Abstract
Background and Purpose
Germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) is the most common neurological problem of premature infants. Despite this, mechanisms of brain injury from IVH are elusive. We hypothesized that GMH-IVH, by induction of NAD(P)H oxidases, might cause oxidative/nitrosative stress contributing to brain injuries and that NAD(P)H oxidase inhibition could offer neuroprotection .
Methods
To test this hypothesis, we exploited our rabbit pup model of glycerol-induced GMH-IVH. We delivered rabbit pups prematurely (E29) by C-section and administered intra-peritoneal glycerol at 2h postnatal age. Free-radical adducts, including nitrotyrosine, 4-hyroxynonenal and 8-Hydroxy-deoxyguanosine [8-OHdG] as well as O2·− and H2O2 levels were measured in the forebrain. To determine the source of free-radical generation, we used inhibitors for NAD(P)H oxidase (apocynin), xanthine oxidase (allopurinol), COX-2 (indomethacin) or NO synthases (L-NAME). IVH pups were treated with apocynin and cell death was compared between apocynin-treated and vehicle-treated pups.
Results
Nitrotyrosine, 4-hyroxynonenal and 8-OHdG levels were higher in pups with IVH than controls. Likewise, O2·− and H2O2 levels were significantly greater in both the periventricular area and cerebral cortex of pups with IVH than controls. In pups with IVH, reactive oxygen species (ROS) production was more in the periventricular area than in cortex. Apocynin, but not allopurinol, indomethacin or L-NAME, inhibited ROS generation. Importantly, apocynin reduced cell death in pups with IVH.
Conclusion
Activation of NAD(P)H oxidase was the predominant mechanism of free-radical generation in pups with IVH. NAD(P)H oxidase inhibition by apocynin might suppress ROS production and confer neuroprotection in premature infants with IVH.
Keywords: Germinal matrix hemorrhage-intraventricular hemorrhage, oxidative stress, reactive oxygen species, reactive nitrogen species, superoxide, nitro-tyrosine, NADPH oxidase, apocynin
INTRODUCTION
Germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) is the most common neurological disorder of premature newborns. GMH-IVH predisposes the preterm infants to cerebral palsy, cognitive deficits and post- hemorrhagic hydrocephalus.1 GMH-IVH is not a substantially preventable disorder; and unfortunately, its treatment is primarily supportive and not therapeutic. Therefore, it is crucial to determine the basis of brain injury from IVH in order to identify a mechanism-based strategy to protect the brain in affected premature infants with IVH. The mechanism of brain injury after intracerebral hemorrhage is complex and involves inflammatory reaction, mass effect of hemorrhage and ischemia around the hemorrhagic areas.2, 3 These responses are associated with the generation of free radicals, cytokines and a number of other immunomodulators. Herein, we ask whether the development of IVH in premature newborns is attended by an enhanced production of reactive oxygen (ROS) and nitrogen species (RNS), and if so, what is the predominant source of free radicals.
In adult rat models, oxidative stress plays a key role in the pathogenesis of cerebral injury after intracerebral hemorrhage.4,5 ROS damages neurons and oligodendrocytes, decreases cerebral blood flow, opens up the blood brain barrier to large molecules like neurotoxins and inflammatory cells, collectively leading to accumulation of oxidized aggregated proteins.6,7 The brain of premature infants, is highly vulnerable to oxidative damage as it is inadequately equipped with antioxidant defense systems.7, 8 Indeed, studies on autopsy specimens of premature infants with white matter (periventricular leukomalacia) and cortical injury have shown evidence of oxidative stress in various regions of the forebrain9. However, free radical generation has neither been evaluated in premature infants with IVH nor in animal models of IVH. Therefore, the present study explores oxidative and nitrosative stress in IVH that might contribute to brain injuries in these premature newborns.
The potential cellular sources of ROS include NAD(P)H oxidase, cyclo-oxygenase, xanthine oxidase or nitric oxide synthase and mitochondria.10, 11 Presently, it is unknown how these mechanisms are affected in GMH-IVH of preterm infants. Interestingly, a recent study in an adult mouse model of brain hemorrhage has shown that oxidative stress resulting from NAD(P)H oxidase largely contributes to the brain injury.12 In addition, NAD(P)H oxidase inhibition is neuroprotective in rodent models of hypoxia-ischemia and surgically-induced brain injury.11, 13 On this basis, we hypothesized that IVH, by induction of NAD(P)H oxidase, might result in formation of ROS in the brain of premature rabbit pups, and that NADPH oxidase inhibition might confer neuroprotection.
Materials and Methods
Animal studies
The animal protocol was approved by the Institutional Animal Care and Use Committee of New York Medical College. We used rabbit model of glycerol-induced IVH in the study. The details of the model have been previously established and published.3 Timed pregnant New Zealand rabbits were obtained from Charles River Laboratories, Inc. (MA, USA). The pups were delivered prematurely by Caesarean-section at a gestational age of 29 days (full-term = 31–32 days). Pups were immediately dried and kept warm in an infant incubator, which was maintained at a temperature of 35°C and 60% humidity. After stabilization, the pups were weighed. Pups were fed 1ml KMR (Kitten Milk Replacer, PETAG Inc., IL, USA) at 4h of age and then 2 ml every 12 h (100ml/kg/day) using a 3.5 French feeding tube. At 2 h postnatal age, the pups alternatively received an intraperitoneal infusion of 50% glycerol (6.5 gm/kg) solution or saline treatment. Glycerol induces intracranial hypotension leading to an increase in the transmural pressure across the vessel walls and thus, causing vessels to rupture. Head ultrasound was performed 4 hours later to assess for the presence and severity of IVH. The pups were euthanized at 6 and 24h age. Although pups were evaluated for IVH both on head ultrasound and after sacrifice, the final determination of the presence and severity of IVH was done on gross examination of the sectioned brain. Based on the presence of IVH, the pups were subdivided as either glycerol-treated IVH or glycerol-treated non IVH.
Rabbit Tissue Collection and Processing
Forebrains were sectioned into 2 mm slices on brain matrix starting from the cranial end. Sections of the second and third slice from the cranial end were used for immunohistochemistry. The second slice had structures including caudate nucleus, medial septal nucleus, GM, internal capsule and others, whereas the third slice had caudate nucleus, hippocampus, thalamus, globus pallidus, internal capsule and others. For immunohistochemisty, the brain slices were fixed in 4% paraformaldehyde in phosphate buffer saline (0.01M PBS; pH 7.4) for 24 h and were cryoprotected by immersing into 20% sucrose in PBS buffer for 18 h followed by 30% sucrose for the next 24 h. We froze tissues after embedding them into Optimum Cutting Temperature Compound (OCT, Sakura). Frozen coronal blocks were cut into 10µ sections using cryostat. For Western blot analysis, we used the second 2 mm slice from the cranial end to make homogenates in the sample buffer. For superoxide and hydrogen peroxide levels, the second 2 mm slice of the forebrain was taken; and a ~1 mm area around the ventricle (periventricular zone) as well as cortical plate was dissected with a surgical blade. The periventricular zone (PVZ) included germinal matrix, caudate nucleus, corona radiata and corpus callosum. The dissected tissue pieces were further chopped into 0.5–1 mm cubes.
Head Ultrasound and Grading of IVH
Head ultrasound was performed on all pups at about 6h postnatal age to determine the presence and severity of IVH using Acuson Sequoia C256, Siemens ultrasound machine.3 As reported before,14 we classified IVH into: a) mild, no gross hemorrhage and hemorrhage detected on microscopy of hematoxylin and eosin (H & E) stained brain sections; b) moderate, gross hemorrhage into lateral ventricles without significant ventricular enlargement (2 separate lateral ventricles discerned); and c) severe, IVH with significant ventricular enlargement (fusion of ventricles into a common chamber) and/or intraparenchymal hemorrhage. In the IVH group, only brains with moderate and severe IVH were included in the study.
Immunohistochemistry
Immunolabeling of coronal brain sections was performed as described to detect markers of oxidative-nitrosative stress in situ.14 4-Hydroxynonenal (trans-4-hydroxy-2-nonenal or 4-HNE) is the primary α, β-unsaturated hydroxyalkenal, which is produced by lipid peroxidation in cells. 8-hydroxydeoxyguanosine (8-OHdG), an oxidized nucleoside of DNA, is a sensitive marker of DNA damage caused by increased cellular production of ROS.15 Reactive nitrogen species such as peroxynitrite can nitrate specific amino acids, such as tyrosine, altering the protein function.16 3-nitrotyrosine is widely used as a sensitive marker of this reaction indicating in vivo nitrosative stress.17 The following primary antibodies were used: mouse monoclonal anti-4 -Hydroxy-2-nonenal (JalCA, Shizuoka, Japan; 1:20), goat polyclonal anti-8-Hydroxydeoxyguanosine (Chemicon, CA, USA; 1:200) and mouse monoclonal anti-3-nitrotyrosine (Invitrogen). Peroxynitrite (Chemicon) was used as positive control and degraded peroxynitrite (Chemicon) was used as negative control. The secondary antibodies (Jackson Immunoresearch) included Cy-3 conjugate of goat anti-mouse, and Cy3 conjugate of mouse anti-goat. After drying and several washes in PBS, the tissue sections were incubated with the primary antibody diluted in PBS at room temperature for one hour. After washing in PBS, the sections were incubated with the secondary antibody diluted in 1% normal goat serum in PBS at room temperature for 40 minutes. Finally, after washes in PBS, sections were mounted with Slow Fade Light Antifade reagent (Molecular Probes) and were visualized under fluorescent microscope (Axioscope 2 Plus, Carl Zeiss Inc. NY).
To evaluate neuronal degeneration in apocynin-treated and vehicle-treated IVH pups, we performed Fluoro-Jade B (Chemicon) staining on fixed brain sections according to manufacturer’s instruction. To detect apoptosis, Fluorescent in situ Detection of DNA Fragmentation (TUNEL) was performed on fixed brain sections as described before.3 The sections were air-dried on slides, hydrated in 0.01M PBS and permeabilized for 5 min in 1:1 ethanol:acetic acid (−20°C). An ApopTag-fluorescein in situ DNA fragmentation detection kit (Chemicon) was used to visualize TUNEL-labeled nuclei. Tissue sections were counterstained with propidium iodide to visualize all the nuclei. We next quantified Fluor-Jade B and TUNEL positive nuclei in apocynin- and vehicle-treated pups. From each brain, a set of 3 to 5 coronal sections were taken as every tenth section at each of 2 levels—medial septal nucleus and posterior ventrolateral thalamic nucleus. From every section, about 5 images were acquired from both periventricular zone (PVZ) and cortex using 40x objective. Thus we performed counting in 60 to 100 (5 images × 3 to 5 sections X× 2 regions × 2 coronal levels) images per brain.
Western blot analysis
Protein extraction and Western blotting were performed under reducing conditions as described before.14 Briefly, frozen brain tissue was homogenized in sample buffer and boiled for 5 minutes. The protein concentration was determined. Total protein was separated by SDS-PAGE. The separated proteins were transferred to polyvinylidene difluoride membrane by electro-transfer. The membranes were then incubated with primary antibodies, washed, incubated with secondary antibody and detected with chemiluminescence ECL system (Amersham).
Ethidium fluorescence
Dihydroethidine (DHE), an oxidative fluorescent dye, was used to measure superoxide levels in situ as we previously reported.18, 19 In brief, thin sections of the brain were incubated with hydroethidine (3×10−6 mol/L; at 37 °C for 30 min), then the samples were washed three times. Unstained sections and samples pre-incubated with PEG-SOD were used for background correction and negative control, respectively. For quantitative measurements the time course of the build-up of ethidium bromide fluorescence in the tissue samples was recorded for 30 min as described.18, 19 The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content/cell mass. The effects of diphenylene iodonium (DPI, 10−6 mol/L, an inhibitor of flavin containing oxidases, including NAD(P)H oxidase), apocynin (3×10−4 mol/L, an inhibitor of NAD(P)H oxidase activation), indomethacin (10−5 mol/L, a specific inhibitor of the cyclooxygenase), allopurinol (10−4 mol/L, a specific inhibitor of the xanthine oxidase) and L-Nω-Nitroarginine methyl ester (L-NAME, 3×10−4 mol/L, a specific inhibitor of nitric oxide synthase) on tissue O2·− production were also determined.
Lucigenin chemiluminescence, NADPH oxidase activity assay
Tissue O2·− levels ex vivo was also assessed by measuring SOD-inhibitable lucigenin (5 µmol/L) chemiluminescence (CL) as we reported before.18 To assess NADPH oxidase activity, samples from the periventricular region and cortex were homogenized and NADPH-driven lucigenin CL was measured as reported.18, 20
Measurement of oxidative stress by DCF and H2O2 production by Amplex Red fluorescence assays
The cell-permeant oxidative fluorescent indicator dye C-H2DCFDA (5 and 6-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate-acetyl ester, abbreviated as DCF throughout the text, Invitrogen, Carlsbad CA) was used to assess oxidative stress in the periventricular region and cortex, as we reported elsewhere.18, 20 Unstained sections and samples pre-incubated with PEG-catalase were used for background correction and negative control respectively. In separate experiments, the time course of the build-up of DCF fluorescence in the tissue samples was recorded for 30 min. The slope factor was calculated and normalized to Hoechst 33258 fluorescence representing DNA content/cell mass.18 In separate experiments, H2O2 production was measured fluorometrically in the periventricular region and the cortex using the Amplex Red/horseradish peroxidase assay according to our published protocols.18 H2O2 generation rate was compared by measuring the time course of the build-up of resorufin fluorescence for 60 minute in the presence and absence of apocynin, DPI, indomethacin, allopurinol or L-NAME.
Quantitative Real-Time Polymerase Chain Reaction (QRT-PCR)
QRT-PCR was used to assess changes in mRNA expression of NOX2 and NOX4 subunit of NAD(P)H oxidase in the periventricular region and cortex of IVH pups compared to saline controls. These subunits have been shown to be induced by intracerebral hemorrhage and hypoxia-ischemia in adult mouse model.21 Total RNA from the periventricular region of rabbit pups with and without IVH was isolated using the Mini RNA isolation kit (Zymo Research, Orange) and was reverse transcribed using Superscript II kit (Invitrogen) as described previously.14 Real-time PCR technique was used to analyze mRNA expression using the Strategen MX3000, as reported before.14 Quantification was performed using efficiency adjusted ΔΔCT method.22 The housekeeping gene GAPDH was used for internal normalization. For the ΔΔCT calculation to be valid, we calculated the efficiency of the target amplification and the efficiency of the reference amplification (the housekeeping gene GAPDH) by constructing a standard curve using dilution series of brain mRNA samples (Efficiency of primers=1.95–2.0). We next normalized target gene to reference gene to control for cDNA loaded into the reaction. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel.
Apocynin treatment
Prematurely delivered (E29) pups were sequentially treated with glycerol or saline at 2h postnatal age. We next alternately assigned the glycerol-treated pups to receive either IV apocynin or vehicle at 3h age. Apocynin (2.5mg/kg/dose) was administered intravenously in the jugular vein at 3h and 12h age using insulin syringe with a 30 gauge needle. Dose of apocynin was based on its previous use in rat experiments.23 At 24h age, the pups were euthanized and evaluated for the presence and severity of IVH. The randomization and stratification procedure insured that 2 groups of pups—apocynin and vehicle treated pups with IVH—were balanced with respect to severity (moderate or severe) of IVH. The three groups of pups including apocynin-treated and vehicle-treated pups with IVH as well as saline treated non IVH pups were compared for the evidence of cell death—apoptosis and neuronal degeneration.
Statistics and analysis
Data are expressed as mean±SEM. The parameters were compared between pups with and without IVH as well as a function of postnatal age. We used t-test (parametric variable) or Mann-Whitney U test (non-parametric variable) to perform pairwise comparisons. A probability value of <0.05 was considered significant.
RESULTS
Higher expression of Free radical adducts in pups with IVH than controls
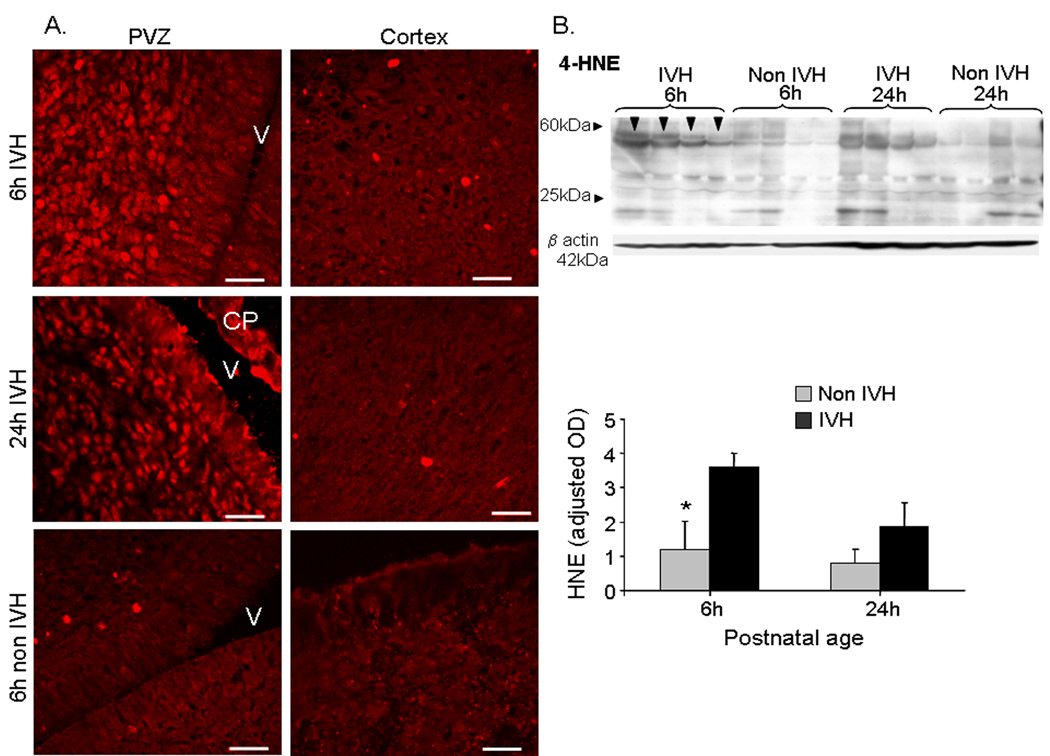
To determine whether differences exist in the presence and location of oxidative stress markers between the brain of premature pups with glycerol-induced IVH and saline treated controls (non-IVH), we evaluated free radical adducts, including 4-hyroxynonenal and 8-Hydroxy-deoxyguanosine (8-OHdG) by immunohistochemistry and Western blot analyses. The cellular content of 3-nitrotyrosine, a marker of nitrosative stress was also assessed. Immuno-labeling of coronal brain sections with 4-HNE specific antibody revealed that 4HNE was more abundant in neural cells of the periventricular zone of pups with IVH than in controls at both 6 (4h after induction of IVH) and 24h (22h after induction of IVH) postnatal age (Fig. 1A). The HNE expression was weaker in the cortex compared to periventricular zone in pups with IVH. Western blot analysis of brain homogenates of full thickness slice at the level of the midseptal nucleus confirmed that 4HNE (25–60kDa) levels were significantly higher in pups with IVH than saline-treated non-IVH controls at 6h (P<0.05), but not at 24h postnatal age (Fig. 1B).
Figure 1. 4HNE levels are greater in pups with IVH than non IVH controls.
A) Representative immunofluorescence of cryosections from brain of premature pups with IVH and saline-treated non-IVH controls labeled with 4HNE specific antibodies. The immunoreactivities were intense in the periventricular region (PVZ) in pups with IVH at both 6 and 24h age, but weak in the cortex. The immunosignals were more abundant at 6h than 24h postnatal age in the periventricular zone of pups with IVH. Scale bar 20µm. V, ventricle. CP, choroid plexus.
B) Representative Western blot analysis of 4HNE in pups with IVH and saline-treated non IVH controls at 6 and 24h. The molecular weight was ranging from 25–60 kDa. The bar graph shows mean ± s.e.m. (n = 6). The values were normalized to β-actin levels. 4HNE levels were higher in pups with IVH than saline-treated non IVH controls at 6h (arrowhead), but not at 24h age. 4HNE levels were 1.5 to 2 fold greater at 6h compared to 24h age in pups with IVH, but the difference was not statistically significant. * P<0.05 for the comparison between IVH pups and non IVH controls at 6h.
We next evaluated the expression of 8-OHdG in pups with IVH compared to saline-treated non-IVH controls. We used human skin as the positive control, while no primary antibody on the section was the negative control. We noted that 8-OHdG immunoreactivities were greater in both the periventricular zone and cortex of pups with IVH compared to controls at 6h and 24h postnatal age (Supplemental Fig 1 online). In addition, the expression of 8-OHdG was higher in periventricular zone than in the cortex of pups with IVH at both 6h and 24h postnatal age. We could not achieve satisfactory immunoblot with the commercially available 8-OHdG specific antibodies and thus, 8-OHdG was not quantified by Western blot analysis.
Immunolabeling of coronal brain sections with nitrotyrosine specific antibody revealed that this was intensely expressed in the neural cells around the ventricle (periventricular zone, PVZ) in pups with IVH at both 6 and 24h postnatal age, but not in pups without IVH (Fig 2A). The immunoreactivities to nitrotyrosine in the cerebral cortex were weaker compared to the periventricular zone in the pups with IVH. We next quantified nitro-tyrosine by Western blot analysis, which showed nitro-tyrosoine bands (20–75kDa) were denser and stronger in pups with IVH than saline-treated non IVH controls (Fig. 2B). The optical density measurement of bands confirmed that nitrotyrosine levels were significantly higher in pups with IVH than non IVH controls at 6h postnatal age (P<0.05), but not at 24h.
Figure 2. Nitrotyrosine levels higher in pups with IVH than without IVH.
A) Cryosections were labeled with nitrotyrosine specific antibody. Positive controls were made by adding peroxynitrite to the section and negative control by adding degraded peroxynitrite. Nitrotyrosine was strongly expressed in the PVZ of pups with IVH at both 6 and 24h age, but weakly expressed or absent in saline-treated controls. Scale bar 20µm. V, ventricle
B) Representative Western blot analysis of nitrotyrosine in pups with IVH and saline-treated controls at 6 h and 24h. The molecular weight was ranging from 20–75 kDa. The bar graph shows mean ± s.e.m. (n = 6). The values were normalized to β-actin levels. Nitrotyrosine levels were significantly higher in pups with IVH than saline-treated non IVH controls (arrowhead) at 6h, but not at 24h age. Nitrotyrosine levels were 1.5 to 2 fold greater at 6h compared to 24h age in pups with IVH, but the difference was not statistically significant. * <0.05 for the comparison between IVH pups and controls at 6h.
Taken together, there was ample evidence of oxidative and nitrosative stress in pups with IVH, in contrast to pups without IVH. In addition, generation of free radical was more around the ventricle than in the cortex of pups with IVH.
Enhanced levels of superoxide (O2·−) and hydrogen peroxide in pups with IVH
Since we observed a consistent elevation in the level of all the biomarkers of oxidative/nitrosative stress in the brains of pups with IVH at 6 h age (4h after induction of IVH), we evaluated O2·− and H2O2 levels at this time point. DHE fluorescence (Fig. 3A) and Lucigenin chemiluminescence (Fig. 3B) measurements demonstrated that O2·− levels were significantly higher in both the periventricular zone and cortex of the pups with IVH than in the respective brain regions of glycerol- and saline-treated non IVH controls. O2·− levels was higher in the PVZ of pups with IVH than cortex, and the difference was statistically significant for O2·− levels measured by dehydroethidium fluorescence (P = 0.03), but not for lucigenin chemiluminescence.
Figure 3. Increased oxidative stress in the brain of premature pups with IVH than saline- and glycerol-treated controls.
A & B) The bar graph shows mean ± s.e.m. O2·− levels measured by dehydroethidium fluorescence and lucigenin chemiluminescence at 6h age. O2 ·− level was significantly higher in both the periventricular zone and cortex of the pups with IVH compared to glycerol- and saline-treated non IVH controls. Among IVH pups, O2·− levels, measured by dehydroethedium fluorescence, was significantly higher in the periventricular zone than in the cortex (P = 0.03).
C) The bar graph shows mean ± s.e.m. Free radicals were assayed by DCF assay. Oxidative stress was significantly higher in PVZ of pups with IVH than glycerol- and saline-treated non IVH controls at 6h postnatal age. * P<0.05, **P<0.01, *** P<0.001 for the comparison between IVH pups vs. non IVH saline treated pups. #P<0.05, ##P<0.01, ###P<0.001 for the comparison between IVH pups vs. non IVH glycerol-treated pups.
Using the DCF fluorescence (Fig. 3C) and amplex red (Fig. 4A) assays we demonstrated that oxidative stress was significantly higher in the PVZ of pups with IVH than glycerol and saline-treated non IVH controls (P < 0.03). Together, IVH results in enhanced generation of O2·− and H202.
Figure 4. NADPH oxidase is the principal source of free radical generation.
A & B) We assayed H2O2 generation by Amplex Red and peroxide levels by DCF assay. Note greater oxidative stress levels in pups with IVH than glycerol- and saline-treated non IVH controls. Apocynin treatment significantly attenuated free radical generation, but not Allopurinol, indomethacin and L-NAME.
C) We assessed O2·− generation by dehydroethidium fluorescence. Note greater O2·− levels in pups with IVH than controls. Apocynin treatment significantly reduced O2·− concentration, but not allopurinol (10−4 mol/L), indomethacin (10−5 mol/L) and L-NAME (3×10−4mol/L).
* P<0.05, **P<0.01 and *** P<0.001 for the comparison between pups with IVH vs. saline and glycerol controls. ## P<0.01 and ### P<0.001 for the comparison between untreated and apocynin-treated IVH brains.
NADPH inhibition suppresses O2·− and H2O2 generation in IVH, but not COX2, XO or NOS suppression
As IVH elicited significant oxidative stress, we sought to determine the main source of cerebral ROS production in the model. Using amplex red, DCF and DHE fluorescence assays, we demonstrated that the NAD(P)H oxidase inhibitor, apocynin, significantly attenuated ROS production in the PVZ in the brains of pups with IVH compared untreated IVH pups (P<0.01, 0.001 and 0.001 respectively, Fig. 4). Similar results were obtained with DPI, which inhibits flavin containing oxidases, including the NAD(P)H oxidase (data not shown). In contrast, we found that allopurinol, indomethacin and L-NAME had no significant effect on cerebral ROS production in IVH. Hence, NAD(P)H oxidase is the primary source of free radical generation in our model of glycerol-induced IVH.
Evaluation of NAD(P)H oxidase activity and gene expression
To further characterize the role of NAD(P)H oxidases in cerebral ROS generation in IVH, we assessed tissue NADPH oxidase activity in vitro. We found that NADPH oxidase activity was significantly increased in both the PVZ and cortex of pups with IVH than glycerol- and saline-treated controls at 6h age, and that it remained substantially elevated at 24h postnatal age (Fig. 5A). Since NOX-2 (gp91phox) and NOX-4 containing NAD(P)H oxidase are abundant in the adult brain with well-recognized roles in free radical production in both surgically induced brain injury and intracerebral hemorrhage in rodent model,12 we evaluated the effect of IVH induction on the expression of NOX-2 and NOX-4. There was no significant difference in their gene expression between IVH pups and non IVH controls (Fig. 5B).
Fig 5. NADPH oxidase activity and NOX-2 &-4 subunit of NADPH oxidase.
A) NADPH oxidase was assayed in the periventricular zone and cortex of pups with IVH as well as in glycerol- and saline-treated non IVH controls. NADPH oxidase levels were significantly higher in both periventricular zone and cortex of pups with IVH at 6h and 24h age compared to glycerol- and saline-treated controls. * P<0.05, **P<0.01 for the comparison between pups with IVH and controls.
B) NOX-2 and NOX-4 gene expression was 1.5 to 2 fold greater in pups with IVH than saline controls at 6h age. However, the comparison was not statistically significant.
Apocynin treatment reduced cell death in pups with IVH
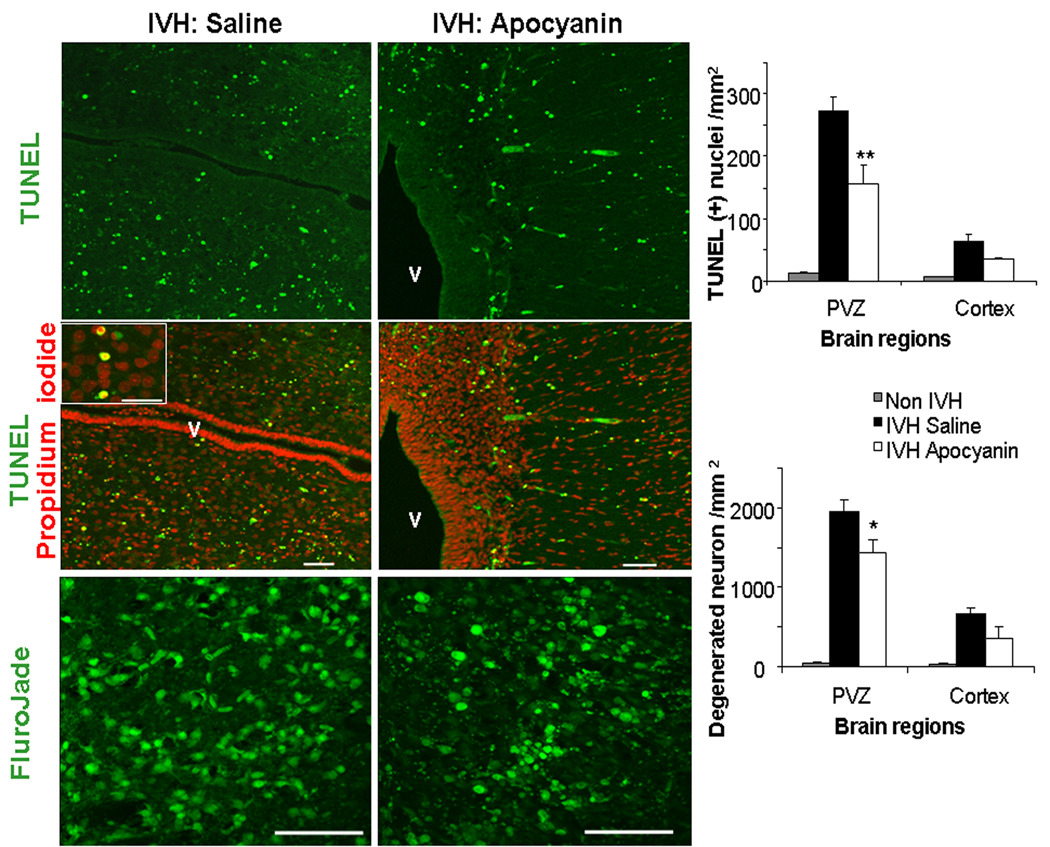
To determine whether apocynin offers neuroprotection, we compared apoptosis (TUNEL staining) and neuronal degeneration among three groups of pups at 24h postnatal age: 1) apocynin-treated pups with IVH, 2) vehicle-treated pups with IVH and 3) saline treated pups without IVH (Fig. 6). Apocynin- and vehicle-treated groups were balanced with respect to the severity of IVH. TUNEL staining showed lesser density of apoptotic cells in apocynin-treated pups with IVH compared to vehicle-treated pups with IVH in the PVZ (P = 0.012) but not in the cortex (P = 0.069 each). Likewise, density of Fluoro-Jade B (+) neurons were lesser in apocynin-treated pups with IVH than vehicle controls in the PVZ (P<0.02 each), but not in the cortex (P<0.09 each). In contrast to pups with IVH, TUNEL and Fluoro-Jade B (+) cells were scarce in saline treated pups without IVH.
Figure 6. Apocynin treatment diminishes cell death in pups with IVH.
A) Representative TUNEL staining of IVH pups with apocynin treatment and vehicle treatment. Sections counterstained with propidium iodide (PI). Note TUNEL (+) nuclei are relatively diminished in number in apocynin-treated pups with IVH compared to vehicle-treated pups. TUNEL (+) nuclei co-localize with propidium iodide positive nuclei. Inset shows high magnification of this co-localization. Scale bar, 50µm. B) Data are mean ± s.e.m. (n = 5 each). There was lesser density of apoptotic cells in apocynin-treated pups with IVH compared to vehicle-treated pups with IVH in the PVZ but not in the cortex. *indicate P=0.05 for the comparison between apocynin and vehicle treated IVH pups. C) Note Fluoro-Jade B (+) neurons are less abundant in apocynin-treated pups with IVH than vehicle controls in the PVZ. D) Data are mean ± s.e.m. (n = 5 each). There was lesser density of Fluoro-Jade B (+) neurons in apocynin-treated pups compared to vehicle treated controls in the PVZ, but not in the cortex. Scale bar, 50µm. *indicate P=0.05 for the comparison between apocynin and vehicle treated IVH pups.
DISCUSSION
GMH-IVH in premature infants is a major public health concern because of its high incidence and the attendant complications.1 Mechanism of brain injury in IVH has not been well elucidated, thus limiting development of therapeutic strategies to protect the brain in affected premature infants. Herein, we evaluated the generation of free radicals in our rabbit model of IVH as well as its mechanism of production. Four principal observations were made. First, induction of IVH in premature pups resulted in oxidative and nitrosative macromolecular damage, as indicated by the higher level of 4-HNE, 8-OHdG and nitrotyrosine in the brain of pups with IVH compared to controls. Second, O2·− and H2O2 levels in brain slices ex-vivo were significantly greater in both the PVZ and the cortical mantle in pups with IVH relative to pups without IVH. Third, apocynin, but not allopurinol, indomethacin or L-NAME, inhibited ROS generation in the brain of preterm pups, suggesting that NAD(P)H oxidase is the predominant source of free radical generation in IVH. Fourth, apocynin treatment reduced apoptosis and neuronal degeneration in pups with IVH. Together, these studies highlight the oxidative and nitrosative stress in premature rabbit pups with IVH, and suggest a mechanism-based strategy for the possibility of future therapeutic intervention to limit brain injury in premature infants with IVH.
The most important and novel observation made in the present study was that induction of IVH in premature pups resulted in significantly enhanced oxidative stress, suggesting key roles of ROS in acute brain injuries. Presently, the mechanisms by which ROS induces neural damage in premature newborns are not well understood. However, several studies in adult animal model of intracerebral hemorrhage have shown that ROS and RNS generation lead to damage in neurons and glia in the brain as well as altered blood brain barrier permeability by several mechanisms.12, 23, 24 ROS induces release of excitatory amino acid and formation of lipid perioxidation products that damages neurons, neurofilaments and glial cells.25 In addition, free radicals oxidize a number of neurotransmitters including norepinephrine, serotonin, glycine and dopamine.25 Reactive species also diminish the synthesis of endothelial tight junction proteins and activate matrix metalloproteinases in the brain.6 These events increase permeability of the blood brain barrier to neurotoxins and inflammatory cells causing accumulation of oxidized aggregated proteins.6,7 Since oligodendrocyte progenitors are more vulnerable to oxidative injury compared to mature oligodendrocytes,26 it is plausible that free radicals mediate white matter injury in premature infants with IVH. Further generation of free radical adducts—nitro-tyrosine and 4HNE–tended to be greater at 6h than 24h despite the fact that NADPH oxidase activity was higher in pups with IVH at both 6 and 24 h age. This apparent temporal decrease in free radical adducts can be attributed to induction of anti-oxidant response in the host and is consistent with the observations made in model of traumatic brain injuries.27 Collectively, ROS and RNS are likely to induce white matter and cortical injury in IVH, and their suppression might confer neuroprotection.
The second key observation made in the present study was that apocynin and DPI attenuated IVH-induced oxidative stress in the brain of preterm pups, suggesting that NAD(P)H oxidase is the predominant source of ROS generation in this model. This finding was further substantiated by the direct demonstration of elevated NADPH oxidase activity in the PVZ of the brains of pups with IVH (Fig. 5). The primary initiating mechanisms for NAD(P)H oxidase activation in IVH of premature infants, albeit unknown, might include reperfusion injury of ischemic regions around the area of hemorrhage and destruction,2 followed by an inflammatory response consisting of neutrophil and macrophage infiltration.3 In contrast, inhibition of cyclooxygenase, xanthine oxidase and nitric oxide synthases had no significant effect on ROS production, suggesting that these sources do not play a key role in IVH-induced oxidative stress in preterm pups. More importantly and consistent with our ex-vivo inhibition of oxidative stress with apocynin, we noted cytoprotection offered by apocynin treatment in pups with IVH compared to vehicle treated controls. The finding of apocynin treatment suppressing generation of ROS and providing neuroprotection in premature pups with IVH is consistent with a number of in vivo studies in adult rodent model of stroke, in which neuroprotective effect of aopcyanin has been demonstrated.28, 29, 30, 31 However, only 20–40% reduction in cell degeneration or death was observed in apocynin-treated IVH pups compared to vehicle-treated IVH pups. Hence, we speculate that postnatal apocynin treatment might offer some neuroprotection in premature infants following IVH.
Importantly, NAD(P)H oxidase activity was significantly elevated in the PVZ as well as in the cortex of premature pups with IVH compared to controls. However, gene expression of NOX-2 and NOX-4, homologs of NAD(P)H oxidase, was not significantly increased in the brain of pups with IVH compared to non IVH controls. A number of other investigators working on adult rodent model of hypoxia-ischemia and cerebral hemorrhage have also observed higher expression of NOX-2(gp91phox) isoform of NAD(P)H oxidase, compared to controls.32, 33,12 NOX enzymes are already known to contribute to pathological processes. Indeed, genetic deficiency of gp91phox subunit of NADPH oxidase reduces the neuropathological consequences of hemorrhage in a mouse model of collagen induced ICH.12 NOX-2 is expressed in neurons, astrocytes and microglia; 33, 34 and NOX-4 is expressed in endothelium and vascular smooth cells in addition to neurons.35, 36NOX-4 has roles in ischemia-induced neoangiogenesis and is upregulated in ischemia in peri-infarct region.36 Together, these findings support the notion that NOX-2 and NOX-4 subunits of NADPH oxidase serve an important function in free radical generation in premature rabbit pups with IVH and by extension, possibly, in human newborns.
Another key finding of the present study is demonstration of substantially elevated levels of 3-nitrotyrosine–a biomarker of increased peroxynitrite (ONOO−) formation–in the PVZ of premature pups with IVH. O2·− reacts with NO (likely derived from nNOS) to form ONOO−, which is a major cytotoxic mediator of neuronal injury during stroke.37 This finding is also important because peroxynitrite in known to activate the poly ADP-ribose polymerase (PARP-1) that suppress neuronal viability in a PARP-dependent fashion.38 PARP(s) plays key roles in post-ischemic, traumatic and other forms of brain injuries and are now considered interesting targets for therapies for neuroprotection.39 Thus, the present work sets stage for future studies on the use of peroxynitrite decomposition catalyst and/or with PARP inhibitors to prevent the neurological sequelae in preterm infants with IVH.
In conclusion, the present study revealed enhanced oxidative-nitrosative stress in the brain regions around the ventricle of prematurely delivered rabbit pups with IVH. In addition, NADPH oxidase was identified as the major source of free radical generation. Accordingly, apocynin treatment in rabbit pups with IVH significantly reduced apoptosis and neuronal degeneration compared to vehicle treated IVH pups. These data suggest that reducing oxidative-nitrosative stress by either suppression of NAD(P)H oxidase , facilitating decomposition of ONOO− or disruption of pathways activated by oxidative stress may remarkably diminish the occurrence and severity of neurological sequelae in premature rabbit pups and possibly, in humans.
Acknowledgement
Supported by New York Medical College Intramural grant (MTZ), grants from the NIH (NS050586 to PB and HL077256 and HL43023 to ZU), Pfizer Inc. and Maria Fareri Children’s hospital Foundation.
Footnotes
Supplemental Figure 1 online. Greater expression of 8-Hydroxy-deoxyguanosine (8-OHdG) in IVH than non IVH pups. Cryosections from the forebrain of rabbit pups with IVH and saline-treated (non IVH) controls were immunolabeled with 8-OHdG specific antibody. 8-OHdG was expressed more in periventricular region (PVZ) than in the cortex of pups with IVH at both 6 and 24h age. However, no such immunoreactivity was observed in non IVH controls. Scale bar 20µm. V, ventricle. CP, choroid plexus.
REFERENCES
- 1.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: Experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 3.Georgiadis PXH, Chua C, Hu F, Collins L, Huynh C, LaGamma EF, Ballabh P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008 doi: 10.1161/STROKEAHA.107.510883. In press. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002;953:45–52. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- 5.Wagner KR. Modeling intracerebral hemorrhage: Glutamate, nuclear factor-kappa b signaling and cytokines. Stroke. 2007;38:753–758. doi: 10.1161/01.STR.0000255033.02904.db. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 7.Frank L, Groseclose EE. Preparation for birth into an o2-rich environment: The antioxidant enzymes in the developing rabbit lung. Pediatr Res. 1984;18:240–244. doi: 10.1203/00006450-198403000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Frank L, Sosenko IR. Prenatal development of lung antioxidant enzymes in four species. J Pediatr. 1987;110:106–110. doi: 10.1016/s0022-3476(87)80300-1. [DOI] [PubMed] [Google Scholar]
- 9.Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 10.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil Granger D, Zhang JH. Role of nadph oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 13.Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. Nadph oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett. 2007;414:228–232. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- 15.Bogdanov M, Matson WR, Wang L, Matson T, Saunders-Pullman R, Bressman SS, Flint Beal M. Metabolomic profiling to develop blood biomarkers for parkinson's disease. Brain. 2008;131:389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- 16.Avila MA, Sell SL, Kadoi Y, Prough DS, Hellmich HL, Velasco M, Dewitt DS. L-arginine decreases fluid-percussion injury-induced neuronal nitrotyrosine immunoreactivity in rats. J Cereb Blood Flow Metab. 2008;28:1733–1741. doi: 10.1038/jcbfm.2008.66. [DOI] [PubMed] [Google Scholar]
- 17.Shigenaga MK, Lee HH, Blount BC, Christen S, Shigeno ET, Yip H, Ames BN. Inflammation and no(x)-induced nitration: Assay for 3-nitrotyrosine by hplc with electrochemical detection. Proc Natl Acad Sci U S A. 1997;94:3211–3216. doi: 10.1073/pnas.94.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and sirt1: Attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 20.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 21.Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time rt-pcr. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titova E, Ostrowski RP, Sowers LC, Zhang JH, Tang J. Effects of apocynin and ethanol on intracerebral haemorrhage-induced brain injury in rats. Clin Exp Pharmacol Physiol. 2007;34:845–850. doi: 10.1111/j.1440-1681.2007.04664.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T, Nagao S, Xi G, Keep RF, Itano T. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39:463–469. doi: 10.1161/STROKEAHA.107.486654. [DOI] [PubMed] [Google Scholar]
- 25.Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radic Biol Med. 2005;39:841–852. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL, Felderhoff-Mueser U, Volpe JJ, Rosenberg PA. Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci. 2008;28:1236–1245. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noh KM, Koh JY. Induction and activation by zinc of nadph oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao HM, Liu B, Hong JS. Critical role for microglial nadph oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 31.Zheng JS, Zhan RY, Zheng SS, Zhou YQ, Tong Y, Wan S. Inhibition of nadph oxidase attenuates vasospasm after experimental subarachnoid hemorrhage in rats. Stroke. 2005;36:1059–1064. doi: 10.1161/01.STR.0000163102.49888.b7. [DOI] [PubMed] [Google Scholar]
- 32.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. Nadph oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 33.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional nadph oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004;1742:81–87. doi: 10.1016/j.bbamcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Griendling KK, Sorescu D, Ushio-Fukai M. Nad(p)h oxidase: Role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 36.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the nadph oxidase nox4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 37.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Endres M, Scott G, Namura S, Salzman AL, Huang PL, Moskowitz MA, Szabo C. Role of peroxynitrite and neuronal nitric oxide synthase in the activation of poly(adp-ribose) synthetase in a murine model of cerebral ischemia-reperfusion. Neurosci Lett. 1998;248:41–44. doi: 10.1016/s0304-3940(98)00224-9. [DOI] [PubMed] [Google Scholar]
- 39.Jagtap P, Szabo C. Poly(adp-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]