Abstract
Background
A retrospective review of pediatric lung transplant recipients at 14 centers in North America and Europe was conducted to characterize the epidemiology and risk factors for cytomegalovirus (CMV) and to explore the impact of preventative antiviral therapy.
Methods
Data were recorded for one year post-transplant. Associations between CMV and continuous and categorical risk factors were assessed using Wilcoxon rank-sum and Chi-square tests, respectively. Associations between time to CMV and risk factors or survival were assessed by multivariable Cox proportional hazards models.
Results
Within 12 months posttransplant, 172 of 577 subjects (29.8%) developed 218 CMV episodes (90 asymptomatic infection, 25 syndrome, 103 disease). Forty-one subjects developed more than one episode of CMV. Donor or recipient CMV seropositivity was associated with increased risk of CMV episodes. Except for decreased prophylaxis in CMV D-/R- subjects, duration of prophylaxis did not vary by D/R serostatus. For CMV D+ subjects, not being on prophylaxis at the time of CMV episode increased the risk of CMV (D+/R+ HR 3.5: 95% CI 1.4, 8.4; D+/R- 1.9: 1.02, 3.7). CMV was associated with increased mortality within the first posttransplant year among those with donor or recipient CMV seropositivity (HR 2.0: 95% CI 1.1, 3.6; p=0.024).
Conclusions
CMV remains a serious complication after pediatric lung transplant, and the impact of prophylaxis is complex.
Keywords: Lung transplantation, Cytomegalovirus, Pediatrics
Introduction
Cytomegalovirus (CMV) after adult lung transplantation is associated with increased morbidity and mortality (1-4), although the the introduction of preventative antiviral medication regimens has significantly improved the natural history of CMV infection and disease after lung transplant (3, 5-9). In pediatrics patients, CMV has been associated with decreased survival (10, 11) in single center studies; however, the scope and risk factors for CMV infection and disease as well as the impact of antiviral regimens has not been explored in a large cohort of pediatric lung transplant recipients.
This retrospective cohort of pediatric lung transplant recipients represents the cumulative experience with CMV at 14 pediatric lung transplant centers in North America and Europe to test the hypothesis that longer duration of prophylaxis is associated with decreased incidence of CMV episodes in pediatric lung transplant recipients.
Materials and Methods
Members of the International Pediatric Lung Transplant Collaborative (IPTLC) were invited to participate in this multi-center retrospective cohort study. Fourteen centers contributed data. Prior to study participation, each center obtained approval from its respective Institutional Review Board or Ethics Committee.
The principal investigator performed a comprehensive chart review on each patient who underwent primary lung transplant at a participating pediatric transplant center between January 1988 and the time of data collection (August 2004-January 2007). Subjects were excluded if survival was less than two weeks. The medical chart review included all clinic records, discharge summaries, visit notes, correspondence, biopsy reports, microbiology and virology results, pulmonary function measurements, and diagnostic imaging up to one year post-transplant or until death or retransplantation if either event occurred before one year post-transplant. Information gathered included demographic data, induction and initial immunosuppressive regimens, prophylaxis regimens, acute rejection episodes, bronchiolitis obliterans/bronchiolitis obliterans syndrome, viral and fungal infections and if applicable, date and cause of death. Patient identifiers were removed from the data at the time of medical chart review.
Therapy Regimen
Pretransplant evaluation of patients was performed according to the standard protocol of each participating institution. Use of induction immunosuppressive therapy varied over the study period and across participating centers, ranging from no induction therapy to receipt of lympholytic agents or IL-2 receptor antagonists. After transplantation, most patients received triple-drug immunosuppression with a calcineurin inhibitor (CNI), prednisone, and either azathioprine or mycophenolate mofetil. Immunosuppressive therapy was gradually reduced as time from transplant increased if no evidence of significant acute or chronic graft rejection was detected on radiographs, transbronchial biopsies, and lung function testing. Target CNI trough serum concentrations varied by center and were not recorded for this study. In addition, prophylaxis for cytomegalovirus and routine transbronchial biopsies to assess for rejection were not standardized across centers and changed over time within centers. At a minimum, surveillance bronchoalveolar lavages with biopsies were performed during the first posttransplant year.
Definitions
Posttransplant cytomegalovirus infection definitions are adapted from the definitions proposed by American Society of Transplantation Infectious Disease Working Group on Infectious Diseases Monitoring and Ljungman et al(12) (13).
CMV infection
Presence of active replicating virus as determined by the standards at each institution by one of the following: conventional viral culture, shell vial viral culture, pp65 antigenemia testing or CMV polymerase chain reaction in whole blood or peripheral blood mononuclear cells.
Asymptomatic CMV infection
Detection of CMV by above methods without associated symptoms or signs of illness. Subjects with CMV infections without clinical documentation near the time of testing were characterized as asymptomatic for this analysis.
CMV syndrome
Constellation of symptoms consisting of fever, fatigue, thrombocytopenia and/or leukopenia in association with proof of CMV infection and without alternate explanations.
Proven CMV invasive disease
Histologic proof of CMV inclusions in biopsy tissue from lung, liver, or other body site with associated clinical signs and symptoms (For example, dyspnea, tachypnea, hypoxemia and radiographic infiltrates with the finding of CMV in biopsy would be proof of CMV pneumonia; diarrhea, blood in stool and or abdominal pain in association with ulceration with CMV inclusions on histology of GI biopsy would be definitive proof of CMV gastroenteritis).
Probable CMV disease
Clinically compatible symptoms, evidence of CMV infection without concurrent infections or rejection but without definitive histopathology on biopsy.
Possible CMV Disease
Clinically compatible symptoms, evidence of CMV infection but also with concurrent infection or rejection.
Other posttransplant outcomes
Posttransplant outcomes were based on definitions proposed by working groups from the International Society for Heart and Lung Transplantation (ISHLT) (14-16).
Acute rejection (AR)
Diagnosis and grading was performed at each individual center without review by a central pathology core using a defined classification scheme for pulmonary allograft rejection (15, 16). In addition, clinical suspicion of acute rejection that resulted in enhanced immunosuppression without histopathological evidence was noted.
Bronchiolitis Obliterans (BO) and Bronchiolitis Obliterans Syndrome (BOS)
Diagnosis and grading of BO used histologic identification (16). Diagnosis of BOS (BOS 1 and greater) was based upon current standards from the ISHLT (14, 17).
Proven and probable pulmonary fungal infections (PFI) were defined as previously reported in the literature (18).
Statistical analysis
Data were entered into an ORACLE database, analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC), and graphics were produced using R version 2.0.1 software (The R Foundation for Statistical Computing, Vienna, Austria). Associations between CMV and continuous and categorical risk factors were assessed using Wilcoxon rank-sum tests and Chi-square and Fisher's exact tests, respectively. Associations between CMV and survival time, and between time to CMV and risk factors were assessed by univariable and multivariable Cox proportional hazards models, censored at death, re-transplant, or one year post-transplant. Left-truncated Cox-proportional hazards models were used to consider risk factors for deaths occurring after 30 days. For each proportional hazards model, the proportional hazards assumption was assessed by entering risk-factor-by-time interactions into the model; this assumption was also assessed graphically using log-log-survival plots. Events that occurred during post-transplant follow-up, such as CMV infection, PFI, and rejection were modeled as time-dependent covariates. The functional form for age and era of transplant was chosen by modeling the quintiles of these variables as categorical variables and assessing the resulting parameter estimates. Multivariable models were chosen by performing backwards selection, with a significance criterion of 0.05, on initial models containing all risk factors while forcing the risk factor of interest into the model. Interactions suggested by the data or by clinical importance were included in the model selection process. All tests were two-tailed and performed at a significance level of 0.05.
Results
Demographics
Data from 577 primary lung or heart-lung transplant recipients were collected at 14 pediatric lung transplant centers in the United States, Canada, Austria, Germany and the United Kingdom. The majority were female (57.2%) and Caucasian (89.7%). Subjects were a median of 12.7 years at transplant (range 17 days to 20.9 years). The underlying etiology for transplantation was cystic fibrosis (53.1%), pulmonary hypertension secondary to congenital heart disease (14.2%), idiopathic pulmonary hypertension (8.9%), bronchiolitis obliterans (4.0%), infantile interstitial lung disease (3%) and pulmonary alveolar proteinosis (2.8%). Bilateral deceased donor (69.4%) and heart-lung transplantation (23.4%) were performed more than living donor transplantation (4.7%) or single lung transplant (2.4%). CMV serostatus was reported on 555 (96%) of subjects with a significant number of D+/R- (30.3%) and D-/R- (38%). Common outcomes within the first year after pediatric lung transplant included death or retransplantation (n=123, 21%), BOS (n=82, 14.2%), PTLD (n=44, 7.6%), fungal infection (n=97, 16.8%) and an episode of acute rejection grade A2 or higher (n=255, 43%). One-year survival was 79.7% (n=460).
Epidemiology
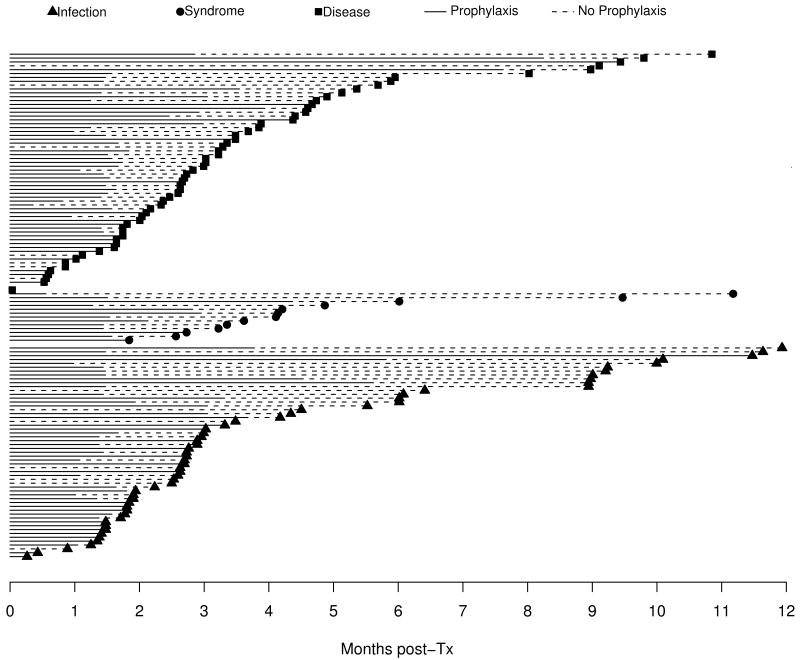
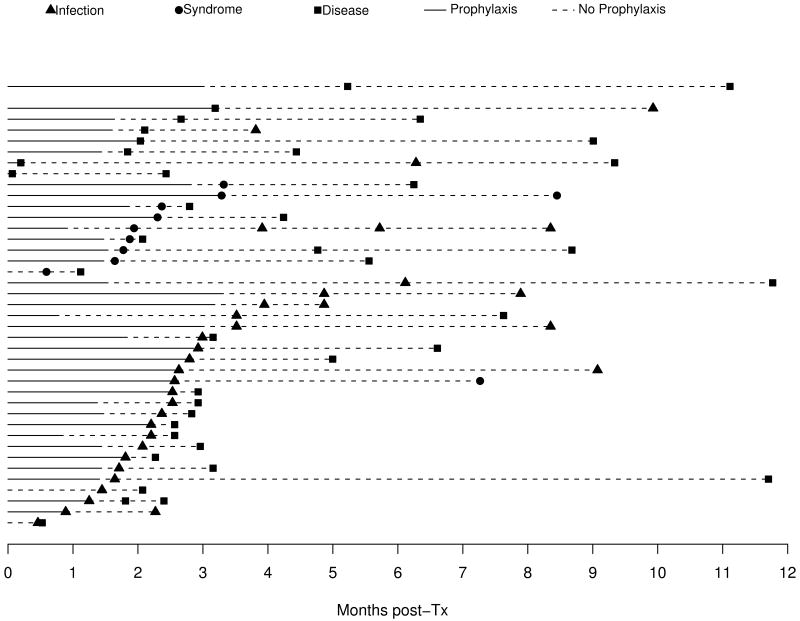
CMV occurred commonly within the first year after pediatric lung transplantation with 172 subjects (29.8%) recording 218 episodes. Asymptomatic CMV infection occurred in 83 subjects, the mean and median time to first infection was 116 and 83 days respectively. Time to first episode of CMV syndrome (n=24) and CMV disease (n=95) occurred at a median of 99 days (mean 113 days) and 86 days (mean 106 days), Figure 1A. CMV disease included pneumonitis (n=90), small bowel or gastric disease (n=3) and retinitis (n=2). CMV disease was categorized as proven (63.7%), probable (17.7%) and possible (18.6%). Forty-one subjects developed more than one episode of CMV, with progression from CMV infection (22% of infected subjects) or CMV syndrome (29% of syndromic subjects) to disease being the most common sequences (Figure 1B).
Figure 1. Patients with Single Episodes N=129.
CMV Diagnosis
The methods used to diagnose CMV were varied and often included more than one technique. Asymptomatic CMV was diagnosed by increased CMV titer (7), antigenemia (16), viral culture (44), or PCR testing (22). Diagnosis of CMV syndrome occurred by antigenemia (3), viral culture (10), and PCR (13). Disease was found on histopathology of lung tissue (64), antigenemia (5), viral culture (57), or PCR (3).
Risks for CMV episodes
Demographic and immunosuppressive regimens were evaluated for association with the development of CMV infection and CMV syndrome/disease (Table 1). Univariable risks for CMV infection included CMV donor or recipient seropositivity (HR 5.7 95% CI 2.4-13.8 for D-/R+; 7.6, 3.5-16.6 for D+/R-; and 12.8, 5.9-27.9 for D+/R+), receipt of induction therapy (1.6, 1.0-2.5) and initial therapy with cyclosporine (2.0, 1.1-3.5). CMV donor or recipient seropositivity was associated with CMV syndrome/disease (HR 4.2 95% CI 1.7-10.4 for D-/R+; 11.5, 5.5-24.1 for D+/R-; and 9.0, 4.1-19.6 for D+/R+). Because there were only minor differences, the episodes of CMV infection and syndrome/disease were pooled to evaluate for risk of any CMV episode. Living donor transplant, older age at transplant, and multiple episodes of A2 rejection prior to CMV were risks for an episode of CMV in univariable analyses in addition to CMV serostatus (Table 2). Era of transplantation, gender, ethnicity, cystic fibrosis, and prior fungal or viral infections were not associated with subsequent CMV episodes.
Table 1.
Demographics
| Infection (never syndrome or disease) | Syndrome or disease | No CMV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median (P25, P75) | P value* | N | Median (P25, P75) | P value* | N | Median (P25, P75) | ||
| Age at transplant (years) | 60 | 13.5 (8.8, 15.3) | 0.20 | 112 | 13.6 (10.5, 16.8) | 0.002 | 403 | 12.5 (5.7, 15.5) | |
| N | (%) | P value** | N | (%) | P value** | N | (%) | ||
| Gender | Female | 36 | 60 | 0.54 | 68 | 60.7 | 0.35 | 226 | 55.8 |
| Race | Caucasian/White | 53 | 88.3 | 0.79# | 101 | 90.2 | 0.63# | 361 | 89.8 |
| Etiology | Cystic fibrosis | 38 | 63.3 | 0.063 | 64 | 57.1 | 0.21 | 204 | 50.5 |
| Combo Transplant type | 0.03# | 0.011# | |||||||
| Single left or right | 2 | 3.3 | 4 | 3.6 | 8 | 2.0 | |||
| Bilateral cadaveric | 41 | 68.3 | 78 | 69.6 | 281 | 69.6 | |||
| Heart and lung | 11 | 18.3 | 20 | 17.9 | 104 | 25.7 | |||
| Living donor | 6 | 10.0 | 10 | 8.9 | 11 | 2.7 | |||
|
| |||||||||
| Donor and recipient CMV status | <0.001 | <0.001 | |||||||
| Donor + / Recipient + | 27 | 45.8 | 31 | 29.0 | 45 | 11.6 | |||
| Donor + / Recipient - | 15 | 25.4 | 57 | 53.3 | 96 | 24.7 | |||
| Donor - / Recipient + | 11 | 18.6 | 11 | 10.3 | 51 | 13.1 | |||
| Donor - / Recipient - | 6 | 10.2 | 8 | 7.5 | 197 | 50.6 | |||
| Donor CMV positive | 42 | 71.2 | <0.001 | 88 | 82.2 | <0.001 | 141 | 36.3 | |
| Donor or recipient CMV positive | 53 | 89.8 | <0.001 | 99 | 92.5 | <0.001 | 192 | 49.4 | |
| Any induction therapy | 31 | 54.4 | 0.04 | 50 | 48.5 | 0.12 | 149 | 40.0 | |
| Home going regimen: cyclosporine | 50 | 83.3 | 0.031 | 81 | 72.3 | 0.62 | 283 | 69.9 | |
| Home going regimen: tacrolimus | 10 | 16.7 | 0.04 | 31 | 27.7 | 0.73 | 119 | 29.4 | |
Comparison against No CMV group. Wilcoxon rank-sum test
Comparison against No CMV group. Chi-square test unless otherwise noted
Fisher's exact test
Table 2.
Univariable risk of CMV Infection or Disease
| Risk factor | N | Hazard ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Donor and recipient CMV status | 555 | <0.001 | ||
| CMV stats D-R- | Reference group | |||
| CMV stats D+R+ | 11.3 (6.3, 20.2) | |||
| CMV stats D+R- | 9.2 (5.2, 16.2) | |||
| CMV stats D-R+ | 5.1 (2.6, 9.9) | |||
|
| ||||
| Transplant type | 576 | <0.001 | ||
| Heart and lung | Reference group | |||
| Single lung | 2.0 (0.84, 4.8) | |||
| Bilateral cadaveric | 1.2 (0.83, 1.8) | |||
| Living Donor | 3.8 (2.1, 6.9) | |||
|
| ||||
| Age at transplant | 575 | 0.027 | ||
| Age quintile 1 (0.0- 5.6 yrs) | Reference group | |||
| Age quintile 2 (5.6-11.6 yrs) | 1.9 (1.1, 3.4) | |||
| Age quintile 3 (11.6-14.2 yrs) | 2.0 (1.2, 3.5) | |||
| Age quintile 4 (14.2-16.9 yrs) | 2.0 (1.2, 3.5) | |||
| Age quintile 5 (16.9-21 yrs) | 2.5 (1.4, 4.4) | |||
|
| ||||
| Era of transplant | 577 | 0.85 | ||
| 2002-2005 | Reference group | |||
| 1985-1993 | 0.99 (0.59, 1.7) | |||
| 1993-1997 | 1.2 (0.75, 1.8) | |||
| 1997-1999 | 0.86 (0.51, 1.5) | |||
| 1999-2002 | 1.04 (0.67, 1.6) | |||
| Female gender | 577 | 1.2 (0.85, 1.6) | 0.34 | |
| Cystic Fibrosis etiology | 576 | 1.3 (0.95, 1.7) | 0.11 | |
| Donor CMV positive | 563 | 5.2 (3.6, 7.5) | <0.001 | |
| Recipient CMV positive | 567 | 2.1 (1.6, 2.8) | <0.001 | |
| Any induction treatment | 533 | 1.4 (1.01, 1.9) | 0.040 | |
| Home going regimen: cyclosporine | 577 | 1.4 (0.95, 1.9) | 0.089 | |
| A2 rejection prior to CMV | 577 | 1.3 (0.98, 1.8) | 0.070 | |
| Second rejection prior to CMV | 577 | 1.5 (1.08, 2.0) | 0.014 | |
| Second A2 rejection prior to CMV | 577 | 1.9 (1.4, 2.8) | <0.001 | |
| PFI prior to CMV | 577 | 1.00 (0.62, 1.6) | 0.99 | |
| RVI prior to CMV | 577 | 0.64 (0.31, 1.3) | 0.22 | |
CMV – cytomegalovirus, D – donor, R – recipient, PFI – pulmonary fungal infection, RVI – respiratory viral infection
Due to the risk imposed by CMV serostatus in the univariable analysis, further evaluation separated CMV D-/R- subjects from those with any CMV seropositivity. In addition, the combined outcome of any CMV episode was employed as sample sizes limited modeling when CMV infection and disease were considered indepently. For D-/R-subjects, time-dependent multivariable models revealed CMV episodes were only associated with the receipt of induction therapy (HR 5.2; 95% CI 1.5, 18.8). For those subjects with either donor or recipient CMV seropositivity, donor CMV seropositivity regardless of recipient status (HR 2.1; 95% CI 1.3, 3.5 for D+/R+, HR 1.9; 95% CI 1.2, 3.0 for D+/R-), receipt of a living donor organ (2.5; 1.4, 4.3), transplant in the earliest transplant era in this study (2.3; 1.5, 3.8) and A2 rejection prior to CMV episode (1.4; 1.00, 1.9) were associated with the development of a CMV episode (Table 3).
Table 3.
Multivariable risk for CMV
| Donor or recipient CMV seropositive (152/344 with CMV episode) | ||
|---|---|---|
| Risk factor | HR (95% CI) | P-value |
| CMV D+/R+ | 2.1 (1.3, 3.5) | 0.003 |
| CMV D+/R- | 1.9 (1.2, 3.0) | 0.010 |
| Transplant type: Living Donor | 2.5 (1.4, 4.3) | 0.001 |
| Years of transplant: 1985-1993 | 2.3 (1.5, 3.8) | <0.001 |
| A2 rejection prior to CMV | 1.4 (1.00, 1.9) | 0.048 |
| Donor and recipient CMV seronegative (14/192 with CMV episode) | ||
|
| ||
| Risk factor | HR (95% CI) | P-value |
|
| ||
| Any induction therapy | 5.2 (1.5, 18.8) | 0.011 |
Prophylaxis
Prophylaxis against CMV included a variety of antiviral agents. 402 of 577 subjects received preventive antiviral therapy. An additional 12 patients received antiviral agents but had missing prescription dates, so they are excluded from all prophylaxis analyses. Ganciclovir (GCV) was most commonly included in a prophylaxis regimen with 372 subjects receiving GCV, intravenously or orally. Other agents used alone or in combination with GCV included acyclovir (52) and valganciclovir (31). The duration of prophylaxis varied considerably from a few weeks to several months (median 50 days). Subjects that were CMV D-/R- were less likely to have prophylaxis administered (P<0.001); prophylaxis duration was not otherwise associated with CMV D/R status.
Impact of prophylaxis duration
The impact of duration of prophylaxis on outcome varied by CMV D/R serostatus. In time-dependent modeling, duration of prophylaxis was not associated with a decrease in CMV episodes for recipients with history of pre-transplant CMV exposure (R+). However, for CMV mismatched subjects (D+/R-) who were naïve to CMV at transplant and received a CMV seropositive organ (n=165), discontinued prophylaxis at the time of CMV episode doubled the risk of CMV (HR 1.9, 95% CI 1.02, 3.7). In the same multivariable model, each month of prophylaxis received was associated with a 30% increase in risk of CMV infection or disease (HR 1.3, 95% CI 1.00-1.6). Evaluation of subjects who received prophylaxis beyond the individual institution's standard of care for clinical reasons revealed that extending prophylaxis was not associated with CMV episodes, Table 4. In addition, for CMV D+/R+ subjects (n=100), discontinued prophylaxis significantly increased the risk of CMV episodes (HR 3.5; 95% CI 1.4, 8.4).
Table 4.
Risk for CMV based on duration of prophylaxis stratified by CMV serostatus
| CMV D+/R+56/100 with CMV | CMV D+/R-71/165 with CMV | CMV D-/R+22/71 with CMV | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Off prophylaxis | 3.5 (1.4, 8.4) | 0.006 | 1.9 (1.02, 3.7) | 0.044 | 3.4 (0.74, 15.3) | 0.12 |
| Risk per month of prophylaxis received | 1.01 (0.80, 1.3) | 0.91 | 1.3 (1.00, 1.6) | 0.049 | 1.3 (0.90, 1.8) | 0.17 |
| Prophylaxis beyond site standard (yes/no) | 0.76 (0.42, 1.4) | 0.39 | 1.7 (0.95, 3.0) | 0.073 | 0.27 (0.08, 0.87) | 0.029 |
| Transplant 1985-1993 | 2.7 (1.2, 5.8) | 0.014 | 4.6 (1.3, 16.4) | 0.018 | ||
| Living Donor | 3.7 (1.7, 8.0) | <0.001 | ||||
Morbidity and mortality related to CMV
An episode of CMV was not associated with increase risk of subsequent morbidity including early BOS, PTLD, PFI or acute rejections during the first posttransplant year in either univariable or multivariable models. In CMV donor or recipient seropositive subjects, a single episode of CMV was associated with death or retransplantation within the first year after pediatric lung transplantation including subjects expiring early posttransplant (HR 2.0; 95% CI 1.1, 3.6; p=0.024) or using left-truncated models excluding subjects who died prior to 30 days (HR 2.0: 95% CI 1.08, 3.6; p=0.027), Table 5.
Table 5.
Risk for Death after pediatric lung transplantation in CMV D+ or R+ subjects
| Risk of death 0-365 days (64/342 with CMV) | Risk of death 30-365 days (56/329 with CMV) | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| CMV (time-dependent) | 2.0 (1.1, 3.6) | 0.024 | 2.0 (1.08, 3.6) | 0.027 |
| PFI (time-dependent) | 2.7 (1.5, 4.9) | <0.001 | 2.7 (1.4, 5.0) | 0.002 |
| Early BOS (time-dependent) | 5.6 (2.4, 13.0) | <0.001 | 4.6 (2.0, 10.4) | <0.001 |
| Living donor transplant | 3.8 (1.7,8.2) | <0.001 | 3.6 (1.5, 8.2) | 0.003 |
| Age < 5 years | 2.2 (1.2,4.0) | 0.009 | 2.7 (1.4, 5.0) | 0.002 |
| RVI (time-dependent) | 2.4 (1.2, 4.6) | 0.012 | 2.5 (1.3, 5.1) | 0.009 |
| A2 rejection (time-dependent) | 0.52 (0.28, 0.95) | 0.033 | ||
Discussion
Cytomegalovirus after pediatric lung transplantation remains a serious complication. The incidence in this cohort is similar (30%) to that reported in previous studies (10, 11). In addition, analogous to prior reports in lung and other organ transplant recipients, CMV donor or recipient seropositivity and prior episodes of acute rejection significantly increase the risk of CMV (19-22). However, other factors previously associated with CMV including immunosuppressive regimen did not increase the risk of CMV in multivariable models in pediatric lung transplant recipients (21, 23). Induction therapy increased the risk of CMV in CMV D-/R- subjects only.
As an immunomodulatory virus, CMV has been associated with increased risk of other complications after transplantation (1, 2). However, in this evaluation from pediatric lung transplant centers, CMV was not associated with an increased risk of posttransplant morbidity including pulmonary fungal infections and respiratory viral infections. The broad use of CMV prophylaxis in this population compared with these early reports of association may have had an impact.
Similar to the prior single-center pediatric lung transplant recipient report (10), CMV is associated with increased mortality after pediatric lung transplantation. Despite the broad use of prophylaxis in this population, a single episode of CMV (asymptomatic infection, syndrome or disease) increases the risk of early mortality.
Duration of prophylaxis and its impact on the development of CMV remains complex. For every month that prophylaxis is continued, the risk of CMV increases. In contrast, longer duration of prophylaxis, although not indefinite extension, has been associated with decreased risk of CMV. The risk for CMV was nearly the same for recipients who received 180, 270 or 365 days of prophylaxis (9). Another study in renal transplant recipients showed decreased CMV antibody seroconversion and inhibition of antibody maturation with prophylaxis (26). In addition, longer prophylaxis may be associated with decreased low-level circulation of CMV which has been reported to stimulate CMV specific T-cell proliferation (24, 25). The increased likelihood that pediatric recipients will be CMV-naïve at transplantation may increase the risk related to prolonged prophylaxis when these two immune-response findings are considered.
More importantly, a period of increased risk for CMV clearly exists after prophylaxis is stopped, with the greatest risk in the first 6 weeks after prophylaxis ends in prior reports (10). The timing of prophylaxis discontinuation after transplant prophylaxis may have less impact than the schedule of and adherence to CMV monitoring during this high risk period. The impact of intensive surveillance after discontinuation of CMV prophylaxis could affect the detection of early CMV and affect progression to CMV disease during this time frame, similar to the intent of preemptive therapy early posttransplant.
As with any retrospective study, there are limitations related to the study design, availability of data, and selection bias. Independent protocols for immunosuppressive therapy, induction therapy, and surveillance for rejection and infection were employed at each participating site. The use of induction therapy and calcineurin inhibitor preference also varied within each site over the study period. To minimize the effects of these temporal, inter-center, and intra-center differences, the principal investigator performed data collection at all participating sites and utilized strict adherence to specific predetermined definitions. Of note, all centers did perform routine surveillance bronchoscopies during the first posttransplant year.
A second limitation of this study involves CMV serostatus, diagnosis and diagnostic testing. Attribution of CMV serostatus in children can be complex, especially for those transplanted under a year of age with passive maternal antibodies, although this subset is small. In addition, the administration of blood products not screened for CMV, especially in the early part of this cohort, may have resulted in an incorrect CMV serostatus categorization or exposed a seronegative donor/recipient pair to CMV. Further, pediatric patients are at risk for disease acquisition from external sources through common contacts including day care and home exposures. Most participating centers did not use routine CMV surveillance systematically during the duration of the study. In addition, testing methods varied both across and within centers during the study period which minimally included the most recent era when molecular diagnostics became prevalent. Either issue may have underestimated the incidence of CMV episodes in this population. Further, a proportion of CMV episodes did not have clinical data associated with the event in the medical record; these episodes were classified as infection only. All episodes of CMV were considered together for evaluation of morbidity and mortality. However, evaluation of the impact of CMV syndrome or disease without infection confirmed a lack of association between CMV and morbidity in this population.
The limited examination of outcomes to one year after transplant also requires mention. Although infection is the most serious complication beyond one month but less than one year post-transplant, it remains a potentially serious complication regardless of the time since transplant and the significance of late-onset CMV is increasingly reported (25). Limiting analysis of outcomes to one year post-transplant may have censored the true occurrence of CMV in the patient population. Furthermore, it is possible that the relationships reported between CMV and negative outcomes, such as BOS and/or retransplantation, may not manifest within the first year posttransplant limiting the ability to identify significant associations. In this case, the hazard ratios of the univariable and multivariable Cox proportional models may be artificially low. Future studies are planned to examine associations between CMV and post-transplant morbidities such as acute and chronic graft rejection beyond one year posttransplant.
Conclusions
The risks for CMV disease after pediatric lung transplantation, primarily CMV donor-recipient mismatch, mirror those reported in adult lung and other solid organ transplant recipients. Interestingly, progression of CMV to more symptomatic disease was found in 22% of infected individuals. CMV is a significant risk for death after 30 days in pediatric lung transplant recipients.
Prophylaxis is a complex issue which includes a balance of immunosuppression, CMV-specific immunity and viral suppression. The balancing point of preventative therapy in this population remains unknown. However, increased surveillance after discontinuation of preventative therapy is warranted given the increased risk when discontinuing prophylaxis. Extended prophylaxis for clinical concern does not seem to decrease the risk for CMV and increases the risk for medication related toxicity and the development of viral resistance. Future studies will require systematic evaluation including monitoring of CMV-specific immunity to discover the optimal duration of CMV prevention therapy after pediatric lung transplantation.
Figure 2. Patients with Multiple Episodes N=41.
Acknowledgments
Funding: Supported by Thrasher Research Fund National Institutes of Health/K23 RR022956 (LDI)
References
- 1.Husni RN, Gordon SM, Longworth DL, et al. Cytomegalovirus infection is a risk factor for invasive aspergillosis in lung transplant recipients. Clin Infect Dis. 1998;26(3):753. doi: 10.1086/514599. [DOI] [PubMed] [Google Scholar]
- 2.Monforte V, Roman A, Gavalda J, et al. Nebulized amphotericin B prophylaxis for Aspergillus infection in lung transplantation: study of risk factors. J Heart Lung Transplant. 2001;20(12):1274. doi: 10.1016/s1053-2498(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 3.Chmiel C, Speich R, Hofer M, et al. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin Infect Dis. 2008;46(6):831. doi: 10.1086/528689. [DOI] [PubMed] [Google Scholar]
- 4.Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81(10):1415. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 5.Maurer JR, Tullis DE, Scavuzzo M, Patterson GA. Cytomegalovirus infection in isolated lung transplantations. J Heart Lung Transplant. 1991;10(5 Pt 1):647. [PubMed] [Google Scholar]
- 6.Ettinger NA, Bailey TC, Trulock EP, et al. Cytomegalovirus infection and pneumonitis. Impact after isolated lung transplantation. Washington University Lung Transplant Group. Am Rev Respir Dis. 1993;147(4):1017. doi: 10.1164/ajrccm/147.4.1017. [DOI] [PubMed] [Google Scholar]
- 7.Patel R, Paya CV. Infections in solid-organ transplant recipients. Clinical Microbiological Reviews. 1997;86 doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan AJ, Dummer JS, Paradis IL, et al. Cytomegalovirus infection and survival in lung transplant recipients. J Heart Lung Transplant. 1991;10(5 Pt 1):638. [PubMed] [Google Scholar]
- 9.Zamora MR, Nicolls MR, Hodges TN, et al. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am J Transplant. 2004;4(10):1635. doi: 10.1111/j.1600-6143.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 10.Danziger-Isakov LA, Delamorena M, Hayashi RJ, et al. Cytomegalovirus viremia associated with death or retransplantation in pediatric lung transplant recipients. Transplantation. 2003;75(9):1538. doi: 10.1097/01.TP.0000061607.07985.BD. [DOI] [PubMed] [Google Scholar]
- 11.Metras D, Viard L, Kreitmann B, et al. Lung infection in pediatric lung transplant: experience in 49 cases. European Journal of Cardio-thoracic Surgery. 1999;15:490. doi: 10.1016/s1010-7940(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 12.Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 13.Ljungman P, Griffiths P, Paya C. Definition of Cytomegalovirus Infection and Disease in Transplant Recipients. Clinical Infectious Diseases. 2002;34:1094. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of the nomenclature and for clinical staging of chronic dysfunction in lung allografts. Journal of Heart and Lung Transplantation. 1993;12:713. [PubMed] [Google Scholar]
- 15.Yousem SA, Berry G, Brunt E, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection. Journal of Heart Transplantation. 1990;9:593. [PubMed] [Google Scholar]
- 16.Yousem SA, et al. Revision of the 1990 working formulation of the classification of pulmonary allograft rejection: Lung rejection study group. Journal of Heart and Lung Transplantation. 1996;15:1. [PubMed] [Google Scholar]
- 17.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 18.Danziger-Isakov LA, Worley S, Arrigain S, et al. Increased mortality after pulmonary fungal infection within the first year after pediatric lung transplantation. J Heart Lung Transplant. 2008;27(6):655. doi: 10.1016/j.healun.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opelz G, Dohler B, Ruhenstroth A. Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(6):928. doi: 10.1111/j.1600-6143.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 20.Kranz B, Vester U, Wingen AM, et al. Acute rejection episodes in pediatric renal transplant recipients with cytomegalovirus infection. Pediatr Transplant. 2008;12(4):474. doi: 10.1111/j.1399-3046.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 21.Kamar N, Mengelle C, Esposito L, et al. Predictive factors for cytomegalovirus reactivation in cytomegalovirus-seropositive kidney-transplant patients. J Med Virol. 2008;80(6):1012. doi: 10.1002/jmv.21176. [DOI] [PubMed] [Google Scholar]
- 22.Kuypers DR, Evenepoel P, Maes BD, Coosemans W, Pirenne J, Vanrenterghem YF. Role of immunosuppressive drugs in the development of tissue-invasive cytomegalovirus infection in renal transplant recipients. Transplant Proc. 2002;34(4):1164. doi: 10.1016/s0041-1345(02)02812-9. [DOI] [PubMed] [Google Scholar]
- 23.Jorge S, Guerra J, Santana A, Mil-Homens C, Prata MM. Mycophenolate mofetil: ten years' experience of a renal transplant unit. Transplant Proc. 2008;40(3):700. doi: 10.1016/j.transproceed.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83(7):1971. [PubMed] [Google Scholar]
- 25.Singh N. Cytomegalovirus infection in solid organ transplant recipients: new challenges and their implications for preventive strategies. J Clin Virol. 2006;35(4):474. doi: 10.1016/j.jcv.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Steininger C, Kundi M, Kletzmayr J, Aberle SW, Popow-Kraupp T. Antibody maturation and viremia after primary cytomegalovirus infection, in immunocompetent patients and kidney-transplant patients. J Infect Dis. 2004;190(11):1908. doi: 10.1086/424677. [DOI] [PubMed] [Google Scholar]