Abstract
Sensorimotor smoking stimuli are important determinants of cigarette use. The present study aimed to determine whether denicotinized cigarettes lose their reinforcing and/or subjective effects over a 9-day outpatient period when they are smoked with or without concurrent transdermal nicotine. After a preferred brand baseline, 68 participants were randomized into one of four conditions based on the dose (mg) of transdermal nicotine and the type of cigarettes (dose/cigarette): 0/nicotine, 0/denicotinized, 7/denicotinized, and 21/denicotinized. Under placebo patch conditions, participants smoked a similar number of nicotine and denicotinized cigarettes and no group differences emerged over repeated testing. The total volume of smoke inhaled was lower in the denicotinized group, although this decrease dissipated over time. Denicotinized cigarettes were rated as having low positive and high negative subjective effects. Compared to placebo, transdermal nicotine decreased the number of denicotinized cigarette smoked, produced a lasting decrease in the total volume of denicotinized cigarette smoke inhaled, but had little effect on the subjective effects of denicotinized cigarettes. Transdermal nicotine attenuated withdrawal during initial smoking abstinence; however, once participants were allowed to smoke withdrawal symptoms were relatively low regardless of patch condition. The persistent use of denicotinized cigarettes may result from the presence of nicotine withdrawal and/or the degree to which smoking becomes somewhat independent of the outcome of the behavior (i.e., habit learning). Additional studies would be useful to determine what factors drive continued use of denicotinized cigarettes, whether their use subsides as withdrawal dissipates, and whether they address motives for smoking distinct from current pharmacotherapy.
Keywords: conditioning, nicotine replacement therapy, treatment, addiction, dependence, smoking
1. Introduction
Nicotine functions as a primary reinforcer in both laboratory animals and humans (Donny et al., 2003; Goldberg et al., 1981; Henningfield and Goldberg, 1983; Perkins et al., 1997; Rose and Corrigall, 1997). As a consequence of classical conditioning, stimuli associated with nicotine develop into conditioned reinforcers (Palmatier et al., 2007) and become an important determinant of cigarette smoking. Disrupting the sensory properties of smoking reduces the rewarding effects of cigarettes (Perkins et al., 2001; Rose et al., 1985); conversely, mimicking the sensory properties of smoking reduces craving and withdrawal (Rose and Behm, 1994). Therefore, cigarette smoke serves, not simply as a nicotine-delivery device, but also as a stimulus that signals nicotine delivery and, consequently, reinforces smoking behavior.
Denicotinized cigarettes are a particularly potent tool for assessing the role of non-nicotine smoking stimuli. Denicotinized cigarettes, which generally contain greatly reduced amounts of nicotine (e.g., <0.1 mg FTC yield), mimic many of the behavioral and sensory aspects of regular smoking, but fail to produce robust nicotinic effects such as an increase in heart rate (Pickworth et al., 1999) and result in less than one-third the binding of the α4β2 nicotinic receptor compared to regular cigarettes (Brody et al., 2008a; Brody et al., 2006). Nevertheless, denicotinized cigarettes have been shown to reinforce behavior in well-controlled laboratory studies (Shahan et al., 2001; Shahan et al., 1999), maintaining similar rates of self-administration as nicotine-containing cigarettes despite the fact that participants prefer nicotine-containing cigarettes when given a choice (Shahan et al., 1999). Our own research has confirmed these reinforcing effects. When only denicotinized cigarettes are available in an inpatient setting, participants will continue to smoke these cigarettes over an 11-day period with only small decreases in the number of cigarettes smoked during periods of unrestricted smoking and in the motivation to smoke during periods of abstinence (Donny et al., 2007).
Recognition of the importance of non-nicotine smoking stimuli has led to the suggestion that denicotinized cigarettes may be useful as an adjunct to nicotine replacement therapy (Rose, 2007). This suggestion relies, in part, on the prediction that repeated administration of denicotinized cigarettes would extinguish the association between smoking stimuli and nicotine and, consequently, reduce the degree to which these stimuli can elicit and support subsequent smoking behavior. In our inpatient research we observed evidence consistent with partial extinction over the 11-day period. Likewise, Rose and colleagues have shown that after smoking denicotinized cigarettes for 2 weeks, participants report that their usual brand cigarettes are less rewarding when they are smoked in a brief laboratory assessments (Rose and Behm, 2004). Achieving extinction in the real world, however, may prove more difficult given the multitude of contexts associated with smoking and the strength of the so-called “renewal effect” which suggests that extinction is context-specific (Bouton, 2004). It is important to note that if extinction occurs it would likely limit exposure to the harmful consequences of smoking denicotinized cigarettes (which still delivery tar, irritants and carbon monoxide) because their reinforcing effects would decrease gradually, limiting their long term use.
Despite the possible utility of denicotinized cigarettes as an adjunct therapy, almost all of the available research on denicotinized cigarettes has been conducted in participants who are not receiving any form of pharmacotherapy. For several reasons, the effects of denicotinized cigarettes may change as a consequence of concurrent exposure to nicotine replacement. First, concurrent use of nicotine replacement therapy might facilitate the extinction process by continuing to present nicotine in a manner that is unassociated with smoking stimuli. Second, denicotinized cigarettes may not be as effective at suppressing craving and withdrawal in individuals receiving nicotine replacement therapy. To the degree to which their reinforcing effects depend on craving/withdrawal-suppression, this effect must be apparent over and above that provided by nicotine replacement therapy. Research suggests that nicotine replacement therapy has little effect on cue-provoked craving and that other interventions that address this type of craving may be beneficial in preventing relapse (Waters et al., 2004). Third, recent research has also suggested that nicotine may directly (i.e., non-associatively) increase the value of other reinforcers in the environment, including nicotine-associated conditioned reinforcers (Donny et al., 2003; Olausson et al., 2003, 2004; Palmatier et al., 2006; Palmatier et al., 2007). For example, animals given a slow, continuous infusion of nicotine respond with markedly greater vigor for modestly reinforcing visual stimulus (Donny et al., 2003). These preclinical data suggest that transdermal nicotine might actually enhance the reinforcing properties of denicotinized cigarettes.
The present study sought to address several questions raised by the research summarized above: 1) How do the subjective and reinforcing effects of denicotinized cigarettes change over repeated exposure in an outpatient setting?; 2) Does transdermal nicotine accelerate the process of extinction?; 3) Does transdermal nicotine influence the magnitude of the reinforcing effects of denicotinized cigarettes?
2. Methods
2.1. Participants
Sixty eight adult volunteers were recruited from the community, completed the study, and are described here. Four additional participants completed the study, but were dropped from the analyses because they were unblinded (i.e., were given cigarettes in which the “Quest” label had not been blacked out; n=2), became ill during the study (n=1), or failed to follow instructions and acted in a threatening manner (n=1). Seventeen non-completing participants withdrew either prior to (n=5) or after (n=12) randomization. The drop out rate among those randomized did not vary significantly by group (χ2=4.0, p=.257)
The following inclusion criteria were employed: 18–65 years of age, self-reported smoking of 10 to 30 cigarettes per day (CPD) for the last year, inhaling while smoking, carbon monoxide (CO) levels greater than 10 ppm, urinary cotinine levels greater than 100 ng/ml and no intention to quit in the next three months. Exclusion criteria included significant medical or psychiatric illness in the past year, alcohol or drug dependence (excluding nicotine and caffeine), pregnancy/lactation, experience smoking Quest brand cigarettes within the past year, use of nicotine replacement therapy within past 3 months, prior adverse reactions to nicotine replacement therapy, and use of any psychotropic medications.
The final sample was 59% female and predominantly Caucasian (72%). Most participants (84%) completed high school or obtained a general equivalency diploma. Participants, on average, were 33.5 years of age (SD: 12.3), smoked 18.8 (SD: 6.4) CPD and scored 5 (SD: 2.1) on the Fagerstrom Test for Nicotine Dependence (FTND) (Fagerstrom, 1078; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). About half (52%) reported that their preferred brand was mentholated.
2.2. Design, Randomization Conditions and Study Overview
This study utilized a multi-dose, placebo-controlled and double-blind experimental design. Volunteers first participated in a telephone and in-person screen during which they provided informed consent. If qualified and interested, participants returned to the research laboratory at the University of Pittsburgh for 12 consecutive days. Participants were compensated for their time and inconvenience.
Table 1 provides a timeline of the experiment. Participants attended a total of 13 laboratory sessions. Days 1 and 2 served as training and baseline assessment days, respectively; on these days, participants were allowed to smoke their preferred brand of cigarettes. Participants were randomly assigned to one of four conditions at the start of the extended laboratory session on Day 3 of the study (described below). After randomization, all participants were instructed to smoke only the research cigarettes provided to them for the remainder of the study. One group was allowed to smoke nicotine-containing cigarettes while wearing a placebo transdermal patch (0/NC; n=17). The remaining three groups were allowed to smoke denicotinized cigarettes while wearing placebo (0/DN; n=14), 7 mg (7/DN; n=18) or 21 mg (21/DN; n=19) nicotine patches. Both participants and research assistants were blind to the nicotine content of the cigarettes and the patches. On Days 3 and 11, CO, the subjective effect of cigarettes, cigarette self-administration and puff topography were assessed (Controlled Puffing and Self-administration assessments described below) in participants who were otherwise required to be abstinent from smoking from midnight that morning until a brief abstinence verification session the following morning. On Days 4–10, data on smoking in the natural environment, CO, withdrawal, mood, puff topography and the subjective effects of cigarettes were assessed during brief laboratory visits. The study ended after the final abstinence verification session and debriefing on Day 12.
Table 1.
Study Overview
| Day | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Conditions | ||||||||||||
| Unrestricted smoking of preferred brand | X | X | ||||||||||
| Naturalistic smoking of research cigarettes | X | X | X | X | X | X | X | |||||
| Restricted smoking of research cigarettes | X | X | ||||||||||
| Patch administration | X | X | X | X | X | X | X | X | X | |||
| Sessions | ||||||||||||
| Orientation and Training Session | X | |||||||||||
| Daily Smoking Assessment | X | X | X | X | X | X | X | X | ||||
| Smoking in the natural environment | X | X | X | X | X | X | X | X | ||||
| CO | X | X | X | X | X | X | X | X | ||||
| Withdrawal and mood | X | X | X | X | X | X | X | X | ||||
| Puff topography | X | X | X | X | X | X | X | X | ||||
| Cigarette subjective effects | X | X | X | X | X | X | X | X | ||||
| Extended Laboratory Sessions | X | X | ||||||||||
| CO | X | X | ||||||||||
| Puff topography | X | X | ||||||||||
| Cigarette subjective effects | X | X | ||||||||||
| Self-administration | X | X | ||||||||||
| Brief abstinence Verification Sessions | X | X | ||||||||||
| CO & urine sample | X | X | ||||||||||
The time line of major experimental conditions is displayed at the top of the table. The time line for experimental sessions is presented in the bottom section of the table. Over the course of 12 days, participants attended 1 orientation and training session, 8 daily afternoon smoking assessment sessions, 2 extended laboratory sessions, and 2 morning sessions verifying overnight abstinence. Italics indicate the major constructs/measures assessed during each session.
2.3. Procedures
All participants started the study on a Monday (Day 1). On Day 1, participants were trained on the topography equipment, CO monitor, electronic cigarettes dispensers, and questionnaires. Nicotine dependence was also assessed.
Day 2 was designed to assess the behavioral and subjective effects of smoking of the participants preferred brand of cigarettes in the natural environment. Participants were instructed to smoke as few or as many cigarettes as they desired throughout the day. During a brief afternoon laboratory visit smoking data were collected and verified, CO and saliva samples were collected (saliva was not analyzed but instead served as a bogus pipeline to encourage honest self-report of nicotine use), and questionnaires were administered to determine withdrawal symptoms, and mood. While in the laboratory, participants smoked a single cigarette through a handheld topography device to assess puff topography and answered questions about the effects of the cigarette smoked.
Day 3 was designed to assess the subjective and reinforcing effects of smoking the participant’s randomly assigned cigarette during a period of abstinence. Participants were instructed to refrain from smoking from midnight prior to the session. Upon arrival, the remainder of the smoking data from Day 2 were collected, abstinence since midnight was verified and the assigned transdermal patch was applied. Three hours later participants smoked a single study cigarette through a desktop topography device that allowed the experimenter to control puff volume, puff duration, number of puffs, and inter-puff interval (Controlled Puffing; see below). The subjective effects of the cigarettes were then determined. Thirty minutes after completing the Controlled Puffing procedure, participants were allowed to freely smoke their assigned cigarettes during a brief self-administration period (1 h; see below). After completing the self-administration period, participants were instructed not to smoke before returning to the laboratory the following day. A brief session was conducted the following morning (i.e., Day 4; average time: 8:44 AM) to verify abstinence. This abstinence period was intended to increase the sensitivity of the self-administration test by restricting access.
Days 4–10 were designed to assess the behavioral and subjective effects of smoking the participant’s randomly assigned cigarette in the natural environment. The procedures were otherwise similar to Day 2 as described above. Only saliva samples taken on Day 10 were analyzed for cotinine and reported here. It is important to note that Day 4 was a somewhat unique day compared to Days 5–10. First, as indicated in the preceding paragraph, participants were required to be abstinent from Day 3 until an additional early morning session on Day 4, while on all other days participants attended only one session and could smoke freely throughout the day. In addition, on Day 4 the withdrawal and mood assessments, which asked the participant to reflect on the past 24 h, are largely indicative of the period of the abstinence required on Day 3, and therefore were analyzed separately from the remaining days in which participants were smoking freely for the entire 24 h period.
Day 11 was identical to Day 3 and designed to determine whether the subjective and reinforcing effects of smoking during a period of abstinence changed as a function of the intervening days of treatment with transdermal nicotine and/or exposure to the study cigarettes.
2.3.1. Controlled puffing
The controlled puffing procedure is a precise way of controlling cigarette self-administration (Fant, Henningfield, Shiffman, Strahs, & Reitberg, 2000) and evaluating the effects of smoking after a fixed dose of tobacco smoke. Participants inhaled eight 40 cc puffs at an interval of 30 seconds, holding each puff for 2 sec. Puffing was controlled by using visual and auditory feedback from the desktop equipment (CReSS, Plowshare Technologies).
2.3.2. Self-administration
The self-administration procedure provided an assessment of smoking reinforcement after a fixed period of transdermal nicotine dosing. During a 1-hr self-administration period on Days 3 and 11 participants were allowed to freely smoke their assigned study cigarettes within a comfortable, laboratory setting. Handheld smoking topography equipment was used to quantify smoking behavior. Participants were instructed that this 1-hr period constituted their last opportunity to smoke until returning to the laboratory the following day.
2.4. Measures
2.4.1. Smoking in the natural environment
Participants used electronic cigarette dispensers (SmokeSignals) to aid in tracking the number of cigarettes smoked on Days 2 and 4–10. Participants also were required to return the unsmoked portion of the cigarettes smoked at each laboratory visit in order to verify that they were smoked and to provide an additional objective measure of smoking (i.e., butt weight). At each visit, the research assistant downloaded the electronic cigarette dispenser data, went through the time-stamped entries with the participant, and compared the total number of cigarettes smoked with both the cigarette butts returned and the number of cigarettes used since the last visit. Discrepancies were discussed with the participant and resolved to the best judgment of the research assistant (e.g., inadvertent dispenser openings were discarded). This corrected value was used as the final naturalistic smoking data. Smoking behavior was compiled into two distinct variables: the total number of cigarettes smoked per day and the pattern of daily smoking (i.e. cigarettes smoked per 2-hour period).
2.4.2. Puff topography
On Days 2 and 4–10, puff topography was assessed using a handheld topography device (CReSSMicro Plowshare Technologies, Baltimore, MD, USA) that measures puff number, puff volume, inter-puff-interval, and time- and date-stamps when each puff/cigarette was smoked. Participants were required to light the cigarette and take at least 1 puff, but otherwise had complete control over their smoking.
2.4.3. Biochemical
Expired air CO was measured at least 2 min after smoking through the portable smoking device (Micro III Smokerlyzer, Bedfont Instruments Ltd., Kent, England). Saliva samples taken on Day 10 were immediately frozen at −80° C and subsequently analyzed for cotinine (Salimetrics, Inc.).
2.4.4. Cigarettes effects
The somatic, sensory, and psychological effects of smoking were assessed using a modified version of the Smoking Effects Questionnaire (SEQ) (Westman et al., 1996). This questionnaire consists of visual analog ratings of Satisfying, Pleasant, Unpleasant, Like taste, Dislike taste, Smoke vs. air (anchored with “mostly smoke” to “mostly air”), Harsh, Strength, High in nicotine, Like drug effect, Dislike drug effect, Like cigarette, Dislike cigarette, Calming, Relaxing, Comforting, Less irritable, Sense of well-being, More awake, Easier to concentrate, Exhilarating, Pleasurable excitement, Dizziness, Lightheaded, Nauseating and Nervous.
2.4.5. Withdrawal
Withdrawal over the past 24 h was assessed with 100 pt visual analog ratings of DSM-IV symptoms of withdrawal based on the Minnesota Nicotine Withdrawal Scale (MNWS; (Hughes and Hatsukami, 1986). Craving was assessed using the Questionnaire for Smoking Urges (Tiffany and Drobes, 1991); however, these data have been omitted because instructions failed to specify whether the questions referred to the participant’s own brand or the assigned cigarette, leading to an inability to interpret the data with confidence.
2.4.6. Mood questionnaires
Mood over the past 24 h was assessed using the Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988) and the Profile of Mood States (POMS) (McNair DM, 1971).
2.4.7. End of study questionnaire
During the last session (Day 12) all participants were asked to estimate how much nicotine was in their study cigarettes and how much nicotine was in their assigned patches. The response options for the cigarettes were 1) nicotine-free: 0 mg; 2) ultra lights: 0.4 mg (e.g. True, Merit Ultra Lights); 3) lights −.08 mg (e.g. Marlboro Light, Vantage); 4) full flavor −1.1 mg (e.g. Marlboro, Camel); and 5) very strong −1.7 mg (e.g. Pall Mall). The response option for the nicotine content of the patches was 1) none; 2) a little; 3) a moderate amount; and 4) a lot.
2.5. Drug/device information
Transdermal nicotine patches that provide 24-h nicotine delivery (Nicoderm CQ) were administered on days 3–11. On days 3, 4 and 11, patches were applied by the research assistant during a morning session. On days 5–10, patches were applied outside the laboratory by the participant at 7 am; patch application was confirmed by a phone call. All placebo patches were labeled as “21 mg nicotine” to match the active 21 mg nicotine patch condition. Staff and participants were unaware that the placebo was matched only to the 21 mg condition (i.e., they were not informed that the 7 mg patch had no placebo matched condition). However, direct comparisons of the placebo and 7 mg patch conditions made below are limited by differences in the transdermal nicotine patch labeling.
Quest brand cigarettes (Vector Tobacco Inc., Research Park Triangle, NC, USA) were used for the research cigarettes. Quest 1 (0.6 mg nicotine; 10 mg tar) was used as the nicotine-containing cigarettes and Quest 3 was used as the denicotinized cigarettes (0.05 mg nicotine; 10 mg tar). Quest branding was hidden from the participants. An ample supply of cigarettes was provided to participants at no cost (including preferred brand cigarettes on days 1 and 2).
2.6. Data analysis
Data obtained on days 4–10 were adjusted for baseline preferred brand smoking (i.e., day 2) by using the difference score. This also provides a reference for evaluating the effects of denicotinized cigarettes with and without transdermal nicotine relative to preferred brand smoking. In the few cases when trends or statistically significant differences between groups were observed at baseline, raw data were also analyzed to provide a more complete picture of the results. Table 2 presents baseline data by group for each measure reported in Table 3 (i.e., trend and significant results post-randomization). Data collected on days 3 and 11 were analyzed as the raw data as no comparable baseline data were available.
Table 2.
Demographics and Baseline Assessments
| 0/DN | 7/DN | 21/DN | 0/NC | P | ||||
|---|---|---|---|---|---|---|---|---|
| Sample Size | 14 | 18 | 19 | 17 | ||||
| Screening Data | ||||||||
| Age | 32.4 (3.1) | 33.7 (2.9) | 33.6 (3.0) | 34.5 (3.2) | .98 | |||
| % Female | 64% | 56% | 53% | 65% | .85 | |||
| Education (yrs) | 13.0 (0.7) | 12.9 (0.5) | 13.2 (0.6) | 13.8 (0.6) | .76 | |||
| % Caucasian | 64% | 72% | 74% | 77% | .90 | |||
| CPD (self-report) | 17.4 (1.5) | 18.9 (1.4) | 19.2 (1.8) | 19.5 (1.4) | .83 | |||
| % Menthol preferred brand | 64% | 44% | 58% | 41% | .52 | |||
| FTND | 4.7 (0.7) | 5.1 (0.4) | 5.1 (0.5) | 5.1 (0.5) | .95 | |||
| Day 2 Baseline Data | ||||||||
| Smoking behavior | ||||||||
| CPD | 16.9 (1.8) | 17.8 (1.4) | 18.8 (1.8) | 18.5 (1.3) | .86 | |||
| Puff volume (per puff) | 44.3 (3.6) | 51.3 (2.7) | 47.3 (2.6) | 43.3 (2.5) | .19 | |||
| Puff volume (total) | 539.1 (45.4) | 704.4 (62.2) | + | 681.5 (74.2) | 548.4 (27.9) | .10 | ||
| Peak flow | 39.1 (2.7) | 44.1 (3.0) | 46.8 (4.1) | 38.0 (2.4) | .17 | |||
| Average flow | 29.0 (1.8) | 30.9 (1.7) | 32.8 (2.5) | 28.2 (1.4) | .35 | |||
| Puff count | 12.4 (0.7) | 13.8 (1.0) | 14.6 (1.4) | 13.2 (0.9) | .52 | |||
| CO (pre-smoking) | 18.9 (2.6) | 15.1 (1.2) | 16.7 (2.1 | 18.8 (1.9) | .47 | |||
| CO (boost) | 3.0 (0.8) | 3.3 (0.7) | 3.8 (1.0) | 4.1 (1.3) | .87 | |||
| MNWS | ||||||||
| Irritability | 14.4 (4.4) | 26.1 (6.8) | 23.9 (7.0) | 33.0 (6.5) | * | .30 | ||
| Difficulty concentrating | 14.1 (4.3) | 26.8 (6.8) | 14.4 (3.5) | 27.8 (6.5) | .14 | |||
| Insomnia | 11.7 (7.4) | 32.6 (8.7) | + | 16.8 (5.6) | 22.1 (7.8) | .25 | ||
| Total withdrawal | 103.2 (22.7) | 173.6 (31.7) | + | 131.4 (24.0) | 183.8 (31.0) | + | .19 | |
| POMS | ||||||||
| Vigor | 15.6 (2.4) | 15.8 (1.6) | 18.0 (1.6) | 12.9 (1.4) | .22 | |||
| PANAS | ||||||||
| Total positive | 21.1 (2.7) | 21.5 (1.7) | 24.0 (1.9) | 19.7 (1.5) | .44 | |||
| SEQ | ||||||||
| Pleasant | 61.1 (5.8) | 68.7 (5.3) | 61.1 (5.6) | 55.2 (5.9) | .40 | |||
| Unpleasant | 18.0 (6.2) | 16.7 (4.5) | 24.1 (5.3) | 28.7 (6.4) | .40 | |||
| Like taste | 64.3 (6.6) | 67.8 (5.6) | 63.4 (3.8) | 55.8 (6.2) | .47 | |||
| Dislike taste | 19.8 (6.7) | 20.4 (5.2) | 25.3 (5.7) | 28.4 (6.3) | .71 | |||
| Harsh | 23.2 (4.6) | 23.7 (6.1) | 27.2 (5.7) | 30.0 (5.8) | .82 | |||
| Sense of wellbeing | 52.4 (7.8) | 51.3 (6.0) | 52.2 (5.3) | 45.8 (6.8) | .87 | |||
| Exhilarating | 34.4 (6.8) | 50.1 (6.3) | 50.8 (6.1) | + | 34.1 (6.6) | .11 | ||
| Pleasurable excitement | 40.8 (7.4) | 58.8 (5.7) | + | 48.9 (6.6) | 38.4 (5.8) | .11 | ||
| Dizziness | 15.6 (5.1) | 11.2 (4.9) | 20.4 (5.2) | 13.2 (5.0) | .58 | |||
| Lightheadedness | 15.4 (4.8) | 11.9 (4.2) | 17.1 (4.7) | 13.9 (5.4) | .88 | |||
| Enjoyable | 65.1 (5.7) | 72.1 (4.6) | 65.3 (5.3) | 57.7 (6.6) | .34 | |||
| Nervous | 7.0 (2.3) | 8.2 (2.4) | 11.9 (3.4) | 19.5 (5.6) | + | .10 | ||
| Calming | 56.0 (6.8) | 68.2 (6.5) | 56.8 (5.6) | 51.4 (6.4) | .27 | |||
| Less irritable | 38.4 (6.8) | 40.4 (6.7) | 35.2 (6.7) | 48.7 (7.9) | .57 | |||
| Like cigarette | 69.6 (5.5) | 69.9 (6.1) | 73.8 (4.7) | 64.7 (6.2) | .71 | |||
| Dislike cigarette | 15.6 (5.4) | 18.4 (6.2) | 17.3 (4.4) | 25.8 (6.7) | .62 | |||
| Relaxing | 62.7 (6.2) | 69.0 (5.4) | 60.1 (5.6) | 58.3 (6.4) | .57 | |||
| Comforting | 59.4 (7.1) | 65.8 (5.2) | 54.5 (6.3) | 53.3 (7.2) | .49 | |||
| Strength | 43.0 (8.8) | 52.0 (6.6) | 52.9 (5.5) | 45.4 (7.6) | .71 | |||
| Like drug effect | 46.6 (8.2) | 46.1 (7.6) | 44.6 (6.7) | 38.7 (6.6) | .86 | |||
| Dislike drug effect | 36.6 (8.1) | 19.3 (5.4) | + | 31.5 (6.2) | 46.9 (7.7) | .04 | ||
| Satisfying | 65.9 (6.6) | 71.0 (5.6) | 63.4 (5.3) | 57.3 (6.2) | .42 |
Age, Education, Race, self-reported CPD, menthol preferred brand and FTND data were collected during the in-person screening. All other data were collected during unrestricted smoking of preferred brand cigarettes on Day 2, prior to randomization. Data presented as mean (SEM) or percent as appropriate. The far right column indicates statistical significance for the overall group effect. Pairwise comparisons between groups were conducted using independent sample t tests with the following symbols representing the results: = trend for differences from 0/DN condition (p<.10)
significantly different from 0/DN condition (p<.05).
Table 3.
Statistical Summary
| 0/DN vs. 0/NC | 0/DN vs. 7/DN | 0/DN vs. 21/DN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | GxD | Group | GxD | Group | GxD | |||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Naturalistic Smoking Days | ||||||||||||
| Smoking behavior (Days 4–10) | ||||||||||||
| CPD | 6.09 | * | 5.69 | * | ||||||||
| Puff volume (per puff) | 3.44 | + | 4.94 | * | ||||||||
| Puff volume (total) | 4.40 | * | 6.09 | * | 4.26 | * | 4.77 | * | 9.88 | ** | ||
| Peak flow | 7.98 | ** | 2.95 | + | 2.98 | + | 6.78 | ** | ||||
| Average flow | 10.80 | ** | 3.74 | + | 6.65 | * | ||||||
| Puff count | 3.95 | + | 4.55 | * | 4.67 | * | ||||||
| CO (boost) | 7.74 | ** | ||||||||||
| CO (post-smoking) | 6.21 | * | ||||||||||
| MNWS (Days 5–10) | ||||||||||||
| Irritability | 5.49 | * | 4.48 | * | 3.74 | + | 3.10 | + | ||||
| Difficulty concentrating | 5.74 | * | ||||||||||
| Insomnia | 5.63 | * | 4.56 | * | ||||||||
| Total withdrawal | 7.44 | * | ||||||||||
| POMS (Days 5–10) | ||||||||||||
| Vigor | 3.66 | + | ||||||||||
| PANAS (Days 5–10) | ||||||||||||
| Total positive | 4.68 | * | ||||||||||
| SEQ (Days 4–10) | ||||||||||||
| Pleasant | 9.39 | ** | 9.51 | ** | ||||||||
| Unpleasant | 13.70 | *** | 5.12 | * | ||||||||
| Like taste | 12.55 | ** | 9.80 | ** | ||||||||
| Dislike taste | 13.57 | *** | 7.70 | ** | ||||||||
| Harsh | 2.79 | + | ||||||||||
| Sense of wellbeing | 7.43 | * | 5.13 | * | ||||||||
| Exhilarating | 3.80 | + | 4.41 | * | ||||||||
| Pleasurable excitement | 6.29 | * | 6.51 | * | ||||||||
| Dizziness | 5.63 | * | ||||||||||
| Lightheadedness | 5.67 | * | ||||||||||
| Enjoyable | 10.36 | ** | 3.13 | + | ||||||||
| Nervous | 3.79 | + | ||||||||||
| Calming | 4.01 | + | 4.22 | * | ||||||||
| Less irritable | 7.03 | ** | ||||||||||
| Like cigarette | 6.56 | * | 4.57 | * | ||||||||
| Dislike cigarette | 12.37 | ** | 5.38 | * | ||||||||
| Relaxing | 6.73 | * | 3.14 | + | ||||||||
| Comforting | 3.75 | + | 3.00 | + | ||||||||
| Strength | 3.17 | + | 4.01 | * | ||||||||
| Like drug effect | 3.79 | + | 10.90 | ** | ||||||||
| Dislike drug effect | 8.13 | ** | ||||||||||
| Satisfying | 9.68 | ** | 3.88 | + | ||||||||
| Restricted Smoking Days | ||||||||||||
| Smoking behavior | ||||||||||||
| IPI (average) | 3.33 | + | ||||||||||
| Puff volume (average) | 3.08 | + | ||||||||||
| CO (boost) | 6.29 | * | ||||||||||
| SEQ | ||||||||||||
| Pleasant | 4.38 | * | 3.18 | + | ||||||||
| Unpleasant | 7.56 | * | 10.51 | ** | ||||||||
| Like taste | 6.14 | * | 3.55 | + | ||||||||
| Dislike taste | 7.45 | * | 5.04 | * | ||||||||
| Smoke vs. Air | 7.33 | * | 4.51 | * | ||||||||
| Sense of wellbeing | 5.63 | * | ||||||||||
| Exhilarating | 8.78 | ** | ||||||||||
| Pleasurable excitement | 3.92 | + | 9.36 | ** | ||||||||
| Dizziness | 3.59 | + | 6.10 | * | 2.92 | + | ||||||
| Lightheadedness | 5.46 | * | 4.23 | * | ||||||||
| Enjoyable | 5.30 | * | 8.22 | ** | ||||||||
| Nervous | 3.64 | + | ||||||||||
| Calming | 4.89 | * | ||||||||||
| Like cigarette | 5.56 | * | 5.42 | * | ||||||||
| Dislike cigarette | 8.67 | ** | 6.71 | * | ||||||||
| Relaxing | 3.40 | + | 3.37 | + | ||||||||
| Strength | 6.45 | * | ||||||||||
| High in nicotine | 6.77 | * | ||||||||||
| Like drug effect | 4.01 | + | ||||||||||
| Dislike drug effect | 4.17 | + | ||||||||||
| Satisfying | 3.28 | + | 4.43 | * | ||||||||
Main effects of Day and dependent measures which failed to reveal significant (p<.05) or trend (p<.10) group differences have been omitted. GxD indicates a group by day interaction. Unrestricted smoking days were analyzed as change from baseline. The degrees of freedom varied slightly across measures depending on group sample size, the number of assessments and/or missing data. As an example, the degrees of freedom for CPD during the Unrestricted Smoking Days were 1,29–31 (Group) and 1,184–196 Group by Day interaction) and the degrees of freedom for the IPI on Restricted Smoking Days were 1,28–30 (Group) and 1,26–27 (Group by Day interaction).
p<.10;
p<.05;
p<.01;
p<.001.
Data were analyzed with a fixed effect model in SAS using Proc Mixed with a compound symmetry covariance structure. The only exception was for baseline analyses of sex, race, and menthol preference which were analyzed using logistic regression. When appropriate, Day was included as a continuous time variable; Group by Day interactions are indicative of group differences in slope. The primary analyses included the following pairwise planned comparisons: 0/DN vs. 0/NC, 0/DN vs. 7/DN, and 0/DN vs. 21/DN. Statistical results from analyses of naturalistic (i.e., days 4–10) and restricted (i.e., days 3 and 11) smoking days are presented in Table 3. Dependent variables in which no significant group differences were observed have been omitted.
3. Results
3.1. Demographic and baseline data
Table 2 presents summary statistics by group for the major demographic variables and all dependent measures for which there were significant group differences after randomization as reported in Table 3. There were no significant differences between the subsequent groups in age, gender, education, race, self-reported CPD, menthol preference, or FTND scores at screening. On day 2, when participants were assessed for preferred brand smoking prior to randomization, there was a significant group differences in ratings of irritability (0/DN vs. 0/NC) and trends for a few additional group differences related to withdrawal, puff volume, and a few subjective cigarette effects. In these cases, the analyses described below were performed on both the raw and difference score data to determine the dependence of any effects observed after randomization on baseline differences.
3.2. Smoking behavior
3.2.1. Daily smoking
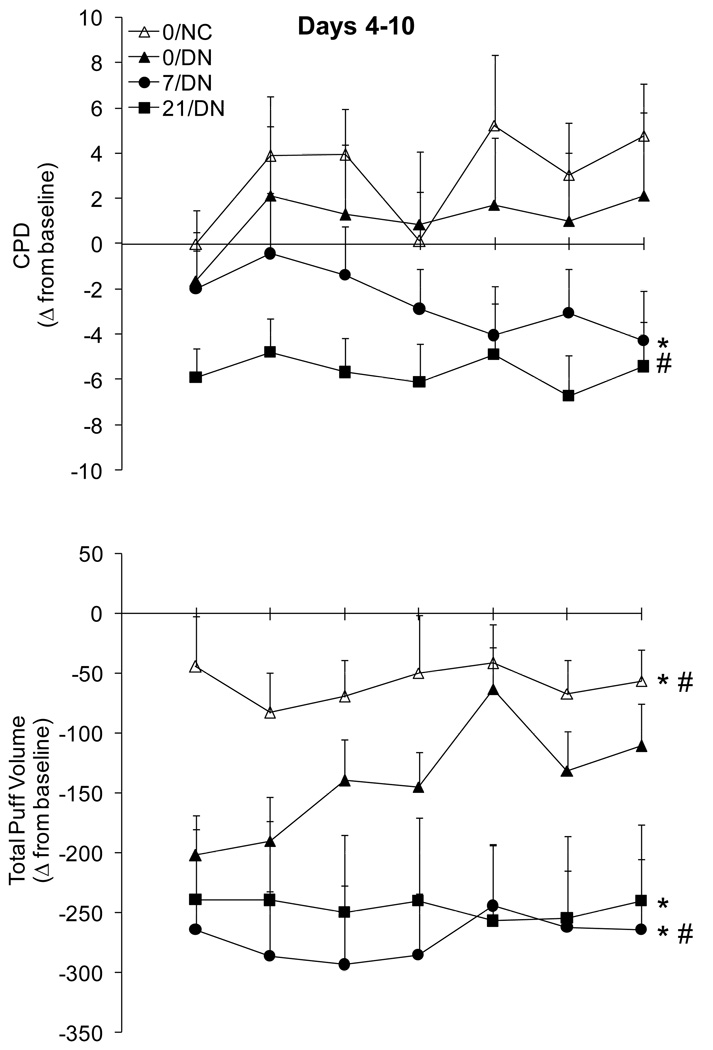
Direct comparison of the 0/DN and 0/NC conditions failed to reveal any significant differences in CPD; both groups tended to smoke at a similar or slightly greater rate than they did at baseline (Figure 1). In participants smoking denicotinized cigarettes, both 7 mg and 21 mg transdermal nicotine reduced the number of CPD relative to placebo patch controls. This effect emerged over days in the 7/DN group and was observed across days in the 21/DN group (Table 3). Examination of the pattern of smoking within day across consecutive 2 h bins failed to reveal any interactions between group and bin, suggesting that the patch/cigarette condition did not change the pattern of smoking within the day but instead the overall smoking intensity.
Figure 1.
Mean (±SEM) change from baseline (i.e., usual brand) for the number of cigarettes smoked per day (CPD; upper panel) and total puff volume (lower panel) from Days 4–10. Baseline data from Day 2 are presented in Table 2. The drop in CPD in the 0/NC condition on Day 7 corresponds to Sunday. *indicates a significant main effect of group when compared to 0/DN; #indicates a significant group by day interaction when compared to 0/DN.
3.2.2. Puff topography
The topography of smoking a single cigarette in the laboratory on days 4–10 varied across cigarette and transdermal nicotine conditions. Total volume, which is the product of the average puff volume and puff count, is presented in Figure 1 as a summary measure of group differences in puff topography. Total volume was clearly suppressed in 0/DN compared to 0/NC, although this difference decreased somewhat over days as total volume increased in the 0/DN group (Table 3). Transdermal nicotine had relatively little effect on the initial decrease in total volume relative to the preferred brand baseline, but attenuated the subsequent increase that was observed in the 0/DN group over days. The differences between 0/DN and 7/DN were largely driven by an emerging difference in average puff volume. In contrast, the differences between 0/DN and 21/DN resulted from an emerging difference in both puff count and average puff volume. Similarly, participants in the 0/NC group initially demonstrated increased peak and average flow compared to the 0/DN group, but these differences disappeared as peak and average flow increased over days in the 0/DN group. The 21 mg transdermal nicotine dose also tended to suppress the increase over days in peak and average flow compared to placebo; similar findings were observed for the 7/DN, but they failed to reach statistical significance.
3.2.3. Biochemical indices of smoking and nicotine exposure
There were no effects of transdermal nicotine on pre-smoking CO during the daily smoking assessments on days 4–10, although consistent with the CPD data described above there was a tendency for participants in the 21/DN group to demonstrate a greater decrease relative to baseline than participants in the 0/DN group (means collapsed across days: −4.56 vs. −0.76 ppm). Analysis of the boost in CO after smoking a cigarette revealed a significantly greater increase in CO in the 0/DN than the 21/DN group which was consistent with the greater total puff volume in the 0/DN condition. As a result, participants in the 21/DN group had a significantly greater drop (relative to preferred brand) in post-smoking CO levels compared to participants in the 0/DN condition (−5.42 vs. 1.84 ppm).
Mean (±SEM) salivary cotinine levels at Day 10 for 0/NC, 0/DN, 7/DN, and 21/DN were 263 (±30) ng/ml, 33 (±9) ng/ml, 194 (±27) ng/ml, and 355 (±32) ng/ml, respectively. Independent sample t tests indicated that 0/DN cotinine levels were significantly lower than all other groups and that 21/DN cotinine levels were significantly higher than all other groups (p<.05).
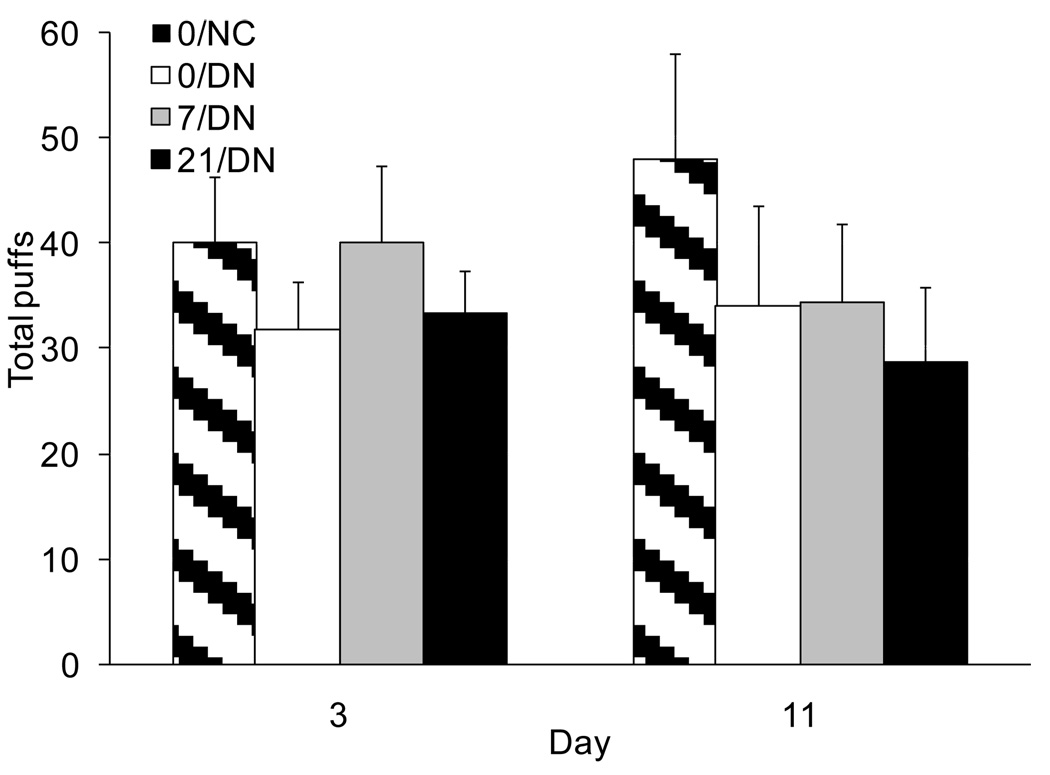
3.2.4. Self-administration test
Few differences emerged between the groups during the laboratory self-administration test after overnight abstinence. Comparison of the 0/DN and 0/NC groups failed to reveal any significant differences for average puff volume, total puff volume and total puff count. There was a trend for a group by day interaction on average puff volume. Average puff volume tended to be slightly higher in the 0/DN compared to the 0/NC group on Day 3 (44.5±2.7 cc vs. 41.3±2.5 cc), but slightly lower on Day 11 (38.9±3.7 cc vs. 42.2±3.2 cc). There were no significant effects of transdermal nicotine on any of these measures of smoking behavior. Similar to the naturalistic smoking days, CO increased more after smoking in the 0/DN than the 21/DN group; no other group differences in CO boost after smoking were significant.
3.3. Subjective effects of smoking
3.3.1. Cigarette ratings
Analyses of the subjective ratings of the assigned cigarettes relative to baseline preferred brand cigarettes are presented in Table 3. It should be noted that these effects were observed after freely smoking the single cigarette in the laboratory when there were effects of both cigarette nicotine content and transdermal nicotine dose on puff topography; therefore, these effects should be considered with caution and are not discussed in detail here. Nevertheless, the pattern of results was generally similar to those observed after a controlled exposure on days 3 and 11 (described below) and suggest that smoking a denicotinized cigarette produces less positive rewarding effects and more negative effects than nicotine cigarettes and that transdermal nicotine has little consistent effect on these ratings.
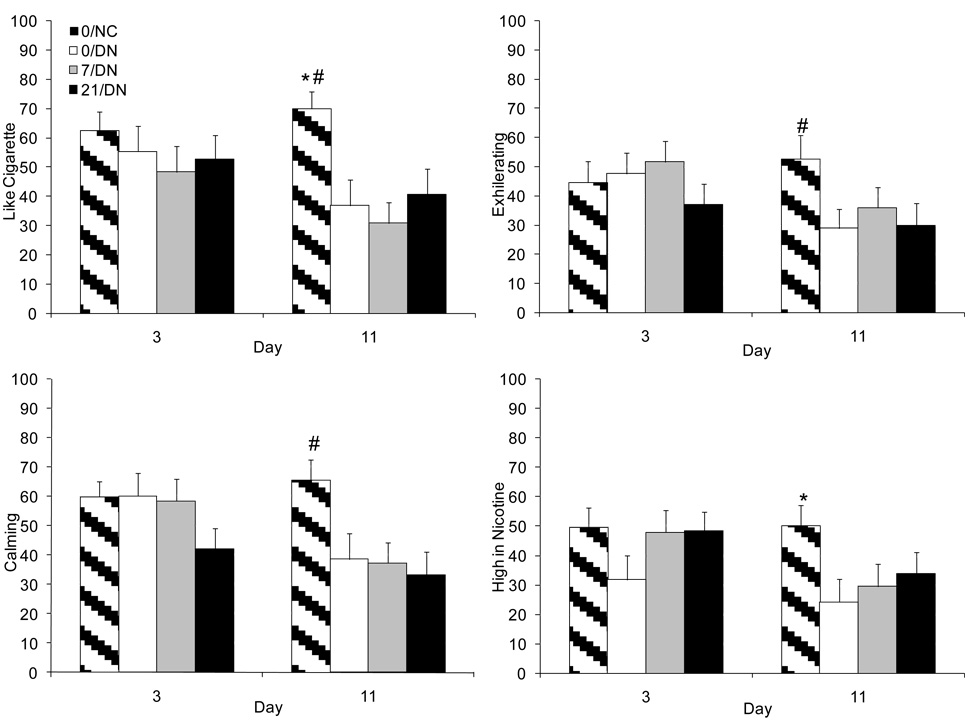
Subjective ratings of the cigarettes during the controlled puffing procedure revealed numerous differences between the 0/DN and 0/NC conditions, some of which varied over days. In contrast, there were relatively few effects of transdermal nicotine on the subjective effects of DN cigarettes. Nicotine-containing cigarettes were rated higher on positive effects (e.g., “pleasant,” “like taste,” “enjoyable,” “like cigarette”; Figure 3) and lower on negative effects (e.g., “unpleasant,” “dislike taste,” “dislike cigarette”). In general, these differences were greater on day 11 than on day 3 because of a decline in the positive and an increase in the negative effects of the denicotinized cigarettes. Other effects such as “sense of well-being,” “pleasurable excitement,” “exhilarating” (Figure 3), “calming” (Figure 3), “relaxing,” and “satisfying” showed a similar decline over days in the 0/DN relative to the 0/NC condition. Nicotine containing cigarettes were also rated as being “higher in nicotine” (Figure 3), causing more “lightheadedness,” and being more like “smoke than air”; these effects did not vary significantly over days. Compared to placebo, the 7 mg patch reduced ratings of “dizziness” and “lightheadedness” attributed to smoking, although these effects were not observed for the 21 mg patch. Similarly, compared to placebo, rating of “strength” decreased from day 3 to 11 in the 7/DN group, but these differences were not observed for the higher nicotine dose. Conversely, compared to placebo, participants in the 21 mg, but not the 7 mg, group rated the denicotinized cigarettes as more like “smoke than air.”
Figure 3.
Mean (+SEM) ratings of “Like cigarette” (upper left panel), “Exhilarating” (upper right panel), “Calming” (lower left panel), and “High in Nicotine” (lower right panel) on Days 3 and 11. All significant differences are reported in Table 3. *indicates a significant main effect of group when compared to 0/DN; #indicates a significant group by day interaction when compared to 0/DN.
3.3.2. Nicotine/tobacco withdrawal
Data from the afternoon session on day 4 (average time of session: 12:56 PM) were analyzed separately because they represent the best data on the effects of transdermal nicotine during smoking abstinence. Smoking was largely restricted during the preceding 24 h period; participants were required to be abstinent from day 3 till the morning session on day 4 (average time of session: 8:44 AM). Consistent with the restriction on smoking, relatively few differences were observed between the 0/DN and 0/NC groups. Analysis of the difference score data revealed greater ratings of irritability and increased appetite in the 0/DN compared to the 0/NC condition (p<.05); however, these differences failed to reach even trend levels (i.e., p>.10) when the raw data were analyzed (see baseline differences in Table 2). Transdermal nicotine tended to decrease withdrawal compared to placebo. Analysis of the difference score data revealed greater total withdrawal (p<.05), irritability (p<.01), difficulty concentrating (p<.05), and insomnia (p<.05) in the 0/DN compared to the 7/DN group. Likewise, participants in the 0/DN group reported greater total withdrawal (p=.07), irritability (p<.05), difficulty concentrating (p<.05), and increased appetite (p<.05) compared to the 21/DN group. Analysis of the raw data also revealed trends for increased irritability (vs. 7 and 21 mg), difficulty concentrating (vs. 21 mg), and appetite (vs. 21 mg) in the 0/DN group; however, these results failed to reach significant.
On days 5–10, when participants were freely smoking their assigned cigarettes during the preceding 24 h, total withdrawal symptoms were similar in the 0/DN compared to the 0/NC group. Difference score analyses of individual symptoms revealed significantly greater irritability in the 0/DN group that dissipated over days; however, this difference was not observed when the raw data were analyzed (see baseline differences in Table 2). Similarly, there were few consistent effects of transdermal nicotine on withdrawal once participants were allowed to smoke. Total withdrawal symptoms, difficulty concentrating and insomnia were significantly suppressed by 7 mg, but not 21 mg, transdermal nicotine compared to the placebo patch. However, these effects of transdermal nicotine appeared to result from relatively high ratings at baseline in the 7/DN group (see Table 2) and were not apparent in analyses of the raw data. Only a group by day interaction on insomnia remained after analyzing the raw data, reflecting a tendency for insomnia to decrease more over days in the 0/DN than the 21/DN condition.
3.3.3. Mood
Data from Day 4, when smoking was largely restricted over the preceding 24 h, indicated that total positive affect scores on the PANAS were similar in the groups receiving placebo transdermal nicotine (0/DN and 0/NC) and reduced compared to baseline (mean±SEM: −6.31±2.14 and −4.65±1.28, respectively). Interestingly, transdermal nicotine attenuated this decrease relative to placebo for both the 7/DN (−2.0±1.5; p<.10) and 21/DN (−1.0±1.5; p<.05) groups. There were no group differences in PANAS total negative affect or in any of the POMS subscales.
On days 5–10, when participants were freely smoking their assigned cigarettes, there was a non-significant trend for higher PANAS positive affect in the 0/NC compared to the 0/DN. Likewise, as seen on day 4, relative to placebo patch, 21 mg transdermal nicotine alleviated the decrease in positive affect during the first few days after randomization but this effect dissipated with time. There were no significant differences between 0/DN and 0/NC on subscales of the POMS, although there was a trend for overall reduced POMS-vigor in the 0/DN compared to the 0/NC group. Neither 7 nor 21 mg nicotine significantly impacted POMS subscale ratings.
3.3.4. End of study questionnaire
Average ratings of the nicotine content of the assigned cigarettes was in the “ultra-light” range. Ratings were somewhat lower in the 0/DN condition (mean ± SEM: 1.86±0.31) than the 0/NC (2.44±0.13), 7/DN (2.39±0.31) and 21/DN (2.42±0.21) conditions. None of these differences reached statistical significance. Ratings of the nicotine content of the patches fell into the “a little” to “a moderate amount” range and were related to dose. Mean (±SEM) ratings were 2.63 (±0.20), 2.21 (±0.30), 2.78 (±0.13), and 3.05 (±0.16) for the 0/NC, 0/DN, 7/DN and 21/DN groups, respectively. Differences between the 0/NC and 0/DN groups failed to reach significance. There was a tendency for higher ratings relative to 0/DN for the 7/DN (p=.07) and 21/DN (p<.05) conditions.
4. Discussion
Denicotinized cigarettes maintained their reinforcing properties throughout the 9-day assessment period. Compared to participants smoking cigarettes with approximately twelve times the nicotine yield, those smoking denicotinized cigarettes and wearing the placebo patch smoked a similar number of cigarettes per day. The only significant change in smoking behavior related to cigarette nicotine yield was in puff topography; the total volume of smoke inhaled from the single cigarette was lower in the denicotinized than the nicotine-containing condition, although this difference faded over days. The failure to observe a decrease in the number of cigarettes smoked is in contrast with a similar inpatient study in which the number of denicotinized cigarettes smoked declined somewhat over time (Donny et al., 2007). This difference highlights the fact that extinction may proceed more slowly in a naturalistic setting, possibly because of the presence of numerous stimuli associated with smoking (Bouton, 2004).
Transdermal nicotine suppressed the number of cigarettes smoked and reduced the volume of smoke inhaled per cigarette, especially over the second half of the study. These behavioral differences resulted in a drop in CO relative to placebo and preferred brand smoking. Interestingly, transdermal nicotine did not appear to facilitate the process of extinction per se. For both cigarettes smoked per day and total puff volume, the clear pattern was an immediate downward shift relative to placebo patch, but little or no change over repeated exposures. In sum, none of the groups assigned to exclusively smoke denicotinized cigarettes, regardless of patch condition, demonstrated signs of a more gradual extinction process.
It is interesting to note that the laboratory-based self-administration test failed to replicate the robust behavioral differences between active and placebo transdermal nicotine. One important difference between these data and those observed on days 4–10 is that the prior were collected after overnight abstinence and while smoking was restricted to the laboratory while the latter was collected when participants were allowed to smoke in their natural environment with little restriction. This suggests that smoking abstinence might suppress the effects of the patch on smoking denicotinized cigarettes, possibly because motivation closely related to cigarette abstinence (e.g., craving) mask or overwhelm motivational influences directly tied to nicotine abstinence (e.g., difficulty concentrating, restlessless) (Buchhalter et al., 2005).
Subjective ratings of the positive rewarding effects of denicotinized cigarettes were relatively low throughout the study. For example, “liking” scores were well below those observed for nicotine cigarettes and were approximately half the value observed for “disliking.” This stands in stark contrast to the persistence of smoking behavior in the absence of nicotine, suggesting that the reason people continue to smoke denicotinized cigarettes is not because these cigarettes produce potent positive rewarding effects. Instead, the persistence of smoking may be more closely related to two other processes: 1) the ability of denicotinized cigarettes to suppress some aspects of craving/withdrawal and/or 2) the relative insensitivity of smoking behavior to manipulations which alter the reward value of smoking (e.g., “habit learning”). These possibilities are discussed in more detail below.
The continued use of denicotinized cigarettes may result from their ability to suppress withdrawal and craving. One limitation of this study is that the craving data could not be used with confidence due to the methodological problems described above. Therefore, we could not determine whether craving-suppression is a viable mediator for the robust reinforcing effects of denicotinized cigarettes observed in this study. However, the withdrawal data were consistent with the hypothesis that denicotinized cigarettes function as negative reinforcers. Withdrawal symptoms increased in the 24 h encompassing the first abstinence day when participants wore their assigned patch, but their smoking was restricted. During this period, transdermal nicotine tended to suppress withdrawal. When participants were subsequently allowed to smoke, withdrawal decreased further, particularly in participants received a placebo patch. Indeed, there were relatively few effects of either cigarette type or transdermal nicotine on withdrawal from days 5–10. Because the study did not include a smoking abstinence condition, it impossible to say definitively that smoking denicotinized cigarettes suppressed withdrawal. However, previous studies that included a smoking abstinence condition have clearly demonstrated that at least some symptoms (desire for sweets, hunger, craving) of withdrawal are suppressed by denicotinized cigarettes when they are smoked over a 5-day period (Buchhalter et al., 2005). These data suggest that both denicotinized cigarettes and transdermal nicotine suppress some aspects of withdrawal and that there may be overlap in the symptoms they treat. In light of the fact that transdermal nicotine also suppressed smoking of denicotinized cigarettes, these data are consistent with the interpretation that denicotinized cigarettes may be negatively reinforcing as a consequence of withdrawal suppression.
Relatedly, we observed decreases in positive affect during nicotine abstinence that were alleviated by transdermal nicotine. Interestingly, however, these effects tended to persist once participants in the 0/DN condition were allowed to smoke, suggesting that they may be specific to nicotine abstinence per se. These data are consistent with data reported by Dawkins and colleagues suggesting that abstinence leads to an attenuated responsivity to reward (i.e., anhedonia) that can be alleviated by acute nicotine replacement (Dawkins et al., 2007; Dawkins et al., 2006). Here, the benefits of transdermal nicotine on positive affect dissipated over days in the present study. To our knowledge, the anhedonic effects of abstinence and the reversal by nicotine replacement has not been investigated beyond a day of abstinence. The possibility that these effects re-emerge despite ongoing nicotine replacement, and in the current study, smoking of denicotinized cigarettes, raises important questions about the duration of this withdrawal-related process and its potential role in relapse after the first few days of abstinence.
Another possibility is that participants continued to smoke denicotinized cigarettes because of so-called “habit” or stimulus-response (S-R) learning (see (Balleine and Ostlund, 2007) for detailed discussion). According to this framework, stimuli can trigger a response even when the outcome of that action has been devalued. In the current analysis, the rewarding value of smoking was reduced by removing nicotine from the cigarettes. Nevertheless, participants continued to smoke at a relatively high rate; even participants in the 21/DN condition only reduced their smoking by approximately 30%. Stimuli which have been associated with nicotine reinforcement (e.g., smoking peers, sight of a cigarette) may continue to trigger smoking behavior even in the absence of a rewarding outcome. This interpretation is similar to the account of drug use proposed by Tiffany in which use is driven largely by automatized action schemata and craving occurs when the action is disrupted (Tiffany, 1990). Indeed, smoking denicotinized cigarettes produces robust craving suppression, an effect that persists even after an extended period of use (Donny et al., 2007). Interestingly, S-R learning may involve neural mechanisms that differ from those involved in action-outcome learning (Faure et al., 2005; Ito et al., 2004; Yin et al., 2004). Several theorists have proposed that this type of shift (i.e., from outcome driven behavior to stimulus driven behavior) is important for understanding the nature of drug dependence (Balfour, 2004; Di Chiara, 2000; Everitt et al., 2001). This may be particularly true for smoking, an act that is closely tied to environmental stimuli and performed hundreds of thousands of times by long-term smokers. Additional research into these processes is clearly needed.
Three other studies have examined the concurrent use of denicotinized cigarettes and transdermal nicotine (Rezaishiraz et al., 2007; Rose and Behm, 2004; Rose et al., 2006). Rose (Rose and Behm, 2004) evaluated treatment-seeking smokers administered 21 mg or placebo transdermal nicotine (double blind) and smoking preferred brand or denicotinized cigarettes (unblind) for 2 weeks prior to quitting. Smoking denicotinized cigarettes for two weeks gradually reduced the rewarding effects of usual brand cigarettes regardless of transdermal nicotine dose. Denicotinized cigarette smoking during the 2-week period tended to be reduced in 21 mg than the placebo condition, although this failed to reach significance. Craving assessed after overnight abstinence declined during the 2-week treatment period, but was not affected by transdermal nicotine dose (Rose et al., 2006). Nevertheless, subsequent follow-up during the quit attempt failed to demonstrate a significant effect of denicotinized cigarettes on abstinence (Rose et al., 2006). In another unblinded study, Rezaishiraz et al (2007) randomized treatment seekers to receive denicotinized cigarettes and 21 mg patch or reduced nicotine cigarettes (0.6 mg FTC) for 2 weeks prior to quitting. They found that the combination of denicotinized cigarettes and 21 mg patch reduced pre-quit and post-quit craving, but that there was no difference in nicotine withdrawal symptoms or quit rates. Finally, a recent study (Becker et al., 2008) demonstrated that the combination of pre-quit denicotinized cigarettes and pre-quit nicotine replacement therapy produced greater 4-week abstinence rates than no pre-quit intervention in participants given post-quit transdermal nicotine. However, the effects of denicotinized cigarettes could not be disentangled from the effects of pre-quit transdermal nicotine as the necessary control conditions were not included. The present study extends this earlier research in several ways. Participants were blind to the nicotine content; therefore, observed differences were not likely the result of expectancies regarding the importance of nicotine. Compliance was likely much higher given the daily visits, bogus pipeline procedure, and salivary cotinine data. More thorough and multi-dimensional assessments are reported including objective verification of self-reported smoking and puff topography. Finally, the present study was aimed at determining change in the effects of the denicotinized cigarettes themselves and not necessarily whether they facilitate a quit attempt; consequently, clear evidence of the persistence of the reinforcing effects of denicotinized cigarettes was observed.
One interesting question not addressed in this study is whether smoking denicotinized cigarettes leads to extinction of the relationship between other “cues” for smoking and nicotine. While the focus of the present study was on the self-administration of smoking stimuli, other stimuli (e.g., an ashtray) are associated with cigarette use and may serve as “triggers” for smoking that are amenable to extinction (Conklin and Tiffany, 2002). Repeatedly smoking denicotinized cigarettes in these contexts may reduce the efficacy of those stimuli to induce craving and relapse. While we did not observe any evidence that the number of cigarettes smoked declined over time, it is possible that a more proximal assessment of cue-induced craving would reveal a different pattern of results.
There has been much discussion as to the consequences of regulating nicotine levels in cigarettes (Hatsukami, 2008; Henningfield et al., 1998; Tengs et al., 2005). The results of the current study provide some information about how reducing nicotine content to very low levels (i.e., 0.05 mg) would impact smoking. One concern is that smokers might compensate for the decrease in nicotine yield by smoking more. The present data do not support this concern. Participants smoked a similar number of denicotinized cigarettes, but reduced the volume of smoke inhaled compared both to their own preferred brand baseline and to participants assigned to nicotine-containing cigarettes. Interestingly, however, differences in total puff volume dissipated as the study progressed suggesting that puff topography may be only temporarily disrupted by the switch to denicotinized cigarettes. This decrease in puff volume is in contrast with a report by Strasser et al (Strasser et al., 2007) indicating a compensatory increase in total puff volume in participants smoking the Quest 3 cigarettes. This discrepancy might be explained by the timing of assessments. Participants in the study by Strasser and colleagues were evaluated in response to their first use of the study cigarettes. Here, participants were first exposed using the controlled puffing procedure which did not allow us to assess changes in puff topography. Data from the next available assessment (self-administration) was more consistent with the data reported by Strasser; average puff volume tended to be higher in the 0/DN compared to the 0/NC group. However, the predominant subsequent pattern observed in the present study was for denicotinized cigarettes to result in reduced (total puff volume) or similar (CO) indices smoke intake. Hence, denicotinized cigarettes may produce a short-lived compensatory increase in smoking, but this effect likely dissipates quickly and is replaced by a down-regulation of smoke intake. Furthermore, concurrent transdermal nicotine reduced the number of cigarettes smoked, the total volume of smoke inhaled per cigarette, and the boost in CO post-smoking.
Finally, it is important to note that these cigarette are not completely free of nicotine. They have an FTC yield of 0.05 mg and while this dose of nicotine may produce reduced nicotinic effects (e.g. greatly reduced tachycardia, striatal dopamine release; Brody et al., 2008b; Pickworth et al., 1999), it may be pharmacologically active. Indeed, recent work by Brody and colleagues suggests that smoking only 1–2 puffs of a regular cigarette results in 50% occupancy of α4β2 nicotinic receptors for over 3 hrs (Brody et al., 2006). Furthermore, smoking a denicotinized cigarette results in 26% occupancy of α4β2 nicotinic receptors, which although substantially lower than both reduced nicotine cigarettes (Brody et al., 2008a) and regular cigarettes (Brody et al., 2006), may nevertheless result in important nicotinic effects.
These data add to a large literature illustrating the importance of smoking stimuli in the maintenance of smoking behavior. Given the observation that smoking persists largely unchanged for an extended period of time despite a dramatic reduction in nicotine yield, it is not surprising that pharmacotherapies for smoking cessation that target nicotinic mechanisms meet with limited success. Improvements in the treatment of tobacco dependence will likely require better understanding of how non-pharmacological processes (e.g., habit learning, conditioned reinforcement) and nicotinic effects on these processes (Chaudhri et al., 2006; Donny et al., 2003; White, 1996) influence relapse. In the end, both behavioral and pharmacological approaches that more directly address these concerns may greatly improve the efficacy of treatment provided to smokers.
Figure 2.
Mean (+SEM) total number of puffs earned during the 1 h self-administration test on Days 3 and 11. None of the pairwise comparisons reached statistical significance.
Acknowledgements
The authors would also like to thank Sindu Ramesh, Purvi Patel, Jessica Siegal, and Melissa Mercincavage for their assistance in data collection and Ron Dahl, M.D., for his medical supervision of the project.
Funding Source
This work was support by a grant from the National Institute on Drug Abuse (NIDA) DA-019626 (ECD). The NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Contributor Information
Eric C. Donny, Department of Psychology, University of Pittsburgh, 3137 Sennott Square, 210 S.Bouquet Street, Pittsburgh, PA 15217
Melissa Jones, CHES, 3705 Fifth Ave., Pittsburgh, PA 15213
References
- Balfour DJ. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus accumbens [corrected] Nicotine Tob Res. 2004;6:899–912. doi: 10.1080/14622200412331324965. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Annals of the New York Academy of Sciences. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. 2008;10:1139–1148. doi: 10.1080/14622200802123294. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2008a:1–12. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Archives of general psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED. Ventral Striatal Dopamine Release in Response to Smoking a Regular vs a Denicotinized Cigarette. Neuropsychopharmacology. 2008b doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction (Abingdon, England) 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction (Abingdon, England) 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addictive behaviors. 2007;32:425–431. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--effects on incentive motivation. Psychopharmacology. 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003 doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction (Abingdon, England) 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science (New York, NY. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK. Nicotine addiction: past, present and future. Marian Fischman lecture given at the 2007 meeting of CPDD. Drug Alcohol Depend. 2008;92:312–316. doi: 10.1016/j.drugalcdep.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tobacco control. 1998;7:281–293. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacology, biochemistry, and behavior. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- McNair DM LM, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology. 2007;195:235–243. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Caggiula A, Wilson AS, Stiller RL. Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacology, biochemistry, and behavior. 1997;56:235–241. doi: 10.1016/s0091-3057(96)00216-x. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1:357–364. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- Rezaishiraz H, Hyland A, Mahoney MC, O'Connor RJ, Cummings KM. Treating smokers before the quit date: can nicotine patches and denicotinized cigarettes reduce cravings? Nicotine Tob Res. 2007;9:1139–1146. doi: 10.1080/14622200701684172. [DOI] [PubMed] [Google Scholar]
- Rose JE. Denicotinized cigarettes: a new tool to combat cigarette addiction? Addiction (Abingdon,England) 2007;102:181–182. doi: 10.1111/j.1360-0443.2006.01727.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug Alcohol Depend. 1994;34:225–229. doi: 10.1016/0376-8716(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: pharmacological and behavioral treatments. Nicotine Tob Res. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R. Sensory blockade of smoking satisfaction. Pharmacology, biochemistry, and behavior. 1985;23:289–293. doi: 10.1016/0091-3057(85)90572-6. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Badger GJ, Giordano LA. Sensitivity of nicotine-containing and de-nicotinized cigarette consumption to alternative non-drug reinforcement: a behavioral economic analysis. Behav Pharmacol. 2001;12:277–284. doi: 10.1097/00008877-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de- nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology. 1999;147:210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Tengs TO, Ahmad S, Savage JM, Moore R, Gage E. The AMA proposal to mandate nicotine reduction in cigarettes: a simulation of the population health impacts. Preventive medicine. 2005;40:170–180. doi: 10.1016/j.ypmed.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of consulting and clinical psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology, biochemistry, and behavior. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction (Abingdon, England) 1996;91:921–949. discussion 951–965. [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. The European journal of neuroscience. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]