Abstract
Sepsis is the leading cause of death in critically ill patients in the United States with over 210,000 deaths annually. One stumbling block to an effective therapy of sepsis has been the lack of a clinically relevant animal model. There are important distinctions in the mouse versus human immune system regarding the host response to invading pathogens. These differences may explain the disappointing results in many sepsis clinical trials despite the clear efficacy of these agents in mouse models of sepsis. The purpose of the present study was to develop a “humanized” mouse model of sepsis and to determine if the model recapitulated the major findings of lymphocyte apoptosis and cytokine response that exist in patients with sepsis. Two-day-old NOD-scid IL2rγnull mice received an adoptive transfer of hCD34+ hematopoietic cord blood stem cells. These mice acquired a functional human innate and adaptive immune system, as evidenced by the development of all lineages of human immune cells as well as by mounting a DTH response. Eight weeks post-transfer, mice were made septic using the highly clinical relevant CLP model of sepsis, and sepsis induced marked elevations in human pro- and anti-inflammatory cytokines as well as a dramatic increase in human T and B cell apoptosis. Collectively, these results show that the humanized mouse model recapitulates many of the classic findings in patients with sepsis. Therefore, it represents an advanced, clinically relevant model for mechanistic studies of sepsis and testing of novel therapies.
Keywords: immune cells, stem cells, inflammation, cell death
Introduction
Sepsis is the systemic inflammatory response that occurs during severe infection, and it remains the most common cause of death in intensive care units [1]. The number of septic patients is increasing every year, and despite medical advances over the past 25 years, the mortality rate from sepsis remains essentially unchanged [2, 3]. A particularly troublesome aspect of sepsis research has been the failure to translate therapies that were successful in animal models to patients [4, 5]. Despite numerous efficacious therapies in murine models of sepsis, virtually no mouse laboratory studies have been replicated in patients with sepsis. This fact is readily reflected in the failure of over 25 clinical trials in patients with sepsis [4]. Part of the problem with the lack of translation of promising animal studies to humans may be related to the many fundamental differences between the mouse and the human immune system. A recent review article listed over 60 key differences between the mouse and human immune systems [6] with many of these relating to key aspects of the host response to pathogens. For example, there are major distinctions in the cellular distribution of Toll receptors, NO production, cytokine expression, and MyD88 signaling [6, 7].
The ability to study human disease is limited as a result of ethical and technical concerns. Given the limitations of mouse models of sepsis, one potential solution is the use of “humanized” mice, which refer to normal immunocompetent mice expressing human genes via transgenesis (for example, HLA or human Ig transgenic mice) or to immunodeficient mice engrafted with human hematopoietic cells. A significant advance in humanized mice occurred with the creation of humanized mice that possess all human myeloid and lymphoid lineages following engraftment with hCD34+ hematopoietic stem cells [8]. In this model, human T and B cells develop from human hematopoietic stem cells that are engrafted into the mice. The T and B cells undergo negative selection in the thymus and are tolerant of the mouse host [9, 10]. Previous models were complicated by poor stem cell engraftment and by development of graft versus host immune abnormalities. Neonatal NOD-scid IL2rγnull mice that receive hCD34+ hematopoietic stem cells develop a complete lineage of human cells of the innate and adaptive immune system including monocytes/macrophages, plasmacytoid and myeloid DC, NK cells, T and B lymphocytes, and others [11]. Thus, this system allows the investigator to study the generation of primary immune responses by a naïve human immune system. These humanized mouse models have been used for the study of human autoimmune diseases [12], human pancreatic islet transplantation [13], infection of hCD4 T cells by HIV [14], and other disorders.
The humanized mouse model is particularly appropriate for sepsis research, as a hallmark of sepsis is the profound apoptosis-induced depletion of cells of the innate and adaptive immune system [15,16,17,18]. Apoptosis of immune effector cells is central to the pathophysiology of sepsis, as multiple independent laboratories have shown that prevention of lymphocyte apoptosis in sepsis improves survival [19,20,21]. Determining if the human lymphocytes undergo apoptosis in the humanized mouse model may provide insights into potential mechanisms for lymphocyte apoptosis. In addition, the immune effector cells induce an initial cytokine-mediated hyperinflammatory response, followed by a hypoimmune or “immunoparalysis” phase [22,23,24,25]. This immunoparalysis is typified by the well-recognized loss of the DTH response [26]. Thus, development of a humanized mouse model of sepsis offers the capability of studying critical aspects of the immunologic response in a manner that more accurately reflects the clinical scenario.
The purpose of the present study was to develop and characterize a humanized mouse model of sepsis. In particular, the effect of sepsis on human lymphocyte apoptosis and human pro- and anti-inflammatory cytokine production were evaluated in a clinically relevant mouse peritonitis model of sepsis. The development of this model will provide keener insight into the pathophysiology of sepsis and serve as a more translational method to evaluate new therapies of this highly lethal disorder.
MATERIALS AND METHODS
Mice
NSG mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA; Stock #005557). Mice were bred to obtain newborn offspring for injection. All animal procedures were performed according to National Institutes of Health guidelines and approved by the Washington University Institutional Animal Care and Use Committee (St. Louis, MO, USA).
Injection of newborn mice
Within 24–36 h post birth, pups received a single radiation dose of 1 Gy in a γ-cell 40 irradiator at a rate of 77 rads/min. Twenty-four hours postradiation, newborn mice were restrained by holding the body between the thumb and forefinger so that the forelimbs were immobilized. The animal’s head was extended back so that the superficial temporal vein formed a straight line [27]. Human-purified CD34+ cord blood cells (1×105; Cambrex Lonza, Allendale, NJ, USA; Cat. #2C-101, or NDRI, Philadelphia, PA, USA; Cat. #OD28329), in a volume of 50 ul saline, were injected using a 30-gauge needle. Engraftment of human cells was determined initially in the peripheral blood by staining for human B cells 8 weeks postadoptive transfer.
Antibodies and reagents
Unless otherwise indicated, all antibodies were purchased from Becton Dickinson (San Diego, CA, USA) or eBioscience (San Diego, CA, USA). The following human antibodies were used for phenotypic analysis of human immune effector cells: eBioscience—CD4 (Cat. #11-0049) for hCD4 T cells, CD8 (Cat. #15-0088) for hCD8 T cells, CD15 (Cat. #11-0159) for human neutrophils and monocytes, CD14 (Cat. #12-0149) for human macrophages, CD20 (Cat. #11-0209) and CD19 (Cat. #12-0199) for human B cells; BD PharMingen (San Jose, CA, USA)—CD86 (Cat. #555659) for hDC, CD34 (Cat. #555823) for human hematopoietic stem cells, CD20 (Cat. #555622) for human B cells, HLA-DR (Cat. #555811) for hDC.
The staining for apoptotic cells via TUNEL was performed using the APO-BRDU™ kit (Phoenix Flow Systems, San Diego, CA, USA). Active caspase-3 was determined by using a rabbit anti-mouse antibody (Cell Signaling Technologies, Beverly, MA, USA; Cat. #9961L), followed by a secondary donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA; Cat. #711-116-152). Cytokine production was determined by BD™ CBA Human Th1/Th2 Cytokine Kit-II (Cat. #551809) and CBA Human Inflammation Kit (Cat. #551811) from BD PharMingen. rhIL-7 was purchased from R&D Systems (Minneapolis, MN, USA; Cat. #207-IL). The IL-7 stabilizing antibody M25 was a kind gift from David Hilderman (Cincinnati Children’s Hospital, Cincinnati, OH, USA). TNBS was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Sepsis model—CLP
The CLP model was used to induce intra-abdominal peritonitis [28]. Mice were anesthetized with isoflurane, and an abdominal incision was performed. The cecum was identified, ligated, and punctured twice with a #27-gauge needle. The abdomen was closed in two layers, and 1 cc 0.9% saline was administered s.c. Sham-operated mice were treated identically, except the cecum was not ligated or punctured. The Animal Studies Committee at Washington University School of Medicine approved the animal experimentation.
Stabilization of rhIL-7
rhIL-7 (2.5 μg) was mixed with 12.5 μg M25/mouse in a small volume. Solution was incubated for 15 min at room temperature. Sterile saline was added to increase the volume to 100 μl/mouse, which was injected into the mouse using the s.c. route [29].
Organ harvest
Mice were killed and organs harvested ∼24 h after CLP or sham surgery. Parts of the organs were stored in formalin for tissue histochemistry. Splenocytes, thymocytes, and lymph nodes were prepared by gently pressing the organs through a 70-μ filter. Bone marrow was flushed out of tibias and femurs. Blood was harvested by cardiac puncture.
Conventional bright-field microscopy of H&E-stained tissue sections
Tissue specimens of spleens and thymi were fixed overnight in 10% buffered formalin. Tissue sections were prepared and stained by H&E in the Morphology Digestive Disease Research Core Center at Washington University School of Medicine. Specimens were examined via bright-field microscopy using a Nikon Eclipse E600 as a nonquantitative confirmatory method of data obtained by flow cytometry. Apoptotic splenocytes exhibit characteristic findings of nuclear compaction (pyknosis) and fragmentation (karyorrhexis). These morphological features are readily apparent on bright-field light microscopy. Five sham and CLP samples were examined.
Flow cytometry staining
Flow cytometry staining for human lymphocytes was performed using FSC and SSC to determine living cells. In engrafted mice, the human lymphocyte population was clearly visible but absent in naïve, nontransplanted mice. Surface marker staining was performed as indicated in the text. Naïve, nontransplanted animals served as negative controls to exclude possible background staining of human surface antibodies on mouse hematopoietic cells.
Determination of cytokine levels
Approximately 24 h postsurgery, mice were anesthetized with isoflurane, and blood was drawn using #25 gauge needles by cardiac puncture. Serum was obtained following clotting by centrifugation and was stored at −80°C. Levels of cytokines in sera were quantified using BD™ CBA Human Th1/Th2 Cytokine Kit-II and CBA Human Inflammation Kit, per the manufacturer’s recommendations. The lower limits of detection were IL-2 (2.6 pg/ml), IL-4 (2.6 pg/ml), IL-6 (3.0 pg/ml), TNF (2.8 pg/ml), IL-10 (2.8 pg/ml), IFN-γ (7.1 pg/ml), IL-8 (3.6 pg/ml), IL-1β (7.2 pg/ml), and IL-12p70 (1.9 pg/ml).
Quantification of apoptosis
Cells were washed and labeled with the human surface antibodies against hCD3 and hCD20. After washing, cells were fixed in 1% paraformaldehyde (30 min at room temperature) and washed again. Cells were permeabilized by treatment with 90% methanol for 30 min on ice. Apoptosis was quantified by flow cytometry using the TUNEL assay APO-BRDU™ kit, following the manufacturer’s instruction and by fluorescent staining for active caspase 3 (Cell Signaling Technologies) as described previously [30]. Flow cytometric analysis (50,000–100,000 events/sample) was performed on FACScan (Becton Dickinson), and CellQuest software was used to analyze the data.
Determination of DTH
One group of mice received 2.5 μg stabilized IL-7 s.c. at the start of the experiment and then every other day for a total of 14 days. The control group received saline. At Day 14, all mice were immunized with injections of 100 μl 10 mM TNBS s.c. At Day 18, all mice had antigenic challenge with 30 μl 10 mM TNBS into the right footpad. PBS was injected into the left footpad as a control. Measurements (μm±se) were taken 24 h later using a Mitutoyo micrometer. Data represent the difference in thickness between the right footpad (antigen challenge) and the left footpad (PBS challenge) [31].
Statistical analysis
Data are reported as the mean ± sem. Data were analyzed using the statistical software program Prism (GraphPad Software, San Diego, CA, USA). Data involving two groups only were analyzed by a Student’s t-test, and data involving more than two groups were analyzed using one-way ANOVA with Tukey’s multiple comparison test. Significance was accepted at P < 0.05.
RESULTS
Phenotypic analysis of transplanted NOD-scid IL2rγnull mice
NSG mice were housed as breeding pairs. One-day-old pups received 1 Gy whole body irradiation and were adoptively transplanted with 1 × 105 purified hCD34+ cord blood cells via the temporal superficial facial vein within 24 h post-irradiation.
The engraftment of human hematopoietic cells in different organs was analyzed 8–12 weeks and at 20 weeks (for lymphocytes only) post-transplantation by histology and flow cytometry. Histological examination of lymphoid tissues (n=5 mice/group) was conducted in 8- to 12-week-old transplanted and nontransplanted NOD-scid IL2rγnull mice, and representative images are presented (Fig. 1). As expected, nontransplanted NSG mice had rudimentary thymi (often difficult to locate) and small spleens (Fig. 1B). Microscopic images of thymi of nonengrafted mice are not shown due to the severe lymphoid depletion and resultant difficulty in anatomic identification. Note that previously, Shultz et al. [9] demonstrated that thymi of NSG mice consist mostly of stromal cells with sporadic cystic structures. Microscopically, spleens from nontransplanted mice were notable for their small size and absence of developed lymphoid follicles and germinal centers (Fig. 1B).
Figure 1.
Human lymphocytes engraft in murine thymus and spleen. NSG 1-day-old pups received a single dose of 1 Gy as a whole body irradiation. One-day post-irradiation, pups were adoptively transplanted with 1 × 105 purified hCD34+ cord blood cells i.v. Eight weeks post-transplantation, mice were killed and thymi and spleen harvested. (A) H&E stainings show abundant thymocytes in two mice (representative for five mice). Note that thymi were rudimentary and difficult to identify in nontransplanted mice (data not shown). (B) H&E stainings of spleens of a nontransplanted (naïve) versus a transplanted (humanized) mouse are shown as representatives of five mice/group. Original magnification, ×40. The naïve mouse has no detectable lymphoid follicles while the spleen from the humanized mouse has abundant lymphoid follicles. The ×100 original magnification shows a large follicle in the humanized mouse but no follicle in the naïve mouse.
In contrast to the findings in nontransplanted mice, NSG mice that received CD34+ hematopoietic stem cells had grossly normal-appearing thymi and spleens. The thymi of transplanted mice had a normal-appearing cortex and medulla (Fig. 1A). Spleens of transplanted mice had well-developed follicles and germinal centers (Fig. 1B). Lymphocyte counts from the thymus, spleen, and bone marrow showed variability, presumably as a result of the different degrees of engraftment of the hematopoietic stem cells. For the flow cytometric analysis of the thymi of three mice, the analysis gate R1 was focused on the thymic lymphocytes (Fig. 2A). Shown are double-positive CD4+/CD8+ T cells (77.5±2.2%), single-positive CD4+ T cells (7.6±2.2%), single-positive CD8+ T cells (6.3±1.9%), and double-negative cells (8.5±1.9%). Thymic lymphocytes are absent in naïve, nontransplanted mice.
Figure 2.
Quantification of human lymphocytes in thymus and spleen. (A) Isolated thymocytes were stained for anti-hCD4 and anti-hCD8 to characterize the distribution of single-positive, double-positive, and double-negative cell populations (images are representative of three mice/group). (B) Spleens were harvested at 8 weeks or 20 weeks postadoptive transfer, respectively. Splenocytes were stained for the human surface markers CD4, CD8, and CD19, and distribution was determined. One representative flow cytometry figure is shown for each stain. Table 1 indicates the number of investigated animals, the average percentage of positive cells for each stain, as well as the maximum and minimum percentage detected.
Splenic T cells showed a pronounced difference in percentages at 8–12 weeks compared to 20 weeks post-transplantation (Table 1). At 8–12 weeks post-transplantation, there was a much higher percentage of CD19+ human B cells (39.6±6.0%) compared with hCD4+ and hCD8+ T cells (2.7±0.6% and 1.4±0.3%, respectively). By 20 weeks post-transplantation, hCD4+ and hCD8+ T cells had increased to 10.1 ± 2.1% and 4.6 ± 0.8%, respectively (Fig. 2B). By 20 weeks, the percentage of hCD3+ T cells averaged 10.7 ± 1.5% in the spleen. B cell percentages increased slightly to 48.0 ± 3.4% at 20 weeks (Table 1).
TABLE 1.
Distribution of Human Hematopoietic Cells
| Spleen
|
8–12 weeks
|
20 weeks
|
||||
|---|---|---|---|---|---|---|
| Cell type | Average in % | Range in % | # of Animals | Average in % | Range in % | # of Animals |
| CD4 T cells | 2.7 ± 0.6 | 0.4–6.5 | 11 | 10.1 ± 2.1 | 0.6–29.3 | 15 |
| CD8 T cells | 1.4 ± 0.3 | 0.4–3.1 | 11 | 4.6 ± 0.8 | 0.4–8.8 | 15 |
| CD3 T cells | n.d. | 10.7 ± 1.5 | 4.1–20.8 | 12 | ||
| B cells | 39.6 ± 6.0 | 1.0–92.4 | 16 | 48.0 ± 3.4 | 7.9–62.9 | 17 |
| DCs | 4.4 ± 1.1 | 1.4–7.1 | 5 | n.d. | ||
| Monocytes | 0.2 ± 0.05 | 0.06–0.33 | 7 | n.d. | ||
| Macrophages | 2.6 ± 0.6 | 0.8–4.6 | 7 | n.d. | ||
| Neutrophils | 0.8 ± 0.3 | 0.4–2.1 | 7 | n.d. | ||
| CD34 progenitors | 3.8 ± 0.2 | 3.5–4.1 | 3 | n.d. | ||
| Bone marrow
|
8–12 weeks
|
20 weeks
|
||||
|---|---|---|---|---|---|---|
| Cell type | Average in % | Range in % | # of Animals | Average | Range in % | # of Animals |
| CD4 T cells | 3.2 ± 1.3 | 0.3–14.9 | 10 | n.d. | ||
| CD8 T cells | 0.9 ± 0.2 | 0.1–2.3 | 10 | n.d. | ||
| CD3 T cells | n.d. | n.d. | ||||
| B cells | 50.9 ± 7.7 | 1.3–77.2 | 10 | n.d. | ||
| DCs | 4.0 ± 0.8 | 0.8–8.3 | 15 | n.d. | ||
| Monocytes | 2.5 ± 0.9 | 0.1–10.1 | 10 | n.d. | ||
| Macrophages | 4.1 ± 0.7 | 2.2–8.6 | 10 | n.d. | ||
| Neutrophils | 4.3 ± 0.9 | 0.1–10.8 | 10 | n.d. | ||
| CD34 progenitors | 6.3 ± 1.0 | 0.6–10.1 | 10 | n.d. | ||
n.d., not determined.
At 8–12 weeks, human monocytes (positive staining for hCD14 and hCD15; 0.2±0.05%), macrophages (positive for hCD14; 2.6±0.6%), and neutrophils (positive for hCD15; 0.8±0.3%) can be detected in the spleen, although percentages were low (Fig. 3A). In contrast, at this time-point, the percentage of monocytes, macrophages, and neutrophils was higher in the bone marrow (2.5±0.9% for monocytes; 4.1±0.7% for macrophages; 4.3±0.9% for neutrophils).
Figure 3.
Quantification of human myeloid cells in spleen and bone marrow. (A) Human macrophages, monocytes, and neutrophils are quantified by anti-hCD14 and anti-hCD15. (B) hDCs were detected by HLA-DR and anti-hCD86. Human hematopoietic stem cells were detected by anti-hCD34. One representative flow cytometry staining is shown per group. Naïve, nontransplanted animals were used as negative controls for spleen and bone marrow stainings. As cells of nontransplanted animals stained similar in spleen and bone marrow, only the spleen staining is shown. Table 1 indicates the number of investigated animals, the average percentage of positive cells for each stain, as well as the maximum and minimum percentage detected.
Cell surface markers for hCD83 and hCD86 as well as HLA-DR were used to determine the presence of hDCs in spleen and bone marrow. As hCD83 showed some background staining in naïve, nontransplanted mice (data not shown), the combination of hCD86 and HLA-DR was used (Fig. 3B). Whereas naïve, nontransplanted mice were negative for hDCs, adoptively transplanted mice showed an average percentage of CD86-HLA-DRhigh of 4.4 ± 1.1% in the spleen and 4.0 ± 0.8% in the bone marrow.
Furthermore, splenocytes and bone marrow cells were stained for the human stem cell marker CD34 to determine the self-renewal capacity of the human stem cell in our mice. Figure 3C shows that high percentages of CD34-positive human stem cells were found in the spleen (3.8±0.2%) and bone marrow (6.3±1.0%), consistent with proliferation and self-renewal. These finding are consistent with the concept that the engraftment of the human hematopoietic stem cells is stable and suitable for long-term studies.
Humanized mice have an intact DTH response
To confirm the functionality of the human immune system in our humanized mice, we investigated their DTH response. The DTH response is dependent on an orchestrated interaction between cells of the innate and adaptive immune system including T cells, DC, and monocytes/macrophages [32]. Thus, it is an effective test of the overall functional status of the host immune response. Humanized mice were immunized with 0.1 ml 10 mM TNBS s.c. Four days later, mice were challenged with TNBS in the right footpad. As an internal control, the left footpad was injected with PBS. The difference in the local tissue swelling was determined 24 h after the challenge. Humanized mice (mice that had received hCD34+ hematopoietic stem cells) showed a significant increase in their DTH response (Fig. 4) while naïve, nontransplanted animals had no response. To further evaluate the DTH response to immune stimulation, mice received treatment with stabilized rhIL-7 (2.5 μg s.c. every other day for 14 days beginning 10 days prior to immunization). Mice that received rhIL-7 had a marked increase in DTH, as revealed by the greatly increased footpad swelling (Fig. 4). IL-7 is essential for the maintenance of naïve and memory T cells, and work from our lab has shown that humanized mice treated with IL-7 have an approximate threefold increase in the percentage of splenic hCD4 T cells by the end of the injection period (data not shown). These results demonstrate that these humanized mice acquired a functional long-term human innate and adaptive immune system.
Figure 4.
Humanized mice demonstrate a DTH response to antigen. Mice received 100 μl 10 mM TNBS s.c. on Day 1. At Day 5, mice were challenged with a 30 μl injection of 10 mM TNBS into the left footpad. The right footpad was used as an internal control and injected with 30 μl PBS. The swelling of the two footpads was measured at Day 6. Shown is the difference between the swellings of the left versus the right control foot. To increase the CD4 T cell counts, mice in the IL-7 group received 2.5 μg stabilized IL-7 s.c. every other day for 14 days. During this time, the percentage of splenic CD4 T cells tripled (data not shown). Naïve mice were nontransplanted mice that had no mouse or human T or B cells and had no response to the antigenic challenge. Humanized mice did have an appropriate DTH response, which was increased in mice receiving rhIL-7.
Humanized mice with sepsis have markedly increased lymphocyte apoptosis
A hallmark of sepsis in animal models and patients is the profound apoptosis-induced depletion of T and B cells [15,16,17,18,19]. We investigated whether human lymphocytes in humanized mice showed the characteristic signs of apoptosis observed in septic human patients. Twenty-four hours post-sham surgery or CLP, mice were killed, and the apoptosis of human T cells and B cells was determined by histology and flow cytometry. Color photomicrographs of H&E-stained splenic sections showed a dramatic increase in apoptotic cells (indicated by the arrows in Fig. 5, B and C) in mice that received CLP surgery (Fig. 5). The apoptotic cells often appear in clusters with the most concentrated density in the lymphoid follicles and germinal centers. Only an occasional, rare, isolated apoptotic lymphocyte was present in sham-operated mice. To quantify lymphocyte apoptosis, human T cells and B cells were analyzed by flow cytometry using two independent methods, i.e., activated caspase-3 and TUNEL (Fig. 5D). Active caspase-3 and TUNEL stainings demonstrated a significant increase in apoptotic human T cells and B cells in response to murine peritonitis. Furthermore, we examined markers for lung and liver injury. Lung tissue sections from humanized mice with sepsis demonstrated mononuclear and polymorphonuclear infiltrates with numerous apoptotic PMNs and (to a much lesser degree) lymphocytes (data not shown). Liver sections of humanized septic mice showed minor evidence of inflammation with only scattered PMNs present.
Figure 5.
Human hematopoietic cells undergo apoptosis in response to murine sepsis. Eight weeks post-transplantation, humanized mice underwent sham or CLP surgery. Twenty-four hours later, mice were killed and spleens harvested. (A–C) Conventional bright-field microscopy of H&E-stained tissue sections shows normal-appearing splenocytes in a sham-operated mouse (representative of five sham mice). For CLP, sections of two representative mice out of five are shown. (B) H&E staining of Mouse 1, which received CLP, demonstrates germinal centers with foci of cells with compacted and fragmented nuclei, diagnostic of apoptotic cell death (×400 original). (C) H&E staining of Mouse 2 with CLP demonstrates apoptotic cells in the region of the periarteriolar lymphoid sheath (×400 original). (D) Flow cytometry detection of apoptosis by TUNEL and active caspase 3. The increase in lymphocyte apoptosis observed in H&E color photomicrographs was confirmed for human T cells and human B cells in mice by two independent staining methods: TUNEL and staining for active caspase-3.
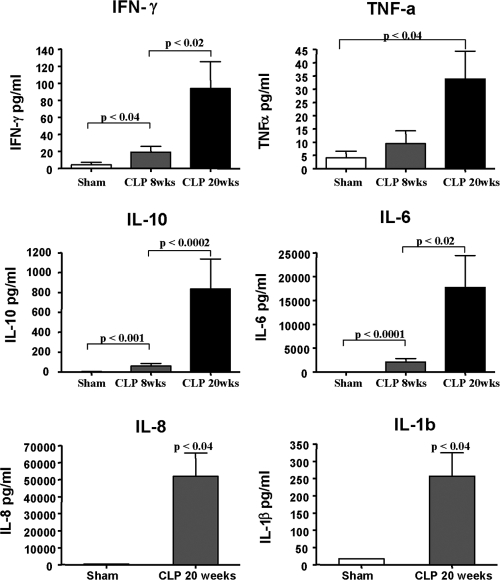
Humanized mice with sepsis make pro- and anti-inflammatory human cytokines
To determine if the human lymphoid cells were activated and functional during murine sepsis, human cytokines were determined in the blood 24 h after the onset of sepsis (Fig. 6). A typical immune response to sepsis in humans and mice includes increased circulating pro- and anti-inflammatory cytokines, i.e., TNF-α, IL-6, IL-10, and IFN-γ. Humanized mice with sepsis did have significant elevations in these human cytokines while the concentrations of the cytokines in sham-operated humanized mice were below the detection level. Again, we detected a profound difference in mice 8–12 weeks post-transplantation versus mice analyzed 20 weeks postadoptive transfer. Whereas human cytokines are clearly induced in CLP at 8–12 weeks post stem cell transplant, there was a much greater increase in circulating cytokines in mice that had received the stem cell transplant 20 weeks prior to CLP. This more robust response likely parallels the increase in CD4 T cells and monocytes/macrophages that occurs with time in the transplanted mice.
Figure 6.
Human pro- and anti-inflammatory cytokines are produced in response to sepsis. Mice that had adoptive transfer of hCD34+ hematopoietic stem cells 8–12 or 20 weeks previously underwent sham or CLP surgery. Twenty-four hours postsurgery, serum was obtained, and hIFN-γ, hTNF-α, hIL-10, and hIL-6 were determined via BD FACSArray. IL-8 and IL-1β were analyzed only at 20 weeks post-transfer. Serum cytokines in sham-operated mice did not differ at 8–12 weeks versus 20 weeks, and therefore, data were pooled. Note the significant increases in pro- and anti-inflammatory human cytokines in septic humanized mice. Also note the increase in cytokines at 8–12 weeks versus 20 weeks.
DISCUSSION
There are a number of important findings in the present study. As noted in numerous other studies, the humanized mice developed a functional innate and adaptive immune system. The H&E stains of spleens and thymi from the immunodeficient mice that received CD34+ human hematopoietic stem cells appeared indistinguishable from tissue sections of normal immune-competent mice (Fig. 1). The thymi had robust cellularity in cortical and medullary regions. Spleens of the humanized mice demonstrated normal-appearing follicles and germinal centers while spleens from immunodeficient mice that did not receive CD34+ human hematopoietic stem cells showed absence of follicles and markedly decreased lymphocyte abundance. Flow cytometry confirmed the presence of all elements of the human innate and adaptive immune system including a bone marrow rich in various cell populations. The DTH response is a complex reaction that requires the coordinated action of T cells, DC, and macrophages. The fact that the humanized mice responded to a repeat antigenic challenge with a vigorous DTH response is strong evidence of a functional innate and adaptive immune system.
A second significant finding of the present study is that the CLP model of sepsis in humanized mice recapitulates many of the hallmarks of sepsis observed in patients. Specifically, humanized mice with sepsis had extensive apoptosis in hCD4 and hCD8 T cells and B cells as well as a marked increase in production of human pro- and anti-inflammatory cytokines, as is typically observed in patients with sepsis. Interestingly, the changes in pro- and anti-inflammatory cytokines in the humanized mice with sepsis are similar to findings in patients with sepsis that report increases in TNF-α, IL-6, and IL-10 [33,34,35].
The fact that humanized mice with sepsis had extensive apoptosis has implications regarding pathogenic mechanisms of apoptosis. If there had been no lymphocyte apoptosis in humanized mice with sepsis, it would have indicated that a particular cytokine or group of cytokines that were produced in patients with sepsis but absent in humanized mice was responsible for the cell death or that human cells are not capable of reacting to apoptogenic factors produced by murine cells. This finding would have provided important clues about the specific cytokines or chemokines that were inducing apoptotic death in lymphocytes in sepsis. The fact that the humanized mice with sepsis have widespread apoptosis suggests that the milieu of the septic mouse contains some unknown factors that cause lymphocyte apoptosis, implying that these factors and their effector pathways are conserved or that the human hematopoietic system itself is producing the apoptogenic factors. These findings are consistent with our previous work, which indicates that myriad stimuli are able to initiate apoptosis in sepsis. Previous studies showed that the death receptor and mitochondrial-mediated apoptotic pathways are activated in sepsis and that a multitude of “triggers” of apoptosis exists [30].
Although the humanized mouse model does represent an advance over the existing CLP model, there remain a number of differences in immune cell-host interactions in this model versus the actual clinical setting, such that results in this model may not reflect the patient situation. For example, immune effector cell activation is in part related to interactions between immune cells and the host endothelial cells, e.g., cell interactions with integrins and/or selectins. These important interactions are likely not occurring in the present model. Also, human immune responses are known to be tempered by proteins and neuroendocrine mechanisms that may differ in the mouse versus the human immune system. Thus, results in this model may not accurately reflect some of the more complex immune effector cell functions in humans with sepsis. Furthermore, we cannot exclude the possibility at this point that differences in the bowel flora between the humanized mice and the wild-type mice may impact differences in immune responses.
Nevertheless, studies involving humanized mice have provided important insights in a number of diseases. In particular, humanized mice that received hCD34+ hematopoietic stem cells as neonates and subsequently developed functional human innate and adaptive immune systems are yielding important translational findings. For example, as these mice develop hCD4+ T cells, they can support HIV infection and allow for testing of novel therapies. Kumar et al. [36] used T cell-specific small interfering RNA delivery to suppress HIV-1 infection in humanized mice. Bente and colleagues [37] demonstrated that humanized mice infected with Dengue virus developed the hallmarks of human infection including rash, fever, and thrombocytopenia. Yajima and associates [38] demonstrated that humanized mice infected with EBV developed B cell lymphoproliferative disorders and histopathologic findings that were highly similar to that occurring in patients. Yu et al. [39] used humanized mice to do preclinical testing of vaccines designed to induce cellular immunity against influenza virus.
The dismal failure of therapies in sepsis may be related in part to the fact that mouse models of sepsis do not reflect the human scenario. This lack of translation of results in murine models of sepsis to clinical studies is likely a result, in part, of the many important differences in the mouse versus the human immune system. One example of key differences in mouse and human immune systems is the pattern of TLR expression. TLR9 recognizes bacterial DNA and activates the host to mount an immune response. TLR9 is expressed on all DCs in mice but only on plasmacytoid DCs in humans [6]. Other important differences in the mouse versus the human host response to sepsis include the robust production of NO, a key inducer of shock in sepsis, by murine but not human macrophages and the role of MyD88, a key downstream adaptor of the TLRs and IL-1Rs. Mice with MyD88 deficiency are highly susceptible to a broad range of pathogens, whereas children with autosomal-recessive MyD88 deficiency are vulnerable to a limited number of pyogenic bacterial but have normal resistance to most pathogens. Given these important distinctions between mouse and human immune systems, it is not surprising that many sepsis trials have failed. One important therapeutic target in sepsis is prevention of apoptosis of immune effector cells. The humanized mice offer an ideal model to evaluate potential strategies to block sepsis-induced apoptosis of immune effector cells. Results examining effects of drugs on blocking apoptosis of activated human immune cells in sepsis in humanized mice are much more likely to be translatable to the clinic.
In conclusion, the use of humanized mice as a preclinical model will provide a powerful new tool in the investigation of sepsis. The current study demonstrates that the humanized mouse model reproduces the classic pathologic and immunologic findings in patients with sepsis. Therefore, humanized mouse models of sepsis likely represent a more clinically relevant model for mechanistic studies of sepsis and testing of novel therapies.
Acknowledgments
This work was supported by the National Institutes of Health grants GM44118, GM55194, and DK072473, the Alan A. and Edith L. Wolff Foundation, and the Juvenile Diabetes Research Foundation. The authors thank Dr. Jonathan McDunn for suggestions regarding testing the DTH response in the humanized mice.
Footnotes
Abbreviations: CBA=cytometric bead array, CLP=cecal ligation and puncture, DC=dendritic cell, DTH=delayed-type hypersensitivity, FSC=forward-scatter, h=human, NSG mice=NOD.Cg-Prkdcscid IL2rγtm1Wjl/Sz mice, PMN=polymorphonuclear leukocyte, SSC=side-scatter, TNBS=2,4,6-trinitrobenzene sulfonic acid
References
- Bone R C, Balk R A, Cerra F B, Dellinger R P, Fein A M, Knaus W A, Schein R M, Sibbald W J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Angus D C, Linde-Zwirble W T, Lidicker J, Clermont G, Carcillo J, Pinsky M R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Murphy S L. Deaths: final data for 1998. Natl Vital Stat Rep. 2000;48:1–105. [PubMed] [Google Scholar]
- Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Natanson C, Hoffman W D, Suffredini A F, Eichacker P Q, Danner R L. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes C C. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku C L, Chrabieh M, Mustapha I B, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor A B, Herrero-Mata M J, Aróstegui J I, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yagüe J, Antón J, Pascal M, Chang H H, Janniere L, Rose Y, Garty B Z, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova J L. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L D, Ishikawa F, Greiner D L. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Shultz L D, Lyons B L, Burzenski L M, Gott B, Chen X, Chaleff S, Kotb M, Gillies S D, King M, Mangada J, Greiner D L, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R γ null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, Kato S, Hotta T. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor γ null mice. J Immunol. 2002;169:204–209. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz L D, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {γ} chain (null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese M A, Jensen L T, Willcox N, Fugger L. Humanized mouse models for organ-specific autoimmune diseases. Curr Opin Immunol. 2006;18:704–709. doi: 10.1016/j.coi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- King M, Pearson T, Shultz L D, Leif J, Bottino R, Trucco M, Atkinson M A, Wasserfall C, Herold K C, Woodland R T, Schmidt M R, Woda B A, Thompson M J, Rossini A A, Greiner D L. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor γ chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan M Z, Yu Z, Ito M, Morio T, Shimizu N, Honda M, Yamamoto N. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rγ null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R S, Swanson P E, Freeman B D, Tinsley K W, Cobb J P, Matuschak G M, Buchman T G, Karl I E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Ayala A, Herdon C D, Lehman D L, DeMaso C M, Ayala C A, Chaudry I H. The induction of accelerated thymic programmed cell death during polymicrobial sepsis: control by corticosteroids but not tumor necrosis factor. Shock. 1995;3:259–267. doi: 10.1097/00024382-199504000-00003. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R S, Tinsley K W, Swanson P E, Schmieg R E, Jr, Hui J J, Chang K C, Osborne D F, Freeman B D, Cobb J P, Buchman T G, Karl I E. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R S, Tinsley K W, Swanson P E, Grayson M H, Osborne D F, Wagner T H, Cobb J P, Coopersmith C, Karl I E. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- Chung C S, Xu Y X, Wang W, Chaudry I H, Ayala A. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis? Arch Surg. 1998;133:1213–1220. doi: 10.1001/archsurg.133.11.1213. [DOI] [PubMed] [Google Scholar]
- Oberholzer C, Oberholzer A, Bahjat F R, Minter R M, Tannahill C L, Abouhamze A, LaFace D, Hutchins B, Clare-Salzler M J, Moldawer L L. Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc Natl Acad Sci USA. 2001;98:11503–11508. doi: 10.1073/pnas.181338198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R S, Tinsley K W, Swanson P E, Chang K C, Cobb J P, Buchman T G, Korsmeyer S J, Karl I E. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer J A, Rodrick M L, Mannick J A. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C, Moldawer L L. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- Ertel W, Kremer J P, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg F W. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–1347. [PubMed] [Google Scholar]
- Docke W D, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk H D, Kox W. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- Meakins J L, Pietsch J B, Bubenick O, Kelly R, Rode H, Gordon J, MacLean L D. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186:241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands M S, Barker J E. Percutaneous intravenous injection in neonatal mice. Lab Anim Sci. 1999;49:328–330. [PubMed] [Google Scholar]
- Chaudry I H, Wichterman K A, Baue A E. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. [PubMed] [Google Scholar]
- Boyman O, Ramsey C, Kim D M, Sprent J, Surh C D. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T cell expansion without lymphopenia. J Immunol. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- Chang K C, Unsinger J, Davis C G, Schwulst S J, Muenzer J T, Strasser A, Hotchkiss R S. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- Griffith T S, Kazama H, VanOosten R L, Earle J K, Jr, Herndon J M, Green D R, Ferguson T A. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- Janeway C A, Travers P, Walport M, Shlomchik M J. London, UK: Garland Science; ImmunobiologyThe Immune System in Health and Disease. 2005 [Google Scholar]
- Debets J M, Kampmeijer R, van der Linden M P, Buurman W A, van der Linden C J. Plasma tumor necrosis factor and mortality in critically ill septic patients. Crit Care Med. 1989;17:489–494. doi: 10.1097/00003246-198906000-00001. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling—regulation of the immune response in normal and critically ill states. Crit Care Med. 2000;28:N3–N12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- Pruitt J H, Welborn M B, Edwards P D, Harward T R, Seeger J W, Martin T D, Smith C, Kenney J A, Wesdorp R I, Meijer S, Cuesta M A, Abouhanze A, Copeland E M, III, Giri J, Sims J E, Moldawer L L, Oldenburg H S. Increased soluble interleukin-1 type II receptor concentrations in postoperative patients and in patients with sepsis syndrome. Blood. 1996;87:3282–3288. [PubMed] [Google Scholar]
- Kumar P, Ban H S, Kim S S, Wu H, Pearson T, Greiner D L, Laouar A, Yao J, Haridas V, Habiro K, Yang Y G, Jeong J H, Lee K Y, Kim Y H, Kim S W, Peipp M, Fey G H, Manjunath N, Shultz L D, Lee S K, Shankar P. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente D A, Melkus M W, Garcia J V, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–13799. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, Nakamura H, Ito M, Shimizu N, Honda M, Yamamoto N, Fujiwara S. A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis. 2008;198:673–682. doi: 10.1086/590502. [DOI] [PubMed] [Google Scholar]
- Yu C I, Gallegos M, Marches F, Zurawski G, Ramilo O, Garcia-Sastre A, Banchereau J, Palucka A K. Broad influenza-specific CD8+ T cell responses in humanized mice vaccinated with influenza virus vaccines. Blood. 2008;112:3671–3678. doi: 10.1182/blood-2008-05-157016. [DOI] [PMC free article] [PubMed] [Google Scholar]