SUMMARY
Specific cone directed therapy is of high priority in the treatment of human hereditary retinal diseases. However, not much information exists about the specific targeting of photoreceptor subclasses. Three versions of the human red cone opsin promoter (PR0.5, 3LCR-PR0.5, and PR2.1), and the human blue cone opsin promoter HB569, were evaluated for their specificity and robustness in targeting green fluorescent protein (GFP) gene expression to subclasses of cones in the canine retina when used in recombinant adeno-associated viral (rAAV) vectors of serotype 5. The vectors were administered by subretinal injection. The promoter PR2.1 led to most effective and specific expression of GFP in the long- and medium-wavelength-absorbing cones (L/M-cones) of normal and diseased retinas. The PR0.5 promoter was not effective. Adding 3 copies of the 35-bp LCR in front of PR0.5 lead to weak GFP expression in L/M-cones. The HB569 promoter was not specific, and GFP was expressed in a few L/M-cones, some rods, and the retinal pigment epithelium. These results suggest that L/M-cones, the predominant class of cone photoreceptors in the retinas of dogs and most mammalian species can be successfully targeted using the human red cone opsin promoter.
Keywords: achromatopsia, canine, cone photoreceptors, opsin promoter, rAAV
INTRODUCTION
Retinal cone photoreceptors can be the primary target of genetic diseases such as achromatopsia,1–4 cone or cone-rod dystrophies (see RetNet for summary: http://www.sph.uth.tmc.edu/RetNet/). As well, concurrent involvement of cones and rods occurs in some forms of X-linked retinitis pigmentosa (XLRP) caused by mutations in the RP GTPase regulator (RPGR) gene,5,6 and it is the early and severe cone involvement that causes the marked visual impairment.7 In many other retinal diseases, however, cones are affected secondarily. This is the case in age-related macular degeneration (AMD),8 and many forms of retinitis pigmentosa (RP) where the primary gene mutation affects the rods, and cone abnormalities represent a secondary bystander effect.9 Although cones represent only about 5% of the human photoreceptor population,10 they are essential for color vision, central visual acuity, and photopic vision. Therefore, preservation of cone structure and function is of critical importance when considering photoreceptor-directed therapies.9
In contrast to man and nonhuman primates that have separate populations of red, green and blue cones, dogs are functional dichromats, with cone populations having combined red/green or blue pigments.11 These are termed long- and medium-wavelength-absorbing cones (L/M-cones) and short-wavelength-absorbing cones (S-cones), and have maximal sensitivities of 555 nm and 429–435 nm, respectively.12,13 Canine diseases with primary cone photoreceptor involvement include achromatopsia and X-linked progressive retinal atrophy (XLPRA).14 Canine achromatopsia is a recessively inherited cone degeneration that is phenotypically similar to human achromatopsia,15,16 and caused by either a genomic deletion or missense mutation of the cone cyclic nucleotide-gated cation channel beta subunit (CNGB3).4 CNGB3 is also mutated in about 50% of human patients with achromatopsia.17 On the other hand, XLPRA is caused by microdeletions in exon ORF15 of RPGR, and the mutation causing a frameshift has a severe, and aggressive early-onset cone/rod disease phenotype similar to what is found in RPGR/XLRP patients.6,18
Previous studies have shown that sequences upstream of the human red or long wavelength (L) opsin gene contain a core promoter with a locus control region (LCR) that confers expression in cones of transgenic mice.19,20 When a truncated version of the upstream sequences was incorporated into a recombinant adeno-associated viral vector (rAAV) of serotype-5, this promoter targeted green fluorescent protein (GFP) expression to cones in the mouse, rat, ferret, guinea pig, and squirrel monkey.21–23 Glushakova and colleagues24 showed that elements of the human S-opsin promoter can preferentially target cones in rats, although specificity is limited and expression is promiscuous.
In preparation to developing a platform for cone-directed therapies in the canine models that could ultimately be translated to the treatment of homologous disorders in humans, we constructed rAAV vectors packaged serotype-5 capsids, and used a GFP reporter gene to monitor transduction efficiency and stability. Different promoters previously shown to confer cone photoreceptor expression were used to target GFP expression to the dog L/M- and S-cones.19–24 Our results demonstrate poor expression levels, and lack of specificity with the human S-opsin promoter. In contrast, the red cone opsin promoters conferred a high degree of specificity, and expression robustness was dependent on the promoter length and content.
RESULTS
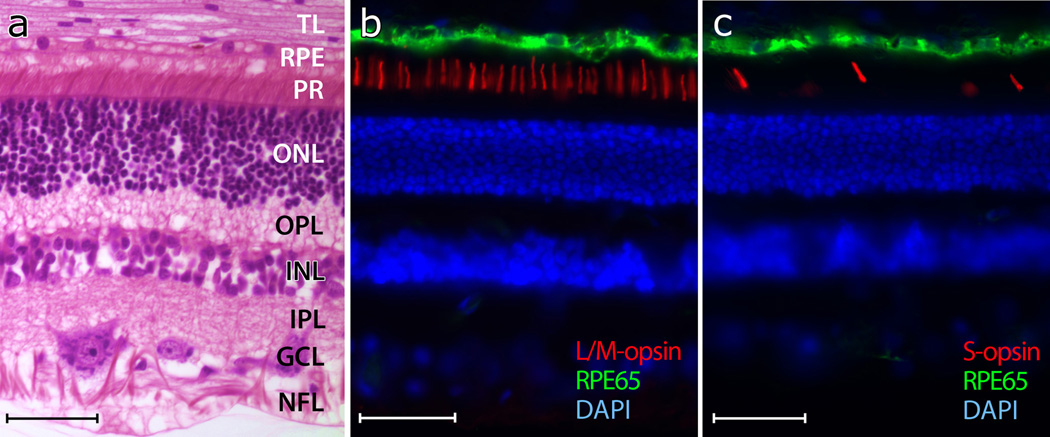
The morphology of the normal canine retina is similar to that of other mammalian species, including man (Figure 1a), but cones, particularly their inner segments, are not as distinct given the high rod/cone ratio. We estimated the ratio of L/M- to S-cones around 13:1 (8% S-cones) in the superior and 8:1 (13% S-cones) in the inferior canine retina (Figures 1b and 1c). These values are similar to previously reported estimates.25
Figure 1. Photomicrographs of normal canine retinas.
(a) Normal retina shown in superior tapetal zone (paraffin section, H&E).
(b) Labeling of L/M-cone outer segments (red) with an L/M-cone opsin antibody.
(c) Labeling of S-cone outer segments (red) with an S-cone opsin antibody. The retinal pigment epithelium (green) is labeled with an RPE65 antibody, and the cell nuclei are shown in blue with DAPI (b, c).
The following layers are identified in (a): Tapetum lucidum (TL), retinal pigment epithelium (RPE), photoreceptors (PR), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), and nerve fiber layer (NFL). Calibration marker = 40 µm.
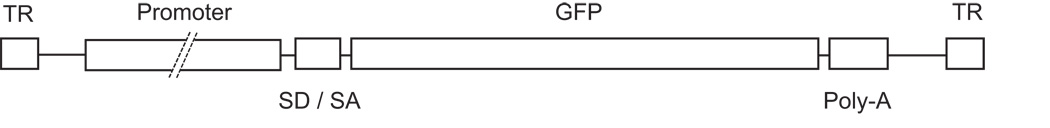
The effectiveness and specificity of rAAV5 and human red (PR0.5, 3LCR-PR0.5, and PR2.1) and blue (HB569) cone opsin promoters to direct cell class-specific GFP expression in cone photoreceptors was assessed in the normal canine retina (Table 1). Schematic illustration of the vectors used (Figure 2) show that they contained AAV2 inverted terminal repeats flanking a promoter, SV40 splice donor / splice acceptor (SD/SA), GFP gene, and a poly-A sequence. A 2.1kb fragment (PR2.1) of the human red cone pigment gene was used. This fragment was composed of bases spanning −4564 to −3009 joined to −496 to 0, and contained a locus control region (LCR) essential for expression of both the L and M pigment genes in human; the sequence is highly conserved among human, cow, and rats.19 The PR0.5 promoter utilized the - 496 to 0 region, and 3LCR-PR0.5 contained the −496 to 0 fragment joined to 3 copies of the 37 bp LCR at the −496 end. The HB569 contained the −557 to +11 region of the human S-opsin gene.24
Table 1.
Summary of dogs injected and promoters used
| Promoter | Dog | Gender | Eye | Age at injection (weeks) | Volume injected (µl) | Vector genomes per ml | Post-injection interval (weeks) | Cone GFP expression | Comment |
|---|---|---|---|---|---|---|---|---|---|
| PR0.5 | M592 | F | OD | 7 | 100 | 7.86 × 1012 | 5 | − | |
| M592 | F | OS | 7 | 80 | 7.86 × 1012 | 5 | − | ||
| M593 | F | OD | 7 | 130 | 4.59 × 1012 | 5 | − | ||
| M578 | M | OS | 13 | 1002 | 2.34 × 1012 | 5 | − | ||
| M579 | F | OD | 13 | 100 | 6.74 × 1012 | 5 | − | ||
| M579 | F | OS | 13 | 1002 | 6.74 × 1012 | 5 | − | ||
| 3LCR-PR0.5 | M571 | F | OD | 28 | 802 | 7.06 × 1012 | 4 | − | |
| M571 | F | OS | 28 | 110 | 7.06 × 1012 | 4 | ++ | L/M-cones only | |
| M572 | F | OD | 28 | 110 | 4.3 × 1012 | 4 | ++ | L/M-cones only | |
| PR2.1 | 18671 | F | OD | 5 | 80 | 8 × 1014 | 4 | +++ | L/M-cones only |
| GS46 | M | OS | 22 | 150 | 1.2 × 1012 | 8 | +++ | L/M-cones only | |
| GS47 | M | OS | 22 | 150 | 1.2 × 1012 | 8 | +++ | L/M-cones only | |
| GS45 | M | OD | 55 | 100 | 1.36 × 1013 | 6 | +++ | L/M-cones only | |
| GS45 | M | OS | 55 | 100 | 1.36 × 1013 | 6 | +++ | L/M-cones only | |
| HB569 | GS46 | M | OD | 22 | 170 | 1.6 × 1012 | 8 | + | Green fluorescence of few L/M-cones (no S-cones), few rods, and all RPE cells |
| GS47 | M | OD | 22 | 170 | 1.6 × 1012 | 8 | + | Green fluorescence of few L/M-cones (no S-cones), few rods, and all RPE cells | |
| HB569 & PR2.1 | GS54 | M | OD | 24 | 70 | 1.4 × 1012 | 8 | +++ | Green fluorescence of all L/M-cones, few rods, all RPE cells |
| GS54 | M | OS | 24 | 170 | 1.4 × 1012 | 8 | +++ | Green fluorescence of all L/M-cones, few rods, all RPE cells | |
| GS55 | M | OD | 24 | 150 | 1.4 × 1012 | 8 | +++ | Green fluorescence of all L/M-cones, few rods, all RPE cells |
PR0.5, 3LCR-PR0.5, and PR2.1: human red cone opsin promoters.
HB569: human blue cone opsin promoter
Dog 1867 is affected with rcd1 resulting from a stop mutation in PDE6B.27
Sub-RPE injections
F = female, M = male, OD = right eye, OS= left eye
Cone GFP expression: −, no signs of GFP expression; +, few green fluorescent cones; ++, few/no green fluorescent cones, but most/all visible cones GFP positive by immunolabeling; +++, most/all visible cones green fluorescent, no immunolabeling necessary to visualize GFP expression.
Figure 2.
Schematic illustration of the rAAV5 vector (not to scale). Inverted terminal repeats (TR) flank a promoter, SV40 splice donor/splice acceptor (SD/SA), GFP gene, and poly-A sequence. Promoters used were PR2.1 (2.1kb fragment composed of bases spanning −4564 to −3009 and −496 to 0 of the human red cone pigment gene promoter that contains a locus control region [LCR]), PR0.5 (the −496 to 0 region), 3LCR-PR0.5 (the −496 to 0 fragment fused to 3 copies of the 37 bp LCR at the −496 end), and HB569 (containing the −557 to +11 region of the human S-opsin gene promoter).
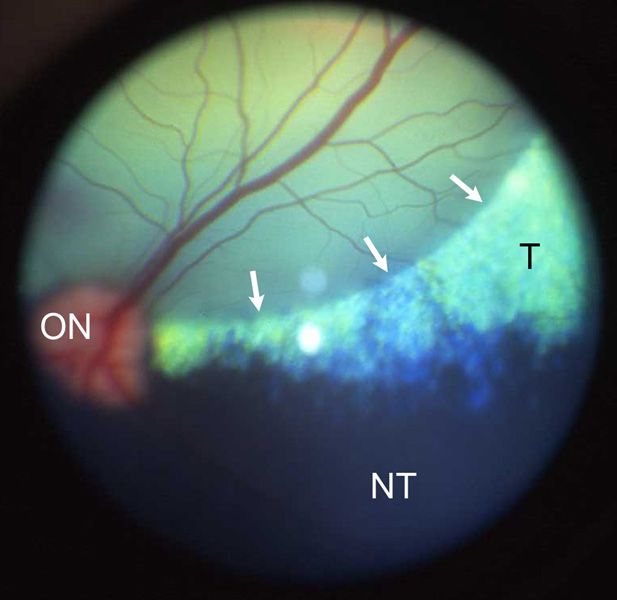
A total of 19 eyes of 12 dogs were injected; 11 dogs (18 eyes) were normal, and one was affected with a PDE6B mutation causing primary, early rod degeneration (Table 1).26,27 In 16 eyes the vector was injected into the subretinal space with visible bleb formation (Figure 3). In the remaining 3 eyes the vector was injected underneath the retinal pigment epithelium (RPE); the rAAV was unable to target the cone photoreceptors following these sub-RPE injections as no GFP expression was detected in these eyes. In all dogs, the mild uveitis induced by the surgery was transient, and controlled with short-term medical treatment.28 In one dog, both eyes developed small intraretinal hemorrhages in the region of the previous bleb within 1 week after injection. These resolved and were not observed in other injected eyes.
Figure 3.
Part of the subretinal bleb is visible immediately after the injection of the vector (arrows). The appearance of the bleb confirmed that the viral vector was administered to the subretinal space, a prerequisite for cone photoreceptor transduction. The green region represents the tapetal (T) zone, and the black region the non-tapetal (NT) zone of the canine ocular fundus. ON, optic nerve head.
Human Red Cone Opsin Promoters
Three versions of the human red cone opsin promoter were used: PR0.5, 3LCR-PR0.5, and PR2.1 (Table 1). The short proximal promoter PR0.5 was not effective in achieving any GFP expression as none of the retinas injected with PR0.5 showed green fluorescence in cones or other retinal cells 5 weeks after injection. Attempts to detect GFP expression by immunocytochemical labeling also failed.
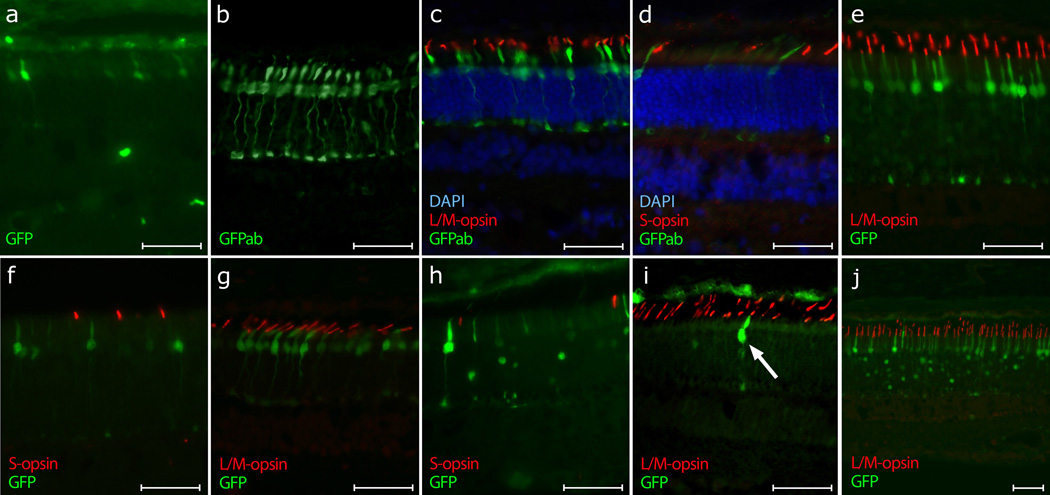
Adding 3 copies of the 35-bp LCR to PR0.5 (3LCR-PR0.5) lead to weak cone-specific GFP expression 4 weeks after injection. A few GFP positive cones could be recognized directly by their green fluorescence (Figure 4a). However, anti-GFP immunolabeling showed that all L/M-cones in the injection area were positive (Figures 4b and 4c). None of the S-cones expressed GFP (Figure 4d). Hence, specific GFP expression was achieved in L/M-cones, but, in general it was weak in that enhancement by immunocytochemical labeling was required for detection.
Figure 4. Fluorescence images showing targeted GFP gene expression in cones. Refer to Table 1 for specific details.
(a) – (c) 3LCR-PR0.5-GFP (dog M571, left eye, 4 weeks post subretinal vector administration).
(a) Native GFP expression visualized by excitation with blue light. Limited transduction and low expression resulted in only a few visible GFP-positive cones. (b) Immunolabeling with anti-GFP antibody (GFPab) identified a larger number of transduced cones. Note: A red fluorophor was used as secondary antibody to visualize GFP fluorescence; for consistency of figures, the color was changed digitally to green without altering the results. (c) All visible L/M-cones (red) in the injected area were positive for GFP when immunolabeled (green). The cell nuclei are shown in blue with DAPI.
(d) 3LCR-PR0.5-GFP (dog M572, right eye, 4 weeks post injection). Area of the retina towards the periphery of the initial bleb with a smaller number of transduced cones. None of the S-cones (red) expressed GFP (green immunolabeling). The cell nuclei are shown in blue with DAPI.
(e) – (f) PR2.1-GFP (dog GS46, left eye, 8 weeks post injection). (e) Strong GFP expression (green) could be seen in all L/M-cones (red) without any immunocytochemical enhancement.
(f) Area of the retina located at the periphery of the bleb with a smaller number of transduced cones. None of the S-cones (red) expressed GFP (green).
(g) PR2.1-GFP (dog 1867, 10-week old, rcd1 affected, right eye, 4 weeks post injection). In the mutant retina the L/M-cones (red) showed a high degree of specific native GFP expression (green).
(h) – (i) HB569-GFP (dog #GS47, right eye, 8 weeks post injection). (h) Strong GFP expression (green) in few cones and rods (with nuclear targeting), and weak GFP expression (green) in the RPE. Immunolabeling of the S-cones (red) showed that they were not transduced by the vector.
(i) Immunolabeling of the L/M-cone outer segments (red) showed that the few GFP positive cones transduced by HB569 (arrow) were indeed L/M-cones.
(j) PR2.1-GFP and HB569-GFP (dog GS54, left eye, 8 weeks post injection). The combination of the two vectors resulted in strong GFP expression (green) in all L/M-cones and few rods (with nuclear targeting). The signal in the RPE was attributed to the unspecific targeting of GFP expression by the HB569 promoter.
Calibration marker = 40 µm.
Ultimately, the longest version of the truncated human red opsin promoter (PR2.1) was used,19,21−23 and produced strong, selective and specific expression of GFP in all L/M-cones 4 to 8 weeks after injection (Figure 4, compare 4e with 4f). No immunocytochemical enhancement was necessary to see the expression of the reporter gene. For all the promoters that lead to the successful cone transduction, cone GFP expression was most intense in the center of the bleb, and tapered to the margins where the relative number of transduced cells gradually decreased (Figures 4d and 4f).
In addition, one eye of a dog affected by rcd1 was injected at 5 weeks of age with rAAV containing the PR2.1 promoter, and the retina was collected at 9 weeks of age. This disease is caused by a mutation in the PDE6B gene, and leads to abnormal development and early degeneration of rod photoreceptors before the canine retina is fully developed.26,27 At 5 weeks, the function and structure of the cone photoreceptors is still normal.26 Strong and specific GFP expression could be seen in all the L/M-cones of the rcd1 affected retina 4 weeks after injection (Figure 4g). At the age examined, the length of the cone photoreceptor cells appeared shorter because of the severe loss of rods which causes the cone inner segments to shorten and broaden.
Human Blue Cone Opsin Promoter
Strong GFP expression could be seen without the use of immunolabeling in only a few cones (approx. 2 %) and rods 8 weeks after injection of vector with the human blue cone opsin promoter HB569 (Figures 4h and 4i). The ratio of GFP positive cones to rods was between 1:5 and 1:3 depending on the area examined. However, immunocytochemical characterization of these cones indicated that they were not S-cones but rather a subgroup of the L/M-cones (Figures 4h and 4i). In addition, we saw green fluorescence in the RPE. Thus the rAAV5 vector with the human blue cone opsin promoter HB569 was not able to specifically target gene expression to canine S-cones, and, moreover, resulted in non-specific GFP expression in different canine retinal neurons.
Combination of Red and Blue Cone Opsin Promoters
With the aim of obtaining GFP expression in both canine L/M- and S-cone classes, we used in a single injection a combination of vectors with PR2.1 and HB569 cone opsin promoters. The results confirmed the previous findings after injection of a single vector. Strong GFP expression was found primarily in L/M-cones; this was attributed to the human red cone opsin promoter PR2.1 (Figure 4j). The strong green fluorescence in some rods and the signal in the RPE were attributed to the unspecific targeting of GFP expression by the HB569 promoter.
DISCUSSION
Cone photoreceptors fulfill critical functions such as central visual acuity, color vision, and photopic vision. Cones can be affected primarily or secondarily by retinal diseases that lead to severe visual deficits in the affected patients. Targeting gene expression to the cones, both for gene replacement therapy or for the synthesis of neurotrophic or cone survival factors, could provide rescue and sustained cone function. For these therapeutic investigations, dogs represent a valuable model for the development of cone-directed gene therapy, especially since two canine achromatopsic lines exist with either a genomic deletion (i.e. functional null) or missense mutation of the CNGB3 gene.4 The work presented herein with human cone opsin promoters provides the groundwork for future cone-directed gene therapy in canine models, and complements recent studies of cone-directed gene expression in the rat, ferret, guinea pig, and primate retina.22,23
Compared to other viral vectors, rAAV provides many advantages, such as its ability to infect both mitotic and post-mitotic growth arrested cells with high efficiency, its ability to accept non-viral regulatory sequences, and the lack of any associated human disease.29 The specific cell targeting is based on the use of cell-type specific promoters, the site of inoculation, and the AAV serotype.30 Currently, nine AAV serotypes are widely available; their AAV capsid proteins influence the cellular tropism as well as the speed of onset and intensity of gene expression.30 The combination of the serotype 2 genome with capsid proteins from other serotypes, i.e., pseudotyped AAV vectors, allow the enhancement of vector transduction characteristics in the retina.31,32 For example, transduction of both RPE and photoreceptor cells can be achieved by subretinal injection of either AAV2 or AAV5; however, the efficiency of transduction appears much greater with AAV5, especially in photoreceptors.33,34
The aim of this project was to evaluate various human cone opsin promoters for their specificity and efficacy to target gene expression to the canine cone photoreceptors. We were able to successfully target gene expression to the canine L/M-cones using the human red cone opsin promoter PR2.1 in rAAV5. The expression of the reporter gene GFP was both specific and effective in that all L/M-cones evaluated histologically were indeed expressing the GFP, and no significant expression of the reporter gene was seen in other cell types. The PR2.1 promoter has recently been used for successful cone-directed gene therapy in a mouse model of achromatopsia caused by a mutation in the cone alpha-transducin gene.21
We were unable to detect expression of GFP when using PR0.5, the shortest version of the human red cone opsin promoter. Adding 3 copies of the 35-bp LCR to the PR0.5 promoter led to detectable GFP expression in the cones. However, the level of GFP expression was lower than with PR2.1, and expression in the canine L/M-cones was detected, almost exclusively, using by immunolabeling. Provided that these low expression levels are stable, such outcome may not be undesirable in cases where overexpression could be detrimental to the cell, and a lower expression level is desired. In addition, the use of a shorter promoter would allow the introduction of a longer gene into the rAAV vector construct.
Unfortunately, the blue cone opsin promoter HB569 did not lead to GFP expression in canine S-cones. Instead, this promoter led to the expression of GFP in a few L/M-cones, rods, and in the RPE. At this point it is unclear why this promoter is not effective in transducing canine S-cones, but targets expression to a subgroup of L/M-cones, rods and RPE cells. The application of the same promoter in rats showed that about 37% of the GFP positive cones were S-cones, about 13% were M-cones, and almost half of the GFP positive photoreceptors in rats were rods.24 The results in our dogs are even less specific as we were not able to identify a GFP positive S-cone. These results are surprising given that the HB569 sequence appears to align better with the S-cone promoter sequence of the dog (75% identity) than the rat (68% identity; data not shown). Modifications will have to be made to the S-cone promoter in the future in order to successfully target the canine S-cones.
Similar to the rat,22 S-cone-specific GFP expression was also not found with the PR2.1 promoter in the dog, even though Wang19 and Alexander and colleagues21 reported that the 2.1 kb human red cone opsin gene promoter directed reporter gene expression in both M- and S-cones in transgenic mice. However, in regards to cone-targeted therapies in higher primates or dogs, such a result is not problematic; S-cones make up only 7% of the human cone population within 4 mm of the foveal center and are missing in a zone about 100 µm (0.35 degrees) in diameter near the site of peak cone density (foveal tritanopia).35 In dogs, we estimated that S-cones make up 8–13% of the cones. Thus we have demonstrated that highly specific targeting of reporter gene expression is possible in the predominant class of cones in the dog, and the results suggest that cone directed gene replacement therapy should be possible with the PR2.1 or 3LCR-PR0.5 promoters.
Finally, we were able to target gene expression to cones in a canine rcd1-affected retina, an example of a disease where cones are secondarily affected by a primary rod disorder. These very preliminary results suggest that cone-targeted therapies may be possible in diseases, where cones are secondarily affected.
In summary, we successfully proved the principle of cone specific gene targeting in a large animal model. Our results open the door for cone-specific gene therapy in canine models of primary and secondary cone disease.
MATERIALS AND METHODS
rAAV vector production and purification
Recombinant adeno-associated virus vectors of serotype 5 (rAAV5) were used. Promoter constructs were based on the human red-cone opsin promoters derived from the pR2.1-LacZ plasmid containing bases spanning −4564 to −3009 and −496 to 0 of the human red cone pigment gene. The pR2.1-LacZ plasmid was digested with NcoI which was then blunt ended by a Klenow polymerase filling reaction, and digested with KpnI to release the 2.1 kb fragment. This fragment was then ligated into a recombinant AAV vector plasmid containing a splice donor/splice acceptor (SD/SA), GFP, and poly adenylation signals, that had been digested with KpnI and XbaI in which the XbaI site was blunted by end-filling, to generate plasmid PR2.1-GFP. The rAAV constructs for PR0.5 and 3LCR PR0.5 driving GFP were created in a similar fashion. Briefly, only the “core” −496 to 0 promoter sequence was used for PR0.5, and three copies of the 37bp locus control region fused to the upstream sequence of the core promoter was used for 3LCR PR0.5 (Figure 2). The HB569-GFP construct was made as previously described using 569-bp PCR products containing human blue cone opsin promoter regions.24 These were directionally cloned into the KpnI and XbaI sites of the AAV proviral plasmids.
Production and purification of rAAV5 was carried out by procedures similar to those previously described.36,37 Real time PCR was used to determine titer, and the final rAAV5 aliquots in balanced salt solution (Alcon Labs, Fort Worth, TX) with 0.014% Tween 20 were stored at −80 °C.
Animal, anesthesia and subretinal injections
Eleven normal crossbred dogs were studied between 7 and 55 weeks of age. Subretinal injections of rAAV5 with different promoter constructs driving GFP were made in 18 eyes, and followed for different time points after injection (Table 1). In addition, 1 eye in a 5-week old dog affected with rcd1 was injected. This dog was genotyped homozygous affected for a PDE6B stop mutation.38 All procedures in this study were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania, and were done in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
The pre-operative and operative procedures for subretinal injections in dogs have been described.28 Briefly, the eyes received multiple applications of topical anti-inflammatory agents (prednisolone acetate 1% and flurbiprofen 0.03%) prior to surgery, and pupils were dilated with topical tropicamide 1%, phenylephrine 10%, and atropine 1%. Systemic antibiotics (amoxicillin trihydrate/clavulanate potassium 14 mg/kg PO q12hrs) and prednisone (0.5 mg/kg PO q12hrs) were administered before and for 3 days after surgery. The RetinaJect™ subretinal injector (SurModics, Inc., Eden Prairie, MN) was used for the injections through a transvitreal approach. Injections consisted of volumes between 80 and 150 µl with vector genome (VG) titers that varied depending on the vector/promoter used (rAAV5.PR0.5.GFP = 2.34–7.86 × 1012 VG/ml; rAAV5.3LCR.PR0.5.GFP = 4.3–7.06 × 1012 VG/ml; rAAV5.PR2.1.GFP = 1.2 × 10 12 – 8.0×1014 VG/ ml; rAAV5.HB569.GFP = 1.6 × 1012 VG/ml). Details of volumes and estimated vector doses use are summarized in Table 1.
The simultaneous retinotomy and separation of the photoreceptors from the RPE was created by the force of the injection. A successful subretinal injection was recognized by the formation of a subretinal bleb (Figure 3). A sub-RPE injection could be recognized by the lack of bleb formation and the immediate pink discoloration of the choroid, particularly the tapetal region. Post-operative management included a subconjunctival injection of 4 mg triamcinolone acetonide, as well as the topical application of neomycin-polymyxin B-dexamethasone 0.1% and atropine sulfate 1% ophthalmic ointments. The systemic antibiotics and steroids were continued for 3 days after surgery as described above. The topical administration of prednisolone acetate 1% and atropine sulfate 1% twice and then once daily was continued for 1 week after surgery. Using this protocol, the uveitis induced by the surgical trauma remained mild, and no signs of ocular pain or periocular swelling were noticed.
Histologic evaluation
Between 4 and 8 weeks after injection, the dogs were euthanatized (Table 1), the eyes enucleated, and fixed for 3 hours in 4% paraformaldehyde in 0.1 M PBS at 4 °C. Then the anterior segment and the vitreous were removed, and the eyecup was fixed for 21 hours in 2% paraformaldehyde in 0.1 M PBS at 4 °C. The eyecups were trimmed in order to have the injected region included in the sections, and tissues sequentially placed in 15% and 30% sucrose for 24 hours each prior to embedding in optimal cutting temperature (OCT) medium. Ten µm cryosections were cut, and the sections included both the area of the previous subretinal bleb and surrounding non-injected regions.
Green fluorescence from the expressed GFP was evaluated in unstained sections with a Zeiss Axioplan microscope (Carl Zeiss Meditec) with and epifluorescence illumination (Filter Set 23 with GFP excitation at 489 nm and emission at 509 nm). Those sections with absent or weak green fluorescence were labeled with GFP polyclonal antibody. In order to identify the 2 classes of cone photoreceptors, immunocytochemical staining was performed using either anti-S-opsin polyclonal antibody (rabbit 1:5,000; Chemicon, Temecula, CA; or goat 1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for the S-cones or anti-L/M-opsin polyclonal antibody for the L/M-cones (rabbit 1:500; Chemicon; or goat 1:100; Santa Cruz). The RPE was labeled with polyclonal anti-RPE65 antibodies (rabbit 1:10,000). Alexa Fluor® labeled goat anti-rabbit IgG or donkey anti-goat IgG (1:200; Molecular Probes, Inc., Eugene, OR) was used as secondary antibody. DAPI stain was used to detect cell nuclei. Additional sections of each eye also were stained with hematoxylin and eosin for routine light microscopic examination. Images were digitally captured (Spot 4.0 camera; Diagnostic Instruments, Inc., Sterling Heights, MI), and imported into a graphics program (Photoshop; Adobe, Mountain View, CA) for display.
ACKNOWLEDGMENTS
Supported by NIH Grants EY06855, EY07132, EY11123, EY13132, K12-EY15398, P30-EY01583, NS36302, Foundation Fighting Blindness, Macular Vision Research Foundation and The ONCE International Price for R & D in Biomedicine and New Technologies for the Blind. The authors thank Jeremy Nathans (Johns Hopkins University – Howard Hughes Medical Institute) for providing the pR2.1-LacZ plasmid; W. Clay Smith (University of Florida) for the GFP antibody; Tom Doyle and Min Ding (University of Florida) for assistance in vector production; T. Michael Redmond (National Eye Institute, Bethesda, MD) for the RPE65 antibody; Barbara Zangerl (University of Pennsylvania) for the promoter alignments; Amanda Nickle, Gerri Antonini, Alice Eidsen, Tracy Greiner and the staff of the Retinal Disease Studies Facility at the University of Pennsylvania for their technical support; and Mary Leonard for the figures.
W.W.H. and the University of Florida have a financial interest in the use of rAAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. All remaining authors declare that they have no competing financial interests.
REFERENCES
- 1.Kohl S, Marx T, Giddings I, Jägle H, Jacobson SG, Apfelstedt-Sylla E, et al. Total colourblindness is caused by mutations in the gene encoding the α-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet. 1998;19:257–259. doi: 10.1038/935. [DOI] [PubMed] [Google Scholar]
- 2.Kohl S, Baumann B, Rosenberg T, Kellner U, Lorenz B, Vadalà M, et al. Mutations in the cone photoreceptor G-protein α -subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet. 2002;71:422–425. doi: 10.1086/341835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundin OH, Yang JM, Li Y, Zhu D, Hurd JN, Mitchell TN, et al. Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet. 2000;25:289–293. doi: 10.1038/77162. [DOI] [PubMed] [Google Scholar]
- 4.Sidjanin DJ, Lowe JK, McElwee JL, Milne BS, Phippen TM, Sargan DR, et al. Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet. 2002;11:1823–1833. doi: 10.1093/hmg/11.16.1823. [DOI] [PubMed] [Google Scholar]
- 5.Demirci FY, Gupta N, Radak AL, Rigatti BW, Mah TS, Milam AH, et al. Histopathologic study of X-linked cone-rod dystrophy (CORDX1) caused by a mutation in the RPGR exon ORF15. Am J Ophthalmol. 2005;139:386–388. doi: 10.1016/j.ajo.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 6.Beltran WA, Hammond P, Acland GM, Aguirre GD. A frameshift mutation in RPGR exon ORF15 causes photoreceptor degeneration and inner retina remodeling in a model of X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006;47:1669–1681. doi: 10.1167/iovs.05-0845. [DOI] [PubMed] [Google Scholar]
- 7.Ebenezer ND, Michaelides M, Jenkins SA, Audo I, Webster AR, Cheetham ME, et al. Identification of novel RPGR ORF15 mutations in X-linked progressive cone-rod dystrophy (XLCORD) families. Invest Ophthalmol Vis Sci. 2005;46:1891–1898. doi: 10.1167/iovs.04-1482. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PT, Lewis GP, Talaga KC, Brown MN, Kappel PJ, Fisher SK, et al. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003;44:4481–4488. doi: 10.1167/iovs.03-0436. [DOI] [PubMed] [Google Scholar]
- 9.Léveillard T, Mohand-Saïd S, Lorentz O, Hicks D, Fintz AC, Clérin E, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 10.Curcio CA, Sloan KR, Kalina RE, Henrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev Camb Philos Soc. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs GH, Deegan JF, Crognale MA, Fenwick JA. Photopigments of dogs and foxes and their implications for canid vision. Vis Neurosci. 1993;10:173–180. doi: 10.1017/s0952523800003291. [DOI] [PubMed] [Google Scholar]
- 13.Neitz J, Geist T, Jacobs GH. Color vision in the dog. Vis Neurosci. 1989;3:119–125. doi: 10.1017/s0952523800004430. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre GD, Acland GM. Models, mutants and man: searching for unique phenotypes and genes in the dog model of inherited retinal degeneration. In: Ostrander EA, Giger U, Lindblad-Toh K, editors. The Dog and Its Genome. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2006. pp. 291–325. [Google Scholar]
- 15.Aguirre GD, Rubin LF. Pathology of hemeralopia in the Alaskan malamute dog. Invest Ophthalmol. 1974;13:231–235. [PubMed] [Google Scholar]
- 16.Aguirre GD, Rubin LF. The electroretinogram in dogs with inherited cone degeneration. Invest Ophthalmol. 1975;14:840–847. [PubMed] [Google Scholar]
- 17.Kohl S, Varsanyi B, Antunes GA, Baumann B, Hoyng CB, Jägle H, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13:302–308. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Acland GM, Wu WX, Johnson JL, Pearce-Kelling S, Tulloch B, et al. Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum Mol Genet. 2002;11:993–1003. doi: 10.1093/hmg/11.9.993. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- 20.Shaaban SA, Deeb SS. Functional analysis of the promoters of the human red and green visual pigment genes. Invest Ophthalmol Vis Sci. 1998;39:885–896. [PubMed] [Google Scholar]
- 21.Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Timmers AM, Guy J, Pang J, Hauswirth WW. Cone-specific expression using a human red opsin promoter in recombinant AAV. Vision Res. doi: 10.1016/j.visres.2007.07.026. in press. [DOI] [PubMed] [Google Scholar]
- 23.Mancuso K, Hendrickson AE, Connor TB, Mauck MC, Kinsella JJ, Hauswirth WW, et al. Recombinant adeno-associated virus targets passenger gene expression to cones in primate retina. J Opt Soc Am A Opt Image Sci Vis. 2007;24:1411–1416. doi: 10.1364/josaa.24.001411. [DOI] [PubMed] [Google Scholar]
- 24.Glushakova LG, Timmers AM, Pang J, Teusner JT, Hauswirth WW. Human blue-opsin promoter preferentially targets reporter gene expression to rat s-cone photoreceptors. Invest Ophthalmol Vis Sci. 2006;47:3505–3513. doi: 10.1167/iovs.05-1670. [DOI] [PubMed] [Google Scholar]
- 25.Gropp KE, Szél A, Huang JC, Acland GM, Farber DB, Aguirre GD. Selective absence of cone outer segment beta 3-transducin immunoreactivity in hereditary cone degeneration (cd) Exp Eye Res. 1996;63:285–296. doi: 10.1006/exer.1996.0117. [DOI] [PubMed] [Google Scholar]
- 26.Aguirre G, Farber D, Lolley R, Fletcher RT, Chader GJ. Rod-cone dysplasia in Irish Setters: a defect in cyclic GMP metabolism in visual cells. Science. 1978;201:1133–1134. doi: 10.1126/science.210508. [DOI] [PubMed] [Google Scholar]
- 27.Ray K, Baldwin VJ, Acland GM, Blanton SH, Aguirre GD. Cosegregation of codon 807 mutation of the rod cGMP phosphodiesterase β gene and rcd1. Invest Ophthalmol Vis Sci. 1994;35:4291–4299. [PubMed] [Google Scholar]
- 28.Komáromy AM, Varner SE, de Juan E, Acland GM, Aguirre GD. Application of a new subretinal injection device in the dog. Cell Transplant. 2006;15:511–519. doi: 10.3727/000000006783981701. [DOI] [PubMed] [Google Scholar]
- 29.Berns KI, Linden RM. The cryptic life style of adeno-associated virus. Bioessays. 1995;17:237–245. doi: 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- 30.Dinculescu A, Glushakova L, Min SH, Hauswirth WW. Adeno-associated virus-vectored gene therapy for retinal disease. Hum Gene Ther. 2005;16:649–663. doi: 10.1089/hum.2005.16.649. [DOI] [PubMed] [Google Scholar]
- 31.Auricchio A, Kobinger G, Anand V, Hildinger M, O'Connor E, Maguire AM, et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10:3075–3081. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- 32.Auricchio A. Pseudotyped AAV vectors for constitutive and regulated gene expression in the eye. Vision Res. 2003;43:913–918. doi: 10.1016/s0042-6989(02)00676-4. [DOI] [PubMed] [Google Scholar]
- 33.Yang GS, Schmidt M, Yan Z, Lindbloom JD, Harding TC, Donahue BA, et al. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J Virol. 2002;76:7651–7660. doi: 10.1128/JVI.76.15.7651-7660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotery AJ, Yang GS, Mullins RF, Russell SR, Schmidt M, Stone EM, et al. Adeno-associated virus type 5: transduction efficiency and cell-type specificity in the primate retina. Hum Gene Ther. 2003;14:1663–1671. doi: 10.1089/104303403322542301. [DOI] [PubMed] [Google Scholar]
- 35.Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB, et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- 36.Zolotukhin S. Production of recombinant adeno-associated virus vectors. Hum Gene Ther. 2005;16:551–557. doi: 10.1089/hum.2005.16.551. [DOI] [PubMed] [Google Scholar]
- 37.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 38.Ray K, Tejero MD, Baldwin VJ, Aguirre GD. An improved diagnostic test for rod cone dysplasia 1 (rcd1) using allele-specific polymerase chain reaction. Curr Eye Res. 1996;15:583–587. doi: 10.3109/02713689609000770. [DOI] [PubMed] [Google Scholar]