Abstract
Background
Sialic acid-binding immunoglobulin-like lectins (Siglecs) are a family of receptors that bind sialic acid and mostly contain immunoreceptor tyrosine-based inhibitory motifs, suggesting that these molecules possess inhibitory functions. We have recently identified Siglec-8 as an eosinophil-prominent Siglec, and cross-linking of Siglec-8 on human eosinophils induces apoptosis. In this article, we address the in vivo consequences of Siglec engagement. We and others have identified mouse Siglec-F as the closest functional paralog of human Siglec-8, based on shared ligand-binding and expression pattern. We therefore hypothesized that Siglec-F engagement would affect levels and viability of eosinophils in vivo.
Methods
Wild type and hypereosinophilic mice were administered Siglec-F antibody and levels of eosinophils in peripheral blood and tissue were measured. Eosinophil apoptosis (in vivo and in vitro) was determined by binding of Annexin-V.
Results
Studies in IL-5 transgenic mice, displaying hypereosinophilia, show that administration of a single dose of Siglec-F antibody results in rapid reductions in quantum of eosinophils in the blood. This decrease was accompanied by reductions in tissue eosinophils. Quantum of eosinophils in blood was decreased using two separate antibodies, as well as in other mouse models (wild type mice and in a mouse model of chronic eosinophilic leukemia). Mechanistic studies demonstrated that Siglec-F antibody administration induced apoptosis of eosinophils in vivo and in vitro.
Conclusion
These data demonstrate that activation of innate immune receptors, like Siglec-F, can significantly reduce mouse eosinophil viability. As such, targeting Siglec-8/F may be a therapeutic approach for eosinophilic disorders.
Keywords: apoptosis, eosinophils, Siglec
Eosinophils are leukocytes (1) that are primarily tissue-dwelling cells found constitutively in several organs such as the gastrointestinal tract (2, 3). However, in inflammatory conditions, eosinophils accumulate in large numbers in other tissues, such as the lung and skin. Persistent inflammatory responses may arise from inefficient mechanisms for resolution of inflammation, including delayed apoptosis. Indeed, several studies suggest that eosinophil apoptosis is delayed in asthma [reviewed in (4)]. Thus, understanding the mechanisms that govern eosinophil survival and death is critical to understanding the pathophysiology of eosinophil-mediated diseases.
The most striking increase in eosinophil burden occurs in hypereosinophilic syndrome (HES). A subset of HES patients has an interstitial deletion leading to the generation of a fusion protein between the platelet-derived growth factor receptor alpha (PDGFRα) gene and Fip1-like1 (FIP1L1). The fusion gene product (F/P) acts as a constitutively active tyrosine kinase (5, 6). This subgroup of HES patients are of late diagnosed, as having chronic eosinophilic leukemia (CEL) according to World Health Organization disease classification criteria. The combined health care burden of eosinophil-associated diseases is significant as asthma is one of the most common diseases of childhood and HES/CEL is an often-fatal disease. Clearly, novel approaches are needed to target these devastating diseases.
Sialic acid-binding immunoglobulin-like lectins (Siglecs) are a family of cell surface lectins (7, 8). The extracellular Ig domain binds specifically to complex carbohydrate structures containing at least one sialic acid residue. Most Siglecs have cytoplasmic immunoreceptor tyrosine-based inhibitory motifs. Thus, it has been suggested that Siglecs have inhibitory effects on cell function. Recently, we identified a novel family member, Siglec-8, using a human eosinophil cDNA library generated from a patient with HES (9, 10). We demonstrated that cross-linking of Siglec-8 on the surface of human eosinophils leads to caspase- and/or reactive oxygen species (ROS)-mediated apoptosis (11–13). Importantly, Siglec-8 is expressed on mast cells and at low levels on basophils, and cross-linking on mast cells leads to inhibition of IgE-dependent histamine release, without affecting viability (14). Thus, Siglec-8 ligation appears to induce eosinophil-specific apoptosis and distinct inhibitory functions on other cells involved in allergic responses. Based on its inhibitory effect on eosinophils, and mast cells, which are considered to be key effector cells in allergic diseases, Siglec-8 is considered a potential target for the development of agents to suppress these cells and generate new treatments for allergic inflammation. However, its functional role in vivo has not been established.
Recently, we and others identified murine Siglec-F as the functional paralog of human Siglec-8, based on its expression on mouse eosinophils (15, 16). Furthermore, recent studies demonstrated that 6′-sulfo-sLex is a specific ligand for human Siglec-8 and mouse Siglec-F (17–19), further supporting the notion that Siglec-F and Siglec-8 are functional paralogs. Recent studies in allergen-challenged mice suggest that Siglec-F may regulate airway eosinophilia (20, 21). However, the consequences of Siglec-F engagement and the function of Siglec-F on mouse eosinophils have not been tested.
In this study, we examine the therapeutic potential of anti-Siglec-F antibodies in vivo and explore the mechanism of action. We demonstrate that treatment of mice with Siglec-F antibodies decreases quantum of eosinophils and suggest that engagement of Siglec-F leads to eosinophil cell death.
Methods
Mice and in vivo treatment
CD2.IL-5 transgenic mice (22) and wild type mice (BALB/c) were used. Initial studies were performed with an affinity-purified polyclonal antibody, raised in sheep (23). As a control, preimmune sheep IgG was used. In other experiments, we used a mouse monoclonal IgG1 antibody against Siglec-F produced from stably transfected CHO cells. As the isotype-matched control, we used an antibody (clone mB86) raised against IgMb (not present in BALB/c mice). For cell-binding control, we treated mice with anti-CCR3 antibody (R&D Systems, Rat IgG2a). Eosinophil and total leukocyte numbers in the peripheral blood were determined using Discombe's and Turk's staining solution, respectively (24).
F/P-mediated HES/CEL model
The F/P-mediated HES/CEL model was induced essentially as described previously (25). Briefly, low-density bone marrow cells from IL-5 transgenic mice were retrovirally transduced with MSCV-F/P-IRES-EGFP and MSCV-IRES-EGFP (mock vector) and transplanted into lethally irradiated BALB/c mice (26). Mice were killed when ill or at day 30.
Flow cytometry
Cells were washed with FACS-buffer (2% BSA, 0.1% Na-azide in PBS), blocked with Fc block (rat anti-mouse CD16/32 antibody, clone 2.4G2; BD Pharmingen, San Diego, CA, USA), and incubated for 30 min at 4°C with anti-CCR3 antibody. For determination of eosinophil apoptosis, cells were washed in Annexin-V binding buffer and incubated with Annexin-V and vital dye 7AAD. Results were analyzed using the cellquest or flowjo (TreeStar) software.
Quantification of tissue eosinophil and mast cell levels
Eosinophils in formalin-fixed paraffin-embedded tissue were differentially stained using antibody against murine major basic protein (anti-MBP) as described earlier (2). Eosinophil levels in the jejunum (normalized for area) were counted by morphometric analysis by an observer blinded to treatment. Similarly, mast cells were identified by chloroacetate esterase (CAE) staining in the jejunum and quantified per high power field by an observer blinded to treatment.
In vitro eosinophil apoptosis assays
Blood was obtained from IL-5 transgenic mice (CD3δ-driven, line NJ.1638). Erythrocytes were lysed hypotonically. This yielded eosinophils of purity ranging from 31% to 46%. Leukocytes (106/ml) were then cultured in RPMI 1640, 10% FBS at 37°C for 4–24 h with or without 10 μg/ml anti-mouse CD44 (Clone IM7, rat IgG2b) or anti-mouse Siglec-F antibody (Clone E50–2440, rat IgG2a); all wells also contained goat anti-rat IgG to further cross-link surface-bound antibodies. Eosinophil apoptosis was determined by flow cytometry, as described above.
Results
Administration of Siglec-F antibodies reduces eosinophil but not mast cell numbers in IL-5 transgenic mice
We first tested the hypothesis that engagement of Siglec-F by antibodies would lower eosinophil levels in vivo. CD2.IL-5 transgenic mice, which have peripheral blood eosinophilia (22) were given Siglec-F antibody intravenously. Initial studies were performed using an affinity-purified polyclonal antibody. As seen in Fig. 1A, administration of 0.5 mg/mouse of the Siglec-F antibody induced a rapid decline in the quantum of eosinophils in the peripheral blood. This decrease was already seen 2 h following infusion of the antibody and was sustained for at least 48 h (84.0 ± 5.4 and 67.0 ± 21.0% decrease at 2 and 48 h, respectively, average of two experiments, n = 8 mice/group). Similar results were seen with 0.1 mg/mouse dose (data not shown, n = 4 mice/group). The level of antibody in the serum of mice was maintained at > 2.5 μg/ml for 48 h following antibody administration.
Figure 1.
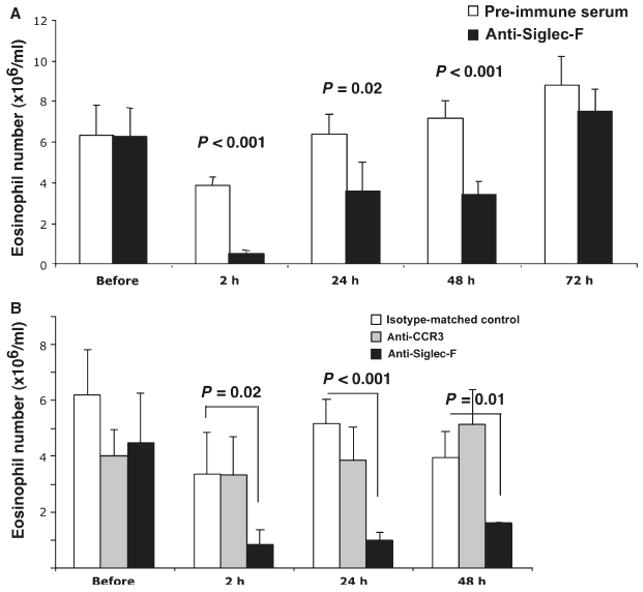
Siglec-F antibody administration leads to a reduction in the quantum of eosinophils. IL-5 transgenic mice were given a single dose of polyclonal Siglec-F antibody or preimmune sera (500 μg/mouse, panel A) or mouse monoclonal Siglec-F antibody vs an isotype-matched control antibody (100 μg/mouse, panel B). In panel B, mice were also given CCR3 antibody (100 μg/mouse). At the indicated time points, the number of eosinophils in the peripheral blood was determined by Discombe's staining. Representative experiments are shown (out of two experiments with the sheep polyclonal and seven with the monoclonal antibody, each performed with 4–8 mice/group). Data are mean ± SD. P-value is determined by two-tailed, equal variance t-test.
The decrease in the quantum of eosinophils was reproduced with a monoclonal Siglec-F antibody (20–280 μg/mouse, Fig. 1B and data not shown). Importantly, total numbers of white blood cells were not significantly altered, demonstrating the eosinophil specificity of the effect (data not shown). This antibody is a mouse IgG1 isotype that does not fix complement (27, 28). We used two controls: an isotype-matched control (mB86) and an eosinophil-binding control (anti-CCR3). Neither led to decreased numbers of circulating eosinophils (Fig. 1B).
Eosinophils are mainly tissue-dwelling cells and are thought to mediate end-organ effects in hypereosinophilic diseases. Thus, we tested the hypothesis that engagement of Siglec-F would decrease quantum of eosinophils in the tissue. We assessed the levels of eosinophils in jejunum as the gastrointestinal tract is a major reservoir of eosinophils. There was a 34.5 ± 9% decrease in the number of eosinophils in the jejunum of mice 48–72 h following administration of Siglec-F antibody, as measured by anti-MBP staining (average of three experiments, P < 0.05 in each individual experiment). Collectively, these data demonstrate that administration of Siglec-F antibody decreases the number of eosinophils in the peripheral blood as well as in the jejunum.
Human Siglec-8 is expressed on eosinophils and mast cells; however, engagement of Siglec-8 does not affect mast cell viability (14). Thus, it was important to determine whether Siglec-F antibody administration affected mouse mast cell numbers. Following treatment of mice with Siglec-F antibodies, peritoneal mast cell numbers were not affected (4630 ± 2790 mast cells and 6280 ± 3010 in preimmune and anti-Siglec-F serum treated mice, respectively). Furthermore, mast cell staining (CAE) of the jejunum did not show any change in the number of mast cells following Siglec-F antibody administration (0.64 ± 0.5 and 0.43 ± 0.3 mast cells/high power field in control and anti-Siglec-F treated mice, respectively, n = 10–11 mice/group). In summary, Siglec-F antibody administration did not affect the number of peritoneal and jejunal mast cells.
Siglec-F antibody administration decreases eosinophils in wild type mice
It remains possible that high levels of circulating IL-5 (as seen in IL-5 transgenic mice) are required for the Siglec-F-mediated effect. To test this possibility, we treated wild type mice with Siglec-F antibodies. This analysis demonstrated a significant decrease in the quantum of eosinophils in the peripheral blood. For instance, eosinophils decreased 69.1 ± 11.7% and 83.3 ± 2.7% at 24 h and 56.8 ± 21.7% and 71.3 ± 5.1% at 48 h following administration of 25 and 250 μg/mouse Siglec-F antibody, respectively (average of three experiments, P < 0.05 in each individual experiment). A representative experiment is shown in Fig. 2A. Furthermore, at the same time points, total white blood cell counts did not change (Fig. 2B). These data demonstrate that Siglec-F engagement by antibodies leads to a specific reduction of blood eosinophils in wild type mice.
Figure 2.
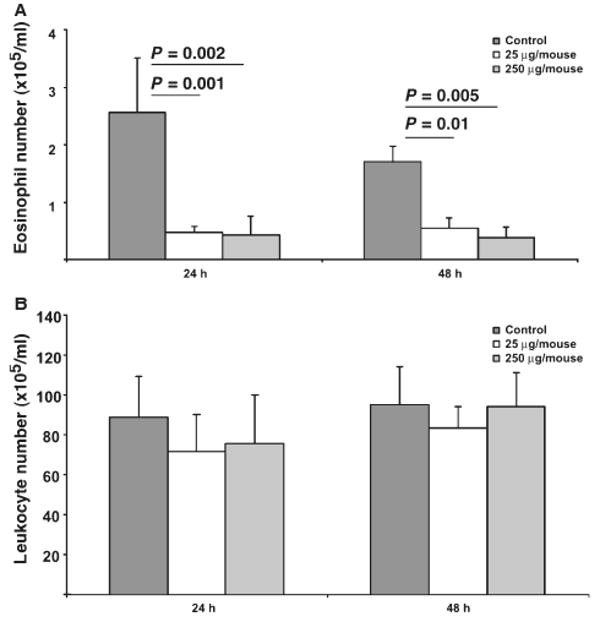
Treatment of wild type mice with Siglec-F antibody decreases numbers of circulating eosinophils. Wild type (Balb/c) mice were treated with indicated doses of the sheep polyclonal Siglec-F antibody and the quantum of eosinophils (panel A) and total leukocytes (panel B) in peripheral blood are shown. Representative data from one of three experiments (each performed with four mice/group) is shown. Data are mean ± SD. P-value is determined by two-tailed, equal variance t-test.
Siglec-F antibody-mediated eosinophil decrease in the model of HES/CEL
We next tested the hypothesis that engagement of Siglec-F in mice transduced with F/P+ bone marrow cells, a mouse model of HES/CEL, would affect the quantum of eosinophils. Mice were treated with Siglec-F antibodies every 48 h, and the quantum of eosinophils in the peripheral blood was monitored. As the disease and the quantum of eosinophils escalated, the dose of antibody was increased from 20 to 100 μg/mouse. As seen in Fig. 3A, Siglec-F antibody treatment consistently decreased the quantum of eosinophils in the peripheral blood. This decrease was again specific for eosinophils, as total white blood cells were not affected by Siglec-F antibody administration (Fig. 3B). Mouse survival time, along with spleen weight and cellularity, were not affected by Siglec-F antibody administration (data not shown).
Figure 3.
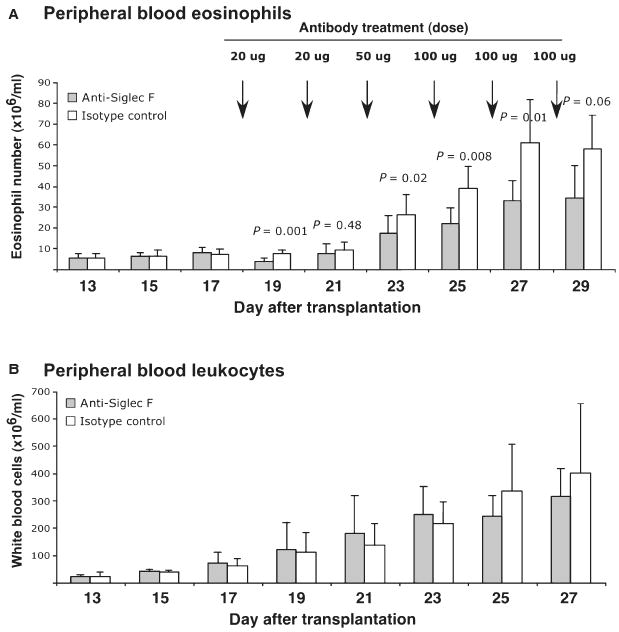
Selective decrease of circulating quantum of eosinophils by Siglec-F antibody administration in the HES/CEL model. HES/CEL is induced by transplanting lethally irradiated wild type mice with FIP1L1/PDGFR-transduced bone marrow cells derived from IL-5 transgenic mice. Once quantum of eosinophils started increasing above the IL-5 transgenic level, mice were treated with Siglec-F antibodies every 48 h, and the quantum of eosinophils in the peripheral blood was monitored (A). As the disease and number of eosinophils escalated, the dose of antibody was increased from 20 to 100 μg/mouse. Simultaneously, the total white blood cell count was monitored by Turk's staining as a control (B). Data are mean ± SD of eight mice/group. P-value is determined by two-tailed, equal variance t-test.
Siglec-F antibody administration leads to eosinophil cell death in vivo
Engagement of Siglec-8 leads to human eosinophil cell death by apoptosis (11, 12). Thus, we hypothesized that the mechanism of decreased quantum of eosinophils in Siglec-F antibody-treated mice involves induction of eosinophil apoptosis. In order to test this hypothesis, mice were treated with Siglec-F antibody. Two hours later, eosinophil death in the peripheral blood was determined by flow cytometry. As seen in Fig. 4, there was a significant increase in apoptotic eosinophils induced by anti-Siglec-F treatment. For instance, the percentage of Annexin-V-positive cells (among the CCR3+ 7AAD− cells) was 28.1 ± 11.3% and 52.3 ± 12.4% in control and Siglec-F antibody treated mice, respectively (n = 4 mice/group, P = 0.028). The average increase in the percent of Annexin-V-positive eosinophils was 76.7 ± 16.2% (average of three experiments with P = 0.001–0.03 in each individual experiment). This was specific for eosinophils as the percent of Annexin-V-positive cells among the CCR3-negative white blood cells was not significantly different between the Siglec-F and control-treated mice (data not shown). By 24 h, the increase in apoptosis observable in the peripheral blood following Siglec-F antibody administration to mice was 34.9 ± 6% with variable statistical significance in each individual experiment (P = 0.02–0.2). Finally, mice were pretreated with FcγRII/III antibody (2.4G2) to exclude a role for Fc-mediated effects in Siglec-F antibody-induced eosinophil apoptosis. Comparable levels of apoptosis were observed with 2.4G2, an isotype-matched control antibody or no pretreatment (data not shown). These data demonstrate that Siglec-F antibody administration leads to decreased eosinophil viability in vivo.
Figure 4.
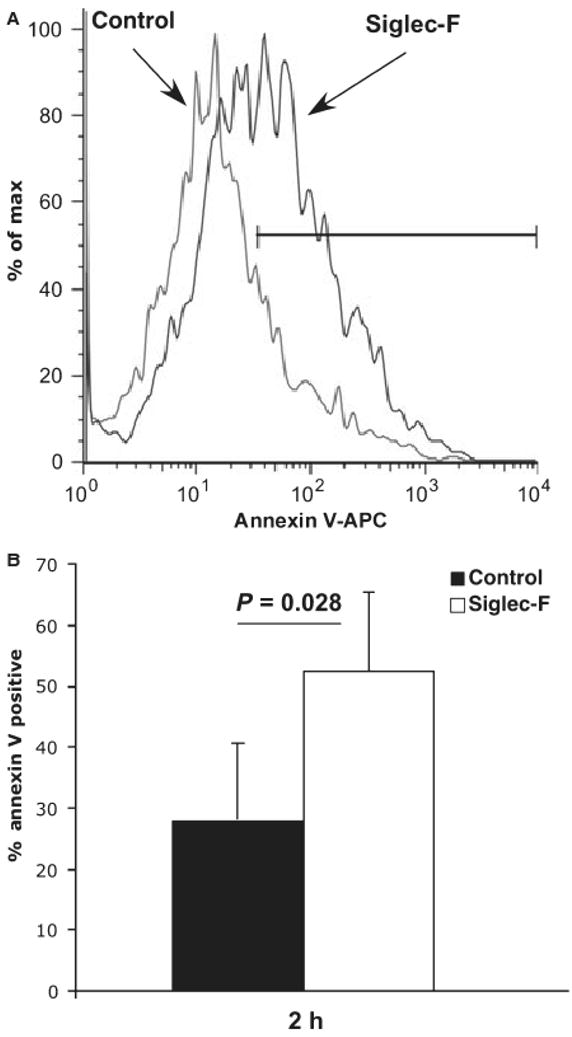
Siglec-F antibody administration leads to eosinophil apoptosis in vivo. IL-5 transgenic mice were treated with Siglec-F antibody (mouse monoclonal, 20 μg/mouse). Eosinophil apoptosis in the peripheral blood was determined by flow cytometry 2 h following the administration of antibody. Apoptotic eosinophils (CCR3+ Annexin-V+ 7AAD- cells) were determined. Panel A shows a representative mouse from each group (isotype-matched control and Siglec-F antibody-treated) and panel B shows quantification from a representative experiment (one out of three experiments, each performed with 4–8 mice/group). P-value was determined by two-tailed, equal variance t-test.
Siglec-F antibody causes eosinophil apoptosis in vitro
It remained possible that in vivo administration of Siglec-F antibody induces eosinophil apoptosis through indirect mechanisms. Thus, we isolated spleen-derived eosinophils from IL-5 transgenic mice and incubated them with Siglec-F antibodies in vitro. As a control, eosinophils were incubated without antibody or with a binding antibody against CD44. Although at 4 h there was no visible difference, 24 h later there was a significant increase in apoptotic eosinophils (Fig. 5). For instance, the percentage of Annexin-V-positive cells (among the CCR3+ cells) increased from 23.5 ± 8.6 (control) and 25.6 ± 8.0% (anti-CD44) to 39.9 ± 11.5% in Siglec-F antibody incubated cells (n = 4, P = 0.02 and 0.05, respectively). These data demonstrate that treatment of eosinophils with anti-Siglec-F antibody in vitro leads to apoptosis.
Figure 5.
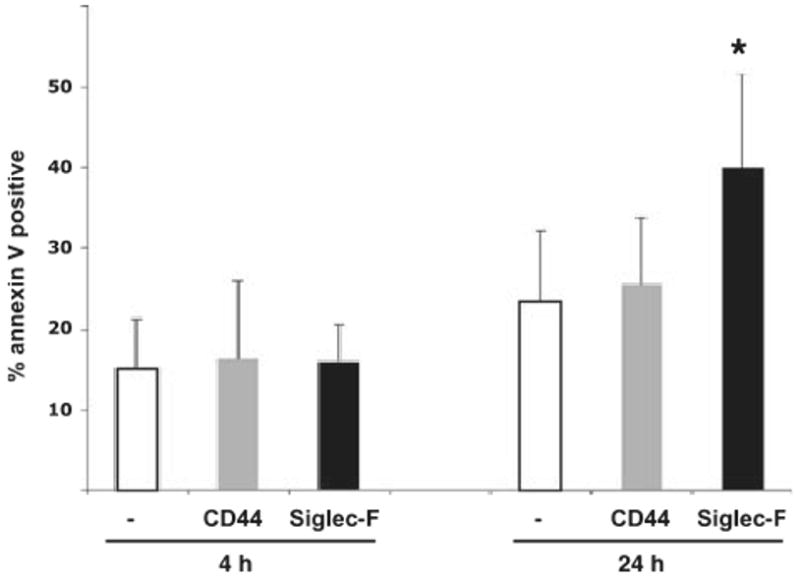
Siglec-F antibody treatment leads to eosinophil apoptosis in vitro. Eosinophils (derived from IL-5 transgenic mice) were incubated with rat anti-Siglec-F or CD44 for 4 or 24 h and apoptosis determined by flow cytometry (CCR3+ Annexin-V+). Data are mean ± SD of 3–4 experiments. *P < 0.05 for Siglec-F vs CD44 and P < 0.02 for Siglec-F vs no antibody (paired t-test).
Discussion
We have demonstrated that Siglec-F is functional on eosinophils. As the CD33/Siglec-3 subgroup of Siglecs is rapidly evolving, the mouse and human counterparts likely did not arise from the same gene. Thus, sequence analysis alone is often not sufficient for the designation of human and mouse orthologs, and other features (such as expression pattern, ligand, and function) must be taken into account. Together with previous studies showing that Siglec-F and Siglec-8 share ligand-specificity (17–19), our data support the hypothesis that mouse Siglec-F is the functional paralog of human Siglec-8.
Based on the inhibitory effect of Siglec-8 on eosinophils and mast cells, Siglec-8 is considered a potential target for the development of agents to suppress these cells. However, a functional role in vivo had not been established. In this study, we have demonstrated that engagement of Siglec-F in vivo, in wild type mice, IL-5-transgenic mice and in mice with experimental HES/CEL leads to decreased numbers of eosinophils. Recently, Siglec-F-deficient mice were developed (21). Although the quantum of eosinophils was not affected at baseline, following allergen-challenge there were significantly increased levels of blood, bone marrow and lung eosinophils, and delayed lung eosinophil apoptosis in Siglec-F-deficient mice. Similarly, a recent study demonstrated decreased quantum of eosinophils in allergen-challenged mice treated with Siglec-F antibody (20). These data are consistent with our finding that administration of antibodies caused a reduction in the quantum of eosinophils and suggest that Siglec-F engagement leads to eosinophil cell death. Importantly, our approach is clinically relevant as one can design therapies, such as agonistic antibodies, targeted at Siglec-8/F for eosinophilic diseases. Other antibodies against Siglecs (CD22 and CD33) are approved or in advanced stages of clinical trials for use in the treatments of B-cell lymphoma and myeloid leukemia, respectively.
Studies in which Siglec-F is engaged by antibodies are limited by potential nonspecific effects including Fc-mediated effects. We have controlled for these effects by using isotype-matched control antibody and preimmune serum in experiments with the mouse monoclonal or sheep polyclonal antibody, respectively. Additionally, we used an antibody against CCR3 or CD44 as cell-binding controls. Furthermore, we also demonstrate that blocking FcγRIIB and FcγRIII by Fc block antibody did not impair the ability of Siglec-F antibodies to induce eosinophil apoptosis. Future studies will aim at directly and selectively activating Siglec-8/F using glycan ligand-based strategies. Indeed, preliminary studies suggest the presence of such endogenous ligands in the airways, particularly at the epithelial cells (21, 29).
We have focused our studies mainly on the eosinophils from IL-5 transgenic mice. Although this is a very robust approach in view of these mice being a good source of large numbers of eosinophils, it remains possible that they are functionally different from naïve eosinophils because IL-5 is an eosinophil survival and priming factor. Indeed, studies in human eosinophils engaged with antibodies to Siglec-8 demonstrate that IL-5 actually enhances the effect of Siglec-8 ligation on eosinophil death (11–13, 30). Even though this finding appears paradoxical, IL-5 has been established as a priming agent for eosinophil responses to opsonized particles (via Fc and complement receptors) and chemokines (31, 32) and as such may have different effects at different stages of development. In summary, IL-5-dependent eosinophilia may be a good model for eosinophilia associated with high levels of IL-5 (such as seen in asthma, parasitic infections, and a subgroup of HES patients), but may not be an ideal representation of wild type eosinophils. Nevertheless, we have also shown that in vivo treatment of wild type mice with Siglec-F antibody is at least as efficient in decreasing the quantum of eosinophils as in the IL-5 transgenic mice.
Engagement of human Siglec-8 with antibodies leads to caspase and/or ROS-dependent apoptosis of eosinophils (11–13, 30). As we have demonstrated that antibodies against Siglec-F lead to reduction in the quantum of eosinophils in the peripheral blood, we hypothesize that the mechanism of reduction in the quantum of eosinophils is via apoptotic removal. Thus, we demonstrate that treatment of mice with Siglec-F antibody leads to eosinophil apoptosis in vivo and in vitro. It remains possible that additional mechanisms contribute to the observed Siglec-F-mediated decrease in eosinophil levels in vivo. One such alternative mechanism includes removal of binding sites for the ligand either by blocking of the ligand-binding site of Siglec-F by the antibodies we used or by Siglec-F internalization (23). This in turn may affect normal eosinophil migration, e.g. egress from bone marrow into the circulation. Another alternative hypothesis is that Siglec-F engagement affects eosinophil homing/migration by inhibiting chemokine-mediated signals. We do not believe this is likely as one would expect an increase in blood eosinophils if their normal egress from the circulation was prevented. It remains possible that the physiological function of Siglec-F engagement is to inhibit signaling pathways of other eosinophil receptors, such as CCR3 and IL-5, rather than cell death induced by engagement of Siglec-F alone. Although the studies concerning other Siglecs would suggest co-engagement as the likely mechanism of action, preliminary data for Siglec-8 on eosinophils and Siglec-9 on neutrophils argue against that mechanism (11–13, 30, 33). Future studies using alternative approaches will ultimately resolve this question.
Our studies show that despite persistent high titers of Siglec-F antibody, a significant portion of eosinophils is not affected suggesting they escape the Siglec-F-induced death pathway. Flow cytometry demonstrates that there is a decreased level of Siglec-F on the surface of eosinophils of mice which were administered the Siglec-F antibody (data not shown). Importantly, control experiments were performed by preincubating the eosinophils with individual Siglec-F antibodies and then staining with different antibodies, and comparing to cells that were not preincubated. Those studies determined that the ’staining‘ by the polyclonal antibody (sheep) is not blocked by preincubating cells with the monoclonal mouse antibody, which was used for in vivo treatment (data not shown). Together, these data suggest that Siglec-F is either internalized or shed and thus not available for engagement by the excess antibody. Alternatively, peripheral blood of Siglec-F-treated mice may be enriched for a subpopulation of eosinophils that has either low or no Siglec-F on the surface or is lacking the cellular machinery (e.g. ROS) to traverse the cell death pathway. Future studies will further delineate the mechanisms of Siglec-F resistance in certain cells.
In vitro treatment of eosinophils with Siglec-F antibody requires 24 h for apoptosis. In contrast, in vivo Annexin-V-positive cells are detected 2 h following antibody administration. The reason for rapid in vivo kinetics may be related to the presence of IL-5, which primes eosinophils for Siglec-8-induced apoptosis (13) or other in vivo factors not present in our in vitro model.
In summary, our study delineates basic in vivo biology of Siglec-F, examines the mechanism of Siglec-F engagement and subsequent cell death and provides therapeutic proof-of-concept for targeting Siglec-8/F in eosinophilic diseases.
Acknowledgments
We thank Drs Patrick Slocombe and Dan Lightwood (UCB Celltech) and Drs Ariel Munitz, David Hildeman, Fred Finkelman, Kimberly Risma, and Simon Hogan (Cincinnati Children's Hospital) for helpful discussions and/or critical review of the article and Leah Kottyan for technical assistance. We also thank Dr Fred Finkelman for antibodies (mB6, 2.4G2, J1.2) and Drs James and Nancy Lee for the anti-MBP antibody and IL-5 transgenic mice. This study was funded in part by the Translational Research Initiative of Cincinnati Children's Hospital (to N.Z.). NIH grant AI41472 (to B.S.B.), and the Dana Foundation grant (to B.S.B. M.E.R. and N.Z.). We are grateful to the Campaign Urging Research for Eosinophilic Disease (CURED) and the Food Allergy Project Foundations for supporting this work, in part. Dr Bochner is a co-inventor on existing and pending Siglec-8-related patents. If Siglec-8-related products are developed in the future, under a licensing agreement between GlaxoSmithKline and the Johns Hopkins University, Dr Bochner may be entitled to a share of royalties received by the University on the potential sales of such products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 2.Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252:418–425. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Kankaanranta H, Moilanen E, Zhang X. Pharmacological regulation of human eosinophil apoptosis. Curr Drug Targets Inflamm Allergy. 2005;4:433–445. doi: 10.2174/1568010054526395. [DOI] [PubMed] [Google Scholar]
- 5.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 6.Griffin JH, Leung J, Bruner RJ, Caligiuri MA, Briesewitz R. Discovery of a fusion kinase in EOL-1 cells and idiopathic hypereosinophilic syndrome. Proc Natl Acad Sci USA. 2003;100:7830–7835. doi: 10.1073/pnas.0932698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varki A, Angata T. Siglecs – the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 8.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 9.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D'Alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105(6 Pt 1):1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 10.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8 A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 11.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 12.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–924. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 13.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8-mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2007;38:121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, et al. Inhibition of FcvarepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 16.Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82:521–530. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 17.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 18.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 19.Guo JP, Nutku E, Yokoi H, Schnaar RL, Zimmermann N, Bochner BS. Siglec-8 and Siglec-F: inhibitory receptors on eosinophils, basophils and mast cells. Allergy Clin Immunol Inter – J World Allergy Org. 2007;19:54–59. [Google Scholar]
- 20.Kearley J, Jones C, McMillan SJ, Cromie K, Crocker PR, Lloyd CM. Anti-Siglec-F antibody treatment during allergen-induced airway inflammation reduces eosinophil numbers but has no effect on airway hyperreactivity in vivo. Am J Respir Crit Care Med. 2007;175:A690. [Google Scholar]
- 21.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, et al. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27:5699–5710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt EB, Rothenberg ME. Eosinophil levels in mice are significantly higher in small blood vessels than in large blood vessels. J Allergy Clin Immunol. 2001;108:142–143. doi: 10.1067/mai.2001.116121. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Rothenberg ME, Lee AW, Saito-Akei H, Brandt EB, Williams DA, et al. The FIP1L1-PDGFRa Fusion Gene Cooperates with IL-5 to induce murine Hypereosinophilic Syndrome (HES)/Chronic Eosinophilic Leukemia (CEL)-like disease. Blood. 2006;107:4071–4079. doi: 10.1182/blood-2005-08-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cools J, Stover EH, Boulton CL, Gotlib J, Legare RD, Amaral SM, et al. PKC412 overcomes resistance to imatinib in a murine model of FIP1L1-PDGFRalpha-induced myeloproliferative disease. Cancer Cell. 2003;3:459–469. doi: 10.1016/s1535-6108(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 27.Leatherbarrow RJ, Dwek RA. Binding of complement subcomponent C1q to mouse IgG1, IgG2a and IgG2b: a novel C1q binding assay. Mol Immunol. 1984;21:321–327. doi: 10.1016/0161-5890(84)90103-2. [DOI] [PubMed] [Google Scholar]
- 28.Burton DR. Immunoglobulin G: functional sites. Mol Immunol. 1985;22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 29.Guo JP, Myers A, Choi O, Lee HS, Zhu Z, Hudson SA, et al. Ligands for Siglec-8 and Siglec-F: binding characteristics and tissue distribution. J Allergy Clin Immunol. 2007;119:S299. [Google Scholar]
- 30.von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–1011. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 31.van der Bruggen T, Kok PT, Raaijmakers JA, Verhoeven AJ, Kessels RG, Lammers JW, et al. Cytokine priming of the respiratory burst in human eosinophils is Ca+ + independent and accompanied by induction of tyrosine kinase activity. J Leuk Biol. 1993;53:347–353. doi: 10.1002/jlb.53.4.347. [DOI] [PubMed] [Google Scholar]
- 32.Warringa RA, Schweizer RC, Maikoe T, Kuijper PH, Bruijnzeel PL, Koendermann L. Modulation of eosinophil chemotaxis by interleukin-5. Am J Respir Cell Mol Biol. 1992;7:631–636. doi: 10.1165/ajrcmb/7.6.631. [DOI] [PubMed] [Google Scholar]
- 33.von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, Seger R, et al. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106:1423–1431. doi: 10.1182/blood-2004-10-4112. [DOI] [PubMed] [Google Scholar]


