Abstract
Purpose
To assess the ability to detect the neural canal opening (NCO) and its characteristics within three-dimensional (3-D) histomorphometric and 3-D spectral domain optical coherence tomography (SD-OCT) reconstructions of the optic nerve head from nonhuman primate (NHP) eyes.
Methods
NCO was delineated within 40 radial, sagittal sections of 3-D histomorphometric reconstructions of 44 normal eyes of 38 NHPs, each perfusion fixed at IOP 10 mm Hg, and 3-D SD-OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) volumes acquired in vivo from a separate group of 33 normal eyes of 24 NHPs. Within all reconstructions, a least-squares ellipse was fitted to the 80 NCO points. For each eye, the dimensions and plane error (a gauge of planarity) of the fitted ellipse were calculated.
Results
The NCO was successfully delineated within every section of each histomorphometric and SD-OCT reconstruction. Median plane error was similar within histomorphometric and SD-OCT volumes (8 μm, range 4–19, histomorphometry, and 10 μm, range 4–26, SD-OCT) and was small relative to the size of the ellipse. Median histomorphometric ellipse dimensions were 1453 μm (major axis, range 1218–1737) and 1066 μm (minor axis, range 808–1263). Median SD-OCT ellipse dimensions were 1512 μm (major axis, range 1191–1865) and 1060 μm (minor axis, range 772–1248).
Conclusions
The NCO is biologically continuous and relatively planar within all 3-D histomorphometric and SD-OCT reconstructions. These characteristics support its further evaluation as a reference plane for cross-sectional and longitudinal measurement of optic nerve head structures using 3-D SD-OCT.
All forms of glaucoma are defined by an optic neuropathy that demonstrates classic and recognizably variable1-3 structural characteristics including “cupping,” focal and diffuse neuroretinal rim loss, retinal nerve fiber layer defects, and splinter hemorrhages. The functional changes caused by the neuropathy are typified by arcuate patterns of visual field loss. Glaucomatous damage to the anatomic visual system includes important pathophysiologies within the retinal ganglion cell soma,4-9 photoreceptors,10-14 lateral geniculate body,15-17 and visual cortex.17 However, there is strong evidence to suggest that damage to the retinal ganglion cell axons within the lamina cribrosa of the optic nerve head18-23 is the central pathophysiology underlying glaucomatous vision loss. Recent studies in the monkey,22-27 rat,28-30 and mouse31 describe profound alterations within the prelaminar, laminar, and peripapillary scleral tissues of the optic nerve head at the earliest detectable stage of the disease.
The commercial introduction of high speed, high-resolution, three-dimensional, spectral domain, optical coherence tomography (SD-OCT) creates the potential for optic nerve head surface and subsurface change detection. These changes may precede either the onset of peripapillary retinal nerve fiber layer thickness changes or visual field loss. However, to date, there has been no rigorous investigation of this technology's ability to image the deep optic nerve head structures and to generate volumetric parameters based on Bruch's membrane, Bruch's membrane opening (BMO), the anterior laminar cribrosa surface, and the anterior scleral surface.
A central requirement for optic nerve head surface and subsurface change detection is a longitudinally stable zero reference plane, from which all structural measurements are defined.32-38 Ideally, the SD-OCT reference plane should be located at, or linked to, a biologically continuous structure (a “reference plane source-structure”) that is both clinically recognizable and easily identifiable in all SD-OCT B-scan images. However, a reference plane source-structure does not necessarily need to be planar or clinically visible as long as it can be segmented reproducibly within SD-OCT images and its position relative to the optic nerve head target structures is relatively stable over time and through the pathophysiology of glaucomatous damage.
We have used BMO previously as a reference plane within quantitative three-dimensional (3-D) histomorphometric reconstructions of the nonhuman primate (NHP) optic nerve head.25-27 The choice of BMO as a reference plane in our histomorphometric reconstructions was predicated on the observations that it was obviously continuous, delineated reproducibly, and altered minimally in the pathophysiology of early experimental glaucomatous damage.25-27
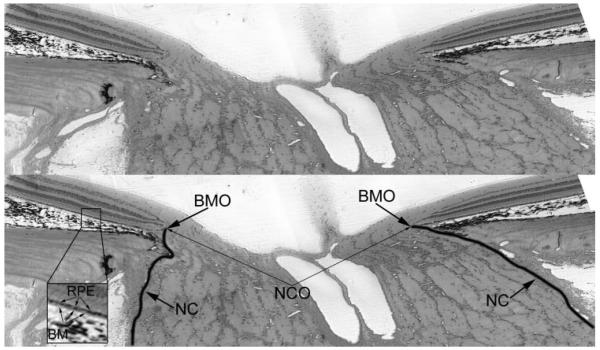
We have previously proposed the term neural canal for the axonal pathway through the eye wall23,25 (Fig. 1), which includes a prescleral region as well as the scleral canal. The neural canal extends from its clinically visible internal opening, the clinically visible optic disc margin, to its clinically invisible external opening (the posterior scleral canal opening). Defining the neural canal in this manner is clinically significant, because at present the relationship between the most anterior (or innermost) portion of the canal and the disc margin within 3-D histomorphometry, clinical confocal scanning laser tomography imaging, clinical OCT imaging, and clinical disc photographs is controversial.25,39-44
Figure 1.
Histologic sagittal section of the optic nerve head of a rhesus macaque, showing the BMO (top, unlabeled; bottom, labeled), NC (boundary demarcated by a black line) and NCO. Inset: RPE and BM.
The neural canal opening (NCO) is defined as the anatomic entrance to the neural canal at the level of the retinal pigment epithelium (RPE) and BM. Within all NHP optic nerve head 3-D histomorphometric reconstructions that have been completed to date, BMO is both the NCO and it colocalizes to the disc margin in color photographs that are registered to the reconstruction.25 However, the anatomy of the NCO in human eyes, and within clinical OCT images of human eyes, is more complicated than the NHP and the ability of OCT to discriminate BM as distinct from the RPE is not resolved.45 At present, most clinical OCT systems suggest that the end of the RPE is being delineated as the zero-order reference plane.45
We propose that in all SD-OCT sagittal ONH sections, there is a discernible NCO that can be manually (and eventually automatically) delineated. Although we believe its underlying histologic and clinical relationships are important, we further propose that the NCO detected by SD-OCT (regardless of its histologic and clinical underpinnings) will serve as a stable anchor for a longitudinal reference plane for SD-OCT imaging of the optic nerve head. Experience using confocal scanning laser ophthalmoscopy (in particular, with the Heidelberg Retina Tomograph; Heidelberg Engineering, Heidelberg, Germany) has shown that the position of the reference plane critically influences the measurement variability of structural parameters.35-37,46 This issue arises particularly when trying to identify structural progression, as one needs to be able to discern true change secondary to disease process from spurious change secondary to measurement noise. An idealized reference plane would therefore have an optimal signal-to-noise ratio, whereby its location is sufficiently stable to minimize measurement variability yet is still capable of identifying “genuine” progression.46 One of the long-term goals of our work is to assess the stability of the NCO as the anatomic anchor for an SD-OCT reference plane within the monkey model of experimental glaucoma and within longitudinal studies in human eyes.
The purpose of the present study was to assess our ability to delineate the NCO within 3-D histomorphometric and SD-OCT reconstructions of normal NHP optic nerve head and to characterize its planarity as a structure. The long-term reproducibility and stability of the SD-OCT-detected NCO within normal and experimental glaucomatous NHP optic nerve heads as well as its colocalization to clinical photographs will be the subject of future reports.
Materials and Methods
Animals
All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All eyes were either SD-OCT imaged in vivo or histomorphometrically reconstructed post mortem as part of other ongoing research studies. Forty-four normal eyes of 38 NHPs (32 rhesus macaque and 6 cynomolgus) were histomorphometrically reconstructed. A separate group of 33 normal eyes from 24 rhesus macaque monkeys were imaged in vivo with SD-OCT. The characteristics of the NHPs included in the study are summarized in Table 1.
Table 1.
A Summary of the Characteristics of the NHP Eyes Included in the Study
| Histomorphometry | SD-OCT | |
|---|---|---|
| Subjects (n) | 38 | 24 |
| Sex (male:female) | 25:13 | 6:18 |
| Eyes (n) | 44 | 33 |
| Laterality (right:left) | 18:26 | 11:22 |
| Age, y (median, range) | 10 (2–32) | 10 (1–19) |
| Condition | Normal | Normal |
| IOP (mm Hg) | 10 | 10 |
IOP shown is the IOP set at perfusion-fixation in the histomorphometry eyes and the IOP at which imaging was performed in the SD-OCT eyes.
Histomorphometry Group: 44 Eyes
Perfusion Fixation at an IOP of 10 mm Hg
The study eyes were cannulated with a 27-gauge needle with the animals under deep pentobarbital anesthesia, and the IOP was set to 10 mm Hg with an adjustable saline reservoir manometer. After a minimum of 30 minutes, the NHP was killed with perfusion fixation via the descending aorta with 1 L of 4% buffered hypertonic paraformaldehyde solution followed by 6 L of 5% buffered hypertonic glutaraldehyde solution. IOP was maintained at 10 mm Hg for 1 hour, after which each eye was enucleated, all extraorbital tissues were removed, and the anterior chamber was removed 2 to 3 mm posterior to the limbus. The posterior scleral shell with intact optic nerve head, choroid, and retina were placed in 5% glutaraldehyde solution for storage.
Generation of the Aligned Serial Sections for 3-D Optic Nerve Head Reconstruction
This procedure has been described in detail in previous reports.23,25 Briefly, the optic nerve head and peripapillary sclera were trephinated (6 mm diameter), pierced with alignment sutures, embedded in paraffin, and mounted on a microtome. The block was stained with a 1:1 (vol/vol) mixture of ponceau S and acid fuchsin stain. Two different imaging/cutting protocols were adopted. In 14 eyes, a first-generation protocol was used whereby cuts were performed at 3.0 μm depth, and imaging of each cut was performed at a resolution of 2.5 μm2 per pixel. The staining and imaging processes were repeated for each cut. A second-generation, high-resolution protocol was used in 30 eyes, wherein cuts were made at a depth of 1.5 μm and the surface imaged at a resolution of 1.5 μm2 per pixel. In both protocols, imaging began at the vitreoretinal interface and then continued approximately 200 μm into the orbital optic nerve. Serial section images were stacked and aligned in the anterior-posterior direction. Image alignment was achieved by using the fiducial sutures for the first-generation reconstructions and laser-displacement sensors for the second-generation reconstructions.
SD-OCT Group: 33 Eyes
All NHPs were imaged under pentobarbital anesthesia. IOP in the imaged eye was set to 10 mm Hg via a manometer connected to a 27-gauge cannula inserted into the temporal anterior chamber. SD-OCT imaging using a commercially available device (Spectralis; Heidelberg Engineering) was performed through a plano rigid gas-permeable contact lens 30 minutes after IOP stabilization.
The technical specifications of the Spectralis device have been covered in detail elsewhere.47,48 Briefly, the Spectralis uses an 870 nm semiluminescent diode light source; its optical resolution is approximately 7 μm in depth and 14 μm transversely. For this study, 290 individual horizontal B-scans were acquired over a 15° retinal window (768 A-scans per B-scan, each B-scan was acquired nine times and averaged for speckle noise reduction). The 290 horizontal B-scans for each OCT data set were then exported from the native Spectralis Explorer software using a “raw” export function into custom-built, Multiview 3-D visualization and delineation software (based on the Visualization Toolkit; VTK, Clifton Park, NY)25-27 that has been modified for Spectralis SD-OCT data sets. Within the Multiview software, the horizontal B-scans were interpolated into a 3-D SD-OCT volume.
3-D Delineation of NCO Points within 3-D Histomorphometric and SD-OCT Volumes
3-D Histomorphometric Reconstructions
Our 3-D delineation technique within histomorphometric reconstructions has been described in detail elsewhere.25-27 Briefly, the 3-D optic nerve head volume was loaded into memory on a remote Linux server by using a suite of custom software (Visualization Toolkit; VTK). Viewing serial digital transverse and/or radial sagittal sections of the volume, the delineator assigned the approximate center of the neural canal to be the center of rotation for 40 radial sagittal slices of the 3-D reconstruction which were generated at 4.5° intervals.
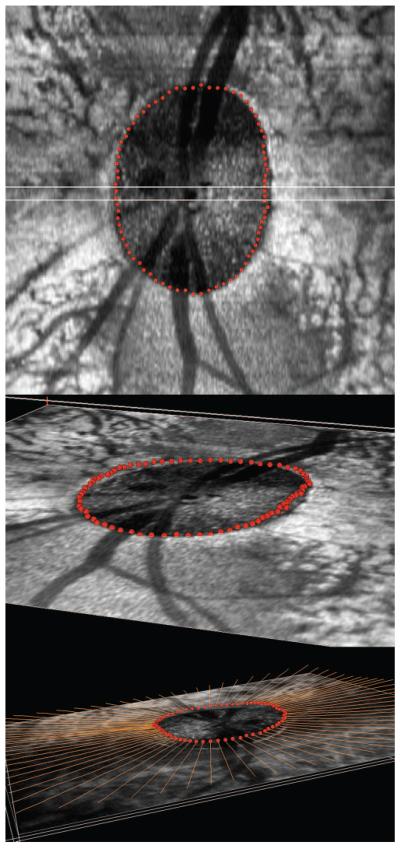
Within each radial section, the delineator marked the NCO on either side of the neural canal (Fig. 2, top image). Delineation of each point was three-dimensional (3-D delineation), in that each sagittal section pixel was linked to its location within a serial transverse (en face) section through the identical location (simultaneously shown on a second monitor). Once the NCO points had been delineated for all 40 sections (80 points in total), the 3-D Cartesian coordinates for each point were saved, allowing a 3-D NCO point cloud to be generated (Fig. 3).
Figure 2.
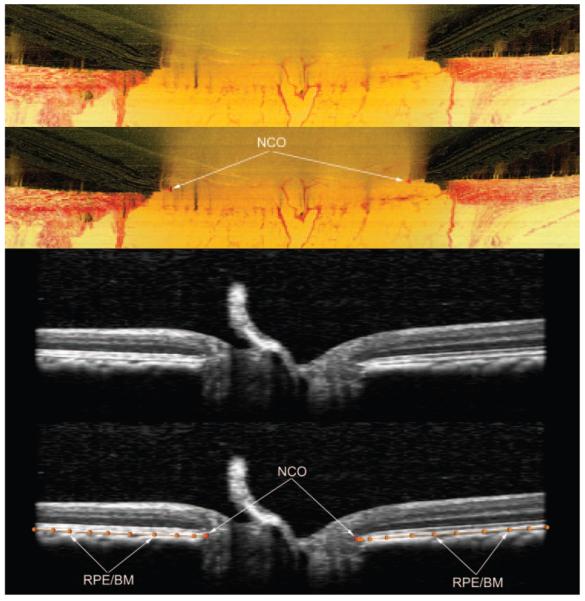
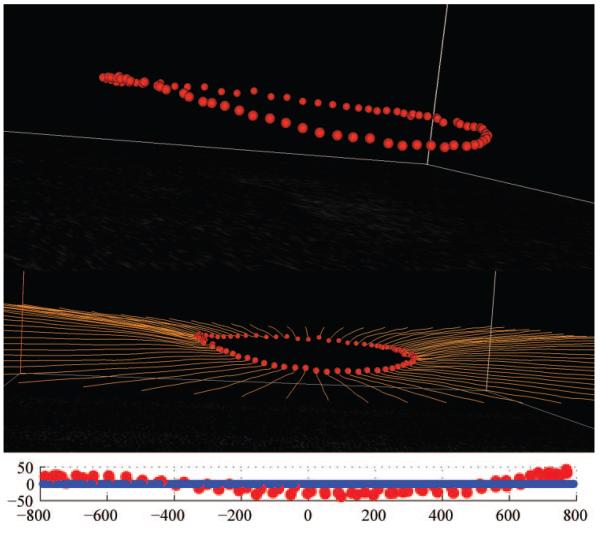
Delineation of the NCO within histomorphometric (top two images) and SD-OCT (bottom two images) reconstructions. These images are Multiview software screen captures. Top image: Digital, radial sagittal section of a histomorphometric reconstruction section (90° or horizontal in location), taken from the right eye of monkey 23540, a 9-year-old female rhesus macaque. Middle top image, red triangles: the location of the NCO (as applied within Multiview software) which in this eye is coincident with the BMO. Middle bottom image: sagittal view of a radial interpolated SD-OCT section (90° location), taken from the left eye of monkey 23511, a 12-year-old male rhesus macaque. Bottom image: the same as the middle image, but with NCO points marked (red glyphs, as applied within Multiview software). The posterior surface of the RPE/BM complex is also marked (orange lines and glyphs). The NCO points are at the innermost aspect of this surface.
Figure 3.
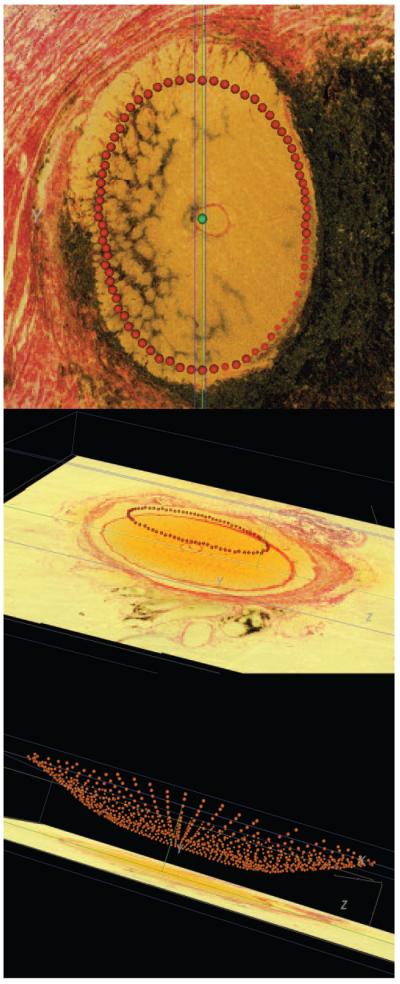
Generation of a histomorphometric NCO point cloud in monkey 23540 (OD eye). Top image: en face (transverse) view of histomorphometric reconstruction. All 80 NCO points (red glyphs) are shown, having been delineated in all the 40 radial sections at 4.5° intervals. The center of rotation is marked by a green glyph. Middle image: the en face view was rotated in space, so as to demonstrate the appearance of the NCO point cloud in three dimensional space. Bottom image: An alternative view of the NCO point cloud in 3-D space; the delineated BM points (orange glyphs) are also shown.
SD-OCT Volumes
Within each SD-OCT volume, interpolated radial sagittal sections at 4.5° intervals (40 in total) were generated as per the histomorphometric reconstructions. In the case of the SD-OCT volumes, the center of rotation was taken as the center of SD-OCT image acquisition. As with the histomorphometric sagittal sections, within each section the delineator marked the location of the NCO (two points per section; Fig. 2, bottom) as the point at which the posterior surface of the RPE/BM complex reached the neural canal. Once the delineator had marked NCO points in all 40 radial sections (80 NCO points in total), the Cartesian coordinates for each delineated point were saved, allowing a 3-D NCO point cloud to be generated (Fig. 4).
Figure 4.
Generation of the OCT NCO point cloud for monkey 23511 (OS). Top image: en face (transverse view of SD-OCT volume). All 80 NCO points (red glyphs) are shown, having been delineated in all the 40 radial sections at 4.5° intervals. Middle image: the en face view has been rotated in space, so as to demonstrate the appearance of the NCO point cloud in three dimensional space. Bottom image: an alternative view of the NCO point cloud in 3-D space; the delineated BM points (orange lines) are also shown.
NCO Ellipse Fitting and Quantification
Plane Error
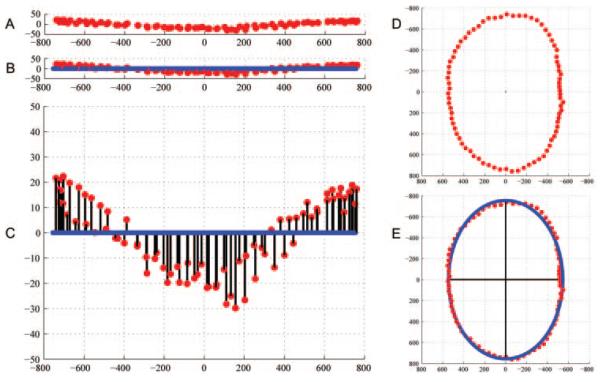
For each histomorphometric and SD-OCT volume, a plane was fitted to the 80 NCO points satisfying a least mean square error restraint (MatLab MathWorks, Natick, MA; Fig. 5). The shortest perpendicular distance (in 3-D space) of each NCO point from the fitted plane was then calculated. Plane error for each histomorphometric and OCT volume was derived from the mean distance of NCO points from the fitted plane. Plane error may thus be regarded as a gauge of the planarity of the observed NCO points, with smaller magnitudes equating to higher planarity.
Figure 5.
Fitted NCO plane and ellipse within an SD-OCT volume from the left eye of monkey 23511. (A) The NCO points are shown in space; (B) a plane has been least-squares fit to the NCO points; (C) the y-axis scale has been magnified to exaggerate the distance of the NCO points from the fitted plane (shown by the black lines). The plane error for each eye is calculated from the mean of these distances. (D) The NCO points have been projected onto the level of the fitted plane; these “planar” NCO points are viewed in the en face orientation. (E) An ellipse has been least-squares fit to the “planar” NCO points. Two black axes are shown within the ellipse, demonstrating the major (longer) and minor (shorter) axes. Eccentricity is derived from the ratio of the major ellipse axis to the minor ellipse axis.
NCO Fitted Ellipse Dimensions
All NCO points were projected onto the level of the fitted plane and an ellipse was least squares fit (NCO fitted ellipse; Fig. 5). The major and minor axes of the fitted ellipse (micrometers) and the area of the ellipse (square micrometers) were then calculated. Within the histomorphometric volumes, the dimensions of the fixed and embedded tissues (each with its own shrinkage artifact) were based on the voxel size dictated by the method. Within the OCT volumes, transverse voxel dimensions were corrected to compensate for the magnification error caused by the optical system of the monkey eye. The power change due to change in axial length (Daxial) was taken as Daxial = (24.46 − L)/0.42,49 where L is the axial length, which was not measured in these eyes, but an estimate of 19 mm was used50-52 giving a Daxial of 13 D. Lateral magnification correction (DM) was taken as DM = 0.018 Daxial + 0.002 Drefraction49 where Drefraction is the power change due to the change in refractive error. As change in axial length has a ninefold greater impact on the magnification error than on refractive error, it was assumed that the influence of refractive error was negligible and therefore not included in the estimation. DM was therefore estimated as:
Thus NCO lateral magnitudes were divided by 1.234, whereas depth magnitudes (z-axis coordinates) were not affected by the optical system and were therefore not adjusted.
The relationship between NHP age and plane error was assessed by using linear regression (P < 0.05) All statistical analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
NCO was detectable and could be manually delineated in all radial sections in both the 3-D histomorphometric reconstructions and the 3-D SD-OCT volumes examined.
The plane errors and fitted NCO ellipse dimensions for both histomorphometric reconstructions and for 3-D SD-OCT volumes are summarized in Table 2. The magnitude of plane error in both categories is small relative to the dimensions of the NCO ellipse, with a median plane error of 8 and 10 μm for histomorphometric reconstructions and SD-OCT volumes, respectively.
Table 2.
NCO Fitted Plane and Fitted Ellipse Characteristics within the 3-D Histomorphometric Reconstructions and SD-OCT Volumes
| Histomorphometry | SD-OCT | |
|---|---|---|
| Eyes (n) | 44 | 33 |
| Major axis of ellipse (μm) | 1,453 (1,218–1,737) |
1,512 (1,191–1,865) |
| Minor axis of ellipse (μnm) | 1066 (808–1,263) |
1,060 (772–1,248) |
| Eccentricity (major/minor) | 1.38 (1.26–1.56) |
1.43 (1.29–1.67) |
| Area of ellipse (μm2) | 1,216,567 (801,287–1,722,124) |
1,305,342 (741,410–1,828,049) |
| Plane error (μm) | 8 (4–19) |
10 (4–26) |
Data are median (range).
Two examples of data sets with a larger magnitude of plane error have been included for histomorphometry (Fig. 6) and for SD-OCT (Fig. 7). It should be noted that the plane errors generated in these two examples (15 μm for monkey 675D OD and 18 μm for monkey 25904 OS) are small relative to the dimensions of their fitted NCO ellipses (1507 μm, major ellipse axis and 1193 μm, minor ellipse axis for 675D OD; 1582 μm, major ellipse axis and 1174 μm, minor ellipse axis for monkey 25904 OS).
Figure 6.
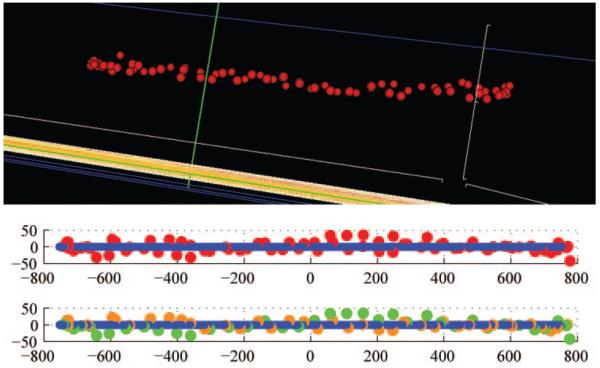
NCO point cloud (top) generated from the histomorphometric reconstruction of the right eye of monkey 675D, a 31-year-old male rhesus macaque. The NCO points relative to their fitted plane viewed from temporal to the disc, (OD configuration, superior left and inferior right) are depicted below. NCO plane error (defined as the average of the shortest distance to the plane for each NCO point) is 15 μm. Although the NCO points appear randomly distributed above and below the plane (middle), when the nasal and temporal points are displayed with different colors (bottom: temporal points [green], nasal points [orange]) their distribution can be seen to continuously follow a structure that is slightly twisted. This finding is easily seen when the points are visualized in 3-D but is difficult to demonstrate in two dimensions. Although method error contributes to plane error, so too does biological nonplanarity. Biological nonplanarity that is delineated reproducibly over time will still provide a stable fitted plane for a zero reference.
Figure 7.
NCO point cloud (top) generated from the SD-OCT volume of the left eye of monkey 25904, a 1-year-old female rhesus macaque. Middle: RPE/BM complex delineated by orange lines. The NCO points relative to their fitted plane viewed from temporal to the disc, (OD configuration, superior left and inferior right) are depicted below. Bottom: NCO plane error (defined as the average of the shortest distance to the plane for each NCO point) is 18 μm. Unlike in Figure 6, in this eye, the NCO points are clearly contiguous suggesting a gently bowed structure that is symmetrical about the vertical axis. Although method error still contributes to plane error in this eye, biological nonplanarity is most likely the principal component. Biological nonplanarity that is delineated reproducibly over time will still provide a stable fitted plane for a zero reference.
A significant association between plane error magnitude and age was observed in the histomorphometric eyes (P = 0.001, linear regression of plane error/age). However, when the two oldest monkeys, a 32-year-old with a plane error of 18 μm and a 31-year-old with a plane error of 15 μm, were removed from the linear regression, the association was no longer significant (P = 0.243, linear regression of plane error/age). There was no significant association between plane error and age in the SD-OCT group (P = 0.565, linear regression of plane error/age).
Discussion
We have previously delineated BMO as the NCO and used it as a reference plane within quantitative 3-D histomorphometric reconstructions of the optic nerve head from both eyes of three NHPs with early experimental glaucoma in one eye.25-27 The purposes of the present report were to examine our ability to detect and delineate manually the NCO within 3-D histomorphometric reconstructions of a larger group of normal NHP optic nerve heads and, second, to perform the first rigorous assessment of NCO detection within interpolated SD-OCT ONH volumes, in a separate group of normal eyes. An additional purpose of this study was to characterize the structure of the NCO in both 3-D histomorphometric reconstructions and in interpolated SD-OCT optic nerve head volumes, with particular reference to planarity.
The principal findings of the study are summarized as follows: First, NCO points can be delineated within all digital, radial sagittal section images from interpolated SD-OCT volumes. Second, NCO plane error appears to be of a similar magnitude in both histomorphometric reconstructions and SD-OCT volumes of normal NHP optic nerve heads. The NCO plane error is similarly small relative to the size of the NCO fitted ellipse. Third, the NCO point clouds of both histomorphometric reconstructions and SD-OCT volumes indicate that the NCO appeared to be a biologically continuous structure.
This is the first report of 3-D visualization and delineation of SD-OCT interpolated optic nerve head volumes. As such, this study establishes the NCO to be clearly discernible and biologically continuous within single SD-OCT volumes of 33 normal NHP eyes. The depth resolution of the 870-nm Spectralis imaging system within the 40 serial radial interpolated SD-OCT sections has been shown to be consistently capable of imaging the NCO, as well as the RPE/BM complex, in all 33 eyes. These observations preliminarily support the concept of applying SD-OCT technology to image the optic nerve head in a volumetric and quantifiable fashion by using the NCO as a reference plane.
The fact that NCO plane error was low in both histomorphometric and SD-OCT data sets is important for several reasons. The histomorphometric data in a much larger number of eyes strengthen our use of an NCO reference plane for quantification within our histomorphometric 3-D reconstructions.25-27 Likewise, the SD-OCT data provides support for an identical application of the NCO within clinically obtained 3-D SD-OCT volumes. Indirectly, one may also extrapolate that, as their magnitude of planarity is similar, in vivo SD-OCT imaging may be capable of capturing the 3-D architecture of the NCO in a clinically meaningful manner that is similar to its postfixed, postembedded, poststained, and postreconstructed architecture. This latter statement is made with the important caveat that the present study does not directly compare in vivo SD-OCT imaging to post mortem 3-D histomorphometry in the same eyes. However, in making this point, one should also note that even if the same optic nerve head had been SD-OCT imaged, then 3-D histomorphometrically reconstructed post mortem, the measurements obtained would still not be directly comparable. Although 3-D histomorphometry is capable of measuring the dimension of structures after they have been processed (in summary, after perfusion-fixation, embedding, staining, sectioning, alignment, 3-D visualization, and delineation), these measurements are influenced by tissue shrinkage and measurement error. Likewise, the accuracy of in vivo measurements obtained by SD-OCT is affected by the image acquisition and alignment software, individual eye magnification correction algorithms, as well as the 3-D visualization and 3-D delineation methods. Given their respective sources of measurement error, the comparison of the SD-OCT data to the 3-D histomorphometric data in this report and its application to in vivo human eyes must remain qualitative and preliminary in nature.
Although a correction for SD-OCT magnification error has been included to compensate for the optical system of the monkey eye, it is at present imprecise. Several methods have been published to correct for eye-camera53,54 and eye55,56 magnification, all of which make assumptions regarding the optics of the eye. In our method, the error is based on axial length; methods using this technique have been shown to result in smaller errors and to be more accurate than those using ametropia and keratometry.57 The magnification error only affects x- and y-axis coordinates, not those within the z-axis. The SD-OCT ellipse dimensions, which are entirely based on x and y coordinates, are likely to be greatly influenced by any measurement error. One should therefore regard the SD-OCT NCO dimensions (namely, major ellipse axis, minor ellipse axis, and ellipse area) as being a best available estimate only and not infer importance from the observed similarity with histomorphometrically derived ellipse dimensions.
Eccentricity, however, which is the ratio of the major to minor ellipse axes, is independent of magnification error in either imaging system. Eccentricity is very similar between histomorphometric reconstructions and SD-OCT volumes (median values of 1.38 and 1.43, respectively), suggesting that the scale of measurements is likely to be similar, regardless of differences in magnification error. Among SD-OCT NCO metrics, plane error is likely to be the least influenced by magnification as a significant component of the normal distance between observed NCO points and the fitted plane will be in the z-axis. Plane error appears to be low relative to ellipse magnitude in both imaging systems, suggesting that NCO is a relatively planar structure in both 3-D histomorphometric reconstructions and in SD-OCT volumes.
As noted in the Methods section, the resolution of our 3-D histomorphometric technique improved from a 3.0- to a 1.5-μm voxel dimension within the group of histomorphometric reconstructions in this report. However, neither the ability to detect the NCO in the 3-D histomorphometric reconstructions nor its plane error (once detected) appeared to be influenced by this improvement. The increase in 3-D resolution achieved by the newer protocol theoretically achieves a greater “biofidelity” to smaller structures such as individual laminar beams and the extension of unpigmented BM beyond the end of the RPE.25
Our data suggest a relationship between the magnitude of plane error and advancing age in the histomorphometric but not the SD-OCT data sets. However, the maximum age of the NHPs imaged by SD-OCT was only 19 years, whereas there were two NHPs more than 30 years old in the histomorphometric data set. The relationship between age and NCO plane error should be studied in large data sets as volumetric SD-OCT imaging of the human optic nerve head begins to be reported.
SD-OCT NCO delineation was performed using interpolated B-scans generated from 290 × 768 horizontal grid B-scan acquisitions. It is likely that our delineation technique will be improved by using interpolated sections generated from higher density acquisitions (for example, 768 A-scans per each of 768 B-scans) or by using actual radial B-scan acquisitions, which will have a better image quality than the current interpolated scans. Despite this, our ability to delineate NCO points in this study is encouraging, with a manageable detrimental influence from motion artifact and/or shadowing from blood vessels.
The current 290 horizontal B-scan acquisition protocol takes 70 seconds in an anesthetized NHP. It is likely that the same scan will take considerably longer in an awake human subject and longer still for a test of higher B-scan density. Acquiring 40 or 80 radial B-scans will likely be of shorter duration and will have the advantage that one will be able to delineate structures within the acquired B-scan, rather than an interpolated SD-OCT section. A disadvantage to this approach is that interpolated 3-D volumes cannot, at present, be generated from radial B-scan acquisition patterns. As a consequence, true 3-D delineation of the NCO points (in which the delineator can cross-reference demarcated points between the location in the transverse and sagittal view before actual delineation), which powerfully aided this study, are not possible using radial B-scan acquisitions in isolation.
NCO visualization with volumetric (or serial B-scan) SD-OCT images may be enhanced with clinical application of a higher wavelength source. In an in vivo study of high-resolution OCT imaging of the human retina, a 1050-nm wavelength source was found to achieve greater depth resolution than an 870-nm source (equivalent to that used in the Spectralis device).58 Visualization beyond the RPE and into the choroidal vasculature was achieved, and this depth resolution was maintained, even in the presence of significant cataract. It will therefore be necessary in the future to investigate the effect of a 1050 nm light source on the ability to delineate NCO and deep optic nerve head structures in the monkey eye.
Although our results show that SD-OCT is capable of reliably discerning the NCO in the monkey eye, this may not necessarily be consistent in the human eye. The reason for potential discrepancy is due to differences in the amount of pigment at the level of the RPE/BM. In normal monkey eyes, RPE and choroidal atrophy is uncommon, whereas it is relatively common in humans, particularly in the aged eye. It will not be apparent whether the NCO is detectable in this context until our methodology can be applied to human eyes.
The location of the reference plane—effectively the landmark from which all structural quantification is measured—is critical in longitudinal imaging of the optic nerve head. The topographical height of the reference plane, determined by tomography (Heidelberg Retina Tomograph [HRT]; Heidelberg Engineering) has been shown to be the major contributor to parameter variability. The default reference plane used in the HRT operational software is the standard reference plane, which is located 50 μm posterior to the temporal disc margin. The choice of the temporal disc margin was based on the assumption that its height would be stable until the latest stages of glaucoma, when papillomacular bundle thinning would be expected to take place.32 However, OCT evidence suggests that retinal nerve fiber layer thinning occurs at a much earlier time frame in the disease process.59 Given this caveat, any measurements generated using a reference plane anchored to the retinal surface height at the disc margin are likely to fluctuate longitudinally as the topographical surface shifts posteriorly as glaucoma progresses.
Studies have shown that HRT reference planes anchored to the retinal surface height at a reference ring located in the image periphery generate less variable stereometric parameters, particularly rim area, and this most likely reflects the more stable topographical surface height at locations distal to the optic disc.35,37,38,46 However, the peripheral surface-anchored reference planes have the shortcoming that they generate erroneous measurements when applied to atypical disc morphology, particularly those with gross tilt or advanced parapapillary atrophy. Indeed, defining the reference plane relative to the topographic surface is always likely to be problematic as the height of the internal limiting membrane alters as the nerve fiber layer thins in progressing glaucoma.
A reference plane source-structure deep within the optic nerve head structure, such as the NCO, is a more logical option, if it is reasonable to assume that this structure will be more stable than the internal limiting membrane through the onset and progression of glaucomatous damage. We propose that, relative to the optic nerve head neural and connective tissues (primary sites of glaucomatous damage and known to be damaged early in the neuropathy),18-23 the position of the NCO will remain relatively stable throughout the course of the neuropathy. If this is true, alterations in the anterior laminar surface and prelaminar neural tissue internal limiting membrane should be more sensitively detected relative to the NCO during periods in which retinal surface–based reference planes are themselves being altered by the neuropathy.
However, the position of the NCO and the same optic nerve head target tissues may change relative to the peripapillary sclera due to glaucomatous (outward) bowing of the peripapillary sclera.26 Since we believe this bowing, when it is present, is part of the neuropathy, it may be advisable to use the NCO as a source-structure for a secondary (peripheral) reference plane that is located at a fixed distance from the NCO on the BM/RPE complex. The advantage of such a peripheral zero-reference plane would be the detection of both glaucomatous scleral deformation and optic nerve head neural and connective tissue alterations at all stages of the neuropathy. The relative stability of NCO and peripheral (NCO-anchored) and internal limiting membrane–based reference planes within longitudinal SD-OCT images of NHPs with early, moderate, and severe experimental glaucoma is currently under study in our laboratories.
Our study is the first in which deep optic nerve head structures were visualized and delineated within SD-OCT 3-D volumes acquired in vivo. The NCO has been shown to be continuous and reasonably planar, which supports its adoption as the SD-OCT reference plane source-structure within the NHP and human optic nerve head.
Acknowledgments
The authors thank Galen Williams for SD-OCT image acquisition and NCO delineation in histomorphometric reconstructions, Jonathan Grimm for software support, Juan Reynaud for software and hardware support, and Stuart K. Gardiner for statistical support.
Supported by National Institutes of Health Grant R01-EY11610, the Legacy Good Samaritan Foundation, a Royal College of Ophthalmologists/Pfizer Travel Fellowship (NGS), and unrestricted instrument support from Heidelberg Engineering (CFB).
Footnotes
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, April 2008.
Disclosure: N.G. Strouthidis, Heidelberg Engineering (F); H. Yang, None; B. Fortune, None; J.C. Downs, None; C.F. Burgoyne, Heidelberg Engineering (F)
References
- 1.Broadway DC, Nicolela MT, Drance SM. Optic disk appearances in primary open-angle glaucoma. Surv Ophthalmol. 1999;43(suppl 1):S223–S243. doi: 10.1016/s0039-6257(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Grundler A. Optic disc morphology in “age-related atrophic glaucoma”. Graefes Arch Clin Exp Ophthalmol. 1996;234:744–749. doi: 10.1007/BF00189355. [DOI] [PubMed] [Google Scholar]
- 3.Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: clinical correlations. Ophthalmology. 1996;103:640–649. doi: 10.1016/s0161-6420(96)30640-4. [DOI] [PubMed] [Google Scholar]
- 4.Asai T, Katsumori N, Mizokami K. Retinal ganglion cell damage in human glaucoma. 2. Studies on damage pattern (in Japanese) Nippon Ganka Gakkai Zasshi. 1987;91:1204–1213. [PubMed] [Google Scholar]
- 5.Garcia-Valenzuela E, Shareef S, Walsh J, et al. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 6.Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 7.Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- 8.Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- 9.Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995;23:85–91. doi: 10.1111/j.1442-9071.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 10.Wygnanski T, Desatnik H, Quigley HA, et al. Comparison of ganglion cell loss and cone loss in experimental glaucoma. Am J Ophthalmol. 1995;120:184–189. doi: 10.1016/s0002-9394(14)72606-6. [DOI] [PubMed] [Google Scholar]
- 11.Panda S, Jonas JB. Decreased photoreceptor count in human eyes with secondary angle- closure glaucoma. Invest Ophthalmol Vis Sci. 1992;33:2532–2536. [PubMed] [Google Scholar]
- 12.Kendell KR, Quigley HA, Kerrigan LA, et al. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci. 1995;36:200–205. [PubMed] [Google Scholar]
- 13.Nork TM, Ver Hoeve JN, Poulsen GL, et al. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol. 2000;118:235–245. doi: 10.1001/archopht.118.2.235. [DOI] [PubMed] [Google Scholar]
- 14.Janssen P, Naskar R, Moore S, et al. Evidence for glaucoma-induced horizontal cell alterations in the human retina. Ger J Ophthalmol. 1996;5:378–385. [PubMed] [Google Scholar]
- 15.Yucel YH, Zhang Q, Gupta N, et al. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–384. doi: 10.1001/archopht.118.3.378. [DOI] [PubMed] [Google Scholar]
- 16.Yucel YH, Zhang Q, Weinreb RN, et al. Atrophy of relay neurons in magno- and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Invest Ophthalmol Vis Sci. 2001;42:3216–3222. [PubMed] [Google Scholar]
- 17.Yucel YH, Zhang Q, Weinreb RN, et al. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 18.Gaasterland D, Tanishima T, Kuwabara T. Axoplasmic flow during chronic experimental glaucoma. 1. Light and electron microscopic studies of the monkey optic nerve head during development of glaucomatous cupping. Invest Ophthalmol Vis Sci. 1978;17:838–846. [PubMed] [Google Scholar]
- 19.Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977;16:426–441. [PubMed] [Google Scholar]
- 20.Quigley HA, Addicks EM, Green WR, et al. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 21.Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–1830. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- 22.Bellezza AJ, Rintalan CJ, Thompson HW, et al. Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2003;44:623–637. doi: 10.1167/iovs.01-1282. [DOI] [PubMed] [Google Scholar]
- 23.Burgoyne CF, Downs JC, Bellezza AJ, et al. Three-dimensional reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. Invest Ophthalmol Vis Sci. 2004;45:4388–4399. doi: 10.1167/iovs.04-0022. [DOI] [PubMed] [Google Scholar]
- 24.Downs JC, Suh JK, Thomas KA, et al. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005;46:540–546. doi: 10.1167/iovs.04-0114. [DOI] [PubMed] [Google Scholar]
- 25.Downs JC, Yang H, Girkin C, et al. Three dimensional histomorphometry of the normal and early glaucomatous monkey optic nerve head: neural canal and subarachnoid space architecture. Invest Ophthalmol Vis Sci. 2007;48:3195–3208. doi: 10.1167/iovs.07-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Downs JC, Girkin C, et al. 3-D Histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci. 2007;48:4597–4607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Downs JC, Bellezza AJ, et al. 3-D Histomorphometry of the normal and early glaucomatous monkey optic nerve head: prelaminar neural tissues and cupping. Invest Ophthalmol Vis Sci. 2007;48:5068–5084. doi: 10.1167/iovs.07-0790. [DOI] [PubMed] [Google Scholar]
- 28.Johnson EC, Morrison JC, Farrell S, et al. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp Eye Res. 1996;62:663–674. doi: 10.1006/exer.1996.0077. [DOI] [PubMed] [Google Scholar]
- 29.Johnson EC, Deppmeier LM, Wentzien SK, et al. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:431–442. [PubMed] [Google Scholar]
- 30.Cepurna WO, Kayton RJ, Johnson EC, et al. Age related optic nerve axonal loss in adult Brown Norway rats. Exp Eye Res. 2005;80:877–884. doi: 10.1016/j.exer.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Howell GR, Libby RT, Jakobs TC, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burk ROW, Vihanninjoki K, Bartke T, et al. Development of the standard reference plan for the Heidelberg retina tomograph. Graefes Arch Clin Exp Ophthalmol. 2000;238:375–384. doi: 10.1007/s004170050368. [DOI] [PubMed] [Google Scholar]
- 33.Chen E, Gedda U, Landau I. Thinning of the papillomacular bundle in the glaucomatous eye and its influence on the reference plane of the Heidelberg retinal tomography. J Glaucoma. 2001;10:386–389. doi: 10.1097/00061198-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Leung CK, Chan WM, Hui YL, et al. Analysis of retinal nerve fiber layer and optic nerve head in glaucoma with different reference plane offsets, using optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:891–899. doi: 10.1167/iovs.04-1107. [DOI] [PubMed] [Google Scholar]
- 35.Strouthidis NG, White ET, Owen VM, et al. Improving the repeatability of Heidelberg retina tomograph and Heidelberg retina tomograph II rim area measurements. Br J Ophthalmol. 2005;89:1433–1437. doi: 10.1136/bjo.2005.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strouthidis NG, White ET, Owen VM, et al. Factors affecting the test-retest variability of Heidelberg retina tomograph and Heidelberg retina tomograph II measurements. Br J Ophthalmol. 2005;89:1427–1432. doi: 10.1136/bjo.2005.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan JC, Garway-Heath DF, Hitchings RA. Variability across the optic nerve head in scanning laser tomography. Br J Ophthalmol. 2003;87:557–559. doi: 10.1136/bjo.87.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan JC, Hitchings RA. Reference plane definition and reproducibility in optic nerve head images. Invest Ophthalmol Vis Sci. 2003;44:1132–1137. doi: 10.1167/iovs.02-0039. [DOI] [PubMed] [Google Scholar]
- 39.Iester M, Mikelberg FS, Drance SM. The effect of optic disc size on diagnostic precision with the Heidelberg retina tomograph. Ophthalmology. 1997;104:545–548. doi: 10.1016/s0161-6420(97)30277-2. [DOI] [PubMed] [Google Scholar]
- 40.Schuman JS, Wollstein G, Farra T, et al. Comparison of optic nerve head measurements obtained by optical coherence tomography and confocal scanning laser ophthalmoscopy. Am J Ophthalmol. 2003;135:504–512. doi: 10.1016/s0002-9394(02)02093-7. [DOI] [PubMed] [Google Scholar]
- 41.Barkana Y, Harizman N, Gerber Y, et al. Measurements of optic disk size with HRT II, Stratus OCT, and funduscopy are not interchangeable. Am J Ophthalmol. 2006;142:375–380. doi: 10.1016/j.ajo.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 42.Neubauer AS, Krieglstein TR, Chryssafis C, et al. Comparison of optical coherence tomography and fundus photography for measuring the optic disc size. Ophthalmic Physiol Opt. 2006;26:13–18. doi: 10.1111/j.1475-1313.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 43.Correnti AJ, Wollstein G, Price LL, et al. Comparison of optic nerve head assessment with a digital stereoscopic camera (discam), scanning laser ophthalmoscopy, and stereophotography. Ophthalmology. 2003;110:1499–1505. doi: 10.1016/S0161-6420(03)00496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung CK, Cheng AC, Chong KK, et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2007;48:3178–3183. doi: 10.1167/iovs.06-1315. [DOI] [PubMed] [Google Scholar]
- 45.Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Poli A, Strouthidis NG, Ho T, et al. Analysis of HRT images: comparison of reference planes. Invest Ophthalmol Vis Sci. 2008;49(9):3970–3975. doi: 10.1167/iovs.08-1764. [DOI] [PubMed] [Google Scholar]
- 47.Heidelberg Engineering . Spectralis Operating Instructions. Heidelberg, Germany; 2007. Ver. 001. [Google Scholar]
- 48.Fortune B, Cull GA, Burgoyne CF. Retinal nerve fiber layer birefringence declines prior to thickness after onset of experimental glaucoma or optic nerve transection in non-human primates. Invest Ophthalmol Vis Sci. 2008 June 19; doi: 10.1167/iovs.08-2255. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakitani Y, Sasoh M, Sugimoto M, et al. Macular thickness measurements in healthy subjects with different axial lengths using optical coherence tomography. Retina. 2003;23:177–182. doi: 10.1097/00006982-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Heickell AG, Bellezza AJ, Thompson HW, et al. Optic disc surface compliance testing using confocal scanning laser tomography in the normal monkey eye. J Glaucoma. 2001;10:369–382. doi: 10.1097/00061198-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Qiao-Grider Y, Hung LF, Kee CS, et al. Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Res. 2007;47:1424–1444. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiao-Grider Y, Hung LF, Kee CS, et al. A comparison of refractive development between two subspecies of infant rhesus monkeys (Macaca mulatta) Vision Res. 2007;47:1668–1681. doi: 10.1016/j.visres.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bengtsson B, Krakau CE. Some essential optical features of the Zeiss fundus camera. Acta Ophthalmol (Copenh) 1977;55:123–131. doi: 10.1111/j.1755-3768.1977.tb06101.x. [DOI] [PubMed] [Google Scholar]
- 54.Bengtsson B, Krakau CE. Correction of optic disc measurements on fundus photographs. Graefes Arch Clin Exp Ophthalmol. 1992;230:24–28. doi: 10.1007/BF00166758. [DOI] [PubMed] [Google Scholar]
- 55.Littmann H. Determination of the real size of an object on the fundus of the living eye (in German) Klin Monatsbl Augenheilkd. 1982;180:286–289. doi: 10.1055/s-2008-1055068. [DOI] [PubMed] [Google Scholar]
- 56.Littmann H. Determining the true size of an object on the fundus of the living eye (in German) Klin Monatsbl Augenheilkd. 1988;192:66–67. doi: 10.1055/s-2008-1050076. [DOI] [PubMed] [Google Scholar]
- 57.Garway-Heath DF, Rudnicka AR, Lowe T, et al. Measurement of optic disc size: equivalence of methods to correct for ocular magnification. Br J Ophthalmol. 1998;82:643–649. doi: 10.1136/bjo.82.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Povazay B, Hermann B, Unterhuber A, et al. Three-dimensional optical coherence tomography at 1050 nm versus 800 nm in retinal pathologies: enhanced performance and choroidal penetration in cataract patients. J Biomed Opt. 2007;12:041211. doi: 10.1117/1.2773728. [DOI] [PubMed] [Google Scholar]
- 59.Park KH, Caprioli J. Development of a novel reference plane for the Heidelberg retina tomograph with optical coherence tomography measurements. J Glaucoma. 2002;11:385–391. doi: 10.1097/00061198-200210000-00003. [DOI] [PubMed] [Google Scholar]