Abstract
During the development of the nervous system embryonic neurons are incorporated into neural networks that underlie behaviour. For example, during embryogenesis in Drosophila, motor neurons in every body segment are wired into the circuitry that drives the simple peristaltic locomotion of the larva. Very little is known about the way in which the necessary central synapses are formed in such a network or how their properties are controlled. One possibility is that presynaptic and postsynaptic elements form relatively independently of each other. Alternatively, there might be an interaction between presynaptic and postsynaptic neurons that allows for adjustment and plasticity in the embryonic network. Here we have addressed this issue by analysing the role of synaptic transmission in the formation of synaptic inputs onto identified motorneurons as the locomotor circuitry is assembled in the Drosophila embryo. We targeted the expression of tetanus toxin light chain (TeTxLC) to single identified neurons using the GAL4 system. TeTxLC prevents the evoked release of neurotransmitter by enzymatically cleaving the synaptic-vesicle-associated protein neuronal-Synaptobrevin (n-Syb) [1]. Unexpectedly, we found that the cells that expressed TeTxLC, which were themselves incapable of evoked release, showed a dramatic reduction in synaptic input. We detected this reduction both electrophysiologically and ultrastructurally.
Results and discussion
The embryonic nervous system of Drosophila is spontaneously active many hours before hatching [2]. To determine whether such activity has a functional role in the development of embryonic neurons and their integration into neural networks, we investigated the consequences of inhibiting synaptic vesicle release in single identifiable neurons that are accessible to electrophysiological investigation [2]. We specifically compared the effects of inhibiting synaptic vesicle release in these single neurons with the effects of inhibiting release in all neurons of the central nervous system (CNS).
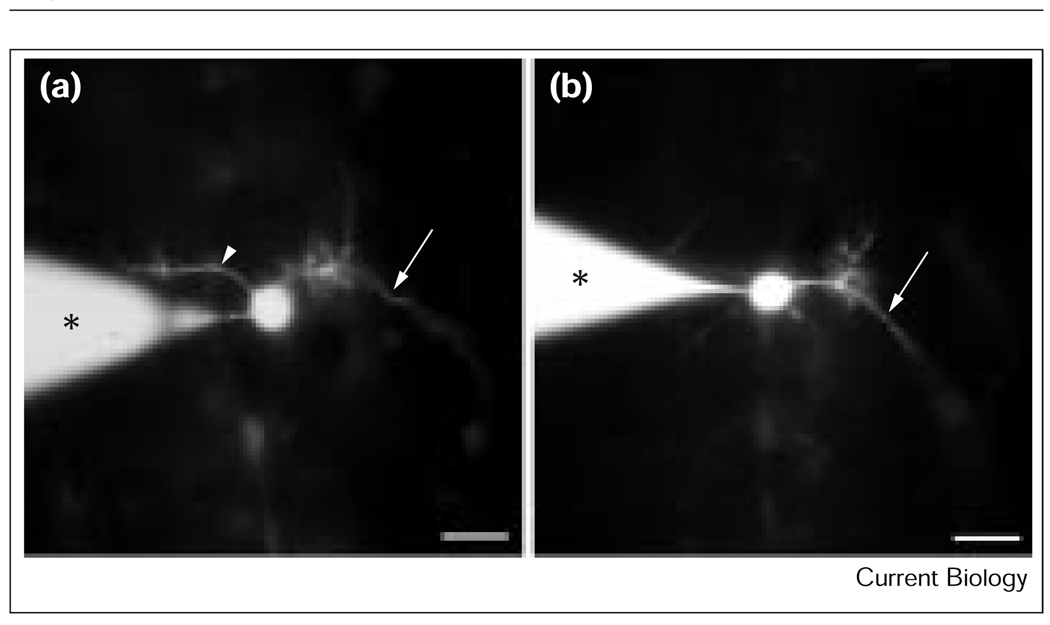
We recorded from two motorneurons in the Drosophila embryonic CNS, namely aCC and RP2. The identity of these cells was confirmed by labelling with carboxy-fluoroscein (Figure 1a,b) and, in addition, by identifying the labelled axon terminals of each neuron on their respective target muscles, DA1 and DA2 [3] (data not shown). In mature embryos at 19–20 hours after egg laying (AEL; hatching occurred at 21 hours), passive recording from aCC and/or RP2, neurons voltage-clamped at a membrane potential (Vh) of −60 mV, revealed that each neuron receives an almost identical pattern of synaptic input. The first type comprises discrete excitatory post-synaptic currents (epscs; Figure 2a). We have previously shown that these currents, which are absolutely dependent on both extracellular calcium and the ability of neurons to support action potentials, are the result of evoked, and not spontaneous (that is, minis), release of presynaptic neurotransmitter [2]. The second type of synaptic input was somewhat slower (lasting up to 2 seconds) and larger (up to 200 pA) than the discrete epscs and generated a sustained inward current that often supported a burst of action potentials (Figure 2b). These sustained events, the frequency of which was approximately 2–3 Hz, might represent the rhythmic activity that underlies larval locomotion. Although we have yet to identify any presynaptic neurons that give rise to either type of synaptic input to aCC and RP2 neurons, both appear to be cholinergic. This is because at the restrictive temperature, both types of input are completely absent in embryos carrying a temperature-sensitive mutation in choline acetyltransferase (Chats2), the synthetic enzyme for acetylcholine [4].
Figure 1.
Identification of the aCC and RP2 motorneurons. (a) The aCC motorneuron has a dendritic process (arrowhead) extending from the cell body in addition to its axon (arrow). (b) The RP2 motorneuron has only a single axonal projection (arrow). The patch electrode (asterisk) is shown in place in both images. The scale bars represent 10 µm.
Figure 2.
TeTxLC expression reduces synaptic activity. (a) Recordings, under voltage clamp (Vh = −60 mV), from an aCC (upper trace) and RP2 (lower trace) neuron show the presence of discrete epscs. (b) Recordings from aCC and RP2 neurons also reveal the presence of relatively slow and large sustained inward currents which often support a burst of action potentials (arrow). (c,d) The proportion of aCC and RP2 neurons that exhibited either (c) epscs or (d) sustained inward currents in the control parental lines (GAL4 and UAS) and when TeTxLC was expressed throughout the CNS. The white bars represent neurons in which these respective currents were observed (labelled +) and the grey bars represent neurons in which currents were not seen (labelled −). The given values represent the frequency per min of observed currents ± standard error (SE).
The targeted expression of TeTxLC throughout the CNS (driven by scabrous GAL4 [5]) significantly reduced the number of aCC and RP2 neurons that showed both epscs and sustained inward currents and, in addition, induced paralysis in the embryo. In the control parental lines (UAS and GAL4), five out of six aCC and three out of three RP2 neurons showed epscs. But, when TeTxLC was expressed, this number was reduced to just two out of ten (one out of five for each neuron type, p ≤ 0.01, Chi squared test). Furthermore, in the two neurons in which epscs were present, their frequency was much reduced (0.6 ± 0.04 per minute, compared with 3.1± 1 per minute in control neurons; Figure 2c). The number of aCC and RP2 neurons that exhibited sustained inward currents following expression of TeTxLC was reduced to zero in the five aCC and four RP2 neurons tested. In contrast, sustained inward currents were seen in five out of six aCC and three out of four RP2 neurons in the control UAS and GAL4 parental cell lines (p ≤ 0.01, Chi squared test; Figure 2d). It would seem, therefore, that TeTxLC, by acting through its only known activity—the proteolytic cleavage of n-Syb [1]—abolishes evoked synaptic communication within the CNS. This effect is presumably mediated by a block in evoked presynaptic release of neurotransmitter, as has been previously demonstrated in both vertebrate central neurons and at the Drosophila neuromuscular junction [1,6,7]. We tested two alternative possibilities, however: first, that expression of TeTxLC in neurons might prevent their reception of synaptic input; and second, that TeTxLC might alter a neuron’s ability to initiate action potentials and thereby release neurotransmitter. Neither was affected by expression of TeTxLC (see Supplementary material).
To determine whether there is a requirement for synaptic activity in the development of individual neurons, we used the GAL4–UAS system to limit TeTxLC expression to specific cells. An enhancer element of the even-skipped gene allowed us to confine expression of GAL4 (RRC-GAL4) to aCC and RP2. A UAS-tau LacZ reporter showed that expression in aCC (two cells per segment in wild type) was strong and consistent (1.8 cells per segment showed expression, n = 63 segments and nine embryos) whereas RP2 showed more variability in both parameters (1.25 cells per segment). A third neuron, pCC, which is an interneuron and the sibling of aCC, expressed GAL4 very weakly in isolated segments (0.25 cells per segment; Figure 3a). Because GAL4 was not expressed strongly in pCC neurons in the majority of segments, we used this neuron, which we are also able to identify and record from, as a control. To show that the expression of TeTxLC in such transgenic embryos specifically disrupts synaptic vesicle release from aCC (RP2 was not used because of its variable expression), we stimulated the axon of aCC and recorded the excitatory junctional current (ejc) evoked in its target muscle (Figure 3b). Expression of TeTxLC abolished ejcs in muscle DA1, but other motorneurons, such as those innervating muscles VL3 (Figure 3b) and VL1 (data not shown) were not affected. Thus, expression of TeTxLC clearly blocks synaptic output from aCC. Because TeTxLC expression was restricted to aCC and RP2 only, we might expect synaptic inputs to aCC from the surrounding non-expressing neurons to be normal.
Figure 3.
Selective expression of TeTxLC in aCC neurons deprives them of synaptic input. (a) Expression pattern of RRC-GAL4 visualised by anti-β-galactosidase immunostaining in three abdominal segments of a late stage 17 embryo (20 h AEL). Two strongly stained aCC neurons are present in all three segments (arrows), whereas staining of RP2 is more variable (arrowheads) and pCC staining is weak (open arrowhead). Only one pCC cell is visible in the three segments shown. The scale bar represents 10 µm. The inset shows a diagrammatic representation of the relative positions of aCC, pCC and RP2 neurons in a single abdominal segment in a late stage 17 embryo. Neurons are shaded to reflect their levels of RRC-GAL4 expression. (b) Electrical stimulation of the axon of an aCC neuron that is expressing TeTxLC under the control of RRC-GAL4 failed to evoke an ejc in its target muscle DA1. Immunostaining of the terminals of aCC using an anti-Fasciclin II antibody revealed no obvious morphological abnormalities (data not shown). Synaptic communication between other motorneurons and their target muscles was not affected (RP3 and muscle VL3 are shown). The RRC-GAL4 line was used as control. (c) Selective expression of TeTxLC results in a significant reduction in the number of aCC neurons that exhibit epscs (white bars, +) compared with expression of inactive toxin (TNT-VIF). (d) The proportion of pCC neurons that exhibit epscs (white bars, +), and their frequency, does not differ significantly in embryos expressing either TeTxLC or TNT-VIF. (e) Expression of TeTxLC similarly results in a significant decrease in the number of aCC neurons that show sustained inward currents compared to TNT-VIF (white bars, +). (f) Expression of TeTxLC does not affect the appearance or frequency of sustained currents in pCC. In each case, the white bars represent those neurons in which these respective currents were observed (+) and the grey bars represent those neurons in which currents were not seen (−). In (c–f) the given values represent the frequency per min of observed currents ± SE.
Surprisingly, however, the expression of TeTxLC in aCC using RRC-GAL4 produced a striking and unexpected reduction in the synaptic input (both epscs and sustained inward currents) that this neuron received compared with the same cell expressing TNT-VIF (an inactive form of TeTxLC). Epscs were present in 14 out of 18 aCC neurons that expressed TNT-VIF, whereas expression of TeTxLC reduced this number to just 5 out of 18 (p ≤ 0.05, Chi squared test). Analysis of epsc characteristics in the five neurons in which they persisted also showed that there was a significant reduction in frequency (1.2 ± 0.2 per minute, compared with 3.0 ± 0.5 per minute in control neurons; Figure 3c). Epsc amplitude, however, did not change (18 ± 1 pA, compared with 17 ± 1.2 pA). In contrast, expression of TeTxLC in pCC neurons did not reduce either the number that exhibited epscs or the epsc frequency recorded in them (Figure 3d). Expression of TeTxLC also produced a significant reduction in the overall number of aCC neurons that showed sustained inward currents (4 out of 18, compared with 14 out of 18 in neurons expressing TNT-VIF, p ≤ 0.05, Chi squared test; Figure 3e). In this instance, however, when sustained currents in aCC persisted, their frequency remained unchanged (2.5 ± 0.6 per minute, compared with 2.4 ± 0.5 in neurons expressing TNT-VIF; see Supplementary material). Recordings from pCC revealed sustained inward currents of similar properties and frequency to those in aCC. The proportion of pCC neurons that showed sustained inward currents was, again, unaffected by expression of TeTxLC (Figure 3f). Thus, the restricted expression of TeTxLC in aCC caused a significant reduction in its synaptic input, both epscs and sustained inward currents, a phenotype identical to that observed when TeTxLC was expressed throughout the entire CNS.
Our results reveal an unexpected consequence of TeTxLC expression in developing neurons. Not only is the release of neurotransmitter blocked, but there is an effective reduction of synaptic input to the cell expressing TeTxLC. This observation might imply that there is an absolute requirement for n-Syb in the postsynaptic neuron for synaptogenesis to occur. Alternatively, there might be a retrograde transfer of TeTxLC across developing synapses in the Drosophila embryo which, if sufficient, might block presynaptic input. There are a number of reasons for supposing that such a retrograde transfer of TeTxLC is unlikely. The strongest of these is the observation that, although diminished in frequency, the remaining epscs observed when TeTxLC was expressed in aCC neurons were identical in amplitude to controls. Thus, those synapses that remained were functioning normally. Furthermore, if a retrograde transfer of TeTxLC was to occur, thus blocking presynaptic input, we might expect the frequency of remaining epscs to resemble that seen when TeTxLC is expressed throughout the CNS. This was not the case: the frequency of epscs seen when TeTxLC was expressed in aCC neurons was approximately twice that seen when expressed throughout the CNS (1.2 ± 0.2, compared with 0.6 ± 0.04). Immunohistochemistry of TeTxLC showed no staining in cells or neuropil other than those in which the transgene was expressed (R.A.B., unpublished observations). As a further test of the possibility of retrograde transfer of TeTxLC, we looked for ultrastructural evidence of synaptic input to aCC. If retrograde transfer of TeTxLC is occurring, then it would be expected to affect only the function, but not the number, of presynaptic inputs to aCC [1,8]. If, on the other hand, postsynaptic TeTxLC expression reduces the establishment or stabilisation of presynaptic input, then a reduction in presynaptic sites should be apparent. To test this, aCC neurons expressing either TeTxLC or TNT-VIF were marked with an electron-dense label and examined in the electron microscope. Sites of presynaptic input were identified on the basis of the presence of a cluster of clear synaptic vesicles, with a requirement that some vesicles appear to be docked to the presynaptic membrane, immediately adjacent to the labelled profile. In control neurons, such synaptic vesicle accumulations were seen immediately adjacent to labelled profiles (that is, profiles belonging to aCC) in about 10% of instances (15 out of 158 profiles; six cells sectioned from five embryos; Figure 4a). In comparison, the frequency of vesicle clusters next to aCC neurons expressing TeTxLC was significantly reduced. About 2% of labelled profiles had putative presynaptic clusters adjacent to them (5 out of 209 profiles, eight cells sectioned from five embryos, p ≤ 0.01, Chi-squared test; Figure 4b).
Figure 4.
Identification of synaptic input to aCC. Electron micrographs showing accumulations of clear synaptic vesicles (arrowheads) immediately adjacent to labelled profiles of aCC neurons (asterisks) in (a) a TNT-VIF and (b) a TeTxLC background (driven by RRC-GAL4). The scale bars represent 200 nm. Potential sites of synaptic input were identified by the accumulation of vesicles, some of which are docked to the presynaptic membrane, immediately adjacent to labelled profiles of aCC. At this stage of development, synaptic elements such as T-bars are not present although increased electron density at the presynaptic membrane is visible in some instances (a). (c) Although similar in appearance, the frequency of putative presynaptic elements adjacent to aCC neurons expressing TeTxLC is significantly reduced (p ≤ 0.01, Chi squared test) compared to control (TNT-VIF).
The mechanisms underlying the reduction of presynaptic input are unknown but are unlikely to involve the retrograde transfer of TeTxLC for the reasons given above. Expression of TeTxLC in a postsynaptic neuron could affect synaptogenesis by altering the characteristics of that cell. Alternatively, it could act by preventing the release of an as yet unknown factor(s) that acts retrogradely to affect the presynaptic element. Thus, far from being the passive output elements of the locomotor circuitry, the motorneurons appear to have an important role in regulating the differentiation of their presynaptic partners.
Materials and methods
The wild-type strain used was Oregon-R. The transgene e5GAL3M–RRC-GAL4 (RRC-GAL4) was used to selectively express UAS-driven transgenes in aCC. To reduce expression in pCC to a minimum, crosses of these flies were maintained at 18°C. Embryos were dissected and whole cell recordings made as previously described [2]. Acetylcholine (0.1 M aqueous) was applied by electrophoresis [2]. Motorneuron axons were stimulated and resultant muscle ejcs recorded as previously described [9]. The aCC neurons were labelled as previously described [3]. Embryos were post-fixed in osmium (1% in dH2O) for 1 h, stained with aqueous uranyl acetate (2%) for 30 min, dehydrated, embedded in Araldite resin and sections taken.
Supplementary Material
Supplementary material including additional methodological detail is available at http://current-biology.com/supmat/supmatin.htm.
Acknowledgements
This work was supported by grants from the Wellcome Trust (052032, M.B.) and NSF (9808931, J.B.J.). We thank S. Sweeney for the gift of UAS–TNT flies, M. Landgraf, H. Skaer, M. Suster and B. Taylor for comments and M. Day for assistance with electron microscopy. We thank M. Landgraf, A. Prokop and J. Uhler for sharing elements of their method for visualising DiI-labelled motorneurons in the electron microscope. We also thank Ian Meinertzhagen for advice.
References
- 1.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 2.Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci. 1998;18:4673–4683. doi: 10.1523/JNEUROSCI.18-12-04673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landgraf M, Bossing T, Technau GM, Bate M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J Neurosci. 1997;15:9642–9655. doi: 10.1523/JNEUROSCI.17-24-09642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvaterra PM, McCaman RE. Choline acetyltransferase and acetycholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. J Neurosci. 1985;5:903–910. doi: 10.1523/JNEUROSCI.05-04-00903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mlodzik M, Baker NE, Rubin GM. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990;4:1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- 6.Link E, Edelman L, Chou JH, Binz T, Yamasaki S, Eisel U, et al. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992;189:1017–1023. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- 7.Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 8.Broadie K, Prokop A, Bellen HJ, O’Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 9.Broadie KS, Bate M. Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J Neurosci. 1993;13:144–156. doi: 10.1523/JNEUROSCI.13-01-00144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material including additional methodological detail is available at http://current-biology.com/supmat/supmatin.htm.