Abstract
This letter reports the use of disulfide linkages to stabilize a β-sheet dimer with a well-defined structure in aqueous and dimethyl sulfoxide solutions. In this dimer, two cyclic β-sheet peptides are connected by disulfide linkages at the non-hydrogen-bonded rings. The cyclic β-sheet “domains” interact through hydrogen bonding to form a four-stranded β-sheet structure. This interaction results in enhanced folding of the cyclic β-sheet peptides.
Disulfide bonds are important in stabilizing protein quaternary structures.1 Our laboratory has previously developed chemical model systems that use hydrogen bonding and hydrophobic interactions to mimic β-sheet quaternary structures.2 Here, we report the use of disulfide linkages to stabilize a β-sheet dimer with a well-defined structure in competitive solvents.
Disulfide bonds in antiparallel β-sheets generally occur at the non-hydrogen-bonded amino acid pairs, rather than the hydrogen-bonded amino acid pairs. This pairing preference has been demonstrated both statistically in proteins3 and empirically in a β-hairpin model system.4 A recent study of a tripeptide cystine dimer has demonstrated that disulfide linkages can induce β-sheet hydrogen-bonding interactions in noncompetitive organic solvents when the cysteine residues form non-hydrogen-bonded pairs of antiparallel β-sheets.5
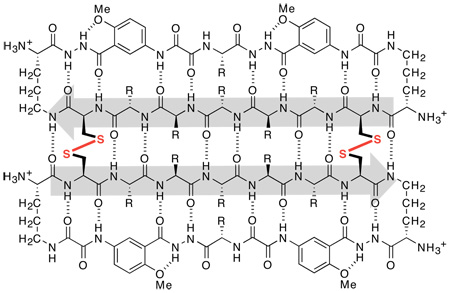
We previously introduced cyclic β-sheet peptide 1a (Figure 1) that self-associates in water through edge-to-edge and face-to-face intermolecular β-sheet interactions to form a tetrameric β-sheet sandwich.6 Cyclic β-sheet peptide 1a consists of a heptapeptide β-strand, a β-strand mimic comprising two unnatural amino acid Hao units,7 and two δ-linked ornithine (δOrn) turn units8 connecting the two strands. Significantly, the antiparallel β-sheet dimer of cyclic peptide 1a is only stable within the tetramer (dimer of dimers) and is not observed as a discrete species. Studies of analogues of this peptide showed that the formation of this tetrameric β-sandwich requires hydrophobic interactions.
Figure 1.
Cyclic β-sheet peptides 1a–1c.
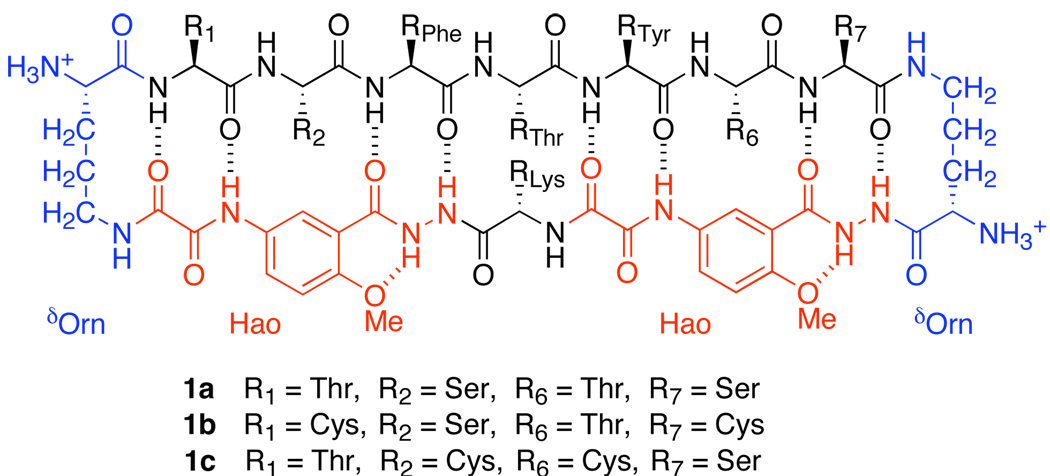
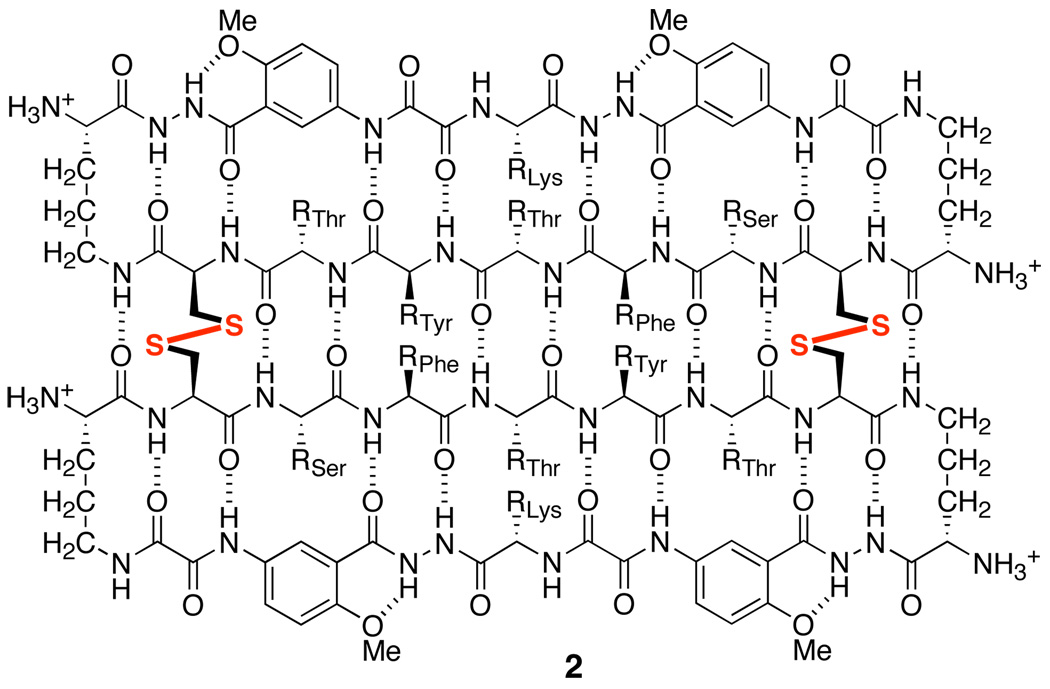
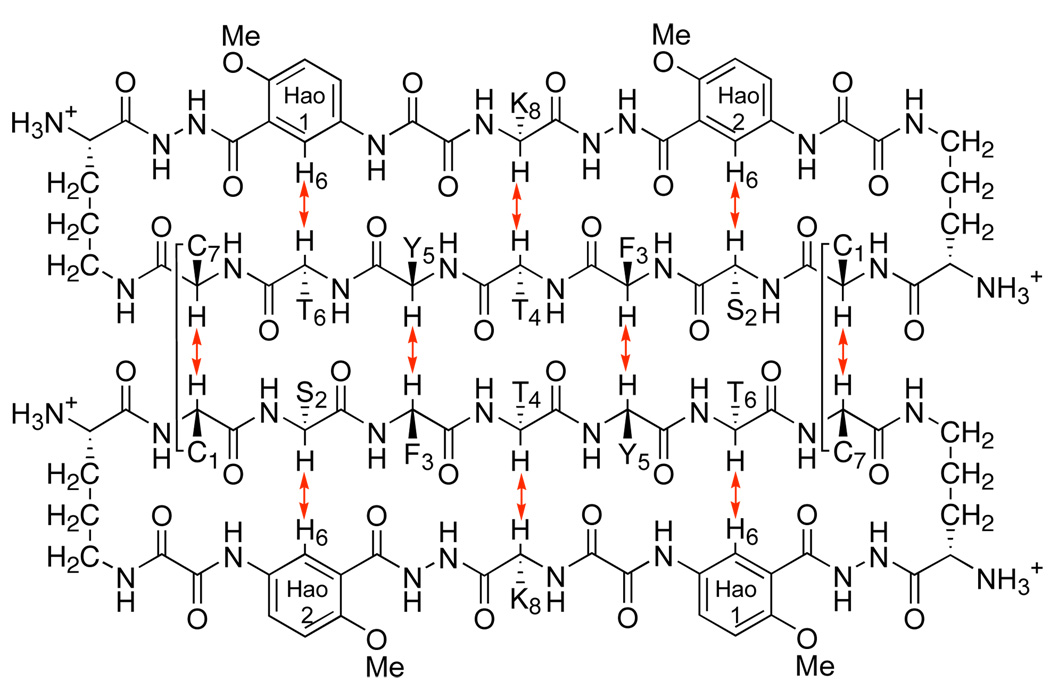
To test whether disulfide linkages can stabilize a hydrogen-bonded β-sheet dimer in competitive solvents, we have now introduced cysteine residues at positions R1 and R7 of peptide 1a, thus creating peptide 1b. Upon oxidation, peptide 1b forms β-sheet dimer 2, with two disulfide linkages (Cys1—Cys7) at these non-hydrogen-bonded positions (Figure 2). These disulfide linkages not only covalently connect the monomeric “domains” in an antiparallel fashion but also substantially stabilize β-sheet folding.
Figure 2.
β-Sheet dimer 2.
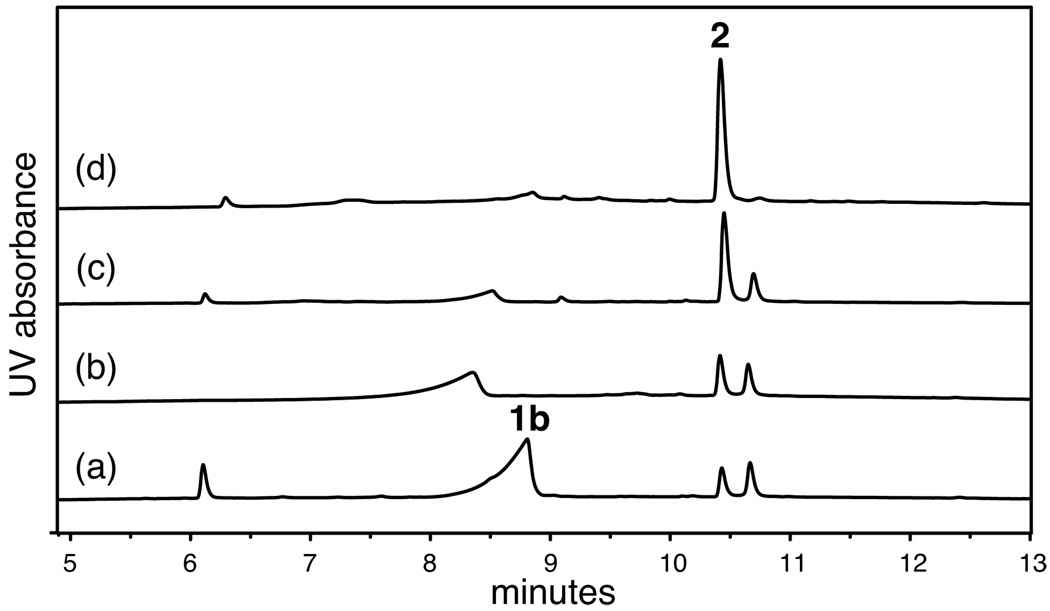
Dimer 2 was synthesized efficiently by oxidation of peptide 1b with a 20% aqueous DMSO solution.9 Oxidation of a 2.5 mM solution of peptide 1b was completed in 24 h and provided dimer 2 as the major product (35–75% isolated yield after HPLC purification). Figure 3 illustrates the progress of the oxidation reaction through analytical RP-HPLC traces of the crude oxidation mixture after 0.25, 1, 6, and 12 h. Although the oxidation could have resulted in two different isomeric bisdisulfides, one parallel and one antiparallel, only the antiparallel isomer was observed upon completion of the reaction.10,11
Figure 3.
Analytical RP-HPLC traces of the oxidation reaction of peptide 1b by a 20% aqueous DMSO solution after: (a) 0.25 h, (b) 1 h, (c) 6 h, and (d) 12 h.
1H NMR studies of dimer 2 were performed in both DMSO-d6 and D2O solutions. The NMR resonances of 2 are sharp and dispersed in DMSO-d6 at room temperature. The NMR resonances of 2 in D2O are broad at room temperature (298 K) and submillimolar concentrations but sharpen at higher temperature (318 K). This broadening may arise from conformational exchange between disulfide bond rotamers occurring at an intermediate rate on the NMR time scale. The NMR resonances of dimer 2 in D2O also sharpen with increasing concentration. This sharpening suggests that covalent dimer 2 forms non-covalent oligomers at millimolar concentrations and that these oligomers likely stabilize a particular conformer.12
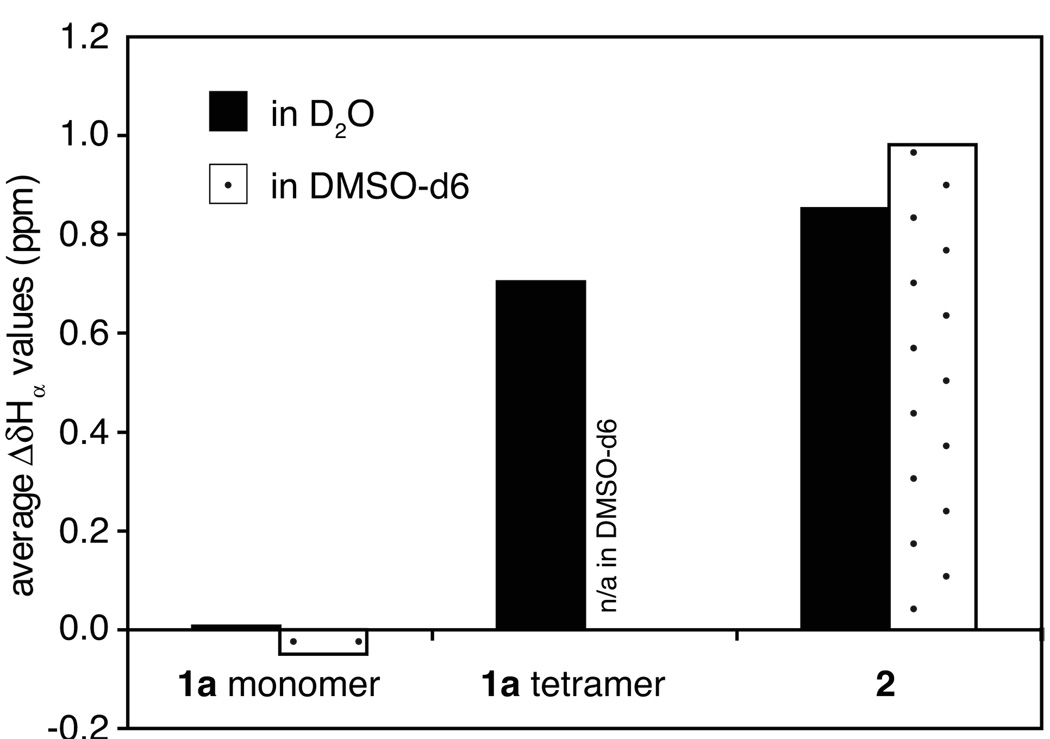
Downfield shifting of the α-proton resonances of dimer 2 suggests that the dimer strongly folds into a β-sheet structure. Figure 4 illustrates the average deviation of the α-proton chemical shifts from published random coil values13 (ΔδHα) of the residues at positions R2 to R6 for covalent dimer 2, the monomer of cyclic peptide 1a, and the tetramer of cyclic peptide 1a in D2O and DMSO-d6 solutions. The average ΔδHα values for dimer 2 in both solvents (0.85 and 0.98 ppm, respectively) are significantly larger than those for the monomer of cyclic peptide 1a (0.01 and −0.05 ppm, respectively) and are comparable to that of the tetramer of cyclic peptide 1a in D2O (0.70 ppm).
Figure 4.
Average ΔδHα values of the residues at positions R2 to R6 for the monomer and tetramer of peptide 1a and for dimer 2. The NMR data were obtained from 0.5 and 8.5 mM D2O solutions of peptide 1a at 280 K, a 0.7 mM DMSO-d6 solution of peptide 1a at 298 K, a 0.2 mM D2O solution of dimer 2 at 318 K, and a 0.7 mM DMSO-d6 solution of dimer 2 at 298 K.
We previously showed that the β-sheet folding is only partial in the monomer of cyclic peptide 1a but strongly increases upon formation of the tetramer.6 The large average ΔδHα value for the tetramer arises form its strong β-sheet folding. The large average ΔδHα values for dimer 2 in DMSO and water also indicate strong β-sheet folding of the dimer in these solvents.
The difference in the chemical shifts of the diastereotopic δ-protons of a δOrn turn unit (ΔδδOrn) reflects the degree of folding of the turn unit, and hence the peptide.8 We have previously observed an average ΔδδOrn value of 0.15 ppm for the partially folded monomer of cyclic peptide 1a and an average ΔδOrn value of 0.69 ppm for the highly folded tetramer of cyclic peptide 1a in D2O.6 Dimer 2 exhibits large average ΔδδOrn values of 0.68 and 0.60 ppm respectively in DMSO-d6 and in D2O. As in the noncovalent tetramer of cyclic peptide 1a, the large average ΔδOrn values for dimer 2 indicate predominant or complete folding.
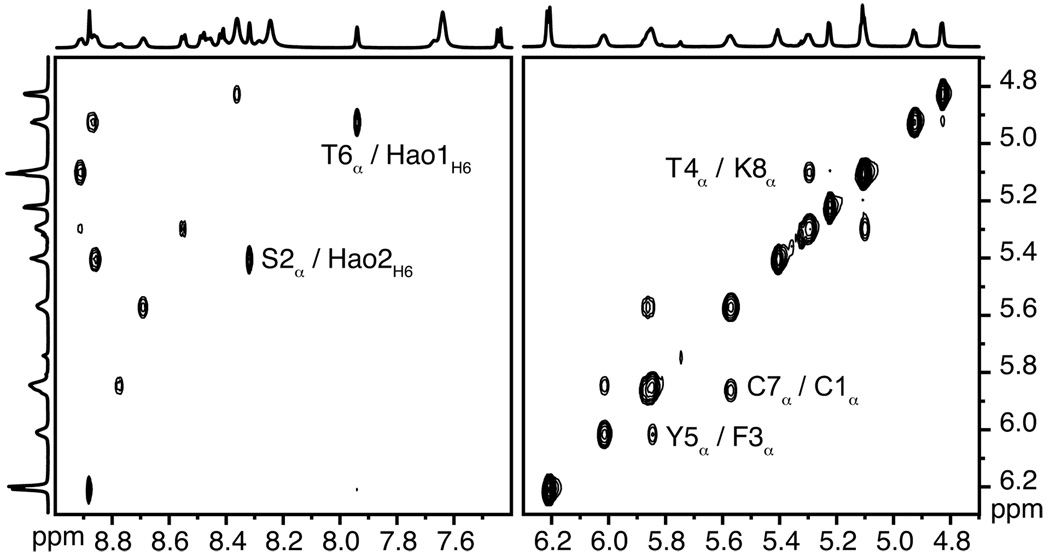
800 MHz NOESY studies of dimer 2 in both D2O and DMSO-d6 reveal a network of NOEs associated with β-sheet folding and antiparallel edge-to-edge interactions. Figure 5 illustrates the key NOEs involving the backbone CHα and HaoH6 protons in these solvents. Figure 6 illustrates these NOE cross-peaks that are observed in DMSO-d6. The intradomain NOEs between the α-protons of Thr4 and Lys8 (T4α/K8α), between the α-proton of Ser2 and H6 of Hao2 (S2α/Hao2H6), and between the α-proton of Thr6 and H6 of Hao1 (T6α/Hao1H6) indicate β-sheet folding within each domain. The interdomain NOEs between the α-protons of Cys1 and Cys7 (C1α/C7α) and between the α-protons of Phe3 and Tyr5 (F3α/Y5α) indicate edge-to-edge dimerization. Collectively, these NOEs establish that dimer 2 folds into a β-sheet structure in which the domains participate in antiparallel edge-to-edge β-sheet interactions.
Figure 5.
Key NOEs associated with the intradomain and interdomain main-chain contacts of dimer 2 observed in D2O and in DMSO-d6.14
Figure 6.
Selected expansions of the 800 MHz NOESY spectrum of 0.7 mM dimer 2 in DMSO-d6 at 298 K.15
The large downfield shifting of the α-proton resonances, the large difference in the chemical shifts of the diastereotopic δ-protons of the δOrn turn units, and the NOEs arising from intradomain and interdomain contacts collectively establish that dimer 2 is strongly folded into β-sheet structure in both water and DMSO.
To evaluate whether the cysteine residues had to be at the non-hydrogen-bonded positions of the β-sheet, we prepared and studied cyclic peptide 1c, which is a sequence isomer of peptide 1b with cysteine at positions R2 and R6, rather than R1 and R7. We attempted to form the dimer of peptide 1c by oxidation and found dimer formation to be strikingly challenging. Oxidation with aqueous DMSO solution provided a bicyclic peptide with an intramolecular Cys2—Cy6 disulfide linkage as the major product. Only prolonged oxidation of a concentrated aqueous solution of the cyclic peptide with air afforded a small quantity of dimeric product. The dimer of 1c proved difficult to characterize by NMR spectroscopy, because it showed broad resonances and multiple sets of peaks. The positions of the α-proton resonances and small ΔδδOrn values of this compound suggest that this dimer is poorly folded. The difficult synthesis, complex NMR spectra, and poor folding suggest that disulfide linkages at hydrogen-bonded positions do not induce and stabilize β-sheet structure.3,4
In conclusion, the Cys—Cys disulfide linkages at non-hydrogen-bonded positions stabilize β-sheet dimers through antiparallel edge-to-edge β-sheet interactions. Covalent dimer 2 in which 54-membered-ring cyclic peptides (domains) are linked by two Cys1—Cys7 disulfide linkages exhibits strong intradomain β-sheet folding and interdomain antiparallel edge-to-edge β-sheet interactions in water and in DMSO. Such disulfide linkages serve as “staples”16 and constitute a new tool for stabilizing β-sheet quaternary structure.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health for grant support (GM-49076) and Dr. Bao Nguyen (UCI) and Dr. Evgeny Fadeev (UCI) for help with the NMR experiments.
Footnotes
Supporting Information Available Experimental procedures and spectroscopic and analytical data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Wedemeyer WJ, Welker E, Narayan M, Scheraga HA. Biochemistry. 2000;39:4207–4216. doi: 10.1021/bi992922o. [DOI] [PubMed] [Google Scholar]; (b) Kadokura H, Katzen F, Beckwith J. Annu. Rev. Biochem. 2003;72:111–135. doi: 10.1146/annurev.biochem.72.121801.161459. [DOI] [PubMed] [Google Scholar]
- 2.(a) Nowick JS. Org. Biomol. Chem. 2006;4:3869–3885. doi: 10.1039/b608953b. [DOI] [PubMed] [Google Scholar]; (b) Nowick JS. Acc. Chem. Res. 2008;41:1319–1330. doi: 10.1021/ar800064f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty K, Thakurela S, Prajapati RS, Indu S, Ali PSS, Ramakrishnan C, Varadarajan R. Biochemistry. 2005;44:14638–14646. doi: 10.1021/bi050921s. [DOI] [PubMed] [Google Scholar]
- 4.Santiveri CM, León E, Rico M, Jiménez MA. Chem. — Eur. J. 2008;14:488–499. doi: 10.1002/chem.200700845. [DOI] [PubMed] [Google Scholar]
- 5.Cashman TJ, Linton BR. Org. Lett. 2007;9:5457–5460. doi: 10.1021/ol7023696. [DOI] [PubMed] [Google Scholar]
- 6.Khakshoor O, Demeler B, Nowick JS. J. Am. Chem. Soc. 2007;129:5558–5569. doi: 10.1021/ja068511u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowick JS, Chung DM, Maitra K, Maitra S, Stigers KD, Sun Y. J. Am. Chem. Soc. 2000;122:7654–7661. [Google Scholar]
- 8.Nowick JS, Brower JO. J. Am. Chem. Soc. 2003;125:876–877. doi: 10.1021/ja028938a. [DOI] [PubMed] [Google Scholar]
- 9.Tam JP, Wu CR, Liu W, Zhang JW. J. Am. Chem. Soc. 1991;113:6657–6662. [Google Scholar]
- 10.A minor monomeric oxidation side product was detected by mass spectrometric analysis of the crude oxidation mixture but was not isolated or further characterized. This product likely contains an intramolecular Cys1—Cys7 disulfide linkage.
- 11.Hydrogen-bonding complementarity between domains may explain the exclusive formation of the antiparallel isomer; a parallel isomer could not form a full complement of interdomain hydrogen bonds while maintaining intradomain hydrogen bonds.
- 12.NMR studies of cyclic peptide 1b (the domain building block) were not performed, because the peptide oxidizes rapidly in aqueous solutions.
- 13.Wishart DS, Sykes BD. Methods Enzymol. 1994;239:363–392. doi: 10.1016/s0076-6879(94)39014-2. [DOI] [PubMed] [Google Scholar]
- 14.The interdomain F3α/Y5α NOE could not be established with certainty in D2O, because the F3α and Y5α resonances are close in this solvent.
- 15.The sample was lyophilized with D2O twice before NMR studies to attenuate resonances associated with water and exchangeable protons.
- 16.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.