Abstract
Detection of LPS in tissues is an integral component of innate immunity that acts to protect against invasion by Gram-negative bacteria. Plasma down-regulates LPS-induced cytokine production from macrophages, thereby limiting systemic inflammation in blood and distant tissues. To identify the protein(s) involved in this process, we used classical biochemical chromatographic techniques to identify fractions of mouse sera that suppress LPS-induced TNF from bone marrow-derived macrophages (BMDMs). Fractionation yielded microgram quantities of a protein that was identified by MS to be hemopexin (Hx). Mouse Hx purified on hemin-agarose beads and rhHx decreased the production of cytokines from BMDMs and peritoneal macrophages induced by LPS. Preincubation of LPS with Hx did not affect the activity of LPS on LAL, whereas preincubation of Hx with macrophages followed by washing resulted in decreased activity of these cells in response to LPS, suggesting that Hx acts on macrophages rather than LPS. Heme-free Hx did not stimulate HO-1 in the macrophages. Purified Hx also decreased TNF and IL-6 from macrophages induced by the synthetic TLR2 agonist Pam3Cys. Our data suggest that Hx, which is an acute-phase protein that increases during inflammation, limits TLR4 and TLR2 agonist-induced macrophage cytokine production directly through a mechanism distinct from HO-1.
Keywords: TLR, agonist, serum, Pam3Cys, innate immunity
Introduction
Much of the morbidity from severe bacterial infections is caused by activation of the innate immune system with secondary inflammation and consequent tissue damage. In the case of Gram-negative bacterial infections, inflammation is induced primarily by release of LPS from bacterial cell walls. LPS makes up a large part of the outer membrane of Gram-negative bacteria and consists of a hydrophobic domain (lipid A), a nonrepeating “core” oligosaccharide, and a polysaccharide chain (the O-antigen) [1]. LPS interacts with the accessory molecule CD14 together with myeloid differentiation protein 2 and TLR4 on the surface of immune recognition cells such as macrophages to transmit the signal across the membrane and into the cell [2]. The signal is then transferred through one of several adaptor molecules to initiate a complex signal transduction cascade, which in turn, activates the NF-κB signaling pathway, ultimately leading to the release of multiple proinflammatory cytokines such as TNF and IL-6 [3]. These and other proinflammatory mediators recruit and activate additional immune cells to expand the inflammation in the microenvironment. If the local bacterial invasion is severe, bacteria and/or free LPS as well as cytokine mediators spill into the blood and induce inflammatory signals at distant sites leading to multiorgan system failure. The clinical manifestation of these processes has been defined by consensus as the sepsis syndrome [4]. Severe sepsis is common, with >700,000 cases in United States each year [5], many of which are caused by infection with Gram-negative bacteria.
The need to generate a sensitive and protective proinflammatory response to Gram-negative bacteria locally in tissues at the same time as limiting potentially deleterious generalized systemic inflammation causing sepsis requires a highly tuned and balanced control system [6, 7]. The interaction and subsequent stimulation of TLR4 on macrophages by LPS are modulated by several proteins that bind to LPS in the microenvironment of cells [8]. For example, LPS binds to LPS-binding protein and CD14 to facilitate activation of TLR4 [9, 10], and this process is opposed by binding of LPS to bacteriocidal permeability-inducing protein [11, 12], which decreases activation of macrophages.
One means of limiting spread of systemic inflammation outside of local tissues is the down-regulation of responsive immune cells in blood and fixed macrophages in the reticuloendothelial system by soluble cellular receptors and negative regulatory cytokines [7, 13]. Collectively, this restraining down-regulation of inflammation has been referred to as the compensatory anti-inflammatory system [7, 14]. In cell culture, the plasma of mice and humans at concentrations >1% acts on macrophages to down-regulate LPS-induced TNF directly [15]. Here, we describe the use of classical biochemical fractionation techniques and a bioassay using murine bone marrow-derived macrophages (BMDMs) to identify the heme-binding protein hemopexin (Hx) as a protein that specifically down-regulates LPS-induced production of TNF and IL-6 from macrophages. This finding was confirmed by using mHx purified on hemin-coupled agarose and rhHx. The data suggest that Hx, which increases in the setting of inflammation in response to IL-6 [16, 17], may limit LPS-induced systemic inflammation directly in settings of bacterial translocation or Gram-negative bacterial infection, irrespective of the presence of free heme.
MATERIALS AND METHODS
Materials
The following TLR agonists were purchased: smooth LPS from Escherichia coli O55:B5 (List Biological Laboratories, Campbell, CA, USA) and Pam3Cys (EMC Microcollections, Germany). C57BL/6 and C3H/HeN mice were from Charles River Labs (Wilmington, MA, USA). rhHx and reagents for the TNF and IL-6 ELISA were from R&D Systems (Minneapolis, MN, USA). Hemin chloride was from Frontier Scientific (Logan, UT, USA). Chicken anti-mHx and HRP-conjugated goat anti-chicken antibodies were from Immunology Consultants Laboratory (Newburg, OR, USA). Rabbit anti-mouse HO-1 and β-actin antibodies were from Abcam (Cambridge, MA, USA). HRP-conjugated goat anti-rabbit antibody was from MP Biomedicals (Aurora, OH, USA).
Preparation of macrophages
BMDMs were prepared from mice, as described [18], with minor modifications [19]. BMDMs were allowed to adhere overnight before use in assays. Thioglycollate-elicited macrophages were prepared as described previously [20] and incubated for 2 h to allow macrophage adherence prior to use.
Macrophage assays and cytokine production
BMDMs or peritoneal macrophages were washed three times in serum-free medium, followed by incubation overnight with LPS or Pam3Cys at indicated concentrations and column fractions to be tested (or Hx) at desired concentrations. Reaction mixtures for fractionation testing in general contained 80 μl media containing LPS or Pam3Cys and 20 μl column fractions or Hx at desired concentrations. Levels of TNF and IL-6 in the supernatants were quantitated by ELISA (R&D Systems). In some experiments, the cells were preincubated with column fractions or Hx for 2 h in serum-free medium and then washed three times, followed by incubation with LPS or Pam3Cys overnight.
Fractionation procedure
Mouse serum (Antibodies Inc., Davis, CA, USA) was precipitated in 60% SAS. After centrifugation, the supernatant was precipitated in 73% SAS, and the resulting precipitate was dialyzed in PBS and frozen in aliquots at −80°C until use. This material was fractionated by chromatofocusing in a decreasing pH 7–4 gradient on a 30-ml Tricorn 10/300 column (Amersham, Piscataway, NJ, USA) packed previously with PBE 94 gel and equilibrated with start buffer (0.025 M imidazole-HCL, pH 7.4). Aliquots that had been buffer-exchanged into 5 ml start buffer were applied to the column followed by elution with polybuffer 74-HCL 1:8 in water, pH 4.0 (Amersham). Fractions were precipitated in SAS to remove polybuffer, dialyzed against PBS, and tested for activity using the BMDM assay. Active fractions (3.6 mg) generated from material eluting at pH 4.3 were pooled and loaded onto a 10-ml anion exchange column (Q SephroseTM fast flow, Amersham), equilibrated with 20 mM Tris, pH 8, and eluted using a linear gradient to 400 mM NaCl. Activity was located in the first peak eluting at 290 mM NaCl. This material was pooled, buffer-exchanged, concentrated, and separated using a size-fractionation column (Superose 6) at a flow rate of 0.18 ml/min. Fractions were pooled, concentrated by microfiltration, split, and tested in the BMDM assay. The other half of the pool or pools from similar fractionations were digested with trypsin and analyzed via liquid chromatography/MS/MS on a Thremo LCQ Deca Ion Trap mass spectrometer followed by database correlational analysis using the Mascot search engine (University of Massachusetts Medical School Laboratory for Proteomic Mass Spectrometry, Shrewsbury, MA, USA).
Purification of Hx from mouse serum
Serum Hx was purified as described [16, 21]. Briefly, 12 ml mouse serum was adjusted to pH 8.0 and mixed gradually with an equivalent volume of cold 1.68% rivanol solution (pH 8.0). The mixture was stirred gently for 5 h at 4°C to precipitate and remove rivanol-abumin complex. Free rivanol in the supernatant was precipitated by centrifugation following addition of 5% NaCl. The above rivanol-supernatant was dialyzed against pyrogen-free PBS and applied to an 8-ml hemin-agarose column (Sigma Chemical Co., St. Louis, MO, USA). In some preparations, protease inhibitors [0.5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 10 μM E-64, 2 μg/ml aprotinin, and 1 μM pepstatin A] were added and interacted with the dialyzed rivanol-supernatant for 15 min by gentle agitation at 4°C before the application to the hemin-agarose column, which was washed thoroughly with 400 ml PBS containing 0.5 M NaCl to remove unbound proteins. Hx bound to the column was eluted by 0.2 M citric acid (pH 2.0), followed by immediate neutralization. Proteins in the eluate were applied to a reverse-phase C4 HPLC column twice, and the middle section of the single peak was collected, lyophilized, and resuspended in PBS.
SDS-PAGE, silver staining, and immunoblot analysis for Hx and HO-1
Hx purified on hemin-agarose and HPLC or the lysate of BMDMs was separated by SDS-PAGE. To analyze HO-1 expression, BMDMs were treated with mHx, rhHx, or hemin chloride at desired concentrations or hemin-Hx complex formed by coincubation of Hx and hemin chloride at the same molar concentrations at room temperature for 20 min. The cells were then removed from tissue culture at various times, washed with PBS three times, and incubated in 50 μl cold radioimmunoprecipitation assay buffer (Cell Signaling, Danvers, MA, USA) containing 1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml pepstatin for 5 min. Cells were next scraped from the plates and sonicated briefly. Cellular debris was removed by centrifugation at 14,000 g 4°C for 10 min. The protein concentrations in the cell lysate were quantified by the MicroBCA™ protein assay kit (Pierce, Rockford, IL, USA). Total proteins in each sample (2–5 μg) were separated by 12% SDS-PAGE under denaturing conditions. The gels were stained with silver, according to the manufacturer’s protocol (Sigma Chemical Co.) or transferred to a nitrocellulose membrane if desired. Membranes were then blocked with 2.5% nonfat dry milk in TTBS and incubated with primary antibody (1:1000) overnight at 4°C, followed by washes in TTBS and visualization using HRP-conjugated goat anti-rabbit IgG (1:1000) or goat anti-chicken IgY (1:5000) and the Western Lightning™ chemiluminescence reagent (PerkinElmer LAS, Boston, MA, USA), according to the manufacturer’s instructions. If desired, the membranes were then stripped and reprobed with rabbit polyclonal anti-β-actin antibody (1:2500).
LAL assay
The LAL assay was performed as described previously [22, 23]. Briefly, 50 μl purified Hx (2 μg/ml) in PBS or PBS alone was mixed with 50 μl serial twofold dilutions of E. coli 0113 LPS in normal saline in 96-well microtiter plates. After incubation for 3 h at 37°C, 100 μl reconstituted LAL (Associates of Cape Cod, Falmouth, MA, USA) was added and mixed. After further incubation at 37°C for 1 h, the plates were read on a microplate reader (BIO-TEC Instruments, Winooski, VT, USA) at 380 nm to measure coagulation of the LAL.
Statistics
Except where indicated, representative data from at least three experiments are presented in the figures. Data are expressed as means, and error bars represent se. The data were analyzed by GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). One-way ANOVA followed by Dunnett’s post hoc test was used to assess cytokine levels produced by macrophages treated with LPS at 20 ng/ml or Pam3Cys at 200 ng/ml and Hx at desired concentrations. Two-way ANOVA followed by Bonferroni’s post hoc test was used to assess cytokine levels from macrophages treated with different concentrations of LPS in the presence of 20 μg/ml mHx or PBS. Values of P < 0.05 were considered to be statistically significant. The following notations have been used to denote P values in the figures: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Fractionation of proteins in mouse serum that down-regulates LPS-induced TNF
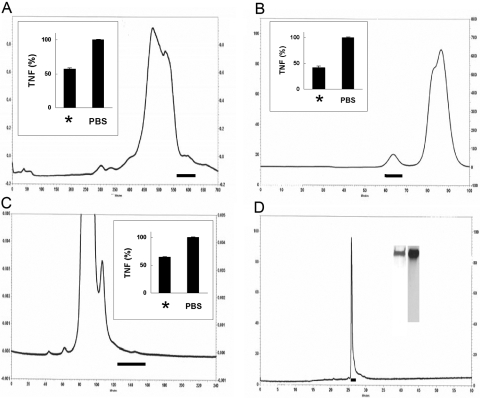
We first developed a sensitive, reproducible, and rapid bioassay that could be used to detect suppression of LPS-induced TNF from BMDMs. This assay was used in combination with classical fractionation techniques to identify fractions from each separation that contained suppressive activity. The final purification sequence consisted of fractionation by salting out, chromatofocusing, anion exchange, and molecular sieving, as shown in Figure 1, A–C. Late eluting material from the molecular sieving column contained a tiny peak on the chromatograph that suppressed LPS-induced TNF in the bioassay (Fig. 1C) and contained only mHx, as assessed by MS. We also identified Hx together with limited numbers of other plasma proteins in four other experimental preparations using similar but not identical fractionation methods.
Figure 1.
Identification, purification, and purity of mHx. (A–C) Chromatograph of active fractions as described. Solid horizontal lines indicate material selected for activity in suppressing LPS-induced TNF from BMDMs. Insets show activity of pooled material from column; left bars are labeled with *, showing LPS-induced TNF in the presence of pooled material indicated by horizontal line, and right bars show LPS-induced TNF in the presence of PBS alone. (A) Chromatogram of 60–73% saturated ammonium sulfate precipitate of mouse serum chromatofocused on a 30-ml Tricorn 10/300 column in a decreasing pH 7 to 4 gradient. (B) Chromatogram of separation by anion exchange of material shown in A. (C) Chromatogram of a molecular sieving column of material shown in B. The material indicated was analyzed by MS and contained mHx. (D) Chromatogram on reverse-phase C4 column of 1 μg mHx purified on hemin-agarose as in Materials and Methods. Horizontal line shows material collected for final preparation. Inset shows SDS-PAGE and silver staining of this material (left lane) and Western blot developed with chicken anti-mHx IgY (right lane).
Purified mHx down-regulates LPS-induced proinflammatory cytokine production from macrophages
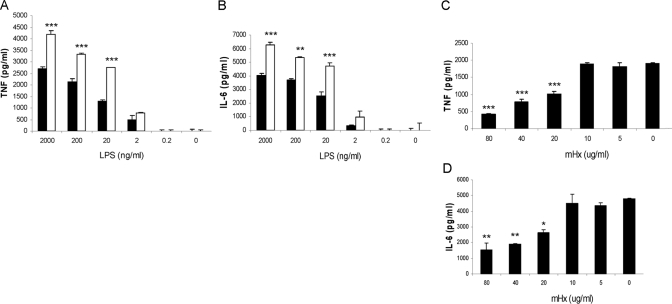
We purified Hx from whole mouse serum using precipitation with rivanol followed by hemin-agarose affinity chromatography [16, 21] and reverse-phase HPLC (Fig. 1D). To examine whether the purified mHx decreased the inflammatory response of macrophages, we treated the BMDMs with 20 μg/ml mHx or PBS and various concentrations of LPS (Fig. 2, A and B) or with 20 ng/ml LPS and various concentrations of Hx (Fig. 2, C and D). Production of TNF and IL-6 was down-regulated in a dose-dependent manner. Similar results were obtained using mouse peritoneal macrophages (see Fig. 3, C and D).
Figure 2.
Effect of mHx on LPS-induced TNF and IL-6 production from BMDMs. Cells were washed three times with serum-free medium and then cultured overnight with 20 μg/ml mHx (solid bars) or PBS (open bars) and indicated concentrations of LPS (A and B) or 20 ng/ml LPS and indicated concentrations of mHx (C and D). Concentrations of TNF and IL-6 in the supernatants were determined by ELISA. The results represent mean ± se and are representative of more than five independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with cells treated with PBS.
Figure 3.
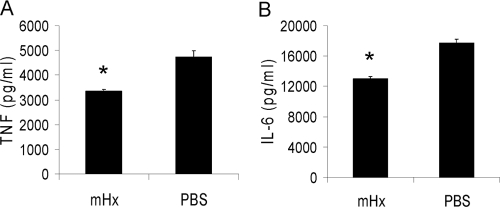
rhHx suppresses LPS-induced TNF and IL-6 from macrophages. BMDMs (A and B) or peritoneal macrophages (C and D) were incubated overnight with 20 ng/ml LPS and the indicated concentrations (A and B) or 40 μg/ml (C and D) mHx and rhHx. Concentrations of TNF and IL-6 in the supernatants were determined by ELISA. The results represent mean ± se and are representative of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with cells treated with PBS.
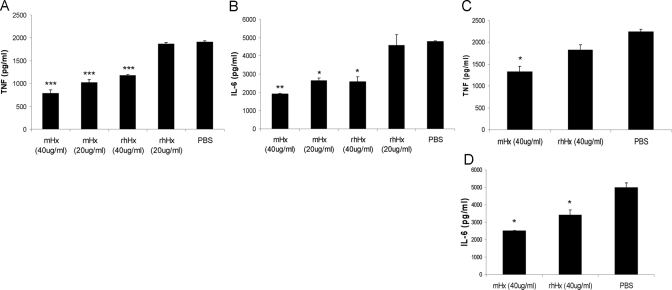
rhHx down-regulates LPS-induced cytokine production from BMDMs and peritoneal macrophages
We estimated the purity of the purified mHx to be >95% (Fig. 1D). To verify that it was the Hx rather than trace amounts of other unknown serum factors that had activity in our assays, we studied the suppressive effect of commercial rhHx on BMDMs (Fig. 3, A and B) and on peritoneal macrophages (Fig. 3, C and D). rhHx down-regulated TNF and IL-6 from BMDMs and IL-6 from peritoneal macrophages. The identity of the sequence of amino acids between hHx and mHx is 74% according to National Center for Biotechnology Information BLAST searching results.
Hx acts on macrophages rather than LPS to suppress cytokine production
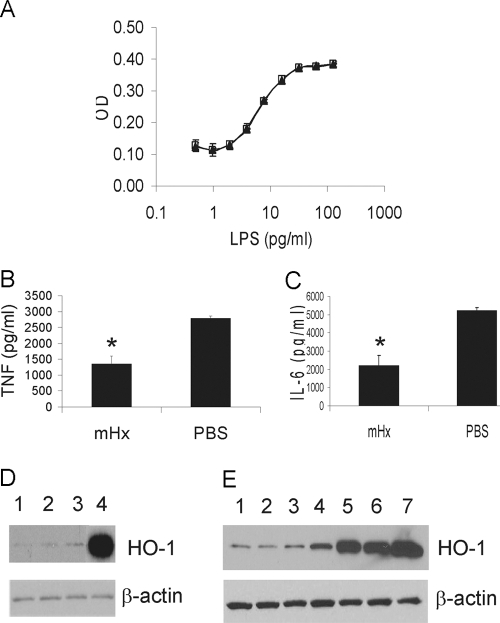
To evaluate if the down-regulation of the LPS-induced inflammatory response from macrophages might be a result of binding and neutralization of LPS by Hx, we studied the ability of Hx to neutralize the effect of LPS on the coagulation of LAL, which is a highly sensitive noncellular assay of LPS activity [24]. There was no significant difference between control PBS and Hx in the assay (Fig. 4A). We also preincubated BMDMs with purified mHx for 2 h, after which, the cells were washed extensively in serum-free medium and stimulated by LPS. Preincubation of the cells with mHx decreased LPS-induced TNF and IL-6 release significantly (Fig. 4, B and C). Preincubation of the cells with rhHx also down-regulated the activity of peritoneal macrophages (data not shown). These data suggest that Hx acts on macrophages rather than the LPS to suppress proinflammatory cytokine production.
Figure 4.
Hx does not neutralize LPS as assessed by Limulus lysate actvity but acts on macrophages in a manner that does not up-regulate the expression of HO-1. (A) LAL assay of LPS preincubated with 2 μg/ml purified mHx (▴) or PBS (□). (B and C) BMDMs were preincubated with mHx (20 μg/ml) for 2 h, followed by extensive washes in serum-free medium. LPS (20 ng/ml) was then added to the culture and incubated overnight. Concentrations of TNF (B) and IL-6 (C) in the supernatants were determined by ELISA. The results represent mean ± se and are representative of five independent experiments. *, P < 0.05, compared with cells treated with PBS. (D and E) BMDMs were incubated overnight with: (D) Lane 1, PBS; Lane 2, mHx (40 μg/ml); Lane 3, rhHx (40 μg/ml); Lane 4, hemin chloride (3 μM), or (E) Lane 1, PBS; Lane 2, mHx (40 μg/ml, equal to 0.67 μM); Lane 3, mHx (200 μg/ml, equal to 3.35 μM); Lane 4, hemin-mHx complex (0.67 μM); Lane 5, hemin-mHx complex (3.35 μM); Lane 6, hemin-chloride (0.67 μM); Lane 7, hemin chloride (3 μM). Cells were then washed three times by PBS. Cell lysates were used for SDS-PAGE. Western blot was performed using anti-mouse HO-1 antibody, followed by stripping and reprobing by anti-β-actin antibody.
Hx does not induce the up-regulation of HO-1 in macrophages
Hx-mediated heme transport into cells induces the up-regulation of HO-1, which has anti-inflammatory effects including suppression of LPS-induced TNF through the heme catabolites carbon monoxide, biliverdin, and bilirubin [17, 25, 26]. The purified mHx and rhHx should be heme-free Hx. To evaluate if HO-1 was involved in the mechanism responsible for the suppressive effect of Hx on LPS-induced cytokine production, we examined if Hx induced HO-1 in macrophages. BMDMs were treated with free Hx, or hemin, or hemin-Hx complexes, and specific anti-mouse HO-1 antibody was used to detect the HO-1 expression in the cells after overnight incubation. Neither purified mHx nor rhHx induced the up-regulation of HO-1 in the absence of heme, even at high concentrations (200 μg/ml; Fig. 4, D and E). In contrast, hemin and hemin-Hx complexes up-regulated HO-1. These results suggest that induction of HO-1 is not involved in the mechanism responsible for the suppressive effect of LPS-induced TNF and IL-6 from macrophages by heme-free Hx.
Hx down-regulates cytokine production induced by synthetic TLR2 agonist Pam3Cys
We next studied the specificity of the macrophage suppression by Hx with respect to TLR agonist stimulation. BMDMs were treated with or without Hx and stimulated with the TLR2 agonist Pam3Cys, a synthetic analog of bacterial lipopeptides. Purified mHx significantly decreased production of TNF and IL-6 induced by stimulation with Pam3Cys (Fig. 5). Similar results were obtained when different concentrations of Hx and Pam3Cys were used. These data suggest that the suppressive effect of Hx is not specific to LPS.
Figure 5.
Effects of Hx on cytokine production from macrophages stimulated by Pam3Cys. BMDMs were incubated overnight with mHx (100 μg/ml) or PBS with Pam3Cys (200 ng/ml). Concentrations of TNF and IL-6 in the supernatants were determined by ELISA. The results represent mean ± se and are representative of more than five independent experiments. *, P < 0.05, compared with cells treated with PBS.
DISCUSSION
The major finding of our studies is that Hx interacts with macrophages to down-regulate LPS-induced TNF and IL-6. The starting point of our studies was mouse serum, which was described to have this property [15]. We purified Hx from mouse serum using classical fractionation procedures and then confirmed its activity using Hx purified on hemin-coupled agarose and commercially available rhHx. Hx is an acute-phase protein with an extremely high binding affinity for heme and is present in blood at concentrations of 0.55–1.25 mg/ml [16, 17]. It is generally considered to be a protective molecule for antioxidative stress caused by free heme [17]. To our knowledge, this is the first study to report that Hx limits LPS- and Pam3Cys-induced TNF and IL-6 from macrophages.
There has been intense interest in the pro- and anti- inflammatory balance of patients with severe Gram-negative bacterial infection because inappropriate, uncontrolled systemic inflammation is believed to be important in the pathophysiology of sepsis [6, 7, 27]. Teleologically, the host might be expected to favor induction of inflammation in the local microenvironment of early bacterial infection, but simultaneously to possess strong mechanisms in place to limit inflammation in blood or distant tissues, which might lead to organ damage and severe sepsis [28]. Our data are consistent with this concept, as Hx in plasma would directly limit systemic cytokine production induced by LPS during endotoxemia or bacteremia and not affect the capacity of macrophages to respond in tissues where levels of Hx in interstitial fluid are presumably low.
It has been known for many years that free hemoglobin potentiates the effects of endotoxemia, increasing TNF and mortality [29, 30]. Indeed, LPS is synergistic with hemoglobin to induce proinflammatory cytokines from macrophages [31]. Hemoglobin [32] and free heme [33] have been described to activate macrophages to produce TNF, even in the absence of LPS. The coexistance of hemoglobin and the potential for free heme together with LPS are common in some clinical settings such as in surgical intensive care units, when there may be simultaneous tissue injury and bleeding, exogenous hemoglobin administration in the form of transfusions, and Gram-negative infection. In these settings, Hx serves to bind and sequester or clear free heme and therefore, diminishes inflammation indirectly by decreasing the synergy of LPS with heme. Our data suggest that Hx may have a much more direct role in limiting inflammation by acting on macrophages to decrease cytokine production in response to LPS.
Our results suggest that Hx may also be important in clinical settings in the absence of liberated heme. It is likely that levels of Hx vary in different disease states. Hx is a Class I acute-phase protein that is responsive to IL-6 [17]. Levels increase in the setting of Gram-negative infections and decrease in settings of catabolism. Therefore, in settings of Gram-negative bacterial translocation or infection, there could be clinical relevance to differing levels of Hx. There is accumulating evidence that there may be endogenous ligands for TLR4 in addition to LPS and that these ligands could play a role in acute or chronic inflammatory disease states [34,35,36,37]. As these TLR4 ligands become better understood, it will be of interest to evaluate if Hx likewise acts to modulate the effect of macrophages on their activity.
There are several potential limitations to our studies. First, we used a mouse system for our studies, in part so that we would eventually be able to study more carefully the serum proteins involved. Plasma levels of Hx in mice and humans are roughly equivalent [16, 38], although mice are relatively resistant to LPS as compared with humans. As in many studies involving mouse proteins or cells, caution will need to be used in extending the results to human conditions.
Second, the molecular signaling mechanisms for our findings remain unclear. Heme as well as heme-Hx complexes stimulate macrophages to increase HO-1, which is broadly anti-inflammatory through generation of biliverdin and carbon monoxide [17, 25, 39]. Pretreatment of cells or animals with heme leads to generation of HO-1, anti-oxidant effects, and protection from LPS [25]. It was therefore logical to evaluate if HO-1 could be part or all of the mechanism by which Hx down-regulates macrophages. However, although we found that while both free hemin and hemin-Hx complexes induced HO-1 in mouse macrophages, neither purified mHx nor rhHx induced HO-1, making stimulation of HO-1 unlikely as a mechanism for the down-regulation. A recent report describes an exquisite specificity of heme to activate macrophages through a mechanism involving TLR4 independently of a LPS-binding site [33]. We considered that the Hx could somehow interact with an unknown source of exogenous or cell-bound heme and thus block a heme-specific site on TLR4 to decrease LPS/heme synergy [31]. However, this seems unlikely, because our experiments were done in the strict absence of heme, because there was no baseline stimulation of the cells in conditions in which LPS was absent and because Hx also down-regulates TLR2 agonist stimulation by Pam3Cys. The low-density receptor-related protein LRP1/CD91 has been reported to scavenge heme-Hx complexes [40], although it is unclear if heme-free Hx also binds to this receptor. More work will be needed to better understand the mechanisms involved.
Third, in some but not all of the biochemical fractionation experiments using mouse serum, we identified plasma proteins in addition to Hx in active fractions. We cannot rule out that some of these proteins could act in concert with Hx or that these or other yet-unidentified proteins may play a contributing role in the net plasma activity.
Our studies were initiated to better understand the plasma proteins involved in limiting systemic inflammation during infections. The kinetics of the release of LPS, the resting level of Hx in plasma, induction of Hx as part of an IL-6-induced inflammatory response, the presence of free heme in blood or tissues, and the induction of HO-1 by heme, heme/Hx, and/or IL-10 may be important determinants of systemic inflammation [25, 26, 41]. The finding that Hx limits the response of macrophages to TLR4 and TLR2 ligands through a mechanism that does not involve heme or HO-1 adds another element to the complicated interplay of these processes and suggests that Hx plays a role to limit generalized inflammation during Gram-negative bacterial infections even in the absence of hemolysis.
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health (AI059010, GM59694), Shriners Hospital for Crippled Children (8720), and institutional funding from the Institute Pasteur. X. L., J.-M. C., and H. S. W. have submitted information contained in this article for patent protection in accordance with institutional policies.
Footnotes
Abbreviations: BMDM=bone marrow-derived macrophage, HO-1=heme oxygenase-1, Hx=hemopexin, LAL=Limulus amoebocye lysate, mHx=mouse Hx, MS=mass spectrometry, Pam3Cys=tripalmitoyl-S-glyceryl-cysteine, rhHx=recombinant human Hx, SAS=saturated ammonium sulfate, TTBS=1% Tween in TBS
References
- Raetz C R, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Bone R C, Balk R A, Cerra F B, Dellinger R P, Fein A M, Knaus W A, Schein R M H, Sibbald W J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Angus D C, Linde-Zwirble W T, Lidicker J, Clermont G, Carcillo J, Pinsky M R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R S, Karl I E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Buras J A, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Gioannini T L, Weiss J P. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res. 2007;39:249–260. doi: 10.1007/s12026-007-0069-0. [DOI] [PubMed] [Google Scholar]
- Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide-binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Gegner J A, Ulevitch R J, Tobias P S. Lipopolysaccharide (LPS) signal transduction and clearance. Dual roles for LPS binding protein and membrane CD14. J Biol Chem. 1995;270:5320–5326. doi: 10.1074/jbc.270.10.5320. [DOI] [PubMed] [Google Scholar]
- Elsbach P, Weiss J. The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against Gram-negative bacteria and lipopolysaccharide. Immunobiology. 1993;187:417–429. doi: 10.1016/S0171-2985(11)80354-2. [DOI] [PubMed] [Google Scholar]
- Tobias P S, Soldau K, Iovine N M, Elsbach P, Weiss J. Lipopolysaccharide (LPS)-binding proteins BPI and LBP form different types of complexes with LPS. J Biol Chem. 1997;272:18682–18685. doi: 10.1074/jbc.272.30.18682. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- Bone R C. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Heumann D, Adachi Y, Le Roy D, Ohno N, Yadomae T, Glauser M P, Calandra T. Role of plasma, lipopolysaccharide-binding protein, and CD14 in response of mouse peritoneal exudate macrophages to endotoxin. Infect Immun. 2001;69:378–385. doi: 10.1128/IAI.69.1.378-385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Eberhard U. Hemopexin. Methods Enzymol. 1988;163:536–565. doi: 10.1016/0076-6879(88)63049-7. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Altruda F. Hemopexin: structure, function, and regulation. DNA Cell Biol. 2002;21:297–306. doi: 10.1089/104454902753759717. [DOI] [PubMed] [Google Scholar]
- Schilling D, Thomas K, Nixdorff K, Vogel S N, Fenton M J. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J Immunol. 2002;169:5874–5880. doi: 10.4049/jimmunol.169.10.5874. [DOI] [PubMed] [Google Scholar]
- Bagchi A, Herrup E A, Warren H S, Trigilio J, Shin H S, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- Hellman J, Roberts J D J, Tehan M M, Allaire J E, Warren H S. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J Biol Chem. 2002;277:14274–14280. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kato H, Sakuma Y, Namiki H. Hemopexins suppress phorbol ester-induced necrosis of polymorphonuclear leucocytes. Cell Struct Funct. 2001;26:235–241. doi: 10.1247/csf.26.235. [DOI] [PubMed] [Google Scholar]
- Novitsky T J, Roslansky P F, Siber G R, Warren H S. Turbidimetric method for quantifying serum inhibition of Limulus amoebocyte lysate. J Clin Microbiol. 1985;21:211–216. doi: 10.1128/jcm.21.2.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H S, Novitsky T J, Ketchum P A, Roslansky P F, Kania S, Siber G R. Neutralization of bacterial lipopolysaccharides by human plasma. J Clin Microbiol. 1985;22:590–595. doi: 10.1128/jcm.22.4.590-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J, Bang F B. The role of endotoxin in the extracellular coagulation of Limulus blood. Bull Johns Hopkins Hosp. 1964;115:265–274. [PubMed] [Google Scholar]
- Otterbein L E, Soares M P, Yamashita K, Bach F H. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Lee T S, Chau L Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Riedemann N C, Guo R F, Ward P A. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- Munford R S. Detoxifying endotoxin: time, place and person. J Endotoxin Res. 2005;11:69–84. doi: 10.1179/096805105X35161. [DOI] [PubMed] [Google Scholar]
- Su D, Roth R I, Levin J. Hemoglobin infusion augments the tumor necrosis factor response to bacterial endotoxin (lipopolysaccharide) in mice. Crit Care Med. 1999;27:771–778. doi: 10.1097/00003246-199904000-00034. [DOI] [PubMed] [Google Scholar]
- Su D, Roth R I, Yoshida M, Levin J. Hemoglobin increases mortality from bacterial endotoxin. Infect Immun. 1997;65:1258–1266. doi: 10.1128/iai.65.4.1258-1266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. Hemoglobin and LPS act in synergy to amplify the inflammatory response. J Dent Res. 2007;86:878–882. doi: 10.1177/154405910708600914. [DOI] [PubMed] [Google Scholar]
- Carrillo E H, Gordon L E, Richardson J D, Polk H C., Jr Free hemoglobin enhances tumor necrosis factor-α production in isolated human monocytes. J Trauma. 2002;52:449–452. doi: 10.1097/00005373-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Figueiredo R T, Fernandez P L, Mourao-Sa D S, Porto B N, Dutra F F, Alves L S, Oliveira M F, Oliveira P L, Graca-Souza A V, Bozza M T. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- Jobin N, Garrel D R, Bernier J. Increased burn-induced immunosuppression in lipopolysaccharide-resistant mice. Cell Immunol. 2000;200:65–75. doi: 10.1006/cimm.2000.1619. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen G, Wyburn K R, Yin J, Bertolino P, Eris J M, Alexander S I, Sharland A F, Chadban S J. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva M V, Inouye K, Tzameli I, Yin H, Flier J S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechl S, Lorenz E, Reindl M, Wiedermann C J, Oberhollenzer F, Bonora E, Willeit J, Schwartz D A. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- Ishiguro T, Imanishi K, Suzuki I. Hemopexin levels in mice. Int J Immunopharmacol. 1984;6:241–244. doi: 10.1016/0192-0561(84)90022-5. [DOI] [PubMed] [Google Scholar]
- Otterbein L E, Bach F H, Alam J, Soares M, Tao L H, Wysk M, Davis R J, Flavell R A, Choi A M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Hvidberg V, Maniecki M B, Jacobsen C, Hojrup P, Moller H J, Moestrup S K. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- Chung S W, Liu X, Macias A A, Baron R M, Perrella M A. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]