal buffer (Millipore). Similarly, the relative abundance of phospho-CD3 (phosphotyrosine), phospho-Lck, phospho-ZAP70, phospho-LAT, and phospho-CREB in cell lysates was assessed with the Beadlyte phosphoprotein detection system (Millipore). Fluorescence measurements using a Luminex 200 system were normalized for total input protein in total cell lysate and expressed as relative MFI.
Phosphatase activity of CN was measured at the cellular level. The cell lysates were obtained by ultracentrifugation (150,000 g) and desalted with resin columns. In the CN activity assay, calmodulin and the cell lysate were incubated with zinc or the CN inhibitor FK506 (A.G. Scientific, San Diego, CA, USA) for 10 min at 30°C. The reaction was initiated by addition of substrate and terminated by addition of malachite green. Phosphate release from the substrate was measured spectrometrically (Calbiochem, San Diego, CA, USA).
Statistical analysis
Data are expressed as means ± sd and were analyzed by one-way or two-way ANOVA (as appropriate) followed by the Student-Newman-Keuls multiple comparisons test or as otherwise indicated in figure legends. Student’s t-test (two-tailed) was used for analysis between two groups. Minimal statistical significance was set at P < 0.05. Data are representative of at least three independent experiments or as otherwise indicated in figure legends.
RESULTS
Zinc influences T cell activation in human subjects
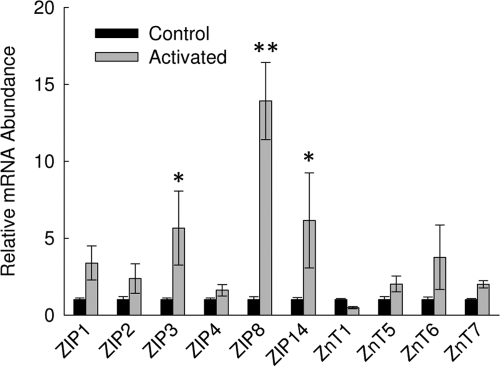
Activation of primary T cells from the human subjects produced increases in IFN-γ transcripts (Table 1). Activation responses were enhanced significantly when an oral zinc supplement was provided to the subjects from whom the cells were isolated. Using qPCR, relative transcript levels for zinc transporters in RNA from these cells were evaluated. ZIP8 mRNA was the most up-regulated upon activation (Fig. 1). Although numerous zinc transporters are expressed in these cells, in addition to ZIP8, only ZIP3 and ZIP14 transcripts were elevated significantly upon activation. Overall, the data show that in human subjects, T cell ZIP8 is highly up-regulated during immune activation, and activation is potentiated by zinc consumption. These data provided a focus for the following studies.
TABLE 1.
Influence of Supplemental Zinc on Activation of T Cells from Human Subjects
| Activation | Zinc | IFN-γ mRNA |
|---|---|---|
| – | – | 1 ± 0.05a |
| – | + | 1.3 ± 0.4a |
| + | – | 107 ± 16b |
| + | + | 197 ± 63c |
Cells were obtained from subjects who were given zinc (15 mg/day) or a placebo for 4 days. The cells were activated for 2 days in vitro. Data are expressed as means ± sd, n = 3, and were analyzed by two-way ANOVA. Values with a different superscript are statistically different (P<0.05−P<0.001).
Figure 1.
Activation of T cells changes zinc transporter expression. Relative ZnT and ZIP transcript levels in total RNA from activated and nonactivated primary human T cells were measured by qPCR. Data are expressed as relative to the nonactivated control and are normalized to 18S rRNA. Representative data are presented. Values shown are means ± sd (n=4). *, P < 0.01; **, P < 0.001, compared with nonactivated control.
T cell activation increases ZIP8 expression
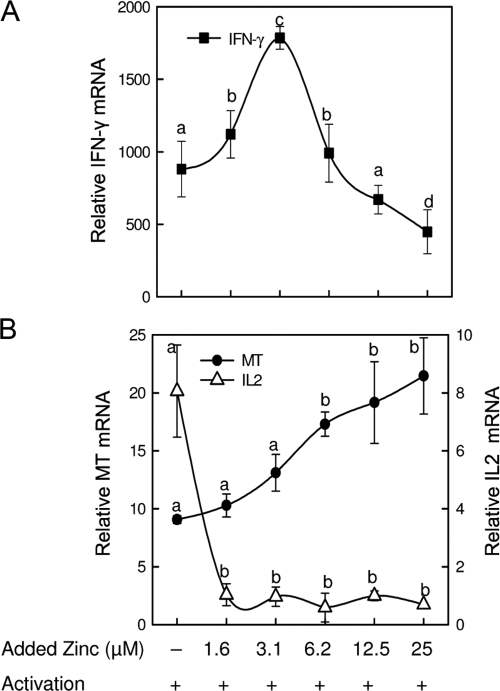
An increase in expression of a zinc transporter in an immune cell upon activation suggests zinc dependency for some activation-related process. As shown in Figure 2, activated T cells are sensitive to zinc added in vitro. Expression of IFN-γ mRNA was biphasic with a maximum at 3.1 μM zinc (Fig. 2A). For comparative purposes, we also examined the zinc responsiveness of MT, a gene that is not a specific marker for activation but is an indicator of cellular zinc status. Particularly relevant is that MT mRNA increased as a progressive trend, significant only when the zinc concentration of the medium was 6.2 μM or above (Fig. 2B). That extracellular zinc concentration suggests it yields an intracellular zinc concentration-threshold necessary for activation of the MTF-1. Also relevant is the extreme sensitivity of IL-2 mRNA levels to extracellular zinc (Fig. 2B). This observation suggests that a pathway leading to IL-2 expression is inhibited strongly by zinc. That IFN-γ mRNA is maximal at 3.1 μM suggests that expression of the gene is sensitive to low but restricted concentrations of extracellular zinc and less than that required for MTF-1 activation.
Figure 2.
In vitro zinc addition influences the effect of activation on IFN-γ, MT, and IL-2 expression in primary T cells. Relative mRNA levels of IFN-γ (A) and MT and IL-2 (B) were measured by qPCR in nonactivated and activated cells. The cells were activated after an initial 2-h incubation with the indicated concentration of zinc. Data are normalized to 18S rRNA and are expressed as relative to the nonactivated control that was adjusted to one. Representative data from three independent experiments are presented. Values shown are means ± sd (n=3–4). A different superscript indicates the means are statistically different (P < 0.05–0.001).
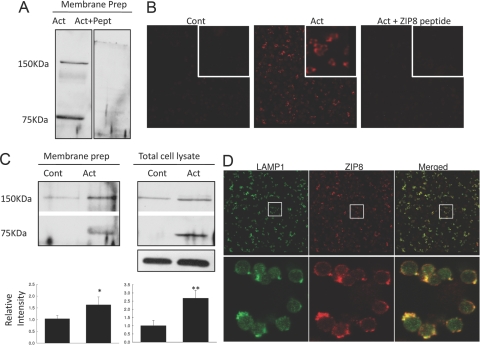
To investigate changes in ZIP8 protein in response to activation, we used Western blot analysis with the polyclonal hZIP8 antibody described above. There were two bands, ∼75 and ∼150 KDa, detected by SDS-PAGE upon activation. To test specificity, the ZIP8 antibody was preincubated with the peptide antigen, and as shown in Figure 3A, both bands were eliminated. PNGase treatment was used to test if ZIP8 protein was glycosylated. We did not observe any reduction in either band with this treatment (data not shown). These two bands were also detected by native gel electrophoresis (data not shown). These data may suggest collectively that in activated T cells, ZIP8 may exist in two forms: a monomer and a dimer. The increase in intensity of both bands in total membrane and total cell lysate preparations is shown in Figure 3B. Ponceau staining was used as the loading control for membrane preparations. Actin was used as a loading control for total cell lysate, and the bar graphs represent the quantification of both ZIP8 band intensities of the two bands after normalization to actin. Densitometry revealed there was a significant increase in ZIP8 protein produced upon T cell activation. In agreement with the Western blot analysis, activation increased ZIP8 abundance (red) markedly, as visualized by confocal microscopy (Fig. 3C). Preincubation of the antibody with the ZIP8 peptide antigen caused the disappearance of ZIP8 fluorescence intensity (Fig. 3C). These data show collectively that activation of T cells increases ZIP8 synthesis.
Figure 3.
ZIP8 protein is up-regulated in response to primary T cell activation and localizes with lysosomes. Western blot analysis (A and B) and confocal images (C and D) from primary T cells. Total membrane proteins and total cell lysates were isolated and used for SDS-PAGE. Blots were probed with an affinity-purified polyclonal hZIP8 antibody. (A) Blots were probed with affinity-purified, polyclonal hZIP8, directly or after preincubation with its peptide antigen (Pept). Act, Activated cell. (B) Representative Western blots from three independent experiments for membrane proteins and total cell lysates. The average values of both ZIP8 band intensities from three independent experiments are shown. Values shown are means ± sd (n=6); *, P < 0.02: **, P < 0.001, compared with nonactivated control (Cont). (C) Immunofluororescence localization of ZIP8 (red) by laser-scanning confocal microscopy. Nonactivated and activated primary T cells probed with the ZIP8 antibody. The area in white boxes represents magnified images. (D) Immunofluororescence localization of ZIP8 (red) and LAMP1 (green), a lysosome-specific membrane marker, in activated T cells by laser-scanning confocal microscopy. The area that was chosen for enlarged images was indicated by white squares. Yellow indicates the extent of colocalization.
It has been reported previously that hZIP8 localizes to lysosomes in ZIP8-transfected human embryo kidney cells [14]. To investigate ZIP8 localization in activated human primary T cells, we used the same polyclonal ZIP8 antibody (red) as above and an antibody against a lysosome-specific membrane marker, LAMP1 (green). Confocal images showed a colocalization between ZIP8 and LAMP1 (Fig. 3D). The localization of LAMP1 to the lysosome shown here is similar to that reported by Shen et al. [7]. This localization target (lysosome) is supported by the images presented in Figure 4C using the lysosomal probe, LysoTracker. Of particular note is the prevalence of yellow when the individual images are merged (Fig. 3D). Colocalization of ZIP8 and LAMP1 upon activation suggests that ZIP8 might function to transport zinc from lysosomal vesicles to the cytoplasm.
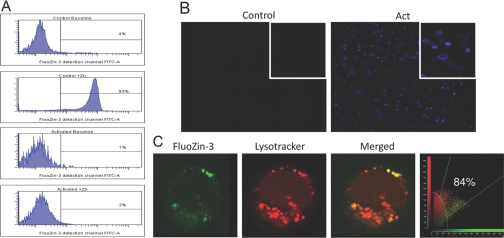
Figure 4.
Decrease in intracellular-free zinc in activated primary T cells. FluoZin-3 fluoresence was used to assess labile zinc changes in nonactivated and activated cells. Flow cytometry experiments were conducted in the absence or presence of 100 μM ZnCl2, which was added for 1.5 min (A). Fluorescence intensity (green) was examined by flow cytometry. T cells were activated for 48 h and probed with MT antibody (blue). Images from nonactivated and activated cells were obtained by laser-scanning confocal microscopy. (B) The area in white boxes represents magnified images. Confocal microscopy was used to visualize localization of FluoZin-3 and lysosomes (C). After initial incubation with FluoZin-3, nonactivated T cells were probed with LysoTracker (red), and colocalization (yellow) with free zinc was shown in merged images.
To investigate labile zinc transitions during activation, we used the highly zinc-specific fluorophore FluoZin-3. The cells were placed in serum-free medium containing FluoZin-3 for 30 min and then in the medium without the fluorophore for an additional 30 min. Flow cytometry was used to monitor fluorescence intensity produced when FluoZin-3 interacts with labile zinc (Fig. 4A). Note that in the upper two panels, only background fluorescence (0–4%) is produced with control and activated cells. The lower two panels show fluorescence 90 s after 100 μM zinc is added in vitro to the same cells. This in vitro addition of zinc is necessary to provide an influx of zinc ions required to bind to FluoZin-3 and produce fluorescence. Of note is the significant fluorescence produced by the control cells. This is shown through the amount of fluorescence (93%) appearing in the FITC channel. Virtually no fluorescence is found with activated cells. We propose that this differential occurs, as control cells have low levels of ZIP8 and do not transport labile zinc from lysosomes. In contrast, activated cells have high ZIP8 abundance and export zinc rapidly to the cytoplasm. In Figures 2B and 4B, we have shown that there is a robust production of MT in T cells upon activation. Competition studies have shown that MT has an apparent stability constant (4×1011 M–1) for binding zinc at pH 7.4, which is 10,000-fold higher than FluoZin-3 (7.1×107 M–1) [19]. Therefore, most likely, the transported zinc produces only a transient increase in cytoplasmic zinc concentrations and is bound to MT rapidly, which quenches the ability of zinc ions to interact with FluoZin-3. Nevertheless, it is important to point out that MT has binding sites for seven atoms of zinc/molecule. Here, we have presented the average apparent stability constant of MT for all zinc-binding sites. However, it should be taken into consideration that ions bind to MT (particularly to β cluster) in a manner that allows them to be physiologically (kinetically) active and potentially able to alter a biochemical process [20].
Furthermore, we used FluoZin-3 and LysoTracker, a lysosomal probe for confocal microscopy, to investigate specific localization of labile zinc in T cells (Fig. 4C). Nonactivated T cells were used in these experiments for detectable zinc-related fluorescence, as activation caused disappearance of the FluoZin-3 fluoresence signal (as above). Merged confocal images showed an 84% colocalization between FluoZin-3 and LysoTracker. The quantitative colocalization was calculated by imaging software. These data obtained by flow cytometry and confocal microscopies suggest collectively that up-regulated ZIP8 during activation is responsible for the reduction of lysosomal labile zinc.
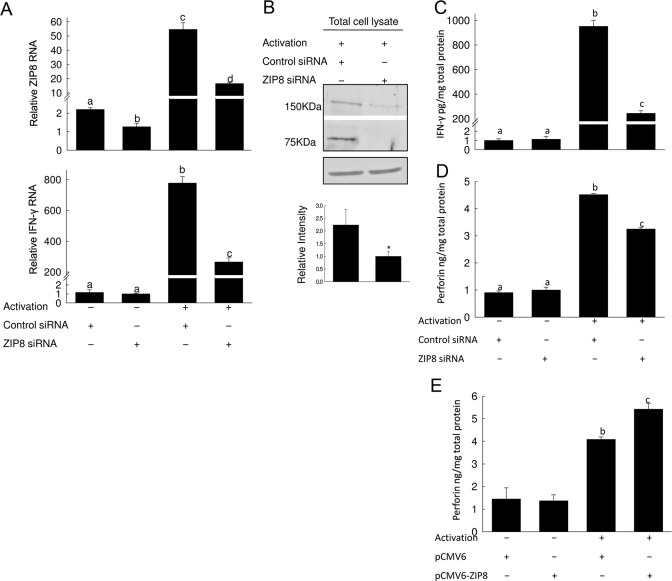
ZIP8 knockdown decreases T cell activation
To test whether translocation of lysosomal zinc through the ZIP8 transporter would affect T cell activation, we knock down ZIP8 with siRNA. We verified knockdown of ZIP8 by qPCR and Western analysis. As shown in Figure 5A, ZIP8 siRNA significantly reduced ZIP8 mRNA levels ∼60% in nonactivated cells. Upon activation, the 25-fold increase in ZIP8 mRNA was decreased 70% with ZIP8 siRNA. Western analysis showed a 55% reduction in ZIP8 upon siRNA transfection (Fig. 5B). Both ZIP8 protein bands showed a comparable reduction. The ZIP8 siRNA did not produce knockdown of ZIP3 or ZIP14 mRNA (data not shown).
Figure 5.
ZIP8 knockdown with siRNA decreases IFN-γ and perforin production in activated T cells. After transfection with ZIP8 siRNA or nontargeting random siRNA for 16 h, the T cells were activated for 48 h. Transcript levels of ZIP8 (A) and IFN-γ (C) were measured by qPCR. Representative data from three independent experiments are expressed as relative to nonactivated control and normalized to 18S rRNA. Values shown are means ± sd (n=3). (B) Representative Western blot of total cell lysates from siRNA-transfected, activated T cells. The average values of both ZIP8 band intensities from three independent experiments are shown. Values shown are means ± sd (n=6). (D) Secretion of IFN-γ into the medium was measured by Luminex multicytokine detection. Representative data are expressed as relative to control and were normalized to total protein in the medium. (E and F) Secretion of perforin into the medium was measured by ELISA and normalized to total protein in the medium. Representative data from three independent experiments are shown for measurement of secreted perforin with ZIP8 knockdown (E) or overexpression (F) conditions. Values shown are means ± sd (n=3) in nonactivated cells compared with activated cells and transfected with ZIP8 siRNA or pCMV6-ZIP8. (A, C–F) Bars with a different superscript indicate the means are statistically different (P<0.001–0.003).
We used enhanced expression of IFN-γ mRNA upon T cell activation to examine the effect of ZIP8 knockdown on activation. Knockdown caused a 66% decrease in IFN-γ mRNA in activated cells, and the basal expression level was not affected in nonactivated cells (Fig. 5C). Similarly, secretion of IFN-γ protein into the culture medium was also diminished significantly (74%) in activated cells by knockdown of ZIP8 (Fig. 5D). To further establish the relationship of T cell activation, lysosomal zinc export, and ZIP8, we measured secretion of perforin. The specific interest in this protein was because its secretion from lysosomes is a characteristic of activated T cells. Importantly, it has been shown that zinc inhibits pore formation [13], leading us to hypothesize that reduction in lysosomal labile zinc might be necessary for optimum lysosomal secretion in T cells during activation. As is shown in Figure 5E, siRNA knockdown of ZIP8 caused a 30% reduction in perforin secretion by activated cells. By contrast, in companion experiments, overexpression of ZIP8 caused a 32% increase in perforin secretion in activated T cells (Fig. 5F). In Figure 5, D–F, the IFN-γ and perforin data are presented on a per mg protein in medium basis to normalize for differences in cell proliferation and/or changes in secretory patterns. Collectively, these data show that ZIP8 is necessary for potentiating IFN-γ production and perforin secretion. These results provide further evidence that a reduction of labile zinc in lysosomes, as shown by FluoZin-3 fluorescence, through up-regulation or overexpression of ZIP8 in T cells, enhances the secretion of perforin, and inhibition of ZIP8 causes a reduction in secretion.
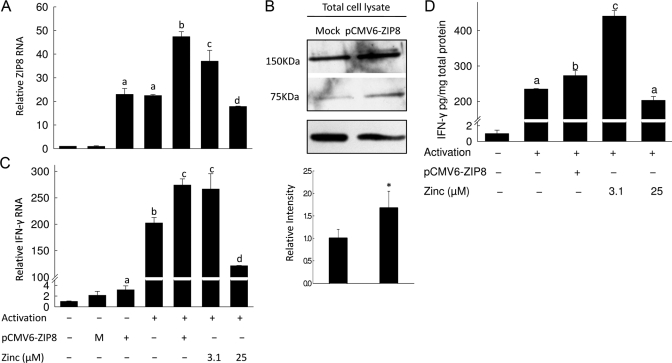
ZIP8 and zinc stimulate T cell activation
ZIP8 is up-regulated during T cell activation (Figs. 1 and 2B). To simulate high expression of ZIP8, we transiently transfected T cells with the ZIP8 plasmid as above (Fig. 5F). There was a 20-fold increase in ZIP8 mRNA and 67% increase in ZIP8 protein levels 48 h after transfection (Fig. 6, A and B). Cells that were transfected and then activated had the highest expression of ZIP8. To test the effect of ZIP8 overexpression on activation, we measured IFN-γ mRNA and protein level (Fig. 6, C and D). The increase of IFN-γ mRNA and protein produced by activation was amplified further when the cells were transfected with ZIP8 cDNA. This shows a direct influence of ZIP8 on activation. In the same experimental setting, we also included low and high zinc conditions to investigate the possibility that ZIP8 is influencing IFN-γ production through a transient alteration of cytoplasmic zinc concentrations. Addition of 3.1 μM zinc enhanced IFN-γ expression to the same extent as ZIP8 overexpression, and 25 μM zinc diminished the enhanced expression of IFN-γ (Fig. 6, C and D). We have also analyzed expression of human MT mRNA by qPCR in ZIP8-overexpressing cells. We found a 3.6-fold increase (data not shown). That finding confirms that overexpression produces increased cytosolic zinc in an amount sufficient to initiate MT synthesis. Overall, the data from Figure 6 show that transfection with ZIP8 yields the same level of enhancement in IFN-γ expression as is achieved through in vitro addition of 3.1 μM zinc during T cell activation (Fig. 2). These data emphasize collectively that a small increase in cytoplasmic zinc provided through the translocation of zinc from lysosome by ZIP8 contributes to enhanced expression of IFN-γ.
Figure 6.
Overexpression of ZIP8 enhances T cell activation. T cells were activated for 48 h after transfection with pCMV6-ZIP8 or empty vector for 2 h. Transcript levels of ZIP8 (A) and IFN-γ (C) were measured by qPCR. Representative data from three independent experiments are expressed as relative to control and normalized by 18S rRNA. Values shown are means ± sd (n=3); P < 0.01–0.001 compared with ZIP8-transfected, nonactivated cells, indicated by a–d. M, Mock transfection. (B) Representative Western blot of total cell lysates from ZIP8-transfected T cells. The average values of both ZIP8 band intensities from three independent experiments are shown. Values shown are means ± sd (n=6); *, P < 0.001, compared with control. (D) Secretion of IFN-γ into the medium was measured by Luminex multicytokine detection. Representative data are expressed as relative to nontransfected cells and normalized by total protein in the medium. Values shown are means ± sd (n=3); Bars with different superscripts indicate the means are statistically different (P < 0.01–0.001).
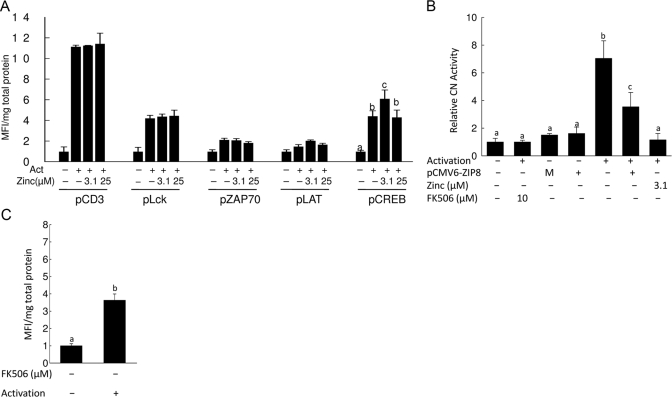
Zinc acts on CREB via CN
We investigated which signaling pathway influencing IFN-γ production in response to TCR activation could be zinc-dependent. CD3, Lck, Zap70, LAT, and CREB proteins were phoshorylated in response to TCR activation for 48 h (Fig. 7A). Of note is that in contrast to Western analysis, which measures the extent of phosphorylation in one time-point, the Luminex-based assay used in these experiments provides a cumulative measure of phosphorylation, as the beads containing the specific antibodies are present throughout the 48 h of incubation. The data are normalized for total protein in each sample. Of note, only CREB phosphorylation was affected by in vitro addition of zinc. Although the preincubation of activated cells with 3.1 μM zinc increased CREB phosphorylation significantly, it was diminished by 25 μM zinc and returned to the basal level. It has been shown in many cell types that the Ca/calmodulin-regulated phosphatase CN dephosphorylates CREB [10]. Zinc also inhibits CN phosphatase activity [11, 12]. To test for a concentration-dependent effect of zinc on CN, we used an in vitro CN assay that measures phosphatase activity (data not shown). The results showed that CN activity was inhibited by zinc in a concentration-dependent manner. At the zinc concentration 3.1 μM, CN activity was inhibited (90%). Further evidence that zinc influences CN activity specifically in T cells was obtained by using a cellular CN activity assay (Fig. 7B). The results showed that CN activity was increased upon activation. When the cells were treated with 10 μM FK506 or 3.1 μM zinc along with activation, CN activity was inhibited (Fig. 7B). We also measured CN activity at 25 μM zinc, and the inhibition was the same as that observed with 3.1 μM zinc (data not shown). To test the effect of ZIP8 on CN activity, we overexpressed ZIP8 in T cells along with activation. Compared with the activated T cells, those overexpressing ZIP8 showed decreased CN activity (Fig. 7B). These results suggest that ZIP8 influences IFN-γ expression via inhibition of CN through a change in the cytoplasmic zinc concentration. Finally, we used the FK506 inhibitor to test whether CN influences CREB dephosphorylation in T cells by using the Beadlyte phospho-protein detection system. When CN activity was inhibited by FK506, CREB phosphorylation was increased further (Fig. 7C).
Figure 7.
Zinc enhances CREB phosphorylation via inhibiting CN activity. Phosphorylation of indicated proteins in the TCR signaling pathway was measured by Luminex phosphoprotein detection (A and C). T cells were activated for 48 h after an initial 2-h incubation with the indicated concentrations of zinc (A) or CN inhibitor FK506 (C). Representative data from three independent experiments are expressed as relative to control and normalized by total input protein in the cell lysate. Values shown are means ± sd (n=3); P < 0.001 compared with nonactivated cells, indicated by a–c. p, Phospho. (B) Representative data from three independent experiments for cellular CN activity assays are shown. Activity of CN was determined spectrophotometrically based on phosphate release from the substrate. Cell cytosol was obtained from lysates by ultracentrifugation and used for detection of cellular CN activity. Lysate was incubated with the indicated concentration of zinc or CN inhibitor FK506 for 10 min prior to addition of substrate. Values shown are means ± sd (n=3); Bars with different superscripts indicate the means are statistically different (P < 0.01–0.001).
DISCUSSION
ZIP transport proteins are believed to control intracellular zinc concentrations in mammalian cells through transport across the plasma membrane or from intracellular vesicles/organelles [2]. The net result is an increase in zinc in the cytoplasm. It is presumed that zinc transport and altered zinc homeostasis have functional outcomes. Our interest in ZIP8 specifically has been stimulated by the relative abundance of ZIP8 transcripts, as markedly higher in T cells than in monocytes or granulocytes, respectively [16]. Furthermore, zinc supplementation of adult human subjects with 15 mg zinc/day, about the recommended dietary allowance, increased IFN-γ mRNA expression markedly in CD3+ primary human T cells upon activation. The high ZIP8 expression in T cells and the evidence that zinc actually has a measurable response under realistic dietary conditions suggested that T cell activation, a major factor in adaptive immunity, has a zinc-dependent component. These findings led to our hypothesis that ZIP8 has an important role in potentiating T cell activation.
Faber et al. [21] have also shown the effect of zinc on IFN-γ production. When they supplement adult human subjects with 80 mg zinc/day (over five times the concentration we used), IFN-γ production was reduced. The different effects of 80 mg and 15 mg zinc/day supplementation may simulate the biphasic effect of zinc on activation that we have shown in our in vitro experiments. The biphasic effect of zinc on IFN-γ expression (Fig. 2) could be the result of activation/inhibition of different signaling pathways that specifically require different concentrations of cytoplasmic zinc. For example, CREB enhances IFN-γ expression in human cells when it is phosphorylated. Several phosphatases and kinases have been shown to regulate the phosphorylation status of CREB. One of the phosphatases that is responsible for dephosphorylation of CREB is CN [10]. Others have also found that CN enzymatic activity is sensitive to zinc. We have found by using an in vitro CN assay that CN activity was inhibited at concentrations of zinc in the range 0.8–25 μM (data not shown). As shown in Figure 2A, 25 μM in vitro addition of zinc inhibited the expression of IFN-γ, and CN activity was totally inhibited at that concentration of zinc. These observations collectively support that although there are multiple mechanisms that regulate the phosphorylation status of CREB, the influence of zinc is primarily on CN. The potential kinases that are responsible for phosphorylation of CREB are mitogen- and stress-activated kinases, calcium/calmodulin-dependent protein kinase type IV, and PKA [22]. It has been shown that AC is necessary for the PKA activation, and its enzymatic activity is inhibited by zinc in a concentration-dependent manner [23]. Interestingly, 10 μM zinc inhibited total AC activity in neuroblastoma cells, and lower concentrations did not have any effect. This suggests that higher concentrations of cytoplasmic zinc may inhibit IFN-γ expression as a result of inhibition of AC activity and that a modest increase in cytoplasmic zinc may enhance the phosphorylation of CREB as a result of inhibition of CN phosphatase activity.
The finding that ZIP3 and ZIP14 are also increased significantly upon activation may relate to a role in specific T cell functions. ZIP3 has been reported as localized to the plasma membrane of mammary epithelial cells [24]. Knockout of murine ZIP3 causes decreased pre-T cell abundance but only upon restriction in dietary zinc [25]. Both observations concur with a role for ZIP3 in T cell zinc transport. Similarly, we observed that ZIP14 expression and localization to the plasma membrane of murine hepatocytes are IL-6-regulated [26]. That observation suggests that there is a cytokine component to ZIP14 regulation that may contribute to zinc transport differences across the plasma membrane of T cells. We did not find any significant change in ZnT transporters in response to T cell activation. ZnT1 was the only known zinc exporter transporter that localizes to the plasma membrane. However, it has been shown recently by Overbeck et al. [27] that ZnT4 also localizes on the plasma membrane of T cell line Molt-4. This new finding, however, should be evaluated carefully, as the expression pattern of proteins might differ between cell lines and primary cells. In our experience, for example, there was no difference in ZIP8 expression in the Jurkat cell line when we used the same stimulants as used to activate primary T cells. Therefore, a specific role of this transporter in response to TCR-mediated T cell activation in primary T cells needs to be investigated further.
The predicted molecular weight for ZIP8 is 49.6 KDa. Different sizes and localizations for ZIP8 protein have been reported by different groups, however. Initial experiments on ZIP8 were with monocyte/macrophages. Using uncharacterized antibody, the lysosome was identified as the cellular site for ZIP8, and the size of the protein was 52 KDa [14]. No functional significance for ZIP8 was reported; however, Chinese hamster ovary cells transfected with ZIP8 exhibited increased fluorescence from Newport Green, suggesting a role in intracellular zinc accumulation. It has been shown by Besecker et al. [28] that in the human primary lung cells, plasma membrane and mitochondria were the cellular sites for ZIP8. They detected two bands for ZIP8 protein: ∼140 and ∼52 KDa. They also have shown by FluoZin-3 that ZIP8 was responsible for intracellular zinc accumulation in response to TNF-α treatment. Our data using FluoZin-3 show that in primary human T cells, the secretory lysosome is the site for ZIP8-mediated zinc release. The sizes of the ZIP8 protein in these T cells as detected by Western blot analysis were ∼150 and ∼75 KDa. We speculate that the 75-KDa protein is newly synthesized, and the 150-KDa band represents the dimer. It has been shown by Lu and Fu [29] that the Escherichia coli YiiP zinc transporter, which belongs to the cation diffusion facilitator transporter family that is homologous to the eukaryotic ZnT zinc transporter family, forms a dimer in the presence of zinc ions. Therefore, we extend our speculation that ZIP8 might be functional in a dimerized form as is the YiiP zinc transporter. In companion experiments, an antibody was produced to mouse ZIP8 by the same approach as that used for the antibody to hZIP8 used in the present experiments [30]. Of note is that mouse ZIP8 from RBC membranes had an apparent size of 60 KDa, and ZIP8 from erythropoietin-induced differentiating erythroid cells had two bands: 120 KDa (major) and 60 KDa (minor). This agrees with our interpretation. The specificity of our hZIP8 antibody is based on the peptide competition shown (Fig. 3A) and complete elimination of both ZIP8 bands in ZIP8 siRNA-treated cells (Fig. 5B). In contrast, in Maden-Darby canine kidney cells, transfected with mouse ZIP8, the transporter was localized to the plasma membrane [31]. These differences in localization may reflect the markedly different function in the cell systems under investigation or atypical localization related to transfection.
Numerous signaling pathways have been reported as being zinc-responsive. It has been proposed that involvement of zinc in intracellular signaling can be classified into two categories: The first is the early zinc signaling pathway, where zinc is provided by intracellular sources, and the second is late zinc signaling, which is dependent on a transcriptional change in zinc transporter expression [32]. The example of the early zinc-signaling pathway could be CD8 or CD4 interaction with Lck in TCR-mediated T cell activation [33, 34]. Zinc provides the interaction with cysteine-containing motifs for assembly of the TCR to the T cell signaling protein Lck. This interaction is the first step in TCR-mediated T cell activation. We have found that there is no change in zinc transporter expression early in the activation process (first 24 h), suggesting that zinc is provided from intracellular sources (data not shown). It was proposed that MT, interacting with oxidizing intracellular conditions, is the source of the zinc ions needed for the Lck/TCR assembly process [34]. Previously, we have discussed the linkage of zinc to cellular signaling and interaction with specific intracellular and physiologically regulated ligands such as MT [35]. Expression of Lck has been shown to up-regulate in zinc-depleted mice [36, 37]. This could represent an attempt to compensate for the lack of zinc binding through overproduction of one of the binding ligands, i.e., Lck.
Downstream of the Lck/TCR interaction, there are many signaling pathways that are activated in T cells (reviewed in ref. [38]). One of the important signaling events is activation of the phospholipase Cγ and subsequent hydrolysis of phosphatidylinositol 4,5-bisphosphate. to generate inositol triphosphate, which causes mobilization of calcium. Increased intracellular Ca2+ will cause activation of CN. A well-known target for CN phosphatase is the NFAT transcription factor. Dephosphorylated NFAT is necessary for IL-2 expression. However, our results (Fig. 7) suggest that CN is also responsible for CREB dephosphorylation. CREB is necessary for IFN-γ expression but in the phosphorylated form. Our specific interest in CN is because of the well-known inhibitory effect of zinc on its phosphatase activity [39]. As we have shown in Figure 2B, IL-2 expression is abolished with even 1.6 μM zinc added to the T cell cultures, and IFN-γ expression is enhanced (Fig. 2A), suggesting that at 48 h of activation, zinc has inhibited CN activity. In the following experiments (Fig. 3), we confirmed that ZIP8 is responsible for an intracellular zinc increase during T cell activation. Therefore, our results suggest that a zinc-dependent process, driven by ZIP8-mediated zinc transport, is responsible for events that culminate in enhanced CREB phosphorylation and increased IFN-γ expression. As the increase in cytoplasmic zinc concentration is caused by a change in a zinc transporter, this effect can be considered as an example of a late zinc signaling pathway.
It has been shown by Haase et al. [40] that intracellular zinc increased immediately after addition of PMA to Jurkat T cells. FluoZin-3 fluorescence was used to monitor immediate mobilization of labile zinc in response to PMA. Therefore, it may serve as another example for the early zinc signaling [32]. In our work, however, we have chosen to use confocal microscopy and flow cytometry to measure fluorescence, as the focus was to detect specific localization and function of ZIP8 and labile zinc in T cells at 48 h of activation. It has been shown by Muylle et al. [41] that laser-scanning confocal microscopy provides sufficient spatial resolution to uncover subcellular fluorescence patterns. Similar to their data from a fish hepatocyte cell line, our confocal microscopy results with FluoZin-3 clearly showed that fluorescence produced by labile zinc mostly resides in lysosomal compartments in nonactivated T cells. Our flow cytometry data revealed that there was no detectable fluorescence signal in activated T cells at 48 h, as zinc has been translocated to cytoplasm by ZIP8 and no longer available for FluoZin-3 interaction. As MT was already high in cytoplasm in response to activation, zinc was likely sequestered by MT and other proteins, as at intracellular redox and pH conditions, protein binding is preferred compared with the free zinc transition process, where the metal can react with the FluoZin-3 fluorophore.
The mobilization of zinc to a labile pool within immune cells has been the subject of much interest and has been well reviewed [3, 4, 42]. Many such experiments are performed with high >25 μM zinc concentrations with frequently variable results. For intramolecular Zn2+ exchanges to occur, oxidizing conditions must be present. The data presented here demonstrate that ZIP8, associated with the lysosomal membrane, may provide the conditions of lower than physiologic pH necessary for zinc transfer to ligands with regulatory influence. It has been estimated that the labile zinc concentration in unstimulated lymphocytes is 0.35 μM [43]. The effect we found with 3 μM zinc added in vitro, as a stimulating influence on activation, is within what would be expected with such a basal intracellular zinc concentration. Of particular relevance to the stimulating effect of zinc found in the present report is that 3 μM is the exact zinc concentration that Huang et al. [11] found recently to produce maximal inhibition of CN activity. Also relevant to the in vitro effects of zinc is that recently, tyrosine phosphorylation in monocytes is sensitive to low μM concentrations of zinc, which regulate LPS-induced signal transduction [40]. These effects have yet to be related to a specific zinc transporter.
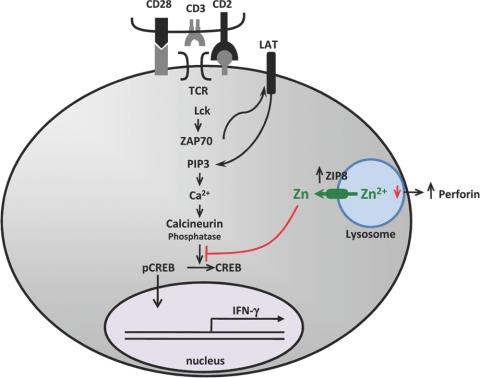
In conclusion, our results demonstrate that ZIP8 is highly expressed in human T cells. ZIP8 expression is up-regulated markedly upon activation. Zinc supplementation of human subjects enhances subsequent T cell activation in vitro and enhances ZIP8 expression. Knockdown of ZIP8 expression in primary human T cells using siRNA decreased IFN-γ and perforin secretion, both signatures of activation. As we propose with our model (Fig. 8), ZIP8 localization to the lysosome suggests that this transporter potentiates cell activation through intracellular zinc translocation. Overexpression of ZIP8 by transient transfection causes enhanced IFN-γ expression and perforin secretion. The lytic properties of T cells have been related to lysosomal development during activation [7]. The mildly increased cytoplasmic zinc produced by ZIP8 inhibits CN phosphatase activity, resulting in increased CREB phosphorylation and CREB binding to the IFN-γ promoter, which simulates IFN-γ synthesis. Although the function of ZIP8 was the focus of the present experiments, the mechanism responsible for up-regulation of ZIP8 upon activation has not been examined. In that regard, it is potentially relevant that hZIP8 has a CREB response element within the first 2000 bp upstream from the start site.
Figure 8.
Antigen presentation activates T cells via signaling pathways that increase IFN-γ production. Zinc potentiates this response through increased production of ZIP8 that exports lysosomal zinc, which increases cytoplasmic zinc, inhibits CN, and leads to increased IFN-γ expression via increased CREB phosphorylation. PIP3, Phosphatidylinositol (3,4,5)trisphosphate.
Our findings may also point to the wider involvement of zinc in signaling that influences T cell development and cell-specific immune functions, particularly those in children and the elderly [44,45,46,47,48]. The mechanism proposed in this report may be related to the success that global zinc supplementation initiatives have had in reducing morbidity and mortality as a result of infectious disease in under-nourished populations [3, 45, 47].
Acknowledgments
This research was supported by National Institutes of Health grant DK 31127 and Boston Family Endowment Funds. We also acknowledge support of the Interdisciplinary Center for Biotechnology Research and funds from the Bankhead-Coley Cancer Research Program. We thank Mitchell D. Knutson, Fikret Aydemir, and Shou-Mei Chang for helpful contributions.
Footnotes
Abbreviations: AC=adenylate cyclase, CN=calcineurin, hZIP8=human ZIP8, LAMP1=lysosome-associated membrane protein, LAT=latency-associated transcript, MFI=mean fluorescence intensity, MT=metallothionein, MTF-1=metal-regulatory transcription factor 1, PKA=protein kinase A, qPCR=quantitative PCR, siRNA=small interfering RNA, TCR=T cell receptor
References
- Liuzzi J P, Cousins R J. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- Eide D J. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–585. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- Beck F, Prasad A, Kaplan J, Fitzgerald J, Brewer G. Changes in cytokine production and T cell subpopulations in experimentally induced zinc deficient humans. Am J Physiol. 1997;272:E1002–E1007. doi: 10.1152/ajpendo.1997.272.6.E1002. [DOI] [PubMed] [Google Scholar]
- Shi H N, Scott M E, Stevenson M M, Goski K G. Energy restriction and zinc deficiency impair the functions of murine T cells and antigen-presenting cells during gastrointestinal nematode infection. J Nutr. 1998;128:20–27. doi: 10.1093/jn/128.1.20. [DOI] [PubMed] [Google Scholar]
- Shen D T, Ma J S Y, Mather J, Vukmanovic S, Radoja S. Activation of primary T lymphocytes results in lysosome development and polarized granule exocytosis in CD4+ and CD8+ subsets, whereas expression of lytic molecules confers cytotoxicity to CD8+ T cells. J Leukoc Biol. 2006;80:827–837. doi: 10.1189/jlb.0603298. [DOI] [PubMed] [Google Scholar]
- Samten B, Howard S T, Weis S E, Wu S, Shams H, Townsend J C, Safi H, Barnes P F. Cyclic AMP response element-binding protein positively regulates production of IFN-γ by T cells in response to a microbial pathogen. J Immunol. 2005;174:6357–6363. doi: 10.4049/jimmunol.174.10.6357. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Siemann G, Blume R, Grapentin D, Oetjen E, Schwaninger M, Knepel W. Inhibition of cyclic AMP response element-binding protein/cyclic AMP response element-mediated transcription by the immunosuppresive drugs cyclosporine A and FK506 depends on the promoter context. Mol Pharmacol. 1999;55:1094–1100. doi: 10.1124/mol.55.6.1094. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang D, Xing W, Ma X, Yin Y, Wei Q, Li G. An approach to assay calcineurin activity and the inhibitory effect of zinc ion. Anal Biochem. 2008;375:385–387. doi: 10.1016/j.ab.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Akaishi E, Hosaka K, Okamura S, Kubohara Y. Zinc ions suppress mitogen-activated interleukin-2 production in Jurkat cells. Biochem Biophys Res Commun. 2005;335:162–167. doi: 10.1016/j.bbrc.2005.07.059. [DOI] [PubMed] [Google Scholar]
- Bashford C L, Menestrina G, Henkart P A, Pasternak C A. Cell damage by cytolysin. Spontaneous recovery and reversible inhibition by divalent cations. J Immunol. 1988;141:3965–3974. [PubMed] [Google Scholar]
- Begum N A, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics. 2002;80:630–645. doi: 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- Calvano S E, Xiao W, Richards D R, Felciano R M, Baker H V, Cho R J, Chen R O, Brownstein B H, Cobb J P, Tschoeke S K, Miller-Graziano C, Moldawer L L, Mindrinos M N, Davis R W, Tompkins R G, Lowry S F, Inflamm and Host Response to Injury Large Scale Collab. Res. Program A network-based analysis of systemic inflammation in human. Nature. 2005;437:1032–1038. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Aydemir T B, Blanchard R K, Cousins R J. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci USA. 2006;103:1699–1704. doi: 10.1073/pnas.0510407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R J, Blanchard R K, Popp M P, Liu L, Cao J, Moore J B, Green C L. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci USA. 2003;100:6952–6957. doi: 10.1073/pnas.0732111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi J P, Bobo J A, Lichten L A, Samuelson D A, Cousins R J. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis for zinc homeostasis. Proc Natl Acad Sci USA. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel A, Maret W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J Am Chem Soc. 2007;129:10911–10921. doi: 10.1021/ja071979s. [DOI] [PubMed] [Google Scholar]
- Zangger K, Oz G, Haslinger E, Kunert O, Armitage I M. Nitric oxide selectively releases metals from the amino-terminal domain of metallothioneins: potential role at inflammatory sites. FASEB J. 2001;15:1303–1305. doi: 10.1096/fj.00-0641fje. [DOI] [PubMed] [Google Scholar]
- Faber C, Gabriel P, Ibs K-H, Rink L. Zinc in pharmacological doses suppresses allogenic reaction without affecting the antigenic response. Bone Marrow Transplant. 2004;33:1241–1246. doi: 10.1038/sj.bmt.1704509. [DOI] [PubMed] [Google Scholar]
- Kaiser M, Wiggin G R, Lightfoot K, Simon J, Arthur C, Macdonald A. MSK regulate TCR-induced CREB phosphorylation but not immediate early gene transcription. Eur J Immunol. 2007;37:2583–2595. doi: 10.1002/eji.200636606. [DOI] [PubMed] [Google Scholar]
- Klein C, Sunahara R K, Hudson T Y, Heyduk T, Howlett A C. Zinc inhibition of cAMP signaling. J Biol Chem. 2002;277:16189–16201. doi: 10.1074/jbc.M108808200. [DOI] [PubMed] [Google Scholar]
- Kelleher S L, Lönnerdal B. Zip3 plays a major role in zinc uptake into mammary epithelial cells and is regulated by prolactin. Am J Physiol Cell Physiol. 2005;288:C1042–C1047. doi: 10.1152/ajpcell.00471.2004. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Huang Z L, Geiser J, Xu W, Andrews G K. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Mol Cell Biol. 2005;25:5607–5615. doi: 10.1128/MCB.25.13.5607-5615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi J P, Lichten L, Rivera S, Blanchard R K, Beker Aydemir T, Knutson M, Gantz T, Cousins R J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeck S, Uciechowski P, Ackland M L, Ford D, Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to Znt-9. J Leukoc Biol. 2008;83:368–380. doi: 10.1189/jlb.0307148. [DOI] [PubMed] [Google Scholar]
- Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell D L. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1127–L1136. doi: 10.1152/ajplung.00057.2008. [DOI] [PubMed] [Google Scholar]
- Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- Ryu M S, Lichten L A, Liuzzi J P, Cousins R J. Zinc transporters ZnT1 (Slc30a1), Zip8 (Slc39a8), and Zip10 (Slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J Nutr. 2008;138:2076–2083. doi: 10.3945/jn.108.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton T P, Reed J, Li H, Soleimani M, Nebert D W. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosak i T, Yamashita S, Tokunaga M, Nishida K, Hirano T. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Eck M J, Harrison S C. A Zn2+ ion links the cytoplasmic tail of CD4 and the N-terminal region of Lck. J Biol Chem. 1998;273:18729–18733. doi: 10.1074/jbc.273.30.18729. [DOI] [PubMed] [Google Scholar]
- Lin R S, Rodriguez C, Veillette A, Lodish H F. Zinc is essential for binding of p56lck to CD4 and CD8α. J Biol Chem. 1998;273:32878–32882. doi: 10.1074/jbc.273.49.32878. [DOI] [PubMed] [Google Scholar]
- Cousins R J, Liuzzi J P, Lichten L A. Mammalian zinc transport trafficking and signals. J Biol Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- Lepage L M, Giesbrecht J-A C, Taylor C G. Expression of T lymphocyte p56lck, a zinc-finger signal transduction protein, is elevated by dietary zinc deficiency and diet restriction in mice. J Nutr. 1999;129:620–627. doi: 10.1093/jn/129.3.620. [DOI] [PubMed] [Google Scholar]
- Moore J B, Blanchard R K, McCormack W T, Cousins R J. cDNA array analysis identifies thymic LCK as upregulated in moderate murine zinc deficiency before T-lymphocyte population changes. J Nutr. 2001;131:3189–3196. doi: 10.1093/jn/131.12.3189. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wange R L. T cell receptor signaling: beyond complex complexes. J Biol Chem. 2004;279:28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- Merten K E, Jiang Y, Kang Y J. Zinc inhibits doxorubicin-activated calcineurin signal transduction pathway in H9c2 embryonic rat cardiac cells. Exp Biol Med (Maywood) 2007;232:682–689. [PubMed] [Google Scholar]
- Haase H, Ober-Blöbaum J L, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- Muylle F A R, Adriaensen D, Coen W D, Timmermans J-P, Blust R. Tracing of labile zinc in live fish hepatocytes using FluoZin-3. Biometals. 2006;19:437–450. doi: 10.1007/s10534-005-4576-y. [DOI] [PubMed] [Google Scholar]
- Ibs K H, Rink L. Zinc-altered immune function. J Nutr. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- Haase H, Hebel S, Engelhardt G, Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Fraker P J, King L E. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- Fischer Walker C, Black R E. Zinc and the risk of infectious disease. Annu Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- Ozgenc F, Aksu G, Kirkpinar F, Altuglu I, Coker I, Kutukculer N, Yagci R V. The influence of marginal zinc deficient diet on post-vaccination immune response against hepatitis B in rats. Hepatol Res. 2006;35:26–30. doi: 10.1016/j.hepres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp (Warsz) 2008;56:15–30. doi: 10.1007/s00005-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstead H H, Prasad A S, Penland J G, Beck F W J, Kaplan J, Egger N G, Alcock N W, Carroll R M, Ramanujam V M S, Dayal H H, Rocco C D, Plotkin R A, Zavaleta A N. Zinc deficiency in Mexican American children: influence of zinc and other micronutrients on T cells, cytokines, and antiinflammatory plasma proteins. Am J Clin Nutr. 2008;88:1067–1073. doi: 10.1093/ajcn/88.4.1067. [DOI] [PubMed] [Google Scholar]