Abstract
GH and IGF-I are important regulators of bone homeostasis and are central to the achievement of normal longitudinal bone growth and bone mass. Although GH may act directly on skeletal cells, most of its effects are mediated by IGF-I, which is present in the systemic circulation and is synthesized by peripheral tissues. The availability of IGF-I is regulated by IGF binding proteins. IGF-I enhances the differentiated function of the osteoblast and bone formation. Adult GH deficiency causes low bone turnover osteoporosis with high risk of vertebral and nonvertebral fractures, and the low bone mass can be partially reversed by GH replacement. Acromegaly is characterized by high bone turnover, which can lead to bone loss and vertebral fractures, particularly in patients with coexistent hypogonadism. GH and IGF-I secretion are decreased in aging individuals, and abnormalities in the GH/IGF-I axis play a role in the pathogenesis of the osteoporosis of anorexia nervosa and after glucocorticoid exposure.
I. Introduction
II. The Growth Hormone (GH)/Insulin-Like Growth Factor-I (IGF-I) Axis
- III. Mechanisms of GH and IGF-I Action in Bone
- A. GH
- B. IGF-I
- C. IGF binding proteins (IGFBPs)
- IV. Skeletal Manifestations of GH Deficiency and Excess in Humans
- A. Adult growth hormone deficiency (GHD)
- B. Skeletal effects of recombinant human GH (rhGH) in adult-onset GHD
- C. Skeletal manifestations of acromegaly
- V. Skeletal Manifestations of Selected Diseases with Abnormal GH/IGF-I Axis
- A. Postmenopausal and senile osteoporosis
- B. Anorexia nervosa
- C. Glucocorticoid-induced osteoporosis
VI. Conclusions
I. Introduction
GH AND IGF-I ARE IMPORTANT regulators of bone homeostasis throughout life (1). During the prepubertal period, GH and IGF-I are determinants of longitudinal bone growth, skeletal maturation, and acquisition of bone mass, whereas in adults they are important in the maintenance of bone mass (2,3).
Longitudinal bone growth is determined by chondrocyte proliferation and differentiation in the epiphyseal growth plate of long bones, leading to endochondral bone formation. Within the growth plate, chondrocyte proliferation, hypertrophy, and differentiation result in chondrogenesis. The newly formed cartilage is invaded by blood vessels, and it is modeled into bone trabeculae. This process, called endochondral ossification, is regulated by genetic and hormonal factors, the cellular environment, and nutrition (4). During embryonic development, IGF-I and IGF-II are key determinants of growth, acting independently of GH (5). Postnatally and throughout puberty, GH and IGF-I play a critical role in determining longitudinal skeletal growth, (6,7,8) and children with GH deficiency (GHD) display short stature.
In addition to the effects on longitudinal growth, GH and IGF-I are anabolic hormones and have the potential to regulate bone modeling and remodeling. Bone remodeling is a temporally regulated process of coordinated bone resorption and bone formation carried out in microscopic basic multicellular units (9,10). There, multinucleated osteoclasts are attracted to specific sites to resorb bone. When resorption is completed, there is a reversal period and mononuclear osteoblasts are attracted to fill the cavity with newly synthesized matrix. This is followed by a resting phase. Bone remodeling is necessary to maintain calcium homeostasis and to remove potentially damaged bone. Bone modeling occurs mostly during growth. In contrast to bone remodeling, bone modeling is a process of uncoupled bone formation and bone resorption (9,10). Often it is regulated by mechanical forces, and it serves to maintain bone shape and mass. GH and IGF-I exert their anabolic effects on trabecular and cortical bone. The latter occurs by periosteal bone apposition, a process of matrix deposition at the outer surface of bone, resulting in increased bone width, and skeletal strength (11). Effects on periosteal apposition by GH or IGF-I may explain the characteristic bone deformities occurring in acromegaly.
The anabolic effects of GH and IGF-I in bone are important for the acquisition of bone mass during adolescence and possibly for the maintenance of skeletal architecture during adult life. Late adolescence and early adulthood are critical periods for the acquisition of bone mass, and the achievement of peak bone mass (12,13,14). This is a critical determinant of future risk of osteoporosis (12,13). The precise time of the attainment of peak bone mass is not certain, and it is skeletal-site dependent. The increase in gonadal steroid synthesis at the time of puberty is an important hormonal regulator of bone accretion. Boys with constitutionally delayed puberty achieve lower peak bone mass than normal boys (15). Systemic and local skeletal IGF-I play a role in bone formation and the maintenance of bone mass (16). This review will highlight the mechanisms of GH and IGF-I actions in bone, skeletal abnormalities occurring in GHD and acromegaly, and the role of GH and IGF-I in the pathophysiology of selected forms of osteoporosis.
II. The Growth Hormone (GH)/Insulin-Like Growth Factor-I (IGF-I) Axis
GH is a single-chain peptide of 191 amino acids. GH was isolated from somatotrophs, cells of the anterior pituitary gland, in 1956 and first used for the treatment of pituitary dwarfism in 1958 (17). The synthesis of GH is under the control of central and peripheral signals (18). The synthesis and release of GH are promoted by GHRH and inhibited by somatostatin and are regulated by a negative feedback mechanism (18). IGF-I, which is secreted by the liver under GH control, inhibits GH secretion directly in somatotrophs and indirectly by stimulating the release of somatostatin (18). Ghrelin, a 28-amino acid peptide synthesized by cells of the gastrointestinal tract, is an endogenous inducer of GH release, acting on somatotrophs and the hypothalamus (19,20,21,22). Ghrelin also was reported to stimulate the proliferation of osteoblasts in vitro (23,24,25,26,27,28). GH secretion is under the influence of additional hormonal signals, and sex steroids and thyroid hormone stimulate, whereas glucocorticoids inhibit, GH secretion (18,29,30,31,32).
Serum GH levels decline with age, reaching a nadir at the sixth decade (18,33). In aged men, the daily GH secretion is 1/5 to 1/20 that observed in young adults (34). GH output decreases twice as rapidly in men as in women so that GH release remains higher in women than in men after the age of 50 (35,36). The age-dependent decline in GH secretion is secondary to a decrease in GHRH and to an increase in somatostatin secretion (18). These changes occur at the hypothalamic level, but their cause is unknown. A reduction in central cholinergic tone leading to an increase in somatostatin release possibly explains the change in GH secretion (37). The decline in the production of sex steroids, physical activity, and the presence of aberrant sleep patterns also may contribute to the decline in GH levels during aging (38). As a consequence of the decline in GH synthesis and release, systemic IGF-I levels decline with advancing age (39). The changes in GH and IGF-I secretion that occur with aging are paralleled by a progressive loss of muscle mass and strength, a decline in physical performance, an increase in body fat, and a decrease in bone mineral density (BMD) (40,41,42,43).
GH circulates bound to a GH-binding protein, which is the extracellular domain of the GH receptor (GHR) (44,45). The GH-binding protein is generated by proteolytic cleavage of the extracellular domain of the GHR or by mRNA splicing (46). GH-binding protein is synthesized primarily by the liver, although synthesis by extrahepatic tissues, such as muscle and adipose tissue, may contribute to the circulating level of GH-binding protein (47,48). The serum levels of the GH-binding protein serve as a marker of GHR expression and GH responsiveness in target tissues (46). The function of the GH-binding protein is incompletely understood, although it may modulate the activity of GH either by prolonging its half-life or by reducing its availability to the GHR.
GH exerts its effects by binding to a single-chain transmembrane glycoprotein receptor. The GHR consists of an extracellular, a transmembrane, and an intracellular domain. Activation of the receptor occurs by ligand-induced dimerization and internalization of the receptor to initiate signaling, primarily by the activation of the Janus tyrosine kinase 2. This leads to autophosphorylation and to phosphorylation of the internalized GHR and recruitment and activation of intracellular proteins of which the signal transducers and activators of transcription (STAT) are the most important, although additional pathways can operate in GH signal transduction (49,50). The GHR is highly expressed in the liver, adipose tissue, heart, kidneys, intestine, lung, pancreas, cartilage, and skeletal muscle. GH acts by inducing the synthesis of IGF-I by the liver. However, the physiology of IGF-I is complex because it acts as a circulating hormone and as a local growth factor (51). Systemic IGF-I is synthesized primarily in the liver, where its synthesis is GH dependent (51). IGF-I also is synthesized in multiple extrahepatic tissues, where it acts as a local growth factor under the control of diverse hormones. IGF-I circulates as part of a 150-kD complex formed by one molecule each of IGF-I, IGF-binding protein (IGFBP)-3, the predominant circulating binding protein, or IGFBP-5, and the acid labile subunit (ALS) (52). There are six IGFBPs, and IGFBP-1, -2, -4, and -6 also can bind IGF-I in the circulation and peripheral tissues but do not form part of the ternary complex. IGFBPs are in concentrations in excess of IGF-I. Consequently, IGF-I circulates mostly bound to the complex, and less than 1% of total serum IGF-I circulates as a free hormone. The 150-kD ternary complex stabilizes IGF-I, prolonging its circulating half-life and regulating its availability to target tissues (52). Consequently, the ternary complex plays an important role in determining the endocrine function of IGF-I. ALS is synthesized in the liver under the control of GH and circulates in excess over the other components of the complex, so that it plays a critical role in the storage and release of IGF-I (52). ALS is absolutely necessary for the accumulation and maintenance of serum IGF-I and IGFBP-3, and als null mice have marked reductions in serum IGF-I and IGFBP-3 levels, because the ternary complex cannot be formed (53). Despite this decrease in serum IGF-I, growth of als null mice is only mildly impaired, but this is consistent with the modest growth deficit found in mice with conditional deletions of igf-1 in the liver and confirms that systemic IGF-I is dispensable for postnatal linear growth (54). In addition to its function as a systemic hormone, IGF-I plays an important role in the autocrine and paracrine regulation of cell metabolism in a variety of tissues, including cartilage and bone. Locally, the availability and activity of IGF-I also is regulated by IGFBPs, and in vitro studies have demonstrated that at the tissue level most of the IGF is bound to IGFBPs, with a small fraction present in the unbound free form. However, in vivo binding interactions between IGF-I and IGFBPs at the tissue level have not been explored. The cellular actions of IGF-I are mediated by the IGF-I receptor (IGF-IR), a receptor tyrosine kinase that is expressed in IGF target tissues (55).
IGF-II shares biochemical and biological properties with IGF-I; it is important in skeletal development, but its function in the adult skeleton is not proven. IGF-II is synthesized by skeletal cells, but its synthesis is not GH dependent. The IGF-II/mannose 6 phosphate receptor does not play a major role in IGF signal transduction and is responsible for clearing IGF-II, regulating its levels, during fetal development (56).
III. Mechanisms of GH and IGF-I Action in Bone
The skeletal effects of GH and IGF are modulated by complex interactions between circulating IGF-I and IGFBPs and the locally produced IGF-I and IGFBPs. IGF-I and IGF-II are the most abundant growth factors present in skeletal tissue, and their synthesis and activity are regulated by systemic hormones, such as GH and PTH (57,58). GH may act by inducing IGF-I in bone or may have direct effects on skeletal cells (Table 1).
Table 1.
Effects of GH on bone
| Functions | Effects |
|---|---|
| Growth plate | |
| Replication of condrocytes | ↑↑ |
| Endochondral bone formation | ↑↑ |
| Bone remodeling unit | |
| Osteoblastogenesis | ↑ |
| Proliferation of osteoblasts | ↑ |
| Function of mature osteoblasts | ↔↑ |
| Production of osteoprotegerin | ↑ |
| Production of RANK-L | ↔ |
| Calcium metabolism | |
| Phosphate retention | ↑ |
Effects of GH on bone. ↔ no effect; ↑ minor stimulating effect; ↑↑ major stimulating effect.
A. GH
1. GHR and signaling.
GHRs are expressed by chondrocytes and osteoblasts (59,60,61,62,63,64). The secretion of GH as well as the expression of GHRs are down-regulated by IGF-I, acting as a systemic and local feedback control mechanism (65). IGFBPs, by binding IGF-I, up-regulate GHR expression (66,67). GH signaling in osteoblasts is mediated by a cascade of protein phosphorylation steps resulting in the activation of transcription factors. GH signals through Janus tyrosine kinase 2/STAT signal transduction pathway (68). GH utilizes STAT 5 to regulate IGF-I expression in the liver, but the function of STAT 5 in bone cells is not clear because stat 5 null mice appear to have normal bone remodeling (69,70). Ghr null mice exhibit decreased bone remodeling, which is rescued by IGF-I, suggesting a role of GH/IGF-I in bone remodeling that is independent of STAT 5. Using a conditional deletion approach, STAT 5 was found to mediate the effects of GH on IGF-I expression in skeletal muscle, but similar experiments have not been conducted in skeletal cells to establish the role of STAT 5 in bone remodeling (71). GH also signals through ERK1 and -2 and MAPKs that regulate osteoblastic cell growth (72,73,74,75). Acting through STATs and ERKs, GH may modulate the activity of runt-related transcription factor-2, which is an intracellular protein required for osteoblast cell differentiation (76,77).
2. In vitro studies.
GH stimulates the proliferation of cells of the osteoblastic lineage (61,78,79,80), although IGF-I is required for selected anabolic effects of GH in osteoblasts (81). GH affects the fate of mesenchymal precursors favoring osteoblastogenesis and chondrogenesis and opposing adipogenesis (82). Mesenchymal precursor cells can differentiate into adipocytes, osteoblasts, and chondrocytes in a tightly controlled process (83). Signals that enhance osteoblastogenesis often suppress adipogenesis, and adipocytes are increased in the bone marrow of patients with osteoporosis and are decreased during bone formation and chondrocyte proliferation (82,84,85). GH down-regulates the expression of fetal antigen-1, which is the soluble form of delta like-1 or Pref-1, and as a consequence may regulate adipogenesis (86,87). GH also stimulates the expression of bone morphogenetic proteins, which are important for the differentiation of osteoblasts and for bone formation (88,89,90).
In addition to its effects on the differentiation of cells of the osteoblast lineage, GH stimulates, either directly or indirectly through IGF-I, the differentiated function of the mature osteoblast. GH also stimulates the carboxylation of osteocalcin, which is a marker of osteoblastic function (91,92).
During bone remodeling, bone formation is coupled to bone resorption so that bone-forming osteoblasts fill resorbed bone surfaces with newly synthesized matrix. In addition, osteoblastic signals are necessary to initiate bone resorption so that bone resorption and formation are highly coordinated processes and agents targeting the osteoblast may influence osteoclast formation and function. Critical to these events are the receptor activator of nuclear factor κB ligand (RANK-L) and its decoy receptor osteoprotegerin (93,94). RANK-L is synthesized by osteoblastic stromal cells and, in the presence of colony-stimulating factor-1, induces osteoclast formation. Osteoprotegerin binds RANK-L and competes with the RANK-L receptor, RANK, present on the surface of osteoclast precursors. As a consequence, osteoprotegerin impairs osteoclastogenesis. GH stimulates the production of osteoprotegerin and its accumulation in the bone matrix (95,96,97,98).
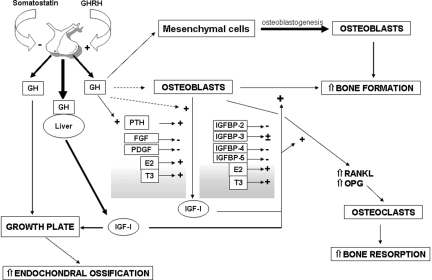
GH stimulates longitudinal bone growth, suggesting either a direct effect of GH on chondrocytes or an effect mediated by the local IGF-I (99,100,101) (Fig. 1). GH may act directly to stimulate the replication of cells in the germinal layer of the epiphyseal plate or indirectly through its stimulatory effect on IGF-I expression, acting at later stages of maturation (102). The growth plate consists of three layers of chondrocytes in various stages of differentiation: the resting zone, the proliferative zone, and the hypertrophic zone (4). In the resting zone, chondrocytes replicate at a slow rate and act to replenish the pool of proliferative chondrocytes. In the proliferative zone, chondrocytes replicate at a high rate, and the resulting daughter cells line up along the long axis of the bone, where they differentiate terminally into hypertrophic chondrocytes in the hypertrophic zone. The hypertrophic zone is invaded by blood vessels and bone cells, the zone is calcified, and new endochondral bone is formed (102). Cell maturation appears to be an important factor determining the response of epiphyseal chondrocytes to GH and IGF-I (103,104,105,106).
Figure 1.
Effects of GH and IGF-I on bone. ---> not consistently demonstrated effect; > minor stimulating effect; > major stimulating effect. FGF, Fibroblast growth factor; E2, estradiol; OPG, osteoprotegerin.
3. In vivo studies.
Mice lacking both the ghr and igf-1 genes exhibit a more severe growth phenotype than either mutant alone, suggesting that GH and IGF-I have independent effects on linear growth (107). Ghr and igf-1 null mice each exhibited a 25–35% reduction in the length and a 35–45% reduction in the width of tibiae, whereas double ghr/igf-1 null mutants exhibited a 50% reduction in the length and 60% reduction in the width of tibiae at 6 months of age (107). Accordingly, ghr null, igf-1 null, and dual mutants exhibited a 28, 35, and 67% reduction in body length, respectively. The phenotype was attributed to decreased proliferation and maturation of growth plate chondrocytes.
GH also influences bone metabolism indirectly by modulating PTH secretion and its circadian levels (108). The effect is mediated in part by changes in serum phosphate levels (109,110). Serum PTH peaks around 1700 h, coinciding with the serum phosphate peak (111,112,113). GH favors phosphate retention by increasing the renal threshold for phosphate, an effect that is independent of PTH and vitamin D activity (114). In addition, GH and IGF-I modulate the activity of the renal 1α-hydroxylase and 24-hydroxylase, activating the former and inhibiting the latter, with an increase in the production of active 1,25 dihydroxyvitamin D3 (115). These mechanisms may contribute to an increase in extracellular calcium-phosphate product, and possibly bone mineralization.
B. IGF-I
1. Regulation of local IGF-I synthesis.
When synthesized by peripheral tissues, IGF-I expression is controlled by diverse hormones and by growth factors. In chondrocytes, IGF-I synthesis is under GH control, whereas in osteoblasts its synthesis is fundamentally under the control of PTH (102,116) (Fig. 1). The igf-1 gene contains six exons and has alternate promoters in exons 1 and 2 (117,118). The igf-1 gene generates multiple heterogeneous transcripts due to the presence of multiple transcription initiation sites in two promoters, alternative splicing, and different polyadenylation signals. IGF-I splice variants have been reported in muscle, where their expression can be regulated by mechanical forces (119). The mature IGF-I peptide is encoded by exons 3 and 4, whereas exons 5 and 6 encode alternate carboxy-terminal extension peptides of undetermined function. Exons 1 and 2 encode mutually exclusive 5′ untranslated regions. The exon 1 promoter has four transcription initiation sites and is responsible for the regulation of IGF-I expression in most extrahepatic tissues including bone (120,121). The IGF-I exon 2 promoter has two transcription initiation sites and is responsible for the transcriptional regulation of IGF-I by GH in the liver (120). IGF-I exon 2 is minimally expressed by osteoblasts, and GH is not a major inducer of IGF-I in these cells (116,120). PTH and other inducers of cAMP in osteoblasts increase IGF-I expression (116). IGF-I mediates selected anabolic actions of PTH in bone in vitro and in vivo (122,123,124,125,126,127). IGF-I can reproduce selected effects of PTH on cell proliferation and survival (128). Estrogens increase and glucocorticoids decrease IGF-I transcription in osteoblasts (129,130,131,132) (Fig. 1), and selected inhibitory effects of glucocorticoids on bone metabolism can be explained by reduced IGF-I levels in the bone microenvironment. However, glucocorticoids have complex effects on osteoblastogenesis and direct effects on osteoblastic gene expression. Glucocorticoids oppose Wnt/β-catenin signaling and activity by destabilizing β-catenin and, as a consequence, oppose Wnt effects on osteoblastogenesis (133). Thyroid hormones are critical regulators of skeletal development and maturation, and they increase bone remodeling. T3 increases IGF-I synthesis by osteoblasts, and IGF-I can mediate anabolic actions of T3 in bone (134,135). In addition to hormones, skeletal growth factors regulate IGF-I synthesis. Growth factors with mitogenic properties, such as platelet-derived growth factor (PDGF) and fibroblast growth factor decrease IGF-I transcripts and polypeptide levels in osteoblasts (136). This inhibition of IGF-I synthesis correlates with their inhibitory actions on osteoblastic differentiated function (136). In contrast, bone morphogenetic protein-2, an agent that enhances osteoblastic differentiation and function, increases IGF-I synthesis in osteoblasts (88).
2. IGF-I receptor and signaling.
IGF-I signals through the IGF-IR, a transmembrane glycoprotein tetramer with ligand-activated tyrosine kinase activity. Upon ligand binding, the IGF-IR dimerizes and undergoes autophosphorylation, leading to the activation of the insulin receptor substrate (IRS)-1 and IRS-2 (55). IRS-1 and -2 mediate the effects of IGF-I in osteoblasts. IGF-I utilizes the phosphatidylinositol-3 kinase pathway, which induces the activation of Akt, and the MAPK pathway, which activates p38, Jun-N-terminal kinases, and ERK 1/2 (137,138,139). The usage of each pathway is dependent on culture conditions and the stage of cell differentiation. IGF-IR number and affinity are regulated by hormones and growth factors. In osteoblasts the IGF-IR is modulated by PDGF, glucocorticoids, and 1,25-dihydroxyvitamin D3 (140,141,142).
3. In vitro studies.
IGF-I and IGF-II are expressed by osteoblasts and exert similar biological actions, but IGF-I is more potent than IGF-II. IGF-I has modest effects on the proliferation of cells of the osteoblastic lineage, and although IGF-I does not direct the differentiation of undifferentiated stromal cells toward cells of the osteoblastic lineage, IGF-I enhances the function of the mature osteoblast (143,144). The fundamental role of IGF-I is the stimulation of osteoblastic function and bone formation. IGF-I up-regulates type I collagen transcription and decreases the synthesis of collagenase 3 or matrix metalloproteinase 13, a collagen-degrading protease (145). This dual action, an increase in collagen synthesis and a decrease in its degradation, is important to maintain appropriate levels of bone matrix and bone mass. In accordance with the effects of IGF-I on osteoblastic function, its synthesis is regulated by factors that regulate the differentiated expression of the osteoblastic phenotype (Fig. 1). It is important to note that whereas IGF-I stimulates the differentiated function of the osteoblast, it does not have a direct effect on the differentiation of stromal cells toward mature osteoblasts (146). Indirectly, IGF-I may favor osteoblastogenesis by stabilizing β-catenin, a signaling molecule used by the Wnt canonical signaling pathway, which is essential for osteoblastogenesis (147,148). When Wnt signals, β-catenin is stabilized and translocates to the nucleus, where it associates with members of the T cell factor/lymphoid enhancer factor family of nuclear proteins to regulate transcription. IGF-I favors stabilization of β-catenin by inducing phosphatidylinositol-3 kinase and activating Akt, which phosphorylates and degrades glycogen synthase 3 kinase, the enzyme that phosphorylates β-catenin for its degradation by ubiquitination (147,148). It is of interest that the IGF-I signaling molecule IRS-1 can associate with β-catenin and regulate its activity (149). The phosphatidylinositol-3 kinase/Akt pathway also is used by IGF-I to decrease osteoblast apoptosis. This effect and the modest mitogenic activity of IGF-I cause an increase in the number of osteoblasts in vitro. Microarray analysis of cells of the osteoblast lineage at various stages of differentiation has demonstrated down-regulation of IGF-I expression from preosteoblastic cells to fully differentiated osteoblasts (150). It would appear that a decline in IGF-I expression is necessary for cellular death to occur and to allow for the terminal differentiation of osteoblasts.
Less clear is the function of IGF-I on osteoclasts than in cells of the osteoblastic lineage. Osteoclasts express IGF-I receptors, and IGF-I has direct effects on their function (151). In vitro, IGF-I induces RANK-L synthesis and, as a consequence, osteoclastogenesis (152,153). The induction of RANK-L by IGF-I may explain the stimulatory effects of IGF-I on bone resorption, whereas the induction of osteoprotegerin by GH may temper these effects. The fact that IGF-I has a dual role enhancing bone formation and bone resorption may explain why it has modest effects on bone mass in vivo.
4. In vivo studies.
IGF-I has been tested for its effects on bone metabolism in experimental animals, where the effects on bone formation observed in vitro have been confirmed. Null mutations of igf-1, igf-2, or igf-1r in mice cause growth arrest (154,155,156). Data from igf-1 and igf-1r gene deletions provide valuable information on the role of IGF-I during development, and conditional deletions of these genes in bone have provided information on the effects of IGF-I in the adult skeleton (154,157). Igf-1 null mutants exhibit impaired chondrocyte maturation and shortened femoral length, confirming the role of IGF-I in the regulation of chondrocyte differentiation (154,156). Mice that survive exhibit reduced cortical, but not trabecular, bone, possibly due to a compensatory increase in GH secretion or due to a decrease in trabecular bone resorption (158). Severe developmental abnormalities and frequent lethality have prevented a detailed analysis of the adult skeletal phenotype of igf-1 null mutants. Igf-1 null mice also exhibit decreased number of functional osteoclasts, indicating that IGF-I is required for normal osteoclastogenesis (159). As a consequence, igf-1 null mice have increased bone volume. The null mutation of the igf-1r also causes severe growth retardation and perinatal lethality (154). In accordance with these observations, irs-1 null mutants display impaired chondrocyte proliferation and early closure of the growth plates, resulting in a marked reduction in growth and weight (160).
The study of genetically engineered mice has provided additional insight into the actions of systemic and locally produced IGF-I in osteoblasts in vivo.
a. Effect of circulating IGF-I.
Mice carrying mutations of the ghrh receptor (lit/lit mouse) or the gh receptor have absent GH secretion or action and consequently low serum IGF-I levels (70,161,162). These models allow for the determination of the contribution of systemic IGF-I to the skeleton, and the phenotype of either mutant is characterized by small growth plates, osteopenia, and reduced cortical bone, but normal trabecular bone. This suggests a more pronounced role of systemic IGF-I on cortical than on trabecular bone. Mice carrying a liver-specific igf-1 deletion display a reduction in total serum IGF-I levels, normal free IGF-I levels, and a modest skeletal phenotype, characterized by a decrease in cortical volume, secondary to a reduction in periosteal bone formation (16,54,163). The normal serum levels of free IGF-I are attributed to IGF-I synthesis by nonhepatic sources (54). Mice carrying deletions of igf-1 and the als display marked reductions in total serum IGF-I, a more significant decrease in cortical bone and a decrease in trabecular bone volume. These observations confirm the contribution of systemic IGF-I to cortical bone integrity and to a lesser extent to trabecular bone. The correlation between IGF-I and bone mass also has been documented in specific mouse strains that have allelic differences at key genomic points. The generation of congenic mice has advanced our understanding of the genetic regulation of selected phenotypes. Two inbred strains C3H/Hej (C3H) and C57BL/6J display differences in BMD, which correlate closely with differences in serum IGF-I levels. Quantitative trait locus analysis revealed the presence of one major quantitative trait locus (Igf1sl-1) in chromosome 6 of the C3H genome with major effects on serum IGF-I concentrations (164). Congenic mice carrying Igf1sl-1 on a C57BL/6J genetic background display a 25% decrease in circulating IGF-I levels and decreased cortical and trabecular bone, confirming the role of circulating IGF-I in the maintenance of murine bone mass (165,166).
b. Effects of locally produced IGF-I.
Transgenic mice overexpressing IGF-I under the control of the osteoblast-specific osteocalcin promoter exhibit transient increases in trabecular bone secondary to an increase in osteoblast function and bone formation (167). Changes in osteoblast number were not observed, confirming the predominant effect of IGF-I on osteoblastic function, and not on mitogenesis.
Igf-1r null mice die shortly after birth and exhibit severe growth retardation. The expression of the Cre recombinase under the control of the osteocalcin promoter has allowed the conditional deletion of igf-1 receptor gene, flanked by loxP sequences, selectively in osteoblasts. Mice carrying this osteoblast-targeted conditional deletion exhibit decreased osteoblast number and function, causing reduced bone formation and trabecular bone volume (157). This observation documents the fundamental role of IGF-I in the maintenance of trabecular bone structure. Current observations suggest that systemic IGF-I is necessary to maintain cortical bone structure, whereas skeletal IGF-I appears to play a more significant role in the maintenance of trabecular bone. The function of IGF-I on skeletal homeostasis was confirmed by the study of mice carrying deletions of the signaling molecules IRS-1 and -2. Irs-1 or -2 null mice exhibit osteopenia (122,168). However, their phenotypes are not identical, and irs-1 null mice display low bone turnover osteopenia and failure to exhibit an anabolic response to PTH, whereas irs-2 null mice have osteopenia with increased bone resorption and display a bone anabolic response to PTH (122,168). The stimulatory effect of PTH on bone formation in vivo is also not observed in igf-1 and igf-1r null mice (122,123,124,169). Igf-1-deficient mice failed to show an increase in BMD at the proximal and distal tibia after PTH administration (170). Moreover, the deletion of igf-1r in osteoblasts leads to impaired stimulatory effects of PTH on osteoprogenitor cell proliferation (170). These observations do not exclude the possibility of other factors mediating selected anabolic actions of PTH on the skeleton. For example, some effects of PTH in the marrow cellular niche are secondary to the induction of Jagged 1 and the activation of Notch signaling. PTH decreases the expression of the Wnt antagonist, sclerostin, potentially leading to enhanced Wnt signaling, although Wnt signaling is not required to detect anabolic effects of PTH in murine models (171,172). PTH also increases and activates skeletal TGF β (173).
C. IGF binding proteins (IGFBPs)
IGFBPs are a family of evolutionary conserved-related proteins, which bind IGF-I and IGF-II (174,175). IGFBPs have differential affinity for IGF-I and IGF-II and modulate the cellular effects of IGFs (174,175). By binding IGFs, IGFBPs may sequester the growth factor and preclude its interactions with cell surface receptors. However, under selected experimental conditions, such as when IGFBPs are associated with the extracellular matrix, there may be an increase in the effective concentrations of IGF-I in the cellular environment, resulting in enhanced IGF-I effects (176,177). IGFBPs also have IGF-independent effects.
1. Regulation of local IGFBP synthesis.
IGFBP synthesis is regulated at the transcriptional, posttranscriptional, and posttranslational level. This regulation occurs by cell-specific mechanisms, and in cells of the osteoblastic lineage the pattern of IGFBP expression is dependent on the stages of osteoblast cell differentiation (178,179). IGFBP-2 and -5 expression is highest in the proliferative phase of rat osteoblastic cell cultures, whereas IGFBP-3, -4, and -6 expression is maximal during terminal cell differentiation (180). The regulation of IGFBP expression during osteoblastic cell differentiation may be related to the relative levels of autocrine and paracrine factors present in the cellular environment. IGFs increase IGFBP-5 expression by the osteoblast, whereas growth factors with mitogenic activity inhibit IGFBP-5 and stimulate IGFBP-4 expression (181,182). Systemic hormones also regulate IGFBP synthesis in a cell- and culture condition-dependent manner (178). GH increases IGFBP-3, and cAMP inducers increase the synthesis of IGFBP-2, -3, -4, and -5 in osteoblasts (179). The induction of binding proteins may be a mechanism to control cell exposure to IGF-I. Conversely, glucocorticoids inhibit the synthesis of IGF-I, IGFBP-3, -4, and -5 and increase the expression of IGFBP-2 in osteoblasts (132,183). This may contribute to a reduction in levels and activity of IGF-I in the bone environment, in addition to the inhibitory effects of glucocorticoids on IGF-I synthesis. 1,25-dihydroxyvitamin D3 increases osteoblast IGFBP-3 and -4 expression (184).
2. In vitro and in vivo studies.
The ubiquitous overexpression of IGFBP-1 in mice causes hyperglycemia, suggesting a role for free IGF-I in glucose homeostasis, but the function of IGFBP-1 in skeletal cells has not been defined (185). IGFBP-2 is important to transport IGFs, and IGFBP-2 serum levels correlate with BMD and bone turnover in humans (186). In vitro, IGFBP-2 prevents the effects of IGF-I on osteoblast function, and the constitutive and indiscriminate overexpression of IGFBP-2 causes impaired growth, decreased bone mass, and failure to respond to the anabolic effects of GH in murine bone (187). However, the effects of IGFBP-2 are complex, and igfbp-2 null mice exhibit gender-specific changes in bone turnover. Female igfbp-2 null mice have increased cortical bone, whereas male null mice display decreased cortical and trabecular bone secondary to decreased bone formation (188). These observations suggest that IGFBP-2 is required for normal bone formation in male mice and are in agreement with clinical observations indicating a correlation between serum IGFBP-2 levels and bone remodeling and an anabolic effect of the administration of IGF-II/IGFBP-2 in disuse osteoporosis (189,190). It is also of interest that mechanical loading up-regulates the expression of IGF-I and IGFBP-2 transcripts in osteocytes (191,192). IGFBP-3 is a major component of the circulating IGF complex, and its concentrations are GH dependent (174,175). In vitro, IGFBP-3 can inhibit or stimulate IGF activity, the latter by up-regulating IGF-I delivery to cell surface receptors (193). However, the constitutive and ubiquitous overexpression of IGFBP-3 in vivo causes growth retardation and osteopenia (194). IGFBP-4 and IGFBP-5 are inhibitors of IGF-I, but under certain experimental conditions they can simulate bone cell function independently of their interactions with IGFs (195). It is important to note that transgenic mice overexpressing either IGFBP-4 or IGFBP-5, under the control of the osteoblast-specific osteocalcin promoter, exhibit osteopenia secondary to decreased bone formation (196,197). The osteopenia is explained by the sequestration of IGF-I present in the bone environment and confirms the inhibitory function of IGFBP-4 and -5 in the skeleton. It is possible that the differential activity of IGFBP-4 and -5 depends on interactions with extracellular matrix proteins or on the levels of the IGFBP present in a specific tissue. The inhibitory effects of IGFBP-5 were documented in vitro using retroviral vectors to overexpress IGFBP-5 in osteoblastic cells. Constitutive overexpression of IGFBP-5 inhibited osteoblastic cell function, whereas the expression of IGFBP-5 fragments had no stimulatory or inhibitory activity (198). The function of IGFBP-6 in skeletal tissue is not known.
3. Regulation of IGFBP action.
The abundance of IGFBPs is regulated by IGFBP proteases. Osteoblasts secrete matrix metalloproteinases and serine proteases, and these cleave IGFBPs (199). Pregnancy-associated plasma protein-A (PAPP-A) is a metalloproteinase expressed by skeletal cells that plays a critical role in osteoblastic function by modulating IGF-I bioavailability (200). PAPP-A cleaves the inhibitory IGFBP-4 in an IGF-dependent manner, and as a consequence the bioavailability of IGF-I is increased (201). IGFBP-2 and -5 also are substrates for PAPP-A. Addition of PAPP-A to osteoblast cultures promotes cell proliferation by increasing IGF-I bioavailability, and transgenic mice overexpressing PAPP-A under the control of the type I collagen promoter exhibit increased bone formation and bone area indicating a bone anabolic effect in vivo (202). Papp-a null mice lack IGFBP-4 proteolytic activity, and at birth they exhibit dwarfism secondary to the sequestration of IGF-II by IGFBP-4 (203). Postnatally, papp-a null mice exhibit decreased trabecular bone volume, low bone turnover osteopenia, and delayed fracture healing (204,205). These observations are consistent with a decrease in bioavailable IGF-I in the bone environment due to IGFBP-4 in excess and confirm the fundamental role of PAPP-A in the bioavailability of IGF pre- and postnatally. The expression of PAPP-A in osteoblasts is enhanced by TGFβ, agents that induce cAMP, such as PTH, and cytokines such as IL-1 and -4 and TNFα (206,207). The synthesis and activity of other metalloproteinases, such as collagenase 3, in osteoblasts also are regulated by cytokines and hormones, such as IL-6 and glucocorticoids (208,209). Limited proteolysis of IGFBPs by a variety of serine proteases control the bioavailability of IGF-I in the circulation and at the cellular level, particularly by cleaving IGFBP-3 (174). In addition, IGFBP-3 is cleaved by plasmin, and the bioavailability of IGF-I in the bone microenvironment can be regulated by activators and inhibitors of plasminogen (210).
IV. Skeletal Manifestations of GH Deficiency and Excess in Humans
A. Adult growth hormone deficiency (GHD)
Adult GHD is a recognized and treatable clinical entity (211,212,213). Severe GHD in adults is associated with adverse changes in body composition, lipid metabolism, insulin sensitivity, and exercise capacity (211,212,214). Adult patients with GHD suffer from low bone turnover osteoporosis leading to increased fracture risk, which may contribute to the increased risk of mortality observed in GHD (1,215).
1. Bone turnover and calcium metabolism in untreated GHD.
GH and IGF-I play an important role in modulating bone remodeling (216). Patients with GHD have a marked reduction in bone turnover. Bone biopsies from male adult patients with GHD reveal decreased osteoid and mineralizing surfaces and decreased bone formation rate (217). Serum levels of osteocalcin and bone resorption markers are decreased, confirming the state of low bone turnover (1,109,218,219,220,221,222). Patients with GHD exhibit renal, skeletal, and intestinal cell insensitivity to PTH, leading to a mild state of PTH resistance and increased serum PTH levels (109,223). Consistent with a decrease in end-organ sensitivity, the calcemic response to PTH is delayed (223). GHD also is accompanied by abnormalities in the circadian rhythm of PTH, which may affect bone remodeling (109,224,225).
2. BMD and fractures in untreated GHD.
Decreased BMD is reported in patients with GHD, either isolated or combined with other pituitary hormone deficiencies (1,215,226,227). Patients with GHD exhibit greater losses of cortical than trabecular bone, and this observation is in accordance with the greater effect of systemic IGF-I on cortical than on trabecular bone (228,229). The degree of bone loss is related to the duration and age of onset of GHD, the severity of the disease, and the age of the patients (230,231,232,233). About half of the patients have normal vertebral BMD (234).
GHD is a heterogeneous disorder, and the clinical manifestations in patients developing GHD in their childhood are different than in patients developing the disease after puberty (235). The etiology of childhood-onset GHD is frequently unknown (i.e., idiopathic GHD); however, it may be secondary to cranial irradiation, neurosurgical removal of craniopharyngioma, or other hypothalamic-pituitary masses and infiltrative diseases (e.g., histiocytosis X). Adult-onset GHD is often secondary to neurosurgery and/or radiotherapy for a pituitary adenoma, but may be also observed in other clinical conditions (e.g., empty sella) (236,237). Patients with childhood-onset GHD are smaller and have a more pronounced decrease in muscle and bone mass and lower IGF-I and IGFBP-3 serum concentrations than subjects with adult-onset GHD (235,236). In childhood-onset GHD, vertebral BMD is markedly reduced with T-scores often between −1 and −2; about one third of the patients have T-scores of −2.5 or less (238). In contrast, patients with adult-onset GHD often have vertebral T-scores of −1 or above (222,225,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253). The reason for the different degrees of bone loss may be because childhood-onset GHD occurs before the achievement of peak bone mass, and because the duration of the disease is longer. In childhood-onset GHD, there may be impaired acquisition of bone mass during childhood and adolescence and during the transition period, a period of life occurring between the closure of the epiphyseal plate and the attainment of peak bone mass (13,254,255,256,257,258,259,260).
Potential confounding factors in the determination of BMD in childhood-onset GHD are the presence of decreased muscle mass and of short stature. The latter occurs in 40% of patients with childhood-onset GHD, even after replacement therapy with GH (261). Differences in BMD between childhood-onset and adult-onset GHD also are found after correction for height (262,263). Consequently, short stature probably plays a minor role in the lower BMD observed in childhood-onset GHD, whereas changes in body composition may play a more significant role (264,265,266).
The degree of bone loss in adult-onset GHD correlates with the age of the patients and the duration and the severity of the disease (222,228,230,239,252,267). Patients with severe GHD, as defined by a GH response in the range of 3.1 to 9.0 μg/liter after GHRH and arginine, or very severe GHD, as defined by a GH response of 3.0 μg/liter or less after GHRH and arginine, display significant reductions in BMD (222,230).
The increased prevalence of osteopenia/osteoporosis in isolated GHD suggests that GHD per se is the cause of bone loss. However, in subjects with multiple hormone deficiencies, one cannot exclude the possibility that the deficiency of other pituitary hormones and hormonal replacement may contribute to the bone loss. In fact, hypogonadism and the use of replacement therapy with thyroid hormone and glucocorticoids in excess may influence the reduction of BMD in patients with multiple pituitary hormone deficiencies (268,269,270). TSH deficiency may also contribute to the bone loss (271,272).
Although untreated GHD can lead to a decrease in bone mass, data demonstrating an increase in the risk of fractures are limited. The risk of nonvertebral fractures is increased about 3-fold in untreated GHD patients (227,273,274). Fractures in GHD are frequently localized to the radius, suggesting a loss of cortical bone (227,274). Patients with GHD also have an increased incidence of vertebral deformities, suggesting an increased incidence of vertebral fractures (234). The prevalence of bone fractures is related to the degree of GHD and seems not to be affected by the presence of other pituitary hormone deficiencies or by hormonal replacement therapy (227,268,275). The different impact of other pituitary hormone deficiencies or hormonal replacement therapy on BMD and fractures is consistent with the finding that fractures do not correlate with BMD in GHD (Fig. 2A) (234,268). This is also the case in other forms of secondary osteoporosis (276,277,278).
Figure 2.
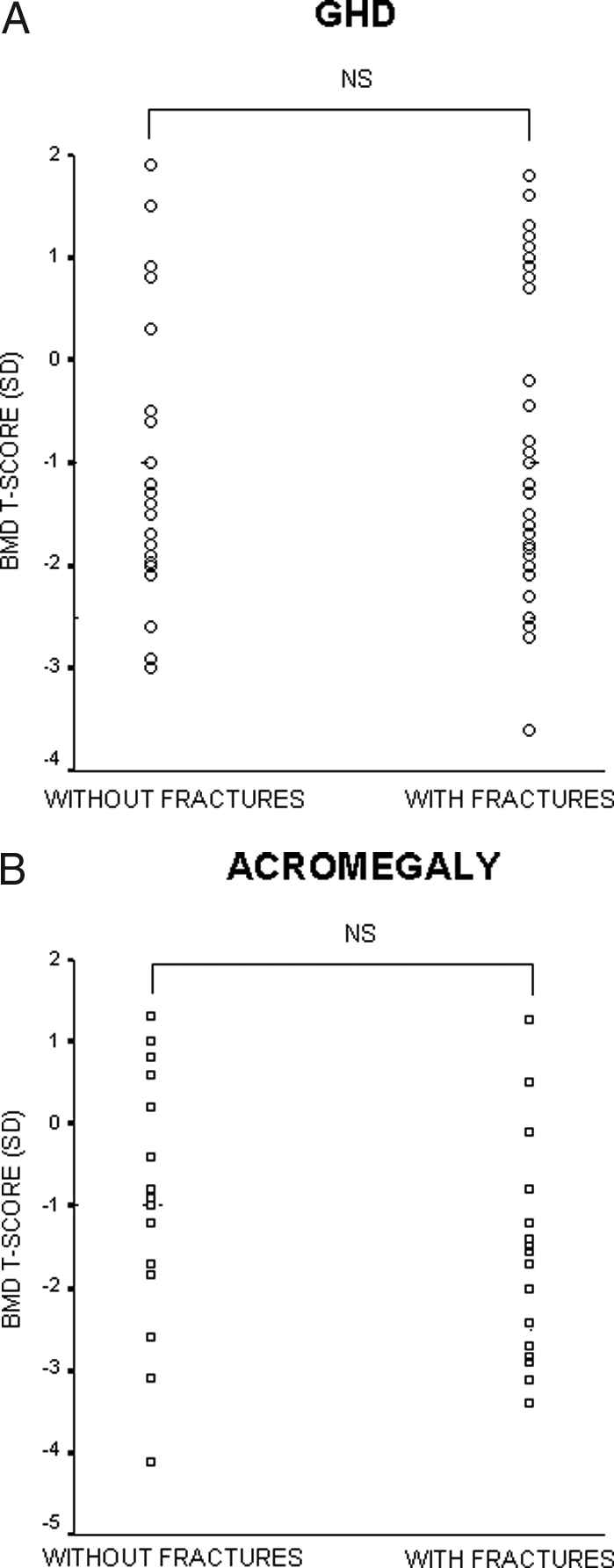
Scatter plots of BMD T-scores in patients with GHD (A) or acromegaly (B), with and without fractures. NS, Not significant. [Adapted from G. Mazziotti et al.: J Bone Miner Res 21:520–528, 2006 (234); and S. Bonadonna et al.: J Bone Miner Res 20:1837–1844, 2005 (276), with permission of the American Society for Bone and Mineral Research.]
An additional risk of fractures in adult-onset GHD is a decrease in muscular strength and, when GHD is secondary to pituitary tumors, impaired vision. Both factors can lead to falls and fractures (227,279).
B. Skeletal effects of recombinant human GH (rhGH) in adult-onset GHD
1. Bone turnover and calcium metabolism in treated GHD.
Replacement therapy with rhGH leads to an increase in bone turnover, as determined by changes in biochemical markers of bone resorption and bone formation (280). The effect of rhGH on bone remodeling is biphasic; rhGH causes a maximal effect on bone resorption after 3 months and on bone formation after 6 months. The effect on bone formation is sustained for prolonged periods of time (1,97,246,280,281,282,283). The effect of rhGH on biochemical markers of bone turnover is dose dependent (221,232,243,284), but not influenced by the modality of administration (i.e., continuous vs. daily administration, and daily vs. administration three times a week) (285,286). rhGH causes an increase in serum and urinary calcium after 3 to 6 months, an effect caused by calcium mobilization from the skeleton, an increase in intestinal calcium absorption and in the renal reabsorption of calcium due to increased sensitivity to PTH (287,288,289,290). rhGH is antiphosphaturic and increases the intestinal absorption of phosphate leading to an increase in serum phosphate levels (115,291,292,293). rhGH may normalize the circadian rhythm of PTH secretion (294).
2. BMD and fractures in treated GHD.
The effect of rhGH on BMD in adult-onset GHD is variable and depends on the duration of the treatment. Short-term studies failed to show an increase in BMD, and a decline in BMD may occur in the initial months of therapy due to an increase in bone resorption occurring after 3 months without an increase in bone formation, which occurs after 6 months (240,250,295,296,297,298,299,300,301). Subsequently, when bone formation is increased, bone mass increases, but an increase in BMD can be documented only after 6 to 12 months in children and after 18 to 24 months in adults receiving rhGH therapy (1,302,303,304,305,306,307,308,309,310,311). The increase in BMD is observed for periods of up to 10 yr in patients receiving continuous rhGH therapy (244,308). Moreover, BMD may even continue to increase 18 months after rhGH discontinuation (312,313). In contrast, the effects on body composition are lost after the discontinuation of rhGH (312,313). rhGH increases bone mineral content to a greater extent than BMD because rhGH also increases bone area (246,299). This is supported by histomorphometric findings demonstrating an increase in periosteal bone formation during rhGH treatment (301). The effects of rhGH on bone may be potentiated by antiresorptive drugs, such as bisphosphonates. The addition of pamidronate or alendronate at the start of rhGH treatment has been shown to prevent the initial decline of BMD induced by rhGH treatment (314). The benefits of alendronate are also observed when it is started in patients receiving long-term rhGH treatment (306,315). It is unknown whether bisphosphonates alone can correct the low-turnover osteoporosis in GHD patients not replaced with rhGH, as they do in other forms of secondary osteoporosis (278).
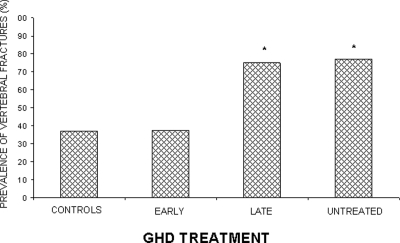
Because measurement of BMD in GHD is not a reliable predictor of fracture risk (234), a direct evaluation of fractures by a radiological morphometric approach is desirable in GHD. A concern is the lack of prospective data documenting a reduction in fractures after rhGH therapy because most studies have used BMD as primary end-point (304). Cross-sectional studies have suggested that rhGH treatment reduces the risk of vertebral and nonvertebral fractures in GHD (Table 2). The effect seems to occur even in patients with untreated hypogonadism (268). The efficacy of rhGH in reducing bone fractures is closely related to the lag-time spanning between the diagnosis of GHD and initiation of therapy, and GH is beneficial only in patients receiving rhGH shortly after the diagnosis is made (Fig. 3) (234).
Table 2.
Risk of fractures in adult-onset GHD patients untreated and treated with rhGH
| Study (Ref.) | No. of patients | Type of fractures | Fracture Risk
|
|
|---|---|---|---|---|
| Untreated | rhGH treated | |||
| Rosen et al. (273) | 107 | Clinical nonvertebral | Increased | N.D. |
| Wuster et al. (227) | 2084 | Clinical nonvertebral | Increased | Decreased |
| Vestergaard et al. (275) | 199 | Clinical nonvertebral | Increased | Unchanged |
| Bouillon et al. (274) | 66 | Clinical nonvertebral | Increased | N.D. |
| Mazziotti et al. (234) | 107 | Morphometric vertebral | Increased | Decreased |
N.D., not determined.
Figure 3.
Prevalence of fractures in adult-onset GHD, untreated and treated (early and late in the course of the disease) with rhGH. *, P < 0.05 untreated vs. late treated or untreated GHD. [Adapted from G. Mazziotti et al.: J Bone Miner Res 21:520–528, 2006 (234) with permission of the American Society for Bone and Mineral Research.]
3. Predictors of rhGH response in bone
a. Sex.
Male and female patients with GHD may display different responses to rhGH in terms of changes in bone turnover and BMD. In men, bone formation and resorption are increased within 1 month of rhGH treatment, whereas in women the increase occurs after 3 months. The increase in markers of bone resorption precedes the change in bone formation markers by about 9 months (316). The early change of bone remodeling may lead to a greater increase in BMD in males than in females, in whom only stabilization of BMD is achieved (253,281,282,305,309,311,317,318,319). These gender-dependent differences may also reflect the different impact of hypogonadism in male and female subjects with GHD (320,321,322). Recently, a decreased incidence of clinical fractures has been reported only in males with treated adult-onset GHD as compared with the normal population (323).
b. Age of onset of GHD.
The effect of rhGH on bone turnover and BMD is different between childhood-onset and adult-onset GHD. In adult-onset GHD, bone formation increases after 6 months of treatment, with little change thereafter (232,324). In contrast, in childhood-onset GHD there is a progressive increase in bone formation for up to 12 months after rhGH, which is followed by a return to baseline values after 18 months of therapy (233). Patients with childhood-onset GHD generally display an increase in BMD after 6 to 12 months of rhGH therapy, whereas patients with adult-onset GHD require 18 to 24 months of rhGH to exhibit a change in BMD (246,305,324,325).
c. Dose of GH.
In childhood-onset GHD, there is a linear correlation between the dose of rhGH and the increase in BMD (326), indicating a need for optimization of the dose of rhGH in children. In contrast, in adult-onset GHD, low of rhGH have an optimal effect on BMD, whereas at high doses rhGH may cause an initial decline in BMD, probably due to an increase in bone resorption (243,324,327,328,329).
d. Concomitant diseases.
The underlying pituitary disease may alter the effects of rhGH on bone mass. A delayed effect of rhGH replacement therapy on BMD is observed in patients with Cushing’s disease and in patients with hyperprolactinemia and hypogonadism, when compared with patients bearing nonsecreting pituitary adenomas (330).
4. Monitoring patients with GHD and the response to GH.
Patients with GHD are at high risk for osteoporosis and fractures (212,213,238). Therefore, it is important to follow the patients and monitor changes in skeletal metabolism. Osteoporosis, when complicated by fractures, contributes to an increased mortality (331). Current guidelines from The Endocrine Society recommend obtaining BMD before initiating therapy with rhGH (238). An independent assessment of fractures also could be useful because BMD is not a good predictor of fractures in GHD. In fact, an early diagnosis of vertebral fractures is important to assess the future risk of osteoporotic fractures (332). After the initiation of rhGH, the measurement of serum calcium, phosphate, alkaline phosphatase activity, and osteocalcin levels may be useful to ensure that a therapeutic response was achieved. After 18 to 24 months of treatment and then every 2 yr, BMD measurements should be considered (238). The measurement of BMD is important in severe GHD patients during the so-called transition period, to monitor the achievement of the normal peak bone mass. The European Society for Pediatric Endocrinology recommends measuring BMD at baseline and at 2- to 5-yr intervals, and the attainment of a normal peak bone mass is defined in the presence of T-score as greater than −1 sd (333).
C. Skeletal manifestations of acromegaly
Acromegaly is a disease caused by the excessive secretion of GH, and in more than 90% of the cases its etiology is a benign monoclonal pituitary adenoma (334). The incidence of acromegaly is approximately three cases per 1 million persons per year, and its prevalence is about 60 cases per million (335). The clinical manifestations of acromegaly range from subtle signs of GH/IGF-I excess, such as acral overgrowth and coarsening of facial features, to significant metabolic, cardiovascular, and respiratory manifestations, leading to an increase in morbidity and mortality (336,337,338).
1. Bone turnover and calcium metabolism in untreated acromegaly.
Patients with acromegaly have increased bone turnover, as determined by changes in biochemical markers, calcium kinetics, and bone histomorphometry (339,340,341,342,343,344,345,346,347,348). Biochemical markers of bone formation and bone resorption are increased, but markers of bone resorption are disproportionately increased in relation to markers of bone formation, and their increase could reflect the degree of bone loss observed. Serum GH and IGF-I levels correlate with markers of bone resorption, whereas only circulating levels of IGF-I correlate with markers of formation (349).
Serum concentrations of PTH, 1,25-dihydroxyvitamin D3, calcium, and phosphorus are increased in active acromegaly (343,350,351). GH stimulates the parathyroid gland and may affect PTH pulsatility, but the contribution of PTH to the skeletal effects observed in acromegaly is not clear (108,351,352).
2. BMD and fractures in untreated acromegaly.
The effects of GH excess on BMD are variable in relation to the skeletal site possibly due to different sensitivity to GH excess of trabecular and cortical bone (1). Decreased BMD has been reported in acromegaly almost exclusively at the lumbar spine, a site rich in trabecular bone, whereas increases in BMD may be observed in the forearm, a site rich in cortical bone (339,340,347,348,353,354,355,356,357,358). The variability of data on BMD in acromegaly may also be explained by the diversity of densitometric techniques used. However, peripheral quantitative computerized tomography and bone biopsies consistently demonstrated differential responses of trabecular and cortical bone to excess GH/IGF-I in humans (359).
Age, gender, and the presence or absence of hypogonadism influence vertebral BMD in acromegaly (346,348,349,355,356,360,361,362); vertebral BMD is inversely correlated with the duration of the hypogonadism (361).
Studies on the prevalence of bone fractures in acromegaly are limited (276,363,364). However, a higher incidence of radiological vertebral deformities is observed in postmenopausal women with active acromegaly than in nonacromegalic postmenopausal women (276). This suggests that acromegaly is associated with an increased risk of osteoporotic vertebral fractures, although they are often not diagnosed or detected (332). The occurrence of vertebral deformities in acromegaly correlates with the duration of the active disease and with serum levels of IGF-I, but not with BMD, and they are found in patients with normal or minimally decreased BMD (Fig. 2B) (276). The mechanisms underlying the metabolic bone disease of acromegaly are multifactorial and possibly include an increase in bone resorption secondary to IGF-I excess and to sex hormone deficiency.
3. Effects of treatment on skeletal abnormalities in acromegaly.
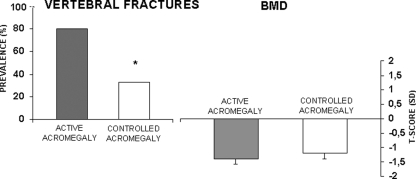
There are limited data on the effects of treatment of acromegaly in bone metabolism, BMD, and fractures. Metabolic abnormalities observed in acromegaly improve after transphenoidal pituitary surgery or pharmacological therapy (365,366,367,368). The effects of the normalization of GH/IGF-I secretion on PTH levels and activity are variable (367,369,370,371). After hormonal control (372), acromegalic patients develop a reduction in PTH target organ sensitivity and a reduced nocturnal rise in PTH (351). Postmenopausal women with controlled acromegaly have reduced risk of radiological vertebral fractures compared with patients with active acromegaly. This reduced risk occurs in the absence of a significant increase in BMD (276) (Fig. 4). It is of interest that after therapy, fractures are observed more often in patients with low levels of serum IGF-I (276).
Figure 4.
Differences in fracture rates and BMD between patients with active acromegaly and those with controlled acromegaly. *, P < 0.05 controlled vs. active acromegaly. [Modified from S. Bonadonna et al.: J Bone Miner Res 20:1837–1844, 2005 (276), with permission of the American Society for Bone and Mineral Research.]
A sustained normalization of bone turnover occurs after treatment of acromegaly with the GH antagonist pegvisomant, a selective antagonist of the GHR, which controls IGF-I secretion (371,373).
4. Monitoring patients with acromegaly.
A consensus statement from the Pituitary Society and European NeuroEndocrine Association for the diagnosis, treatment, and follow-up of acromegaly and its complications was published in 2003 (336). The determination of BMD was recommended only in hypogonadal acromegalic patients. Consequently, hypogonadal and postmenopausal patients should be screened with BMD. Radiological evaluation of the spine is advisable to exclude morphometric prevalent fractures (276). As recommended in the cited consensus statement, patients with uncontrolled active acromegaly or with established osteoporosis should be monitored with periodic BMD measurements, particularly when they are hypogonadal.
V. Skeletal Manifestations of Selected Diseases with Abnormal GH/IGF-I Axis
A. Postmenopausal and senile osteoporosis
Postmenopausal osteoporosis occurs in approximately 35% of postmenopausal white women and 19% of white elderly men (374). Estrogen deficiency plays a role in the pathogenesis of postmenopausal osteoporosis and possibly male osteoporosis (375,376). A number of other factors have been implicated in the etiology of bone loss, including secondary hyperparathyroidism, vitamin D deficiency, and decreased IGF-I levels, which could lead to impaired osteoblastic function in an elderly population (377,378).
There are some analogies between the clinical features of adult GHD and of advancing age, and a relative GH-deficient state, the somatopause, may occur during aging. A decline in GH and IGF-I secretion may play a role in the pathogenesis of osteoporosis (379,380,381). There is a correlation between serum IGF-I levels and BMD in postmenopausal women, and igf-1 promoter polymorphisms have been linked to bone mass (382,383,384,385,386). The content of IGF-I in human cortical bone decreases with age, a decline that parallels the one observed in serum concentrations of IGF-I (387). Consequently, changes in IGF-I content in the cortical bone may be due to a decrease in skeletal IGF-I accumulation from the systemic circulation, or due to a decrease in the synthesis of IGF-I by the aging skeleton.
rhGH increases bone turnover in normal subjects and improves bone mineral metabolism in postmenopausal females, males with idiopathic osteoporosis and elderly patients, but the effect of rhGH on BMD is controversial (388,389,390,391,392,393,394,395,396,397,398,399). Some studies have reported an increase in BMD after rhGH treatment of normal subjects and osteoporotic patients, whereas some have shown a lack of an effect (395,396,397,398,399,400). The lack of a response is observed despite increases in serum IGF-I. A recent meta-analysis has demonstrated no beneficial skeletal effect of rhGH in older subjects. This finding may be influenced by the scarcity of controlled trials suitable for the meta-analysis, as well as by the heterogeneity of the subjects examined (401).
An orally active GH secretagogue (MK677) combined with alendronate determined an improvement of BMD only at femoral neck in postmenopausal osteoporosis (402). Recombinant human IGF-I can influence bone metabolism in humans, but the lack of skeletal specificity and potential side effects would limit its possible use in osteoporosis (391,403,404,405,406,407,408).
B. Anorexia nervosa
Anorexia nervosa is a severe eating disorder that leads to progressive malnutrition (409). The prevalence of the disease in adolescents and young adults is between 0.5 and 1%, with an incidence of five to 10 new cases per 100,000 women between 15 and 19 yr of age per year (410). Osteopenia and osteoporosis are important features of anorexia nervosa, occurring in about 92 and 38% of the patients, respectively (411,412,413,414). Anorexia nervosa is associated with an increased prevalence of fractures (415,416). The bone loss is severe and rapid with an average annual loss of BMD of 2.5% (412). Bone loss in anorexia nervosa is due to a reduction in bone formation and an increase in bone resorption (406,412,416,417). The pathogenesis of the bone loss is multifactorial, and decreased body weight is the most important predictor of osteoporosis (418). Peak bone mass is not achieved during adolescence (417,418,419,420,421,422,423). Body weight recovery leads to skeletal improvement, and lean body mass is the most important component determining skeletal recovery (412,417). Anorexia nervosa is associated with profound metabolic abnormalities, including amenorrhea, GH resistance with decreased IGF-I synthesis, hypercortisolism, and decreased serum leptin levels. These abnormalities may play a role in the pathogenesis of the bone loss, which occurs mostly at sites rich in trabecular bone (424). Although hypogonadism may be a factor in the development of osteoporosis, estrogen deficiency alone does not explain the extreme degree of bone loss observed in anorexia nervosa (412,425,426). In fact, bone loss is more severe in young women with anorexia nervosa than in age-matched women of normal weight suffering from hypothalamic amenorrhea and with an equivalent degree of estrogen deficiency (427). Preservation of gonadal function does not protect against the bone loss, although the resumption of menstrual function is an important predictor of vertebral BMD recovery in amenorrheic women with anorexia nervosa (412,425). Replacement therapy with estrogen/progestin in women with anorexia nervosa does not increase BMD, confirming that factors other than hypogonadism are important determinants of the bone loss (428,429).
A state of GH resistance characterized by increased GH and decreased IGF-I serum levels is present in anorexia nervosa and may be related to the nutritional state of the patient (424,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447). Serum IGF-I, IGFBP-2 and -3 levels are useful nutritional indicators, and in anorexia nervosa, serum levels of IGF-I and IGFBP-3 are low, whereas serum IGFBP-2 is elevated (448,449,450,451,452,453,454,455,456). The decreased serum levels of IGF-I and IGFBP-3 and the increased levels of IGFBP-2 may contribute to the bone loss observed in anorexia nervosa. In fact, these parameters correlate with markers of bone formation, and IGFBP-3 is an independent determinant of hip BMD (457,458,459,460). Moreover, an improvement in the nutritional status of patients with anorexia nervosa leads to an increase in serum IGF-I levels, followed by a progressive increase in markers of bone formation (457,460,461,462).
IGF-I was assessed for its effect on bone turnover and bone mass in anorexia nervosa. Short-term rhIGF-I treatment increases bone turnover in a dose-dependent fashion (407). Interestingly, low doses of rhIGF-I induced an increase in bone formation without a stimulation of bone resorption (407). Low doses of rhIGF-I in combination with estrogens increase BMD (429). These results are encouraging, but appropriate trials to establish the role of rhIGF-I in the treatment of anorexia nervosa are needed.
C. Glucocorticoid-induced osteoporosis
Glucocorticoid-induced osteoporosis is the most common form of secondary osteoporosis (278). Glucocorticoids have direct and indirect effects on the skeleton. Glucocorticoids impair the replication, differentiation and function of osteoblasts and induce the apoptosis of mature osteoblasts and osteocytes (278). These effects lead to a suppression of bone formation, a central feature in the pathogenesis of glucocorticoid-induced osteoporosis. Glucocorticoids inhibit GH secretion by increasing the somatostatin tone in the hypothalamus, and glucocorticoids reduce the GH response to GHRH (18,463,464,465,466). This also is observed in asthmatic patients using inhaled glucocorticoids, although serum levels of IGF-I and biochemical markers of bone remodeling are not altered by inhaled glucocorticoids (467). In vitro, glucocorticoids suppress IGF-I transcription in bone cells (468).
The abnormal GH secretion in glucocorticoid-induced osteoporosis is associated with abnormal bone turnover and ultrasonometric data (469). An increase in serum osteocalcin, carboxy-terminal propeptide of type I procollagen, and carboxy-terminal telopeptide of type I collagen was observed after short-term rhGH treatment in a selected group of patients on chronic corticosteroid therapy (470). Combined rhGH and rhIGF-I therapy counteract selective negative effects of short-term glucocorticoid therapy in healthy volunteers (471). Theoretically, due to the kinetics of bone markers in subjects treated with glucocorticoids and GH, a window of opportunity exists for GH treatment in patients with glucocorticoid-induced low bone turnover osteoporosis. However, bone cells are resistant to the effects of GH and IGF-I in the presence of glucocorticoids (133,472), and there is little evidence for a beneficial effect of GH or IGF-I in glucocorticoid-induced osteoporosis (473). Observational and controlled studies in children receiving glucocorticoid therapy for juvenile idiopathic arthritis showed that rhGH restores normal height velocity with a concomitant improvement in bone mineralization (474,475,476). Potential side effects of chronic rhGH administration in long-term glucocorticoid users are hyperglycemia and hypertension, although additional benefits on body composition by GH administration are possible (214).
VI. Conclusions
GH and IGF-I are anabolic hormones with an important role in the regulation of bone remodeling. GH and IGF-I are necessary to achieve and maintain bone mass throughout life. IGF-I mediates most of the effects of GH on skeletal metabolism, IGF-I increases bone formation by regulating the differentiated function of the osteoblast, and as a consequence GH and IGF-I increase bone remodeling. Diseases affecting the GH/IGF-I axis are frequently associated with significant alterations in bone metabolism that often lead to bone loss.
Footnotes
This work was supported by Ministero Italiano dell’Istruzione, dell’Università e della Ricerca Scientifica, by Centro di Ricerca sull’Osteoporosi-University of Brescia/EULO and by Grants DK 42424 and DK 45227 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Statement: A.G. consults for Novartis, Ipsen, Italfarmaco, and Merck Sharp and Dohme and received lecture fees from Eli Lilly Italy, Procter and Gamble, Merck Sharp and Dohme, and Pfizer. G.M. has nothing to declare. E.C. consults for Eli Lilly and Acceleron Pharma and received lecture fees from Procter and Gamble Pharmaceuticals, GlaxoSmithKline, Roche, Sanofi-Aventis, and Novartis.
First Published Online April 24, 2008
Abbreviations: ALS, Acid labile subunit; BMD, bone mineral density; GHD, GH deficiency; GHR, GH receptor; IGFBP, IGF-binding protein; IGF-IR, IGF-I receptor; IRS, insulin receptor substrate; PAPP-A, pregnancy-associated plasma protein-A; PDGF, platelet-derived growth factor; RANK, receptor activator of nuclear factor κB; RANK-L, RANK ligand; rhGH, recombinant human GH; STAT, signal transducers and activators of transcription.
References
- Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC 1998 Growth hormone and bone. Endocr Rev 19:55–79 [DOI] [PubMed] [Google Scholar]
- Baroncelli GI, Bertelloni S, Sodini F, Saggese G 2003 Acquisition of bone mass in normal individuals and in patients with growth hormone deficiency. J Pediatr Endocrinol Metab 16(Suppl 2):327–335 [PubMed] [Google Scholar]
- Monson JP, Drake WM, Carroll PV, Weaver JU, Rodriguez-Arnao J, Savage MO 2002 Influence of growth hormone on accretion of bone mass. Horm Res 58(Suppl 1):52–56 [DOI] [PubMed] [Google Scholar]
- Nilsson O, Marino R, De Luca F, Phillip M, Baron J 2005 Endocrine regulation of the growth plate. Horm Res 64:157–165 [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hubner C, Savage MO, Clark AJ 1996 Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335:1363–1367 [DOI] [PubMed] [Google Scholar]
- Isaksson OG, Lindahl A, Nilsson A, Isgaard J 1987 Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev 8:426–438 [DOI] [PubMed] [Google Scholar]
- Guler HP, Zapf J, Scheiwiller E, Froesch ER 1988 Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci USA 85:4889–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Wit JM 2003 Systemic and local regulation of the growth plate. Endocr Rev 24:782–801 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 2001 The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res 16:1583–1585 [DOI] [PubMed] [Google Scholar]
- Canalis E 2005 The fate of circulating osteoblasts. N Engl J Med 352:2014–2016 [DOI] [PubMed] [Google Scholar]
- Seeman E 2003 Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med 349:320–323 [DOI] [PubMed] [Google Scholar]
- Bangor JP, Theists G, Bushes B, Solomon D, Rizzoli R 1991 Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 73:555–563 [DOI] [PubMed] [Google Scholar]
- Matkovic V, Jelic T, Wardlaw GM, Ilich JC, Goel PK, Wright JK, Andon MB, Smith KT, Heaney R 1994 Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis: inference from a cross-sectional model. J Clin Invest 93:799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, Bonjour JP 1992 Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab 75:1060–1065 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Neer RM, Biller BM, Crawford JD, Klibanski A 1992 Osteopenia in men with a history of delayed puberty. N Engl J Med 326:600–604 [DOI] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D 2002 Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben MS 1958 Treatment of a pituitary dwarf with human growth hormone. J Clin Endocrinol Metab 18:901–903 [DOI] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD 1998 Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- Conley LK, Teik JA, Deghenghi R, Imbimbo BP, Giustina A, Locatelli V, Wehrenberg WB 1995 Mechanism of action of hexarelin and GHRP-6: analysis of the involvement of GHRH and somatostatin in the rat. Neuroendocrinology 61:44–50 [DOI] [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt Jr MJ, Fisher MH, Nargund RP, Patchett AA 1997 Peptidomimetic regulation of growth hormone secretion. Endocr Rev 18:621–645 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K 2000 Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911 [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschöp M, Heiman ML, Ghigo E 2004 Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 25:426–457 [DOI] [PubMed] [Google Scholar]
- Cocchi D, Maccarinelli G, Sibilia V, Tulipano G, Torsello A, Pazzaglia UE, Giustina A, Netti C 2005 GH-releasing peptides and bone. J Endocrinol Invest 28(Suppl 8):11–14 [PubMed] [Google Scholar]
- Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, Takeda S, Takeuchi Y, Fukumoto S, Kangawa K, Nagata K, Kojima M 2005 Ghrelin directly regulates bone formation. J Bone Miner Res 20:790–798 [DOI] [PubMed] [Google Scholar]
- Maccarinelli G, Sibilia V, Torsello A, Raimondo F, Pitto M, Giustina A, Netti C, Cocchi D 2005 Ghrelin regulates proliferation and differentiation of osteoblastic cells. J Endocrinol 184:249–256 [DOI] [PubMed] [Google Scholar]
- Kim SW, Her SJ, Park SJ, Kim D, Park KS, Lee HK, Han BH, Kim MS, Shin CS, Kim SY 2005 Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3–E1 cells. Bone 37:359–369 [DOI] [PubMed] [Google Scholar]
- Delhanty PJ, van der Eerden BC, van der Velde M, Gauna C, Pols HA, Jahr H, Chiba H, van der Lely AJ, van Leeuwen JP 2006 Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol 188:37–47 [DOI] [PubMed] [Google Scholar]
- Wehrenberg WB, Giustina A 1996 Feedback regulation of growth hormone. In: Kostyo JL, ed. Handbook of physiology (Endocrinology, Vol. 5): hormonal control of growth. Bethesda, MD: American Physiological Association; 1101–1129 [Google Scholar]
- Wehrenberg WB, Giustina A 1992 Basic counterpoint: mechanisms and pathways of gonadal steroid modulation of growth hormone secretion. Endocr Rev 13:299–308 [DOI] [PubMed] [Google Scholar]
- Giustina A, Wehrenberg WB 1992 The role of glucocorticoids in the regulation of growth hormone secretion. Trends Endocrinol Metab 3:306–311 [DOI] [PubMed] [Google Scholar]
- Giustina A, Wehrenberg WB 1995 Influence of thyroid hormones on the regulation of growth hormone secretion. Eur J Endocrinol 133:646–653 [DOI] [PubMed] [Google Scholar]
- Zadik Z, Chalew SA, McCarter Jr RJ, Meistas M, Kowarski AA 1985 The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab 60:513–516 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Bowers CY 2003 Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest 26:799–813 [DOI] [PubMed] [Google Scholar]
- van den Berg G, Veldhuis JD, Frolich M, Roelfsema F 1996 An amplitude-specific divergence in the pulsatile mode of GH secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab 81:2460–2466 [DOI] [PubMed] [Google Scholar]
- Ho KKY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO 1987 Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58 [DOI] [PubMed] [Google Scholar]
- Coiro V, Volpi R, Bertoni P, Finzi G, Marcato A, Caiazza A, Colla R, Giacalone G, Rossi G, Chiodera P 2002 Effect of potentiation of cholinergic tone by pyridostigmine on the GH response to GHRH in elderly men. Gerontology 38:217–222 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A 1996 Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: predominant impact of age, obesity, gonadal function, and sleep. Sleep 19:S221—S224 [DOI] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosén T, Lindstedt G, Lundberg PA, Bengtsson B-Å 1996 Serum insulin-like growth factor I in a random population of men and women: Relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin Endocrinol (Oxf) 41:2209–2215 [DOI] [PubMed] [Google Scholar]
- Savine R, Sonksen P 2000 Growth hormone—hormone replacement for the somatopause? Horm Res 53(Suppl 3):37–41 [DOI] [PubMed] [Google Scholar]
- Young A 1998 Muscle function in old age. New Iss Neurosci I:141–156 [Google Scholar]
- Skelton DA, Grieg CA, Davies JM, Young A 1994 Strength, power and related functional ability of healthy people aged 60–89 years. Age Ageing 23:371–377 [DOI] [PubMed] [Google Scholar]
- Bohannon RW 1997 Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing 26:15–19 [DOI] [PubMed] [Google Scholar]
- Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI 1987 Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature 330:537–543 [DOI] [PubMed] [Google Scholar]
- Clark RG, Mortensen DL, Carlsson LM, Spencer SA, McKay P, Mulkerrin M, Moore J, Cunningham BC 1996 Recombinant human growth hormone (GH)-binding protein enhances the growth-promoting activity of human GH in the rat. Endocrinology 137:4308–4315 [DOI] [PubMed] [Google Scholar]
- Amit T, Youdim MB, Hochberg Z 2000 Does serum growth hormone (GH) binding protein reflect human GH receptor function? J Clin Endocrinol Metab 85:927–932 [DOI] [PubMed] [Google Scholar]
- Fisker S, Vahl N, Jorgensen JO, Christiansen JS, Orskov H 1997 Abdominal fat determines growth hormone-binding protein levels in healthy nonobese adults. J Clin Endocrinol Metab 82:123–128 [DOI] [PubMed] [Google Scholar]
- Ballesteros M, Leung KC, Ross RJ, Iismaa TP, Ho KK 2000 Distribution and abundance of messenger ribonucleic acid for growth hormone receptor isoforms in human tissues. J Clin Endocrinol Metab 85:2865–2871 [DOI] [PubMed] [Google Scholar]
- Fisker S 2006 Physiology and pathophysiology of growth hormone-binding protein: methodological and clinical aspects. Growth Horm IGF Res 16:1–28 [DOI] [PubMed] [Google Scholar]
- Lanning NJ, Carter-Su C 2006 Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7:225–235 [DOI] [PubMed] [Google Scholar]
- Melmed S 1999 Insulin-like growth factor I—a prototypic peripheral-paracrine hormone? Endocrinology 140:3879–3880 [DOI] [PubMed] [Google Scholar]
- Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT 2001 The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol 170:63–70 [DOI] [PubMed] [Google Scholar]
- Ueki I, Ooi GT, Tremblay ML, Hurst KR, Bach LA, Boisclair YR 2000 Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proc Natl Acad Sci USA 97:6868–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D 1999 Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96:7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams TE, Epa VC, Garrett TP, Ward CW 2000 Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci 57:1050–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett-Solberg PF, Cohen P 2000 Genetics, chemistry, and function of the IGF/IGFBP system. Endocrine 12:121–136 [DOI] [PubMed] [Google Scholar]
- Canalis E, McCarthy T, Centrella M 1988 Isolation and characterization of insulin-like growth factor I (somatomedin-C) from cultures of fetal rat calvariae. Endocrinology 122:22–27 [DOI] [PubMed] [Google Scholar]
- Mohan S, Jennings JC, Linkhart TA, Baylink DJ 1988 Primary structure of human skeletal growth factor: homology with human insulin-like growth factor-II. Biochim Biophys Acta 966:44–55 [DOI] [PubMed] [Google Scholar]
- Barnard R, Haynes KM, Werther GA, Waters MJ 1988 The ontogeny of growth hormone receptors in the rabbit tibia. Endocrinology 122:2562–2569 [DOI] [PubMed] [Google Scholar]
- Werther GA, Haynes K, Edmonson S, Oakes S, Buchanan CJ, Herington AC, Waters MJ 1993 Identification of growth hormone receptors on human growth plate chondrocytes. Acta Paediatr Suppl 82(Suppl 391):50–53 [DOI] [PubMed] [Google Scholar]
- Barnard R, Ng KW, Martin TJ, Waters MJ 1991 Growth hormone (GH) receptors in clonal osteoblast-like cells mediate a mitogenic response to GH. Endocrinology 128:1459–1464 [DOI] [PubMed] [Google Scholar]
- Kassem M, Mosekilde L, Eriksen EF, Kassem M, Mosekilde L, Eriksen EF 1994 Growth hormone stimulates proliferation of normal human bone marrow stromal osteoblast precursor cells in vitro. Growth Regul 4:131–135 [PubMed] [Google Scholar]
- Nilsson A, Swolin D, Enerback S, Ohlsson C 1995 Expression of functional growth hormone receptors in cultured human osteoblast-like cells. J Clin Endocrinol Metab 80:3483–3488 [DOI] [PubMed] [Google Scholar]
- Morales O, Lindgren U, Haldosen LA 2000 Growth hormone-regulated intracellular signaling in UMR 106 osteosarcoma cells. J Bone Miner Res 15:2284–2290 [DOI] [PubMed] [Google Scholar]
- Leung K, Rajkovic IA, Peters E, Markus I, Van Wyk JJ, Ho KK 1996 Insulin-like growth factor I and insulin down-regulate growth hormone (GH) receptors in rat osteoblasts: evidence for a peripheral feedback loop regulating GH action. Endocrinology 137:2694–2702 [DOI] [PubMed] [Google Scholar]
- Slootweg MC, Ohlsson C, Salles JP, de Vries CP, Netelenbos JC 1995 Insulin-like growth factor binding proteins-2 and -3 stimulate growth hormone receptor binding and mitogenesis in rat osteosarcoma cells. Endocrinology 136:4210–4217 [DOI] [PubMed] [Google Scholar]
- Slootweg M, Ohlsson C, van Elk E, Netelenbos J, Andress D 1996 Growth hormone receptor activity is stimulated by insulin-like growth factor binding protein 5 in rat osteosarcoma cells. Growth Regul 6:238–246 [PubMed] [Google Scholar]
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- Zhu T, Goh EL, Graichen R, Ling L, Lobie PE 2001 Signal transduction via the growth hormone receptor. Cell Signal 13:599–616 [DOI] [PubMed] [Google Scholar]
- Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA 2000 Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest 106:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klover P, Hennighausen L 2007 Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148:1489–1497 [DOI] [PubMed] [Google Scholar]
- Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL 2001 Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem 276:14443–14450 [DOI] [PubMed] [Google Scholar]
- Huang Z, Cheng SL, Slatopolsky E 2001 Sustained activation of the extracellular signal-regulated kinase pathway is required for extracellular calcium stimulation of human osteoblast proliferation. J Biol Chem 276:21351–21358 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC 2003 Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 111:1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT 2002 Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3–E1 cells. J Bone Miner Res 17:101–110 [DOI] [PubMed] [Google Scholar]
- Ziros PG, Georgakopoulos T, Habeos I, Basdra EK, Papavassiliou AG 2004 Growth hormone attenuates the transcriptional activity of Runx2 by facilitating its physical association with Stat3β. J Bone Miner Res 19:1892–1904 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B 2002 The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29 [DOI] [PubMed] [Google Scholar]
- Slootweg MC, van Buul-Offers SC, Herrmann-Erlee MP, Duursma SA 1988 Direct stimulatory effect of growth hormone on DNA synthesis of fetal chicken osteoblasts in culture. Acta Endocrinol 118:294–300 [DOI] [PubMed] [Google Scholar]
- Slootweg MC, van Buul-Offers SC, Herrmann-Erlee MP, van der Meer JM, Duursma SA 1988 Growth hormone is mitogenic for fetal mouse osteoblasts but not for undifferentiated bone cells. J Endocrinol 116:R11–R13 [DOI] [PubMed] [Google Scholar]
- Kassem M, Blum W, Ristelli J, Mosekilde L, Eriksen EF 1993 Growth hormone stimulates proliferation and differentiation of normal human osteoblast-like cells in vitro. Calcif Tissue Int 52:222–226 [DOI] [PubMed] [Google Scholar]
- Digirolamo DJ, Mukherjee A, Fulzele K, Gan Y, Cao X, Frank SJ, Clemens TL 2007 Mode of growth hormone action in osteoblasts. J Biol Chem 282:31666–31674 [DOI] [PubMed] [Google Scholar]
- Gevers EF, Loveridge N, Robinson IC 2002 Bone marrow adipocytes: a neglected target tissue for growth hormone. Endocrinology 143:4065–4073 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR 1999 Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA 2007 Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J Biol Chem 282:14515–14524 [DOI] [PubMed] [Google Scholar]
- Nuttall ME, Gimble JM 2000 Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 27:177–184 [DOI] [PubMed] [Google Scholar]
- Abdallah BM, Ding M, Jensen CH, Ditzel N, Flyvbjerg A, Jensen TG, Dagnaes-Hansen F, Gasser JA, Kassem M 2007 Dlk1/FA1 is a novel endocrine regulator of bone and fat mass and its serum level is modulated by growth hormone. Endocrinology 148:3111–3121 [DOI] [PubMed] [Google Scholar]
- Abdallah BM, Boissy P, Tan Q, Dahlgaard J, Traustadottir GA, Kupisiewicz K, Laborda J, Delaisse JM, Kassem M 2007 dlk1/FA1 regulates the function of human bone marrow mesenchymal stem cells by modulating gene expression of pro-inflammatory cytokines and immune response-related factors. J Biol Chem 282:7339–7351 [DOI] [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E 2003 Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 24:218–235 [DOI] [PubMed] [Google Scholar]
- Li H, Bartold PM, Zhang CZ, Clarkson RW, Young WG, Waters MJ 1998 Growth hormone and insulin-like growth factor-1 induce bone morphogenetic proteins 2 and 4: a mediator role in bone and tooth formation. Endocrinology 139:3855–3862 [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V 2006 BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38:1424–1429 [DOI] [PubMed] [Google Scholar]
- Hubina E, Lakatos P, Kovacs L, Szabolcs I, Racz K, Toth M, Szucs N, Goth MI 2004 Effects of 24 months of growth hormone (GH) treatment on serum carboxylated and undercarboxylated osteocalcin levels in GH-deficient adults. Calcif Tissue Int 74:55–59 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kawai S 2001 Carboxylation of osteocalcin may be related to bone quality: a possible mechanism of bone fracture prevention by vitamin K. J Bone Miner Res 19:146–149 [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ 1997 Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319 [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ 1998 Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176 [DOI] [PubMed] [Google Scholar]
- Rubin J, Ackert-Bicknell CL, Zhu L, Fan X, Murphy TC, Nanes MS, Marcus R, Holloway L, Beamer WG, Rosen CJ 2002 IGF-I regulates osteoprotegerin (OPG) and receptor activator of nuclear factor-κB ligand in vitro and OPG in vivo. J Clin Endocrinol Metab 87:4273–4279 [DOI] [PubMed] [Google Scholar]
- Ueland T, Bollerslev J, Flyvbjerg A, Hansen TB, Vahl N, Mosekilde L 2002 Effects of 12 months of growth hormone (GH) treatment on cortical and trabecular bone content of insulin like growth factors (IGF) and osteoprotegerin in adults with acquired GH deficiency: a double-blind, randomized, placebo-controlled study. J Clin Endocrinol Metab 87:2760–2763 [DOI] [PubMed] [Google Scholar]
- Ueland T, Odgren PR, Yndestad A, Godang K, Schreiner T, Marks SC, Bollerslev J 2003 Growth hormone substitution increases gene expression of members of the IGF family in cortical bone from women with adult onset growth hormone deficiency—relationship with bone turn-over. Bone 33:638–645 [DOI] [PubMed] [Google Scholar]
- Mrak E, Villa I, Lanzi R, Losa M, Guidobono F, Rubinacci A 2007 Growth hormone stimulates osteoprotegerin expression and secretion in human osteoblast-like cells. J Endocrinol 192:639–645 [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Hall K, Raben MS, Salmon Jr WD, van den Brande JL, van Wyk JJ 1972 Somatomedin: proposed designation for sulphation factor. Nature 235:107 [DOI] [PubMed] [Google Scholar]
- Green H, Morikawa M, Nixon T 1985 A dual effector theory of growth-hormone action. Differentiation 29:195–198 [DOI] [PubMed] [Google Scholar]
- Zezulak KM, Green H 1986 The generation of insulin-like growth factor-1—sensitive cells by growth hormone action. Science 233:551–553 [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson O, Lindahl A 1992 Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci USA 89:9826–9830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl A, Isgaard J, Nilsson A, Isaksson OG 1986 Growth hormone potentiates colony formation of epiphyseal chondrocytes in suspension culture. Endocrinology 118:1843–1848 [DOI] [PubMed] [Google Scholar]
- Lindahl A, Nilsson A, Isaksson OG 1987 Effects of growth hormone and insulin-like growth factor-I on colony formation of rabbit epiphyseal chondrocytes at different stages of maturation. J Endocrinol 115:263–271 [DOI] [PubMed] [Google Scholar]
- Lindahl A, Isgaard J, Carlsson L, Isaksson OG 1987 Differential effects of growth hormone and insulin-like growth factor I on colony formation of epiphyseal chondrocytes in suspension culture in rats of different ages. Endocrinology 121:1061–1069 [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Isaksson O, Lindahl A 1994 Clonal analysis of rat tibia growth plate chondrocytes in suspension culture—differential effects of growth hormone and insulin-like growth factor I. Growth Regul 4:1–7 [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A 2001 Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- Lancer SR, Bowser EN, Hargis GK 1976 The effect of growth hormone on parathyroid function in rats. Endocrinology 98:1289–1293 [DOI] [PubMed] [Google Scholar]
- Ahmad AM, Hopkins MT, Fraser WD, Ooi CG, Durham BH, Vora JP 2003 Parathyroid hormone secretory pattern, circulating activity, and effect on bone turnover in adult growth hormone deficiency. Bone 32:170–179 [DOI] [PubMed] [Google Scholar]
- el-Hajj Fuleihan G, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA 1997 The parathyroid hormone circadian rhythm is truly endogenous—a general clinical research center study. J Clin Endocrinol Metab 82:281–286 [DOI] [PubMed] [Google Scholar]
- Estepa JC, Aguilera Tejero E, Lopez I, Almaden Y, Rodriguez M, Felsenfeld AJ 1999 Effect of phosphate on parathyroid hormone secretion in vivo. J Bone Miner Res 14:1848–1854 [DOI] [PubMed] [Google Scholar]
- Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L, Campistol JM, Torres A, Rodriguez M 1998 High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9:1845–1852 [DOI] [PubMed] [Google Scholar]
- Kilav R, Silver J, Naveh-Many T 1995 Parathyroid hormone gene expression in hypophosphatemic rats. J Clin Invest 96:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner JM, Horst RL, Broadus AE, Rasmussen H, Genel M 1979 Parathyroid function and vitamin D metabolism during human growth hormone replacement. J Clin Endocrinol Metab 49:185–188 [DOI] [PubMed] [Google Scholar]
- Wei S, Tanaka H, Kubo T, Ono T, Kanzaki S, Seino Y 1997 Growth hormone increases serum 1,25-dihydroxyvitamin D levels and decreases 24,25-dihydroxyvitamin D levels in children with growth hormone deficiency. Eur J Endocrinol 136:45–51 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E 1990 Cyclic AMP induces insulin-like growth factor I synthesis in osteoblast-enriched cultures. J Biol Chem 265:15353–15356 [PubMed] [Google Scholar]
- Hall LJ, Kajimoto Y, Bichell D, Kim SW, James PL, Counts D, Nixon LJ, Tobin G, Rotwein P 1992 Functional analysis of the rat insulin-like growth factor I gene and identification of an IGF-I gene promoter. DNA Cell Biol 11:301–313 [DOI] [PubMed] [Google Scholar]
- Kim SW, Lajara R, Rotwein P 1991 Structure and function of a human insulin-like growth factor-I gene promoter. Mol Endocrinol 5:1964–1972 [DOI] [PubMed] [Google Scholar]
- Cheema U, Brown R, Mudera V, Yang SY, McGrouther G, Goldspink G 2005 Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J Cell Physiol 202:67–75 [DOI] [PubMed] [Google Scholar]
- Adamo ML, Ben Hur H, Roberts Jr CT, LeRoith D 1991 Regulation of start site usage in the leader exons of the rat insulin-like growth factor-I gene by development, fasting, and diabetes. Mol Endocrinol 5:1677–1686 [DOI] [PubMed] [Google Scholar]
- Pash JM, Delany AM, Adamo ML, Roberts Jr CT, LeRoith D, Canalis E 1995 Regulation of insulin-like growth factor I transcription by prostaglandin E2 in osteoblast cells. Endocrinology 136:33–38 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H 2005 Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology 146:2620–2628 [DOI] [PubMed] [Google Scholar]
- Canalis E, Centrella M, Burch W, McCarthy TL 1989 Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 83:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP 2002 Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res 17:1570–1578 [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Laukhuf F, Muller-Beckmann B, Blum WF, Pfister T, Ziegler R 1995 Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor β 1 in rat bone. J Clin Invest 96:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Lazowski D, Han V, Fraher L, Steer B, Hodsman A 1995 Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor I gene expression in ovariectomized rats. Bone 16:357–365 [DOI] [PubMed] [Google Scholar]
- Linkhart TA, Mohan S 1989 Parathyroid hormone stimulates release of insulin-like growth factor-I (IGF-I) and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology 125:1484–1491 [DOI] [PubMed] [Google Scholar]
- Yeh LC, Adamo ML, Olson MS, Lee JC 1997 Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology 138:4181–4190 [DOI] [PubMed] [Google Scholar]
- Ernst M, Rodan GA 1991 Estradiol regulation of insulin-like growth factor-I expression in osteoblastic cells: evidence for transcriptional control. Mol Endocrinol 5:1081–1089 [DOI] [PubMed] [Google Scholar]
- Ernst M, Heath JK, Rodan GA 1989 Estradiol effects on proliferation, messenger ribonucleic acid for collagen and insulin-like growth factor-I, and parathyroid hormone stimulated adenylate cyclase activity in osteoblastic cells from calvariae and long bones. Endocrinology 125:825–833 [DOI] [PubMed] [Google Scholar]
- Kassem M, Okazaki R, Harris SA, Spelsberg TC, Conover CA, Riggs BL 1998 Estrogen effects on insulin-like growth factor gene expression in a human osteoblastic cell line with high levels of estrogen receptor. Calcif Tissue Int 62:60–66 [DOI] [PubMed] [Google Scholar]
- Delany AM, Durant D, Canalis E 2001 Glucocorticoid suppression of IGF-I transcription in osteoblasts. Mol Endocrinol 15:1781–1789 [DOI] [PubMed] [Google Scholar]
- Smith E, Frenkel B 2005 Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3β-dependent and -independent manner. J Biol Chem 280:2388–2394 [DOI] [PubMed] [Google Scholar]
- Lakatos P, Caplice MD, Khanna V, Stern PH 1993 Thyroid hormones increase insulin-like growth factor-I content in the medium of rat bone tissue. J Bone Miner Res 8:1475–1481 [DOI] [PubMed] [Google Scholar]
- Huang BK, Golden LA, Tarjan G, Madison LD, Stern PH 2000 Insulin-like growth factor I production is essential for anabolic effects of thyroid hormone in osteoblasts. J Bone Miner Res 15:188–197 [DOI] [PubMed] [Google Scholar]
- Canalis E, Pash J, Gabbitas B, Rydziel S, Varghese S 1993 Growth factors regulate the synthesis of insulin-like growth factor-I in bone cell cultures. Endocrinology 133:33–38 [DOI] [PubMed] [Google Scholar]
- Chuang LM, Myers Jr MG, Seidner GA, Birnbaum MJ, White MF, Kahn CR 1993 Insulin receptor substrate 1 mediates insulin and insulin-like growth factor I-stimulated maturation of Xenopus oocytes. Proc Natl Acad Sci USA 90:5172–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers Jr MG, Sun XJ, Cheatham B, Jachna BR, Glasheen EM, Backer JM, White MF 1993 IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3′-kinase. Endocrinology 132:1421–1430 [DOI] [PubMed] [Google Scholar]
- Grey A, Chen Q, Xu X, Callon K, Cornish J 2003 Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology 144:4886–4893 [DOI] [PubMed] [Google Scholar]
- Bennett A, Chen T, Feldman D, Hintz RL, Rosenfeld RG 1984 Characterization of insulin-like growth factor I receptors on cultured rat bone cells: regulation of receptor concentration by glucocorticoids. Endocrinology 115:1577–1583 [DOI] [PubMed] [Google Scholar]
- Kurose H, Yamaoka K, Okada S, Nakajima S, Seino Y 1990 1,25-Dihydroxyvitamin D3 [1,25-(OH)2D3] increases insulin-like growth factor I (IGF-I) receptors in clonal osteoblastic cells. Study on interaction of IGF-I and 1,25-(OH)2D3. Endocrinology 126:2088–2094 [DOI] [PubMed] [Google Scholar]
- Rubini M, Werner H, Gandini E, Roberts Jr CT, LeRoith D, Baserga R 1994 Platelet-derived growth factor increases the activity of the promoter of the insulin-like growth factor-1 (IGF-1) receptor gene. Exp Cell Res 211:374–379 [DOI] [PubMed] [Google Scholar]
- Canalis E 1980 Effect of insulin-like growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest 66:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E 1989 Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology 124:301–309 [DOI] [PubMed] [Google Scholar]
- Canalis E, Rydziel S, Delany AM, Varghese S, Jeffrey JJ 1995 Insulin-like growth factors inhibit interstitial collagenase synthesis in bone cell cultures. Endocrinology 136:1348–1354 [DOI] [PubMed] [Google Scholar]
- Thomas T, Gori F, Spelsberg TC, Khosla S, Riggs BL, Conover CA 1999 Response of bipotential human marrow stromal cells to insulin-like growth factors: effect on binding protein production, proliferation, and commitment to osteoblasts and adipocytes. Endocrinology 140:5036–5044 [DOI] [PubMed] [Google Scholar]
- Playford MP, Bicknell D, Bodmer WF, Macaulay VM 2000 Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of β-catenin. Proc Natl Acad Sci USA 97:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, MacDougald OA 2006 Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu A, Sun H, Drakas R, Garofalo C, Cascio S, Surmacz E, Baserga R 2005 Functional significance of type 1 insulin-like growth factor-mediated nuclear translocation of the insulin receptor substrate-1 and β-catenin. J Biol Chem 280:29912–29920 [DOI] [PubMed] [Google Scholar]
- Kalajzic I, Staal A, Yang WP, Wu Y, Johnson SE, Feyen JH, Krueger W, Maye P, Yu F, Zhao Y, Kuo L, Gupta RR, Achenie LE, Wang HW, Shin DG, Rowe DW 2005 Expression profile of osteoblast lineage at defined stages of differentiation. J Biol Chem 280:24618–24626 [DOI] [PubMed] [Google Scholar]
- Hou P, Sato T, Hofstetter W, Foged NT 1997 Identification and characterization of the insulin-like growth factor I receptor in mature rabbit osteoclasts. J Bone Miner Res 12:534–540 [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Hakeda Y, Wakatsuki N, Usui N, Akashi S, Sato T, Tanaka K, Kumegawa M 1992 Insulin-like growth factor-I supports formation and activation of osteoclasts. Endocrinology 131:1075–1080 [DOI] [PubMed] [Google Scholar]
- Niu T, Rosen CJ 2005 The insulin-like growth factor-I gene and osteoporosis: a critical appraisal. Gene 361:38–56. [DOI] [PubMed] [Google Scholar]
- Liu P, Baker J, Perkins AS, Robertson EJ, Efstratiadis A 1993 Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- De Chiara TM, Efstratiadis A, Robertson EJ 1990 A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345:78–80 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou J, Bondy CA 1999 Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J 13:1985–1990 [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL 2002 Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277:44005–44012 [DOI] [PubMed] [Google Scholar]
- Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B 2001 The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res 16:2320–2329 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD 2006 Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res 21:1350–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi K, Ogata N, Shimoaka T, Terauchi Y, Kadowaki T, Kenmotsu S, Chung UI, Ozawa H, Nakamura K, Kawaguchi H 2004 Deficiency of insulin receptor substrate-1 impairs skeletal growth through early closure of epiphyseal cartilage. J Bone Miner Res 19:214–223 [DOI] [PubMed] [Google Scholar]
- Beamer WH, Eicher EM 1976 Stimulation of growth in the little mouse. J Endocrinol 71:37–45 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ 1997 A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA 94:13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaogren K, Sheng M, Moverare S, Liu JL, Wallenius K, Tornell J, Isaksson O, Jansson JO, Mohan S, Ohlsson C 2002 Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res 17:1977–1987 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Shultz KL, Donahue LR, Churchill GA, Sen S, Wergedal JR, Baylink DJ, Rosen CJ 2001 Quantitative trait loci for femoral and lumbar vertebral bone mineral density in C57BL/6J and C3H/HeJ inbred strains of mice. J Bone Miner Res 16:1195–1206 [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Rosen CJ, Turner CH, Ackert CL, Shultz KL, Donahue LR, Churchill G, Adamo ML, Powell DR, Turner RT, Muller R, Beamer WG 2002 Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res 17:570–579 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Ckert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M 2004 Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35:1046–1058 [DOI] [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL 2000 Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141:2674–2682 [DOI] [PubMed] [Google Scholar]
- Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K, Kawaguchi H 2000 Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest 105:935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, Yamaguchi A 1997 Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest 99:2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD 2007 The IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res 22:1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL 2005 Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583 [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT 2003 Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846 [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Mundy GR 1987 Modulation of type β transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci USA 84:2024–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR 1995 Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- Hwa V, Oh Y, Rosenfeld RG 1999 The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev 20:761–787 [DOI] [PubMed] [Google Scholar]
- Andress DL, Birnbaum RS 1992 Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem 267:22467–22472 [PubMed] [Google Scholar]
- Jones JI, Gockerman A, Busby Jr WH, Camacho-Hubner C, Clemmons DR 1993 Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol 121:679–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassager C, Fitzpatrick LA, Spencer EM, Riggs BL, Conover CA 1992 Basal and regulated secretion of insulin-like growth factor binding proteins in osteoblast-like cells is cell line specific. J Clin Endocrinol Metab 75:228–233 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Casinghino S, Centrella M, Canalis E 1994 Complex pattern of insulin-like growth factor binding protein expression in primary rat osteoblast enriched cultures: regulation by prostaglandin E2, growth hormone, and the insulin-like growth factors. J Cell Physiol 160:163–175 [DOI] [PubMed] [Google Scholar]
- Birnbaum RS, Wiren KM 1994 Changes in insulin-like growth factor-binding protein expression and secretion during the proliferation, differentiation, and mineralization of primary cultures of rat osteoblasts. Endocrinology 135:223–230 [DOI] [PubMed] [Google Scholar]
- Canalis E, Gabbitas B 1995 Skeletal growth factors regulate the synthesis of insulin-like growth factor binding protein-5 in bone cell cultures. J Biol Chem 270:10771–10776 [DOI] [PubMed] [Google Scholar]
- Dong Y, Canalis E 1995 Insulin-like growth factor (IGF) I and retinoic acid induce the synthesis of IGF-binding protein 5 in rat osteoblastic cells. Endocrinology 136:2000–2006 [DOI] [PubMed] [Google Scholar]
- Okazaki R, Riggs BL, Conover CA 1994 Glucocorticoid regulation of insulin-like growth factor-binding protein expression in normal human osteoblast-like cells. Endocrinology 134:126–132 [DOI] [PubMed] [Google Scholar]
- Moriwake T, Tanaka H, Kanzaki S, Higuchi J, Seino Y 1992 1,25-Dihydroxyvitamin D3 stimulates the secretion of insulin-like growth factor binding protein 3 (IGFBP-3) by cultured human osteosarcoma cells. Endocrinology 130:1071–1073 [DOI] [PubMed] [Google Scholar]
- Rajkumar K, Barron D, Lewitt MS, Murphy LJ 1995 Growth retardation and hyperglycemia in insulin-like growth factor binding protein-1 transgenic mice. Endocrinology 136:4029–4034 [DOI] [PubMed] [Google Scholar]
- Amin S, Riggs BL, Atkinson EJ, Oberg AL, Melton III LJ, Khosla S 2004 A potentially deleterious role of IGFBP-2 on bone density in aging men and women. J Bone Miner Res 19:1075–1083 [DOI] [PubMed] [Google Scholar]
- Eckstein F, Pavicic T, Nedbal S, Schmidt C, Wehr U, Rambeck W, Wolf E, Hoeflich A 2002 Insulin-like growth factor-binding protein-2 (IGFBP-2) overexpression negatively regulates bone size and mass, but not density, in the absence and presence of growth hormone/IGF-I excess in transgenic mice. Anat Embryol (Berl) 206:139–148 [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ 2008 Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology 149:2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S, Riggs BL, Melton 3rd LJ, Achenbach SJ, Atkinson EJ, Khosla S 2007 High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. J Bone Miner Res 22:799–807 [DOI] [PubMed] [Google Scholar]
- Conover CA, Johnstone EW, Turner RT, Evans GL, John Ballard FJ, Doran PM, Khosla S 2002 Subcutaneous administration of insulin-like growth factor (IGF)-II/IGF binding protein-2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Horm IGF Res 12:178–183 [DOI] [PubMed] [Google Scholar]
- Lean JM, Jagger CJ, Chambers TJ, Chow JW 1995 Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol 268:E318–E327 [DOI] [PubMed] [Google Scholar]
- Reijnders CM, Bravenboer N, Holzmann PJ, Bhoelan F, Blankenstein MA, Lips P 2007 In vivo mechanical loading modulates insulin-like growth factor binding protein-2 gene expression in rat osteocytes. Calcif Tissue Int 80:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L, Torello M, Buckway C, O'Rear L, Horton WA, Hwa V, Roberts Jr CT, Chiarelli F, Rosenfeld RG, Spagnoli A 2003 A novel insulin-like growth factor (IGF)-independent role for IGF binding protein-3 in mesenchymal chondroprogenitor cell apoptosis. Endocrinology 144:1695–1702 [DOI] [PubMed] [Google Scholar]
- Silha JV, Mishra S, Rosen CJ, Beamer WG, Turner RT, Powell DR, Murphy LJ 2003 Perturbations in bone formation and resorption in insulin-like growth factor binding protein-3 transgenic mice. J Bone Miner Res 18:1834–1841 [DOI] [PubMed] [Google Scholar]
- Richman C, Baylink DJ, Lang K, Dony C, Mohan S 1999 Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology 140:4699–4705 [DOI] [PubMed] [Google Scholar]
- Devlin RD, Du Z, Buccilli V, Jorgetti V, Canalis E 2002 Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology 143:3955–3962 [DOI] [PubMed] [Google Scholar]
- Zhang M, Faugere MC, Malluche H, Rosen CJ, Chernausek SD, Clemens TL 2003 Paracrine overexpression of IGFBP-4 in osteoblasts of transgenic mice decreases bone turnover and causes global growth retardation. J Bone Miner Res 18:836–843 [DOI] [PubMed] [Google Scholar]
- Durant D, Pereira RM, Canalis E 2004 Overexpression of insulin-like growth factor binding protein-5 decreases osteoblastic function in vitro. Bone 35:1256–1262 [DOI] [PubMed] [Google Scholar]
- Conover CA 1995 Insulin-like growth factor binding protein proteolysis in bone cell models. Prog Growth Factor Res 6:301–309 [DOI] [PubMed] [Google Scholar]
- Boldt HB, Conover CA 2007 Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 17:10–18 [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Hilliker S, Baylink DJ, Mohan S 1994 Evidence that human bone cells in culture produce insulin-like growth factor-binding protein-4 and -5 proteases. Endocrinology 134:383–392 [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S 2006 Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology 147:5653–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale LK, Conover CA 2005 Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol 86:325–331 [DOI] [PubMed] [Google Scholar]
- Tanner SJ, Hefferan TE, Rosen CJ, Conover CA 2008 Impact of pregnancy-associated plasma protein-a deletion on the adult murine skeleton. J Bone Miner Res 23:655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BS, Bronk JT, Nishiyama T, Yamagiwa H, Srivastava A, Bolander ME, Conover CA 2007 Pregnancy associated plasma protein-A is necessary for expeditious fracture healing in mice. J Endocrinol 192:505–513 [DOI] [PubMed] [Google Scholar]
- Ortiz CO, Chen BK, Bale LK, Overgaard MT, Oxvig C, Conover CA 2003 Transforming growth factor-β regulation of the insulin-like growth factor binding protein-4 protease system in cultured human osteoblasts. J Bone Miner Res 18:1066–1072 [DOI] [PubMed] [Google Scholar]
- Conover CA, Chen BK, Resch ZT 2004 Regulation of pregnancy-associated plasma protein-A expression in cultured human osteoblasts. Bone 34:297–302 [DOI] [PubMed] [Google Scholar]
- Franchimont N, Durant D, Canalis E 1997 Interleukin-6 and its soluble receptor regulate the expression of insulin-like growth factor binding protein-5 in osteoblast cultures. Endocrinology 138:3380–3386 [DOI] [PubMed] [Google Scholar]
- Rydziel S, Delany AM, Canalis E 2004 AU-rich elements in the collagenase 3 mRNA mediate stabilization of the transcript by cortisol in osteoblasts. J Biol Chem 279:5397–5404 [DOI] [PubMed] [Google Scholar]
- Lalou C, Silve C, Rosato R, Segovia B, Binoux M 1994 Interactions between insulin-like growth factor-I (IGF-I) and the system of plasminogen activators and their inhibitors in the control of IGF-binding protein-3 production and proteolysis in human osteosarcoma cells. Endocrinology 135:2318–2326 [DOI] [PubMed] [Google Scholar]
- de Boer H, Block GJ, Van der Veen EA 1995 Clinical aspects of growth hormone deficiency in adults. Endocr Rev 16:63–86 [DOI] [PubMed] [Google Scholar]
- Doga M, Bonadonna S, Gola M, Mazziotti G, Giustina A 2006 Growth hormone deficiency in the adult. Pituitary 9:305–311 [DOI] [PubMed] [Google Scholar]
- Doga M, Bonadonna S, Gola M, Solerte SB, Amato G, Carella C, Giustina A 2005 Current guidelines for adult GH replacement. Rev Endocr Metab Disord 6:63–70 [DOI] [PubMed] [Google Scholar]
- Gola M, Bonadonna S, Doga M, Giustina A 2005 Growth hormone and cardiovascular risk factors. J Clin Endocrinol Metab 90:1864–1870 [DOI] [PubMed] [Google Scholar]
- Doga M, Bonadonna S, Gola M, Mazziotti G, Nuzzo M, Giustina A 2005 GH deficiency in the adult and bone. J Endocrinol Invest 28(8 Suppl):18–23 [PubMed] [Google Scholar]
- Canalis E, Giustina A, Bilezikian JP 2007 Medical progress: mechanisms of anabolic therapies for osteoporosis. N Engl J Med 357:905–916 [DOI] [PubMed] [Google Scholar]
- Bravenboer N, Holzmann P, de Boer H, Blok GJ, Lips P 1996 Histomorphometric analysis of bone mass and bone metabolism in growth hormone deficient adult men. Bone 18:551–557 [DOI] [PubMed] [Google Scholar]
- Hitz MF, Jensen JE, Eskildsen PC 2006 Bone mineral density in patients with growth hormone deficiency: does a gender difference exist? Clin Endocrinol (Oxf) 65:783–791 [DOI] [PubMed] [Google Scholar]
- Sartorio A, Conti A, Monzani M, Morabito F, Faglia G 1993 Growth hormone treatment in adults with GH deficiency: effects on new biochemical markers of bone and collagen turnover. J Endocrinol Invest 16:893–898 [DOI] [PubMed] [Google Scholar]
- Delmas PD, Chatelain P, Malaval L, Bonne G 1986 Serum bone GLA-protein in growth hormone deficient children. J Bone Miner Res 1:333–338 [DOI] [PubMed] [Google Scholar]
- Nielsen HK, Jorgensen JO, Brixen K, Christiansen JS 1991 Serum osteocalcin and bone isoenzyme alkaline phosphatase in growth hormone-deficient patients: dose-response studies with biosynthetic human GH. Calcif Tissue Int 48:82–87 [DOI] [PubMed] [Google Scholar]
- Toogood AA, Adams JE, O'Neill PA, Shalet SM 1997 Elderly patients with adult-onset growth hormone deficiency are not osteopenic. J Clin Endocrinol Metab 82:1462–1466 [DOI] [PubMed] [Google Scholar]
- Ahmad AM, Hopkins MT, Thomas J, Durham BH, Fraser WD, Vora JP 2004 Parathyroid responsiveness to hypocalcemic and hypercalcemic stimuli in adult growth hormone deficiency after growth hormone replacement. Am J Physiol Endocrinol Metab 286:E986–E993 [DOI] [PubMed] [Google Scholar]
- Ledger GA, Burritt MF, Kao PC, O'Fallon WM, Riggs BL, Khosla S 1995 Role of parathyroid hormone in mediating nocturnal and age-related increases in bone resorption. J Clin Endocrinol Metab 80:3304–3310 [DOI] [PubMed] [Google Scholar]
- White HD, Ahmad A, Durham B, Peter R, Prabhakar V, Corlett P, Vora J, Fraser W 2007 PTH circadian rhythm and PTH target-organ sensitivity is altered in adult growth hormone deficiency patients with low bone mineral density. J Bone Miner Res 22:1798–1807 [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Taelman P, Vermeulen A, Vandeweghe M 1992 Bone mineral status in growth hormone-deficient males with isolated and multiple pituitary deficiencies of childhood onset. J Clin Endocrinol Metab 74:118–123 [DOI] [PubMed] [Google Scholar]
- Wuster C, Abs R, Bengtsson BA, Bennmarker H, Feldt-Rasmussen U, Hernberg-Stahl E, Monson JP, Westberg B, Wilton P, KIMS Study Group and the KIMS International Board. Pharmacia, Upjohn International Metabolic Database 2001 The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J Bone Miner Res 16:398–405 [DOI] [PubMed] [Google Scholar]
- Murray RD, Adams JE, Shalet SM 2006 A densitometric and morphometric analysis of the skeleton in adults with varying degrees of growth hormone deficiency. J Clin Endocrinol Metab 91:432–438 [DOI] [PubMed] [Google Scholar]
- Kosowicz J, El Ali Z, Ziemnicka K, Sowinski J 2007 Abnormalities in bone mineral density distribution and bone scintigraphy in patients with childhood onset hypopituitarism. J Clin Densitom 10:332–339 [DOI] [PubMed] [Google Scholar]
- Colao A, Di Somma C, Pivonello R, Loche S, Aimaretti G, Cerbone G, Faggiano A, Corneli G, Ghigo E, Lombardi G 1999 Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab 84:1919–1924 [DOI] [PubMed] [Google Scholar]
- White HD, Ahmad AM, Durham BH, Patwala A, Whittingham P, Fraser WD, Vora JP 2005 Growth hormone replacement is important for the restoration of parathyroid hormone sensitivity and improvement in bone metabolism in older adult growth hormone-deficient patients. J Clin Endocrinol Metab 90:3371–3380 [DOI] [PubMed] [Google Scholar]
- Janssen YJ, Hamdy NA, Frolich M, Roelfsema F 1998 Skeletal effects of two years of treatment with low physiological doses of recombinant human growth hormone (GH) in patients with adult-onset GH deficiency. J Clin Endocrinol Metab 83:2143–2148 [DOI] [PubMed] [Google Scholar]
- Lissett CA, Shalet SM 2000 Effects of growth hormone on bone and muscle. Growth Horm IGF Res 10(Suppl B):S95–S101 [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Bianchi A, Bonadonna S, Nuzzo M, Cimino V, Fusco A, De Marinis L, Giustina A 2006 Increased prevalence of radiological spinal deformities in adult patients with GH deficiency: influence of GH replacement therapy. J Bone Miner Res 21:520–528 [DOI] [PubMed] [Google Scholar]
- Attanasio AF, Lamberts SWJ, Matranga AM, Birkett MA, Bates PC, Valk NK, Hilsted J, Bengtsson BA, Strasburger CJ 1997 Adult growth hormone-deficient patients demonstrate heterogeneity between childhood onset and adult onset before and during human GH treatment. J Clin Endocrinol Metab 82:82–88 [DOI] [PubMed] [Google Scholar]
- Lissett CA, Shalet SM 2002 Childhood-onset growth hormone (GH) deficiency in adult life. Best Pract Res Clin Endocrinol Metab 16:209–224 [DOI] [PubMed] [Google Scholar]
- De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A 2005 Primary empty sella. J Clin Endocrinol Metab 90:5471–5477 [DOI] [PubMed] [Google Scholar]
- Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML; Endocrine Society’s Clinical Guidelines Subcommittee; Stephens PA 2006 Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91:1621–1634 [DOI] [PubMed] [Google Scholar]
- Murray RD, Columb B, Adams JE, Shalet SM 2004 Low bone mass is an infrequent feature of the adult growth hormone deficiency syndrome in middle-age adults and the elderly. J Clin Endocrinol Metab 89:1124–1130 [DOI] [PubMed] [Google Scholar]
- Degerblad M, Bengtsson BA, Bramnert M, Johnell O, Manhem P, Rosen T, Thoren M 1995 Reduced bone mineral density in adults with growth hormone (GH) deficiency: increased bone turnover during 12 months of GH substitution therapy. Eur J Endocrinol 133:180–188 [DOI] [PubMed] [Google Scholar]
- Fernholm R, Bramnert M, Hagg E, Hilding A, Baylink DJ, Mohan S, Thoren M 2000 Growth hormone replacement therapy improves body composition and increases bone metabolism in elderly patients with pituitary disease. J Clin Endocrinol Metab 85:4104–4112 [DOI] [PubMed] [Google Scholar]
- Kann P, Piepkorn B, Schehler B, Andreas J, Lotz J, Prellwitz W, Beyer J 1998 Effect of long-term treatment with GH on bone metabolism, bone mineral density and bone elasticity in GH-deficient adults. Clin Endocrinol (Oxf) 48:561–568 [DOI] [PubMed] [Google Scholar]
- Abrahamsen B, Hangaard J, Horn HC, Hansen TB, Gregersen G, Hansen-Nord M, Vahl N, Junker P, Andersen M, Hagen C 2002 Evaluation of the optimum dose of growth hormone (GH) for restoring bone mass in adult-onset GH deficiency: results from two 12-month randomized studies. Clin Endocrinol (Oxf) 57:273–281 [DOI] [PubMed] [Google Scholar]
- Arwert LI, Roos JC, Lips P, Twisk JW, Manoliu RA, Drent ML 2005 Effects of 10 years of growth hormone (GH) replacement therapy in adult GH-deficient men. Clin Endocrinol (Oxf) 63:310–316 [DOI] [PubMed] [Google Scholar]
- Hoffman AR, Kuntze JE, Baptista J, Baum HB, Baumann GP, Biller BM, Clark RV, Cook D, Inzucchi SE, Kleinberg D, Klibanski A, Phillips LS, Ridgway EC, Robbins RJ, Schlechte J, Sharma M, Thorner MO, Vance ML 2004 Growth hormone (GH) replacement therapy in adult-onset GH deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab 89:2048–2056 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Rosen T, Bosaeus I, Sjostrom L, Bengtsson BA 1996 Two years of growth hormone (GH) treatment increases bone mineral content and density in hypopituitary patients with adult onset GH deficiency. J Clin Endocrinol Metab 81:2865–2873 [DOI] [PubMed] [Google Scholar]
- Johannsson AG, Burman P, Westermark K, Ljunghall S 1992 The bone mineral density in acquired growth hormone deficiency correlates with circulating insulin-like growth factor. J Intern Med 232:447–452 [DOI] [PubMed] [Google Scholar]
- Bing-You RG, Denis MC, Rosen CJ 1993 Low bone mineral density in adults with previous hypothalamic-pituitary tumors: correlation with GH responses to GHRH, IGF-I and IGFBP-3. Calcif Tissue Int 52:183–187 [DOI] [PubMed] [Google Scholar]
- Rosen T, Hansson T, Granhed H, Szucs J, Bengtsson BA 1995 Reduced bone mineral content in adult patients with growth hormone deficiency. Eur J Endocrinol 132:727–729 [DOI] [PubMed] [Google Scholar]
- Thoren M, Soop M, Degerblad M, Saaf M 1993 Preliminary study of the effects of growth hormone substitution therapy on bone mineral density and serum osteocalcin levels in adult growth hormone deficiency. Acta Endocrinol 128(Suppl 2):41–43 [PubMed] [Google Scholar]
- Beshyah SA, Freemantle C, Thomas E, Rutherford O, Page B, Murphy M, Johnston DG 1995 Abnormal body composition and reduced bone mass in growth hormone deficient hypopituitary adults. Clin Endocrinol (Oxf) 42:179–189 [DOI] [PubMed] [Google Scholar]
- Holmes SJ, Economou G, Whitehouse RW, Adams JE, Shalet SM 1994 Reduced bone mineral density in patients with adult onset growth hormone deficiency. J Clin Endocrinol Metab 78:669–674 [DOI] [PubMed] [Google Scholar]
- Bex M, Abs R, Maiter D, Beckers A, Lamberigts G, Bouillon R 2002 The effects of growth hormone replacement therapy on bone metabolism in adult-onset growth hormone deficiency: a 2-year open randomized controlled multicenter trial. J Bone Miner Res 17:1081–1094 [DOI] [PubMed] [Google Scholar]
- Baroncelli GI, Bertelloni S, Sodini F, Saggese G 2004 Longitudinal changes of lumbar bone mineral density (BMD) in patients with GH deficiency after discontinuation of treatment at final height; timing and peak values for lumbar BMD. Clin Endocrinol (Oxf) 60:175–184 [DOI] [PubMed] [Google Scholar]
- Rapaport R, Cook DM 2006 Transition of childhood-onset growth hormone-deficient patients to adult healthcare. Pediatr Endocrinol Rev 4(Suppl 1):82–90 [PubMed] [Google Scholar]
- Radovick S, DiVall S 2007 Approach to the growth hormone-deficient child during transition to adulthood. J Clin Endocrinol Metab 92:1195–1200 [DOI] [PubMed] [Google Scholar]
- Mauras N, Pescovitz OH, Allada V, Messig M, Wajnrajch MP, Lippe B; Transition Study Group 2005 Limited efficacy of growth hormone (GH) during transition of GH-deficient patients from adolescence to adulthood: a phase III multicenter, double-blind, randomized two-year trial. J Clin Endocrinol Metab 90:3946–3955 [DOI] [PubMed] [Google Scholar]
- Sabatier JP, Guaydier-Souquières, Benmalek A, Marcelli C 1999 Evolution of bone mineral content during adolescence and adulthood: a longitudinal study in 395 healthy females 10–24 years of age and 206 premenopausal women. Osteoporos Int 9:476–482 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C 2000 Peak bone mass. Osteoporos Int 11:985–1009 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Travers R, Rauch F, Glorieux FH 2000 Structural and cellular changes during bone growth in healthy children. Bone 27:487–494 [DOI] [PubMed] [Google Scholar]
- Attanasio AF, Howell S, Bates PC, Frewer P, Chipman J, Blum WF, Shalet SM 2002 Body composition, IGF-I and IGFBP-3 concentrations as outcome measures in severely GH-deficient (GHD) patients after childhood GH treatment: a comparison with adult onset GHD patients. J Clin Endocrinol Metab 87:3368–3372 [DOI] [PubMed] [Google Scholar]
- Trevisan C, Ortolani S, Bianchi ML, Caraceni MP, Ulivieri FM, Gandolini G, Polli EE 1991 Age, time since menopause, and body parameters as determinants of female spinal bone mass: a mathematical model. Calcif Tissue Int 49:1–5 [DOI] [PubMed] [Google Scholar]
- Baroncelli GI, Bertelloni S, Ceccarelli C, Saggese G 1998 Measurement of volumetric bone mineral density accurately determines degree of lumbar undermineralization in children with growth hormone deficiency. J Clin Endocrinol Metab 83:3150–3154 [DOI] [PubMed] [Google Scholar]
- Tothill P 1989 Methods of bone mineral measurement. Phys Med Biol 34:543–572 [DOI] [PubMed] [Google Scholar]
- Farrel TJ, Webber CE 1989 The error due to fat inhomogeneity in lumbar spine bone mineral mass measurements. Clin Phys Physiol Measurement 10:57–64 [DOI] [PubMed] [Google Scholar]
- Tothill P, Pye DW 1992 Errors due to non-uniform distribution of fat in dual x-ray absorptiometry of the lumbar spine. Br J Radiol 65:807–813 [DOI] [PubMed] [Google Scholar]
- Rosen T, Hansson T, Granhed H, Szucs J, Bengtsson BA 1993 Reduced bone mineral content in adult patients with growth hormone deficiency. Acta Endocrinol 129:201–206 [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Bianchi A, Cimino V, Bonadonna S, Martini P, Fusco A, De Marinis L, Giustina A 2008 Effect of gonadal status on bone mineral density and radiological spinal deformities in adult patients with growth hormone deficiency. Pituitary 11:55–61 [DOI] [PubMed] [Google Scholar]
- Stall GM, Harris S, Sokoll LJ, Dawson-Hughes B 1990 Accelerated bone loss in hypothyroid patients over-treated with l-thyroxine. Ann Intern Med 113:265–269 [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A 2006 Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab 17:144–149 [DOI] [PubMed] [Google Scholar]
- Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, Iqbal J, Eldeiry L, Rajendren G, Blair HC, Davies TF, Zaidi M 2003 TSH is a negative regulator of skeletal remodeling. Cell 115:151–162 [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Sorvillo F, Piscopo M, Cioffi M, Pilla P, Biondi B, Iorio S, Giustina A, Amato G, Carella C 2005 Recombinant human TSH modulates in vivo C-telopeptides of type-1 collagen and bone alkaline phosphatase, but not osteoprotegerin production in postmenopausal women monitored for differentiated thyroid carcinoma. J Bone Miner Res 20:480–486 [DOI] [PubMed] [Google Scholar]
- Rosen T, Wilhelmsen L, Landin-Wilhelmsen K, Lappas G, Bengtsson BA 1997 Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol 137:240–245 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Koledova E, Bezlepkina O, Nijs J, Shavrikhova E, Nagaeva E, Chikulaeva O, Peterkova V, Dedov I, Bakulin A, Oganov V, Attanasio AF 2004 Bone status and fracture prevalence in Russian adults with childhood-onset growth hormone deficiency. J Clin Endocrinol Metab 89:4993–4998 [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Jorgensen JO, Hagen C, Hoeck HC, Laurberg P, Rejnmark L, Brixen K, Weeke J, Andersen M, Conceicao FL, Nielsen TL, Mosekilde L 2002 Fracture risk is increased in patients with GH deficiency or untreated prolactinomas—a case-control study. Clin Endocrinol (Oxf) 56:159–167 [DOI] [PubMed] [Google Scholar]
- Bonadonna S, Mazziotti G, Nuzzo M, Bianchi A, Fusco A, De Marinis L, Giustina A 2005 Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in postmenopausal women. J Bone Miner Res 20:1837–1844 [DOI] [PubMed] [Google Scholar]
- Vestergaard P 2007 Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 18:427–444 [DOI] [PubMed] [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP 2007 Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328 [DOI] [PubMed] [Google Scholar]
- Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S 2006 The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr Rev 27:287–317 [DOI] [PubMed] [Google Scholar]
- Hansen TB, Brixen K, Vahl N, Jorgensen JO, Christiansen JS, Mosekilde L, Hagen C 1996 Effects of 12 months of growth hormone (GH) treatment on calciotropic hormones, calcium homeostasis, and bone metabolism in adults with acquired GH deficiency: a double-blind, randomized, placebo-controlled study. J Clin Endocrinol Metab 81:3352–3359 [DOI] [PubMed] [Google Scholar]
- Gotherstrom G, Svensson J, Koranyi J, Alpsten M, Bosaeus I, Bengtsson B, Johannsson G 2001 A prospective study of 5 years of GH replacement therapy in GH-deficient adults: sustained effects on body composition, bone mass, and metabolic indices. J Clin Endocrinol Metab 86:4657–4665 [DOI] [PubMed] [Google Scholar]
- Drake WM, Rodriguez-Arnao J, Weaver JU, James IT, Coyte D, Spector TD, Besser GM, Monson JP 2001 The influence of gender on the short- and long-term effects of growth hormone replacement on bone metabolism and bone mineral density in hypopituitary adults: a 5-year study. Clin Endocrinol (Oxf) 54:525–532 [DOI] [PubMed] [Google Scholar]
- Sneppen SB, Hoeck HC, Kollerup G, Sorensen OH, Laurberg P, Feldt-Rasmussen 2002 Bone mineral content and bone metabolism during physiological GH treatment in GH-deficient adults—an 18-month randomised, placebo-controlled, double-blinded trial. Eur J Endocrinol 146:187–195 [DOI] [PubMed] [Google Scholar]
- Jensen LT, Jorgensen JOL, Risteli J, Christiansen JS, Lorenzen I 1991 Type I and III procollagen propeptides in growth hormone-deficient patients: effects of increasing doses of GH. Acta Endocrinol 124:278–282 [DOI] [PubMed] [Google Scholar]
- Laursen T, Gravholt CH, Heickendorff L, Drustrup J, Kappelgaard AM, Jorgensen JO, Christiansen JS 2001 Long-term effects of continuous subcutaneous infusion versus daily subcutaneous injections of growth hormone (GH) on the insulin-like growth factor system, insulin sensitivity, body composition, and bone and lipoprotein metabolism in GH-deficient adults. J Clin Endocrinol Metab 86:1222–1228 [DOI] [PubMed] [Google Scholar]
- Amato G, Mazziotti G, Di Somma C, Lalli E, De Felice G, Conte M, Rotondi M, Pietrosante M, Lombardi G, Bellastella A, Carella C, Colao A 2000 Recombinant growth hormone (GH) therapy in GH-deficient adults: a long-term controlled study on daily versus thrice weekly injections. J Clin Endocrinol Metab 85:3720–3725 [DOI] [PubMed] [Google Scholar]
- Nilsson AG 2000 Effects of growth hormone replacement therapy on bone markers and bone mineral density in growth hormone-deficient adults. Horm Res 54(Suppl 1):52–57 [DOI] [PubMed] [Google Scholar]
- Beshyah SA, Kyd P, Thomas E, Fairney A, Johnston DG 1995 The effects of prolonged growth hormone replacement on bone metabolism and bone mineral density in hypopituitary adults. Clin Endocrinol (Oxf) 42:249–254 [DOI] [PubMed] [Google Scholar]
- Beshyah SA, Thomas E, Kyd P, Sharp P, Fairney A, Johnston DG 1994 The effect of growth hormone replacement therapy in hypopituitary adults on calcium and bone metabolism. Clin Endocrinol (Oxf) 40:383–391 [DOI] [PubMed] [Google Scholar]
- Saggese G, Baroncelli GI, Bertelloni S, Cinquanta L, Di Nero G 1993 Effects of long-term treatment with growth hormone on bone and mineral metabolism in children with growth hormone deficiency. J Pediatr 122:37–45 [DOI] [PubMed] [Google Scholar]
- Caversazio J, Bonjour JP, Fleisch H 1989 Adaptation of tubular phosphate transport: relation between phosphate requirement as influenced by growth and supply. Adv Exp Med Biol 128:107–111 [DOI] [PubMed] [Google Scholar]
- Bouillon R 1991 Growth hormone and bone. Horm Res 36(Suppl 1):49–55 [DOI] [PubMed] [Google Scholar]
- Burstein S, Chen IW, Tsang RC 1983 Effects of growth hormone replacement therapy on 1,25-dihydroxyvitamin D and calcium metabolism. J Clin Endocrinol Metab 56:1246–1251 [DOI] [PubMed] [Google Scholar]
- Ahmad AM, Thomas J, Clewes A, Hopkins MT, Guzder R, Ibrahim H, Durham BH, Vora JP, Fraser WD 2003 Effects of growth hormone replacement on parathyroid hormone sensitivity and bone mineral metabolism. J Clin Endocrinol Metab 88:2860–2868 [DOI] [PubMed] [Google Scholar]
- Holmes SJ, Whitehouse RW, Swindell R, Economou G, Adams JE, Shalet SM 1995 Effect of growth hormone replacement on bone mass in adults with adult onset growth hormone deficiency. Clin Endocrinol (Oxf) 42:627–633 [DOI] [PubMed] [Google Scholar]
- Binnerts A, Swart GR, Wilson JHP, Hoogerbrugge N, Pols HA, Birkenhager JC, Lamberts SW 1992 The effect of growth hormone administration in growth hormone-deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as body composition. Clin Endocrinol (Oxf) 37:79–87 [DOI] [PubMed] [Google Scholar]
- Whitehead HM, Boreham C, McIlrath EM, Sheridan B, Kennedy L, Atkinson AB, Hadden DR 1992 Growth hormone treatment of adults with growth hormone deficiency: results of a 13-month placebo-controlled cross-over study. Clin Endocrinol (Oxf) 36:45–52 [DOI] [PubMed] [Google Scholar]
- Amato G, Carella C, Fazio S, La Montagna G, Cittadini A, Sabatini D, Marciano-Mone C, Sacca L, Bellastella A 1993 Body composition, bone metabolism, and heart structure and function in growth hormone-deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab 77:1671–1676 [DOI] [PubMed] [Google Scholar]
- Vandeweghe M, Taelman P, Kaufman JM 1993 Short and long-term effects of growth hormone treatment on bone turnover and bone mineral content in adult growth hormone-deficient males. Clin Endocrinol (Oxf) 39:409–415 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arnao J, James I, Jabbar A, Trainer PJ, Perrett D, Besser GM, Ross RJ 1998 Serum collagen crosslinks as markers of bone turn-over during GH replacement therapy in growth hormone-deficient adults. Clin Endocrinol (Oxf) 48:455–462 [DOI] [PubMed] [Google Scholar]
- Bravenboer N, Holzmann P, de Boer H, Roos JC, van der Veen EA, Lips P 1997 The effect of growth hormone (GH) on histomorphometric indices of bone structure and bone turnover in GH-deficient men. J Clin Endocrinol Metab 82:1818–1822 [DOI] [PubMed] [Google Scholar]
- Finkenstedt G, Gasser RW, Hofle G, Watfah C, Fridrich L 1997 Effects of growth hormone (GH) replacement on bone metabolism and mineral density in adult onset of GH deficiency: results of a double-blind placebo-controlled study with open follow-up. Eur J Endocrinol 136:282–289 [DOI] [PubMed] [Google Scholar]
- Kotzmann H, Riedl M, Bernecker P, Clodi M, Kainberger F, Kaider A, Woloszczuk W, Luger A 1998 Effect of long-term growth hormone substitution therapy on bone mineral density and parameters of bone metabolism in adult patients with growth hormone deficiency. Calcif Tissue Int 62:40–46 [DOI] [PubMed] [Google Scholar]
- Davidson P, Milne R, Chase D, Cooper C 2004 Growth hormone replacement in adults and bone mineral density: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 60:92–98 [DOI] [PubMed] [Google Scholar]
- ter Maaten JC, de Boer H, Kamp O, Stuurman L, van der Veen EA 1999 Long-term effects of growth hormone (GH) replacement in men with childhood-onset GH deficiency. J Clin Endocrinol Metab 84:2373–2380 [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Hamdy NA, Pereira AM, Romijn JA, Roelfsema F 2004 Long-term skeletal effects of recombinant human growth hormone (rhGH) alone and rhGH combined with alendronate in GH-deficient adults: a seven-year follow-up study. Clin Endocrinol (Oxf) 60:568–575 [DOI] [PubMed] [Google Scholar]
- Clanget C, Seck T, Hinke V, Wuster C, Ziegler R, Pfeilschifter J 2001 Effects of 6 years of growth hormone (GH) treatment on bone mineral density in GH-deficient adults. Clin Endocrinol (Oxf) 55:93–99 [DOI] [PubMed] [Google Scholar]
- Gotherstrom G, Bengtsson BA, Bosaeus I, Johannsson G, Svensson J 2007 Ten-year GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. Eur J Endocrinol 156:55–64 [DOI] [PubMed] [Google Scholar]
- Johansson AG, Edén Engström B, Ljunghall S, Karlsson FA, Burman P 1999 Gender differences in the effects of long term growth hormone (GH) treatment on bone in adults with GH deficiency. J Clin Endocrinol Metab 84:2002–2007 [DOI] [PubMed] [Google Scholar]
- Bravenboer N, Holzmann PJ, ter Maaten JC, Stuurman LM, Roos JC, Lips P 2005 Effect of long-term growth hormone treatment on bone mass and bone metabolism in growth hormone-deficient men. J Bone Miner Res 20:1778–1784 [DOI] [PubMed] [Google Scholar]
- Valimaki MJ, Salmela PI, Salmi J, Viikari J, Kataja M, Turunen H, Soppi E 1999 Effects of 42 months of GH treatment on bone mineral density and bone turnover in GH-deficient adults. Eur J Endocrinol 140:545–554 [DOI] [PubMed] [Google Scholar]
- Holmes SJ, Whitehouse RW, Economou G, O'Halloran DJ, Adams JE, Shalet SM 1995 Further increase in forearm cortical bone mineral content after discontinuation of growth hormone replacement. Clin Endocrinol (Oxf) 42:3–7 [DOI] [PubMed] [Google Scholar]
- Biller BMK, Sesmilo G, Baum HBA, Hayden D, Schoenfeld D, Klibanski A 2000 Withdrawal of long-term physiological growth hormone (GH) administration: differential effects on bone density and body composition in men with adult-onset GH deficiency. J Clin Endocrinol Metab 85:970–976 [DOI] [PubMed] [Google Scholar]
- Valk NK, Erdtsieck RJ, Algra D, Lamberts SW, Pols HA 1995 Combined treatment of growth hormone and the bisphosphonate pamidronate, versus treatment with GH alone, in GH-deficient adults: the effects on renal phosphate handling, bone turnover and bone mineral mass. Clin Endocrinol (Oxf) 43:317–324 [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Hamdy NA, Janssen YJ, Roelfsema F 2001 Additional beneficial effects of alendronate in growth hormone (GH)-deficient adults with osteoporosis receiving long-term recombinant human GH replacement therapy: a randomized controlled trial. J Clin Endocrinol Metab 86:3079–3085 [DOI] [PubMed] [Google Scholar]
- White HD, Ahmad AM, Syed AA, Clewes A, Peter R, Vora JP, Fraser WD 2004 Gender variation in PTH sensitivity and rhythmicity following growth hormone replacement in adult growth hormone-deficient patients. Clin Endocrinol (Oxf) 60:516–526 [DOI] [PubMed] [Google Scholar]
- Hubina E, Kovacs L, Szabolcs I, Szucs N, Toth M, Racz K, Czirjak S, Gorombey Z, Goth MI 2004 The effect of gender and age on growth hormone replacement in growth hormone-deficient patients. Horm Metab Res 36:247–253 [DOI] [PubMed] [Google Scholar]
- Burman P, Johansson AG, Siegbahn A, Vessby B, Karlsson FA 1997 Growth hormone (GH)-deficient men are more responsive to GH replacement therapy than women. J Clin Endocrinol Metab 82:550–555 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Biller BM, Zagar A, Jackson I, Arafah BM, Nippoldt TB, Cook DM, Mooradian AD, Kwan A, Scism-Bacon J, Chipman JJ, Hartman ML 2007 Effect of growth hormone replacement on BMD in adult-onset growth hormone deficiency. J Bone Miner Res 22:762–770 [DOI] [PubMed] [Google Scholar]
- Giustina A, Licini M, Bussi AR, Girelli A, Pizzocolo G, Schettino M, Negro-Vilar A 1993 Effects of sex and age on the growth hormone response to galanin in healthy human subjects. J Clin Endocrinology Metab 76:1369–1372. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD 1996 Gender differences in secretory activity of the human somatotropic (growth hormone) axis. Eur J Endocrinol 134:287–295 [DOI] [PubMed] [Google Scholar]
- Giustina A, Scalvini T, Tassi C, Desenzani P, Poiesi C, Wehrenberg WB, Rogol AD, Veldhuis JD 1997 Maturation of the regulation of growth hormone secretion in young males with hypogonadotropic hypogonadism pharmacologically exposed to progressive increments in serum testosterone. J Clin Endocrinol Metab 82:1210–1219 [DOI] [PubMed] [Google Scholar]
- Holmer H, Svensson J, Rylander L, Johannsson G, Rosén T, Bengtsson BA, Thorén M, Höybye C, Degerblad M, Bramnert M, Hägg E, Engström BE, Ekman B, Thorngren KG, Hagmar L, Erfurth EM 2007 Fracture incidence in GH-deficient patients on complete hormone replacement including GH. J Bone Miner Res 22:1842–1850 [DOI] [PubMed] [Google Scholar]
- Baum HB, Biller BM, Finkelstein JS, Cannistraro KB, Oppenhein DS, Schoenfeld DA, Michel TH, Wittink H, Klibanski A 1996 Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset of growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med 125:883–890 [DOI] [PubMed] [Google Scholar]
- Balducci R, Toscano V, Pasquino AM, Mangiantini A, Municchi G, Armenise P, Terracina S, Prossomariti G, Boscherini B 1995 Bone turnover and bone mineral density in young adult patients with panhypopituitarism before and after long-term growth hormone therapy. Eur J Endocrinol 132:42–46 [DOI] [PubMed] [Google Scholar]
- Underwood LE, Attie KM, Baptista J; Genentech Collaborative Study Group 2003 Growth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: a two-year, multicenter, multiple-dose, placebo-controlled study. J Clin Endocrinol Metab 88:5273–5280 [DOI] [PubMed] [Google Scholar]
- Boguszewski CL, Meister LH, Zaninelli DC, Radominski RB 2005 One year of GH replacement therapy with a fixed low-dose regimen improves body composition, bone mineral density and lipid profile of GH-deficient adults. Eur J Endocrinol 152:67–75 [DOI] [PubMed] [Google Scholar]
- Gaillard R-C, Giusti V 2004 Dosing and individual responsiveness to growth hormone replacement therapy. In: Abs R, Feldt-Rasmussen U, eds. Growth hormone deficiency in adults: 10 years of KIMS. Edition 1, Chap 10. Oxford, UK: Oxford Pharmagenesis Ltd; 103–116 [Google Scholar]
- Amato G, Izzo G, La Montagna G, Bellastella A 1996 Low dose recombinant human growth hormone normalizes bone metabolism and cortical bone density and improves trabecular bone density in growth hormone-deficient adults without causing adverse effects. Clin Endocrinol (Oxf) 45:27–32 [PubMed] [Google Scholar]
- Colson A, Brooke AM, Walker D, Besser GM, Chew SL, Grossman AB, Jenkins PJ, Drake WM, Monson JP 2006 Growth hormone deficiency and replacement in patients with treated Cushing’s disease, prolactinomas and non-functioning pituitary adenomas: effects on body composition, glucose metabolism, lipid status and bone mineral density. Horm Res 66:257–267 [DOI] [PubMed] [Google Scholar]
- Jalava T, Sarna S, Pylkkanen L, Mawer B, Kanis JA, Selby P, Davies M, Adams J, Francis RM, Robinson J, McCloskey E 2003 Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 18:1254–1260 [DOI] [PubMed] [Google Scholar]
- Siris ES, Genant HK, Laster AJ, Chen P, Misurski DA, Krege JH 2007 Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int 18:761–770 [DOI] [PubMed] [Google Scholar]
- Clayton PE, Cuneo RC, Juul A, Monson JP, Shalet SM, Tauber M 2005 Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol 152:165–170 [DOI] [PubMed] [Google Scholar]
- Melmed S 2006 Medical progress: acromegaly. N Engl J Med 355:2558–2573 [DOI] [PubMed] [Google Scholar]
- Holdaway IM, Rajasoorya C 1999 Epidemiology of acromegaly. Pituitary 2:29–41 [DOI] [PubMed] [Google Scholar]
- Giustina A, Casanueva FF, Cavagnini F, Chanson P, Clemmons D, Frohman LA, Gaillard R, Ho K, Jaquet P, Kleinberg DL, Lamberts SW, Lombardi G, Sheppard M, Strasburger CJ, Vance ML, Wass JA, Melmed S; The Pituitary Society and the European Neuroendocrine Association 2003 Diagnosis and treatment of acromegaly complications. J Endocrinol Invest 26:1242–1247 [DOI] [PubMed] [Google Scholar]
- Colao A, Ferone D, Marzullo P, Lombardi G 2004 Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 25:102–152 [DOI] [PubMed] [Google Scholar]
- Maison P, Tropeano AI, Macquin-Mavier I, Giustina A, Chanson P 2007 Impact of somatostatin analogs on the heart in acromegaly: a metaanalysis. J Clin Endocrinol Metab 92:1743–1747 [DOI] [PubMed] [Google Scholar]
- Aloja JF, Roginsky MS, Jowsey J, Dombrowski CS, Shukla KK, Cohn SH 1972 Skeletal metabolism and body composition in acromegaly. J Clin Endocrinol Metab 35:543–551 [DOI] [PubMed] [Google Scholar]
- Halse J, Melsen F, Mosekilde L 1981 Iliac crest bone mass and remodelling in acromegaly. Acta Endocrinol 97:18–22 [DOI] [PubMed] [Google Scholar]
- Halse J, Gordeladze JO 1981 Total and non-dialyzable urinary hydroxyproline in acromegalics and control subjects. Acta Endocrinol 96:451–457 [DOI] [PubMed] [Google Scholar]
- de la Piedra C, Larranaga J, Castro N, Horcajada C, Rapado A, Herrera Pombo JL, Carbo E 1988 Correlation among osteocalcin, growth hormone, and somatomedin C in acromegaly. Calcif Tissue Int 43:44–45 [DOI] [PubMed] [Google Scholar]
- Ezzat S, Melmed S, Endres D, Eyre DR, Singer FR 1993 Biochemical assessment of bone formation and resorption in acromegaly. J Clin Endocrinol Metab 76:1452–1457 [DOI] [PubMed] [Google Scholar]
- Halse J, Gordeladze JO 1978 Urinary hydroxyproline excretion in acromegaly. Acta Endocrinol 89:483–491 [DOI] [PubMed] [Google Scholar]
- Kotzmann H, Bernecker P, Hubsch P, Pietschmann P, Woloszczuk W, Svoboda T, Geyer G, Luger A 1993 Bone mineral density and parameters of bone metabolism in patients with acromegaly. J Bone Miner Res 8:459–465 [DOI] [PubMed] [Google Scholar]
- Kayath MJ, Vieira JG 1997 Osteopenia occurs in a minority of patients with acromegaly and is predominant in the spine. Osteoporos Int 7:226–230 [DOI] [PubMed] [Google Scholar]
- Bolanowski M, Daroszewski J, Medras M, Zadrozna-Sliwka B 2006 Bone mineral density and turnover in patients with acromegaly in relation to sex, disease activity, and gonadal function. J Bone Miner Metab 24:72–78 [DOI] [PubMed] [Google Scholar]
- Scillitani A, Chiodini I, Carnevale V, Giannatempo GM, Frusciante V, Villella M, Pileri M, Guglielmi G, Di Giorgio A, Modoni S, Fusilli S, Di Cerbo A, Liuzzi A 1997 Skeletal involvement in female acromegalic subjects: the effects of growth hormone excess in amenorrheal and mestruating patients. J Bone Miner Res 12:1729–1736 [DOI] [PubMed] [Google Scholar]
- Ueland T, Fougner SL, Godang K, Schreiner T, Bollerslev J 2006 Serum GH and IGF-I are significant determinants of bone turnover but not bone mineral density in active acromegaly: a prospective study of more than 70 consecutive patients. Eur J Endocrinol 155:709–715 [DOI] [PubMed] [Google Scholar]
- Halse J, Haugen HN 1980 Calcium and phosphate metabolism in acromegaly. Acta Endocrinol 94:459–467 [DOI] [PubMed] [Google Scholar]
- White HD, Ahmad AM, Durham BH, Chandran S, Patwala A, Fraser WD, Vora JP 2006 Effect of active acromegaly and its treatment on parathyroid circadian rhythmicity and parathyroid target-organ sensitivity. J Clin Endocrinol Metab 91:913–919 [DOI] [PubMed] [Google Scholar]
- Mazziotti G, Cimino V, De Menis E, Bonadonna S, Bugari G, De Marinis L, Veldhuis JD, Giustina A 2006 Active acromegaly enhances spontaneous parathyroid hormone pulsatility. Metabolism 55:736–740 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Randall RV, Wahner HW, Jowsey J, Kelly PJ, Singh M 1972 The nature of the metabolic bone disorder in acromegaly. J Clin Endocrinol Metab 34:911–918 [DOI] [PubMed] [Google Scholar]
- Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL 1982 Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J Clin Invest 69:1302–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond T, Nery L, Posen S 1989 Spinal and peripheral bone mineral densities in acromegaly: the effects of excess growth hormone and hypogonadism. Ann Intern Med 111:567–573 [DOI] [PubMed] [Google Scholar]
- Lesse GP, Fraser WD, Farquharson R, Hipkin L, Vora JP 1998 Gonadal status is an important determinant of bone density in acromegaly. Clin Endocrinol (Oxf) 48:59–65 [DOI] [PubMed] [Google Scholar]
- Chiodini I, Trischitta V, Carnevale V, Liuzzi A, Scillitani A 2001 Bone mineral density in acromegaly: does growth hormone excess protect against osteoporosis? J Endocrinol Invest 24:288–291 [DOI] [PubMed] [Google Scholar]
- Longobardi S, Di Somma C, Di Rella F, Angelillo N, Ferone D, Colao A, Merola B, Lombardi G 1998 Bone mineral density and circulating cytokines in patients with acromegaly. J Endocrinol Invest 21:688–693 [DOI] [PubMed] [Google Scholar]
- Ueland T, Ebbesen EN, Thomsen JS, Mosekilde L, Brixen K, Flyvbjerg A, Bollerslev J 2002 Decreased trabecular bone biomechanical competence, apparent density, IGF-II and IGFBP-5 content in acromegaly. Eur J Clin Invest 32:122–128 [DOI] [PubMed] [Google Scholar]
- Tutuncu NB, Erbas T 2004 Factors associated with bone metabolism in acromegalic patients: hypogonadism and female gender. Exp Clin Endocrinol Diabetes 112:328–332 [DOI] [PubMed] [Google Scholar]
- Scillitani A, Battista C, Chiodini I, Carnevale V, Fusilli S, Ciccarelli E, Terzolo M, Oppizzi G, Arosio M, Gasperi M, Arnaldi G, Colao A, Baldelli R, Ghiggi MR, Gaia D, Di Somma C, Trischitta V, Liuzzi A 2003 Bone mineral density in acromegaly: the effect of gender, disease activity and gonadal status. Clin Endocrinol (Oxf) 58:725–731 [DOI] [PubMed] [Google Scholar]
- Zgliczynski W, Kochman M, Misiorowski W, Zdunowski P 2007 In acromegaly, increased bone mineral density (BMD) is determined by GH excess, gonadal function and gender. Neuro Endocrinol Lett 28:621–628 [PubMed] [Google Scholar]
- Vestergaard P, Mosekilde L 2004 Fracture risk is decreased in acromegaly—a potential beneficial effect of growth hormone. Osteoporos Int 15:155–159 [DOI] [PubMed] [Google Scholar]
- Lowenthal MN, Galinsky D, Fried V, Castel H, Shany S, Gazit D 1990 Acromegaly and proximal femoral fracture in an aged person: bone histomorphometry, vitamin D metabolities and parathormone. Isr J Med Sci 26:280–283 [PubMed] [Google Scholar]
- Marazuela M, Astigarraga B, Tabuenca MJ, Estrada J, Marin F, Lucas T 1993 Serum bone Gla protein as a marker of bone turnover in acromegaly. Calcif Tissue Int 52:419–421 [DOI] [PubMed] [Google Scholar]
- Terzolo M, Piovesan A, Osella G, Pia A, Reimondo G, Pozzi C, Raucci C, Torta M, Paccotti P, Angeli A 1993 Serum levels of bone Gla protein (osteocalcin BGP) and carboxyterminal propeptide of type I procollagen (PICP) in acromegaly: effects of long-term octreotide treatment. Calcif Tissue Int 52:188–191 [DOI] [PubMed] [Google Scholar]
- Legovini P, De Menis E, Breda F, Billeci D, Carteri A, Pavan P, Conte N 1997 Long-term effects of octreotide on markers of bone metabolism in acromegaly: evidence of increased serum parathormone concentrations. J Endocrinol Invest 20:434–438 [DOI] [PubMed] [Google Scholar]
- Piovesan A, Terzolo M, Reimondo G, Pia A, Codegone A, Osella G, Boccuzzi A, Paccotti P, Angeli A 1994 Biochemical markers of bone and collagen turnover in acromegaly or Cushing’s syndrome. Horm Metab Res 26:234–237 [DOI] [PubMed] [Google Scholar]
- Takamoto S, Tsuchiya H, Onishi T, Morimoto S, Imanaka S, Mori S, Seino Y, Uozumi T, Kumahara Y 1985 Changes in calcium homeostasis in acromegaly treated by pituitary adenomectomy. J Clin Endocrinol Metab 61:7–11 [DOI] [PubMed] [Google Scholar]
- Fredstorp L, Pernow Y, Werner S 1993 The short and long-term effects of octreotide on calcium homeostasis in patients with acromegaly. Clin Endocrinol (Oxf) 39:331–336 [DOI] [PubMed] [Google Scholar]
- Parkinson C, Kassem M, Heickendorff L, Flyvbjerg A, Trainer PJ 2003 Pegvisomant-induced serum insulin-like growth factor-I normalization in patients with acromegaly returns elevated markers of bone turnover to normal. J Clin Endocrinol Metab 88:5650–5655 [DOI] [PubMed] [Google Scholar]
- Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S 2000 Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 85:526–529 [DOI] [PubMed] [Google Scholar]
- Fairfield WP, Sesmilo G, Katznelson L, Pulaski K, Freda PU, Stavrou S, Kleinberg D, Klibanski A 2002 Effects of a growth hormone receptor antagonist on bone markers in acromegaly. Clin Endocrinol (Oxf) 57:385–390 [DOI] [PubMed] [Google Scholar]
- Melton III LJ, Chrischilles EA, Cooper C, Lane AW, Riggs BL 1992 Perspective. How many women have osteoporosis? J Bone Miner Res 7:1005–1010 [DOI] [PubMed] [Google Scholar]
- Albright F, Smith PH, Richardson AM 1941 Postmenopausal osteoporosis. JAMA 116:2465–2474 [Google Scholar]
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Wiske PS, Epstein S, Bell NH, Queener SF, Edmondson J, Johnston CC 1979 Increases in immunoreactive parathyroid hormone with age. N Engl J Med 300:1419–1421 [DOI] [PubMed] [Google Scholar]
- Ueland T 2004 Bone metabolism in relation to alterations in systemic growth hormone. Growth Horm IGF Res 14:404–417 [DOI] [PubMed] [Google Scholar]
- Dennison EM, Hindmarsh PC, Kellingray S, Fall CH, Cooper C 2003 Growth hormone predicts bone density in elderly women. Bone 32:434–440 [DOI] [PubMed] [Google Scholar]
- Dennison EM, Syddall HE, Rodriguez S, Voropanov A, Day IN, Cooper C; Southampton Genetic Epidemiology Research Group 2004 Polymorphism in the growth hormone gene, weight in infancy, and adult bone mass. J Clin Endocrinol Metab 89:4898–4903 [DOI] [PubMed] [Google Scholar]
- Patel MB, Arden NK, Masterson LM, Phillips DI, Swaminathan R, Syddall HE, Byrne CD, Wood PJ, Cooper C, Holt RI; Hertfordshire Cohort Study Group 2005 Investigating the role of the growth hormone-insulin-like growth factor (GH-IGF) axis as a determinant of male bone mineral density (BMD). Bone 37:833–841 [DOI] [PubMed] [Google Scholar]
- Zhao HY, Liu JM, Ning G, Zhao YJ, Chen Y, Sun LH, Zhang LZ, Xu MY, Chen JL 2008 Relationships between insulin-like growth factor-I (IGF-I) and OPG, RANKL, bone mineral density in healthy Chinese women. Osteoporos Int 19:221–226 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Nishiyama K, Kuribayashi F, Chihara K 1997 Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res 12:1272–1279 [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP 1998 Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J Clin Endocrinol Metab 83:4257–4262 [DOI] [PubMed] [Google Scholar]
- Rivadeneira F, Houwing-Duistermaat JJ, Vaessen N, Vergeer-Drop JM, Hofman A, Pols HA, Van Duijn CM, Uitterlinden AG 2003 Association between an insulin-like growth factor I gene promoter polymorphism and bone mineral density in the elderly: the Rotterdam Study. J Clin Endocrinol Metab 88:3878–3884 [DOI] [PubMed] [Google Scholar]
- Salminen H, Sääf M, Ringertz H, Strender LE 2008 The role of IGF-I and IGFBP-1 status and secondary hyperparathyroidism in relation to osteoporosis in elderly Swedish women. Osteoporos Int 19:201–209 [DOI] [PubMed] [Google Scholar]
- Canalis E 1994 Skeletal growth factors and aging. J Clin Endocrinol Metab 78:1009–1010 [DOI] [PubMed] [Google Scholar]
- Brixen K, Nielsen HK, Mosekilde L, Flyvbjerg A 1990 A short course of recombinant human growth hormone treatment stimulates osteoblasts and activates bone remodeling in normal human volunteers. J Bone Miner Res 5:609–618 [DOI] [PubMed] [Google Scholar]
- Kassem M, Brixen K, Blum WF, Mosekilde L, Eriksen EF 1994 Normal osteoclastic and osteoblastic responses to exogenous growth hormone in patients with postmenopausal spinal osteoporosis. J Bone Miner Res 9:1365–1370 [DOI] [PubMed] [Google Scholar]
- Brixen K, Kassem M, Nielsen HK, Loft AG, Flyvbjerg A, Mosekilde L 1995 Short-term treatment with growth hormone stimulates osteoblastic and osteoclastic activity in osteopenic postmenopausal women: a dose response study. J Bone Miner Res 10:1865–1874 [DOI] [PubMed] [Google Scholar]
- Johansson AG, Lindh E, Blum WF, Kollerup G, Sorensen OH, Ljunghall S 1996 Effects of growth hormone and insulin-like growth factor I in men with idiopathic osteoporosis. J Clin Endocrinol Metab 81:44–48 [DOI] [PubMed] [Google Scholar]
- Bianda T, Hussain MA, Glatz Y, Bouillon R, Froesch ER, Schmid C 1997 Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man. J Intern Med 241:143–150 [DOI] [PubMed] [Google Scholar]
- Kassem M, Brixen K, Mosekilde L, Blum WF, Flyvbjerg A 1998 Effects of growth hormone treatment on serum levels of insulin-like growth factors (IGFs) and IGF binding proteins 1–4 in postmenopausal women. Clin Endocrinol (Oxf) 49:747–756 [DOI] [PubMed] [Google Scholar]
- Joseph F, Ahmad A, Ul-Haq M, Durham B, Whittingham P, Fraser W, Vora J 2008 Effects of growth hormone administration on bone mineral metabolism, parathyroid hormone sensitivity and parathyroid hormone secretory rhythm in postmenopausal women with established osteoporosis. J Bone Miner Res 23:721–729 [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE 1990 Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Nakaoka D, Nasu M, Kanzawa M, Sugishita T, Chihara K 1999 Effect of recombinant human growth hormone in elderly osteoporotic women. Clin Endocrinol (Oxf) 51:715–724 [DOI] [PubMed] [Google Scholar]
- Saaf M, Hilding A, Thoren M, Troell S, Hall K 1999 Growth hormone treatment of osteoporotic postmenopausal women—a one-year placebo-controlled study. Eur J Endocrinol 140:390–399 [DOI] [PubMed] [Google Scholar]
- Gillberg P, Mallmin H, Petren-Mallmin M, Ljunghall S, Nilsson AG 2002 Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. J Clin Endocrinol Metab 87:4900–4906 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Kaji H, Nakaoka D, Yamauchi M, Yano S, Sugishita T, Baylink DJ, Mohan S, Chihara K 2002 Effect of low-dose of recombinant human growth hormone on bone metabolism in elderly women with osteoporosis. Eur J Endocrinol 147:339–348 [DOI] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Nilsson A, Bosaeus I, Bengtsson BA 2003 Growth hormone increases bone mineral content in postmenopausal osteoporosis: a randomized placebo-controlled trial. J Bone Miner Res 18:393–405 [DOI] [PubMed] [Google Scholar]
- Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR 2007 Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med 146:104–115 [DOI] [PubMed] [Google Scholar]
- Murphy MG, Weiss S, McClung M, Schnitzer T, Cerchio K, Connor J, Krupa D, Gertz BJ; MK-677/Alendronate Study Group 2001 Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab 86:1116–1125 [DOI] [PubMed] [Google Scholar]
- Johansson AG, Lindh E, Ljunghall S 1992 Insulin-like growth factor I stimulates bone turnover in osteoporosis. Lancet 339:1619 [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Jones JD, O'Fallon WM, Janes CH, Riggs BL 1993 Short-term effects of recombinant human insulin-like growth factor I on bone turnover in normal women. J Clin Endocrinol Metab 77:1384–1387 [DOI] [PubMed] [Google Scholar]
- Rubin CD, Reed B, Sakhaee K, Pak CY 1994 Treating a patient with the Werner syndrome and osteoporosis using recombinant human insulin-like growth factor. Ann Intern Med 121:665–668 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A 1996 Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab 81:3864–3870 [DOI] [PubMed] [Google Scholar]
- Mauras N, Doi SQ, Shapiro JR 1996 Recombinant human insulin-like growth factor I, recombinant human growth hormone, and sex steroids: effects on markers of bone turnover in humans. J Clin Endocrinol Metab 81:2222–2226 [DOI] [PubMed] [Google Scholar]
- Boonen S, Rosen C, Bouillon R, Sommer A, McKay M, Rosen D, Adams S, Broos P, Lenaerts J, Raus J, Vanderschueren D, Geusens P 2002 Musculoskeletal effects of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with proximal femoral fracture: a double-blind, placebo-controlled pilot study. J Clin Endocrinol Metab 87:1593–1599 [DOI] [PubMed] [Google Scholar]
- DSM IV 1994 Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association [Google Scholar]
- Hsu LK 1996 Epidemiology of the eating disorders. Psychiatr Clin North Am 19:681–760 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A 2000 Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med 133:790–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Grinspoon S, Ciampa J, Hier J, Herzog D, Klibanski A 2005 Medical findings in outpatients with anorexia nervosa. Arch Intern Med 165:561–566 [DOI] [PubMed] [Google Scholar]
- Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, Gleysteen S, Mickley D, Herzog D, Klibanski A 2006 Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab 91:2931–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Klibanski A 2006 Anorexia nervosa and osteoporosis. Rev Endocr Metab Disord 7:91–99 [DOI] [PubMed] [Google Scholar]
- Lucas AR, Melton 3rd LJ, Crowson CS, O'Fallon WM 1999 Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc 74:972–977 [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K 2002 Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders—a nationwide register study. Int J Eat Disord 32:301–308 [DOI] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Sato K, Demura H 1998 The importance of body weight history in the occurrence and recovery of osteoporosis in patients with anorexia nervosa; evaluation by dual x-ray absorptiometry and bone metabolic markers. Eur J Endocrinol 139:276–283 [DOI] [PubMed] [Google Scholar]
- Galusca B, Bossu C, Germain N, Kadem M, Frere D, Lafage-Proust MH, Lang F, Estour B 2006 Age-related differences in hormonal and nutritional impact on lean anorexia nervosa bone turnover uncoupling. Osteoporos Int 17:888–896 [DOI] [PubMed] [Google Scholar]
- Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A 1989 Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 68:548–554 [DOI] [PubMed] [Google Scholar]
- Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R 1990 Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics 86:440–447 [PubMed] [Google Scholar]
- Riggotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR 1991 The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA 265:1133–1138 [PubMed] [Google Scholar]
- Hay PJ, Delahunt JW, Hall A, Mitchell AW, Harper G, Salmond C 1992 Predictors of osteopenia in premenopausal women with anorexia nervosa. Calcif Tissue Int 50:498–501 [DOI] [PubMed] [Google Scholar]
- Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A 2002 Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 87:4177–4185 [DOI] [PubMed] [Google Scholar]
- Munoz MT, Argente J 2002 Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances. Eur J Endocrinol 147:275–286 [DOI] [PubMed] [Google Scholar]
- Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, Herzog DB, Klibanski A 2004 Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab 89:4434–4438 [DOI] [PubMed] [Google Scholar]
- Cachelin FM, Maher BA 1998 Is amenorrhea a critical criterion for anorexia nervosa? J Psychosom Res 44:435–440 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A 1999 Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab 84:2049–2055 [DOI] [PubMed] [Google Scholar]
- Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC 1995 The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 80:898–904 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A 2002 Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 87:2883–2891 [DOI] [PubMed] [Google Scholar]
- Masuda A, Shibasaki T, Hotta M, Suematsu H, Shizume K 1988 Study on the mechanism of abnormal growth hormone (GH) secretion in anorexia nervosa: no evidence of involvement of a low somatomedin-C level in the abnormal GH secretion. J Endocrinol Invest 11:297–302 [DOI] [PubMed] [Google Scholar]
- Brambilla F, Ferrari E, Cavagnini F, Invitti C, Zanoboni A, Massironi R, Catalano M, Cocchi D, Muller EE 1989 α2-Adrenoceptor sensitivity in anorexia nervosa: GH response to clonidine or GHRH stimulation. Biol Psychiatry. 25:256–264 [DOI] [PubMed] [Google Scholar]
- Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GBJ 1992 The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab 75:762–767 [DOI] [PubMed] [Google Scholar]
- Golden NH, Kreitzer P, Jacobson MS, Chasalow FI, Schebendach J, Freedman SM, Shenker IR 1994 Disturbances in growth hormone secretion and action in adolescents with anorexia nervosa. J Pediatr 125:655–660 [DOI] [PubMed] [Google Scholar]
- Scacchi M, Pincelli AI, Caumo A, Tomasi P, Delitala G, Baldi G, Cavagnini F 1997 Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J Clin Endocrinol Metab 82:3225–3229 [DOI] [PubMed] [Google Scholar]
- Argente J, Caballo N, Barrios V, Munoz MT, Pozo J, Chowen JA, Morande G, Hernandez M 1997 Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab 82:2084–2092 [DOI] [PubMed] [Google Scholar]
- De Marinis L, Mancini A, Valle D, Fiumara C, Conte G, Bianchi A, Perrelli M, Gentilella R, Giustina A 1997 Physiological role of the opioid-cholinergic interaction in growth hormone neuroregulation: effect of sex and food intake. Metabolism 46:740–744 [DOI] [PubMed] [Google Scholar]
- Stoving RK, Flyvbjerg A, Frystyk J, Fisker S, Hangaard J, Hansen-Nord M, Hagen C 1999 Low serum levels of free and total insulin-like growth factor I (IGF-I) in patients with anorexia nervosa are not associated with increased IGF-binding protein-3 proteolysis. J Clin Endocrinol Metab 84:1346–1350 [DOI] [PubMed] [Google Scholar]
- De Marinis L, Mancini A, Valle D, Bianchi A, Gentilella R, Milardi D, Mascadri C, Giustina A 2000 Effects of galanin on growth hormone and prolactin secretion in anorexia nervosa. Metabolism 49:155–159 [DOI] [PubMed] [Google Scholar]
- De Menis E, Gola M, Giustina A 2006 Development of acromegaly in a patient with anorexia nervosa: pathogenetic and diagnostic implications. J Endocrinol Invest 29:821–825 [DOI] [PubMed] [Google Scholar]
- Stoving RK, Chen JW, Glintborg D, Brixen K, Flyvbjerg A, Horder K, Frystyk J 2007 Bioactive IGF-I and IGF-binding protein-1 in anorexia nervosa. J Clin Endocrinol Metab 92:2323–2329 [DOI] [PubMed] [Google Scholar]
- Dieguez C, Page MD, Scanlon MF 1989 Growth hormone neuroregulation and its alterations in disease states. Clin Endocrinol (Oxf) 28:109–121 [DOI] [PubMed] [Google Scholar]
- Tamai HT, Komaki G, Matsubayashi S, Kobayashi N, Mori K, Nakagawa T, Truong MP, Walter Jr RM, Kumagai LF 1990 Effect of cholinergic muscarinic receptor blockade on human growth hormone (GH)-releasing hormone-(1–44)-induced-GH secretion in anorexia nervosa. J Clin Endocrinol Metab 70:738–741 [DOI] [PubMed] [Google Scholar]
- Rolla M, Andreoni A, Belliti D, Cristofani R, Ferdeghini M, Muller EE 1991 Blockade of cholinergic muscarinic receptors by pirenzepine and GHRH induced GH secretion in the acute and recovery phase of anorexia nervosa and atypical eating disorders. Biol Psychiatry 29:1079–1091 [DOI] [PubMed] [Google Scholar]
- Ghigo E, Arvat E, Gianotti L, Nicolosi M, Valetto MR, Avagnina S, Bellitti D, Rolla M, Muller EE, Camanni F 1994 Arginine but not pyridostigmine, a cholinesterase inhibitor, enhances the GHRH-induced GH rise in patients with anorexia nervosa. Biol Psychiatry 36:689–695 [DOI] [PubMed] [Google Scholar]
- Gianotti L, Arvat E, Valetto MR, Ramunni J, Di Vito L, Maccagno B, Camanni F, Ghigo E 1998 Effects of β-adrenergic agonists and antagonists on the growth hormone response to growth hormone-releasing hormone in anorexia nervosa. Biol Psychiatry 43:181–187 [DOI] [PubMed] [Google Scholar]
- Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C 1999 Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab 84:2056–2063 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR 1999 Leptin, anorexia nervosa, and anorexia of acute and chronic disease. Nutrition 15:943–945 [DOI] [PubMed] [Google Scholar]
- Rappaport R, Prevot C, Czernichow P 1980 Somatomedin activity and growth hormone secretion. I. Changes related to body weight in anorexia nervosa. Acta Paediatr Scand 69:37–41 [DOI] [PubMed] [Google Scholar]
- Unterman TG, Vazquez RM, Slas AJ, Martyn PA, Phillips LS 1985 Nutrition and somatmedin. XIII. Usefulness of somatomedin-C in nutritional assessment. Am J Med 78:228–234 [DOI] [PubMed] [Google Scholar]
- Soliman AT, Hassan AE, Aref MK, Hintz RL, Rosenfeld RG, Rogol AD 1986 Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr Res 20:1122–1130 [DOI] [PubMed] [Google Scholar]
- Donahue SP, Phillips LS 1989 Response of IGF-I to nutritional support in malnourished hospital patients: a possible indicator of short-term changes in nutritional status. Am J Clin Nutr 50:962–969 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Snyder DK, Busby Jr WH 1991 Variables controlling the secretion of insulin-like growth factor binding protein-2 in normal human subjects. J Clin Endocrinol Metab 73:727–733 [DOI] [PubMed] [Google Scholar]
- Smith WJ, Underwood LE, Clemmons DR 1995 Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab 80:443–449 [DOI] [PubMed] [Google Scholar]
- Newman MM, Halmi KA 1988 The endocrinology of anorexia nervosa and bulimia nervosa. Neurol Clin 6:195–212 [PubMed] [Google Scholar]
- Hochberg Z, Hertz P, Colin V, Ish-Shalom S, Yeshurun D, Youdim MB, Amit T 1992 The distal axis of growth hormone (GH) in nutritional disorders: GH-binding protein, insulin-like growth factor-I (IGF-I), and IGF-I receptors in obesity and anorexia nervosa. Metabolism 41:106–112 [DOI] [PubMed] [Google Scholar]
- Muller EE, Locatelli V 1992 Undernutrition and pituitary function: relevance to the pathophysiology of some neuroendocrine alterations of anorexia nervosa. J Endocrinol 132:327–329 [DOI] [PubMed] [Google Scholar]
- Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K 2000 The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab 85:200–206 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog DB, Johnson ML, Klibanski A 2003 Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 88:5615–5623 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Miller K, Herzog D, Clemmons D, Klibanski A 2003 Effects of recombinant human insulin-like growth factor (IGF)-I and estrogen administration on IGF-I, IGF binding protein (IGFBP)-2, and IGFBP-3 in anorexia nervosa: a randomized-controlled study. J Clin Endocrinol Metab 88:1142–1149 [DOI] [PubMed] [Google Scholar]
- Heer M, Mika C, Grzella I, Heussen N, Herpertz-Dahlmann B 2004 Bone turnover during inpatient nutritional therapy and outpatient follow-up in patients with anorexia nervosa compared with that in healthy control subjects. Am J Clin Nutr 80:774–781 [DOI] [PubMed] [Google Scholar]
- Compston JE, McConachie C, Stott C, Hannon RA, Kaptoge S, Debiram I, Love S, Jaffa A 2006 Changes in bone mineral density, body composition and biochemical markers of bone turnover during weight gain in adolescents with severe anorexia nervosa: a 1-year prospective study. Osteoporos Int 17:77–84 [DOI] [PubMed] [Google Scholar]
- Viapiana O, Gatti D, Dalle Grave R, Todesco T, Rossini M, Braga V, Idolazzi L, Fracassi E, Adami S 2007 Marked increases in bone mineral density and biochemical markers of bone turnover in patients with anorexia nervosa gaining weight. Bone 40:1073–1077 [DOI] [PubMed] [Google Scholar]
- Manelli F, Carpinteri R, Bossoni S, Burattin A, Bonadonna S, Agabiti Rosei E, Giustina A 2002 Growth hormone in glucocorticoid-induced osteoporosis. Front Horm Res 30:174–183 [DOI] [PubMed] [Google Scholar]
- Giustina A, Girelli A, Doga M, Bodini C, Bossoni S, Romanelli G, Wehrenberg WB 1990 Pyridostigmine blocks the inhibitory effect of glucocorticoids on growth hormone releasing hormone stimulated growth hormone secretion in normal man. J Clin Endocrinol Metab 71:580–584 [DOI] [PubMed] [Google Scholar]
- Giustina A, Bossoni S, Bodini C, Girelli A, Balestrieri GP, Pizzocolo G, Wehrenberg WB 1992 Arginine normalizes the growth hormone (GH) response to GH-releasing hormone in adult patients receiving chronic daily immunosuppressive glucocorticoid therapy. J Clin Endocrinol Metab 74:1301–1305 [DOI] [PubMed] [Google Scholar]
- Terzolo M, Bossoni S, Ali A, Doga M, Reimondo G, Milani G, Peretti P, Manelli F, Angeli A, Giustina A 2000 Growth hormone (GH) responses to GH-releasing hormone alone or combined with arginine in patients with adrenal incidentaloma: evidence for enhanced somatostatinergic tone. J Clin Endocrinol Metab 85:1310–1315 [DOI] [PubMed] [Google Scholar]
- Malerba M, Bossoni S, Radaeli A, Mori E, Bonadonna S, Giustina A, Tantucci C 2005 Growth hormone response to growth hormone-releasing hormone is reduced in adult asthmatic patients receiving long-term inhaled corticosteroid treatment. Chest 127:515–521 [DOI] [PubMed] [Google Scholar]
- Jux C, Leiber K, Hugel U, Blum W, Ohlsson C, Klaus G, Mehls O 1998 Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-I production and expression of GH- and IGF-I-receptor in cultured rat chondrocytes. Endocrinology 139:3296–3305 [DOI] [PubMed] [Google Scholar]
- Malerba M, Bossoni S, Radaeli A, Mori E, Romanelli G, Tantucci C, Giustina A, Grassi V 2006 Bone ultrasonometric features and growth hormone secretion in asthmatic patients during chronic inhaled corticosteroid therapy. Bone 38:119–124 [DOI] [PubMed] [Google Scholar]
- Giustina A, Bussi AR, Jacobello C, Wehrenberg WB 1995 Effects of recombinant human growth hormone (GH) on bone and intermediary metabolism in patients receiving chronic glucocorticoid treatment with suppressed endogenous GH response to GH-releasing hormone. J Clin Endocrinol Metab 80:122–129 [DOI] [PubMed] [Google Scholar]
- Berneis K, Oehri M, Kraenzlin M, Keller U 1999 Effects of IGF-1 combined with GH on glucocorticoid-induced changes of bone and connective tissue turnover in man. J Endocrinol 162:259–264 [DOI] [PubMed] [Google Scholar]
- Delany A, Dong Y, Canalis E 1994 Mechanisms of glucocorticoid action in bone cells. J Cell Biochem 56:295–302 [DOI] [PubMed] [Google Scholar]
- Simon D, Prieur AM, Quartier P, Charles Ruiz J, Czernichow P 2007 Early recombinant human growth hormone treatment in glucocorticoid-treated children with juvenile idiopathic arthritis: 3-year randomized study. J Clin Endocrinol Metab 92:2567–2573 [DOI] [PubMed] [Google Scholar]
- Simon D, Lucidarme N, Prieur AM, Ruiz JC, Czernichow P 2003 Effects on growth and body composition of growth hormone treatment in children with juvenile idiopathic arthritis requiring steroid therapy. J Rheumatol 30:2492–2499 [PubMed] [Google Scholar]
- Bechtold S, Ripperger P, Bonfig W, Schmidt H, Bitterling H, Häfner R, Schwarz H 2004 Bone mass development and bone metabolism in juvenile idiopathic arthritis: treatment with growth hormone for 4 years. J Rheumatol 31:1407–1412 [PubMed] [Google Scholar]
- Bechtold S, Ripperger P, Bonfig W, Dalla Pozza R, Häfner R, Schwarz H 2005 Growth hormone changes bone geometry and body composition in patients with juvenile idiopathic arthritis requiring glucocorticoid treatment: a controlled study using peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:3168–3173 [DOI] [PubMed] [Google Scholar]