Abstract
Although stressors are believed to impair memory, experimental studies with humans have provided inconsistent support for this conclusion. The current study was designed to examine the effect of an acute psychosocial stressor, and subsequent reactivity, on episodic memory. One hundred participants completed a list-recall task before and after random assignment into a stressor or nonstressor condition. Participants assigned to the stressor condition exhibited both impaired delayed and immediate recall, and also exhibited increasesin the commission of intrusions and perseverations. The experience of off-task thoughts and intentional suppression of such thoughts, were associated with greater impairment of immediate recall. Changes in state anxiety, negative mood, and heart rate were unrelated to changes in memory. These data indicate that exposure to a stressor impaired the recall of previously learned information, and compromised the recall of newly acquired information. Furthermore, cognitive interference is an important factor regarding stress-related impairments of episodic memory. memory.
Keywords: Stress, Stress reactivity, Episodic memory
Chronic exposure to stress, and high-levels of stress-related hormones (e.g., cortisol), are known to impair memory function (Caswell et al., 2003;, Lee Kawachi, & Grodstein, 2004; Lupien et al., 1994) and functioning of the hippocampus (Lupien et al., 1998), a brain structure critical for episodic memory function (Squire, 1992). Although the results of studies demonstrating the deleterious effects of chronic stress on episodic memory are fairly consistent (although see, Isaac Cushway, & Jones, 2006), results demonstrating the effects of acute stressful experiences on episodic memory are inconsistent. Some studies have found evidence for stress-related impairments of episodic memory (Kuhlmann, Piel, & Wolf, 2005; Lupien et al., 1997; Wolf, Kudielka, Hellhammer, Hellhammer, & Kirschbaum, 1998), whereas others show no such effects (Hoffman & al’Absi, 2004; Nater et al., 2007; Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, 2001). Moreover, the mechanism(s) responsible for the effects of acute stress on episodic memory are not well understood. The current study was conducted to extend previous research, focusing on two main objectives. First, we sought to examine whether episodic memory performance for nonemotional information is impaired by the experience of a stressor, and determine whether such effects are observed during the recall of previously learned information (i.e., delayed recall) and during recall when new information must be acquired (i.e., immediate recall). Our second objective was to examine potential psychological mediators of the effects of stress on episodic memory performance, in an effort to complement previous research which has examined physiological mediators such as cortisol.
Given that the aims of the current study are to examine the effects of a psychosocial stress manipulation on episodic memory performance and potential psychological mediators of such effects, we devote our review of the existing literature to studies which employed a psychosocial stress manipulation. Furthermore, our review will focus exclusively on the effects of psychosocial stress manipulations on emotionally neutral, or nonemotional information. There is an abundance of recent research devoted to examining stress effects on memory for emotional information, which has provided an elegant examination of emotion–cognition linkages under conditions of stress (see, Wolf Kuhlmann, Buss, Hellhammer, & Kirschbaum, 2004, for review). Considering this vast and growing literature, however, is beyond the scope of the current study. For the purposes of the current study, and in reviewing relevant literature, we use terminology consistent with, Cohen Kessler, and Gordon (1997) whereby the term stressor refers to the actual psychosocial manipulation, and stress reactivity refers to biological, emotional, and cognitive changes observed in response to a stressor.
A growing body of literature exists that has examined the effects of stressors on episodic memory performance. A number of studies have examined the effects of a stressor on memory retrieval by exposing participants to a stressor, then asking them to recall information learned prior to the stressor. Lupien et al. (1997) found that a stressor manipulation impaired older adults’ delayed recall, whereas a nonstressor control task had no effect on delayed recall. However, the stressor condition always followed the control condition, and the observed deficits could be due to increased proactive interference, or fatigue from the demands of the protocol (Wixted, 2004). Wolf et al. (1998) similarly observed that exposure to a stressor impaired older adults’ delayed recall performance on a verbal-visual picture memory task; however, no control group was included, so it is unclear whether the observed effect was due to stress, forgetting, or a combination of both. In contrast, Kuhlmann et al. (2005) found that exposure to a stressor impaired delayed recall, but only for emotional information, not neutral information. Together, these studies indicate that stress might impair delayed recall, but alternative explanations including increased proactive interference, fatigue, and forgetting cannot be ruled out. Furthermore, studies that did observe impairments of delayed recall had samples comprised of older adults, who are more susceptible to proactive interference and forgetting (Lustig, May, & Hasher, 2001; MacDonald, Stigsdotter-Neely, Derwinger, & Bäckman, 2006); thus, if stress is responsible for the observed impairments, the results may not generalise to younger adults.
Research examining the effects of stress on immediate recall has also been inconsistent. Four studies (Domes, Heinrichs, Reichwald, & Hautzinger, 2002; Hoffman & al’Absi, 2004; Nater et al., 2007; Wolf et al., 2001) have examined immediate recall performance, assessing recall after exposing participants to a stressor. The studies by Domes et al. (2002), Nater et al. (2007), and Wolf et al. (2001) all found that there was no main effect of the stressor condition on memory performance. That is, the recall performance of participants exposed to the stressor was not significantly different to that of participants in the control condition.1 One study did, however, find that participants exposed to a stressor exhibited worse immediate recall (Jelicic, Geraerts, Merckelbach, & Guerrieri, 2004). In general, these results provide some, but not strong evidence that exposure to a stressor adversely affects immediate recall performance. Variations in the assessment of memory, such as making semantic judgements during encoding could account for the observed discrepancies. What these results do indicate is that, in addition to the impairing effects of a stressor on memory retrieval, exposure to a stressor compromises recall when performance depends upon the successful acquisition of new information; however, the robustness of this effect must be taken with caution.
Given that there is evidence of stressors affecting memory, it is of interest to examine indices of stress reactivity to identify and understand the mechanisms responsible for such effects. Much research examining the stress–episodic memory link has focused on physiological mechanisms, such as cortisol (Lupien & Lapage, 2001; Lupien & McEwen, 1997; Wolf, 2003). Comparatively little research has been conducted examining the role of psychological mechanisms in the stress–episodic memory link. Three primary psychological mechanisms have been advanced to account for stressor-related impairments of cognitive function: negative mood (Ellis & Ashbrook, 1988), state anxiety (Eysenck & Calvo, 1992), and cognitive interference (Klein & Boals, 2001a; Klein & Boals, 2001b; Sarason, Pierce, & Sarason, 1996). Relatively little research, however, has linked these variables to stressor-related episodic memory impairments.
Ellis and Ashbrook (1988) hypothesised that increases in negative mood lead individuals to focus on their current mood state, leaving fewer resources available for other forms of cognitive processing. If this is true, stressor-related increases in negative mood should explain any observed impairments in memory performance. To our knowledge, Jelicic et al. (2004) is the only study that has explicitly examined negative mood as a predictor of stressrelated episodic memory impairment. Consistent with Ellis and Ashbrook’s hypothesis, higher poststress levels of negative mood were associated with poorer recall (Jelicic et al., 2004). These results, however, do not indicate whether increases in negative mood explain the effects of stressor exposure on memory performance.
Eysenck and Calvo (1992) hypothesised that stressors result in increased anxiety, resulting in increased self-evaluation (i.e., concern about well-being and task performance), which in turn reduces resources that can be used for cognitive processing. According to this hypothesis, stressor-related increases in state anxiety should drive stress-related impairments of memory. Previous studies have shown that high trait anxiety is associated with stress-related impairment of cognitive function (Darke, 1988; Sorg & Whitney, 1992). These studies, however, do not show how stress-related changes in state anxiety are related to stress-related impairment of episodic memory. We are unaware of any studies which have examined this explicitly.
Klein and Boals (2001b) have argued that cognitive interference, including intrusive thoughts and the suppression of intrusive thoughts about stressors, reduces the resources necessary for cognitive processing. The evidence supporting this hypothesis has come largely from individual differences research showing that individuals with higher levels of stressor-related cognitive interference also exhibit poorer working memory (Klein & Boals, 2001b), processing speed and episodic memory (Stawski, Sliwinski, & Smyth, 2006). Although this previous research links stress-related cognitive interference to poorer cognitive function, the correlational designs of these studies do not provide insight into the direction of this effect. To our knowledge, no studies have demonstrated the presence of cognitive reactivity to an acute stressful experience and subsequently linked this reactivity to impaired cognitive function.
Taken together, negative mood, state anxiety, and cognitive interference are each hypothesised to explain stress-related impairments of episodic memory, yet have received little empirical attention. Cognitive interference may, however, be the most likely explanatory mechanism, as it represents one type of psychological stress reactivity, separate from but implicated in both Ellis and Ashboork’s (1988) and Eysenck and Calvo’s (1992) hypotheses (i.e., the conscious awareness of emotional and anxious states). These hypotheses both suggest that increases in negative mood and anxiety lead to introspection and processing of current states, which will subsequently affect memory. Thus, a portion of cognitive interference in response to a stressor may reflect cognitive representations of negative mood and anxiety. Therefore, cognitive interference may be a more direct measure of the mechanism and processes that Ellis and Ashbrook and Eysenck and Calvo advocate. Nonetheless, there is a clear need for a more comprehensive examination of the psychological mechanisms underlying stress-related impairments of episodic memory.
THE CURRENT STUDY
The present study was designed to accomplish two goals. First, we sought to extend previous research by examining the effects of an acute psychosocial stressor on episodic memory performance both for when previously learned information must be retrieved (i.e., delayed recall), and when recall depends on the acquisition of new information (i.e., immediate recall). With regards to this first goal, assessing the immediate recall of a list of words prior to the psychosocial stressor, and once again after the stressor (i.e., delayed recall) provides a test of whether stressor exposure compromises processes involved in the successful retrieval of previously learned information. In contrast, comparing the immediate recall of a list of words learned prior to the psychosocial stressor with the immediate recall of a second list of words learned after the stressor demonstrates the extent to which stressor exposure compromises recall when new information must be encoded or acquired. Such a distinction is important because previous research has shown asymmetries between encoding and retrieval at behavioural and neural levels (Braver et al., 2001; Naveh-Benjamin, Kilb, & Fisher, 2006), and it is important to understand which aspects of episodic memory are susceptible to stressor-related impairments. Second, we wanted to determine the extent to which various psychological markers of stress reactivity including negative mood, state anxiety, and cognitive interference, are implicated in the impairment of episodic memory performance.
METHOD
Study design
The design of the current study is a 2 × 2 mixed design with two repeated measures (time of memory assessment: premanipulation and postmanipulation recall performance) and one between-subjects factor (condition: stressor, control). Assignment into the between-subjects factor was random.
Participants
One hundred (26 males, 74 females) undergraduate students from an introductory psychology course at Syracuse University participated for course credit. Participants were randomly assigned to either a stressful (10 males, 40 females) or nonstressful control (16 males, 34 females) condition. The mean age was 18.94 (SD = 1.02, range = 18–24). All participants gave informed consent prior to beginning the study protocol, and were fully debriefed as to the purpose of the study at the end of the session.
Materials
Manipulations
In the stressor condition, participants were asked to complete the Trier Social Stress Test (TSST: Kirschbaum, Pirke, & Hellhammer, 1993). The TSST is a 20 minute procedure in which participants prepare (10 minutes) and deliver an impromptu speech (5 minutes), as well as perform a serial subtraction task (5 minutes), in front of confederates, while being recorded on video. In the control condition, individuals were asked to provide written descriptions of five neutral pictures adapted from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1997). Participants were given 20 minutes to write descriptions of each of the five pictures. They were instructed to describe everything they saw in the picture. Furthermore, participants were asked to refrain from making up stories about the pictures or from writing about how the pictures made them feel.
Episodic memory
The episodic memory task consisted of the free recall of a list of 30 nouns. All words were common nouns, no more than two syllables in length, semantically unrelated, and emotionally neutral. Participants were given 2 minutes to study the list of words, and were then given 2 minutes to immediately recall the words. All words were presented simultaneously on a computer screen. Performance was rated on three dimensions: number of correctly recalled words, number of recalled words not appearing on the list (intrusions), and the repetition of correctly recalled words (perseverations). Two lists of words were used. The lists were counterbalanced for word frequency, and the order the lists were presented in was counterbalanced across participants.
State anxiety
State anxiety was assessed using Spielberger, Gorusch, Lushene, Vagg, and Jacobs’ (1983) State-Trait Anxiety Inventory. Participants responded to 20 state-based questions such as “I feel satisfied” and “I feel frightened”, and trait-based questions including “I feel secure” and “I feel like a failure”. Responses were made on a 4-point scale (“quite untrue of you”, “somewhat untrue of you”, “somewhat true of you”, “quite true of you”). Negatively worded items were reverse coded and a total score was obtained by summing the responses of all 20 items. Higher scores reflected greater state anxiety.
Negative mood
Negative mood was measured using items from the Positive and Negative Affect Scales (Watson, Clark, & Tellegen, 1988). Individuals responded to five items: irritated, depressed, worried, annoyed, and sad, using a 5-point scale (“very slightly or not at all”, “a little”, “moderately”, “quite a bit”, and “extremely”) indicating how they were feeling right now, at this very moment. Scores were obtained by summing participant’s responses to all the items, with higher scores indicating greater negative mood.
Cognitive interference
Four items were generated to assess cognitive interference, including the self-reported experience of distracting (intrusive) thoughts and the intentional suppression of off-task thoughts during the postmanipulation memory tasks. Responses were made on a 7-point Likert scale with values ranging from 0 (“not at all”) to 6 (“a lot”). The first two items were administered to individuals in both conditions, and probed general levels of intrusive thoughts and intentional thought suppression during the post-manipulation memory tasks: “How distracted by your thoughts did you feel while you performed the last series of memory tasks?” and “How much did you feel yourself intentionally suppressing off-task thoughts while you performed the last series of memory tasks?”. The second two items assessed intrusive thoughts and intentional suppression of thoughts related to the specific manipulation. Individuals randomly assigned to the nonstressor condition responded to: “How distracted did you feel by thoughts about the writing task while performing the last series of memory tasks?” and “How much did you feel yourself intentionally suppressing off-task thoughts about the writing task while you were performing the last series of memory tasks?”. The stressor condition participants responded to the following questions: “How distracted did you feel by thoughts about the public speaking task, while performing the last series of memory tasks?”, and, “How much did you feel yourself intentionally suppressing off-task thoughts about the public speaking task, while you were performing the last series of memory tasks?”.
Heart rate
Heart rate (in beats per minute: bpm) was obtained using a Welch Allyn Vital Signs Monitor (Welch Allyn, Inc.). The cuff for the vital signs monitor was applied to the participant’s nonwriting hand.
Procedure
Table 1 displays the procedure and timing of the assessments. Testing began with participants giving informed consent. After providing consent, a baseline heart rate assessment was taken. Heart rate was measured every 3 minutes for 12 minutes. Following the heart rate assessment, participants completed a baseline report of state anxiety and negative mood, and were then asked to study and immediately recall the first list words (List 1). Following the recall task, half of the participants were randomly assigned to complete the TSST (stressor) protocol; the other half were assigned to complete the writing (nonstressor) protocol. Immediately after completing the TSST or writing protocol, heart rate, state anxiety, and negative mood were measured once again. Next, participants were asked to perform a delayed recall task of the words from List 1. Then, after a brief (~8 minute) period of completing a simple number identification filler task, participants were presented with a new list of words (List 2) to study for an immediate recall test. After, this last recall task, participants completed the cognitive interference questions, and were fully debriefed as to the purpose of the study. All testing was conducted during the afternoon between 12:00 p.m. and 6:00 p.m.
TABLE 1.
Time course for study protocol
| Time in protocol (minutes) | |
|---|---|
| Consent | 0 |
| Heart rate State anxiety Negative mood | +4 |
| Presentation (List 1) | +24 |
| Immediate recall (List 1) | +26 |
| TSST/writing manipulation | +30 |
| Heart rate State anxiety Negative mood | +50 |
| Delayed recall (List 1) | +60 |
| Number identification task | +62 |
| Presentation (List 2) | +70 |
| Immediate recall (List 2) | +72 |
| Cognitive interference | +76 |
| Debriefing | +80 |
RESULTS
Descriptive statistics
Equivalent proportions of each gender (males = .20 and .32 for nonstressor and stressor conditions, respectively) were randomly assigned to each of the conditions, χ2(1, N = 100) = 1.87, ns. Four participants randomly assigned to the stressor condition withdrew from the study prior to completing the study protocol, reporting that they were either uncomfortable completing the TSST or were unable to continue after having completed the TSST. Table 2 contains descriptive statistics for the heart rate, state anxiety, negative mood, and cognitive interference measures, by time and condition. Independent samples t-tests were conducted comparing baseline heart rate, negative mood, state anxiety, and cognitive interference. No group differences were observed (all ps >.20).
TABLE 2.
Pre- and postmanipulation descriptive statistics for stress reactivity variables
| Condition |
||||
|---|---|---|---|---|
| Nonstress (N=50) |
Stress (N=46) |
|||
| Pre Mean (SD) | Post Mean (SD) | Pre Mean (SD) | Post Mean (SD) | |
| Heart rate(bpm) | 77.47 (9.01)* | 75.44 (9.63) | 80.08 (11.02)* | 90.00 (11.62) |
| Negative mood | 7.38 (2.35) | 7.48 (2.21) | 7.54 (3.06) | 11.04 (4.25) |
| State anxiety | 38.66 (11.02) | 39.46 (9.98) | 37.59 (11.64) | 48.59 (12.25) |
| CI (Dist-Gen.) | — | 2.84 (1.62) | — | 3.15 (1.89) |
| CI (Supp-Gen.) | — | 3.26 (1.72) | — | 3.15 (1.87) |
| CI (Dist-Man.) | — | 1.76 (1.48) | — | 3.91 (1.62) |
| CI(Supp-Man) | — | 1.94 (1.47) | — | 3.56 (1.70) |
Average of 4 values taken at baseline (bpm = beats per minute). CI (Dist – Gen.) = Cognitive Interference: Experience Distracting Thoughts (General), CI (Supp – Gen.) = Cognitive Interference: Intentionally Suppress Thoughts (General), CI (Dist – Man.) = Cognitive Interference: Experience Distracting Thoughts (Manipulation Specific), CI (Supp – Man.) = Cognitive Interference: Intentionally Suppress Thoughts (Manipulation Specific).
Manipulation check
Heart rate, negative mood, and state anxiety were subjected to a 2 × 2 mixed analysis of variance (ANOVA), with condition (stressor, control) as a between-subjects factor and time (pre, post) as a within-subjects factor. Results of the ANOVAs revealed a significant main effect of time for heart rate, F(1, 94) = 95.71, MSE = 7.81, p <.01, negative mood, F(1, 94) = 24.16, MSE = 6.43, p <.01, and state anxiety, F(1, 94) = 33.47, MSE = 49.83, p < .01. There were also significant main effects of condition for heart rate, F(1, 94) = 17.17, MSE = 205.66, p < .01, negative mood, F(1, 94) = 13.70, MSE = 12.14, p < .01, and state anxiety, F(1, 94) = 3.85, MSE = 202.02, p = .05. These main effects were qualified by significant Condition × Time interactions for heart rate, F(1, 94) = 219.01, MSE = 7.81, p < .01, negative mood, F(1, 94) = 21.55, MSE = 6.42, p < .01, and state anxiety, F(1, 94)= 25.01, MSE = 49.83, p < .01. The interactions indicated participants in the stressor condition, on average, exhibited increases in heart rate, negative mood, and state anxiety after the speaking task, whereas there was little change in any of these three variables in the control condition.
Independent samples t-tests were conducted to test for group difference in the general and task specific cognitive interference items. For the general items, there were no group differences in either reported levels of experiencing distracting thoughts, t(94)= −0.87, ns, or the intentional suppression of such thoughts, t(94) = 0.29, ns. There were, however, significant group differences in the manipulation-specific cognitive interference items. The experience of distracting thoughts specific to the manipulation was significantly higher in the stressor condition, t(94) = 6.82, p < .01, as was the intentional suppression of the distracting thoughts, t(94) = 5.02, p < .01.
Effects of stressor exposure on episodic memory
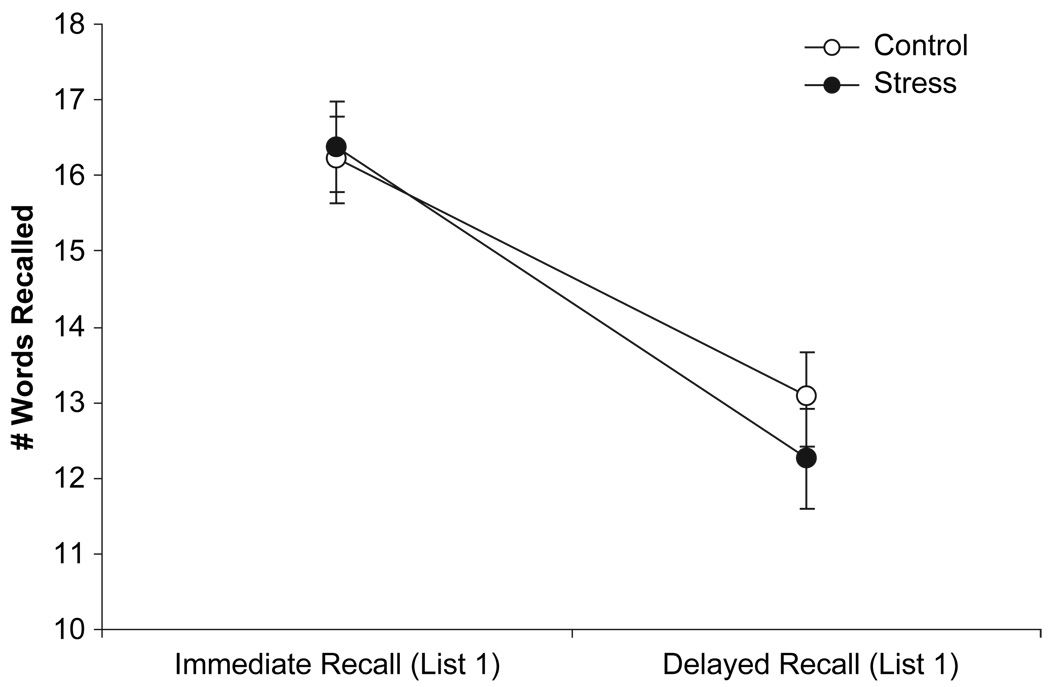
2 × 2 mixed ANOVAs were used to analyse the effects of stress on episodic memory recall performance. Analyses of differences between premanipulation (List 1) immediate free recall and delayed recall revealed a main effect of time, F(1, 94)=258.01, MSE = 4.91, p < .01, but not of condition, F(1, 94) = 0.18, MSE = 31.61, ns. The main effect of time was qualified by a significant Time × Condition interaction, F(1, 94) = 4.40, MSE = 4.91, p = .04. As can be seen in Figure 1, participants in both the stressor and control conditions recalled fewer words compared to their initial immediate recall occurring prior to the manipulation, indicative of forgetting. The decline in recall, however, was significantly greater in the stressor condition, indicating that the stressor manipulation had an additional negative effect.
Figure 1.
Number of words correctly recalled (±SEM) during immediate recall of List 1 and delayed recall of List 1 by condition.
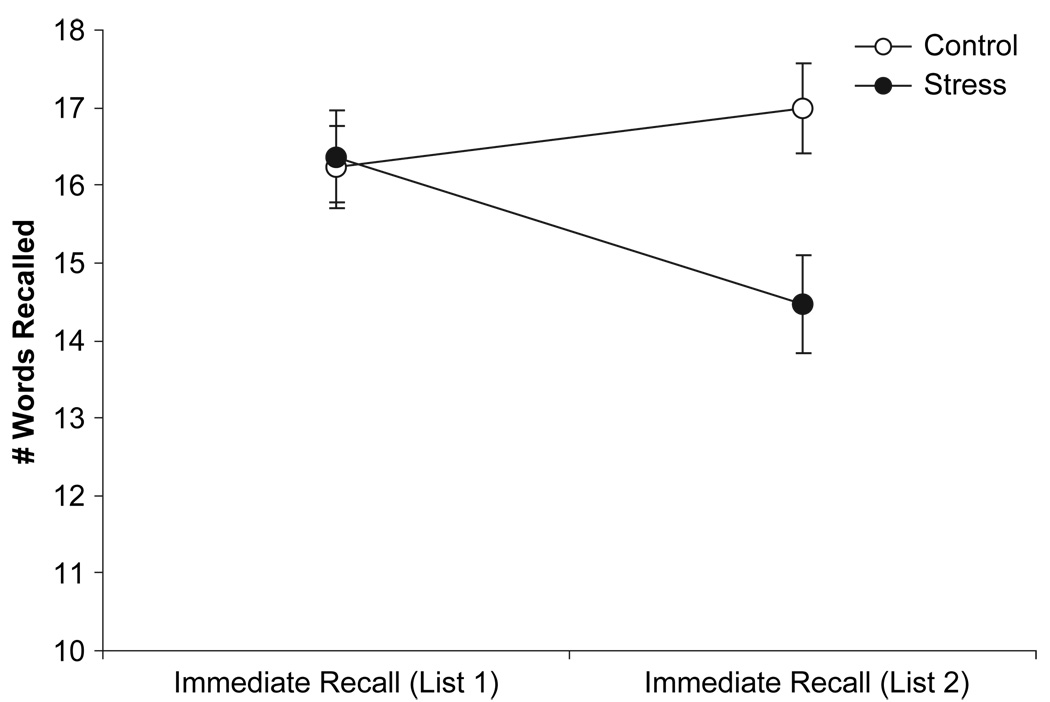
Analysis of differences between premanipulation (List 1) and postmanipulation (List 2) immediate free recall showed a main effect of time, F(1, 94) = 4.39, MSE = 3.75, p =.04, but not condition, F(1, 94) = 2.35, MSE = 29.19, ns, and a significant Time × Condition interaction, F(1, 94) = 22.48, MSE = 3.75, p < .01. Figure 2 shows the stressor group displayed a significant decrease in the number of words they could correctly immediately recall, F(1, 45) = 22.04, MSE = 3.82, p < .01, whereas the control group exhibited a moderately significant increase in the number of words recalled correctly, F(1, 49) = 3.71, MSE = 3.69, p = .06, indicative of a practice effect. These effects indicate exposure to a stressor compromised episodic memory when performance depended on the acquisition of new information.
Figure 2.
Number of words correctly recalled (±SEM) during immediate recall of List 1 and immediate recall of List 2 by condition.
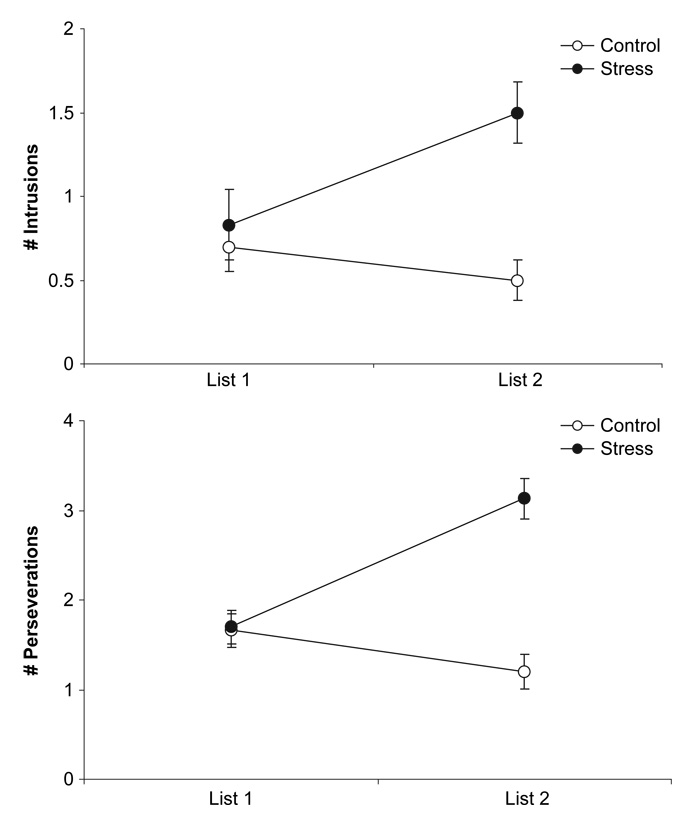
Next we analysed the effects of the stressor manipulation on the commission of intrusions and perseverations. The distribution of intrusions and perseverations were considerably positively skewed. We analysed the data using both repeated measures ANOVAs and Poisson regression for repeated measures using the generalised estimating equation (GEE). The two methods yielded identical results, and we present only the results from the ANOVAs. Analyses comparing intrusion commissions during immediate recall for List 1 and List 2 revealed a significant main effect of condition, F(1, 94) = 9.64, MSE = 1.58, p < .01, but not time, F(1, 94) = 2.55, MSE = 1.05, ns, and a significant Time × Condition interaction, F(1, 94) = 8.68, MSE = 1.05, p < .01. A similar pattern of results emerged for perseverations. Results of the ANOVA revealed significant main effects of condition, F(1, 94) = 18.58, MSE = 2.49, p < .01, and time, F(1, 94) = 9.23, MSE = 1.23, p < .01, which were qualified by a significant Condition × Time interaction, F(1, 94) = 34.89, MSE = 1.23, p < .01. The top and bottom panels of Figure 3 show that the exposure to the stressor resulted in an increase in the number of intrusions and perseverations committed, respectively.
Figure 3.
Top: number of intrusions committed (±SEM); bottom: number of perseverations committed (±SEM) during recall of List 1 and List 2 by condition.
Mediators of the effect of stressor exposure on episodic memory performance
We also sought to identify potential psychological indices of stress reactivity that might explain the observed stressor-related impairments of episodic memory performance. To test such possibilities, we followed procedures outlined by Baron and Kenny (1986). If the observed group differences in memory performance are attenuated after including one of the indices of stress reactivity (e.g., negative mood), this would suggest that the observed impairments can be explained by the observed group differences in changes in negative mood. If, however, the group differences in memory performance remain significant or largely unchanged, then the observed impairment in memory performance cannot be attributed, completely, or in part, to the effects of the stressor on negative mood. Such tests were conducted for negative mood, state anxiety, and the cognitive interference measures, and each index was considered in a separate model. The inclusion of negative mood, state anxiety, or cognitive interference into the statistical model did not alter the effect of the stressor manipulation on recall performance. The effect of condition (stress vs. control) when comparing List 1 immediate recall to List 1 delayed recall remained statistically significant (p < .05) regardless of which index of stress reactivity was included. Similarly, the effect of condition when comparing List 1 immediate recall to List 2 immediate recall remained statistically significant (p < .01) regardless of which index of reactivity was entered into the model. For all models, any reduction of the stressor effect, after inclusion of each potential mediator, was minimal. Additionally, none of the direct effects of the potential mediators on the change in memory performance was statistically significant (all ps >.10), rendering even partial mediation untenable. Together, effects of the stressor manipulation on immediate and delayed recall could not be explained by the effects of the stressor manipulation on negative mood, state anxiety, or cognitive interference.
Correlations among indices of stress reactivity and episodic memory performance
Negative mood, state anxiety, and cognitive interference failed to mediate the effect of the stressor on episodic memory performance, so we conducted exploratory analyses to examine whether reactivity to the stressor was associated with changes in episodic memory performance. We estimated Pearson correlations between the change scores (calculated as post minus pre) for recall, intrusion, and perseveration commissions, and change scores for heart rate, negative mood, and state anxiety. Change scores for the cognitive interference items could not be obtained as they were only assessed once.
Results showed that the general measures of cognitive interference were the only variables significantly correlated with the stress-related impairment of pre–post immediate recall. Individuals reporting higher levels of distracting thoughts experienced a greater decrease in immediate recall performance, r(44)= .35, p < .05, as did individuals reporting intentionally suppressing their distracting thoughts, r(44) = .30, p < .05. Evidence of a similar association was present in the data between manipulation-specific intentional thought suppression and impairment of immediate recall; however, the correlation only approached significance, r(44) = .25, p = .09. No other correlations were statistically significant.
DISCUSSION
Three main findings emerged from the current study. First, exposure to an acute psychosocial stressor impaired both immediate and delayed performance. Second, exposure to an acute psychosocial stressor resulted in increased commission of intrusions and perseverations. Third, cognitive interference, including the experience of distracting thoughts and intentionally suppressing such thoughts, was significantly greater among participants exposed to the stressor, and was associated with the greatest impairment in immediate recall performance.
The current results are consistent with Lupien et al. (1997) and Wolf et al. (1998), and extend these results to show stressor-related deficits in delayed recall performance among younger adults. The results, however, stand in contrast with Kuhlmann et al. (2005), who failed to observe a deficit in delayed recall after exposure to a stressor. One possible reason for the discrepancy is that our sample had a high proportion of females, whereas Kuhlmann et al.’s sample comprised of only males. Thus, fundamental ender differences in stress processes may account for these discordant results2 (Taylor et al., 2000). Whereas Domes et al. (2002), Hoffman and al’Absi (2004), and Wolf et al. (2001) showed no effects of a stressor on immediate recall, our results are consistent with Jelicic et al. (2004) who did observe a stressor-related decrement in immediate recall. Together, these results, in concert with previous findings provide strong evidence that exposure to psychosocial stress compromises both the recall of previously learned information (i.e., delayed recall), and recall dependent on the acquisition of new information (i.e., immediate recall). Furthermore, these results are consistent with studies of pharmacological stress and memory, which have shown detrimental effects of increasing glucocorticoids on memory performance (Het, Ramlow, & Wolf, 2005; Lupien & McEwen, 1997).
Exposure to the stressor not only impaired immediate and delayed recall performance, but also resulted in increased intrusion and perseveration commissions. Exposure to stress resulted in approximately a 50% increase in the number of intrusions and perseverations committed. Some researchers have argued that the commission of intrusions and perseverations reflects working memory or executive attentional deficits (Rosen & Engle, 1997, 1998). Given that such behaviours are thought to be mediated by the frontal lobes (i.e., Rosen & Engle, 1998), these findings are consistent with the assertion that tasks reflective of frontal lobe function are likely susceptible to the deleterious effects of stress on cognitive performance (Lupien & Lapage, 2001).
The current findings, in concert with the results of previous studies, raise several questions about the nature of the effects of stress on episodic memory. Previous studies have demonstrated stressor-related impairment of episodic memory performance, but alternative explanations including increased forgetting and proactive interference could not be discounted. The results of the current study can discount such explanations as the pattern of performance in the control group provides evidence of changes in memory performance without exposure to a stressor. Given that the stress manipulation had a demonstrable effect on both immediate and delayed recall performance, these results provide strong evidence supporting the hypothesis that an acute stressor can compromise episodic memory performance. Furthermore, the effects observed in the current study indicate that exposure to the stressor impaired the recall of previously learned information as well as when new information must be acquired. If the stress only impaired retention and retrieval processes, then the effect of stress on immediate and delayed recall should be equivalent, if not greater for delayed recall. Given that the effect of stress on immediate recall was larger than the effect on delayed recall, this suggests that stress has a specific impairing effect when new information must be acquired.
Our results are consistent with Jelicic et al. (2004), who observed stressor-related impairments of immediate recall, but not Domes et al. (2002) and Wolf et al. (2001), who observed no such effects. One potential reason for the observed differences could be due to the time of testing. Domes et al. and Wolf et al. conducted assessments during morning hours (between 9:00 a.m. and 12:00 p.m.), whereas we, along with Jelicic et al., conducted assessments during the afternoon (between 12:00 p.m. and 6:00 p.m.). There may be vulnerabilities to psychosocial stressors that are more pronounced as the day progresses, that are, as of yet, unexplained. Furthermore, given that both Jelicic et al.’s sample and our sample were primarily female, these findings could also further reflect some fundamental underlying gender differences in stress processes (Taylor et al., 2000). Another explanation for the discrepant results could be the method of memory assessment. Domes et al. and Wolf et al. both had participants make semantic judgements while they learned their word lists, whereas no such judgements were made in either our study or that of Jelicic et al. Previous research has shown that when attention is divided during the initial learning of information, episodic memory performance is compromised (Mangels, Picton, & Craik, 2001; Naveh-Benjamin & Guez, 2000; Naveh-Benjamin, Guez, & Marom, 2003), and that support during encoding is beneficial for memory performance (Naveh-Benjamin, Craik, & Ben-Shual, 2002). Therefore, providing support during the learning of new information after stressor exposure may help counteract any negative effects that occur as a result of the stressor. Future research could be conducted to examine both of these possibilities.
Although we sought to identify psychological indices of stress reactivity that could explain stressor-related impairments of episodic memory, we failed to do so. Increases in negative mood, state anxiety, and cognitive interference did not mediate the effects of stress on immediate recall. These findings do not support theories that psychological mechanisms such as negative mood (Ellis & Ashbrook, 1988), state anxiety (Eysenck & Calvo, 1992), and cognitive interference (Klein & Boals, 2001a,b; Stawski et al., 2006) underlie stressor-related impairments of episodic memory. However, such effects are purported to limit resources, such as working memory and attention, which are used for information processing. Thus, the episodic memory task used in the current study does not provide a direct test of these theories. It is possible that stressor-related increases in negative mood, state anxiety, and cognitive interference might mediate stress-related impairments of working memory and attention, but not true, long-term episodic memory. Additionally, it is possible that the effects of acute psychosocial stressor exposure on episodic memory also depend on activation of physiological systems linked to the hippocampus, such as the hypothalamic pituitary adrenal axis (Kuhlmann et al., 2005; Lupien & Lapage, 2001). Future research examining negative mood, state anxiety, and cognitive interference as mediators of the effects of stress on working memory and attention performance, as well as physiological mediators of the effects of stress on episodic memory, could prove fruitful.
Exploratory analyses revealed that cognitive interference, including the experience of distracting thoughts and the intentional suppression of such thoughts compound the effects of stress on immediate recall. As the measures that were correlated with memory impairment assessed cognitive interference in general, not those specific to the manipulation, it appears that a general proclivity to experience intrusive, off-task thoughts, and to intentionally suppress such thoughts may lead to greater susceptibility to stressor-related impairments of cognitive performance. These results are consistent with psychological accounts of the stress–cognition link, which hypothesise that intrusive thoughts about stress and the suppression of such thoughts consume the same cognitive resources used to perform complex cognitive tasks (Klein & Boals, 2001a; Klein & Boals, 2001b; Sarason et al., 1996; Stawski et al., 2006). They also suggest that such individual differences in cognitive interference are predictive of acute stressor-related impairments of episodic memory. It remains unclear, however, whether the amplified deficit among individuals reporting higher levels of cognitive interference reflects a limitation of the amount of information that can be remembered or a limitation of the attentional resources that can be allocated for information processing during acquisition. It is possible that intrusions may limit storage capacity, while intentional thought suppression limits the allocation of attentional resources for rehearsal, consolidation, and ultimately successful long-term storage. This evidence of associations between cognitive interference and stressor-related impairments of episodic memory does present an interesting and potentially insightful direction for future psychological stress research.
With respect to stress effects on delayed recall, we failed to observe any associations between memory performance and indices of stress reactivity. This pattern of results is not surprising given that negative mood, state anxiety and cognitive interference are hypothesised to impair cognitive functioning by reducing the attentional resources that can be allocated for information processing (Ellis & Ashbrook, 1988; Eysenck & Calvo, 1992; Sarason et al., 1996). Memory retrieval is much less susceptible to the effects of divided attention, and thus would likely be unaffected by attentional constraints due to increased negative mood, state anxiety, or cognitive interference (Naveh-Benjamin et al., 2006).
Limitations
One limitation of the current study is that the data cannot determine whether the observed impairments are truly hippocampal-mediated (i.e., specific to episodic memory processes), or mediated by the impairment of basic cognitive processes necessary for episodic memory, such as attentional processes or working memory. Recent behavioural (Naveh-Benjamin et al., 2006) and neuroimaging (Braver et al., 2001) data suggest that attention/working memory and episodic memory are not orthogonal at behavioural or neural levels, but could present an interesting avenue for future research.
The current data cannot dissociate the effects of the stressor manipulation on encoding and retrieval processes. Although the data are certainly suggestive of stressor-related deficits in the acquisition and recall new information, as well as retrieval of previously learned information, it cannot speak to the precise magnitude of stressor-related deficits in encoding, storage, consolidation, and retrieval processes. Future studies manipulating when information is encoded and the duration between learning and recall phases will help to address these issues.
Although we observed more pronounced stressor effects on immediate recall, our failure to include a brief distractor period before recall cannot conclusively rule out the possibility that exposure to a stressor compromised short-term memory or primary memory processes. Future studies including a sufficient distraction period before immediate recall will help better understand stressor effects on short-term and long-term memory. Despite this limitation, previous research using similar methodologies have failed to demonstrate that exposure to acute psychosocial stressors compromises short-term memory (Hoffman & al’Absi, 2004; Kuhlmann et al., 2005), suggesting such an explanation is unlikely. The possibility does remain that other primary cognitive mechanisms such as controlled attentional processes and coordinative functions needed for episodic memory are compromised by stressors (cf. Braver et al., 2001; Naveh-Benjamin et al., 2006).
Another limitation of the current study is the small sample size. The study was powered a priori for examining the treatment (stressor) effect, not for examining correlations between indices of stress reactivity and the magnitude of memory performance impairment. Furthermore, the current data are underpowered for examining the predictive strength of the stress reactivity markers simultaneously. Future research specifically examining individual differences in physiological and psychological markers of stress reactivity and episodic memory impairment could be quite informative. Finally, we cannot rule out that the observed group differences are attributable to differences in mental exertion (Wixted, 2004) as the stressor protocol was possibly more cognitively challenging and complex than the writing task used in the control condition.
CONCLUSION
The current study was successful in demonstrating that the experience of experimentally induced stress can impair episodic memory performance, whereas previous studies’ results were inconclusive. This study has also extended previous research to demonstrate that functions thought to be governed by the frontal lobe are susceptible to the effects of stressor exposure. Much research to date has focused on physiological markers of stress on cognitive performance, but the current data suggest that psychological mechanisms may play an important role in stressor-related impairment of episodic memory performance, in particular cognitive interference. Although the current study supports the argument that acute psychosocial stressors can impair episodic memory, this is only a first step in understanding the effects of stress on cognitive performance. Exposure to an acute stressor was shown to have a substantial effect compromising the ability to immediately recall new information as well as recall previously learned information. The results of this study suggest that continued examination of the link between stress and cognitive performance is warranted to understand what aspects of cognitive functioning are most susceptible to the experience of stressors, as well as what aspects of stress reactivity (i.e., physiological and/or psychological reactivity) underlie such compromise.
Acknowledgments
This study was conducted by the first author in partial fulfilment of the Master of Science degree at Syracuse University. We would like to acknowledge William Hoyer and Peter Vanable for valuable comments on earlier versions of this manuscript, as well as Tyera Eulberg and Christopher Terry for their help with data collection. This research was supported by grants from the NIMH (T32MH018904) and NIA (R01AG12448).
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
It is worth noting, however, that while no group differences in memory performance were observed as a function of stressor exposure, Nater et al. (2007) observed that among participants exposed to the stressor, those exhibiting high levels of stress reactivity, as indexed by increases in salivary cortisol, were associated with significantly better recall performance, whereas Wolf et al. (2001) observed a negative relationship. Thus, the effects of the manipulation may be specific to a subsample of participants, in both of these studies.
We did test for gender differences in the effects of stressor exposure on recall performance, as well as gender differences in the correlations between our indices of stress reactivity and the magnitude of memory impairment. No significant gender differences were observed in any of the analyses (all ps > .25, full results not reported). The current study, however, was not designed to test for gender differences so these findings should be viewed cautiously.
Contributor Information
Robert S. Stawski, The Gerontology Center, Pennsylvania State University, University Park, PA, USA
Martin J. Sliwinski, Department of Psychology and Center for Health and Behavior, Syracuse University, New York, USA
Joshua M. Smyth, Department of Psychology and Center for Health and Behavior, Syracuse University, New York, USA
REFERENCES
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RM, Cohen NJ, Miezin FM, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. NeuroImage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Caswell LW, Vitiliano PP, Croyle KL, Scanlan JM, Zhang J, Daruwala A. Negative associations of chronic stress and cognitive performance in older adults spouse caregivers. Experimental Aging Research. 2003;29:303–318. doi: 10.1080/03610730303721. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Measuring stress: A guide for health and social scientists. New York: Oxford University Press; 1997. [Google Scholar]
- Darke S. Anxiety and working memory capacity. Cognition and Emotion. 1988;2:145–154. [Google Scholar]
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic-pituitary-adrenal axis reactivity to stress and memory in middle-aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 2002;27:843–853. doi: 10.1016/s0306-4530(01)00085-3. [DOI] [PubMed] [Google Scholar]
- Ellis HC, Ashbrook PW. Resource allocation model of the effects of depressed mood states. In: Fiedler K, Forgas J, editors. Affect, cognition and social behaviour. Toronto, Ontario, Canada: Hogrefe; 1988. pp. 25–43. [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The Processing Efficiency Theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hoffman R, al’Absi M. The effect of acute stress on subsequent neuropsychological test performance. Archives of Clinical Neuropsychology. 2004;19:497–506. doi: 10.1016/j.acn.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clinical Psychology Review. 2006;26:939–955. doi: 10.1016/j.cpr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Jelicic M, Geraerts E, Merckelbach H, Guerrieri R. Acute stress enhances memory for emotional words, but impairs memory for neutral words. International Journal of Neuroscience. 2004;114:1343–1351. doi: 10.1080/00207450490476101. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klein K, Boals A. Expressive writing can increase working memory capacity. Journal of Experimental Psychology: General. 2001a;130:520–533. doi: 10.1037//0096-3445.130.3.520. [DOI] [PubMed] [Google Scholar]
- Klein K, Boals A. The relationship of life events stress and working memory capacity. Applied Cognitive Psychology. 2001b;15:565–579. [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical manual and affective ratings. Gainesville, FL: Center for Research in Psychophysiology, University of Florida; 1997. [Google Scholar]
- Lee S, Kawachi I, Grodstein F. Does caregiving stress affect cognitive function in older women? Journal of Nervous and Mental Diseases. 2004;192:51–57. doi: 10.1097/01.nmd.0000106000.02232.30. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NPV, et al. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. Journal of Clinical Endocrinology and Metabolism. 1997;82:2070–2075. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lapage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioural Brain Research. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NPV, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. Journal of Neuroscience. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Research Reviews. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology: General. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Stigsdotter-Neely A, Derwinger A, Bäckman L. Rate of acquisition, adult age, and basic cognitive abilities predict forgetting: New views on a classic problem. Journal of Experimental Psychology: General. 2006;135:368–390. doi: 10.1037/0096-3445.135.3.368. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Picton TW, Craik FIM. Attention and successful episodic encoding: An event-related potential study. Cognitive Brain Research. 2001;11:77–95. doi: 10.1016/s0926-6410(00)00066-5. [DOI] [PubMed] [Google Scholar]
- Nater UM, Moor C, Okere U, Stallkamp R, Martin M, Ehlert U, Kleigel M. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology. 2007;32:758–763. doi: 10.1016/j.psyneuen.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik FIM, Ben-Shual L. Age-related differences in cued recall: Effects of support at encoding and retrieval. Aging, Neuropsychology, and Cognition. 2002;9:276–287. [Google Scholar]
- Naveh-Benjamin M, Guez J. Effects of divided attention on encoding and retrieval processes: Assessment of attentional costs and a computational analysis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1461–1482. doi: 10.1037//0278-7393.26.6.1461. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Marom M. The effects of divided attention at encoding on item and associative memory. Memory and Cognition. 2003;31:1021–1035. doi: 10.3758/bf03196123. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Kilb A, Fisher T. Concurrent task effects on memory encoding and retrieval: Further support for an asymmetry. Memory and Cognition. 2006;34:90–101. doi: 10.3758/bf03193389. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology: General. 1997;126:211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. Working memory capacity and suppression. Journal of Memory and Language. 1998;39:418–436. [Google Scholar]
- Sarason IG, Pierce GR, Sarason BR. Cognitive interference: Theories, methods, and findings. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1996. [Google Scholar]
- Sorg BA, Whitney P. The effect of trait anxiety and situational stress on working memory capacity. Journal of Research in Personality. 1992;26:235–241. [Google Scholar]
- Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Sliwinski MJ, Smyth JM. Stress-related cognitive interference predicts cognitive function in old age. Psychology and Aging. 2006;21:535–544. doi: 10.1037/0882-7974.21.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Watson DA, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;47:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annual Review of Psychology. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Practice and Research: Clinical Endocrinology and Metabolism. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroimmunology. 1998;23:617–629. doi: 10.1016/s0306-4530(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kuhlmann S, Buss C, Hellhammer DH, Kirschbaum C. Cortisol and memory retrieval in humans: Influence of emotional valence. Annals of the New York Academy of Sciences. 2004;1032:195–197. doi: 10.1196/annals.1314.019. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]