Abstract
Human mesenchymal stem cells (MSC) are immunosuppressive and poorly immunogenic, but may act as antigen-presenting cells (APC) for CD4+ T cell responses; here we have investigated their ability to serve as APC for in vitro CD8+ T cell responses.
MSC pulsed with peptides from viral antigens evoked IFN-γ and Granzyme B secretion in specific CTL and were lysed, although with low efficiency. MSC transfected with tumor mRNA or infected with a viral vector carrying the Hepatitis-C virus NS3Ag gene induced cytokine release, but were not killed by specific CTL, even following pre-treatment with IFN-γ.
To investigate the mechanisms involved in MSC resistance to CTL mediated lysis, we analysed expression of HLA class I related antigen-processing machinery (APM) components and of immunosuppressive HLA-G molecules in MSC. The LMP7, LMP10 and ERP-57 components were not expressed and the MB-1 and zeta molecules were downregulated in MSC either unmanipulated or pre-treated with IFN-γ.
Surface HLA-G was constitutively expressed on MSC, but not involved in their protection from CTL-mediated lysis. MSC supernatants containing soluble (s)HLA-G inhibited CTL-mediated lysis, whereas those lacking sHLA-G did not. The role of sHLA-G in such inhibition was unambiguously demonstrated by partial restoration of lysis following sHLA-G depletion from MSC supernatants.
In conclusion, human MSC can process and present HLA class I restricted viral or tumor antigens to specific CTL with a limited efficiency, likely due to some defects in APM components. However, they are protected from CTL-mediated lysis through a mechanism that is partly sHLA-G dependent.
Keywords: antigen presenting cells, antigen processing machinery, CTL, immunogenicity, MSC
INTRODUCTION
Human mesenchymal stem cells (MSC) are a rare subset of non-hematopoietic stem cells localized around the vasculature and trabeculae in the bone marrow (BM), representing 0.01-0.001 % of total BM cells. MSC have been also isolated from many other human tissues1. Due to the limited stemness of MSC, a recent consensus conference has proposed to denominate them “mesenchymal stromal cells”2.
In the BM, MSC contribute to the formation of the hemopoietic stem cell (HSC) niche, supporting growth, maturation, differentiation and survival of HSC3. On this ground, human MSC have been successfully administered to breast cancer patients to improve engrafment after allogeneic HSC transplantation4, 5.
MSC can differentiate into cells of mesodermal origin in vivo (osteoblasts, adipocytes and condrocytes) thus representing a promising tool for tissue repair6, 7, and into cells of other lineages in vitro (muscle cells, hepatocytes, endothelial cells, neurons), through a process called “transdifferentiation”8.
MSC mediate immunoregulatory activities by inhibiting the funtions of different cell types9, 10. As far as the effects on T lymphocytes is concerned, MSC i) inhibit proliferation in response to mitogens11, 12, anti-CD3 and anti CD28 specific antibodies13, or alloantigens14, 15, ii) induce anergy in naFve T cells11, 15, 16, iii) induce expansion of regulatory T cells 14, 17, and iv) inhibit CTL mediated cytotoxicity against allogeneic cells18, 19. As far as the effects on NK cells is concerned, MSC i) inhibit cytotoxicity against virus-infected cells 20, ii) inhibit IL-2 driven NK cell IFN-γ secretion and proliferation12, 21, 22 and iii) exert “veto” function for allogeneic cells18. In dendritic cells (DC), MSC i) downregulate expression of co-stimulatory molecules 17, 23, 24, ii) inhibit in vitro differentiation of DC from monocytes and CD34+ progenitors25, 26, iii) reduce pro-inflammatory cytokine secretion (IL-12, IFN-γ, TNF-α) and increase IL-10 secretion 14, 23, 25. Furthermore, human MSC are poorly immunogenic, in spite of constitutive HLA-class I expression and IFN-γ inducible HLA-class II expression27.
The immunoregulatory functions of human MSC coupled with their low immunogenicity provide a rationale for the use of allogeneic MSC to treat severe GVH disease28 and, possibly, autoimmune disorders29, 30. Encouraging results have been obtained in patients with GVH31, whereas in two murine HSC transplantation models32, 33 MSC did not prevent GVH disease34 or were immunopriviliged.
It has been reported that, in a narrow window of IFN-γ concentration, human MSC can exert APC functions for HLA-class II restricted recall antigens, such as Candida albicans and Tetanus toxoid. MSC up-regulated their HLA-class II antigen expression by autocrine secretion of low IFN-γ levels; however, when IFN-γ concentration in culture increased, HLA-class II antigen expression was down-regulated and the APC function was inhibited 35. Furthermore, IFN-γ induced up-regulation of class II major histocompatibility molecules on both murine and human MSC was found to be modulated by TGF-β, serum factors and cell density in vitro36.
Recently, it has been reported that MSC do not trigger effector functions in activated CTL, inducing an abortive activation program in the latter cells 37.
Here, we have investigated for the first time i) the ability of MSC to process and present viral or tumor associated antigens to HLA-class I restricted CD8+ T cells; ii) the expression of HLA-class I related APM components in human MSC and iii) the expression of immunoregulatory molecules, such as HLA-G, HLA-E and the Granzyme B inhibitor Protease inhibitor 9 (PI-9) in human MSC.
MATERIALS AND METHODS
Monoclonal and polyclonal antibodies
MSC were characterized using the following mAbs: anti-CD105, anti-CD73, anti-CD14 (BD Biosciences, San Jose, CA, USA), anti-CD34, anti-CD45 and anti-CD44 (Caltag Laboratories, Burlingame, CA, USA).
The mAb HC-10, which recognizes a determinant of β2m-free HLA-B heavy chain (HC) and HLA-A10, -A28, -A29, -A30, -A31, -A32 and -A33 HC 38, 39; the anti-β2m mAb L368 40; the mAb TP25.99, which recognizes a conformational determinant of β2m-associated HLA-A,B and C HC and a linear determinant expressed of β2m-free HLA-B (except HLA-B73), HLA-A1, -A3, -A9, -A11 and -A30 HC 41 were developed and characterized as described 42.
The anti-MB1 mAb SJJ-3, the anti-delta mAb SY-4, the anti-zeta mAb NB1, the anti LMP-2 mAb SY-1, the anti-LMP-7 mAb SY-3, the anti-LMP10 mAb TO-7, the anti-TAP-2 mAb SY-2, the anti-calnexin mAb TO-5, the anti ERp-57 mAb TO-2, the anti-calreticulin mAb TO-11 and the anti-tapasin mAb TO-3 were developed and characterized as described 43.
MEM-G/9 and 87G 44 mAbs (anti-HLA-G) were purchased from Exbio (Vestec, CZ). 7G3 and 3D12 45 mAbs, which recognize HLA-E HC, were kindly provided by Dr.Daniel E.Geraghty. 7D8 mAb (anti-PI-9) was purchased from Serotec (Oxford, UK).
All mAbs are of the IgG1 isotype, except HC-10 mAb, which is an IgG2a and 7G3 mAb, which is a IgG2b. Irrilevant isotype-matched mouse immunoglobulins (Southern Biotechnology Associates, Birmingham, AL, USA) were used as controls. FITC-conjugated F(ab’)2 fragments of rabbit anti-mouse IgG antibodies (Dako, Glostrup, Denmark) or PE-conjugated F(ab’)2 fragments of goat anti-mouse IgG1 antibody (Southern) were used as secondary reagents.
Cell separation and culture
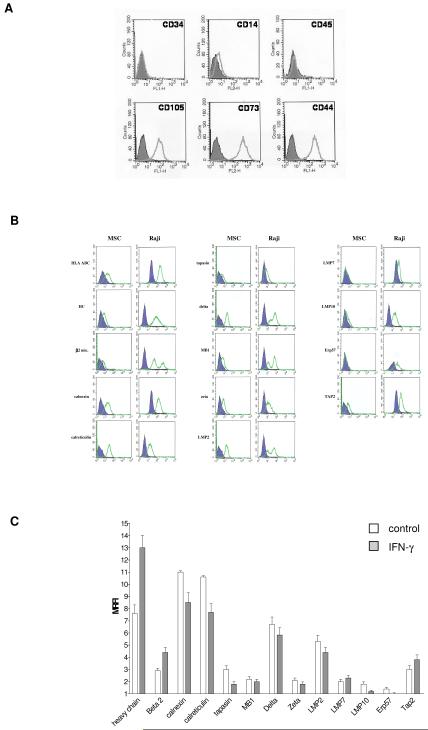
Human MSC were expanded in vitro from healthy donors’ BM obtained after informed consent. Mononuclear cells were isolated by Ficoll-Hystopaque (Sigma, St. Louis, MO, USA; 1077 g/mL density) gradient centrifugation at 2500 rpm for 30 minutes (Sigma, St. Louis, MO), washed twice with phosphate-buffered saline (PBS; Sigma), counted and plated at 20-30×106 cells/75-cm2 flask in Mesen-cult basal medium supplemented with mesenchymal Stem Cell Stimulatory Supplement (StemCell Technologies, Vancouver, BC, Canada). After 1 week culture at 37°C and 5% CO2, non adherent cells were removed, and medium was replaced every other day. MSC were trypsinized (Trypsin-EDTA solution, Cambrex Bio Science, Verviers, Belgium) when cultures reached 80-100% confluence. The purity of MSC suspensions was assessed by flow cytometry based on the expression of CD105, CD73 and CD44, and the absence of CD34, CD45 and CD14 (Figure 4, panel A). MSC were cultured in vitro for 1-2 passages.
Figure 4. Immunophenotypic characterization of MSC and expression of APM components.
Panel A Flow cytometric analysis of MSC immunophenotype.
Panel B Intracellular expression of APM components was evaluated by flow cytometric analysis of MSC and Raji Burkitt’s lymphoma cell line, used as positive control. Empty profiles represent staining with specific antibodies, whereas filled profiles represent staining with isotype-matched controls. One representative experiment out of five performed is shown.
Panel C The intracellular expression of APM components in MSC was evaluated by flow cytometry, in basal conditions (white bars) and after 48 h treatment with IFN-γ (grey bars). Results are expressed as mean relative fluorescence intensity (MRFI). Means ± SD of five different experiments are shown.
MSC supernatants were collected after 24-48h of culture. Depletion of soluble HLA-G was performed using Dynabeads Pan Mouse IgG (Dynal Biotech, Oslo, Norway), coated with anti-HLA-G1/-G5 mAb MEM-G/9 (Exbio) for 1h at 4°C, following manufacturer’s protocol.
The TAP deficient HLA-A2+ lymphoma T2 cell line, the EBV-positive human B cell lymphoma Jy cell line (purchased from American Type Culture Collection, Rockville, MD, USA), the EBV-infected Burkitt Raji lymphoma cell line and the LCL cell line 721.221.G1 (kindly provided by Dr. Francesco Puppo, University of Genoa, Italy) were cultured in RPMI 1640 medium (Euroclone, Wetherby, UK) supplemented with 10% fetal bovine serum (GIBCO, Carlsbad, CA, USA), HEPES buffer, non essential aminoacids and antibiotics (Cambrex).
Flow cytometry
The intracellular staining43and the surface staining46 of MSC was performed as previously described. Cells were subsequently subjected to flow cytometry using FACScalibur (BD Biosciences).
Cell Quest software (BD Biosciences) was used for data analysis. Results are expressed as percentage of positive cells or as mean relative fluorescence intensity (MRFI) obtained as a ratio between mean fluorescence intensity (MFI) of cells stained with specific mAb and MFI obtained with isotype control.
MSC transfection and infection
mRNA was extracted from four human neuroblastoma (NB) cell lines (GI-ME-N, SKNBE, SHSY5Y and IMR-32) or from normal donor peripheral blood mononuclear cells (PBMNC) using mRNA Isolation Kit (Roche Diagnostics Gmbh, Mannheim, Germany) according to manufacturer’s protocol, pooled in equal ratio at 200 μg/ml and stored at -80° C until use. MSC transfection was performed using Transmessenger Transfection Reagent (Qiagen, Chatworth, CA, USA) following manufacturer’s protocol. After transfection, MSC were cultured for additional 24 h in fresh medium before being used, as reported47.
Jy cell line and MSC were infected with NS3Ag-expressing vaccinia virus (VV) (5 plaque-forming units [PFU]/cell) for 1 h at 37°C, then washed twice and cultured overnight in fresh medium before being used, as reported48, 49.
Peptides
The peptides GILGFVFTL50 (Flu Matrix 58-66 peptide from Influenza A virus) and CINGVCWTV51 (NS3 1073-1081 peptide from Hepatitis-C virus HCV) were manually synthesized using the standard method of solid phase peptide synthesis which follows the 9-fluorenylmethoxycarbonyl (Fmoc) strategy with minor modifications52.
The HLA-A2 binding of the selected peptides was tested by in vitro cellular binding assay using T2 cell line, as described 53. Cells were pulsed with peptides (10 μM) for 2 h at 37°C, washed twice and then used as APC for in vitro assays.
CTL generation
NB specific CTL were generated by weekly restimulation of freshly isolated CD8+ T cells from normal donors with autologous monocyte-derived DC transfected with NB mRNA, as previously described 47.
Flu-specific CTL were generated by weekly restimulation of freshly isolated CD8+ T cells with autologous DC pulsed with Flu peptide.
A CTL clone specific for NS3 peptide 1073-1081 was generated by weekly restimulation of freshly isolated CD8+ T cells with autologous DC pulsed with NS31073-1081 peptide, followed by limiting dilution cloning.
Soluble (s)HLA-G ELISA
sHLA-G ELISA was performed using MaxiSorp Nunc-Immuno 96 microwell plates (Nunc A/S, Roskilde, Denmark) coated overnight at 4°C with mAb MEM-G/9 (Exbio; 10 μg/ml) in 0.001 M PBS, pH 7.4. After three washes with PBS 0.05% Tween 20 (washing buffer), plates were saturated with 200 μl/w of PBS 2% BSA for 30 min at RT.
100 μl of samples (supernatants from MSC) or standards (serial dilutions of calibrated 721.221.G1 cell line supernatant) were added to each well and incubated at RT for 1h. Plates were washed three times with washing buffer, and then incubated with 100 μl/w of HRP-conjugated anti-β2m mAb NAMB-1 (1 μg/ml) at RT for 1h. After three washes, plates were incubated with the substrate (3′-3′-5′-5′ Tetramethylbenzidine, Sigma) for 30 min at RT. H2SO4 5 M (100 μl/w) was added, and optical densities were measured at 450 nm. The assay’s lowest threshold was 1,95 ng/ml of sHLA-G. Each sample was tested in duplicate.
ELISPOT assays
IFN-γ and Granzyme B ELISPOT assays were carried out using Multiscreen-IP Millipore plates (Millipore, Bedford, MA) coated overnight at 4°C with anti-IFN-γ (clone 1-DK-1, 1 μg/ml, Mabtech, Nacka, Sweden) or anti-Granzyme B (clone GB10, 15 μg/ml, Mabtech) mAbs, respectively. Plates were then washed and blocked with PBS 2% human albumin (Kedrion SpA, Lucca, Italia). 3×104 specific CTL were cultured together with 6×104 target cells (1:2 cell ratio) in 200 μl of RPMI 1640 5% human AB serum. The T2 cell line, pulsed with Flu peptide (10 μM), or MSC (unmanipulated, pulsed with Flu peptide or transfected with NB mRNA) were used as targets. All targets were γ-irradiated (45 Gy) before being plated. Blocking experiments were performed by adding 10 μg/ml of anti-HLA class I mAb TP25.99 to target cells 30 min before culture with lymphocytes. After 20h incubation at 37°C and 5% CO2, ELISPOT were developed according to manufacturer’s protocol. Spots were counted using Bioreader 2000 (Biosys, Karben, Germany).
NS3 antigen presentation
Cells of a CTL clone specific for the HLA-A2 restricted peptide NS31073-1081 (CINGVCWTV) were stimulated for 4h at 37°C and 5% CO2 with peptide-pulsed APC (Jy or MSC), or NS3Ag-VV infected APC in U-bottom microculture wells at 6×104 APCs/3×104 T cells / well in 0.2 mL of RPMI 1640 10% FBS.
Brefeldin-A (10 μg/mL, Sigma-Aldrich) was added after 2h of culture. Cells were washed and stained with anti-CD8 FITC (Caltag Laboratories, Burlingame, CA) for 15 min at 4°C, fixed, permeabilized using Cytofix/Cytoperm solution (BD PharMingen) at 4°C for 20 min, rewashed with Perm Wash Buffer (BD PharMingen), intracellularly stained with PE-labeled anti-IFN-γ antibody (BD PharMingen) for 15 min at 4°C and finally subjected to flow cytometry.
Cytotoxicity assays
CTL-mediated cytotoxicity was evaluated by standard 4 h 51Cr release assay. Effector to target (E:T) cell ratio ranged from 100:1 to 1:1. A 10 fold excess of unlabeled K562 cells was added to minimize NK-like activity. Blocking experiments were performed by adding anti-HLA class I TP25.99, anti-HLA-G 87G or anti-HLA-E 7G3 and 3D12 mAbs (10 μg/ml) to target cells, 30 min before culture with lymphocytes.
Cold target inhibition was performed only for Flu-specific CTL by adding 10 fold excess of unlabelled Flu-pulsed T2 cell line.
Specific lysis was determined by the formula: % specific lysis = cpm (sample-spontaneous) / cpm (total-spontaneous) x 100.
Assay for PI-9 expression
MSC (unmanipulated, transfected with NB mRNA or pulsed with Flu peptide) were tryspinized, washed, fixed and permeabilized using Cytofix/Cytoperm solution (BD PharMingen) at 4°C for 20 min, rewashed with Perm Wash Buffer (BD PharMingen), and intracellularly stained with anti-PI-9 mAb (1 μg/106 cells) in permeabilization buffer. Cells were then washed twice in permeabilization buffer and intracellularly stained with anti-mouse IgG1 PE mAb (Serotec), then subjected to flow cytometry. Human tonsil mononuclear cells were used as positive control. Results were expressed as MRFI.
Statistical analysis
Data were analyzed by PRISM 3.0 (Graphpad software), using two-tailed Wilkoxon ranked test and Kolmogorov-Smirnov test. p values < 0.01 or < 0.05 were considered as significant.
RESULTS
MSC act as APC for HLA-class I restricted viral peptides
To investigate whether HLA-class I molecules on the surface of MSC can present exogenously loaded HLA-class I restricted peptides derived from viral antigens to specific CTL, HLA-A2+ MSC from four different donors were pulsed with Flu peptide or with an irrelevant peptide, and used as APC for Flu-specific HLA-A2+ CTL. T2 cells pulsed with the same peptides were tested as positive control.
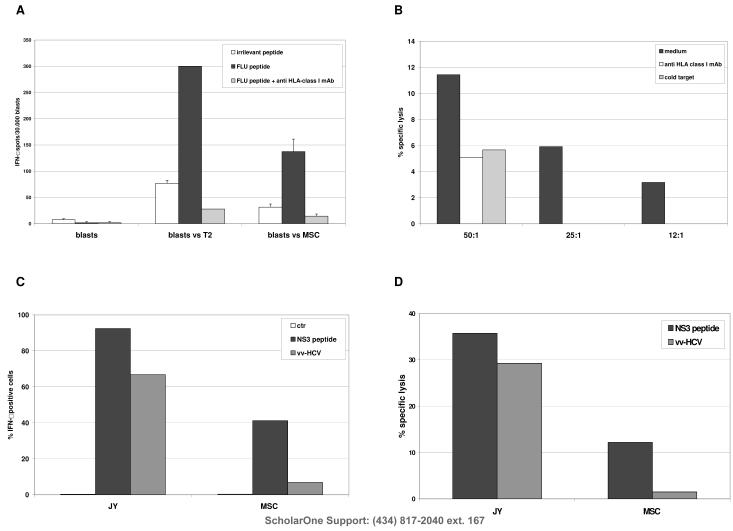
CTL efficiently recognized MSC pulsed with Flu peptide (137,3 spots/30.000 blasts) in IFN-γ ELISPOT assay (Figure 1, Panel A). The recognition was specific, since the number of IFN-γ producing CTL detected was significantly lower against MSC pulsed with an irrelevant peptide (31,5 spots/30.000 blasts, p=0.0135). Furthermore the recognition is HLA class I -restricted, since addition of the HLA class I -specific mAb TP25.99 significantly reduced the number of IFN-γ producing CTL (14.33 spots/30,000 blasts, p=0.0095).
Figure 1. Processing and presentation of viral antigens by MSC to CTL.
Panel A HLA-A2+ Flu specific CTL were tested in IFN-γ ELISPOT assay against HLA-matched MSC or T2 cell line pulsed with Flu peptide (black bar) or with an irrelevant peptide (white bar). Grey bar indicate the inhibition of IFN-γ secretion obtained by adding anti-HLA class I mAb TP25.99 to target cells before co-culture with CTL. Results are means ± SD from five different experiments.
Panel B HLA-A2+ Flu specific CTL were tested in cytotoxicity assays against HLA-matched Flu-pulsed MSC (black bar) at three different E/T ratios. Blocking experiments were performed by the addition of anti HLA-class I mAb (white bar) or by cold target inhibition with unlabelled Flu-pulsed T2 cell line (grey bar). One representative experiment out of the three performed is shown.
Panel C IFN-γ secretion by NS3 specific HLA-A2+ CTL clone was investigated by flow cytometry after culture with HLA-matched Jy cell line or MSC, both pulsed with NS3 peptide (black bars) or infected with NS3-Ag VV (grey bars). White bars indicate the percentage of IFN-γ+ cells obtained incubating the CTL clone with unmanipulated Jy or MSC cells. One representative experiment out of the four performed is shown.
Panel D HLA-A2+ NS3 specific CTL clone was tested in cytotoxicity assays against HLA-matched MSC or Jy cell line, pulsed with NS3 peptide (black bars) or infected with NS3-Ag VV (grey bars). One representative experiment out of the four performed is shown.
In cytotoxicity assays, Flu-pulsed MSC were lysed by specific CTL (specific lysis 11.43 % at 50:1 E/T ratio) in HLA-class I restricted manner, as demonstrated by blocking experiments (specific lysis 5% with anti HLA-class I mAb, p=0.0002, and 5,67% with cold target inhibition, p=0.0003) (Figure 1, Panel B). CTL-mediated lysis of peptide-pulsed MSC was lower than that obtained with T2 cell line (specific lysis 98,4 % at 50:1 E/T ratio).
Next, to compare the efficiency of MSC to present an endogenously processed antigen vs an exogenously loaded peptide derived from the same antigen to a specific CTL clone, MSC and Jy cells (tested as positive control) were pulsed with NS31073-1080 peptide or infected with NS3Ag-VV, and then used as APC for NS3 specific CTL clone.
The CTL clone specifically secreted IFN-γ in response to infected or peptide pulsed Jy cells (86.7% and 92.4% IFN-γ + cells, respectively). Peptide-pulsed MSC were recognized more efficiently than infected MSC (41.2 % IFN-γ + cells vs 6.8 % IFN-γ + cells, respectively, p = 0.0474) (Figure 1, Panel C). Peptide-pulsed MSC were lysed by the CTL clone, whereas NS3-VV infected MSC were not (specific lysis 14.2 % and 0.75 %, respectively, at the E/T ratio of 10:1; p=0.0008). The Jy cell line, either peptide pulsed or infected, was recognized and lysed at 10:1 E/T ratio (specific lysis 35.7% and 29.29%, respectively) (Figure 1, Panel D).
Tumor-associated antigen processing and presentation by MSC
To test the ability of MSC to process and present tumor associated antigens in a HLA-class I restricted manner, HLA-A2+ MSC were transfected with NB mRNA. Unmanipulated or transfected MSC were used as APC for HLA-matched NB-specific CTL.
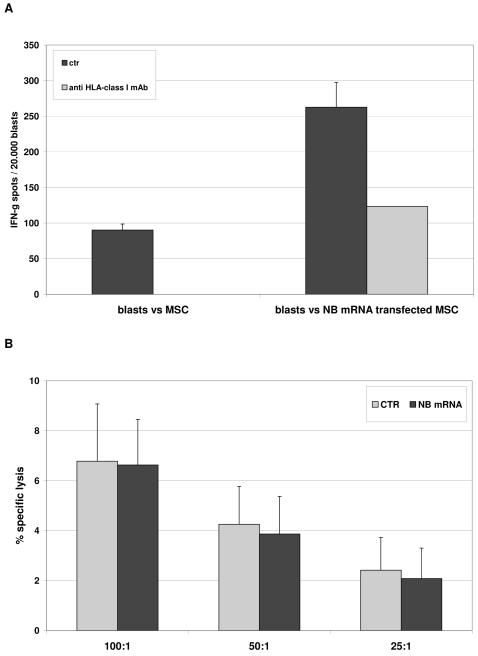
CTL efficiently recognized transfected (262.7 spots/30.000 blasts), but not unmanipulated MSC (90.3 spots/30.000 blasts p<0.0001) in IFN-γ ELISPOT assay. Such recognition occurred in an HLA-class I restricted manner, as demonstrated by inhibition with anti-HLA class I mAb (123.3 spots/30.000 blasts, p=0.0001) (Figure 2, Panel A).
Figure 2. Processing and presentation of tumor-associated antigens by MSC to CTL.
Panel A HLA-A2+ NB specific CTL were tested in IFN-γ ELISPOT assay against HLA-matched MSC, unmanipulated or transfected with NB mRNA (black bars). Grey bars indicate the inhibition of IFN-γ secretion obtained by adding anti-HLA class I mAb to target cells before being cultured with lymphocytes. Results are means ± SD from five different experiments.
Panel B HLA-A2+ NB specific CTL were tested in cytotoxicity assay against HLA-matched MSC, either unmanipulated (grey bars) or transfected with NB mRNA (black bars). Results are means ± SD from five different experiments.
When tested in cytotoxicity, CTLs did not lyse either transfected or unmanipulated MSC at any E/T ratio (Figure 2, panel B).
Immunogenicity of MSC is not affected by IFN-γ
MSC from three different HLA-A2+ donors, either unmanipulated or transfected with NB mRNA, were cultured for 72h with medium alone or 1000 U/ml of rhIFN-γ, that up-regulated surface expression of HLA class I on MSC (not shown and ref. 23). These cells were used as APC for HLA-matched NB-specific CTL in IFN-γ ELISPOT assay.
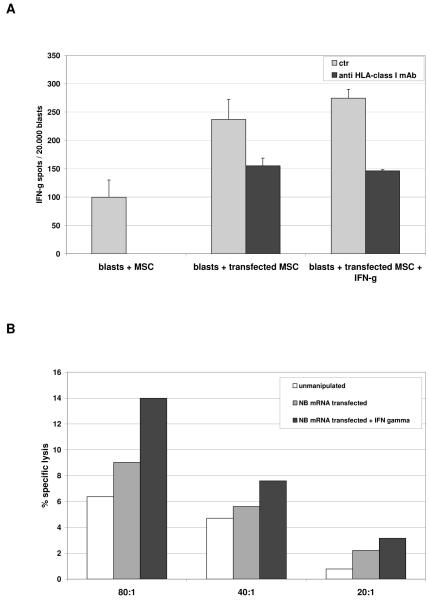
CTL efficiently recognized transfected, but not unmanipulated MSC (237 spots/30.000 blasts and 99 spots/30.000 blasts, respectively, p=0.0008) in HLA-restricted manner, as demonstrated by inhibition with anti-HLA class I mAb (155 spots/30.000 blasts, p=0.01). IFN-γ treatment did not significantly increase specific recognition of transfected MSC (274,5 spots/30.000 blasts, p=0.4957) (Figure 3, Panel A).
Figure 3. Effects of IFN-γ on APC function of MSC.
Panel A HLA-A2+ NB specific CTL were tested in IFN-γ ELISPOT assays against HLA-matched MSC, either unmanipulated or transfected with NB mRNA, following treatment with IFN-γ or medium alone (grey bars). Black bars indicate the inhibition of IFN-γ secretion obtained by adding anti-HLA class I mAb to target cells before being cultured with lymphocytes. Results are means ± SD from five different experiments.
Panel B HLA-A2+ NB specific CTL were tested in cytotoxicity assays at three different E/T ratios against HLA-matched MSC, unmanipulated (white bars), transfected with NB mRNA (grey bars) or transfected with NB mRNA and pre-treated with IFN-γ (black bars). One representative experiment out of the four performed is shown.
In cytotoxicity assay, no lysis of unmanipulated or transfected MSC was observed up to 80:1 E/T ratio, either after IFN-γ treatment of MSC (Figure 3, Panel B).
Taken together, the above results demonstrated that MSC are able to present exogenously loaded peptides to specific CTL, to process endogenous antigens and present them, although with lower efficiency, in HLA-class I restricted manner. Antigen-loaded MSC evoked IFN-γ secretion in specific CTL but were resistant to CTL-mediated lysis in all experimental conditions.
Expression of HLA class I related APM components in MSC
To analyze the completeness of APM in MSC, intracellular expression of APM components was investigated by flow cytometry in MSC isolated from the BM of normal individuals and expanded in culture, following 48 h incubation with 1000 U/ml IFN-γ or medium alone. Raji cell line was tested as positive control. As shown in Figure 4 (panel B), the chaperon ERP-57 and the immunoproteasomal components LMP7 and LMP10 were virtually undetectable in MSC cultured with medium alone. β2 microglobulin (βm), β2m free HC and the proteasomal components MB-1 and zeta were downregulated as compared to control. The chaperons calnexin, calreticulin and tapasin, the proteasomal component delta, the immunoproteasomal component LMP2 and the transporter Tap-2 were consistently expressed in untreated MSCs. IFN-γ treatment strongly enhanced expression of β2m free HC, β2-microglobulin and, at lesser extent, Tap2, whereas had no effect on the expression of the remaining APM components (Figure 4, panel C).
Expression of the immunosuppressive molecules HLA-G and HLA-E in MSC
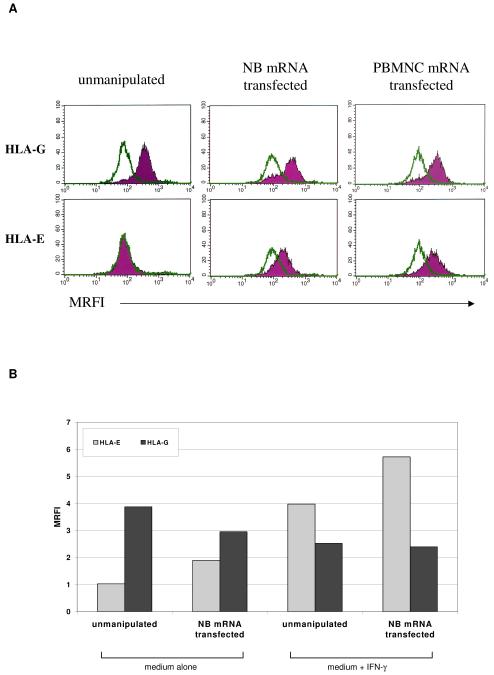
To further analyze the mechanism(s) involved in the inhibition of CTL-mediated killing of antigen-loaded MSC, the expression of two immunosuppressive HLA-class Ib molecules, HLA-G and HLA-E, was investigated by flow cytometry on MSC expanded in culture from 5 different donors, in the following experimental conditions: unmanipulated (control), transfected with NB mRNA, transfected with normal PBMNC mRNA or infected with NS3-Ag VV.
As shown in Figure 5 (panel A), HLA-G was expressed on the surface of unmanipulated MSC (MRFI 3.13 ± 1.05) and its expression was not increased after transfection or infection (MRFI 2.69 ± 0.37). In contrast, HLA-E was virtually undetectable in unmanipulated MSC or infected MSC (MRFI 1.19 ± 0.18 and 0.66 ± 0.01). Its expression was significantly induced after transfection, either with NB or PBMNC mRNA (MRFI 3.15 ± 1.68, p = 0.022). This latter control excluded that HLA-E was encoded by tumor mRNA.
Figure 5. Expression of HLA-G and HLA-E in MSC.
Panel A HLA-G and HLA-E surface expression was evaluated by flow cytometry on MSC from 5 different donors in basal conditions, after NB mRNA transfection or after NS3Ag-VV infection. One representative experiment out of five performed is shown.
Panel B HLA-G (black bars) and HLA-E (grey bars) expression was evaluated by flow cytometry on MSC in the following experimental conditions: unmanipulated or transfected, both cultured with medium alone or with IFN-γ. One representative experiment out of five performed is shown.
Next, HLA-G and HLA-E expression was evaluated by flow cytometry on MSC in the same experimental conditions described above, following culture with IFN-γ. IFN-γ treatment strongly up-regulated HLA-E but not HLA-G expression (Figure 5, Panel B).
The presence of HLA-E only in transfected MSC argued against its role in CTL inhibition. Blocking the interaction between HLA-G and its receptors using anti-HLA-G mAb 87G did not restore CTL-mediated cytotoxicity, ruling out a role of surface HLA-G in CTL inhibition (data not shown).
sHLA-G secreted by MSC is involved in the resistance of MSC to CTL-mediated cytotoxicity
Additional experiments tested whether sHLA-G released by MSC played a role in their resistance to CTL-mediated cytotoxicity.
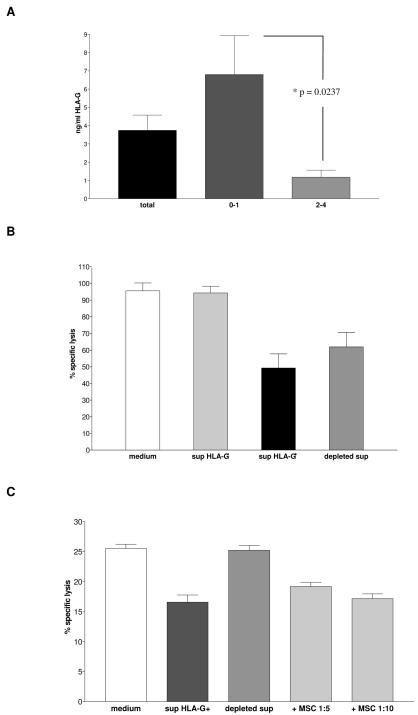
The concentration of sHLA-G in MSC supernatants, tested by ELISA, ranged from 0.17 to 24.3 ng/ml, with significantly higher levels in earlier than in later passages of culture (passages 0-2, 6.81 ± 2.12 ng/ml, passages 3-4, 1.18 ± 0.38 ng/ml, p=0.0237) (Figure 7, panel A).
Figure 7. sHLA-G secretion by MSC and its role in CTL-mediated lysis inhibition.
Panel A sHLA-G levels in supernatants from MSC cultures were evaluated by ELISA. sHLA-G concentration was measured in all MSC supernatants tested (black bar), in supernatants from MSC cultures at passages 0-2 (grey bar) and from MSC cultures at passages 3-4 (light grey bar). Results are means ± SD from fifty experiments.
Panel B HLA-A2+ Flu-specific CTL were cultured with fresh medium (white bar), with MSC supernatants lacking sHLA-G (light grey bar), with MSC supernatants containing sHLA-G (black bar) or with MSC supernatants containing sHLA-G after antibody-mediated depletion of the latter molecule (grey bar). Effector cells were then tested in cytotoxicity assay against T2 cell line pulsed with Flu peptide. Means ± SD from four different experiments are shown.
Panel C HLA-A2+ NB-specific CTL were cultured with fresh medium (white bar), with MSC supernatants containing sHLA-G (black bar), with MSC supernatants containing sHLA-G after depletion of the latter molecule (grey bar) or in the presence of MSC at 1:5 or 1:10 ratio with effector cells (light grey bars). Effector cells were then tested in cytotoxicity assay against autologous DC transfected with NB mRNA. Means ± SD from four different experiments are shown.
To analyze the role of soluble factors secreted by MSC in CTL-mediated cytotoxicity inhibition, we selected twenty supernatants, half of them containing sHLA-G (range 3.28 - 24 ng/ml) and the others lacking sHLA-G.
Flu-specific or NB-specific HLA-A2+ CTL were incubated with MSC supernatants or fresh medium for 2h at 37°C, and then cultured with target cells (Flu-pulsed T2 cell line or autologous DC transfected with NB mRNA, respectively) in cytotoxicity assay.
As shown in Figure 7 (panel B) Flu-specific CTL treated with fresh medium caused 51Cr release by 96.2%, that was significantly reduced when CTL were incubated with MSC supernatants containing sHLA-G (specific lysis 49.66 %, p<0.0001), but not with supernatants lacking sHLA-G (specific lysis 94.9%). Depletion of sHLA-G from MSC supernatants by immunomagnetic beads coated with anti HLA-G1/-G5 mAb partially restored CTL-mediated lysis (specific lysis 62.3 %, p=0.019).
Same results were obtained with NB-specific CTL (Figure 7, panel C). Specific lysis of target cells was 25.5% when CTL were treated with fresh medium, and it decreased at 16.6% when CTL were treated with MSC supernatants containing sHLA-G (p=0.0079). sHLA-G depleted supernatants did not inhibit lysis of target cells (specific lysis 25.24%, p=0.0143).
In this experiment, lysis of target cells was also significantly reduced when MSC were added in the assay, at 1:5 or 1:10 effector/MSC ratio (specific lysis 19.2% and 17.7% respectively, p=0.004). These data proved unambiguously that soluble factors secreted by MSC inhibited CTL-mediated lysis, and that sHLA-G played a role in this inhibition.
Expression of Granzyme B inhibitor PI-9 in MSC
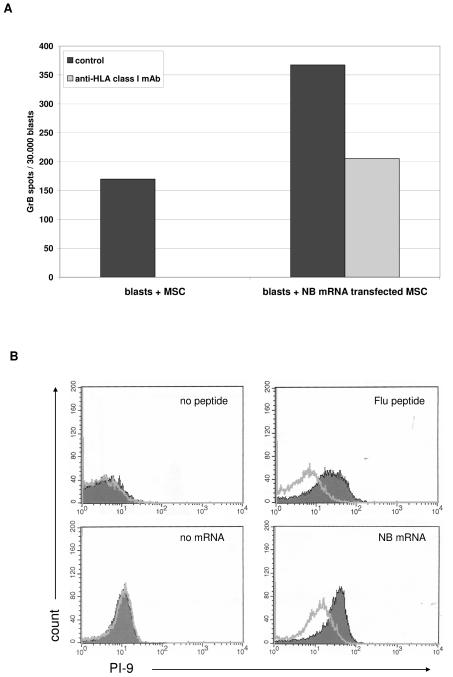
When tested in ELISPOT assay, antigen-loaded MSC induced Granzyme B secretion in antigen specific CTL. Figure 6, Panel A, shows a representative experiment performed with NB-mRNA transfected MSC cultured with HLA-matched NB-specific CTL.
Figure 6. Granzyme B secretion in CTL upon incubation with MSC and PI-9 expression in antigen loaded MSC.
Panel A HLA-A2+ NB specific CTL were tested in Granzyme B (GrB) ELISPOT assay against HLA-matched MSC, either unmanipulated or transfected with NB mRNA (black bars). Grey bar indicates the inhibition of GrB secretion obtained by adding anti-HLA class I mAb to target cells before being cultured with lymphocytes. One representative experiment out of five performed is shown.
Panel B. The expression of the Granzyme B inhibitor PI-9 was evaluated by flow cytometry in MSC transfected with NB mRNA or pulsed with Flu peptide, using unmanipulated MSC cultured in the same experimental conditions as negative control. One representative experiment out of three performed is shown.
PI-9 is the only known physiological antagonist of Granzyme B54. We tested by flow cytometry the intracellular expression of PI-9 in MSC transfected with NB mRNA or pulsed with Flu peptide, using unmanipulated MSC (cultured in the same experimental conditions) as control.
As shown in Figure 6, Panel B, PI-9 was not expressed in the cytoplasm of unmanipulated MSC (MRFI 1.14 and 1, respectively), but was significantly induced in NB mRNA transfected or Flu-pulsed MSC (MRFI 2,78 and 3.76 respectively, p < 0.01), suggesting a potential role of PI-9 in MSC protection from CTL mediated lysis.
DISCUSSION
MSC are immunosuppressive and poorly immunogenic and these features make them attractive candidates as therapeutic agents for diseases characterized by abnormal activation of the immune system, such as graft versus host disease (GVHD) and autoimmune disorders14-16, 55-58. LeBlanc and coworkers paved the way to the clinical use of MSC in allogeneic HSC transplanted patients undergoing severe GVHD31, 59. The successful control of EAE, a model of multiple sclerosis, and collagen arthritis, a model of rheumatoid arthritis, achieved following MSC infusion holds promise for the treatment of different human autoimmune disorders29, 60.
In this setting, the issue of whether or not MSC can act as non-professional APC is of crucial importance. Recently, it has been demonstrated that MSC can act as APC for HLA-class II restricted antigens depending on the IFN-γ concentration in their microenvironment35, 36.
The direct interactions between MSC and CTL have been partially characterized. Rasmusson et al. recently demonstrated that MSC pulsed with an HLA-class I restricted viral peptide did not trigger effector functions in specific CTL, downregulating TCR and CD25 expression, and damping protein phosphorylation37.
However, the mechanism(s) utilized by MSC to inhibit CTL function have not been clarified. Moreover, the expression of HLA-class I related APM components in human MSC, their ability to process HLA-class I restricted antigens and to present them to specific CTL have not yet been analysed.
Here we addressed the APC function of MSC, using two different experimental systems, i.e. i) pulsing of MSC with HLA-A2 restricted peptides from Influenza A virus (Flu) or HCV (NS3) and ii) transfection of MSC with the NS3 gene, to dissect exogenous peptide presentation from endogenous antigen processing.
MSC pulsed with viral peptides induced ifn-γ release by specific CTL in an HLA-A2 restricted and antigen specific manner. When different aliquots of the same MSC suspensions were either transfected with the HCV NS3 gene or pulsed with the NS3 peptide and subsequently incubated with the same specific CTL suspension, the latter displayed a significantly reduced IFN-γ production following challenge with transfected vs peptide pulsed MSC. These experiments demonstrated that MSC are partly defective in their ability to process a viral antigen, whereas they are competent at presenting an exogenously loaded peptide from the same antigen to CTL. This latter observation is in contrast with the report by Rasmusson and coworkers37, who showed that MSC loaded with an EBV peptide did not induce IFN-γ release in specific CTL. The reasons for this discrepancy are not easily apparent and may be related to the different experimental conditions. Notably, in the present study, two different viral peptides were tested using two different assays with similar results.
Cytotoxicity experiments against NS3 peptide or Flu peptide pulsed MSC showed that lysis of the latter cells by specific CTL was detectable at E/T ratios ranging from ten:1 to 50:1. This finding is in agreement with the results of Rasmusson et al., who showed in their study that antigen specific CTL did not lyse EBV peptide pulsed MSC when tested at a 3:1 ratio. While NS3 peptide pulsed MSC were killed by specific CTL, NS3 gene transfected MSC were not.
MSC injected systemically in tumor bearing mice are attracted to tumor site where they can inhibit or stimulate malignant cell growth, depending on the model and the experimental conditions tested61-64. The ability of human MSC to present tumor-associated antigens to specific CTL has not been investigated so far. To address this issue we transfected MSC with pooled mRNA from four NB cell lines. This protocol has been used successfully with professional APC, i.e. myeloid dendritic cells and B cells, to generate NB specific CTL47, 65. NB mRNA transfected MSC stimulated IFN-γ and Granzyme B production by specific CTL, but were completely protected from lysis, similarly to that observed with NS3 gene transfected MSC, even at very high E/T ratios.
In this study, CTL mediated lysis of MSC was low to absent in all experimental system tested, and it was not enhanced by IFN-γ treatment, at variance with that reported for HLA-class II restricted-antigen presentation by MSC35. The protection of MSC from CTL-mediated lysis can be due to i) defects in the HLA-class I related APM, that transforms native proteins into small peptides, which are loaded onto nascent HLA class I molecules and then exported to the cell surface and presented to specific CTL66, or ii) the expression of immunosuppressive molecules by MSC.
Here we demonstrate for the first time that human MSC display several defects in the expression of some APM components. MSC lacked expression of the chaperon Erp-57, that assists in intracellular trafficking of newly generated peptides and in their loading onto HLA class I molecules, and showed down-regulated MB1 and zeta, that form the cylinder backbone of the 20S proteasome where protein degradation takes place67. Furthermore, the immunoproteasomal components LMP7 and LMP10, that are induced in professional APC by IFN-γ treatment68, were not expressed. However, MSC incubation with IFN-γ did not up-regulate MB1 or zeta nor induced Erp57, LMP7 or LMP10 expression. Taken together, these findings suggested that protein antigen processing and peptide generation may be impaired in MSC irrespective of IFN-γ stimulation.
The non polymorphic HLA class Ib molecule HLA-G exerts many immunosuppressive activities, including inhibition of cell mediated cytotoxicity69. Surface HLA-G was expressed constitutively by MSC and not up-regulated by IFN-γ stimulation. Blocking of surface HLA-G did not reinstate lysis of NB mRNA transfected MSC by specific CTL. In contrast, MSC in early culture passages were found to release sHLA-G in their supernatants, that inhibited significantly the lysis of target cells by specific CTL. sHLA-G depletion from the latter supernatants reduced significantly such inhibition, thus demonstrating that HLA-G is one of the factors involved in protection of MSC from CTL mediated killing. Other investigators have shown that MSC release constitutively sHLA-G in culture supernatants and that the latter molecule inhibits T cell proliferation in response to alloantigens70.
Another potential mechanism involved in MSC resistance to CTL mediated lysis is related to de novo induction of the expression of the Granzyme B inhibitor PI-9 in MSC pulsed with peptide or transfected with NB mRNA. PI-9 is an intracellular serpin expressed predominantly in lymphocytes and monocyte-derived cells, that protects them from lysis54.
MSC express HLA-class I, but not co-stimulatory molecules, such as CD80 and CD86, that are essential for antigen stimulation of naïve T cells15. Accordingly, it has been previously reported that, in the absence of co-stimulation, MSC induce anergy in naïve T cells15, 16, 23.
However, MSC could act as APC for antigen-specific memory CD8+ T cells, as the expression of co-stimulatory molecules by APC is not a crucial requirement for these cells71, 72.
Human BM contains a microenvironment that allows interactions between APC and circulating naïve antigen specific T cells, leading to the induction of primary memory CD8+ T cell responses55. Zhang et al. have demonstrated the presence of “effector memory” CTL specific for viral antigens with potent recall function in the BM, that could be re-stimulated and clonally expanded 73. Moreover, the APC function of infected non-hematopoietic cells and their role in amplifying clonal expansion of effector CD8+ T cells have been emphasized by Thomas et al.74 We speculate that MSC or their progeny in the BM act as APC for CD8+ “effector memory” T cells in response to antigenic stimulation, for example in the course of viral infections. In vivo interactions between MSC and CTL may result into cytokine production leading to recruitment and/or activation of other cell types to the BM. Since MSC appear to be rather resistant to CTL mediated lysis, they can perform different cycles of antigen presentation to CTL. In this respect, mice immunized with ovalbumin-pulsed, IFN-γ treated MSC have been previously shown to develop antigen specific CTL and acquire protection against ovalbumin-expressing tumors75.
CONCLUSION
Human MSC can process and present HLA class I restricted viral or tumor antigens to specific CTL. The limited efficiency of antigen processing and presentation is likely due to defects in the expression of some APM components, whereas MSC resistance to CTL-mediated lysis appears to be partly sHLA-G secretion.
ACKNOWLEDGEMENTS
We thank Mrs. Chiara Bernardini for excellent secretarial assistance.
This study has been supported by grants from Fondazione Carige, Genova, and Ministero della Salute, Progetto di Ricerca Finalizzata 2006 “Cellule staminali e terapie cellulari rigenerative” to V.P., and PHS grants RO1CA67108, RO1CA110249 and PO1CA109688 awared by the National Cancer Institute, DHHS to S.F. F.M. was the recipient of a fellowship from Fondazione Italiana Ricerca sul Cancro. L.R. was the recipient of a fellowship from Fondazione Italiana per la Lotta al Neuroblastoma.
REFERENCES
- 1.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006 Jun 1;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 2.Dominici M, Le Blanc K, Mueller I, et al. The International Society for Cellular Therapy position statement Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006 Feb;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 4.Ball LM, Bernardo ME, Roelofs H, et al. Co-transplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem cell transplantation. Blood. 2007 Jul 16; doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 5.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000 Jan;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997 Apr 4;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006 Nov;13(6):419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007 Jul 30; doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 10.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006 Oct;36(10):2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 11.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002 May 15;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 12.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006 Feb;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 13.Bocelli-Tyndall C, Bracci L, Spagnoli G, et al. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology (Oxford) 2007 Mar;46(3):403–408. doi: 10.1093/rheumatology/kel267. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005 Feb 15;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 15.Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 16.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003 May 1;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 17.Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005 Apr;90(4):516–525. [PubMed] [Google Scholar]
- 18.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003 Oct 1;171(7):3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 19.Chang CJ, Yen ML, Chen YC, et al. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006 Nov;24(11):2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 20.Kang HS, Habib M, Chan J, et al. A paradoxical role for IFN-gamma in the immune properties of mesenchymal stem cells during viral challenge. Exp Hematol. 2005 Jul;33(7):796–803. doi: 10.1016/j.exphem.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006 Feb 15;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 22.Poggi A, Prevosto C, Massaro AM, et al. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J Immunol. 2005 Nov 15;175(10):6352–6360. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 23.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005 Mar 1;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004 Jun;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 25.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005 May 15;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 26.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006 Aug 15;177(4):2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003 Oct;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 28.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004 May 1;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 29.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005 Sep 1;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 30.Uccelli A, Zappia E, Benvenuto F, Frassoni F, Mancardi G. Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Expert Opin Biol Ther. 2006 Jan;6(1):17–22. doi: 10.1517/14712598.6.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006 May 27;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 32.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006 Sep 15;108(6):2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005 Dec 15;106(13):4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 34.Sudres M, Norol F, Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006 Jun 15;176(12):7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 35.Chan JL, Tang KC, Patel AP, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006 Jun 15;107(12):4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romieu-Mourez R, Francois M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007 Aug 1;179(3):1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- 37.Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007 Jul 3; doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 38.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- 39.Puppo F, Bignardi D, Contini P, et al. Beta2-micro-free HLA class I heavy chain levels in sera of healthy individuals. Lack of association with beta2-micro-associated HLA class I heavy chain levels and HLA phenotype. Tissue Antigens. 1999 Mar;53(3):253–262. doi: 10.1034/j.1399-0039.1999.530305.x. [DOI] [PubMed] [Google Scholar]
- 40.Lampson LA, Fisher CA, Whelan JP. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- 41.Desai SA, Wang X, Noronha EJ, et al. Structural relatedness of distinct determinants recognized by monoclonal antibody TP25.99 on beta 2-microglobulin-associated and beta 2-microglobulin-free HLA class I heavy chains. J Immunol. 2000 Sep 15;165(6):3275–3283. doi: 10.4049/jimmunol.165.6.3275. [DOI] [PubMed] [Google Scholar]
- 42.Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S. Characterization of anti-HLA class II monoclonal antibody LGII-612.14 reacting with formalin fixed tissues. J Immunol Methods. 1993 May 26;161(2):239–256. doi: 10.1016/0022-1759(93)90300-v. [DOI] [PubMed] [Google Scholar]
- 43.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003 Nov;62(5):385–393. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 44.Rebmann V, Lemaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Report of the Wet Workshop for Quantification of Soluble HLA-G in Essen, 2004. Hum Immunol. 2005 Aug;66(8):853–863. doi: 10.1016/j.humimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998 May 15;160(10):4951–4960. [PubMed] [Google Scholar]
- 46.Morandi F, Levreri I, Bocca P, et al. Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release. Cancer Res. 2007 Jul 1;67(13):6433–6441. doi: 10.1158/0008-5472.CAN-06-4588. [DOI] [PubMed] [Google Scholar]
- 47.Morandi F, Chiesa S, Bocca P, et al. Tumor mRNA-transfected dendritic cells stimulate the generation of CTL that recognize neuroblastoma-associated antigens and kill tumor cells: immunotherapeutic implications. Neoplasia. 2006 Oct;8(10):833–842. doi: 10.1593/neo.06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapenta C, Santini SM, Spada M, et al. IFN-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8(+) T cells against exogenous viral antigens. Eur J Immunol. 2006 Aug;36(8):2046–2060. doi: 10.1002/eji.200535579. [DOI] [PubMed] [Google Scholar]
- 49.Gomez CE, Vandermeeren AM, Garcia MA, Domingo-Gil E, Esteban M. Involvement of PKR and RNase L in translational control and induction of apoptosis after Hepatitis C polyprotein expression from a vaccinia virus recombinant. Virol J. 2005 Sep 12;2:81. doi: 10.1186/1743-422X-2-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang HG, Pang XW, Shang XY, Xing Q, Chen WF. Functional supertype of HLA-A2 in the presentation of Flu matrix p58-66 to induce CD8+ T-cell response in a Northern Chinese population. Tissue Antigens. 2003 Oct;62(4):285–295. doi: 10.1034/j.1399-0039.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 51.Cucchiarini M, Kammer AR, Grabscheid B, et al. Vigorous peripheral blood cytotoxic T cell response during the acute phase of hepatitis C virus infection. Cell Immunol. 2000 Aug 1;203(2):111–123. doi: 10.1006/cimm.2000.1683. [DOI] [PubMed] [Google Scholar]
- 52.Wellings DA, Atherton E. Standard Fmoc protocols. Methods Enzymol. 1997;289:44–67. doi: 10.1016/s0076-6879(97)89043-x. [DOI] [PubMed] [Google Scholar]
- 53.Casati C, Dalerba P, Rivoltini L, et al. The apoptosis inhibitor protein survivin induces tumor-specific CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res. 2003 Aug 1;63(15):4507–4515. [PubMed] [Google Scholar]
- 54.Classen CF, Bird PI, Debatin KM. Modulation of the granzyme B inhibitor proteinase inhibitor 9 (PI-9) by activation of lymphocytes and monocytes in vitro and by Epstein-Barr virus and bacterial infection. Clin Exp Immunol. 2006 Mar;143(3):534–542. doi: 10.1111/j.1365-2249.2006.03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003 Sep;9(9):1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 56.Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004 Sep;60(3):307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 57.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004 Mar;33(6):597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 58.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003 Feb 15;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 59.Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007 Aug;21(8):1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 60.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007 Apr;56(4):1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 61.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005 Apr 15;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 62.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007 Feb;21(2):304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 63.Khakoo AY, Pati S, Anderson SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006 May 15;203(5):1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007 Oct 4;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 65.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004 Mar 15;103(6):2046–2054. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 66.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000 Sep;21(9):455–464. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 67.Kloetzel PM, Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004 Feb;16(1):76–81. doi: 10.1016/j.coi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Bandoh N, Ogino T, Cho HS, et al. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005 Sep;66(3):185–194. doi: 10.1111/j.1399-0039.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 69.Le Gal FA, Riteau B, Sedlik C, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol. 1999 Aug;11(8):1351–1356. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 70.Nasef A, Mathieu N, Chapel A, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007 Jul 27;84(2):231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 71.Zhou P, Seder RA. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with histoplasma capsulatum. J Exp Med. 1998 Apr 20;187(8):1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997 Nov 3;186(9):1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Dong H, Lin W, et al. Human bone marrow: a reservoir for “enhanced effector memory” CD8+ T cells with potent recall function. J Immunol. 2006 Nov 15;177(10):6730–6737. doi: 10.4049/jimmunol.177.10.6730. [DOI] [PubMed] [Google Scholar]
- 74.Thomas S, Kolumam GA, Murali-Krishna K. Antigen presentation by nonhemopoietic cells amplifies clonal expansion of effector CD8 T cells in a pathogen-specific manner. J Immunol. 2007 May 1;178(9):5802–5811. doi: 10.4049/jimmunol.178.9.5802. [DOI] [PubMed] [Google Scholar]
- 75.Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006 Mar 15;107(6):2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]