Both commercial and non-profit research organizations seek to capture the value of their inventions through filing their own patent applications and licensing those inventions to others. Until now, however, researchers have not systematically studied how large academic institutions patent and license their DNA-based inventions. The study of licensing practices presents difficulties for researchers because license information, in contrast to patents and most patent applications, is non-public. In fact, the information is often considered to be proprietary. Thus, respondents to a licensing survey – even within academic non-profit settings – must be assured that the financial information that they disclose will remain confidential unless public disclosure is explicitly authorized.
In this article, we describe the academic DNA patent universe and present the results of a federally funded survey of licensing practices at 19 of the 30 U.S. academic institutions that have received the largest number of DNA patents. Our analysis reveals not only that large U.S. research universities are active participants in DNA patenting and licensing, but also that common assumptions about licensing strategies often fail to capture the nuances and complexities of technology transfer in practice.
DNA and intellectual property
We define DNA patents as patents those containing at least one claim that includes a nucleic-acid-specific term. Gene patents, a subset of DNA patents that contain protein encoding nucleic acid sequences, have been of particular interest, as many have proven to be commercially important1,2. In a recent article, Kyle Jensen and Fiona Murray identified 4,270 U.S. patents that make claims on human DNA sequences; sequences from almost 20% of catalogued human genes are found in the claims of U.S. patents (4,382 of 23,688 genes in the National Center for Biotechnology Information RefSeq and Gene databases)3.
Diagnostic gene patents have sometimes raised concern about both cost and access4-6. Several reports from national and international bodies note that genetic testing applications require far less investment after initial gene discovery than development of therapeutic proteins, and so the rationale for exclusive intellectual property rights may be less compelling7-11.
DNA patents have generated other concerns, as well. In 1998, Michael Heller and Rebecca Eisenberg from the University of Michigan, posited that a profusion of patents upstream from final products can create an “anticommons” effect—creating a thicket of patents that encumber research progress and access to resources thus making it difficult to aggregate sufficient IP and stifling innovation12. In this context, however, a recent survey by John Walsh, Charlene Cho and Wesley Cohen of biomedical scientists found minimal research-blocking effects from patents, but somewhat more “friction” caused by material transfer agreements13 (contracts between institutions governing the transfer of research material). Transfers from industry to academe were more likely to come with conditions on publication, rights to improvements, or royalties, and took longer on average to complete than transfers among academic institutions13. The same survey found that few academic scientists check for patents related to their research13,14.
Another aspect of the debate about whether intellectual property fosters or hinders biomedical research relates to “research tools” – ideas, data, materials or methods used to conduct research. Many such materials and methods are disclosed or claimed in DNA patents. The gene patent subset of DNA patents has also been drawn into the research tools debate. This is because genes are not only inputs to developing genetic tests and therapeutic proteins—and are thus directly relevant to commercially valuable products and services—but also crucially important tools for ongoing research. Patent claims based on DNA sequences can be infringed by research activities that entail making or using the claimed sequence, not just by selling products or services.
The role of the NIH
As the primary source of biomedical research funding in the United States, the NIH has been concerned that patenting and licensing practices not impede advances in biomedical research, particularly for inventions arising from NIH funding. To promote broad access, NIH developed guidelines for grantee institutions about how to license biomedical research resources arising from federally funded research. In February 1996, the US National Research Council (NRC) convened a workshop on the patenting and licensing of such resources, or “research tools,” in molecular biology15. Even as the report from that workshop was being prepared, then-NIH Director Harold Varmus invited University of Michigan Law Professor Rebecca Eisenberg, to chair a working group to recommend policies NIH might pursue to ensure maximum social benefit from NIH-funded inventions. The working group addressed exchanges of data and research materials, as well as patenting and licensing of research tools in its June 1998 report16. In turn, the working group report was one of the sources of input to a December 1999 set of NIH guidelines for the sharing of NIH-funded biomedical research resources17. Compliance with those guidelines subsequently became an explicit consideration in the award of NIH grants and contracts. The ‘research tool’ guidelines are now regarded by at least some technology transfer officers as de facto federal policy. The NIH guidelines urge recipients of NIH grants and contracts to license or otherwise share research tools with all biomedical researchers who request them.
In 2000, the NIH began developing ‘Best Practices’ guidelines for all genomic inventions. These inventions were broadly construed to include “cDNAs; expressed sequence tags (ESTs); haplotypes; antisense molecules; small interfering RNAs (siRNAs); full length genes and their expression products; as well as methods and instrumentation for the sequencing of genomes, quantification of nucleic acid molecules, detection of single nucleotide polymorphisms (SNPs), and genetic modifications.”18 NIH's best practices thus included, but were not restricted to, inventions covered by claims of DNA patents. The guidelines also discussed unpatented methods and materials, which can also be licensed. The best practices guidelines proposed for grantee institutions were based largely on how NIH's own technology transfer office licensed inventions arising from NIH laboratories. Early drafts of the best practices guidelines were presented to several audiences, including a February 2004 meeting of the Association of University Technology Managers (AUTM).19-21
The revised, final guidelines were published in the Federal Register in April 200518. In general, these guidelines recommended that recipients of NIH funding strongly consider broad and nonexclusive licensing of genomic inventions, with allowance for cases when exclusive licensing was needed to induce large investment in post-discovery commercial development.
Survey inception and conception
In late 2002 and early 2003, we designed a survey to address concerns about the possibility that DNA patents were impeding genomic research, taking into account frequently proposed remedies, including those adopted or floated informally by the NIH before the survey got underway.19-21 One of our goals was to determine the degree to which US academic institutions were already practicing or anticipating the NIH technology transfer guidelines.
In 2003, NIH also funded the NRC to study IP in genome and protein research. We presented preliminary survey data to the NRC committee, which began work in February 2004 and whose final report was released in November 2005.22. The methodology of our survey are presented in Box 1 and Fig. 1 (see also Supplementary Methods; and Supplementary Figs. 1 and 2); detailed results are presented below.
Figure 1.
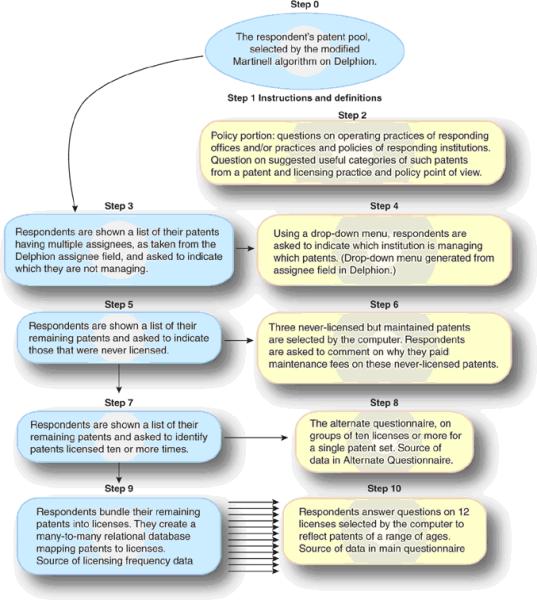
Steps undertaken by survey respondents.
Portrait of the patents
Table 1 provides a sample of the types of diverse types of DNA patents identified by our search algorithm (see Supplementary Methods). These include inventions describing genes, recombinant DNA technology, DNA sequencing methods and instruments, DNA labeling, DNA synthesis, bioinformatics software and hardware, and many other genomic technologies.
Table 1.
Examples of DNA patents captured by the search algorithm.
| US patent number | Title |
|---|---|
| 4613566 | Hybridization assay and kit |
| 4643969 | Novel cloning vehicles for polypeptide expression in microbial hosts |
| 4948733 | Zoogloea transformation using exopolysaccharide non-capsule producing strains |
| 4970154 | Method for inserting foreign genes into cells using pulsed radiofrequency |
| 5097025 | Plant promoters |
| 5169939 | Chimeric antibodies |
| 5215904 | Method for producing a recombinant mammal in vivo |
| 5266459 | Gaucher's disease: detection of a new mutation in intron 2 of the glucocerebrosidase gene |
| 5348878 | Class I major histocompatibility complex (MHC)-restricted T-T hybridomas, and a CD8-transfected BW5147, fusion partner |
| 5521071 | Soluble LDL receptor and gene |
| 5558998 | DNA fragment sizing and sorting by laser-induced fluorescence |
| 5569824 | Transgenic mice containing a disrupted p53 gene |
| 5571671 | Method for detecting Alzheimer disease |
| 5624823 | DNA encoding procine interleukin-10 AU:PROCRINE? IS THIS TYPO FOR PORCINE?, yes, on the patent |
| 5681934 | 47-kilodalton antigen of Treponema pallidum |
| 5750347 | In situ polymerase chain reaction |
| 5785965 | Vascular endothelial growth factor gene transfer into endothelial cells for vascular prosthesis |
| 5837244 | Oncoprotein protein kinase |
| 5874304 | Humanized green fluorescent protein genes and methods |
| 5917025 | Human telomerase |
| 5981842 | Production of water stress or salt stress tolerant transgenic cereal plants |
| 5998145 | Method to determine predisposition to hypertension |
| 6017524 | Inhibiting the growth p53 deficient tumor cells by administering the p53 gene |
| 6027882 | Patched genes and their use for diagnostics |
| 6107027 | Ribozymes for treating hepatitis C |
| 6146593 | High density array fabrication and readout method for a fiber optic biosensor |
| 6159745 | Interdigitated electrode arrays for liposome-enhanced immunoassay and test device |
| 6207392 | Semiconductor nanocrystal probes for biological applications and process for making and using such probes |
| 6261786 | Screening assays for hedgehog agonists and antagonists |
| 6319709 | Tumor cells with increased immunogenicity and uses |
| 6340567 | Genomics via optical mapping with ordered restriction maps |
| 6383754 | Binary encoded sequence tags |
As of November 9, 2005, we identified 38,929 DNA patents that had been issued. Four hundred and eighty-one fewer DNA patents were issued in 2004 than in 2003; indeed, historical data reveals that the number of DNA patents issued each year has declined since 200123 (see also Fig. 2). Our projected total for 2005 (2954) suggests this decline will continue.
Figure 2.
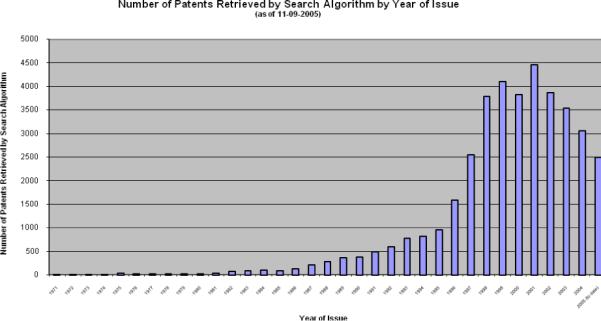
Number of US DNA patents issued 1971−2005*
*Through 11/30/05
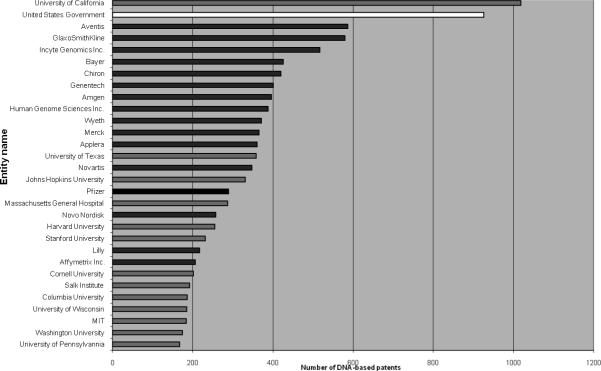
Roughly 78% of US DNA patents are owned by for-profit entities and 22% by nonprofits. Among the top 30 entities holding the largest number of DNA patents in the United States, 16 are for-profit companies, 13 are non-profit academic institutions, and 1 is the US government (mainly but not exclusively from the intramural research program at NIH; see Fig. 3). These top 30 entities own 10,824 (or 28%) of all DNA patents that had been issued by the USPTO through September 14, 2005. Of the 10,824 patents held by the top 30 entities, 3,772 (35%) are owned by the 13 academic institutions included in Fig. 3.
Figure 3.
The top 30 entities holding the largest number of DNA-based US patents.
A government interest, indicating that the invention arose in whole or in part from federally funded research, is acknowledged in almost 5,300 (15%) of US DNA patents. Among academic institutions, government interest was acknowledged in approximately half of their DNA patents (see Box 2).
Academic institutions are major players in the patenting and licensing of DNA-based inventions (see Fig. 3). The top 30 academic institutions assigned the largest numbers of DNA patents (75 DNA patents or more) are shown in Table 2. Nineteen technology transfer offices responded. These 19 offices contributed information to a relational database containing linked records for the approximately 2,600 DNA patents assigned to, and managed by, those institutions (see Table 2).
Table 2.
Academic institutions selected for the survey (based on 75 or over DNA patents).
| Institution | Number of patents (1/13/03)a | Number of patents (9/14/05) | Response |
|---|---|---|---|
| University of California System | 659 | 1018 | Completed by phone |
| University of Texas | 251 | 358 | No |
| Johns Hopkins University | 251 | 331 | No |
| Harvard University | 212 | 255 | Complete |
| Massachusetts General Hospital | 211 | 287 | No |
| Stanford University | 180 | 231 | Complete |
| Massachusetts Institute of Technology | 146 | 184 | Complete |
| Washington University in St. Louis | 140 | 174 | Complete |
| Cornell University | 138 | 202 | Complete |
| Columbia University | 134 | 186 | Complete |
| University of Wisconsin | 134 | 185 | Complete |
| Salk Institute | 133 | 192 | Complete |
| University of Pennsylvania | 133 | 167 | Complete |
| Rockefeller University | 125 | 146 | Complete |
| Baylor College of Medicine | 112 | 146 | No |
| Dana Farber Cancer Institute | 112 | 143 | No |
| Scripps | 110 | 151 | No |
| Yale University | 106 | 161 | Complete |
| Thomas Jefferson University | 103 | 122 | No |
| University of Michigan | 100 | 146 | Partial |
| California Institute of Technology | 91 | 132 | Complete |
| University of Washington | 88 | 114 | No |
| University of Chicago | 86 | 137 | Complete |
| University of Utah | 85 | 113 | Complete |
| State University of New York | 83 | 101 | Partial |
| New York University | 82 | 124 | No |
| Michigan State University | 78 | 93 | No |
| Duke University | 77 | 106 | No |
| University of Florida | 77 | 97 | Complete |
| Whitehead | 75 | 95 | Complete |
Cut-off date for survey
Patent type and licensing approach
The policy portion of the survey was designed to tease apart respondents’ views on useful categories and subcategories of patents pertinent to licensing policies. The subcategories, which were not mutually exclusive, covered patents with a range of utilities. One group of questions addressed specifically the subset patents on genes, gene fragments and DNA markers. At one end of the spectrum are patents on fully sequenced human genes encoding therapeutic proteins of known function. At the other end of the spectrum are patents on DNA sequences that are markers for phenotypes. In the middle are patents on genes encoding proteins that are potential drug targets, but that are not themselves therapeutic drugs.
DNA sequences that encode therapeutic proteins
For a fully sequenced gene that encodes a therapeutic protein, the utility and the development risks are both generally acknowledged to be high, and survey respondents largely agreed that they would patent and license such inventions exclusively. For example, Robin Rasor from the University of Michigan's Office of Technology Transfer indicated that the type of license her institution would seek would be “probably exclusive”. Kathleen Denis at the Rockefeller University Office of Technology Transfer indicated her institution would “patent and license, perhaps exclusively”, and Katharine Ku of the Stanford University Office of Technology Licensing responded, “We would be more likely to file a patent application on this and license it exclusively”. Richmond Wolf from the Caltech Office of Technology Transfer “we would almost certainly file a patent on such a human gene if it had strategic value for us – as well as on the protein and method of its use clinically. Caltech would likely license such a candidate exclusively if that were the best way to transfer the technology and if it were within our policy guidelines”.
DNA sequences that are markers only
For a partial DNA sequence used only as a phenotypic marker, where the utility is much less clear and the development costs and risks are generally modest or low, respondents reported that patenting was less likely, and that if the invention were patented, nonexclusive licensing would be more likely.. The survey question did not specify whether phenotypic markers had clinical utility. Joyce Brinton of the Harvard University Office for Technology and Trademark Licensing wrote “where disease-linked mutations that may be useful in clinical diagnostics assays are identified, they sometimes are patented; this decision depends somewhat on the diagnostic kit and service market, which is less robust than the therapeutics market”. The importance of market forces in determining licensing strategy was noted by others. For example, Caltech's Wolf responded, “If the phenotype had a significant clinical diagnostic application, we would be likely to patent. Our licensing strategy would depend on market demand”. According to Ku, Stanford “would be less likely to file a patent application, unless there seemed to be enough of a market for the marker. Most likely this type of DNA-based invention would be licensed nonexclusively, unless there were a significant investment and justifiable reason for exclusivity.”
Rockefeller's Denis, perhaps assuming no diagnostic utility for the marker, wrote that such DNA sequences have been “patented in the past but now resist [the] occasional push to patent—these are not worth the time and the money”.
DNA sequences comprising genes encoding drug targets
Perhaps because the survey instrument did not specify whether the therapeutic lead against the target had also been discovered, there was the least consensus among the technology transfer offices on how to handle a fully sequenced human gene, where the encoded protein is a target for drug discovery. Jon Soderstrom of the Yale Office of Cooperative Research indicated his university's tech transfer officer “[would] not file. Handle the transfer through [a] material transfer agreement.” At Stanford, Ku noted “at the end of the day, we are less likely to file on this than on other biotech inventions.” Nevertheless, Stanford might still consider patent depending on the “commercial interest of the target.”
There is also considerable flexibility in the licensing strategies adopted for such patents. At Stanford's tech transfer office, “licensing would depend on what type of company was interested in commercializing the technology—larger companies would probably want to be licensed nonexclusively, small companies may likely need exclusivity to generate investment for working with the target,” wrote Ku. Elsewhere, at the Office of Technology Management at the Salk Institute for Biological Studies, Polly Murphy commented “in the past we would probably have licensed exclusively, but now usually nonexclusively”. Caltech's Wolf provided a helpful refinement to the survey question, indicating that his institution's tech transfer personnel “would very likely file such a patent if we could get claims on molecules that bind to the target as a therapeutic application. We would likely license such a patent exclusively if it were the best way to transfer the technology and if it were within our policy guidelines”.
DNA discoveries or inventions representing research tools
Another group of survey questions addressed ‘research tools,’ which included, but went well beyond, patents on genes, gene fragments and DNA markers. Respondents were asked how they defined research tools, what their policies and practices regarding the licensing of research tools were, and whether these practices would differ if the research tools were patented. Respondents frequently referred to the NIH guidelines. Some noted the difficulty of defining a research tool, saying that at times tools also required development incentives. For example, Alan Paau from the University of California San Diego (UCSD) commented: “If you know its specific utility, it is not a research tool. If you are using the method to produce a commercial product, then it is not a research tool.” He went on to give an example of how the same patent could be licensed at two different rates, under two different licensing agreements, one for research use and the other for commercial use. Others indicated that for most research tool DNA patents, nonexclusive licensing was the preferable route. Thus, Richard Cahoon of Cornell University wrote, “We prefer to license the tools nonexclusively”, and the University of Pennsylvania's Lou Berneman responded that his institution opted for “broad nonexclusive licensing, regardless of patent status.”
Retained, and transferable research-use rights
We accumulated evidence of a strong and expanding retained, and transferable research-use right, even within Exclusive, All Fields of Use licenses. The 19 large respondent academic institutions retain research-use rights themselves and insist on the right to transfer this research-use right to other nonprofit institutions. For example, Joyce Brinton wrote that “In our licenses we reserve the right to use the invention in our research and the right to grant such licenses to other nonprofits” [Harvard]. At UCSD, Alan Paau reported that for over six years the right to use the work by other non-profits had been included in license agreements (expanding the research exemption beyond the initial licensor). Andrew Neighbour of UCLA commented, “They [university technology transfer officers] always insist on a research exemption not only for themselves, but for other non-profit institutions; adding the other non-profits into the research exemption has been a trend”. In a follow-up phone interview, Joel Kirschbaum of UCSF stated that the institution's licensing agreements “retain a full shop-right to use [the institution's] technology for research and education purposes, and the right to share those rights with other research scientists for their noncommercial research and education purposes as well”. Carol Mimura of UC Berkeley commented that about six years ago [the institution] had expanded its “shop rights,” which had previously been reserved for internal institutional use, to include an ongoing right to transfer the materials required to practice the invention to others in the non-profit sector.
De facto research exemption, or “rational forbearance”24
The assertion of patent rights by for-profit firms against academic research institutions or individual scientists working within their institutions was discussed in three follow-up telephone interviews. Representatives of all three technology transfer offices acknowledged current policies of forbearance on the part of private companies, tempered by awareness that these policies could change. For-profit firms generally do not threaten infringement litigation against academic research institutions (a de facto research exemption), in part because such academic use may improve their invention, and they wish to maintain good will and to ensure access to future academic inventions—and because the damages are likely to be very small The question of such complaints about infringement arose in part because of a 2002 Appeals Court decision that interprets the common law “research exemption” in such a way that it will rarely apply to academic research.25
Licensing frequency and exclusivity
The 2,607 patents managed by the 19 responding tech transfer offices were associated with approximately 1,200 license agreements. (See Figure 4 and the accompanying table.) Some patents in the 1−9 group (comprised of 1,787 patents) were licensed together with others, while some patents were licensed multiple times. Approximately 70% of the 2,607 managed patents have either been licensed in the past or are still under license. Seven hundred and seventy-five of the managed patents were never licensed. Approximately 2% of the patents (45) were licensed more than nine times; this patent group includes the 400+ licenses granted to the Cohen-Boyer trio of patents and the licenses granted for the Axel patents. The correspondence between patents and licenses is clearly not one-to-one; rather it is a many-to-many relationship. For this reason no conclusions can be drawn about the number of distinct inventions that are licensed or commercialized.
Figure 4.
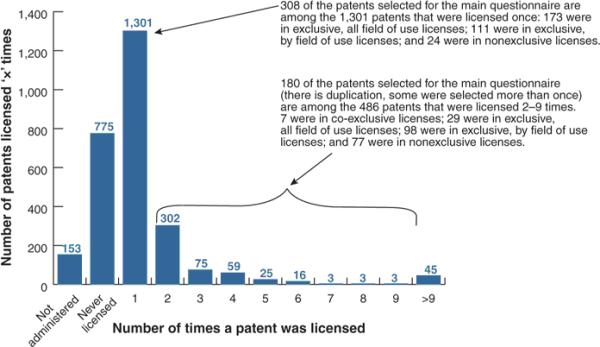
Number of times a DNA patent was licensed.
Our survey results show that frequency of licensing is an imprecise proxy for license exclusivity, and vice versa (Fig. 4). Having been licensed once is associated with ‘exclusive, all fields of use’ licensing only 56% of the time, whereas having been licensed 2−9 times is associated with nonexclusive licensing only 36% of the time (30 patents were selected twice, and one patent was selected 3 times). Some patents are exclusively licensed many times in different fields of use. Exclusive licenses terminate for a variety of reasons, and an invention may be subsequently re-licensed. Some licenses are renegotiated and their exclusivity modified from initial licensing, and some ‘exclusive’ licenses are sublicensed by the licensee.
The term ‘exclusive’ needs to be used with precision and care. Avoiding exclusivity is often the focus of discussions on how to improve access to biotechnology-related inventions. Exclusivity is, however, a more nuanced property than often supposed. The three categories of exclusivity used in this survey—‘exclusive, all fields of use,’ ‘exclusive, by field of use,’ and ‘co-exclusive’ — are all lumped together as ‘exclusive’ in the AUTM survey (an annual survey conducted by academic licensing professionals26). In our view, the absence of important subcategories in the AUTM data can be a source of misinterpretation or confusion. Exclusivity is frequently limited in duration as part of the license itself or by license termination. It can also be limited by geographical area, by field of use, and by the licensor's agreeing to grant only a limited number of additional licenses (co-exclusivity).
The 29 patents licensed more than once, and characterized as having been licensed ‘exclusive, all fields of use’ merited further investigation. When we dug deeper, many instances were explained by sequential licensing, where at least one of the licenses was ‘exclusive, all fields of use’. In other cases, respondents explained the multiple ‘exclusive, all fields of use’ licenses by noting that their database tracked sublicenses granted by their ‘exclusive, all field of use’ licensees, and that they reported the sublicense, and its terms, as a separate license. Not all respondents track such sublicenses, however; this was an area in which the survey instructions were unclear. Thus, the data on sublicenses should be regarded as serendipitous and not systematic. The serendipitous sublicensing data we did garner reinforce the observation that exclusivity alone is not a reliable indicator of availability and utilization.
Broadly licensed patents
Data on the 45 patents licensed 10 or more times are rich and interesting, but well beyond the scope of this report. One feature worth noting, however, is that ‘diligence clauses,’ which contain the contractual requirement that licensees develop the invention or lose rights to it, are generally not regarded as essential in such nonexclusive licenses. That is, the patent holder does not require the multiple licensees to develop the invention further.
Some of these sets of patent licenses generated considerable income, including the well known Cohen-Boyer trio of patents27, now expired, that were licensed by Stanford and the University of California. As a group, the 45 frequently-licensed patents were licensed in 21 separate bundles, containing from one to five patents each. The Cohen-Boyer patent trio, for example, was licensed over 400 times28. Most of the 21 bundles were licensed a few dozen times. Columbia University declined to report further on its Axel co-transformation patents29 beyond noting that they had been licensed 10 or more times. A case study in the 2004 book Ivory Tower and Industrial Innovation30 reports that the Axel patents were licensed to 34 firms and generated over $370 million (1996 dollars) between 1983 and 2000. These broadly licensed patents are thus important both to universities and licensees. However, failure to ‘work’ the invention is less a concern because licensing is nonexclusive (see Supplementary Tables 1, 2).
Licensee influence on exclusivity
Start-ups have, in nearly all cases, exclusive licenses, although only about two-thirds have ‘exclusive, all fields of use’ licenses (Fig. 5). Exclusive licensing is consistent with the need to lower the perceived risk of investing in unproven technology to attract private risk capital. Thus, there are trade-offs between optimizing licensing terms solely for startup formation and job creation on the one hand, and making the technology freely available at all times to all potentially interested parties via the intentional avoidance of exclusive or partly exclusive license grants on the other.
Figure 5.
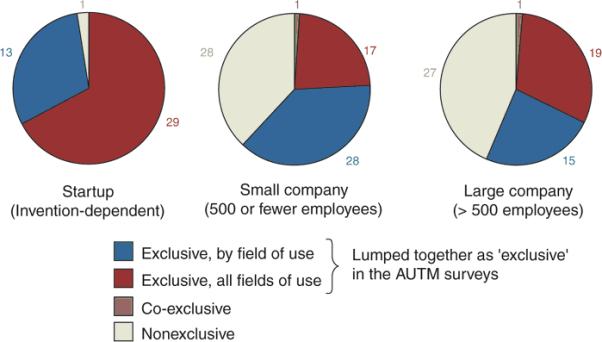
Exclusivity patterns of university patents by company type
We also asked whether there were competing interested parties at the time the license was signed and found that university licensors are responsive to the “market” – that is, their tendency to grant exclusive licenses is reduced in response to increased outside interest. (Fig. 6). Note that the sensitivity to the market also plays a role in patenting decisions, where respondents cited patent cost and outside market interest as factors in patenting decisions.
Figure 6. Percent of time there were competing interests at the time the license was signed.
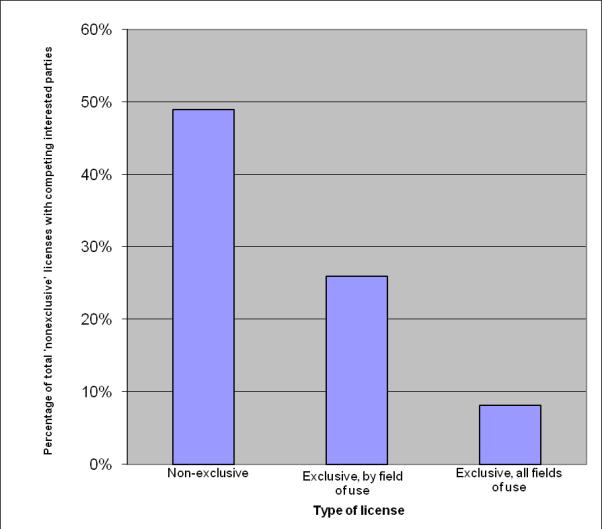
In the case of the 56 ‘nonexclusive’ licenses for which there are detailed data, in 24−31 (or 43−56%; range due to incomplete responses), there were competing bidders at the time the licensing was signed. In the case of 56 ‘exclusive, by field of use’ licenses, respondents reported that in 13−19 cases (23−34%; range due to incomplete responses), there were competing interested parties at the time the license was signed. In the case of the 65 ‘exclusive, all field of use’ licenses, respondents reported multiple interested parties for 5−9 (8−14%; range due to incomplete responses). Both the co-exclusive licenses had competing interested parties.
License age and termination
Of the 179 licenses for which there is detailed information (these licenses were selected by computer algorithm, so as to contain patents of representative ages in them, as described in the Supplementary Materials), 50 (28%) were terminated, and in only two cases was such termination a result of patent expiration. In three cases, the terminated licenses were characterized as ‘terminated by the institution,’ and in 35 cases as ‘terminated by the licensee.’ Many other interesting examples of termination were given, including ‘conversion to a nonexclusive’ and ‘by mutual agreement’ (see Supplementary Table 2).
There are no obvious patterns in termination by exclusivity: 16 of 65 (26%) of the ‘exclusive, all field of use’ licenses terminated, compared with 16 of 56 (29%) of the ‘exclusive, by field of use’ and 15 of 56 (27%) of the ‘nonexclusive’ licenses. The terminated licenses lasted, on average, fewer years—about 4.5—than the licenses that are still active. Active licenses are, on average, about eight years old, but the age distribution of both terminated and active licenses is very broad.
Diligence milestones
Building milestones into a license that demonstrate progress toward commercializing the invention, which, if not met, provide the licensor an opportunity to terminate or modify the license, is a common strategy that allows a university to recapture intellectual property rights. Such “diligence” (use it or lose it) provisions in the license play an important role in US academic licensing transactions. In this section “diligence” will refer to contractual requirements beyond paying the patent owner a fee, as paying a fee to the licensor is not necessarily correlated with making progress toward commercializing an invention. Diligence was measured in the survey by six questions on whether certain kinds of defaults, other than paying the licensor a fee, would result in termination or loss of rights. Sample diligence milestone include a requirement that the licensee spend money toward commercializing the invention, or that they submit a potential product for regulatory approval. One or more diligence milestones is included in approximately 80% of licenses with some degree of exclusivity, but only about 45% of nonexclusive licenses. All respondents use contractual requirements to actively commercialize licensed inventions some of the time. As it was somewhat surprising that diligence milestones were included in only approximately 80% of licenses with a degree of exclusivity, certain institutions were asked to comment on their diligence practices. Two institutions commented that in the past they negotiated stringent requirements beyond payment of a fee for continuation of a license, but that they had more recently opted for a combination of financial payments and the licensee's desire to maintain a cordial relationship with the licensing institution – a policy that they thought worked better than specific diligence milestones.
For patents licensed nonexclusively ten or more times, we found that few diligence clauses were included. During telephone interviews, multiple respondents mentioned mandatory sublicensing as another technique to make technology broadly available. A mandatory sublicense requires the licensee, under certain conditions, to license an invention to a third party if the third party is also qualified and prepared to develop the invention. One phone interviewee referred to “license audits” and “mutual termination” as techniques for taking back rights from current holders and thus renewing broader access to technology not being adequately commercialized
Conclusions
A central finding of our survey is that simple reports on exclusive and nonexclusive licensing miss important nuances of licensing practice, nuances that are infrequently discussed in the literature on patenting and licensing. An invention licensed just once is not necessarily the object of an exclusive license, and some exclusive licenses are restricted by field of use or in duration or are terminated by one party or the other. Technologies can remain available, while exclusively licensed, if the exclusivity is for a particular field of use, or if research or humanitarians use exemptions have been included in the license. In addition, an exclusive license may, under certain circumstances, be renegotiated to be nonexclusive, or the licensee may sublicense, or conditions for sublicensing may be stipulated in the exclusive license.
Evidence of diligence provisions in license agreements in the quantitative responses was enriched by comments about how different offices monitor and deal with such provisions, such as requirements to raise investment money, to spend money on development, to submit products for US Food and Drug Administration approval and to sell products and services. Mandatory sublicensing is a type of diligence and was described by a few respondents, as were a “license audits,” “mutual termination” agreements and the mutual desire on the part of both parties to maintain good relations.
Licenses are commonly exclusive in certain fields of use – a type of licensing that is often described with the shorthand phrase “exclusive by field of use”. Figure 5 illustrates that this “exclusive by field of use licensing” is roughly as prevalent, as a whole, across all types of licensees, as exclusive licenses in all fields of use, or non exclusive licenses. Thus, the quantitative portion of the survey corroborates the qualitative portion, where respondents report using such field of use licensing in their contracts. Our findings suggest that licensing practices at the large and experienced academic institutions studied in this survey are largely in agreement with the NIH guidelines for research tools and genomic inventions.17, 18
A third important survey finding is the market sensitivity observed in both patenting and licensing behavior. The number of DNA patents has declined each year since 200123 (See Figure 2.) In the qualitative portion of the questionnaire, respondents noted patent costs as a factor. Patent prosecution, maintenance, and management costs--estimated by respondents at between $20,000 and $30,000 per patent--militate against patenting inventions that are unlikely to recover those costs, and encourage greater selectivity in what gets patented. Figure 6 illustrates how exclusivity in license is reduced in response to increased outside interest, and suggests a fruitful area for future work, understanding the timing of licenses relative to publication of the inventions.
The quantitative data and qualitative written and oral responses paint a picture of practices evolving in light of experience. These practices appear to be designed pragmatically to accommodate both economic goals, such as revenue generation and new company formation, and social goals, such as ensuring utilization and availability of federally funded inventions.
Box 1. Methodology and survey design.
Our first task was to create a working definition of DNA patents and to collect these patents into a searchable database. For this purpose, we used an algorithm (for details, see Supplementary Methods) that two of us (R.M.C.-D. and J.M) had refined between 1994 and 2002 from one initially developed by James Martinell at the US Patent and Trademark Office (USPTO, Washington, DC) to identify all the patents in the Delphion Patent Database (http://www.delphion.com) whose claims explicitly mention DNA- or RNA-specific terms. Patents identified in this manner were then collated into a publicly accessible database, the DNA Patent Database (DPD), which is maintained online and updated weekly (http://dnapatents.georgetown.edu/).
We then searched the DNA patent database for patents assigned to each of the top 100 academic institutions on the National Science Foundation's (NSF) list of federal R&D funding recipients for FY 2001 (ref. 24). The 30 institutions assigned the largest numbers of DNA patents were selected for the survey (Table 1; 75 DNA patents was assigned as the survey cutoff). Technology licensing offices at each of these 30 institutions were subsequently invited to participate in a survey covering their patent licensing practices, policies and outcomes.
The survey questions were developed with input from the members of the project's Advisory Board (for the survey instrument and a list of the Advisory Board, see Supplementary Methods.) Respondents were promised that their licensing information would remain confidential in a secure server. The survey was reviewed and approved in advance by the Georgetown University Social Science Institutional Review Board.
The survey focused on the details of a subset of license agreements containing patents with ages representative of all DNA patents held by the institution, from the oldest to the youngest. (See Supplementary Figs 1, 2). Detailed license data were obtained for this representative subset of approximately 500 patents in approximately 200 license agreements, supplemented by qualitative written responses to open-ended policy questions on the web-based survey, and followed up in semi-structured telephone interviews.
The design of the survey (Fig. 1) resulted in the respondent-created relational database mapping patents to licenses. Linking of specific patents to specific licenses made possible important analyses, previously not possible, such as the timing of licensing relative to publication or the elapsed time between licensing and revenue and product milestones. (For a more detailed explanation of the survey methodology, see Supplementary Methods.)
Box 2. Government interest in US DNA patents.
US national policy to enable federal grantees and contractors to patent the results of their work was already emerging when the Bayh-Dole Act passed in 1980. But the Act clarified the rules, made them more uniform among and within funding agencies as well as reinforced the mandate to encourage commercial application of inventions arising from federally funded research1. Thus, Bayh-Dole made it easier for commercial firms to license the biotech IP arising from universities and other academic research institutions. On the other hand, it reserved certain rights to the government agency funding research leading to patented invention. Grantees and contractors are obligated by law to report those rights when applying for a patent. Our search algorithm (see Supplementary Methods) allows us to capture the acknowledgement of a patent's federal funding or government interest by identifying terms common in such acknowledgement.
On the basis of our results, almost 14% of DNA patents report government interest compared with 1.3% of all patents (Table 3). Of the DNA patents owned by the top 30 academic institutions and the 19 survey respondents, government interest is acknowledged by 50% and 52%, respectively. Studies from Offices of the Inspector General and the General Accounting Office (OIG; Washington, DC) have shown significant under-reporting of such ‘government interest,’ so these numbers may be underestimates of the number of DNA patents linked to federally funded R&D28,29. For example, the Scripps Institute (La Jolla, CA) had reported 54 of 125 patents to NIH as having been developed with its funds; in contrast, the OIG judged that 94 patents had actually involved such funding.
Supplementary Material
Table 3.
Percentage of DNA patents with government interest (as of 09/09/05).
| Patent type | Total | Government interest | Percentage government interest |
|---|---|---|---|
| Total Patents | 3593421 | 45823 | 1.28% |
| Total DNA patents | 38482 | 5288 | 13.70% |
| Total patents owned by top 30 academic institutions | 28426 | 12725 | 44.77% |
| Total DNA patents owned by top 30 academic institutions | 5702 | 2891 | 50.70% |
| Total patents owned by 19 survey respondents | 21764 | 10000 | 45.95% |
| Total DNA patents owned by 19 survey respondents | 3857 | 2008 | 52.06% |
Acknowledgments
The authors wish to thank the respondents at the 19 academic institutions that participated in this survey. The authors gratefully acknowledge the support of the National Human Genome Research Institute (Grant No. 1 R03 HG02683) and the Department of Energy (Grant No. DE-FG02-01ER63171) to Georgetown University (LeRoy Walters, PI) and of both NHGRI and DOE for a Centers of Excellence for ELSI Research grant (CEER Grant No. P50 HG003391) to Duke University (Robert M. Cook-Deegan, PI). We also thank our Advisory Board members for expert suggestions as we designed the survey instrument, Bi Ade for her capable research assistance, and Marian Nella Daggett for her helpful comments on an earlier draft of this article.
A survey of technology transfer of DNA inventions at 19 top US research universities reveals consensus, diversity, and flexibility in intellectual property management. Patent filing and license terms are influenced by intended uses of inventions, outside market interest, and NIH guidelines.
Footnotes
Publisher's Disclaimer: First published as Pressman L, Burgess R, Cook-Deegan RM, McCormack S, Nami-Wolk I, Soucy M, Walters L. The licensing of DNA patents by large U.S. academic institutions: an empirical survey. Nat Biotechnol 2006 Jan;24(1):31−39. doi:10.1038/nbt0106−31 Final publication available at http://www.nature.com/nbt/journal/v24/n1/full/nbt0106-31.html
References
- 1.Rai AK, Eisenberg RS. Bayh-Dole reform and the progress of biomedicine. Law and Contemporary Problems. 2003;66:289–314. [Google Scholar]
- 2.Eisenberg RS. Structure and function in gene patenting. Nat. Genet. 1997;15:125–130. doi: 10.1038/ng0297-125. [DOI] [PubMed] [Google Scholar]
- 3.Jensen K, Murray F. The intellectual property landscape of the human genome. Science. 2005;310:239–240. doi: 10.1126/science.1120014. [DOI] [PubMed] [Google Scholar]
- 4.Cho MK, Illangasekare S, Weaver MA, Leonard DGB, Merz JF. Effects of patents and licenses on the provision of clinical genetic testing services. J. Mol. Diagn. 2003;5:3–8. doi: 10.1016/S1525-1578(10)60444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paradise J, Andrews L, Holbrook T. Patents on human genes: an analysis of scope and claims. Science. 2005;307:1566–1567. doi: 10.1126/science.1105162. [DOI] [PubMed] [Google Scholar]
- 6.Andrews LB, Paradise J. Gene patents: the need for bioethics scrutiny and legal change. Yale J Health Policy Law Ethics. 2005;5:403–412. [PubMed] [Google Scholar]
- 7.Nuffield Council on Bioethics . The Ethics of Patenting DNA. Nuffield Council on Bioethics; London, England: 2002. http://www.nuffieldbioethics.org/go/screen/ourwork/patentingdna/introduction. [Google Scholar]
- 8.Commission on Intellectual Property Rights . Integrating Intellectual Property Rights and Development Policy. Commission on Intellectual Property Rights; London, UK: Sep, 2002. [13 October 2005]. http://www.iprcommission.org/papers/pdfs/final_report/CIPRfullfinal.pdf. [Google Scholar]
- 9.Human Genetics Programme, World Health Organization . Genetics, Genomics, and the Patenting of DNA. WHO; Geneva, Switzerland: 2005. [13 October 2005]. http://www.who.int/genomics/patentingDNA/en/ [Google Scholar]
- 10.Australian Law Reform Commission . Genes and Ingenuity: Gene Patenting and Human Health. Report 99. Australian Law Reform Commission; Sydney, Australia: Jun, 2004. [Google Scholar]
- 11.Ontario Report to the Provinces and Territories . Genetics, Testing and Gene Patenting: Charting New Territory in Healthcare. Toronto: Jan, 2002. [13 October 2005]. http://www.health.gov.on.ca/english/public/pub/ministry_reports/geneticsrep02/report_e.pdf. [Google Scholar]
- 12.Heller MA, Eisenberg RA. Can patents deter innovation? The anticommons in biomedical research. Science. 1998;280:698–701. doi: 10.1126/science.280.5364.698. [DOI] [PubMed] [Google Scholar]
- 13.Walsh J, Cho C, Cohen WM. Material Transfers and Access to Research Inputs in Biomedical Research. Final Report to the National Academy of Sciences’ Committee [on] Intellectual Property Rights in Genomic and Protein-Related Research Inventions; Sep 20, 2005. [21 November 2005]. Patents. [URL: http://tigger.uic.edu/~jwalsh/WalshChoCohenFinal050922.pdf;] [Google Scholar]
- 14.Walsh JP, Cho C, Cohen WM. View from the bench: patents and material transfers. Science. 2005;309:2002–2003. doi: 10.1126/science.1115813. [DOI] [PubMed] [Google Scholar]
- 15.National Research Council . Intellectual Property Rights and Research Tools in Molecular Biology. Summary of a Workshop Held at the National Academy of Sciences, February 15−16, 1996. National Academy Press; Washington, DC, USA: 1997. [PubMed] [Google Scholar]
- 16.National Institutes of Health Working Group on Research Tools . Report of the National Institutes of Health (NIH) Working Group on Research Tools, Presented to the Advisory Committee to the Director, June 4, 1998. NIH; Bethesda, MD, USA: 1998. [14 October 2005]. http://www.nih.gov/news/researchtools/ [Google Scholar]
- 17.Anonymous Principles and guidelines for recipients of NIH research grants and contracts on obtaining and disseminating biomedical research resources. Federal Register. 1999;64:72090–72096. [Google Scholar]
- 18.Anonymous Best practices for the licensing of genomic inventions. Federal Register. 2005;70:18413–18415. [Google Scholar]
- 19.Claire Driscoll at the University of Pennsylvania; Mar 3, 2003. http://www.genome.duke.edu/centers/gelp/downloads; PowerPoint presentation by. [Google Scholar]
- 20.the AUTM annual meeting; San Antonio. Jack Spiegel, NIH Office of Technology; Feb 7, 2004. [11 November 2005]. http://www.genome.duke.edu/centers/gelp/downloads; draft guidelines distributed by. Transfer on. [Google Scholar]
- 21.Malakoff D. NIH roils academe with advice on licensing DNA patents. Science. 2004;303:1757–1758. doi: 10.1126/science.303.5665.1757a. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council, Committee on Intellectual Property Rights in Genomics and Protein-Related Research, [re-check final name of committee], Science, Technology and Economic Policy Board and the Science, Technology, and Law Committee Intellectual Property Rights in Genomic and Proteomic Research: Access to Discoveries, Inventors’ Rights, and Public Health. [report released 17 November 2005; URL:]
- 23.Finnegan, Henderson, Farabow, Garrett & Dunner, LLP . The Finnegan Henderson 2005 Biotechnology Innovation Report. Finnegan, Henderson, Farabow, Garrett & Dunner, LLP; Washington, DC, USA: 2005. http://www.bio.org/events/2005/speaker/sessionlist.asp?id=16. [Google Scholar]
- 24.Rosenberg L, Major pharmaceutical company. In National Research Council . Intellectual Property Rights and Research Tools in Molecular Biology. Summary of a Workshop Held at the National Academy of Sciences, February 15−16, 1996. National Academy Press; Washington, DC, USA: 1997. p. 63. [Note: The entire work was cited as reference 15 above.]
- 25.U.S. Court of Appeals for the Federal Circuit Madey v. Duke. 2002 October 3; decided ( http://cyber.law.harvard.edu/people/tfisher/2002Madeyedit.html)[307 F.3d 1351; 2002 U.S. App. LEXIS 20823; 64 U.S.P.Q.2D (BNA) 1737]
- 26.Association of University Technology Transfer Managers . AUTM Licensing Survey. FY 2004. AUTM; Northbrook, IL: 2005. [23 November 2005]. http://www.autm.net/events/File/Surveys/03_Abridged_Survey.pdf. [Google Scholar]
- 27.US4237224 (Process for producing biologically functional molecular chimeras; 2 December 1980). US4468464 (Biologically functional molecular chimeras; 28 August 1984); and US4740470 (Biologically functional molecular chimeras; 26 April 1988).
- 28.Hughes SS. Making dollars out of DNA. The first major patent in biotechnology and the commercialization of molecular biology. Isis. 2002;92:541–575. doi: 10.1086/385281. [DOI] [PubMed] [Google Scholar]
- 29.US4399216 (Processes for inserting DNA into eucaryotic cells and for producing proteinaceous materials; 16 August 1983); US4634665 (Processes for inserting DNA into eucaryotic cells and for producing proteinaceous materials; 6 January 1987); US5149636 (Method for introducing cloned, amplifiable genes into eucaryotic cells and for producing proteinaceous products; 22 September 1992); US5179017 (Processes for inserting DNA into eucaryotic cells and for producing proteinaceous materials; 12 January 1993); and US6455275 (DNA construct for producing proteinaceous materials in eucaryotic cells; 24 September 2002).
- 30.Mowery DC, Nelson RR, Sampat BN, Ziedonis AA. Ivory Tower and Industrial Innovation. University-Industry Technology Transfer Before and After the Bayh-Dole Act. Stanford University Press; Stanford, CA, USA: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.