Abstract
It has been difficult to find conditioned preference for tactile cues paired with ethanol intoxication in rats. Toward understanding the ontogeny of ethanol reinforcement, we aimed at establishing a simple and reliable procedure for: (i) assessing primary appetitive conditioning to ethanol in infant rats and (ii) discerning the role the opioid system plays in ethanol-mediated conditioning at this age. Experiment 1 determined the parameters (i.e., dose, interval of conditioning) for assessing ethanol-mediated conditioning. Pups were then trained with differential Pavlovian conditioning (Experiments 2 and 3) in which ethanol intoxication (1.0 – 2.0 g/kg, intragastrically or intraperitoneally delivered) was paired with a tactile stimulus (sandpaper) while an alternative texture signaled the absence of ethanol’s effects. Unpaired control conditions were also employed. Tactile preferences were assessed after two conditioning sessions. Paired rats spent significantly more time on sandpaper than unpaired controls, an effect that was greater following intragastric administration of 1.0 than 2.0 g/kg ethanol. This effect was replicated in Experiments 4a and 4c and found to be inhibited by pretreatment with general (naloxone) or specific (D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 [CTOP] and naltrindole) opioid antagonists. Blood ethanol levels at conditioning were not altered by naloxone (Exp. 4b). The study outlines a procedure that reveals appetitive conditioning to ethanol by infant rats. The results are discussed in terms of a potential ethanol-induced activation of the endogenous opioid system during the onset of the intoxication process.
Keywords: ethanol, appetitive conditioning, infant rat, opioid system, naloxone, naltrindole
Introduction
Early exposure to ethanol increases the likelihood of later alcohol abuse and dependence, as found in both human (Alati et al., 2006; Baer et al., 2003) and animal studies (Chotro et al., 2007). It has been suggested (Spear and Molina 2005; Pautassi et al., 2009) that ethanol-mediated motivational learning may underlie this association. The young organism would learn that the taste and flavor of ethanol—or any other stimuli paired with its administration—predicts the appetitive, positive reinforcing properties of the drug. Later re-exposure to these stimuli would increase the probability of ethanol seeking and self-administration. Hence, it is important to analyze how infant rats learn about ethanol’s motivational properties and the neurobiology underlying this phenomenon (for a review on the relevance of ontogenetic analysis for understanding ethanol-related problems, see Pautassi et al., 2009).
It has been difficult to detect first-order, ethanol-mediated appetitive conditioning in the heterogeneous, non-selected adult or infant rat. The majority of studies have found avoidance of tactile or taste cues previously paired with ethanol's effects (Cunningham et al., 1993; Hunt et al., 1991; Molina et al., 1996; Pautassi et al., 2002; Schechter and Krimmer, 1992). To further explore ethanol’s appetitive motivational properties, and the mechanisms modulating them, it seems important to develop rat models of primary positive ethanol reinforcement during early ontogeny.
High -ethanol doses usually support conditioned taste aversion in infant rats (Pautassi et al., 2002, 2005). Recent reports, however, also indicate that experience with ethanol doses of 2.0 g/kg or higher can result in appetitive learning in infant and adolescent rats (Molina et al., 2006, 2007; Pautassi et al., 2008) when assessed in terms of second order conditioning (Molina et al., 2006, 2007) or revaluation procedures (Pautassi et al., 2006; 2007). Furthermore, infant rats administered 2.5 g/kg ethanol exhibit drug-induced motor activation, which is often regarded as an index of the appetitive reinforcing effects of drugs of abuse (Arias et al., 2008). Hence, it is also conceivable that higher ethanol doses can produce appetitive reinforcement. It is important to point out that none of these examples (i.e., Molina et al., 2006; 2007; Pautassi et al., 2006; 2007) used first order conditioning procedures. First order appetitive conditioning to ethanol’s pharmacological effects has been found in a very limited set of studies and usually has required additional experimental manipulations, such as pre-exposure to the pharmacological properties of ethanol (20 days or more: Bienkowski et al., 1996, Reid et al., 1985; intraoral ethanol infusions: Pautassi et al., 2008) or a concurrent stress (Matsuzawa et al., 1998, 1999). One explanation for the disparity between the expression of ethanol-mediated learning in primary conditioning (expressed as aversions, Molina et al., 1996; Pautassi et al., 2002; Asin et al., 1985) and other conditioning methods (second order conditioning or devaluation, Molina et al, 2006: 2007; Pautassi et al., 2006; 2007) may be that in methods of primary conditioning in rats the CS has been introduced during a late phase of ethanol intoxication (Molina et al., 1996; Pautassi et al. 2002). In contrast, the second order conditioning and devaluation methods have included pairing of the early effects of ethanol with their respective CS or US. Hence, it could be postulated that the early and late phases of the blood ethanol curve may be associated, respectively, with appetitive and aversive effects of ethanol (Conrod et al., 1998; 2001).
The mechanisms responsible for the appetitive effects of ethanol are still not fully understood. It is clear, however, that several families of opioid receptors (including μ, κ and δ) have been implicated in ethanol intake and reinforcement. In adult rodents voluntary intake of ethanol is reduced by general opioid antagonists ( e.g., naloxone and naltrexone; Bienkowski et al., 1999; Davidson & Amit, 1997; Froehlich et al., 1991) as well as by selective kappa receptor agonists and delta and mu receptor antagonists (Hyytia & Kiianmaa, 2001; Krishnan-Sarin et al., 1995; Lindholm et al., 2001). Ethanol-mediated conditioned reinforcement also involves the opioid system. Naloxone in conjunction with moderate (1.5 g/kg) or high (3.0 g/kg) doses of ethanol has been shown to increase conditioned taste aversion (Broadbent et al., 1996). Injections of a general opioid antagonist prior to testing blocks conditioned place preference (CPP) and facilitates extinction of both CPP and operant responding for ethanol in rodents (Bechtholt & Cunningham 2005; Bienkowski et al., 1999; Cunningham et al., 1995; 1998; Kuzmin et al., 2008).
Yet, in adult rats, naloxone seems to have little or no effect on conditioned place aversions (CPA) produced by ethanol. Borman and Cunningham (1997) found that naloxone (0, 1.5, or 10 mg/kg) did not alter the expression of CPA induced by ethanol (1.8 g/kg, ip). When given during acquisition, naloxone seemed to directly support CPA and to enhance ethanol-mediated CPA. These results, however, do not discount the possibility that, in the rat, the opioid system may be involved in ethanol’s appetitive reinforcing properties. The learning procedure employed by Borman and Cunningham (1997) tested only aversive conditioning. A model of first order appetitive reinforcement to ethanol would help the investigation of the control of the opioid system over the positive reinforcing properties of ethanol. For infants the data on opioid involvement in ethanol’s motivation properties are not as abundant as for adults. Nizhnikov and colleagues (2006a; b) have demonstrated that, when testing neonatal rats with an age-specific conditioning procedure (i.e., “surrogate nipple technique”, Petrov et al., 2003) kappa and mu opioid receptor antagonists disrupt appetitive conditioning to ethanol administered intraperitoneally (i.p.), intracisternally (IC) or orally. It has also been shown that although infant rats (postnatal days 7 to 8, PD 7–8) exposed to ethanol exhibit increased acceptance of ethanol, this effect is blocked by co-administration of naloxone with ethanol during the early exposure (Chotro & Arias, 2007). Naloxone treatment also blocks the heightened palatability to ethanol that follows prenatal exposure to the drug (Arias & Chotro, 2005) as well as the motor activating effects induced by high doses of ethanol (2.5 g/kg; Arias et al., 2009).
The present study aimed at establishing a simple, yet reliable procedure for assessing: (i) primary appetitive conditioning to ethanol in infant rats and (ii) the possible involvement of the opioid system in ethanol’s appetitive motivational effects. A first experiment determined parameters for assessing ethanol-mediated appetitive conditioning. After establishing ethanol induced conditioned preference, subsequent experiments replicating this conditioning tested variables likely to affect its expression, including manipulations of the opioid system
In detail, Experiment 1 employed specific combinations of ethanol dose, post-administration time (PAT) and route of drug administration to identify a time course of blood ethanol levels (BELs) that would allow testing for ethanol-mediated motivational learning. Ethanol content in blood was tested at several time points in pups given 1 or 2 g/kg ethanol, delivered either i.p. or i.g. Experiment 2 analyzed the expression of ethanol-mediated first order tactile conditioning in infant rats. Animals were trained in a conditioning procedure in which a tactile stimulus was paired with a specific phase of ethanol intoxication (derived from the results of Experiment 1) whereas an alternative stimulus (CS-) signaled the absence of ethanol’s effects. Experiment 3 tested whether exposure to the CS− at training is a necessary element for acquisition of ethanol-mediated motivational learning. Finally, the role of the endogenous opioid system on the acquisition of ethanol-mediated motivational learning was assessed (Experiment 4), by training pups in tactile conditioning following administration of ethanol alone or in conjunction with general (naloxone, Exp. 4a) or specific opioid antagonists (D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 [CTOP] or Naltrindole, Exp. 4c). Since previous studies suggest that naloxone can alter ethanol absorption in adult rats (Linseman & Le, 1997) a separate group of animals (Experiment 4b) was used to determine BALs following co-administration of ethanol and naloxone.
Materials and Methods
General Procedures
Subjects
A total of 476 Sprague-Dawley rat pups (13 days old at the beginning of the procedures), representative of 59 litters born and reared at the Center for Development and Behavioral Neuroscience (Binghamton University, USA), were employed. Number of animals and litter representation in each experiment was as follows. Experiment 1, 144 animals (18 litters); Experiment 2, 88 animals (12 litters); Experiment 3, 31 animals (4 litters); Experiment 4a, 64 animals (8 litters); Experiment 4b, 48 animals (6 litters); Experiment 4c, 101 animals (11 litters). Births were examined daily and the day of parturition was considered as postnatal day 0 (PD 0). Pups were housed with the dam in standard maternity cages with free access to water and food. The colony was kept at 22 – 24 °C and a 12-hour light-dark cycle was used with light onset occurring at 0700 AM. Experimental procedures complied with the Guide for Care and Use of Laboratory Animals (NIH, Institute of Laboratory Animal Resources, 1996) and were also approved by the Institutional Animal Care and Use Committee within an AAALAC-accredited facility (vivarium of the Center for Development and Behavioral Neuroscience, Binghamton University, Binghamton, NY).
Determination of Blood Ethanol Concentrations (BELs) (PD 13)
Infant rats used for determination of blood ethanol concentration (Experiments 1 and 4b) were naive to any experimental manipulation until being food and fluid deprived for 60 min prior to administration of the corresponding ethanol dose on PD13. In Experiment 4b, an opioid antagonist (naloxone) was intraperitoneally (i.p.) injected 15 min prior to ethanol administration.
Animals were placed in same-sex pairs in pine-shaving lined containers and kept warm through the use of heating pads, for 60 minutes. Following the deprivation period subjects were intragastrically or intraperitonealy administered 1.0 or 2.0 g/kg ethanol. BELs were derived from blood samples taken at PAT 5, 10, 20, 40, 80 or 120 min (Experiment 1). Subjects in experiment 4b received injections of naloxone (0.0, 0.25, 0.75 and 1.5 mg/kg, i.g.) 15 minutes prior to ethanol administration (1.0 g/kg, i.g.) and blood samples were obtained 25 minutes post alcohol exposure. This time period corresponds with the mid point of the tactile conditioning procedure. Blood samples consisted of trunk blood (2 ml samples) obtained through decapitation, employing a heparinized capillary tube, and centrifuged at high speed (15 min / 3000 rpm; Micro-Haematocrit Centrifuge, Hawksley & Sons LTD, Sussex, England). The vials containing the plasma phase were stored at −70° C for later analysis. BELs were obtained by means of an AM5 Alcohol Analyzer (Analox Instruments, Lunenburg, MA). The apparatus estimates BELs by oxidizing ethanol to acetaldehyde in the presence of ethanol oxidase. BELs were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg %).
Conditioning procedures (PD 13–14)
In Experiments 2, 3 and 4, daily conditioning trials were conducted on PDs 13–14. At the start of each day (08:30 AM, approximately) animals were removed from their mother and placed in pairs in holding cages warmed by means of a heating pad. Conditioning started 60 min later.
In Experiment 2, animals were sequentially exposed to two different tactile conditioned stimuli (CSs), a smooth and a rough surface (CS− and CS+, respectively). The rough surface was made of sandpaper (coarse: 50, Gatorgrit, USA) while the smooth floor was composed of cardboard (i.e., the backside of a piece of sandpaper). A temporal gap occurred between presentations of these CSs. Smooth was always presented first and sandpaper always followed. In general terms, Paired animals were drug-free when exposed to the CS− but experienced ethanol’s post-absorptive effects when exposed to the CS+. Unpaired animals were drug free when exposed to both the CS− and the CS+ and were given ethanol only 90 minutes after the termination of the CS+ exposure. In other words, CS− exposure preceded ethanol administration by 20 min for paired and 120 min or 150 for unpaired subjects (i.e., unpaired groups administered 1.0 or 2.0 g/kg ethanol, respectively).
More specifically, at the start of each conditioning trial animals were placed into clear Plexiglas boxes (15 × 7 × 14 cms) lined with a smooth surface (cardboard, CS-). They remained in contact with the CS− for 10 min. Twenty minutes following CS-exposure, paired pups (P) were weighed to the nearest 0.01 g (Sartorius, Gottingen, Germany) and administered ethanol (1.0 or 2.0 g/kg), either intragastrically or intraperitoneally (IG and IP groups respectively). Pups were then returned to the holding chambers. Following the waiting period (20 or 40 min, depending on whether they had been assigned to the 1.0 or 2.0 g/kg dose groups), all pups (both P and UP) were placed into Plexiglas chambers lined with a rough surface (sandpaper; CS+) for 10 min, before being returned to their corresponding holding chambers. Conditioning with the CS+ began at 20 min post-administration for pups given 1.0 g/kg ethanol and 40 min post-administration for those that received 2.0 g/kg (trial duration: 10 min). Unpaired controls were exposed to the CS+ drug free and received ethanol administration 90 minutes following CS+ exposure. A previous study (Nizhnikov et al., presented at SFN meeting, 2008) indicated that a 90 min delay between CS+ and ethanol administration is enough to prevent the development of associative learning when employing the ethanol doses used in the present experiments and provides an effective, conservative control.
Moreover, several experiments conducted in our lab indicate that 13 to 15 day old pups show equal preference for sandpaper and the smooth surface when assessed in a two-way test (i.e., Molina et al., 2006; 2007; Pautassi et al., 2008). In other words, it seems that at this age pups do not have a bias for the textures employed. To ensure this, a pilot study was conducted just before the present Experiments. It was found that, when employing the two-way tactile preference test, naive animals showed approximately 50% selection of the sandpaper surface.
The rationale for using unpaired animals was that this type of control condition addresses several caveats, such as the possibility of non-specific changes (i.e., sensitization, habituation) resulting from the mere exposure to the US or the CS. These caveats --which are not accounted for when using saline or CS-only controls-- need to be seriously addressed in pharmacological studies (see Cunningham, 1993), as long as psychoactive substances can exert lingering effects, that can still be present at test (e.g., thermal alterations, Spiers and Fusco, 1992). By using an unpaired condition all animals are equated in terms of exposure to the CS and US, and differences between the groups could be related to the specific CS-US contingency experienced by the paired group. In the present study all specific statements about ethanol-induced preferences for sandpaper are made by contrasting animals that experienced the explicit pairings between a tactile cue and ethanol’s effects, against animals exposed to both sandpaper and ethanol but in an explicitly unrelated manner (unpaired group).
In Experiment 3, half of the animals were exposed to the differential CS−/CS+ conditioning procedure as in Experiment 2 while the remaining animals were given training with exposure to the CS+ only. The procedure for conditioning was identical to that of Experiment 2 except no CS− was presented to subjects in the CS+ only group. All other handling of the subjects was identical to that of the CS−/CS+ group. Ethanol dose and route of administration was restricted to the condition that seemed to yield the most effective appetitive reinforcement in Experiment 2 (i.e., 1.0 g/kg; i.g.). This Experiment aimed partially at assessing if training with a CS− was necessary for the emergence of ethanol-mediated learning. Since the CS− turned out not to be necessary (see results section), conditioning in the remaining behavioral experiments (i.e., Exps. 4a and 4c), included only exposure to the CS+.
In Experiment 4a, animals were given a general opioid antagonist (naloxone, 0.25, 0.75 or 1.5 mg/kg) fifteen minutes prior to ethanol intubation (1.0 g/kg, i.g.), which equated to thirty-five minutes before pairings of ethanol’s postingestive effects and the CS+ (sandpaper). In Experiment 4c ethanol-sandpaper pairings were preceded by intraperitoneal injection of varying doses of CTOP (μ antagonist; 0.10 or 1.0 mg/kg), naltrindole (δ antagonist; 1.0 or 5.0 mg/kg), or saline. All conditioning procedures were identical to the CS+ only group in Experiment 3. Naloxone doses previously shown to facilitate extinction of ethanol-mediated conditioned place preference in mice were chosen for use in Experiment 4a (Cunningham et al., 1995). Doses of CTOP were chosen due to their effectiveness in blocking the reinforcing effects of ethanol in infant rat pups (Nizhnikov et al., 2006). Unpaired groups in Experiments 4a and 4c also received doses of the antagonist. These doses were given at the same time as in paired subjects; that is, thirty-five minutes prior to CS+ exposure.
Across all Experiments all animals were returned to the mother 120 min after delivery of ethanol in UP pups. These manipulations allowed maternal deprivation to be equated across paired and unpaired groups.
Assessment of conditioned texture preferences (PD 15)
Pups were separated from the dam and placed in pairs in heated holding chambers. Sixty minutes later subjects were placed into a clear Plexiglas split-floor box (28 × 12 × 15 cm). Half of the floor was lined with sandpaper while the other half was covered with a smooth cardboard paper. Both textures were replaced in each new test. The testing procedure lasted 5 min and was conducted under red light. The test started by gently placing the animal in the juncture of the two textures; namely in the middle section of the apparatus. To ensure that the rat was indeed over a given surface, this middle section (15 % of the entire surface) was considered as a neutral area.
Time spent over each particular tactile section of the apparatus was recorded. As mentioned, the middle section of the apparatus featured both textures and, hence, was not taken into account for data collection nor analysis (for a similar procedure, see Pautassi et al, 2002; 2007; 2008).
Tactile preference scores were expressed as (a) total time (in seconds) spent over the sandpaper section of the apparatus and (b) percent time spent on sandpaper. The latter index was calculated as follows: [(total time spent over sandpaper × 100) / (total time spent over sandpaper + total time spent over smooth]. Total duration of forward locomotion (s) during the 5-min test and frequency of wall climbing were also registered. Wall climbing was measured when pups stood on their rear limbs with the forepaws placed on the walls of the chamber. Locomotion was defined as the movement of the 4 paws at a given time. The dependent variables were registered in real time by experimenters blind to the training conditions of the animals.
Drug preparation and drug administration procedures
Ethanol
The ethanol doses of 0.5, 1.0 and 2.0 g/kg were achieved by administering 0.015 mls of an ethanol solution at 4.2, 8.4 and 16.8 % v/v, respectively, per gram of body weight (190-proof Ethanol, Pharmaco, Brookfield; vehicle: distilled water). Ethanol i.g. administration was conducted by means of an 8-cm section of PE 10 polyethylene tubing (Clay-Adams). The tubing, which was connected to a 1cc syringe mounted with a 27-½ gauge needle, was gently introduced into the pup’s oral cavity and guided into the subjects’ stomach, prior to the actual delivery of ethanol. Intraperitoneal ethanol injections were executed, in about 10 sec, with a needle (30 G, Becton Dickinson & Co., Rutheford, N.J) attached to a 1cc syringe.
Naloxone
Naloxone solution was prepared by dissolving 10.0 mg of the drug (Sigma– Aldrich, St. Louis, MO) in 100 ml of 0.9% saline. Naloxone doses of 0.25, 0.75 and 1.5 were achieved by varying injection volume (animal weight in grams × 0.0025, 0.0075 or 0.015, for each dose respectively).
CTOP and Naltrindole
CTOP doses of 0.1 and 1.0 mg/kg were achieved by diluting 1 mg of this μ antagonist (Sigma–Aldrich, St. Louis, MO) in either 100 or 10 ml of 0.9% saline, respectively. Naltrindole doses of 1.0 and 5.0 mg/kg were achieved by diluting either 10 or 50 mg of the δ antagonist (Sigma–Aldrich, St. Louis, MO) in 100 ml of 0.9% saline, respectively. Injection volumes for each dose of either CTOP or Naltrindole were calculated by multiplying the weight of the animal by 0.01.
Experimental Designs and Data Analysis
The orthogonal factors defining the experimental design of Experiment 1 were dose, route of administration and post-administration time (PAT). Animals were intragastrically or intraperitonealy administered 1.0 or 2.0 g/kg ethanol. BELs were derived as described in the methods section. Each of the 24 experimental groups in Experiment 1 included six animals. Experiment 2 employed a factorial design with three between-group factors: ethanol dose (1.0 and 2.0 g/kg), conditioning procedure [the CS+ was presented during a given ethanol post-administration time (P groups), or was explicitly unpaired with ethanol’s effects (UP)] and route of ethanol administration (IP or IG). Each of the eight groups had 9–12 animals. In Experiment 3, animals were randomly assigned to four groups (n = 7–8), as a function of the two factors: conditioning treatment (paired or unpaired) and training procedures (sequential CS−/CS+ exposure or CS+ only).
A 2 (Conditioning treatment: paired or unpaired) X 4 (naloxone dose: (0.0, 0.25, 0.75, or 1.5 mg/kg) factorial was used in Experiment 4a. Experiment 4b consisted of a one-factor, four-categories design (1.0 g/kg ethanol, i.g., in conjunction with 0.0, 0.25, 0.75, or 1.5 mg/kg naloxone). Finally, Experiment 4c employed a 2 (Conditioning: paired or unpaired) × 5 (antagonist treatment) factorial. During conditioning, animals could receive injections of CTOP (0.10 or 1.0 mg/kg), naltrindole (1.0 or 5.0 mg/kg), or vehicle. We will refer to these groups as: CTOP/.10, CTOP/1.0, NALT/1.0, NAL/5.0 and 0.0/VH, respectively. Across Experiments 4a, b and c, groups had 8–12 rats.
The dependent variables were processed through fixed factor Analyses of Variance (ANOVA). The loci of significant main effects or interactions were further examined through follow-up ANOVAs and by means of pair-wise post-hoc comparisons (Fisher’s Least Mean Significant tests, alpha level set at 0.05). Planned comparisons were also conducted if justified by previous hypotheses. To confirm that pups in a given group were exhibiting a sandpaper preference above chance (and hence exhibiting ethanol-mediated appetitive learning), percent preference scores were further analyzed. Specifically, the lower limit for the 95% confidence interval (CI) was computed and a t-test for single means against a user-defined constant was performed. In this case, the constant was a theoretical 50% percent of preference for the CS+.
In order to avoid overrepresentation of litters within each specific group, no more than two animals per litter (one male and one female) were assigned to each particular treatment. Sex was also considered as a factor, but since it failed to exert significant main effects or interact with the remaining factors data were collapsed across sex for all figures. It needs to be noted, though, that across Experiments, n per sex was low in some groups. Hence, in these occasions, it may have been difficult to detect significant effects (either main effects or significant interactions) of sex.
Results
Experiment 1
BELs were analyzed by means of a 2 (route of administration) × 2 (ethanol dose) × 6 (PAT) factorial ANOVA. The analysis yielded significant main effect for each of these factors: F(1, 120) = 87.81, F(1, 120) = 556.50 and F (5, 120) = 7.11, respectively, all ps < 0.0001. The interactions between route and PAT, route and dose as well as the one comprising PAT and dose, also achieved significance: F(5, 120) = 18.17, F(1, 120) = 17.93 and F (5, 120) = 2.96, respectively, all ps < 0.05. The three-way interaction (mode × dose × PAT) was also significant, F (5, 120) = 4.56, p < 0.001.
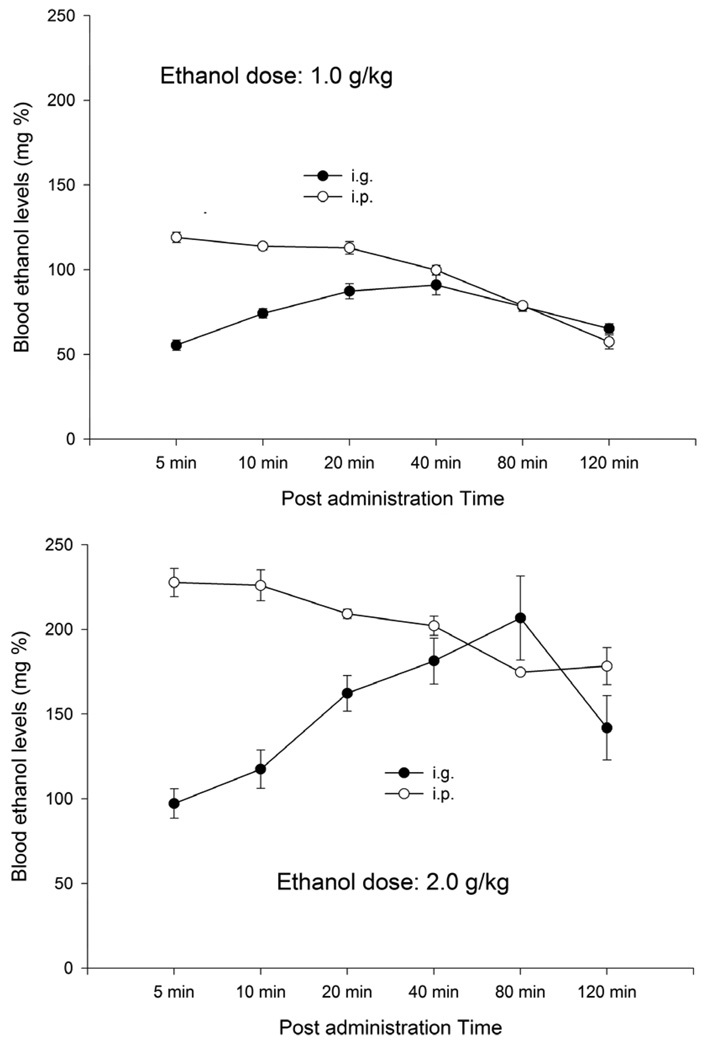
As observed in Fig.1, the higher ethanol dose induced higher BELs. Furthermore, peak BELs were achieved much faster by means of i.p. than i.g. administration, a phenomenon particularly noticeable at the highest dosage. Post-hoc comparisons (p < 0.05) also indicated that, at PAT 40 for 2.0 g/kg, i.p. and i.g. ethanol delivery induced BELs that were not significantly different (roughly 190 mg%). Administration of 1.0 g/kg ethanol resulted in similar (yet statistically different) BELs at PAT 20 min (87.3 +/− 4.5 and 112.9 +/− 3.7, for i.g., and i.p., respectively). At the point of BEL equivalence, BELs derived from i.g. delivery of both 1.0 and 2.0 g/kg seemed to be still rising, whereas BELs associated with i.p. drug delivery had reached peak or seemed to be descending. In regards with the latter statement, the post-hocs indicated that BELs derived from i.g. administration were significantly higher at 20 and 40 min (1.0 and 2.0 g/kg, respectively) when compared to each corresponding initial 5 min sampling time. With regards to the 2.0 g/kg ethanol when given i.p, the analysis revealed significantly lower BEL at 40 than at 5 min PAT. This was not the case for 1.0 g/kg. Intraperitoneal delivery of this dose yielded similar BELs at 5 and 20 min PAT. In light of these results, it could be proposed that i.p. and i.g. administration of low and high ethanol dose (i.e., 1.0 and 2.0 g/kg) at 20 and 40 min PAT, respectively, may represent different sections of the ethanol absorption/elimination curve. Hence, Experiment 2 tested motivational consequences associated with these combinations of mode, dose and PAT
Figure 1.
Blood ethanol content of subjects administered 1.0, or 2.0 g/kg ethanol (top and bottom panels, respectively) either i.p. or i.g. in Experiment 1. Blood ethanol concentration was measured at 5, 10, 20, 40, 80 and 120 minutes post administration. Each experimental group was composed by 6 animals.
Experiment 2
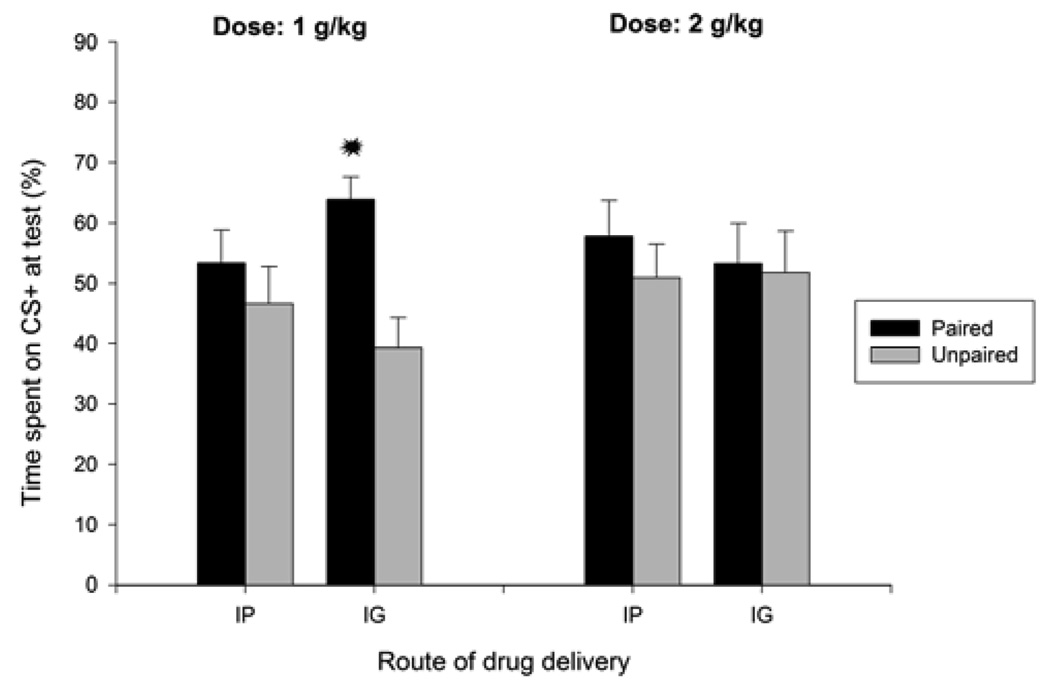
The ANOVA revealed a significant main effect of conditioning, for both absolute and percent time spent on the rough surface [F(1, 80) = 6.36, F(1, 80) =6.06, both ps < 0.05]. CS+ preference scores were significantly greater in paired pups than in unpaired controls (Figure 2). No other significant main effects or significant interactions were detected.
Figure 2.
Percent time spent on sandpaper (conditioned stimulus, CS+) as a function of conditioning procedures [sandpaper paired or unpaired with i.p or i.g. administration of ethanol (1.0 or 2.0 g/kg)]. Each of the eight groups had 9–12 animals. Asterisks indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars represent the standard error of the means (S.E.M.).
As can be observed in Figure 2, however, it seems that route of administration exerted an effect on time spent on the CS+ in pups given 1.0 g/kg but not in those given 2.0 g/kg. Among subjects administered 1.0 g/kg, the i.g. route seems to promote better appetitive conditioning (i.e., higher CS+ preference scores) than i.p. drug delivery. Under the guidance of our a-priori hypotheses, planned comparisons were conducted between paired and unpaired groups, for each combination of dose and mode of administration. No significant difference was found among pups treated with 2.0 g/kg. This was also the case between paired and unpaired subjects given 1.0 g/kg intraperitoneally. The planned comparison did reveal a significant difference, however, between P and UP pups intragastrically treated with 1.0 g/kg. Therefore, it seems prudent and conservative to suggest that ethanol-mediated appetitive conditioning was mainly driven by the i.g. delivery of 1.0 g/kg ethanol. One could cogitate whether level of sandpaper preference in the latter paired group was significantly different from a theoretical baseline of 50%. The lower limit for the CI fell above 50% (55.92). Moreover, the t test revealed a significant difference between the observed mean and the theoretical value of 50% of preference, t(11) = 3.83, p < 0.005.
Neither forward locomotion nor wall climbing at test was affected by the conditioning treatment or the route of administration experienced by the pups on PDs 13–14. The ANOVA for these variables indicated no significant main effects or significant interactions. Locomotion and wall climbing at test are presented in table 1.
Table 1. Locomotion and wall-climbing during the two-way preference test (Experiments 2, 3, 4a and 4b).
Behavioral activation [total duration of forward locomotion (s) and frequency of wall climbing] at test (a 5-min, two-way tactile preference test) as a function of conditioning treatment (sandpaper paired or unpaired with ethanol’s pharmacological consequences; delivered intragastrically, i.g., or intraperitoneally, i.p.). Unless indicated, ethanol dose at training was 1 g/kg. In Experiment 3 animals (15-day old rats) were trained with a sequential CS−/CS+ differential conditioning procedure or with exposure to the CS+ only. In Experiments 4a and 4c, rats were pretreated with either a general (naloxone) or a specific opioid receptor antagonist (CTOP and naltrindole). Across Experiments, the pertinent ANOVAs indicated no significant main effects or significant interactions comprising these variables. Values represent mean +/− SEMs.
| Experiment 2 | Wall-Climbing | Locomotion | ||
|---|---|---|---|---|
| 1.0 g/kg | 2.0 g/kg | 1.0 g/kg | 2.0 g/kg | |
| Paired i.p | 8.00 +/−1.41 | 5.00 +/−2.52 | 16.58 +/−1.14 | 13.20 +/−1.23 |
| Paired i.g | 6.50 +/−1.23 | 7.28 +/−1.06 | 15.66 +/−1.82 | 15.29 +/−2.70 |
| Unpaired i.p | 5.25 +/−1.30 | 5.38 +/−1.50 | 12.72 +/−1.26 | 13.13 +/−1.57 |
| Unpaired i.g | 5.42 +/−1.63 | 6.78 +/−1.24 | 14.66 +/−0.94 | 13.89 +/−0.77 |
| Experiment 3 | Wall-Climbing | Locomotion | ||
|---|---|---|---|---|
| Paired i.g CS−/CS+ | 9.75 +/−2.05 | -- | 13.63 +/−1.53 | -- |
| Paired i.g CS+ only | 5.12 +/−1.39 | -- | 14.25 +/−2.68 | -- |
| Unpaired i.g CS−/CS+ | 6.13 +/−1.96 | -- | 15.25 +/−1.95 | -- |
| Unpaired i.g CS+only | 6.00 +/−1.71 | -- | 14.25 +/−2.84 | -- |
| Experiment 4a | Wall-Climbing | Locomotion | ||
|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | |
| Naloxone 0.0 mg/kg | 7.50 +/−2.11 | 6.86 +/−2.77 | 12.12 +/−2.03 | 13.42 +/−1.42 |
| Naloxone 0.25 mg/kg | 7.50 +/−3.45 | 5.25 +/−2.17 | 12.25 +/−1.54 | 14.25 +/−2.21 |
| Naloxone 0.75 mg/kg | 6.87 +/−3.73 | 8.88 +/−1.85 | 13.12 +/− 1. 64 | 12.87 +/−1.26 |
| Naloxone 1.5 mg/kg | 4.38 +/−1.85 | 6.25 +/−3.25 | 13.87 +/−1.67 | 11.75 +/−1.40 |
| Experiment 4c | Wall-Climbing | Locomotion | ||
|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | |
| Vehicle | 7.13 +/−1.26 | 9.11 +/−2.18 | 15.00 +/−1.11 | 13.22 +/−0.72 |
| CTOP(0.10 mg/kg) | 5.70 +/−1.65 | 2.88 +/−1.38 | 10.70 +/−0.97 | 10.87 +/−1.43 |
| CTOP(1.0 mg/kg) | 5.63 +/−1.98 | 4.89 +/−1.26 | 11.75 +/−1.46 | 11.55 +/−1.51 |
| Naltrindole (1.0 mg/kg) | 4.10 +/−1.57 | 9.56 +/−2.29 | 12.60 +/−1.18 | 12.44 +/−1.46 |
| Naltrindole (5.0 mg/kg) | 2.38 +/−0.77 | 5.00 +/−1.02 | 9.62 +/−1.66 | 12.00 +/−0.53 |
Experiment 3
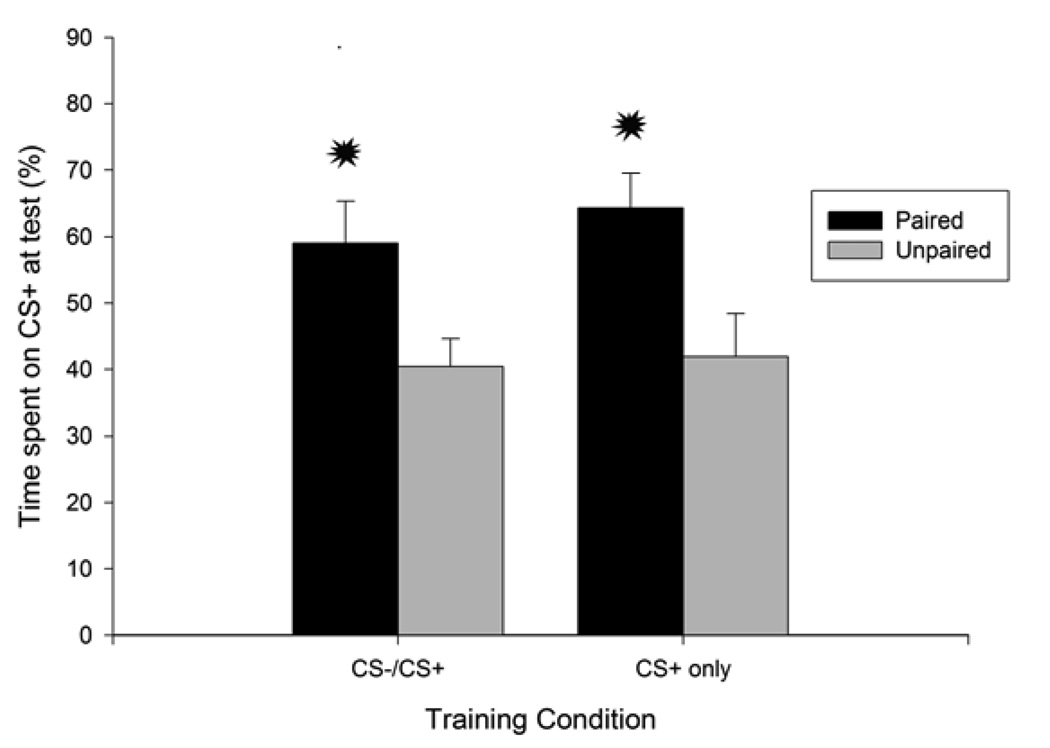
The 2 × 2 ANOVA (conditioning treatment x training procedure) indicated a significant main effect of conditioning, for both absolute and percent time on the CS+ [F (1, 27) = 14.09 and F (1, 27) = 12.75, both ps < 0.05]. Neither a significant main effect nor a significant interaction involving training procedure (CS−/CS+ or CS+ only) was detected by the analysis. As can be observed in Figure 3, the results replicated those found in Experiment 2: pairings of i.g. 1.0 g/kg ethanol and a tactile cue promoted heightened preference for this cue at test when compared to unpaired controls given unrelated exposure to both the CSs and the US (UP pups). Magnitude of this appetitive conditioned response was not altered by the presence or absence of a non-reinforced alternative tactile cue (smooth surface, CS−) preceding the CS-US pairing. The latter statement was further confirmed by subsequent post-hoc comparisons: for both training conditions (i.e., CS−/CS+ and CS+ only), paired animals spent significantly more time on sandpaper than their unpaired counterparts. Paired animals spent significantly more than 50% of the test on the CS+, as indicated by a pertinent t-test, t(15) = 2.91, p < 0.05. The lower limit for the 95% CI for percent time on CS+ was also above 50% (53.14). Separate 2 × 2 ANOVAs revealed that conditioning treatment and training procedures had no effect on wall climbing and locomotion scores during the 5-min tactile preference test. No significant main effect or significant interaction involving these variables was observed. Locomotion and wall climbing scores (mean and SEM) can be inspected in table 1.
Figure 3.
Percent time spent on sandpaper (conditioned stimulus, CS+) as a function of conditioning procedures. Subjects were exposed to a tactile cue (sandpaper CS+) either paired or unpaired with i.g. administration of 1.0 g/kg ethanol on 2 consecutive days. During conditioning, they were trained with a sequential CS−/CS+ differential conditioning procedure or with exposure to the CS+ only. Each of the four groups had 7–8 animals. Asterisks indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars represent the standard error of the means (S.E.M.).
Experiment 4
Experiment 4a
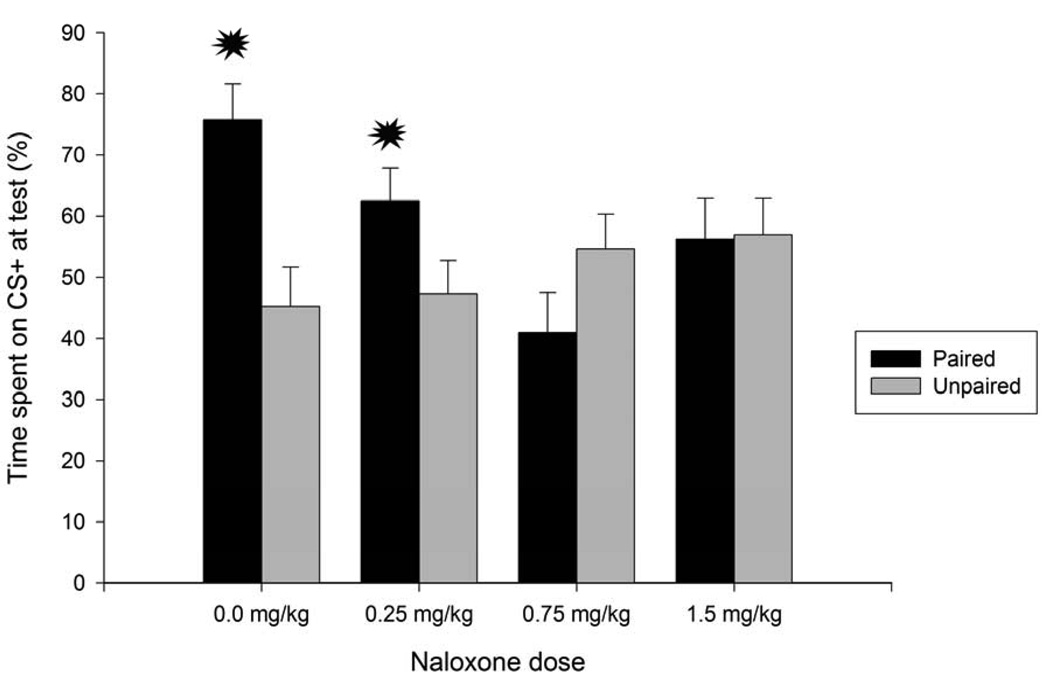
Examination of Figure 4 indicates that ethanol’s appetitive properties were transferred onto the sandpaper (CS). Pups given sandpaper-ethanol pairings (P-0.0 group) displayed greater preference for the CS than unpaired controls, but only if they did not receive the opioid antagonist. Pre-conditioning injection of 0.75 or 1.5 mg/kg naloxone abolished expression of this ethanol-mediated appetitive conditioning. The lower naloxone dose (0.25 mg/kg), in turn, had an intermediate effect, not differing from P/0.0, P/0.75 or P/1.5. These observations were confirmed by statistical analyses. Total time spent on sandpaper (absolute and percent) was analyzed through ANOVAs, which considered conditioning treatment (CS paired or unpaired with ethanol’s pharmacological consequences) and NAL dose (0.0, 0.25, 0.75 and 1.5) as between-group factors. The ANOVAs revealed a significant conditioning x NAL dose interaction [F(3, 56) = 5.20 and F(3, 56) = 5.10; both ps <.005, for absolute and percent time, respectively]. Post-hoc comparisons indicated that animals pretreated with vehicle and subsequently given pairings of ethanol and sandpaper (group P/0.0) spent more absolute and percent time on sandpaper than any other group but paired animals given vehicle and subsequently 0.25 g/kg ethanol. Similar comparisons also indicated that paired pups pretreated with 0.25 mg/kg naloxone spent more time on sandpaper than their unpaired controls (UP/0.25 mg/kg) as well as groups P/0.75 and UP/0.0. Percent preference in paired animals treated with vehicle was significantly above 50%, as indicated by a t test for a single mean against a given constant, t(7) = 4.40, p < 0.005. The lower limit for the 95% CI of this mean was 61.92.
Figure 4.
Percent time spent on sandpaper (conditioned stimulus, CS+) as a function of conditioning procedures [sandpaper paired or unpaired with i.g. administration of 1.0 g/kg ethanol] and naloxone dose given prior to conditioning (0.0, 0.25, 0.75 or 1.5 mg/kg). Each group was composed by eight animals. Asterisks indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars represent the standard error of the means (S.E.M.).
Naloxone effects during training seemed to be specifically related to an inhibition of ethanol’s effects and not aversive properties of the antagonist, as unpaired pups given naloxone did not differ from vehicle treated controls. A one-way ANOVA conducted on unpaired conditions (independent between group factor: naloxone dose) confirmed this statement: F(3, 28) = 1.21 and F(3, 28) = 0.92; both ps > 0.30, for absolute and percent CS preference, respectively.
Independent ANOVAs (conditioning treatment x NAL dose) indicated no significant main effect or significant interaction in terms of behavioral activation at test, either when focusing on wall-climbing or on overall locomotion. Mean and standard error values for each condition are presented in Table 1
Experiment 4b
A one-way between factor ANOVA indicated that treatment with naloxone did not significantly affect BELs, F(3, 44)= 0.52, p > 0.60. In other words, it seems that BELs at time of conditioning were not altered by the general opioid antagonist. Mean and SEM for each group were as follows: ethanol + 0.0 mg/kg NAL: 70.49 +/− 4.05; ethanol + 0.25 mg/kg NAL: 67.57 +/− 5.08; ethanol + 0.75 mg/kg NAL: 64.70 +/− 5.91; ethanol + 1.50 mg/kg NAL: 62.97 +/− 2.50.
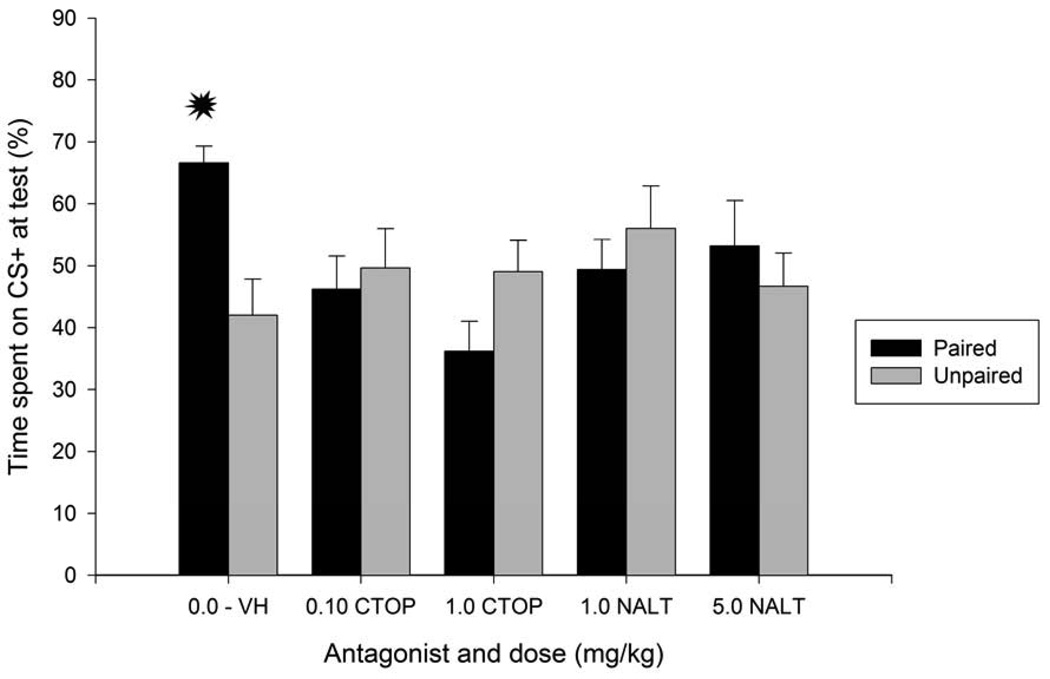
Experiment 4c
The ANOVAs indicated a significant interaction between conditioning (paired or unpaired) and antagonist treatment for both absolute and percent preference for sandpaper [F(4, 91) = 3.99 and F(4, 91) = 3.95; both ps <.05, respectively]. As shown in Figure 5 and confirmed by post-hoc analyses, pups given saline followed by ethanol-sandpaper pairings (P-0.0/VH group) exhibited significantly greater sandpaper preference at test than their controls. The post-hocs also indicated heightened CS preference in the P-0.0/VH group when compared to the remaining paired and unpaired conditions treated with CTOP (0.1 or 1.0 mg/kg) or 1.0 naltrindole. On the other hand, no sign of conditioned responding was found in pups given opioid antagonists (either μ or δ) prior to CS-US pairings. A t-test indicated that pups in the P-0.0/VH group had a greater than chance CS+ percent preference, t(9) = 6.17, p < 0.0005. The lower limit for the 95% CI of the mean was 66.61.Within unpaired conditions, there was no sign of conditioned preference or aversion as a function of antagonist treatment. A one-way ANOVA revealed that neither CTOP nor naltrindole exerted a significant effect on sandpaper preference scores, F(4, 46) = 1.29 and F(4, 46)= 0.77; both ps > 0.25, for absolute and percent CS preference, respectively.
Figure 5.
Percent time spent on sandpaper (conditioned stimulus, CS+) as a function of conditioning procedures [sandpaper paired or unpaired with i.g. administration of 1.0 g/kg ethanol] and antagonist treatment prior to conditioning: animals could receive injections of CTOP (0.10 or 1.0 mg/kg), naltrindole (NALT, 1.0 or 5.0 mg/kg), or vehicle (0.0, vehicle). Groups were composed by 9 to 12 animals. Asterisks indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars represent the standard error of the means (S.E.M.).
Wall-climbing and overall locomotion during the 5-min test were not affected by conditioning procedures or opioid antagonism treatment. The ANOVAs revealed a lack of significant main effects or significant interactions. Behavioral scores, in terms of mean and standard error values for each condition, are presented in Table 1
Discussion
The main findings of this study are that, in the rat, a robust and reliable tactile preference to a CS paired with ethanol’s pharmacological effects is possible and that activation of the endogenous opioid system is critical in the acquisition of this first-order, ethanol-mediated appetitive conditioning indicative of ethanol reinforcement.
Experiment 1 showed that, when administering 2.0 g/kg ethanol via i.p. or i.g., relatively similar BELs were achieved at post-administration time 40 min. Twenty minutes following i.p. or i.g administration of 1.0 g/kg, BELs were found to be similar, yet still significantly different across ethanol route. At these post-administrations times, the BELs resulting from i.g delivery apparently were still ascending while BELs associated with i.p. delivery had reached peak or were descending. Experiment 2 employed these parameters and revealed primary appetitive conditioning by ethanol. The possibility that this motivational learning would be regulated by ethanol dose did not receive full confirmation from the statistical analysis. However, it seems prudent and conservative to suggest that ethanol-mediated appetitive conditioning was mainly driven by those groups given i.g. delivery of 1.0 g/kg ethanol. Therefore, the subsequent experiments only employed pups given 1.0 g/kg i.g.
It could be hypothesized that better conditioning would have been observed if conditioning to the CS+ had occurred sooner after ethanol administration than the 20 PAT employed in the present experiments. The greater capability of 1.0 g/kg i.g. to induce tactile preferences when compared to i.p. administration could also be influenced by the fact that, at 20 min PAT, BELs across dose were slightly, yet significantly, different. However, conditioning trial duration in animals given 1.0 g/kg ethanol continued until post-administration time 30 min. In this time point of the blood ethanol curve, BEL differences across route of administration probably were negligible (see Figure 1). The rationale for conditioning at 20 PAT was that, although not optimal, it was the point of the curve in which 1 g/kg i.p. and i.g. were most proximal.
It is conceivable that attention to the sandpaper cue at training was impaired in paired conditions due to detrimental effects of ethanol upon cognitive processing, which might have resulted in sandpaper being preferred at test due to its novelty. Previous experiments, however, indicate that infant rats can perceive tactile and/or olfactory cues even when testing takes place under the effects of ethanol doses higher than those employed here (Molina et al., 1987; Molina et al., 1996). This suggests that it is unlikely that subjects given 1.0 g/kg ethanol were sedated or unable to attend to the CS+ in the present set of studies.
Experiment 3 revealed that employment of a CS− signaling the absence of ethanol is not necessary for acquisition of ethanol-mediated tactile preference. This is not a complete surprise. Employment of discriminative training procedures benefits acquisition of Pavlovian learning in some circumstances, but the importance and generality of the “CS− effect” for appetitive procedures is still unclear, particularly when employing ethanol as a US (Spear et al., 1989). In Experiments 4a and 4c ethanol-mediated preference was completely inhibited when pups were pretreated with either a general opioid antagonist (naloxone, 0.75 and 1.5 mg/kg) or with drugs tailored to block mu or delta opioid receptors (CTOP 0.1 and 1.0 mg/kg and naltrindole 1.0 and 5.0 mg/kg, respectively). Blood ethanol levels at the time of conditioning were not altered by naloxone (Exp. 4b), indicating that its effect is not confounded by pharmacokinetic differences.
Human and animal studies indicated that ethanol’s positive hedonic effects may be greater shortly after intoxication, when blood ethanol levels are rising and linked the appetitive properties of the drug during the rising portion of the BEL to an ethanol-induced activation of the endogenous opioid system (Conrod et al., 1998; 2001; Froehlich et al. 1991; Risinger & Cunningham, 1992). In adult humans naltrexone blocks the acute psychomotor stimulant effects of alcohol during the rising limb of the intoxication (Peterson et al., 1996; King et al., 1997). Similarly, Arias et al (2008) found that ethanol (2.5 g/kg) induced motor behavioral activation in 14-day old rats when tested soon after administration (5–10 min). This activation effect was blocked by naloxone (Arias et al., 2009). In the present study ethanol-mediated appetitive learning (i.e., paired pups given 1.0 g/kg, i.g.) was strongest when the sandpaper CS had been present during the rising phase of the BEL. Hence, it is possible that the inhibition of primary appetitive learning following opioid antagonism is due to a blunting of the early psychomotor stimulant effects of ethanol.
The present results suggest that both mu and delta opioid receptors must function in concert for ethanol-mediated appetitive learning to occur. If it were the case that activity at either the mu or delta opioid receptor alone were sufficient to induce this learning, then blockade of either receptor alone should not eliminate expression of ethanol-mediated conditioning during test. But in fact either the mu or delta receptor antagonist essentially eliminated reinforcement. Matsuzawa and colleagues (1999) have previously reported similar results when using ethanol-induced conditioned place preference in adult rats. Antagonists for either mu or delta opioid receptors attenuated place preference induced by ethanol. Bals-Kubik and colleagues (1990) also described what they called a “mu-delta opioid receptor complex” which is necessary for beta-endorphin reinforcement. Finally, similar results were observed in infant rats showing preference for a lemon odor paired with ethanol. Specifically, either mu (CTOP) or kappa (nor-BNI) receptor antagonists disrupted the reinforcing effects of the drug (Nizhnikov et al., 2006). These results are in agreement with the current set of data suggesting that at least two classes of opioid receptors need to function together for the expression of ethanol-mediated appetitive conditioning. Disruption of either class of opioid receptor (mu or delta) alone is sufficient to eliminate the positive reinforcing effects of ethanol.
The present findings may have implications for the use of opioid-antagonist treatments in clinical settings. The suppressing effects of opioid antagonists on ethanol intake have been the rationale for their use in the treatment of alcohol abuse and dependence (Davidson and Amit, 1997). Naltrexone also reduces craving and relapse in humans, processes presumably regulated by conditioned motivational effects of ethanol. Cunningham et al. (1995) found that naloxone (0.0, 0.15, 1.5, 3.0, or 10.0 mg/kg) did not interfere with the acquisition of CPP by ethanol in mice but, when given before testing, dose-dependently reduced the CPP during the course of a 60-min session. Cunningham concluded that naltrexone may facilitate extinction of the association between environmental stimuli and the positive motivational effects of ethanol. The present rat study adds new information indicating that, when employing infants and a tactile conditioning procedure sensitive to the appetitive effects of the drug, opioid treatment can also block the acquisition of ethanol-mediated appetitive conditioning.
Similarly to the present study but at a much younger age (newborn, 3-hr old rats), Nizhnikov et al. (2006a; 2007) found that conditioned preference mediated by ethanol’s pharmacological effects was blocked by CTOP. The authors acknowledged that a general inhibition of appetitive learning due to the antagonist could not be discounted. However, it should be noted that unpublished experiments by these authors have shown that opioid antagonists do not block all learning, since aversion conditioned by intraoral infusion of quinine persisted even when conditioning occurred under the effects of CTOP. Bormann and Cunningham (1997) found that naloxone (1.5 – 10 mg/kg) did not alter acquisition or expression of CPA induced by ethanol in adult rats. The author’s interpretation was that the opioid system might not be related to the conditioned motivational effects of ethanol in rats. When trying to reconcile the apparent discrepancy between Bormann and Cunningham (1998) findings and those reported here, an important difference should be noted. In adult rats, place conditioning with ethanol consistently yields aversions (Asin et al., 1985; Cunningham, 1981) although rarely preferences. That may not be the case in infants (Pautassi et al., 2008), particularly when using the combination of dose, age and post-administration time that defined the present place conditioning paradigm.
In conclusion, the current set of studies puts forward a reliable and simple procedure capable of detecting primary appetitive conditioning in infant rats. Furthermore, the present study adds new information indicating that, in the rat, the mu and delta family of receptors play a role in the acquisition of ethanol-mediated appetitive learning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alati R, Almamum A, Williams GM, O'Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch. Gen. Psych. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro M. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharm. Biochem. Behav. 2005;82:434–442. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewsky C, Pautassi RM, Molina JC, Spear NE. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharm. Biochem. Behav. 2008;89:608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewsky C, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol’s activating effects in preweanling Sprague-Dawley rats. Behav. Neurosci. 2009;123:172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Asin K, Wirtshafter D, Tabakoff B. Failure to Establish A Conditioned Place Preference with Ethanol in Rats. Pharm. Biochem. Behav. 1985;22:169–173. doi: 10.1016/0091-3057(85)90372-7. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Conner PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch. Gen. Psych. 2003;60:377–386. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Shippenberg TS, Herz A. Involvement of central mu and delta opioid receptors in mediating the reinforcing effects of beta-endorphin in the rat. Eur. J. Pharmacol. 1990;175:63–69. doi: 10.1016/0014-2999(90)90153-w. [DOI] [PubMed] [Google Scholar]
- Bechtholt A, Cunningham C. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav. Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W, Koros E. Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur. J. Pharmacol. 1999;374:321–327. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kuca P, Piasecki J, Kostowski W. Low dose of ethanol induces conditioned place preference in rats after repeated exposures to ethanol or saline injections. Alcohol Alcohol. 1996;31:547–553. doi: 10.1093/oxfordjournals.alcalc.a008190. [DOI] [PubMed] [Google Scholar]
- Bormann NM, Cunningham CL. The effects of naloxone on expression and acquisition of ethanol place conditioning in rats. Pharmacol. Biochem. Behav. 1997;58:975–982. doi: 10.1016/s0091-3057(97)00304-3. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Linder H, Cunningham C. Genetic differences in naloxone enhancement of ethanol-induced conditioned taste aversion. Psychopharmacology. 1996;126:147–155. doi: 10.1007/BF02246350. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci. Biobehav. Rev. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcohol. Clin. Exper. Res. 1998;22:585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- Cunningham C. Spatial aversion conditioning with ethanol. Pharm. Biochem. Behav. 1981;14:263–264. doi: 10.1016/0091-3057(81)90255-0. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Pavlovian drug conditioning. In: Van Haaren F, editor. Methods in Behavioral Pharmacology. BV: Elsevier Science Publishers; 1993. pp. 349–381. [Google Scholar]
- Cunningham C, Gremel C, Groblewski P. Drug-induced conditioned place preference and aversion in mice. Nature Prot. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, Okorn DM. Naloxone facilitates extinction but does not affect acquisition or expression of ethanol-induced conditioned place preference. Exp. Clin. Psychopharm. 1995;3:330–343. [Google Scholar]
- Cunningham C, Niehus J, Noble D. Species difference in sensitivity to ethanol's hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Davidson D, Amit Z. Effect of ethanol drinking and naltrexone on subsequent drinking in rats. Alcohol. 1997;14:581–584. doi: 10.1016/s0741-8329(97)00052-9. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Bocco G, Chotro MG, Spear NE, Molina JC. Operant responding controlled by milk or milk contaminated with alcohol as positive reinforcers in infant rats. Pharm. Biochem. Behav. 1993;44:403–409. doi: 10.1016/0091-3057(93)90482-9. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology. 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin. Exp. Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Spear LP, Spear NE. An Ontogenetic Comparison of Ethanol-Mediated Taste Aversion Learning and Ethanol-Induced Hypothermia in Preweanling Rats. Behav. Neurosci. 1991;105:971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O'Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK, Froehlich JC. The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology. 1995;120:177–185. doi: 10.1007/BF02246191. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Stenback T, Liljequist S. Memantine enhances the inhibitory effects of naltrexone on ethanol consumption Eur. J. Pharmacol. 2008;584:352–356. doi: 10.1016/j.ejphar.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brené S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav. Brain Res. 2001;120:137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Linseman MA, Lê AD. Effects of opioids on the absorption of alcohol. Pharmacol. Biochem. Behav. 1997;58:79–84. doi: 10.1016/s0091-3057(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Involvement of mu- and delta-opioid receptors in the ethanol-associated place preference in rats exposed to foot shock stress. Brain Res. 1998;803:169–177. doi: 10.1016/s0006-8993(98)00679-9. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Different roles of mu-, delta-and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur. J Pharmacol. 1999;368:9–16. doi: 10.1016/s0014-2999(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Molina JC, Bannoura MD, Chotro M, Mckinzie DL, Arnold H, Spear NE. Alcohol-Mediated Tactile Conditioned Aversions in Infant Rats: Devaluation of Conditioning through Alcohol-Sucrose Associations. Neurobiol. Learn. Mem. 1996;66:121–132. doi: 10.1006/nlme.1996.0053. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J, Ponce L, Truxell E, Spear N. Infantile sensitivity to ethanol's motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin. Exp. Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the Care and Use of Laboratory Animals (DHEW Publication No. 86-23) Washington, DC.: US Government Printing Office; 1986. [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing Properties of Ethanol in Neonatal Rats: Involvement of the Opioid System. Behav. Neurosci. 2006a;120:267–280. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Spear NE. Reinforcing effects of central ethanol injections in newborn rat pups. Alcohol Clin. Exp. Res. 2006b;30:2089–2096. doi: 10.1111/j.1530-0277.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- Pautassi R, Godoy J, Spear N, Molina J. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin. Exp. Res. 2002;26:644–654. [PubMed] [Google Scholar]
- Pautassi R, Nizhnikov M, Molina J, Boehm S, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41:421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov M, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci. Biobehav. Rev. 2009;33:953–974. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi R, Ponce L, Molina J. Effects of early exposure to ethanol on subsequent learning mediated by the unconditional attributes of the drug. Revista Latinoamericana de Psicología. 2005;37:149–166. [Google Scholar]
- Pautassi R, Sanders S, Miller S, Spear N, Molina J. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin. Exp. Res. 2006;30:448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Pautassi R, Molina J, Spear N. Infant rats exhibit aversive learning mediated by ethanol's orosensory effects but are positively reinforced by ethanol's post-ingestive effects. Pharmacol. Biochem. Behav. 2008;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, LeMarquand DG, Bruce KR. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcohol Clin. Exp. Res. 1996;20:1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Petrov E, Varlinskaya E, Spear N. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin. Exp. Res. 2003;27:1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Reid L, Hunter G, Beaman C, Hubbell C. Toward understanding ethanol's capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharm. Biochem. Behav. 1985;22:483–487. doi: 10.1016/0091-3057(85)90051-6. [DOI] [PubMed] [Google Scholar]
- Risinger F, Cunningham C. Ethanol produces rapid biphasic hedonic effects. Ann. N Y Acad. Sci. 1992;654:506–508. doi: 10.1111/j.1749-6632.1992.tb26014.x. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Krimmer EC. Difference in response to the aversives properties and activity effects of low doses of ethanol in LAS and HAS selectively bred rats. Psychopharmachology. 1992;107:564–568. doi: 10.1007/BF02245271. [DOI] [PubMed] [Google Scholar]
- Spear N, Kucharski D, Miller JS. The CS− effect in simple conditioning and stimulus selection during development. Animal Lear. Behav. 1989;17:70–82. [Google Scholar]
- Spear N, Molina J. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin. Exp. Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Spiers DE, Fusco LE. Delayed thermoregulatory changes in the immature rat following a single injection of ethanol. Alcohol Clin. Exp. Res. 1992;16:41–47. doi: 10.1111/j.1530-0277.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]