Abstract
Recombinant adenoviral vectors (AdV) are potent vehicles for antigen engineering of dendritic cells (DC). DC engineered with AdV to express full length tumor antigens are capable stimulators of antigen-specific polyclonal CD8+ and CD4+ T cells. To determine the impact of AdV on the HLA class I antigen presentation pathway, we investigated the effects of AdV transduction on antigen processing machinery (APM) components in human DC. Interactions among AdV transduction, maturation, APM regulation and T cell activation were investigated. The phenotype and cytokine profile of DC transduced with AdV was intermediate, between immature (iDC) and matured DC (mDC). Statistically significant increases in expression were observed for peptide transporters TAP-1 and TAP-2, and HLA class I peptide-loading chaperone ERp57, as well as co-stimulatory surface molecule CD86 due to AdV transduction. AdV transduction enhanced the expression of APM components and surface markers on mDC, and these changes were further modulated by the timing of DC maturation. Engineering of matured DC to express a tumor-associated antigen stimulated a broader repertoire of CD8+ T cells, capable of recognizing immunodominant and subdominant epitopes. These data identify molecular changes in AdV-transduced DC (AdV/DC) that could influence T cell priming and should be considered in design of cancer vaccines.
Keywords: Dendritic cells, Human, Antigen presentation/processing, Gene therapy, Tumor immunity
Introduction
For immunotherapy of cancer, optimal antigen presenting cells (APC) must be utilized to present tumor antigen epitopes to multiple CD8+ and CD4+ T cells, to activate and expand tumor antigen-specific CD8+ effectors, and to provide for cognate help from CD4+ T cells. Dendritic cells (DC) have been shown to be the most potent APC. DC can be exogenously pulsed with peptides, loaded with proteins and lysates, or transfected with DNA, RNA or viruses. Loading DC with full length tumor antigen, for endogenous processing and presentation of multiple peptides restricted by MHC class I and II, has proved technically difficult. DNA transfection is inefficient and many viruses have a negative impact on DC function. Adenovirus (AdV) is a safe, well studied viral vector which has proven to be a useful vehicle for genetic engineering of DC, with >95% efficiency of gene transfer [1].
AdV transduction has biological impact on human DC function. We and others have shown that immature monocyte-derived DC, transduced with AdV (at multiplicity of infection of 1,000 pfu:1 DC), become more mature by phenotypic criterion (increased CD83, CD86, HLA-DR), decreased secretion of IL-10 and increased IL-12p70, as well as by efficient stimulation of antigen-specific, IFN γ-producing T cells [2–5]. AdV can also reduce antigen uptake via macropinocytosis, consistent with maturation [6]. The intracellular pathways involved in positive and negative regulation of these functional changes in human DC are now beginning to be characterized; the PKR and PI3 K signaling pathways are activated in human DC transduced with AdV [7].
Many comparisons of exogenous peptide pulsing and tumor antigen transfection have recently been performed, supporting the superiority of DC transfection with full length tumor antigen genes for optimal peptide-specific T cell activation [8–11]. Plasmid DNA transfection can be more efficient with newer methods [12] but in head-to-head comparisons, AdV was superior to plasmid DNA [13, 14] and to mRNA [13]. In continuing investigations, we have also demonstrated broad and potent activation of multiple CD8+ T cell specificities by AdV engineered DC [15] as well as strong type 1 cytokine production by CD4+ T cells activated by AdV/DC (vs. protein-loaded DC [16]). The mechanism of this superior T cell activation is unknown, but may be related to antigen expression duration, DC maturation effects, or other impact of AdV on DC.
The impact of AdV and maturation on DC and the resultant repertoire of presented peptide epitopes is critical, but to the best of our knowledge, no information is available on the molecular basis for this interaction. Specifically, it has not been investigated whether AdV transduction modulates the expression of antigen processing machinery (APM) components which play a crucial role in the generation of peptides from mostly, although not exclusively, endogenous proteins and their presentation to cognate cytotoxic T lymphocytes (CTL).
The HLA class I antigen processing and presentation pathway starts with proteins being marked for ubiquitination and subsequent degradation by the proteasome. The proteasome, which consists of multiple catalytic subunits (Delta, MB1 and Z, and their IFN-γ-inducible counterparts LMP-2, LMP-7 and LMP-10), is involved in the degradation of proteins and the generation of antigenic peptide fragments. These peptides are then translocated across the endoplasmic reticulum (ER) membrane via the ATP-dependent heterodimeric transporter associated with antigen-processing subunits TAP-1 and TAP-2. The HLA class I heavy chain is synthesized in the ER where it associates with β2-microglobulin (β2m) with the assistance of multiple chaperone proteins (calnexin, calreticulin, and ERp57) that ensure the proper folding of HLA class I molecules. The HLA class I–β2m complex then associates with tapasin, which allows the dimeric complex to interact with TAP-associated peptides and facilitates peptide loading into HLA class I–β2m complex. The tri-molecular HLA class I/β2m/peptide complexes are then transported to the cell surface [17, 18].
Previous studies of APM in DC demonstrated that immature DC (iDC) had a significantly lower expression of MB1, LMP-7, LMP-10, TAP-1, and tapasin than cytokine-matured DC [19, 20]. Furthermore, it was shown that APM expression in iDC could be inhibited by tumor cells, but that ex vivo maturation of DC derived from patients with cancer restored normal expression of APM components and their antigen-processing capability. This tumor cell-mediated inhibition was subsequently shown to involve tumor cell-derived gangliosides [21]. Also, heat shock has been shown to have positive effects on APM and epitope-specific antigen presentation in antigen presenting cells (APC) [22]. In tumor cells, chemotherapy has been demonstrated to modulate APM and TAA immunogenicity [23], and evidence suggests that tumor-driven alterations in APM components may be a key element of immune escape [20, 24].
Because analysis of APM components in AdV-transduced DC may contribute to the understanding of antigen processing in these candidate vaccines, in the present study, we have investigated whether AdV transduction of DC affects the levels of multiple APM components. We have also tested the effects on DC cell surface maturation phenotype and cytokine production, as these elements are also critical to antigen presentation and its milieu. Lastly, we have tested the TAA-specific CD8+ T cell response to these DC. Importantly, we find that the combination of DC transduction and maturation further modulates APM component expression and modifies the resultant activated T cell repertoire.
Materials and methods
Recombinant adenoviral vectors
The enhanced green fluorescent protein expressing vector (Ad.EGFP) was a kind gift of University of Pittsburgh Vector Core Facility. The AFP-expressing vector (AdVhAFP), which encodes the human AFP cDNA driven by the CMV promoter, has been previously described [25]. These are E1- and E3-deleted AdV encoding the EGFP and the human AFP cDNA, respectively. To inactivate the virus, infectious Ad.EGFP was heat-inactivated at 65°C for 1 h (hiAd.EGFP). Viral titers were tested using the Adeno-X Rapid Titer Kit (Clontech Laboratories Inc., Mountain View, CA, USA); multiplicity of infection (MOI) used to infect DC was based on the values obtained using this procedure.
APM and cell surface antibodies
Intracellular staining for APM components was performed using the following unconjugated mouse monoclonal antibodies: β2m (NAMB-1), Delta (SY-5), MB-1 (SJJ-3), Z (NB1), LMP-2 (SY-1), LMP-7 (HB2), LMP-10 (TO-6), TAP-1 (NOB1), TAP-2 (NOB2), Calnexin (TO-5), Calreticulin (TO-11), ERp57 (TO-2), Tapasin (TO-3) [26–28]. As a secondary antibody, PE anti-mouse IgG1 (BD Pharmingen) was used. PE-conjugated HLA-DR (Caltag/Invitrogen), HLA-ABC (BioLegend, San Diego, CA, USA), CCR7 (R&D Systems, Minneapolis, MN, USA), CD40, CD80, CD86, and CD83 (BD Pharmingen, San Jose, CA, USA) were used to analyze DC cell surface maturation markers.
Isolation of normal donor PBMC
Peripheral blood was obtained from normal donors by venipuncture. Peripheral blood “buffy coats” were procured from Central Blood Bank, Pittsburgh, PA, USA (approved by IRB # 0403105 and UPCI # 04-001). Blood was diluted 1:2 with PBS, applied to a Ficoll-Hypaque gradient (Cellgro, Mediatech Inc., Herndon, VA, USA), and centrifuged at 550×g for 25 min at room temperature. Peripheral blood mononuclear cells (PBMC) were recovered from the buoyant interface and washed three times with PBS in order to remove residual platelets and Ficoll-Hypaque.
DC generation
Following separation, PBMC were plated in T75 culture flasks (COSTAR, Cambridge, MA, USA) in AIM-V (GIBCO-BRL, Gaithersburg, MD, USA) medium for 60 min at 37°C. Following incubation, non-adherent (NA) cells were removed from flasks by gentle washing with PBS and cryo-preserved in freezing media [10% v/v DMSO (Sigma-Aldrich, St Louis, MO, USA), 20% v/v human AB serum (HuAB, Omega Scientific, Tarzana, CA, USA), 70% v/v RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA)] using a controlled-rate freezing technique in a −80°C freezer. Adherent monocytes were cultured at 37°C (5% CO2) in DC medium [AIM-V supplemented with 1,000 U/ml GM-CSF (Sargramostim, Amgen, Thousand Oaks, CA, USA) and 1,000 U/ml IL-4 (Schering Plough, Kenilworth, NJ, USA)] for 5 days. iDC were matured by adding 1,000 U/ml IFN-γ (PeproTech Inc., Rocky Hill, NJ, USA), and 250 ng/ml LPS (Sigma) on day 5, and incubated for an additional 24 h at 37°C.
In vitro stimulation (IVS)
For these experiments, NA cells isolated from HLA-A2+ donors were used. On the day of establishing DC-T cell cultures, the cryopreserved NA cells were thawed at 37°C and washed in AIM-V medium. DC were used as stimulators of autologous lymphocytes and were either peptide pulsed (10 μg/ml) or transduced with recombinant adenoviral vectors (MOI = 500), using a previously described protocol [5]. The impact of viral transduction on DC maturation was assessed by FACS analyses (indicated by upregulation of CD83, CCR7, MHC class I and class II antigens, CD80, and CD86). DC were incubated with AdV for 3 h in 1 ml of AIM-V. Afterwards, virus was washed out with PBS and DC were co-cultured with autologous NA cells at a 1:10 (DC:T cell) ratio in T cell media (AIM-V+ 5% HuAB). One round of stimulation (over a 7-day period) was performed.
T cell isolation
On the day of testing, CD8+ T cells were isolated from the T cell cultures by magnetic cell sorting (MACS, Miltenyi Biotech, Auburn, CA, USA) according to the manufacturer’s protocol.
ELISPOT
After a single round of IVS for 7 days, the frequency of peptide-specific CD8+ T cell responders was measured using autologous cells as antigen-presenting cells in a human IFN-γ assay (antibody pair from Mabtech Inc., Cincinnati, OH, USA; MultiScreen™ HTS plates from Millipore, Bedford, MA, USA), as previously described [29, 30]. The number of peptide-specific CD8+ T cell responders was compared to the background number of spots produced against APCs alone. Positive control wells contained T cells cultured in the presence of 10 μg/ml phytohemagglutinin (PHA, Sigma-Aldrich).
FACS staining
Intracellular staining for APM components was performed as previously described [20]. Prior to antibody staining, cells were washed in FACS buffer (PBS + 0.2% w/v BSA + 0.02% w/v NaN3). Staining was performed at room temperature for 30 min in the dark, followed by washing cells in FACS buffer and resuspending in 2% w/v paraformaldehyde in PBS. Samples were analyzed on Beckman Coulter Epics XL using the Expo32 ADC XL 4 Color software.
Cytokine multiplex assays
Cell free supernatants from ELISPOT assays were stored at −80°C until assayed for appropriate cytokines and chemokines by Luminex-based bead arrays (Biosource). We tested iDC, iDC.EGFP, and mDC supernatants for inflammatory (IL-8), Th1 (TNF-α, IL-15, IL-12p70), and Th2 (IL-10, IL-13) cytokine and chemokine profiles. Assays were performed in the UPCI Luminex Core facility, and UPCI Immunological Monitoring Laboratory.
Statistical analysis
A two-sided paired t test was used to compare the marker and cytokine expression levels of DC treated under different conditions; prior to analysis, the data were log-transformed. P values ≤0.05 were considered to be statistically significant. No correction for multiple comparisons was applied. Data presented in Figs. 5, 6, 7 and 8 is from single donors and these studies were exploratory.
Fig. 5.
Evaluation of the ability of heat inactivated AdV to induce phenotypic changes in DC. Surface phenotypic markers and APM components were compared by flow cytometry 48 h after treatments among iDC, iDC.EGFP, mDC, and iDC transduced with heat-inactivated Ad.EGFP (iDC.hiEGFP) as described in Fig. 1. MFI values are shown for each group. These data are representative of one of three similar experiments performed. 1. iDC+ IgG; 2. iDC; 3. iDC.EGFP; 4. iDC.hiEGFP; 5. mDC
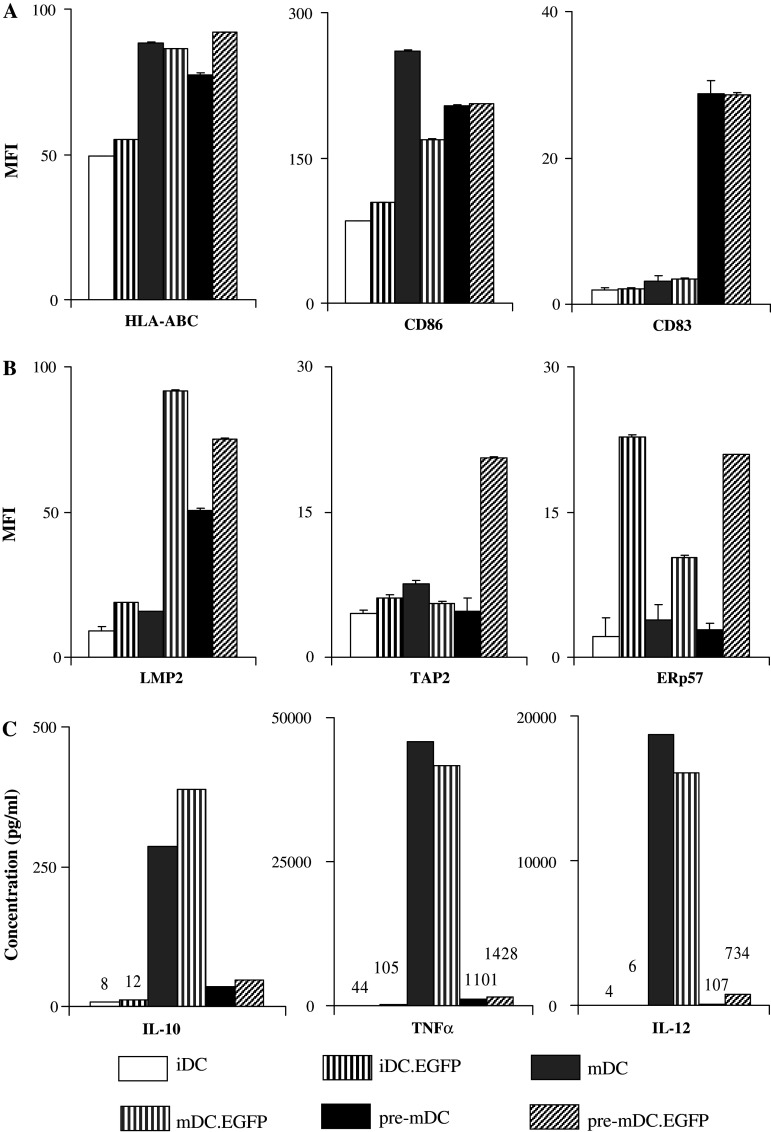
Fig. 6.
Assessment of maturation effect on AdV-transduced DC. iDC, iDC.EGFP, mDC and DC matured post infection (mDC.EGFP) were evaluated 48 h after treatments for a surface phenotypic marker expression, b APM component expression, and c cytokine secretion patterns. Surface and APM evaluations were performed by flow cytometry as described in Fig. 1, while cytokine analysis by Luminex as described in Fig. 4. These data are representative of one of three experiments performed in different healthy donors
Fig. 8.
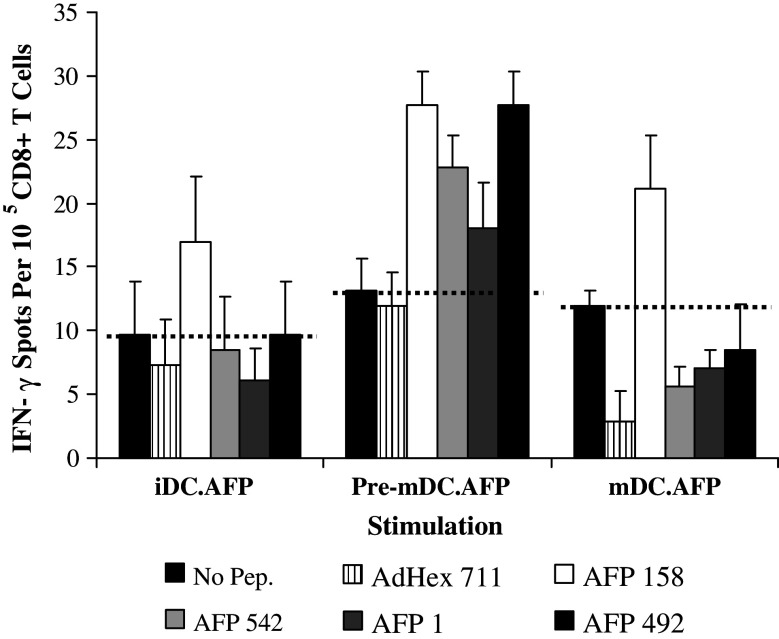
Impact of the timing of maturation signals on AdVhAFP-transduced DC stimulation of CD8+ T cells. A single round of IVS of HLA-A2+ healthy donor T cells was performed, using iDC, pre-mDC, and mDC infected with AdVhAFP (iDC.AFP, pre-mDC.AFP, and mDC.AFP respectively) as APC. Purified CD8+ T cells selstimulated in these cultures were tested against HLA-A2-restricted AFP158–166 (AFP158), AFP542–550 (AFP542), AFP1–9 (AFP1) and AFP492–500 (AFP492) AFP-derived epitopes, as well as AdHex711–721 (AdHex 711; adenovirus hexon peptide) in an ELISPOT assay. Tests were performed in triplicate wells, and the average T cell frequencies are shown. The dotted line represents background levels of IFN-γ spots. These data are representative of one of two similar experiments performed in different healthy donors
Results
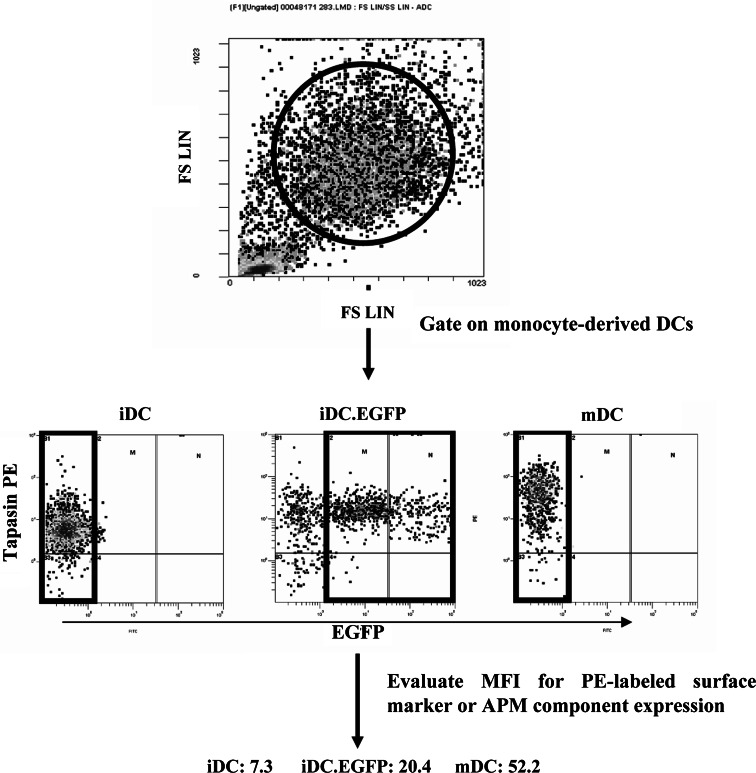
Recently, we observed an increase in breadth of the repertoire of MHC class I antigen-restricted peptide-specific CD8+ T cells activated by AdV-transfected DC [15]. Based on this work, and the studies of APM component regulation in DC [20] we wished to investigate how AdV transduction could impact not only DC maturation, but also peptide presentation. For these experiments we infected human monocyte-derived iDC with Ad.EGFP (iDC.EGFP). We used the multiplicity of infection (MOI) of 500 particle forming units (pfu) per 1 DC as this viral load yielded high level transduction efficacy and viability (data not shown) while allowing for a range of transgene expression levels in DC. The expression of EGFP allowed us to specifically follow by flow cytometry, the extent of modulation of surface markers and intracellular APM components in AdV-infected DC (Fig. 1) and compare these values to those derived from analysis of IFN-γ/LPS-matured mDC and of iDC.
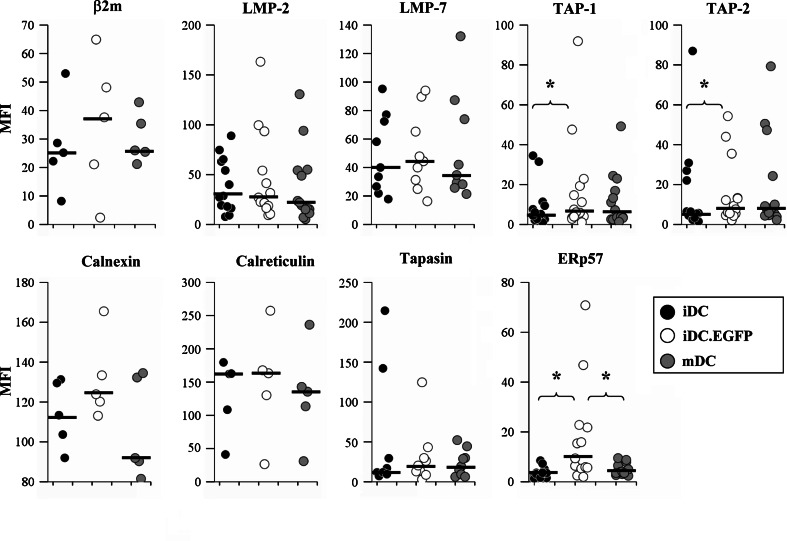
Fig. 1.
Strategy for surface marker and APM component analysis. Monocyte-derived iDC were either untreated, transduced with Ad.EGFP (iDC.EGFP) or matured with IFN-γ and LPS (mDC); 48 h after treatment, DC were collected and analyzed by flow cytometry against a number of PE-labeled antibodies against surface markers and APM components. Top on the forward scatter vs. side scatter plot, DC were gated on as large, granular cells. Middle the relative level of protein expression was evaluated by measuring and correlating mean fluorescent intensity (MFI) values between groups. Bottom MFI was measured for all the iDC and mDC, while for iDC.EGFP only EGFP positive cells were analyzed
AdV transduction induces an ‘intermediate’ maturation phenotype in human DC
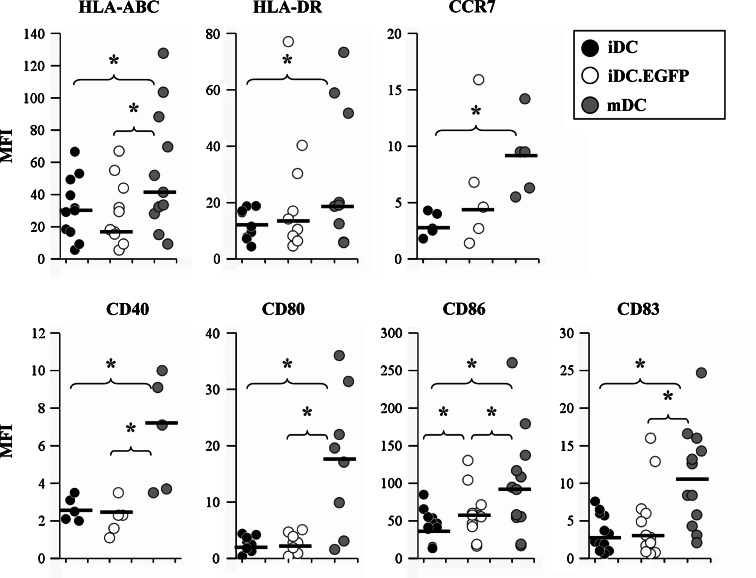
First, we wished to confirm our previous results that AdV transfection causes upregulation of maturation markers in DC, and extend these findings to incorporate additional molecules important for DC antigen presentation. Expression of HLA-ABC, HLA-DR, costimulatory markers CD40, CD80 and CD86, the lymph node trafficking marker CCR7, and the DC maturation marker CD83 were evaluated (Fig. 2). Surface staining for these ‘classical’ maturation markers showed that iDC.EGFP expressed an intermediate level of maturation (Fig. 2). In AdV-transduced DC, a statistically significant increase in expression was observed for CD86. A higher median MFI level was detected for HLA-DR, CD83, and CCR7, although these differences did not reach statistical significance. In contrast to HLA-DR, no clear modulation pattern was observed for HLA-ABC levels in AdV-transduced DC. A statistically significant increase in HLA-ABC expression was detected on mDC (mMFI = 41.4), and previously, an moi of 1,000 was required to induce any increase in MHC class I [1], indicating an moi-dependence for modulation of some cell surface molecules [5].
Fig. 2.
Evaluation of surface phenotypic markers expressed on DC from healthy donors. iDC, iDC.EGFP, and mDC were labeled for flow cytometry using PE-conjugated antibodies for HLA-ABC, HLA-DR, CCR7, CD40, CD80, CD86 and CD83 as described in “Materials and methods”. Data was analyzed as described in Fig. 1. Black bars represent mMFI for the donors analyzed. Asterisks represent statistically significant differences (P ≤ 0.05) between two analyzed groups according to the 2-sided paired t test
We also evaluated CD80 and CD40 surface expression. Unlike IFN-γ/LPS-mediated maturation, AdV did not significantly modulate CD80, and might reduce CD40 expression (Fig. 2). There was no change in the expression of CD80 in AdV-transduced DC (mMFI = 2.8 for infected cells compared to mMFI = 2.75 for iDC), but an increase in mDC (mMFI = 18.35). AdV-transduced DC had a lower expression of CD40 (mMFI = 2.3) than mDC (mMFI = 7.1), but similar to that by iDC (mMFI = 2.5). We observed a decrease in CD40 expression in 3/5 donors.
APM component modulation in AdV-transduced DC
Next we evaluated direct effects of AdV infection on the expression of APM molecules. We evaluated a broad panel of APM components, and analysis of AdV-transduced DC showed strong staining for β2m, the IFN-γ-inducible proteasomal subunits LMP-2 and LMP-7, endoplasmic reticulum peptide transporters TAP-1 and TAP-2, and MHC class I antigen folding and stabilizing chaperones calnexin, calreticulin, ERp57 and tapasin. The results were reproducible across multiple donors.
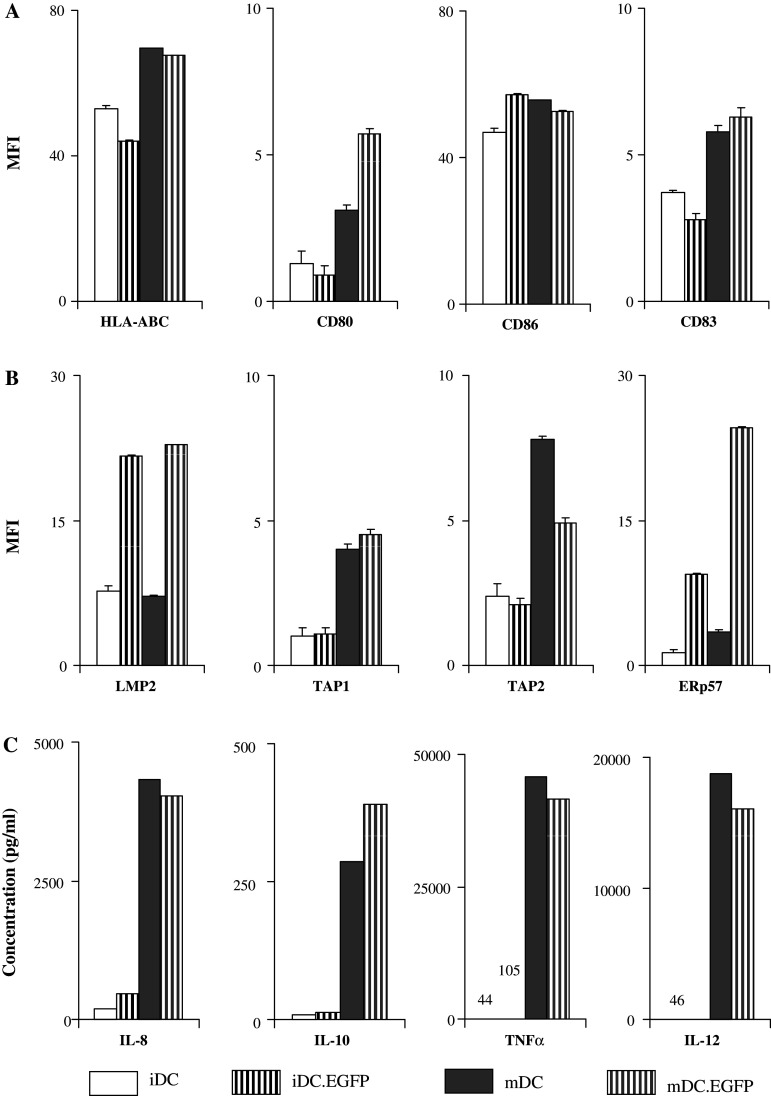
The most striking and significant change associated with AdV transduction (and not cytokine-driven maturation) was an increase in ERp57 (Fig. 3). This increase was observed in 11/13 donors tested, while maturation increased expression of ERp57 in 10/13 donors, but not to the same extent. This increase was the highest magnitude increase observed among tested APM markers, with mMFI = 3.5 for iDC, mMFI = 9.4 for iDC.EGFP, and mMFI = 3.9 for mDC.
Fig. 3.
Assessment of APM component expression in DC. iDC, iDC.EGFP, and mDC were labeled for flow cytometry using mouse monoclonal antibodies against β2m, LMP-2, LMP-7, TAP-1, TAP-2, calnexin, calreticulin, tapasin, and ERp57, and then stained with PE-labeled anti-mouse secondary antibody. The data were analyzed as described in Fig. 1. Black bars represent mMFI for the donors analyzed. Asterisks represent statistically significant differences (P ≤ 0.05) between two analyzed groups according to the two-sided paired t test
Significant AdV transduction-mediated increases were also observed for the expression of the peptide transporters TAP-1 (mMFI = 6.0 vs. mMFI = 4.85 for iDC) and TAP-2 (mMFI = 7.6 vs. mMFI = 5.7 for iDC). These increases were seen in 12/16 (TAP-1) and 10/15 (TAP-2) donors tested. The median MFI levels for these molecules were similar to those found in IFN-γ/LPS-driven maturation of DC (mMFI = 5.6 for TAP-1 and mMFI = 7.8 for TAP-2).
Several other APM components were upregulated in response to AdV transduction, although not significantly, including β2m (3/5 donors positive, mMFI = 37.6 vs. mMFI = 25.2 for iDC), LMP-2 (8/14 donors, mMFI = 25.45 vs. mMFI = 27.9 for iDC), LMP-7 (6/9 donors, mMFI = 44.4 vs. mMFI = 40 for iDC), calnexin (2/5 donors, mMFI = 123.9 vs. mMFI = 113.4 for iDC), and tapasin (5/9 donors, mMFI = 20.4 vs. mMFI = 11.9 for iDC). Of these, LMP-2 and β2m were over-expressed more frequently and at higher magnitudes in iDC.EGFP than in mDC (5/15 donors, mMFI = 20.65 for LMP-2; 2/5 donors, mMFI = 26 for β2m).
Together, these results suggest that AdV transduction can specifically modulate APM components, and does so differently from the changes observed after cytokine maturation. Importantly, several changes (ERp57, LMP-2, LMP-7, β2m) were specific to AdV transduction and were less affected by the IFN-γ/LPS-driven maturation process. Changes in the expression of this subset of APM components could also potentially affect the repertoire of epitopes being presented.
DC cytokine production
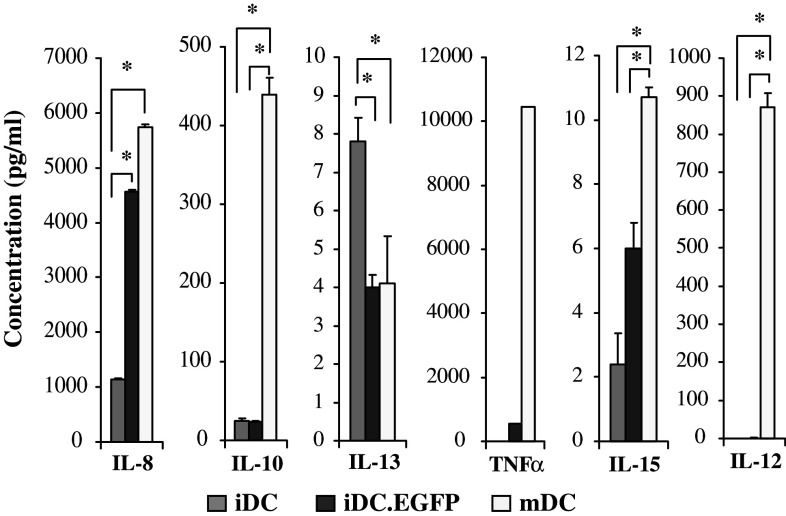
Both AdV and maturation can also impact the production of cytokines by DC, and this impacts the local environment in which antigen presentation occurs. Therefore, we tested the cytokines secreted by the three DC populations evaluated. Supernatants from five tested donors were evaluated by Luminex bead array. Average values for the five donors are shown in Fig. 4. Similar to flow cytometry results, the cytokine profile in iDC.EGFP appeared to be intermediate between that in iDC and in mDC. Cytokines IL-8 and IL-15 were significantly upregulated in AdV-infected cells when compared to iDC; mDC produced even higher levels. TNFα was also increased by AdV transduction, and further increased by maturation. Only minute amounts of IL-12p70 were produced by iDC.EGFP, but significant amounts were produced mDC. Interestingly, IL-13 was significantly down-regulated in iDC.EGFP to the same extent as in mDC. Importantly, mDC, unlike iDC and iDC.EGFP, produced significantly high levels of IL-10 in agreement with our previous data [5].
Fig. 4.
DC cytokine secretion. Supernatants collected from 48 h iDC, iDC.EGFP and mDC cultures were analyzed by Luminex bead array for IL-8, IL-10, IL-13, TNFα, IL-15, and IL-12. Data presented represents average cytokine profiles established for five healthy donors tested. Asterisks represent statistically significant differences (P ≤ 0.05) between two analyzed groups according to the two-sided paired t test
Structural AdV proteins are responsible for AdV-induced changes of DC
The recombinant viruses used in this study are E1-deleted and E3- mutated or deleted, and, therefore, replication-defective. However, these viruses are known to be “leaky”, with some residual level of viral gene expression [31]. In order to determine whether this low level of AdV gene expression might be responsible for the changes in expression levels of cell surface markers or of intracellular APM components as opposed to the process of infection itself (mediated by structural viral proteins [31]) we tested heat-inactivated virions. Specifically, we compared surface markers and APM component expression in iDC.EGFP and heat-inactivated Ad.EGFP “transduced” iDC (iDC.hiEGFP). The lack of viral gene expression was confirmed by the lack of EGFP expression in iDC.hiEGFP and by the lack of virus production in E1+ permissive cells by viral titer plaque assay (data not shown). For this study, we focused on a subset of APM and cell surface molecules that were most responsive to the treatments. The changes observed in surface marker (HLA-ABC, CD86, CD83) and APM component (TAP-1, TAP-2, ERp57) expression levels (Fig. 5) and cytokine secretion patterns (data not shown) were similar in iDC infected with active or heat-inactivated vectors. These findings indicate that inactivated virions were competent to induce the changes we observed.
Combining AdV transduction with maturation signals yields superior DC that are potent stimulators of antigen-specific CD8+ T cells
Given that mDC expressed higher levels of most tested cell surface markers, cytokines, and several APM components, we next investigated the combination of AdV transduction and DC maturation. Previously, we found that different DC maturation treatments used after AdV transduction did not improve T cell activation compared to AdV-transduced DC alone [5]. In view of our experience with IFNγ and LPS-matured DC [32, 33] we revisited this issue, and also tested AdV transduction after the initiation of DC maturation for the first time.
We initially compared DC that were matured post-transduction (mDC.EGFP) to iDC, iDC.EGFP, and mDC (Fig. 6). We focused our assessment on the four most highly regulated surface (HLA-ABC, CD80, CD86, CD83) and APM (LMP-2, TAP-1, TAP-2, ERp57) molecules. The expression of surface markers that were minimally affected by AdV-transduction alone (HLA-ABC, CD80, CD83) was upregulated with subsequent maturation, to a level similar to (or greater than) mDC (CD80, CD83), indicating that AdV did not inhibit the ability of DC to fully mature with subsequent treatment. It was noteworthy that while AdV did not inhibit subsequent DC maturation, AdV-specific modulations observed for APM continued to be positive post-maturation, and were further enhanced (LMP-2, ERp57). AdV transduction and maturation of DC resulted in similarly high levels of IL-8, IL-10, IL-12, and TNFα (Fig. 6c).
Another important question was whether the order of transduction and maturation treatment affects expression patterns of surface and APM molecules (Fig. 7). In general, DC matured prior to AdV transduction (pre-mDC.EGFP) had surface marker (Fig. 7a) and APM component (Fig. 7b) expression patterns similar to mDC.EGFP. Maturing DC prior to AdV transduction did enhance the expression of surface CD83 and intracellular TAP-2. The most notable difference between pre-mDC.EGFP and mDC.EGFP was the level of cytokine secretion (Fig. 7c). Similar to mDC, pre-mDC cytokine secretion was enhanced post AdV transduction. However, while mDC and mDC.EGFP secreted high levels of IL-10, IL-12, and TNFα, pre-mDC and pre-mDC.EGFP secreted significantly lower levels. However, these levels were still higher than those secreted by iDC and iDC.EGFP.
Fig. 7.
The order of transduction and maturation treatment affects expression patterns of surface markers, APM components, and cytokine secretion. iDC, iDC.EGFP, mDC and mDC.EGFP, as well as DC matured on day 4 (pre-mDC) and transduced with Ad.EGFP on day 5 (pre-mDC.EGFP) are compared; 48 h after treatments (day 7), all the groups are evaluated for a surface phenotypic marker expression, b APM component expression, and c cytokine secretion patterns. Surface and APM evaluation was performed by flow cytometry as described in Fig. 1; cytokine analysis by Luminex was performed as described in Fig. 4. These data are representative of one of three experiments performed in different healthy donors
While surface phenotype and cytokine production are useful approximations of DC maturation state, the ability to stimulate antigen-specific CD8+ T cell responses is the best measure of DC function. To evaluate the impact of the timing of DC maturation and AdV transduction, we performed a single 7-day stimulation of healthy donor cells, using iDC, pre-mDC, and mDC infected with AdV encoding the human AFP [AdVhAFP; iDC.AFP, pre-mDC.AFP, and mDC.AFP respectively]. CD8+ T cells selected from these cultures were tested against two immunodominant [AFP158–166 (AFP158), AFP542–550 (AFP542)] and two subdominant [AFP1–9 (AFP1), AFP492–500 (AFP492)] AFP-derived epitopes in an ELISPOT assay (Fig. 8). In agreement with our previous data, a single stimulation with iDC.AFP was unable to expand detectable AFP peptide-specific T cells from any healthy donors tested [15, 34]. However, AFP-specific CD8+ T cell responses were elicited after only one round of stimulation with pre-mDC.AFP or mDC.AFP. Importantly, while mDC.AFP stimulation induced a significant response to the immunodominant epitope AFP158 only (1.7-fold over background), pre-mDC.AFP stimulated responses to both of the immunodominant epitopes AFP158 (2.1-fold) and AFP542 (1.7-fold) as well as to the subdominant epitope, AFP492 (2.1-fold). AdV hexon peptide-specific cells were also detected with pre-mDC.AFP, but at a lower frequency than AFP antigen-specific T cells, indicating that responses to viral antigens do not overwhelm the encoded transgene TAA responses. These data suggest that maturing DC prior to AdV transduction significantly enhanced the ability of DC to stimulate a broad repertoire of antigen-specific T cells.
Discussion
For efficient stimulation of Type-1 CD4+ and CD8+ T cell responses, three signals are required. Signal 1 is the antigen-specific signal mediated by the ligation of T cell receptor (TCR) and MHC-peptide complex. The specificity of T cell responses is dependent on the repertoire of peptides processed and presented by an APC. Signal 2 is antigen independent, and is delivered by ligation of co-stimulatory receptors and ligands, such as CD28 on T cells by CD80/CD86 or CD40 by CD40 ligand (CD40L). The lack of signal 2 induces anergy in T cells. As professional APC, DC are capable of providing strong signals 1 and 2 due to high levels of expression of MHC class I and multiple costimulatory molecules. Signal 3 is provided by the cytokine milieu, and is essential for the development of type 1/type 2 skewed T cell responses [35]. For tumor immunotherapy, it was demonstrated that Type-1 responses are important for the rejection of tumors, as well as long term survival of patients [36, 37]. Cytokines such as IL-12p70 were shown to skew these responses [38]. DC are also known to exhibit a high degree of plasticity, and are highly responsive to various environmental cues, including virus-mediated signals. In this manuscript, we analyzed the effect of AdV transduction on all three signals in DC.
First we analyzed the effect of AdV transduction on the expression of DC surface markers. Surface staining showed that iDC.EGFP express an intermediate level of maturation. These data confirmed our previous results that the expression levels of maturation markers fall between iDC and fully mDC [5]. We observed a statistically significant increase of CD86 mediated by AdV transduction when compared to iDC, as well as increasing trends for HLA-DR, CD83, and CCR7 (a chemokine receptor associated with leukocyte migration into draining lymph nodes). However, HLA-ABC and CD80 were not significantly increased and AdV-transduced DC had a slightly reduced expression of CD40, compared to iDC. Such alterations could potentially affect the ability of DC to stimulate TAA-specific T cell responses. Co-stimulatory molecules CD40, CD80, and CD86 are independently capable of co-stimulating human T cells, although with differing kinetics [39, 40]. Each molecule has been found to be dominant at different time points of T cell stimulation, and for different subtypes of T cells (naïve or memory) [39–42]. In the case of AdV-transduced DC, the optimal delivery of MHC class I/peptide epitope complexes required maturation with AdV, which resulted in upregulation of MHC class I, stronger enhancement of multiple co-stimulatory pathways (Figs. 6a, 7a) and could generate more potent TAA-specific responses that could be applicable to cancer therapies.
We observed that AdV transduction can specifically modulate APM components, some of which are also impacted by cytokine maturation (TAP-1, TAP-2, tapasin), and others (ERp57, LMP-2) were not as affected by cytokine maturation which could also potentially affect the repertoire of epitopes being presented. The most significant change specifically associated with AdV transduction, was an increase of ERp57 expression. ERp57 is the soluble thiol oxidoreductase that forms a heterodimer with tapasin which is crucial for enhancing the loading and editing of peptides loaded onto MHC class I molecules. Both ERp57 and tapasin are stable components of the peptide-loading complex found inside the ER which also includes MHC class I heavy chain, β2m, calreticulin, TAP-1 and TAP-2. Oxidoreductases such as ERp57 can generate or destroy disulfide bonds in their substrates to enable their proper folding. ERp57 was shown to regulate both the construction and destruction of peptide-MHC class I antigen complexes and that covalent formation of dimers with tapasin is the key to these two opposing functions [43, 44]. It was shown that if ERp57 cannot form a proper heterodimer with tapasin, it can reduce the α2 disulfide bond in MHC class I molecules. In other words, tapasin must sequester ERp57 to prevent its reduction of the disulfide bond in the α2 domain and destruction of the peptide-binding groove [43]. ERp57 and tapasin are both upregulated post-transduction, ERp57 expression is three fold, while tapasin is twofold greater in iDC.EGFP than the iDC baseline. These observations suggest that some of the induced ERp57 might not be sequestered by tapasin due to concentration differences, and thusly negatively affect the formation of properly folded/peptide-loaded MHC class I molecules. If so, that could explain the lack of upregulation observed for HLA-ABC in iDC.EGFP.
Other statistically significant increases in AdV-transduced DC were in TAP-1 and TAP-2 expression. While this upregulation will most likely lead to an increase in efficiency of peptide uptake into ER, it will not necessarily impact the repertoire of peptides presented. Modulations in the expression of inducible proteasomal subunits LMP-2 and LMP-7 could impact the presented repertoire. The immunoproteasome is more likely to generate peptides with hydrophobic and basic C-terminal residues and less likely to generate peptides with acidic C-terminal residues [45–47]. A number of antigenic epitopes are differentially processed by immunoproteasome-expressing cells [48, 49], hence, AdV transduction-mediated increase of expression of LMP-2 or LMP-7 could lead to differential antigen processing by the immunoproteasome. This effect was further enhanced by maturing DC pre- or post-transduction.
Even though IFNγ and LPS were both reported to independently enhance LMP-2 expression [50, 51], IFNγ and LPS together did not upregulate LMP-2 in DC in our system. Similar to several other studies, we have observed that LMP-2 are expressed at high constitutive levels in iDC [52, 53], and that the expression is decreased in mDC [52]. These results, however, somewhat differ from our group’s previous publication where maturation with TNF-α, IL-1β and IL-6 induced higher expression of immunoproteasomal subunits [20]. These differences could potentially be attributed to differences in the DC maturation stimuli used (here we used IFN-γ/LPS), or in the time-point of analysis (here, 48 h post-transduction; previously, 24 h after treatment). Along with increased expression levels of TAP-1 and TAP-2, these results suggest that AdV-mediated APM modulations could alter the repertoire of processed and presented epitopes and impact the breadth of T cell clones activated.
We next evaluated the third signal provided by DC-secreted cytokines (Fig. 4). A key difference between iDC.EGFP and IFN-γ/LPS matured DC was that transduced DC secreted little or no IL-10 and IL-12. Higher cytokine levels may require MOI 1000 [5] instead of the MOI 500 used here. Maturation following transduction induced the secretion of high levels of IL-10 and IL-12, similar to or greater than those secreted by mDC. This expression appeared decreased in pre-mDC and pre-mDC.EGFP. However, Th1-skewing IL-12 and TNF-α cytokines were still being produced by pre-mDC.EGFP. Low levels of these cytokines produced locally may be sufficient for effective stimulation of T cell responses.
It was critical to test the efficacy and timing of maturation on the ability of AdV-transduced DC to stimulate antigen-specific CD8+ T cells. We used AdVhAFP to transduce DC, and used them for a single round of IVS. Previously, we have found that healthy donor PBMC require at least 3 weeks of IVS to expand detectable frequencies of AFP-specific CD8+ T cells [15, 25, 34]. While iDC.AFP were again ineffective at stimulating T cell responders in a 7-day culture, DC first matured and then transduced were the most efficient APC capable of stimulating CD8+ T cell responses against a range of immunodominant and subdominant AFP-derived peptides (Fig. 8). DC matured post-transduction were superior to iDC.AFP, but appeared to have a more limited repertoire than pre-mDC.AFP. These results suggest that maturing DC prior to AdV transduction might be optimal for polyclonal type-1 T cell stimulation.
Recently, it was reported that AdV fiber shaft is critical for AdV activation of DC [54], but the exact mechanism has yet to be fully elucidated. Here we have shown that DC maturation and APM upregulation was not dependent on AdV replication, gene expression or function, but rather on AdV structural proteins and viral DNA (possibly the fiber shaft). Taken together, phenotypic maturation markers, APM component molecules, and cytokine profiles suggest that AdV transduction of DC alone leads to acquisition of an intermediary level of DC maturation. We find that optimal DC treatment with IFN-γ/LPS maturation signals preceding AdV leads to upregulation of specific APM components and also positively impacts the breadth of peptide-specific CD8+ T cells (which could be impacted by the efficiency of peptide presentation related to APM regulation, improved costimulation, as well as modulated cytokine milieu). In order to optimize DC vaccines for future clinical trials, antigen engineering and additional maturation signals may be needed to upregulate all signals needed for efficient migration and priming of immunodominant and subdominant antigen-specific T cells.
Acknowledgments
This work was supported by the University of Pittsburgh Cancer Institute and the Henry L. Hillman Foundation (LHB).
References
- 1.Arthur JF, et al. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4(1):17–25. [PubMed] [Google Scholar]
- 2.Butterfield LH, et al. Generation of melanoma-specific cytotoxic T lymphocytes by dendritic cells transduced with a MART-1 adenovirus. J Immunol. 1998;161(10):5607–5613. [PubMed] [Google Scholar]
- 3.Diao J, et al. Human PBMC-derived dendritic cells transduced with an adenovirus vectorinduce cytotoxic T-lymphocyte responses against a vector-encoded antigen in vitro. Gene Ther. 1999;6(5):845–853. doi: 10.1038/sj.gt.3300899. [DOI] [PubMed] [Google Scholar]
- 4.Lundqvist A, et al. Recombinant adenovirus vector activates and protects human monocyte-derived dendritic cells from apoptosis. Hum Gene Ther. 2002;13(13):1541–1549. doi: 10.1089/10430340260201635. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher L, et al. Human dendritic cell maturation by adenovirus transduction enhances tumor antigen-specific T-cell responses. J Immunother. 2004;27(3):191–200. doi: 10.1097/00002371-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Perreau M, et al. Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: implications for clinical efficacy. J Virol. 2007;81(7):3272–3284. doi: 10.1128/JVI.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan PH, et al. Immunolipoplexes: an efficient, nonviral alternative for transfection of human dendritic cells with potential for clinical vaccination. Mol Ther. 2005;11(5):790–800. doi: 10.1016/j.ymthe.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.He Y, et al. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174(6):3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 9.Metharom P, Ellem KA, Wei MQ. Gene transfer to dendritic cells induced a protective immunity against melanoma. Cell Mol Immunol. 2005;2(4):281–288. [PubMed] [Google Scholar]
- 10.Nakamura M, et al. Dendritic cells transduced with tumor-associated antigen gene elicit potent therapeutic antitumor immunity: comparison with immunodominant peptide-pulsed DCs. Oncology. 2005;68(2–3):163–170. doi: 10.1159/000086770. [DOI] [PubMed] [Google Scholar]
- 11.Yuan J, et al. Langerhans cells derived from genetically modified human CD34+ hemopoietic progenitors are more potent than peptide-pulsed Langerhans cells for inducing antigen-specific CD8+ cytolytic T lymphocyte responses. J Immunol. 2005;174(2):758–766. doi: 10.4049/jimmunol.174.2.758. [DOI] [PubMed] [Google Scholar]
- 12.Curti A, et al. Interleukin-12 production by leukemia-derived dendritic cells counteracts the inhibitory effect of leukemic microenvironment on T cells. Exp Hematol. 2005;33(12):1521–1530. doi: 10.1016/j.exphem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Lotem M, et al. Presentation of tumor antigens by dendritic cells genetically modified with viral and nonviral vectors. J Immunother (1997) 2006;29(6):616–627. doi: 10.1097/01.cji.0000211312.36363.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Leeuwen EB, et al. Transduction with a fiber-modified adenoviral vector is superior to non-viral nucleofection for expressing tumor-associated Ag mucin-1 in human DC. Cytotherapy. 2006;8(1):36–46. doi: 10.1080/14653240500508166. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, et al. Hierarchy of AFP-specific T cell responses in subjects with AFP-positive hepatocellular cancer. J Immunol. 2006;177(1):712–721. doi: 10.4049/jimmunol.177.1.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evdokimova VN, et al. AFP-specific CD4+ helper T-cell responses in healthy donors and HCC patients. J Immunother. 2007;30(4):425–437. doi: 10.1097/CJI.0b013e31802fd8e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch J, Tampe R. The macromolecular peptide-loading complex in MHC class I-dependent antigen presentation. Cell Mol Life Sci. 2006;63:653–662. doi: 10.1007/s00018-005-5462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishito M, et al. Immunoproteasomes and immunosenescence. Ageing Res Rev. 2003;2:419–432. doi: 10.1016/S1568-1637(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 19.Connolly N, et al. Levels of antigen processing machinery components in dendritic cells generated for vaccination of HIV-1+ subjects. Aids. 2007;21(13):1683–1692. doi: 10.1097/QAD.0b013e32825eabbc. [DOI] [PubMed] [Google Scholar]
- 20.Whiteside TL, et al. Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol. 2004;173(3):1526–1534. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 21.Tourkova IL, et al. Restoration by IL-15 of MHC class I antigen-processing machinery in human dendritic cells inhibited by tumor-derived gangliosides. J Immunol. 2005;175(5):3045–3052. doi: 10.4049/jimmunol.175.5.3045. [DOI] [PubMed] [Google Scholar]
- 22.Callahan MK, et al. Heat shock up-regulates lmp2 and lmp7 and enhances presentation of immunoproteasome-dependent epitopes. J Immunol. 2006;177(12):8393–8399. doi: 10.4049/jimmunol.177.12.8393. [DOI] [PubMed] [Google Scholar]
- 23.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 24.Meissner M, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11(7):2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 25.Butterfield LH, et al. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59(13):3134–3142. [PubMed] [Google Scholar]
- 26.Bandoh N, et al. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005;66(3):185–194. doi: 10.1111/j.1399-0039.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 27.Ogino T, et al. Association of tapasin and HLA class I antigen down-regulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clin Cancer Res. 2003;9(11):4043–4051. [PubMed] [Google Scholar]
- 28.Wang X, et al. A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods. 2005;299(1–2):139–151. doi: 10.1016/j.jim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Tatsumi T, et al. MAGE-6 encodes HLA-DRbeta1*0401-presented epitopes recognized by CD4+ T cells from patients with melanoma or renal cell carcinoma. Clin Cancer Res. 2003;9(3):947–954. [PubMed] [Google Scholar]
- 30.Tatsumi T, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196(5):619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philpott NJ, et al. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc Natl Acad Sci USA. 2004;101(16):6200–6205. doi: 10.1073/pnas.0308368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vujanovic L, et al. A mycoplasma peptide elicits heteroclitic CD4+ T cell responses against tumor antigen MAGE-A6. Clin Cancer Res. 2007;13(22 pt 1):6796–6806. doi: 10.1158/1078-0432.CCR-07-1909. [DOI] [PubMed] [Google Scholar]
- 33.Vujanovic L, et al. IL-12p70 and IL-18 gene-modified dendritic cells loaded with tumor antigen-derived peptides or recombinant protein effectively stimulate specific Type-1 CD4+ T-cell responses from normal donors and melanoma patients in vitro. Cancer Gene Ther. 2006;13(8):798–805. doi: 10.1038/sj.cgt.7700964. [DOI] [PubMed] [Google Scholar]
- 34.Butterfield LH, et al. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J Immunol. 2001;166(8):5300–5308. doi: 10.4049/jimmunol.166.8.5300. [DOI] [PubMed] [Google Scholar]
- 35.Stager S, Kaye PM. CD8+ T-cell priming regulated by cytokines of the innate immune system. Trends Mol Med. 2004;10(8):366–371. doi: 10.1016/j.molmed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Lowes MA, et al. T helper 1 cytokine mRNA is increased in spontaneously regressing primary melanoma. J Invest Derm. 1997;108:914–919. doi: 10.1111/1523-1747.ep12292705. [DOI] [PubMed] [Google Scholar]
- 37.Schwaab T, et al. A randomized phase II trial comparing two different sequence combinations of autologous vaccine and human recombinant interferon gamma and human recombinant interferon alpha2B therapy in patients with metastatic renal cell carcinoma: clinical outcome and analysis of immunological parameters. J Urol. 2000;163:1322–1327. doi: 10.1016/S0022-5347(05)67771-3. [DOI] [PubMed] [Google Scholar]
- 38.Ahn HJ, et al. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- 39.Rogers NJ, et al. Cross-species costimulation: relative contributions of CD80, CD86, and CD40. Transplant. 2003;75(12):2068–2076. doi: 10.1097/01.TP.0000069100.67646.08. [DOI] [PubMed] [Google Scholar]
- 40.Rogers NJ, et al. CD40 can costimulate human memory T cells and favors IL-10 secretion. Eur J Immunol. 2003;33:1094–1104. doi: 10.1002/eji.200323475. [DOI] [PubMed] [Google Scholar]
- 41.Fields PE, et al. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naive CD8+ TCR-transgenic T cells. J Immunol. 1998;161:5268–5275. [PubMed] [Google Scholar]
- 42.Manzotti CN, et al. Integration of CD28 and CTLA-4 Function Results in Differential Responses of T Cells to CD80 and CD86. Eur J Immunol. 2006;36:1413–1422. doi: 10.1002/eji.200535170. [DOI] [PubMed] [Google Scholar]
- 43.Kienast A, et al. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat Immunol. 2007;8:864–872. doi: 10.1038/ni1483. [DOI] [PubMed] [Google Scholar]
- 44.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol. 2007;8:873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 45.Fruh K, et al. Displacement of housekeeping proteasome subunits by MHC-encoded LMPs: a newly discovered mechanism for modulating the multicatalytic proteinase complex. EMBO J. 1994;13:3236–3244. doi: 10.1002/j.1460-2075.1994.tb06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaczynska M, et al. Proteasome subunits X and Y alter peptidase activities in opposite ways to the interferon-γ-induced subunits LMP2 and LMP7. J Biol Chem. 1996;271:17275–17280. doi: 10.1074/jbc.271.29.17275. [DOI] [PubMed] [Google Scholar]
- 47.Gaczynska M, et al. Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proc Natl Acad Sci USA. 1994;91:9213–9217. doi: 10.1073/pnas.91.20.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz K, et al. Overexpression of the proteasome subunits LMP2, LMP7, and MECL-1, but not PA28 α/β, enhances the presentation of an immunodominant lymphocytic choriomeningitis virus T cell epitope. J Immunol. 2000;165:768–778. doi: 10.4049/jimmunol.165.2.768. [DOI] [PubMed] [Google Scholar]
- 49.Sijts AJAM, et al. MHC class I antigen processing of an adenovirus CTL epitope is linked to the levels of immunoproteasomes in infected cells. J Immunol. 2000;164:4500–4506. doi: 10.4049/jimmunol.164.9.4500. [DOI] [PubMed] [Google Scholar]
- 50.Macagno A, et al. Pronounced up-regulation of the PA28α/β proteasome regulator but little increase in the steady-state content of immunoproteasome during dendritic cell maturation. Eur J Immunol. 2001;31:3271–3280. doi: 10.1002/1521-4141(200111)31:11<3271::AID-IMMU3271>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Marques L, et al. STAT1 regulates lipopolysaccharide- and TNF-alpha-dependent expression of transporter associated with antigen processing 1 and low molecular mass polypeptide 2 genes in macrophages by distinct mechanisms. J Immunol. 2004;173(2):1103–1110. doi: 10.4049/jimmunol.173.2.1103. [DOI] [PubMed] [Google Scholar]
- 52.Li J, et al. Bipartite regulation of different components of the MHC class I antigen-processing machinery during dendritic cell maturation. Int Immunol. 2001;13(12):1515–1523. doi: 10.1093/intimm/13.12.1515. [DOI] [PubMed] [Google Scholar]
- 53.Macagno A, et al. Dendritic cells up-regulate immunoproteasomes and the proteasome regulator PA28 during maturation. Eur J Immunol. 1999;29:4037–4042. doi: 10.1002/(SICI)1521-4141(199912)29:12<4037::AID-IMMU4037>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 54.Cheng C, et al. Mechanism of ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 2007;3(2):e25. doi: 10.1371/journal.ppat.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]