Abstract
Induction of proinflammatory mediators by alveolar macrophages exposed to ambient air particulate matter has been suggested to be a key factor in the pathogenesis of inflammatory and allergic diseases in the lungs. However, receptors and mechanisms underlying these responses have not been fully elucidated. In this study, we examined whether TLR2, TLR4, and the key adaptor protein, MyD88, mediate the expression of proinflammatory cytokines and chemokines by mouse peritoneal macrophages exposed to fine and coarse PM. TLR2 deficiency blunted macrophage TNF-α and IL-6 expression in response to fine (PM2.5), while not affecting cytokine-inducing ability of coarse NIST Standard Reference Material (SRM 1648) particles. In contrast, TLR4−/− macrophages showed inhibited cytokine expression upon stimulation with NIST SRM 1648 but exhibited normal responses to PM2.5. Preincubation with polymyxin B markedly suppressed the capacity of NIST SRM 1648 to elicit TNF-α and IL-6, indicating endotoxin as a principal inducer of cytokine responses. Overexpression of TLR2 in TLR2/4-deficient human embryonic kidney 293 cells imparted PM2.5 sensitivity, as judged by IL-8 gene expression, whereas NIST SRM 1648, but not PM2.5 elicited IL-8 expression in 293/TLR4/MD-2 transfectants. Engagement of TLR4 by NIST SRM 1648 induced MyD88-independent expression of the chemokine RANTES, while TLR2-reactive NIST IRM PM2.5 failed to up-regulate this response. Consistent with the shared use of MyD88 by TLR2 and TLR4, cytokine responses of MyD88−/− macrophages to both types of air PM were significantly reduced. These data indicate differential utilization of TLR2 and TLR4 but shared use of MyD88 by fine and coarse air pollution particles.
Keywords: TLR, LPS, inflammation, signal transduction, cytokines
Introduction
Particular matter (PM), a term used to describe particles present in ambient air, is a mixture of solid and liquid particles [1]. Solid particles are composed of a core of carbonaceous material and contain nitrates and sulfates, metals, polycyclic aromatic hydrocarbons, alkanes and alkanoic acids, and biological components (endotoxin, pollen, fungal spores, viruses, and bacteria) [1,2,3,4]. When characterized by size, PM falls into 3 categories: ultrafine (median aerodynamic diameter 0.1 μm or less, PM0.1), fine (median aerodynamic diameter 2.5 μm or less, PM2.5), and coarse (median aerodynamic diameter 10 μm or less, PM10) particles [5]. The fine PM2.5 fraction is composed primarily of carbonaceous material, soluble metals, sulfate, nitrate, and ammonium, and the coarse PM10 fraction consists of insoluble minerals and biologic aerosols, with smaller contributions from primary and secondary aerosols and sea salts [4,5,6]. PM can originate as a result of geographical conditions, weather, and seasonal patterns, or come from anthropogenic sources, including catalytic converters in cars, power plant emissions, and vehicle engine exhaust [7,8,9].
Experiments in animals and studies with human volunteers and human populations affected by air pollution have demonstrated that PM affects the respiratory, cardiovascular, and nervous systems [4, 5]. In epidemiological studies, ambient PM concentrations have been linked to increased incidence of respiratory infections, bronchitis, chronic pulmonary obstructive disease, asthma exacerbation, and higher morbidity and mortality [1, 10,11,12,13,14,15]. Air particles have been shown to stimulate cells to produce proinflammatory cytokines and chemokines [1, 4, 9, 16, 17], with both PM-associated endotoxin and soluble metals implicated in this response [2, 3, 6, 18]. In addition to endotoxin and soluble metals, organics content of PM, such as polycyclic aromatic hydrocarbons (PAHs; e.g., benzo[a]pyrene, benzo[b]fluoranthene, and pyrene), PAH-like compounds, quinolines, and others, are thought to induce proinflammatory responses in lung cells, including macrophages [19, 20]. Typically, the organic components have been associated with the man-made combustion products, which are primarily present in the PM2.5 class of air particles [20]. Although many studies suggest that lung inflammation plays a key role in the pathogenesis of pulmonary diseases caused by ambient air particles [4], the receptors and intracellular signaling mechanism(s) underlying the health effects of PM are poorly understood.
TLRs are principal innate immune sensors recognizing microbial pathogen-associated molecular patterns and endogenous “danger” molecules released from host cells as a consequence of infection, inflammation, or cell stress [21,22,23]. Thirteen mammalian TLRs show a similar structural organization and express a leucine-rich repeats-containing ectodomain implicated in ligand recognition, a transmembrane domain, and a cytoplasmic tail containing the TIR-signaling domain [22]. TLRs can be classified as receptors primarily recognizing lipids (e.g., TLR4 sensing LPS and TLR2 recognizing lipoproteins) [24,25,26,27], proteins (e.g., TLR5-flagellin recognition) [28, 29], and bacterial or viral nucleic acids (TLR3, TLR7-9) [30,31,32,33,34,35], and sense their ligands either on the cell surface (TLR2-5) or in intracellular endosomes (TLR3, TLR7-9) [36, 37].TLR signaling proceeds via recruitment of adaptor proteins MyD88 (shared by all TLRs except of TLR3), TIR domain containing adaptor activating IFN-β (TRIF) (used by TLR3 and TLR4 only) [37], and kinases IL-1R-associated kinase [38]-1-4, TANK binding kinase (TBK)-1 and IκB-kinase (IKK)-ε [39,40,41,42,43]. In addition to signaling adapters MyD88 and TRIF, TLR2, and TLR4 employ “bridging” adapters MyD88-like adaptor (Mal) (also called TIR domain-containing adaptor protein, TIRAP) to couple receptors with MyD88 [44], and TLR4 uses TIR-domain-related adaptor molecule to engage TLR4 with TRIF [42].
Only a few reports have been published that examine the mechanisms by which ambient air particles utilize TLRs to elicit cellular responses. Becker et al. [6] suggested the involvement of both TLR2 and TLR4 in recognition of PM10, which was primarily based on studies in CHO cells coexpressing CD14 and TLR2 or TLR4 and application of the synthetic TLR4 antagonist (E5531) to inhibit PM-induced IL-6 production from alveolar macrophages. In contrast to macrophage responses, primary human airway epithelial cells were reported to respond to air PM10 via TLR2, most likely due to low TLR4 expression by these cells [45]. Interestingly, exposure of respiratory epithelial cells to an aqueous-trapped solution of diesel exhaust has been shown to modulate susceptibility to viral (influenza) infections by increasing the expression levels and functional activities of TLR3, as evidenced by increased the poly(I:C)-induced expression of IL-6, nuclear translocation of IFN regulatory factor-3, and the expression of IFN-β [46]. Thus, utilization of TLRs by air particulate matter of different sizes and compositions is not fully understood, and signaling pathways involved in PM-mediated inflammatory reactions are still poorly characterized. In the present study, we examined the involvement of TLR2, TLR4, and the principal adaptor protein, MyD88, in the initiation of inflammatory macrophage cytokine responses by a NIST IRM PM2.5 and NIST SRM 1648, using wild-type and gene-deficient mouse macrophages, as well as human embryonic kidney 293 (HEK293) cell lines overexpressing the corresponding TLRs.
MATERIALS AND METHODS
Reagents and cell culture
In this study, we used standard air PM samples collected by the NIST (Gaithersburg, MD, USA), and they were characterized according to metal content. The PM2.5 sample (obtained as a gift) was collected by NIST from 1998 to 2001 in Baltimore, MD, USA, to create an interim standard reference material of fine urban particulate matter (NIST IRM PM2.5) and was collected as described by Heller-Zeisler et al. [47], www.cstl.nist.gov/projects/fy05/e&e05schantz2.pdf. Briefly, a bulk sample of PM2.5 was collected at an established EPA site in the Clifton Park area of Baltimore on an 8 in × 10 in Teflon filter array, using a high volume 1-m cyclone sampler. The 50% cutpoint for the sampler was 1.8 μm @ 400 cfm. The bulk PM2.5 sample was removed mechanically as a dry sample. The PM10 sample, collected by NIST in St. Louis, MO, USA, in the 1970s, was purchased as a NIST SRM 1648 (urban particulate matter). AlamarBlue was purchased from BioSource (Camarillo, CA, USA) and was used to measure cell viability. The synthetic lipopeptide Pam3Cys (S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-Lys4-OH trihydrochloride), a TLR2 ligand, was purchased from EMC Microcollections GmbH (Tübingen, Germany). LPS from Escherichia coli K12 W3100, a TLR4 ligand, and polymyxin B, an LPS binding protein [48, 49], which were used to examine the contribution of LPS present in PM fractions to cytokine production, were purchased from Sigma (St. Louis, MO, USA). The LPS was repurified to remove contaminating lipoproteins, as described previously [50]. HEK 293T cells, HEK293T cells stably expressing either the YFP-TLR2 (293/TLR2), or untagged TLR4/Flag-MD-2 (293/TLR4/MD-2) were a kind gift from Dr. Douglas T. Golenbock (University of Massachusetts School of Medicine; Worcester, MA, USA). HEK293T and 293/TLR4/MD-2 cells were cultured in DMEM medium (Mediatech, Herndon, VA, USA) supplemented with 2 mM L-glutamine, 10% FBS (HyClone, Logan, UT, USA), penicillin (100 U/ml), and streptomycin (100 μg/ml) (complete DMEM). 293/TLR2 cells were maintained in complete DMEM supplemented with 1 mg/ml G418 (neomycin, Sigma). Mouse peritoneal macrophages were cultured in RPMI 1640 medium (Cellgro, Herndon, VA, USA) supplemented with 2 mM L-glutamine, 10% FBS, 10 mM HEPES (BioWhittaker), penicillin (100 U/ml), and streptomycin (100 μg/ml) (BioWhittaker), 5 × 10−5 M β-mercaptoethanol (Sigma).
Analysis of metals in NIST IRM PM2.5
Suspensions (1 mg/g) of NIST Interim Reference Material (IRM) PM2.5 were prepared in high purity water (NIST subboiling distillation) and ultrasonicated prior to analysis. Total metal content of PM2.5 was measured by instrumental neutron activation analysis, using established procedures in a sequence of irradiation and counting steps of samples, controls, and multielement standards appropriate to the analytical task [51]. For the assay of elements with short-lived nuclides, individual samples were irradiated together with multielement standards and metal flux monitor foils (Al or Ti) for 300 s at the pneumatic tube facility RT-1 of the NIST Center for Neutron Research (thermal neutron flux 1.3 × 1014 cm-2 s-1). Irradiation was followed by counting for 300 s after the 100-s decay and for 600 s after the 1000-s decay. For the assay of elements with longer-lived nuclides, sets of 10 to 30 of the previously analyzed samples were irradiated together with multielement standards and Fe flux monitor foils for 16 h at the RT-4 facility (thermal neutron flux 3.4 × 1013 cm-2 s-1), followed by 2-h counts after the 3- to 5-day decay and 8-h counts after the 20-day decay. The quantitative evaluation was carried out with automated neutron activation analysis software (GENIE, Canberra, Australia).
Determination of endotoxin content
Separate 1 mg/ml stock suspensions of both Interim IRM PM2.5 and SRM 1648 PM were prepared in sterile, endotoxin-free water (Baxter, Deerfield, IL, USA) and diluted to 100 μg/ml and 10 μg/ml, respectively. Endotoxin measurements were carried out on each sample using a kinetic, chromogenic Limulus polyphemus amoebocyte lysate assay, according to the manufacturer’s instructions (BioWhittaker, Walkersville, MD, USA). Results are reported as endotoxin units (EU) per milligram of particles, the limit of detection for the Limulus endotoxin test was 0.1 pg/ml [52].
Exposure of cells to NIST IRM PM2.5 and SRM 1648 PM samples
Stock suspensions of NIST IRM PM2.5 and NIST SRM 1648 PM were prepared at a concentration of 1 mg/ml in complete DMEM and sonicated for 1 h at room temperature prior to each experiment, using the Branson 1510 ultrasonic water bath (LabX, Midland, Canada). Following sonication, cells were treated with medium alone, NIST IRM PM2.5 (7.8, 31.2, and 125 μg/ml), or NIST SRM 1648 (31.2, 125, and 500 μg/ml). Pam3Cys (1 μg/ml) or LPS (100 ng/ml) were used as positive controls for activation of TLR2 and TLR4, respectively. Triton X-100 was added to the cultured cells as a positive control to ensure complete cell lysis when assessing cell viability. Cells were incubated for 24 h at 37°C in air supplemented with 5% CO2 in the presence of both standard reference materials and control treatments. After 24 h, supernatants were collected and used for cytokine analysis by ELISA. Cell viability was measured following control or experimental treatments using the AlamarBlue assay (Biosource International, Camarillo, CA, USA). At the end of the 24-h exposure period, the cell media were removed, the cells were washed, and 200 μl of fresh media containing 20 μl of AlamarBlue were added to each well. Cells were then incubated at 37°C/5% CO2 for an additional 3 h. After the incubation period, the cell medium was removed to a fresh 96-well plate, in order to prevent the NIST particles in each well from quenching the fluorescent signal of the AlamarBlue. The fluorescence (F; λex=530 nm/25 nm, λem = 580 nm/50 nm) of reduced AlamarBlue, a measure of overall metabolic activity, was determined using a Cytofluor 4000/TC fluorometer (Applied Biosystems, Foster City, CA, USA). Percent cell viability was calculated comparing the metabolic activity of treated cells relative to cells incubated for 24 h with medium alone.
Mice and isolation of macrophages
TLR2−/−, TLR4−/−, and MyD88−/− mice were a kind gift from Dr. Shuzio Akira (Osaka University, Osaka, Japan) and were produced as described previously [53,54,55]. In brief, the respective genes were disrupted by replacement of the first and second exons with a neomycin-resistance cassette, resulting in the complete abrogation of TLR2, TLR4, or MyD88 protein expression. These mice were backcrossed into a C57BL/6 background for at least seven generations prior to use, to produce the respective heterozygous (TLR2+/−, TLR4+/−, MyD88+/−) and knockout (TLR2−/−, TLR4−/−, MyD88−/−) mice. No differences were observed in response to Pam3Cys or LPS between homozygous WT mice and heterozygous mice. Heterozygous mice were used as positive controls for all experiments. To obtain mouse peritoneal exudate cells, mice were intraperitoneallly injected with 3 ml sterile 3% thioglycollate medium, peritoneal lavage fluid was harvested 3 days after injection, and macrophages were prepared according to a previously published procedure [56] based on their plastic adherence. All experiments using mice and murine macrophages were conducted with the approval by the Institutional Animal Care and Use Committee, University of Maryland, Baltimore.
Genotyping of mice
DNA was extracted from tail snips using the Qiagen DNeasy Tissue Kit (Valencia, CA, USA) according to manufacturer’s instructions. The TLR2, TLR4, and MyD88 genotypes were identified by an allele-specific PCR assay using the following primer sets: TLR2: GTTTAGTGCCTGTATCCAGTCAGTGCG (forward), AATGGGTCAAGTCA ACACTTCTCTGGC (reverse); TLR4: CGTGTAAACCAGCCAGGTTTTGAAGGC (forward), TGTTGCCCTTCAGTACAGAGA CTCTG (reverse); MyD88: GTCAGAAACAACCACCA CCATGC (forward), TGGCATGCCTCCATCATAGTTAACC (reverse); and neocassette: ATCGCCTTCTATCGCCTTCTTGACGAG. PCR reactions for TLR2 were carried out in 25 μl at 30 cycles of 94°C denaturing for 45 s, 63°C annealing for 1 min, and 72°C extension for 1 min. TLR4 samples were amplified at 30 cycles of 94°C denaturing for 45 s, 66°C annealing for 1 min, and 72°C extension for 1 min, and MyD88 PCR was done at 32 cycles of 95°C denaturing for 45 s, 65°C annealing for 1 min, and 74°C extension for 1 min.
RNA isolation, reverse transcription, and real-time PCR
RNA was isolated using RNeasy kits (Qiagen) following the manufacturer’s protocol, treated with DNase (Roche, Pleasanton, CA, USA) to remove residual genomic DNA, repurified and quantified spectrophotometrically. cDNA was prepared from 1 μg RNA using Reverse Transcription System (Promega, Madison, WI, USA) and subjected to real-time PCR with gene-specific primers for human HPRT and IL-8 on a MyiQ real-time PCR machine (Bio-Rad, Hercules, CA, USA). PCR amplification was performed using 5 μl of cDNA, 0.3 μM forward and reverse primers, and SYBR Green Supermix (Bio-Rad). The following gene-specific primers were used: IL-8, 5′-CACCGGAA GGAACCATCTCACT-3′ (forward), 5′-TGCACCTTCACACAGAGCTGC-3′ (reverse); and HPRT: 5′-ACCAGTCAACAGGGGACATAAAAG-3′ (forward), 5′-GTCTGCATTGTTTTGCCAGT GTC-3′ (reverse). PCR was carried out for 40 cycles of 95°C denaturing for 10 s, 55°C annealing for 1 min, and 72°C extension for 30 s. Melting curve analysis was carried out to ensure specific amplification of the desired mRNA, as recommended by the manufacturer. Real-time PCR data were processed using 2-ΔΔCT method, as described previously [57].
Measurement of cytokine production by ELISA
Following exposure to PM particles, cell media samples were collected and centrifuged (250 g for 10 min) to remove any residual cellular or particle debris. Concentrations of the proinflammatory cytokines IL-6 and TNF-α and the chemokine RANTES were measured using ELISA kits (R&D Systems, Minneapolis, MN, USA), according to manufacturer’s instructions in the Cytokine Core Facility (University of Maryland, Baltimore). The lower detection limits for these assays were 3.9 pg/ml, 6.25 pg/ml, and 24.9 pg/ml for TNF-α, IL-6, and RANTES, respectively.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 program for Windows (GraphPad Software, San Diego, CA, USA). Statistical differences among experimental groups were evaluated a one-way ANOVA with repeated measures, followed by post hoc comparisons using Tukey’s multiple paired comparison test (GraphPad PRISM 5 program for Windows). Values are expressed as means ± sd.
RESULTS
NIST IRM PM2.5 and NIST SRM 1648 initiate the production of proinflammatory cytokines and chemokines without significantly affecting cell viability
Previous studies [3, 6] and our results indicate that ambient air PM2.5 and PM10 are characterized by differences in their size, content of metals, and LPS. For instance, the PM2.5 used in this study (NIST IRM PM2.5) had higher concentrations of many oxidatively active metals (e.g., Fe, Cr, and Cu) than the NIST SRM 1648 sample (Table 1), while the NIST SRM 1648 PM had ∼5 times higher levels of LPS compared with NIST IRM PM2.5 (0.075±0.006 EU/mg vs. 0.016±0.006 EU/mg). Hence, it is plausible that they may use distinct receptors and/or signaling pathways to trigger proinflammatory immune responses.
TABLE 1.
Percent Metal Concentrations (w/w) and Endotoxin Concentrations in NIST SRM 1648 PM10 and IRM PM2.5
| Metal Constituent | NIST SRM 1648a Mass Concentration (%) | NIST IRM PM2.5 Mass Concentration (%) |
|---|---|---|
| Iron | 3.91 ± 0.10 | 19.5 ± 0.4 |
| Aluminum | 3.42 ± 0.11 | 5.40 ± 0.04 |
| Chromium | 0.040 ± 0.001 | 4.680 ± 0.080 |
| Manganese | 0.079 ± 0.001 | 0.280 ± 0.002 |
| Copper | 0.061 ± 0.003 | 0.276 ± 0.064 |
| Titanium | 0.40d | 0.151 ± 0.013 |
| Zinc | 0.476 ± 0.014 | 0.063 ± 0.017 |
| Cobalt | 0.002d | 0.0472 ± 0.0009 |
| Vanadium | 0.013 ± 0.001 | 0.0316 ± 0.0005 |
| Molybdenum | -c | 0.022 ± 0.003 |
| Arsenic | 0.012 ± 0.001 | 0.0040 ± 0.0002 |
| Cadmium | 0.0075 ± 0.001 | -b |
| Lead | 0.655 ± 0.008 | -c |
| Nickel | 0.0082 ± 0.0003 | -c |
| Selenium | 0.0027 ± 0.0001 | -c |
| EU/mg | EU/mg | |
| Endotoxin | 0.075 ± 0.006 | 0.016 ± 0.006 |
NIST SRM 1648 certified values (http://ts.nist.gov/MeasurementServices/ReferenceMaterials/archived_certificates/1648.pdf).
Below limit of detection.
Unable to be measured.
Not certified.
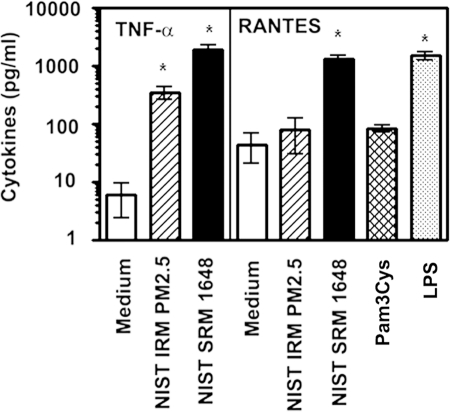
In our first set of experiments, we examined the capacities of NIST IRM PM2.5 and NIST SRM 1648 PM to trigger cytokine release from murine peritoneal macrophages. We used IRM PM2.5 at 31.2 μg/ml and 125 μg/ml, and SRM 1648 PM at 125 μg/ml and 500 μg/ml that partially correspond to calculated PM concentrations deposited in the alveolar regions of the human lung [58, 59]. Peritoneal macrophages were obtained from control mice that showed heterozygous (+/−) expression of WT and mutated alleles of TLR2, TLR4, or MyD88 (e.g., TLR2+/−, TLR4+/−, MyD88+/−), and whose cytokine production was indistinguishable from responses exhibited by WT (+/+) mice (data not shown). Figure 1 shows that stimulation of control, heterozygous (TLR4+/−) macrophages with 125 μg/ml NIST SRM 1648 resulted in a potent induction of TNF-α and RANTES secretion. NIST IRM PM2.5 exhibited a lower, although significant capacity to increase TNF-α release over the control levels but failed to up-regulate RANTES expression (Fig. 1). Consistent with the literature [60], stimulation of macrophages with a TLR4 agonist (LPS) led to a significant increase in the production of RANTES over untreated cells (1,514 ± 211 vs. 45 ± 23 pg/ml), while Pam3Cys, a TLR2 agonist, failed to stimulate expression of RANTES (Fig. 1). Measurements of cell viability with AlamarBlue demonstrated that macrophages exhibited 82.1 ± 15.5% viability following 24-h treatment with the highest (125 μg/ml) concentration of NIST IRM PM2.5, and 95.1 ± 3.2% viability after exposure to the highest concentration (500 μg/ml) of NIST SRM 1648, whereas lower concentrations of air PM did not affect macrophage viability (average values were not lower than 95±3.4%). These results indicate that NIST SRM 1648 and, to a lesser extent, NIST IRM PM2.5 induce production of TNF-α in a manner that cannot be attributed to their cytotoxic effects. In addition, our data show that NIST SRM 1648, but not NIST IRM PM2.5 up-regulate the expression of the MyD88-independent, TRIF-dependent RANTES [60,61,62].
Figure 1.
The cytokine-inducing capacities of air pollution particles NIST IRM PM2.5 and NIST SRM 1648. Peritoneal macrophages obtained from control (TLR4+/−) mice were plated in 12-well plates (1×106 cells per well) and treated for 24 h with medium, ambient air particles NIST IRM PM2.5 (PM2.5), NIST SRM 1648 (PM10) (125 μg/ml each), 1 μg/ml Pam3Cys, and 100 ng/ml LPS. Following stimulation, supernatants were collected to analyze secretion levels of TNF-α and RANTES by ELISA. Data are presented as mean ± sd from 5 independent experiments. *, P < 0.05 compared with medium-treated cells.
Endotoxin contributes to cytokine induction in response to NIST SRM 1648
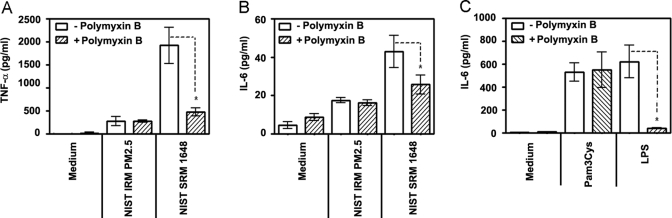
Next, we sought to delineate the possible involvement of LPS in mediating biological effects exerted by ambient air PM. To this end, we used polymyxin B, an endotoxin binding protein capable of inhibiting LPS activity [48, 49]. Polymyxin B was preincubated with air PM, Pam3Cys, or LPS prior to addition to macrophages, and the levels of proinflammatory cytokines in these cultures were compared with those in cell cultures without polymyxin B. NIST IRM PM2.5-mediated TNF-α and IL-6 cytokine production in control (+/−) macrophages was not inhibited by polymyxin B (Fig. 2A and B), confirming that cytokine induction in response to NIST IRM PM2.5 cannot be attributed to LPS. In contrast, prior exposure to polymyxin B suppressed much of the ability of NIST SRM 1648 to induce both TNF-α and IL-6 secretion (Fig. 2A and B). Consistent with reported data [48, 49], polymyxin B suppressed induction of IL-6 (Fig. 2C) and TNF-α (data not shown) following stimulation with LPS, whereas Pam3Cys-mediated cytokine responses were not affected. These results indicate that the cytokine-inducing capacity of NIST IRM PM2.5 is not due to the negligible amount of LPS present in this particle fraction, whereas NIST SRM 1648-induced proinflammatory cytokine production is largely dependent on its endotoxin content.
Figure 2.
The role of endotoxin in air particle-induced cytokine production. Macrophages obtained from control mice (+/−) (106 cells per well of 12-well plates) were treated for 24 h with medium, NIST IRM PM2.5, NIST SRM 1648 (125 μg/ml each) (A and B), 1 μg/ml Pam3Cys and 100 ng/ml LPS (C). Polymyxin B (10 μg/ml) was incubated with the stimuli 30 min prior to cell stimulation, cells were stimulated for 24 h as indicated, and cell-free supernatants were collected to measure TNF-α (A) and IL-6 (B) secretion levels by ELISA. Data of 3 independent experiments are presented (mean ± sd). *, P < 0.05.
The effect of TLR2 and TLR4 deficiencies on cytokine producing capacities of NIST IRM PM2.5 and NIST SRM 1648
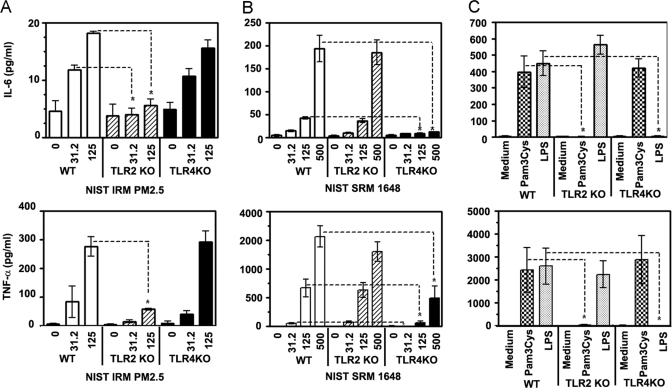
PM2.5 comprises many components, including bacterial or mycoplasmal lipoproteins, both of which are known activators of TLR2 [63, 64]. In addition, the high content of metals in NIST IRM PM2.5 (Table 1) suggests that oxidatively active metals may also contribute to TLR2-dependent responses. In contrast, cellular responses mediated by the NIST SRM 1648 fraction, which has higher endotoxin concentrations than NIST IRM PM2.5 and whose cytokine-inducing activities are inhibited by the LPS binding protein, polymyxin B, are likely to be primarily TLR4 dependent. Because TLR2−/− and TLR4−/− mice represent a straightforward model to analyze the involvement of TLRs, these mice were used to examine the role of TLR2 and TLR4 in air pollution particle-mediated cytokine responses. TLR2−/− macrophages showed a significant decrease in IL-6 (top) and TNF-α (bottom) production upon exposure to 31.2 μg/ml and 125 μg/ml NIST IRM PM2.5, compared with the cytokine response of control (+/−) cells stimulated with the same concentrations of air PM2.5 (Fig. 3A). In contrast, NIST IRM PM2.5 caused similar levels of IL-6 (top) and TNF-α (bottom) secretion in TLR4−/− and control (TLR4+/−) macrophages (Fig. 3B). NIST SRM 1648-mediated IL-6 (top) and TNF-α (bottom) secretion levels were comparable in TLR2−/− macrophages and control TLR2+/− cells, while exerting a markedly diminished cytokine response in TLR4−/− macrophages (Fig. 3B). As shown in Fig. 3C, TLR2 KO macrophages were unable to produce IL-6 (top) or TNF-α (bottom) in response to Pam3Cys, whereas comparable cytokine responses were observed in TLR4−/− and TLR2+/− cells. LPS failed to induce IL-6 and TNF-α release from TLR4 KO macrophages, and comparable cytokine responses were seen in TLR2−/− and TLR4+/− control cells (Fig. 3C). These data illustrate the specificity of the loss of functional phenotype exhibited by TLR2 and TLR4 KO macrophages and indicate that induction of IL-6 and TNF-α in response to NIST IRM PM2.5is TLR2-dependent, whereas NIST SRM 1648 activate macrophages to produce cytokines largely by a TLR4-dependent, TLR2-independent fashion.
Figure 3.
Utilization of TLR2 and TLR4 by air particles NIST IRM PM2.5 and NIST SRM 1648 for the induction of cytokine responses in mouse macrophages. Peritoneal macrophages obtained from control, heterozygous mice (TLR2+/− as a control for TLR2 KO, and TLR4+/− as a control for TLR4 KO) or mice deficient for TLR2 or TLR4 were plated in 12-well plates (1×106 cells per well) and treated for 24 h with medium or the indicated concentrations of air particles NIST IRM PM2.5 (A), NIST SRM 1648 (B), or LPS and Pam3Cys (C). Culture supernatants were collected and levels of IL-6 (top panels) and TNF-α (bottom panels) were determined by ELISA. Shown are data (mean ± sd) of eight independent experiments. *, P < 0.05.
Overexpression of TLR2 and TLR4 in HEK293 cells imparts differential sensitivity to NIST IRM PM2.5 vs. NIST SRM 1648
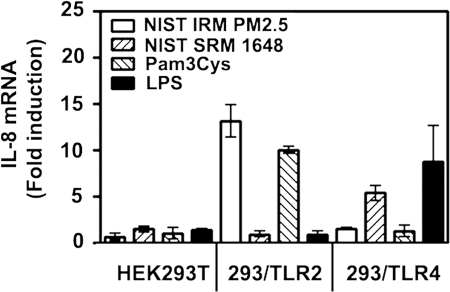
To confirm and extend our results on utilization of TLRs by ambient PM, we took advantage of the TLR2-, TLR4-, and MD-2-negative HEK293T cell line [67, 68] to delineate whether introduction of TLR2 or TLR4/MD-2 would impart sensitivity to NIST IRM PM2.5 or NIST SRM 1648. Since MD-2 is associated with TLR4 on the cell surface and enables TLR4 to respond to LPS [65, 66], it was transfected into HEK293 cells along with TLR4. HEK cell lines were treated for 3 h with medium, NIST IRM PM2.5 or NIST SRM 1648 (125 μg/ml each), 1 μg/ml Pam3Cys or 100 ng/ml LPS, RNA was isolated, and relative expression levels of IL-8 mRNA were determined by quantitative real-time PCR. Untransfected HEK293T cells failed to up-regulate IL-8 mRNA expression in response to any concentration of NIST IRM PM2.5 or NIST SRM 1648, nor was IL-8 induced by TLR2 (Pam3Cys) or TLR4 (LPS) agonists used as controls for TLR-mediated signaling (Fig. 4). Stable transfection of TLR2 in HEK293T cells (the 293/TLR2 cell line) resulted in IL-8 mRNA expression upon cell stimulation with NIST IRM PM2.5 and Pam3Cys, while no IL-8 gene expression was observed upon treatment with NIST SRM 1648 or LPS (Fig. 4). In contrast, overexpression of TLR4/MD-2 enabled HEK cells to respond to NIST SRM 1648 and LPS with ∼5-fold and ∼8-fold increases in IL-8 mRNA expression, but there was no response to NIST IRM PM2.5 or Pam3Cys (Fig. 4). These data confirm our results obtained in mouse macrophages and indicate that TLR2 and TLR4 expression is sufficient to confer responsiveness to NIST IRM PM2.5 and NIST SRM 1648 air PM, respectively.
Figure 4.
Effect of TLR2 and TLR4/MD-2 overexpression in HEK cells on the ability of air particles to induce IL-8 mRNA expression. HEK293T cells or the 293/TLR2 or 293/TLR4/MD-2 stable cell lines were treated for 24 h with medium, NIST IRM PM2.5, NIST SRM 1648 (125 μg/ml each), 1 μg/ml Pam3Cys or 100 ng/ml LPS. Cells were lysed, RNA was isolated and subjected to real-time PCR analysis of IL-8 and (HPRT) mRNA expression with the respective gene-specific primers. IL-8 mRNA expression were normalized to HPRT values according to 2-ΔΔCT method [57], and the results are expressed and fold induction is compared with IL-8 levels detected in medium alone. Shown are data (mean±sem) of a representative (n=2) experiment.
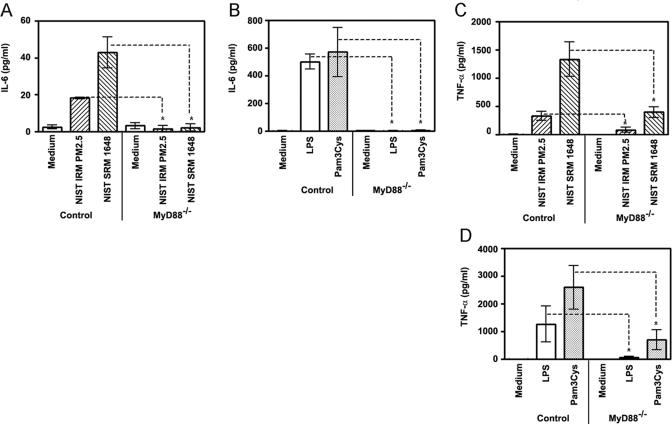
Importance of MyD88 in the proinflammatory response to ambient PM
Because MyD88 is an adaptor protein used by both TLR2 and TLR4 signaling pathways [54], we studied whether MyD88 deficiency would affect the ability of air NIST IRM PM2.5 and NIST SRM 1648 to trigger secretion of proinflammatory cytokines. Macrophages obtained from MyD88−/− and control (MyD88+/−) mice were treated for 24 h with medium or stimulated with ambient PM, and levels of proinflammatory cytokines in culture supernatants were determined by ELISA. As a control, defined TLR2 (Pam3Cys) and TLR4 (LPS) agonists were employed for stimulation of control and MyD88 KO macrophages. As shown in Fig. 5, production of IL-6 (A) and TNF-α (C) in response to NIST IRM PM2.5 and NIST SRM 1648 was significantly decreased in MyD88 KO macrophages when compared with control cells. Similar inhibition of IL-6 and TNF-α production from MyD88 KO macrophages was observed when macrophages were stimulated with a higher (500 μg/ml) concentration of NIST SRM 1648 (data not shown). In line with the involvement of MyD88 in TLR2- and TLR4-mediated expression of proinflammatory cytokines [54], a significant decrease in IL-6 (Fig. 5B) and TNF-α (Fig. 5D) levels was observed in MyD88−/− macrophages stimulated with Pam3Cys and LPS, while control macrophages showed robust Pam3Cys- and LPS-inducible cytokine secretion. Collectively, these results indicate that the MyD88 signaling pathway is involved in macrophage activation by both NIST IRM PM2.5 and NIST SRM 1648, consistent with the utilization of MyD88 by TLR2- and TLR4-mediated signaling pathways that are activated by PM2.5 and PM10, respectively.
Figure 5.
Involvement of MyD88 in macrophage cytokine production elicited by ambient air particles. Peritoneal macrophages obtained from control (MyD88+/−) and MyD88−/− mice were plated in 12-well plates (1×106 cells/well in triplicates) and treated for 24 h with medium, NIST IRM PM2.5 or NIST SRM 1648 (125 μg/ml each, A and C), 1 μg/ml Pam3Cys and 100 ng/ml LPS (B and D). Following stimulation, supernatants were collected to analyze IL-6 (A and B) and TNF-α (C and D) secretion levels by ELISA. *, P < 0.05.
DISCUSSION
Previous reports by us and others suggest a strong correlation between cytokine-inducing abilities of ambient air particles and airway inflammation [1, 6, 9, 11, 17, 18, 67,68,69,70]. However, the extent to which metal, organic (e.g., PAHs, PAH-like components, quinolines), and endotoxin constituents of PM2.5 and PM10 contribute to eliciting inflammatory cytokine production and the receptors/signaling cascades they utilize for triggering these responses are poorly characterized. In this study, we employed macrophages obtained from wild-type and gene-deficient mice, and used transfection-based complementation of TLR2/4-negative HEK293T cells to elucidate the effect of NIST IRM PM2.5 and NIST SRM 1648 on cytokine production and to delineate receptors and signaling adapters involved. These air PM were employed at concentrations comparable to reported environmental concentrations of ambient air particles [71], calculated PM concentrations deposited in the alveolar regions of the human lung [58, 59] and our data (Table 1), as well as at higher concentrations to approximate to the levels of air PM reported at environmental pollution disasters. Consistent with previous findings [4, 17, 68], both NIST SRM 1648 and, to a lesser extent, NIST IRM PM2.5, were found to elicit secretion of MyD88-dependent [54] TNF-α (Fig. 1). In contrast, only NIST SRM 1648, but not NIST IRM PM2.5, induced the production of RANTES (Fig. 1), a TRIF-dependent cytokine [60,61,62]. Because TLR2 and TLR4 share the ability to signal expression of proinflammatory cytokines via the common MyD88 signaling cascade, but only TLR4 induces RANTES via the TRIF pathway [21], these data suggest that PM2.5 and NIST SRM 1648 differ in their utilization of TLR2 and TLR4.
To directly identify specific signaling receptors involved in stimulation of macrophage cytokine release in response to ambient air particles, we used two experimental approaches. First, control heterozygous (TLR2+/− or TLR4+/−) or TLR2−/− and TLR4−/− mice were employed to examine the effect of TLR2 or TLR4 deficiency on the ability of NIST IRM PM2.5 or NIST SRM 1648 to induce TNF-α and IL-6 from peritoneal macrophages. Our results confirm differential utilization of TLRs by air pollution particles, as TLR2 KO macrophages showed decreased cytokine production in response to NIST IRM PM2.5 compared with control macrophages, whereas no changes in cytokine production between TLR4 KO macrophages and control cells were observed (Fig. 3). In contrast, proinflammatory cytokine release induced by NIST SRM 1648 is TLR4-dependent, as evidenced by inhibited TNF-α and IL-6 release in TLR4 KO macrophages stimulated with this PM but preserved cytokine responses in TLR2 KO cells. Because NIST SRM 1648 PM have higher concentrations of endotoxin than the PM2.5 [6, 45] and Table 1) and because neutralization of endotoxin with polymyxin B led to inhibition of NIST SRM 1648-induced IL-6 and TNF-α release (Fig. 2), the primary component responsible for the dependency on TLR4 is likely to be LPS.
The conclusion that NIST IRM PM2.5 induces cytokine production through TLR2, while NIST SRM 1648-induced cytokine induction is dependent on TLR4 was confirmed by using human embryonic kidney (HEK) 293 cells. HEK293 cells lack endogenous TLR2, TLR4, CD14, and MD-2, but become responsive to TLR2 and TLR4 ligands upon transfection of the respective TLRs [72, 73]. Therefore, we used untransfected HEK293 cells and 293/TLR2 and 293/TLR4/MD-2 stable transfectants to confirm the involvement of TLRs in cytokine responses induced by ambient air particles. No responses to air PM, LPS, or Pam3Cys were observed in HEK293T cells, stressing the requirement for TLR2/4 expression for induction of cell activation by NIST SRM 1648 and NIST IRM PM2.5. Treatment of 293/TLR2 cells with NIST IRM PM2.5 and Pam3Cys, a control TLR2 ligand, but not with NIST SRM 1648 or LPS, led to robust induction of IL-8 mRNA (Fig. 4), further supporting the conclusion that proinflammatory cytokine expression in response to NIST IRM PM2.5 is TLR2-dependent and TLR4-independent. Conversely, overexpression of TLR4/MD-2 in TLR2/4/MD-2-negative HEK293T cells imparted sensitivity to NIST SRM 1648 and LPS, while responses to NIST IRM PM2.5 and Pam3Cys were not inducible, confirming that this PM10-containing NIST SRM 1648 sample induces cell activation in a TLR4-dependent manner. These results, coupled with the cytokine production data in mouse macrophages, strongly indicate that proinflammatory cytokine release from macrophages in response to components of NIST IRM PM2.5 is dependent on TLR2, while the NIST SRM 1648-induced cytokine production is TLR4 dependent. This clear difference between the two particle samples is particularly interesting in light of the fact that the NIST SRM 1648 sample contains a fraction of particles in the PM2.5 range. However, the larger (>0.25 μm) particles are the primary components of this sample on a mass basis. Our data on differential induction of MyD88-dependent (TNF-α) and TRIF-dependent (RANTES) cytokines, polymyxin B-mediated blocking of cytokine-inducing ability and differential utilization of TLR2 vs. TLR4 by NIST SRM 1648 vs. PM2.5 strongly suggest that the functional contribution of the larger particles in the NIST SRM 1648 sample appears to be major and dominant.
Candidate TLR2 ligands triggering macrophage cytokine responses to NIST IRM PM2.5 are currently unknown. It is plausible that NIST IRM PM2.5 contains bacterial or mycoplasma lipoproteins, which are known activators of TLR2 [26, 55, 61, 65]. Because PM2.5 contain high concentrations of a large number of metals (refs [6, 45]. and Table 1), one or more of these metals could be involved in increased TLR2-mediated cytokine production triggered by microbial lipoprotein constituents of the NIST IRM PM2.5. In this regard, it is interesting that TLR2 has been implicated in proinflammatory cytokine expression by airway epithelial cells stimulated with air PM containing high amounts of metals [45]. Another possibility is that PM2.5 cytokine-inducing effects are mediated by an indirect mechanism, via the generation of endogenous “danger” host molecules activating macrophages via TLR2. Noteworthy, a number of endogenous “danger” molecules have been reported to activate cells via TLR2, including heat shock proteins 60 and 70, high mobility group box-1 protein, and Gp96 (rev. in [22, 74]. Although NIST IRM PM2.5 exerted only moderate cytotoxic effects on macrophages (82.1±15.5% viability) at the highest exposure concentration, we cannot completely rule out the possibility that ∼18% macrophages that underwent apoptosis or necrosis may release endogenous “danger” TLR2 agonists. Studies are currently underway to examine a possible role of transition metals in mediating and modulating PM2.5-initiated macrophage cytokine responses and to address a role of endogenous “danger” molecules in this process.
Having established that NIST IRM PM2.5 and NIST SRM 1648 activate macrophages via TLR2 and TLR4, we next sought to determine the importance of MyD88 in proinflammatory macrophage cytokine responses induced by air PM. Considering the utilization of TLR2 and TLR4 by ambient air particles, it was hypothesized that MyD88 deficiency would have a similar effect on the ability of NIST IRM PM2.5 and NIST SRM 1648 to induce TNF-α and IL-6 cytokine release from macrophages. Indeed, we observed very similar impairment of TNF-α and IL-6 secretion by MyD88−/− macrophages in response to NIST IRM PM2.5 and NIST SRM 1648, compared with cytokine responses observed in control cells (Fig. 5). Residual TNF-α production observed in MyD88−/− macrophages stimulated with TLR4-engaging NIST SRM 1648 and LPS is reminiscent of the house dust extracts-mediated cytokine responses of MyD88−/− dendritic cells [75] and could be explained by the dual dependency of TNF-α on both MyD88 and TRIF [37, 54, 76, 77]. It is also plausible that, in addition to TLR2 and TLR4 ligands, ambient air particles contain components capable of signaling through other TLRs. In this respect, it is noteworthy that residual TNF-α production was also observed in TLR2 KO and TLR4 KO macrophages stimulated with NIST IRM PM2.5 and NIST SRM 1648. Organic constituents such as PAHs, PAH-like compounds, and quinolines are primarily present in the PM2.5 and have been implicated in eliciting and/or modulating proinflammatory responses in macrophages [19, 20]; therefore, differences between NIST IRM PM2.5 and NIST SRM 1648 in the content of organics should also be taken into account and warrant further studies. Finally, metals that constitute NIST IRM PM2.5 may also be responsible for triggering TNF-α secretion in MyD88 KO macrophages through either TLR-dependent or TLR-independent particle uptake by macrophages, which, in turn, generate reactive oxygen species that can then activate transcription factors and initiate production of cytokine genes [2, 16, 17, 58, 69]. Relevant to this idea, Dostert et al. [78] have recently reported that Nalp3, a member of the NLR family, senses the particulate asbestos and silica, leading to the induction of IL-1. Furthermore, it is plausible that fine and course ambient PM collected from different geographical locations may contain combinations of microbial, organic, and metal constituents specific for these sites that may be sensed by different macrophage receptors (e.g., TLRs, NLRs, or scavenger receptors). Thus, further investigation will be required to identify additional TLRs, and other pattern recognition receptors, that are triggered by components of fine and course ambient PM. In addition, it will be interesting to determine whether air PM simply represent vehicles for microbial, organic and metal constituents that are primarily responsible for eliciting inflammatory responses, or they may evoke specific inflammatory pathways or modulate signaling cascades induced by their constituents.
ACKNOWLEDGMENTS
This study was partially supported by the National Institutes of Health RO1 grant AI059524 (to A. E. M.)
Footnotes
Abbreviations: EU=endotoxin units; HEK=human embryonic kidney; HPRT=hypoxantin phosphoribosyltransferase; IRF=IFN regulatory factor; NIST= National Institute for Standards and Technology; NLR=NOD-like receptor; PAHs=polycyclic aromatic hydrocarbons; PM=particulate matter, TIR=Toll-IL-1R; TLRs=Toll-like receptors; WT=wild-type
References
- Schwartz J. Air pollution and daily mortality: a review and meta analysis. Environ Res. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- Dye J A, Adler K B, Richards J H, Dreher K L. Role of soluble metals in oil fly ash-induced airway epithelial injury and cytokine gene expression. Am J Physiol Lung Cell Mol Physiol. 1999;277:L498–L510. doi: 10.1152/ajplung.1999.277.3.L498. [DOI] [PubMed] [Google Scholar]
- Adamson I Y, Prieditis H, Hedgecock C, Vincent R. Zinc is the toxic factor in the lung response to an atmospheric particulate sample. Toxicol Appl Pharmacol. 2000;166:111–119. doi: 10.1006/taap.2000.8955. [DOI] [PubMed] [Google Scholar]
- Hopke P K, Rossner A. Exposure to airborne particulate matter in the ambient, indoor, and occupational environments. Clin Occup Environ Med. 2006;5:741–771. doi: 10.1016/j.coem.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Brook R D, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith S C., Jr Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Becker S, Fenton M J, Soukup J M. Involvement of microbial components and Toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- Allen J O, Mayo P R, Hughes L S, Salmon L G, Cass G R. Emissions of size-segregated aerosols from on-road vehicles in the Caldecott tunnel. Environ Sci Technol. 2001;35:4189–4197. doi: 10.1021/es0015545. [DOI] [PubMed] [Google Scholar]
- Kavouras I G, Koutrakis P, Cereceda-Balic F, Oyola P. Source apportionment of PM10 and PM2.5in five Chilean cities using factor analysis. J Air Waste Manag Assoc. 2001;51:451–464. doi: 10.1080/10473289.2001.10464273. [DOI] [PubMed] [Google Scholar]
- Li N, Wang M, Oberley T D, Sempf J M, Nel A E. Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. J Immunol. 2002;169:4531–4541. doi: 10.4049/jimmunol.169.8.4531. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Slater D, Larson T V, Pierson W E, Koenig J Q. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis. 1993;147:826–831. doi: 10.1164/ajrccm/147.4.826. [DOI] [PubMed] [Google Scholar]
- Schwartz J. What are people dying of on high air pollution days? Environ Res. 1994;64:26–35. doi: 10.1006/enrs.1994.1004. [DOI] [PubMed] [Google Scholar]
- Delfino R J, Murphy-Moulton A M, Burnett R T, Brook J R, Becklake M R. Effects of air pollution on emergency room visits for respiratory illnesses in Montreal, Quebec. Am J Respir Crit Care Med. 1997;155:568–576. doi: 10.1164/ajrccm.155.2.9032196. [DOI] [PubMed] [Google Scholar]
- Atkinson R W, Bremner S A, Anderson H R, Strachan D P, Bland J M, de Leon A P. Short-term associations between emergency hospital admissions for respiratory and cardiovascular disease and outdoor air pollution in London. Arch Environ Health. 1999;54:398–411. doi: 10.1080/00039899909603371. [DOI] [PubMed] [Google Scholar]
- Ilabaca M, Olaeta I, Campos E, Villaire J, Tellez-Rojo M M, Romieu I. Association between levels of fine particulate and emergency visits for pneumonia and other respiratory illnesses among children in Santiago, Chile. J Air Waste Manag Assoc. 1999;49:154–163. doi: 10.1080/10473289.1999.10463879. [DOI] [PubMed] [Google Scholar]
- Norris G, Young Pong S N, Koenig J Q, Larson T V, Sheppard L, Stout J W. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect. 1999;107:489–493. doi: 10.1289/ehp.99107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Soukup J M, Gilmour M I, Devlin R B. Stimulation of human and rat alveolar macrophages by urban air particulates: Effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–648. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- Quay J L, Reed W, Samet J, Delvin R B. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-κB activation. Am J Respir Cell Mol Biol. 1998;19:98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- Kodavanti U P, Schladweiler M C, Ledbetter A D, Hauser R, Christiani D C, Samet J M, McGee J, Richards J H, Costa D L. Pulmonary and systemic effects of zinc-containing emission particles in three rat strains: multiple exposure scenarios. Toxicol Sci. 2002;70:73–85. doi: 10.1093/toxsci/70.1.73. [DOI] [PubMed] [Google Scholar]
- Goulaouic S, Foucaud L, Bennasroune A, Laval-Gilly P, Falla J. Effect of polycyclic aromatic hydrocarbons and carbon black particles on pro-inflammatory cytokine secretion: impact of PAH coating onto particles. J Immunotoxicol. 2008;5:337–345. doi: 10.1080/15476910802371016. [DOI] [PubMed] [Google Scholar]
- Happo M S, Hirvonen M R, Halinen A I, Jalava P I, Pennanen A S, Sillanpaa M, Hillamo R, Salonen R O. Chemical compositions responsible for inflammation and tissue damage in the mouse lung by coarse and fine particulate samples from contrasting air pollution in Europe. Inhal Toxicol. 2008;20:1215–1231. doi: 10.1080/08958370802147282. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- O'Neill L A. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock D T, Latz E, Czura C J, Fenton M J, Tracey K J, Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu M Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- Hajjar A M, O'Mahony D S, Ozinsky A, Underhill D M, Aderem A, Klebanoff S J, Wilson C B. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, Equilis O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001;167:987–994. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- Gewirtz A T, Navas T A, Lyons S, Godowski P J, Madara J L. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith K D, Ozinsky A, Hawn T R, Yi E C, Goodlett D R, Eng J K, Akira S, Underhill D M, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci USA. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Hemmi H, Miyamoto H, Moriishi K, Tamura S, Takaku H, Akira S, Matsuura Y. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol. 2005;79:2847–2858. doi: 10.1128/JVI.79.5.2847-2858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt A C, Medzhitov R, Flavell R A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Lund J M, Alexopoulou L, Sato A, Karow M, Adams N C, Gale N W, Iwasaki A, Flavell R A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad-Nejad P, Kacker H, Rutz M, Bauer S, Vabulas R M, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- O'Neill L A, Bowie A G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Ruffini P A, Leifer C A, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa A K, Farber J M, Segal D M, Oppenheim J J. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Ninomiya-Tsuji J, Kishida S, Li X, Stark G, Matsumoto M. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of tak 1 by inducing tab2 translocation in the IL-1 signaling pathway. Mol Cell Biol. 2001;21:2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strelow A, Fontana E J, Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K A, Rowe D C, Barnes B J, Caffrey D R, Visintin A, Latz E, Monks B, Pitha P M, Golenbock D T. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K A, McWhirter S M, Faia K L, Rowe D C, Latz E, Golenbock D T, Coyle A J, Liao S M, Maniatis T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Kagan J C, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Becker S, Dailey L, Soukup J M, Silbajoris R, Delvin R B. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol Appl Pharmacol. 2005;203:45–52. doi: 10.1016/j.taap.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Ciencewicki J, Brighton L, Wu W D, Madden M, Jaspers I. Diesel exhaust enhances virus- and poly(I:C)-induced Toll-like receptor 3 expression and signaling in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1154–L1163. doi: 10.1152/ajplung.00318.2005. [DOI] [PubMed] [Google Scholar]
- Heller-Zeisler S F, Ondov J M, Zeisler R. Collection and characterization of a bulk PM2.5 air particulate matter material for use in reference materials. Biol Trace Elem Res. 1999;71:195–202. doi: 10.1007/BF02784205. [DOI] [PubMed] [Google Scholar]
- Birkenmeier G, Nicklisch S, Pockelt C, Mossie A, Steger V, Gläser C, Hauschildt S, Usbeck E, Huse K, Sack U. Polymyxin B-conjugated α2-macroglobulin as an adjunctive therapy to sepsis: modes of action and impact on lethality. J Pharmacol Exp Ther. 2006;318:762–771. doi: 10.1124/jpet.106.104265. [DOI] [PubMed] [Google Scholar]
- Bhor V M, Thomas C J, Surolia N, Surolia A. Polymyxin B: an ode to an old antidote for endotoxic shock. Mol Biosyst. 2005;1:213–222. doi: 10.1039/b500756a. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Greenberg R R. Elemental characterization of the National Bureau of Standards Milk Powder Standard Reference Material by instrumental and radiochemical neutron activation analysis. Anal Chem. 1986;58:2511–2516. doi: 10.1021/ac00125a034. [DOI] [PubMed] [Google Scholar]
- Zhang G H, Baek L, Koch C. New microassay for quantitation of endotoxin using Limulus amebocyte lysate combined with enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:1464–1470. doi: 10.1128/jcm.26.8.1464-1470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Medvedev A E, Kopydlowski K M, Vogel S N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Li N, Hao M, Phalen R F, Hinds W C, Nel A E. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Turpin B J, Weisel C P, Morandi M, Colome S, Stock T, Eisenreich S, Buckley B. Relationships of indoor, outdoor, and personal air (RIOPA): part II. Analyses of concentrations of particulate matter species. Res Rep Health Eff Inst. 2007;130:79–92. [PubMed] [Google Scholar]
- Foster S L, Hargreaves D C, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Toshchakov V, Jones B W, Lentschat A, Silva A, Perera P Y, Thomas K, Cody M J, Zhang S, Williams B R, Major J. TLR2 and TLR4 agonists stimulate unique repertoires of host resistance genes in murine macrophages: interferon-β-dependent signaling in TLR4-mediated responses. J Endotoxin Res. 2003;9:169–175. doi: 10.1179/096805103125001577. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Weis J J, Toshchakov V, Salkowski C A, Cody M J, Ward D C, Qureshi N, Michalek S M, Vogel S N. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmuller K H, Jung G, Brock R, Akira S, Ulmer A J. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem. 2006;281:9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- Wieland C W, Knapp S, Florquin S, de Vos A F, Takeda K, Akira S, Golenbock D T, Verbon A, van der Poll T. Non-mannose-capped lipoarabinomannan induces lung inflammation via toll-like receptor 2. Am J Respir Crit Care Med. 2004;170:1367–1374. doi: 10.1164/rccm.200404-525OC. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Wang Q, Miyake K, Kirschning C J, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938–1944. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- Devalia J L, Bayram H, Rusznak C, Calderon M, Sapsford R J, Abdelaziz M A, Wang J, Davies R J. Mechanisms of pollution-induced airway disease: In vitro studies in the upper and lower airways. Allergy. 1997;52:45–51. doi: 10.1111/j.1398-9995.1997.tb04870.x. [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook R D, Aguinaldo J G, Fayad Z A, Fuster V, Lippmann M. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Schaumann F, Borm P J, Herbrich A, Knoch J, Pitz M, Schins R P, Luettig B, Hohlfeld J M, Heinrich J, Krug N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med. 2004;170:898–903. doi: 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- Stoeger T, Reinhard C, Takenaka S, Schroeppel A, Karg E, Ritter B, Heyder J, Schulz H. Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ Health Perspect. 2006;114:328–333. doi: 10.1289/ehp.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat S E, Suh H H, Coull B A, Schwartz J, Stone P H, Gold D R. Ambient particulate air pollution and cardiac arrhythmia in a panel of older adults in Steubenville, Ohio. Occup Environ Med. 2006;63:700–706. doi: 10.1136/oem.2006.027292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev A E, Vogel S N. Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J Endotoxin Res. 2003;9:60–64. doi: 10.1179/096805103125001360. [DOI] [PubMed] [Google Scholar]
- Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor 4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Medvedev A E, Sabroe I, Hasday J D, Vogel S N. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- Boasen J, Chisholm D, Lebet L, Akira S, Horner A A. House dust extracts elicit Toll-like receptor-dependent dendritic cell responses. J Allergy Clin Immunol. 2005;116:185–191. doi: 10.1016/j.jaci.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Covert M W, Leung T H, Gaston J E, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman B T, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]