Summary
A critical but seldom-studied component of life history theory is how behavior and age affect whole-organism performance. To address this issue we compared the flight performance of honey bees (whose behavioral development and age can be assessed independently via simple manipulations of colony demographics) between distinct behavioral castes (in-hive nurse bees vs out-of-hive foragers) and across lifespan. Variable-density gases and high-speed video were used to determine the maximum hovering flight capacity and wing kinematics of age-matched nurse bees and foragers sampled from a single-cohort colony over a period of 34 days. The transition from hive work to foraging was accompanied by a 42% decrease in body mass and a proportional increase in flight capacity (defined as the minimum gas density allowing hovering flight). The lower flight capacity of hive bees was primarily due to the fact that in air they were functioning at a near-maximal wing angular velocity due to their high body masses. Foragers were lighter and when hovering in air required a much lower wing angular velocity, which they were able to increase by 32% during maximal flight performance. Flight performance of hive bees was independent of age, but in foragers the maximal wingbeat frequency and maximal average angular velocity were lowest in precocious (7–14 day old) foragers, highest in normal-aged (15–28 day old) foragers and intermediate in foragers older than 29 days. This pattern coincides with previously described age-dependent biochemical and metabolic properties of honey bee flight muscle.
Keywords: flight, development, senescence, Apis mellifera
INTRODUCTION
A critical issue in life history theory is how behavior and age affect the lifetime patterns of whole-organism performance (Roff, 2007; Rose et al., 2007). Studies of this issue should ideally separate the effects of age and behavior without ambiguity, focus on performance traits that are ecologically relevant, and utilize free-living animals, whose behavior and physiology may be quite different from those of laboratory-reared counterparts (Ricklefs and Wikelski, 2002). These challenges can be met by comparing the flight performance of honey bees (Apis mellifera, whose behavioral development and age can be assessed independently via simple manipulations of colony demographics) among distinct behavioral castes and across lifespan. Flight is a principal trait (along with eusociality, memory, communication and navigation) contributing to honey bee fitness and success via colony-level resource acquisition. Flight is unique among these traits in that its capacity is subject to a suite of physiological changes during development, yet chronic performance of this behavior entails exposure to stressors (e.g. high temperature, reactive oxygen species, mechanical wear) that may hinder these same beneficial physiological traits and cause senescence (Roberts and Elekonich, 2005).
Adult honey bees proceed through behaviorally defined life-history stages as they age, a process of behavioral development called temporal polyethism. These insects increasingly rely on flight ability during this process, which normally involves in-hive tasks such as brood care (nursing) and hive maintenance during the first 2–3 weeks of adult life followed by a transition to tasks outside the hive, predominantly foraging, which typically last for 2–3 weeks prior to death (Dukas, 2008). Among the many physiological and biochemical changes occurring between eclosion and the onset of foraging are a 10-fold increase in cytochrome concentrations (Herold and Borei, 1963), a doubling of thoracic glycogen levels (Fewell and Harrison, 2001; Harrison, 1986), and increased citrate synthase levels and troponin T (TnT) 10A expression (Schippers et al., 2006) that combine to yield an 8-fold increase in flight metabolic rate (up to 800 W kg–1) during this period (Harrison and Fewell, 2002; Roberts and Harrison, 1999).
For many metabolically expensive behaviors such as insect flight, peak capacity is transient and progressively senesces (Carey et al., 2006; Grotewiel et al., 2005; Leffelaar and Grigliatti, 1984; Miller et al., 2008), presumably due in large part to oxidative stress and the impairment of mechanisms resisting it (Amdam and Omholt, 2002; Golden et al., 2002; Martin and Grotewiel, 2006; Seehuus et al., 2006; Sun and Tower, 1999; Vieira et al., 2000; Yoon et al., 2002; Yu and Chung, 2006). In Drosophila melanogaster, the frequency and duration of flight bouts as well as wing kinematic performance decrease with age beginning 1–2 weeks after eclosion (Carey et al., 2006; Leffelaar and Grigliatti, 1984; Miller et al., 2008). In house flies (Musca domestica), flight behavior accelerates age-dependent oxidative damage including the accrual of mitochondrial peroxide, carbonylation of select mitochondrial enzymes, and mitochondrial DNA damage, while preventing flight prevents such damage and increases longevity (Agarwal and Sohal, 1994; Sohal and Buchan, 1981; Sohal and Dubey, 1994; Yan et al., 1997; Yan and Sohal, 1998; Yan and Sohal, 2000).
Oxidative stress produced by the intense aerobic demands upon honey bee foragers is likely mitigated by upregulation of flight muscle Hsp70, catalase and CuZn superoxide dismutase (Williams et al., 2008; Wolschin and Amdam, 2007). However, the diurnal upregulation of Hsp70 and catalase (along with total antioxidant capacity) in the flight muscles of foragers subsides with age (Williams et al., 2008), and honey bee mortality sharply increases following 12–14 days of foraging experience (Dukas, 2008). Thus, oxidative stress that accrues with age, especially following the transition to foraging behavior, may accelerate senescence of flight capacity in honey bees.
The present study investigates how age and behavioral development
independently affect honey bee flight capacity. We hypothesized that changes
in flight capacity reflect physiological and biochemical changes in flight
muscle that occur during behavioral development and with age as described
above. We predicted that, independent of age, bees collecting pollen and
nectar (foragers) will have greater flight capacity than bees performing brood
care (nurses). We also predicted that the flight capacity of foragers will
initially improve with age, reach some maximum level in intermediate-aged
individuals, and senesce in older individuals. To separate the effects of age
and behavioral development on normal vs maximal hovering flight
capacity, we created a single-cohort colony (SCC) composed only of 1–2
day old honey bees. About 10% of bees in a SCC will transition to foraging
precociously (i.e. about 1 week after eclosion) while others remain
normal-aged nurses. In the following 1–2 weeks more bees transition into
foraging behavior at a typical age while others remain in the hive as
over-aged nurses. Thus, a SCC allows for comparisons of flight performance
between age-matched groups of nurses and foragers, to assess the effects of
behavior independently of age, and within behavioral castes, to assess the
effects of age independently of behavior. We assayed maximal flight capacity
by permitting bees to hover in a series of normoxic, variable-density gasses
to determine the minimal gas density (MGD) that allowed for hovering flight
(Roberts et al., 2004). A
high-speed (4348 frames s–1) digital video camera was used to
record hovering sequences, from which we derived the following kinematics:
wingbeat frequency (n), wing stroke amplitude (Φ) and wing
angular velocity (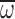 ). We found that honey bee flight
capacity is limited and age independent in nurses but greatly improves at the
transition to foraging behaviors. Moreover, flight capacity further improves
with age if the transition to foraging is premature, and then senesces in very
old foragers.
). We found that honey bee flight
capacity is limited and age independent in nurses but greatly improves at the
transition to foraging behaviors. Moreover, flight capacity further improves
with age if the transition to foraging is premature, and then senesces in very
old foragers.
MATERIALS AND METHODS
SCC: sampling and weighing
A SCC containing 2240 European honey bee (Apis mellifera L.) workers was created from six frames of immature bees from three different source colonies (each derived from multiply mated queens) at the University of Nevada, Las Vegas apiary during late June, 2007. The frames were placed in an incubator (32°C, 75% relative humidity RH, 24 h dark cycle) and newly eclosed adult bees were removed every 24 h. The SCC was founded from adult bees that eclosed on 2 consecutive days. On the first of these 2 days, 1000 bees were fitted with small, unique, color- and number-coded tags (Opallitplätchen, Graze, KG, Endersbach, Germany) glued to the dorsal thorax for the purpose of individual identification. Of these bees, 400 were individually weighed immediately following tagging. The SCC was provided with an unrelated queen bee, one frame each of honey and pollen, and three empty frames for egg laying/brood development. The SCC was kept closed in an environmental chamber (30°C and 30% RH) for 5 days post-eclosion to allow the queen to lay eggs and for maturation of the workers before being moved to the outdoor apiary to permit normal colony activity. Only tagged nurses and foragers were collected for assessment of flight capacity.
Maximal flight capacity and wing kinematics
Forager and nurse bees were assessed for maximal flight capacity. Foragers generally exit the hive at a relatively high velocity (relative to bees performing guarding behavior, or in-hive bees performing orientation or defecation flights) and in a straight-line trajectory towards the perimeter of the apiary. We intercepted individual out-going foragers (N=57, ranging in age from 8 to 40 days) as they flew into a 1 quart (∼1 l), clear plastic bag held approximately 30 cm from the entrance of the hive. Nurses (N=40, ranging in age from 8 to 27 days old) were collected from the comb using light forceps after they performed the caste-specific behavior of repeatedly sticking their heads into cells that contained larvae. We were unable to collect nurses older than 27 days from the original cohort of tagged bees because these individuals were gradually replaced by younger bees from the brood laid by the resident queen. Bees were transported to an environmental chamber maintained at a temperature of 30°C where maximal flight capacity was determined. Bees were weighed to the nearest 0.0001 g following assessment of maximal flight capacity.
The methods used to assess individual flight capacity were similar to those described previously (Roberts et al., 2004). Forager and nurse bees were immediately transferred to a flight chamber which consisted of a 5 l Erlenmeyer flask fitted with an inlet port at the base for gas perfusion and a lucite cover to prevent the bees from escaping. Bees were exposed to variable density, normoxic gas mixtures which consisted of oxygen and nitrogen and/or helium, and ranged from normodense air (21% O2, 79% N2; 1.21 kg m–3) to hypodense heliox (21% O2, 79% He; 0.41 kg m–3) in 0.16 kg m–3 increments. The gasses were mixed using calibrated bi-metal thermo-actuated valves (low flow: Tylan FC-260; San Diego, CA, USA) and solenoid-actuated valves (high flow: Tylan FC-2910), and mixtures and flow rates were metered by an electronic flow controller (Sable Systems MFC-4; Las Vegas, NV, USA). When assessing maximal flight capacity and filming hovering flight, total gas flow rate was maintained at 1 l min–1. Each trial began with air and the five hypodense gas mixtures were then administered in random order. In between gas mixtures, the flight chamber was flushed with the new gas mixture at a flow rate of 25 l min–1 for 1 min to ensure complete washout. Bees were flown in each gas mixture until: (1) sustained hovering flight was observed and recorded; (2) hovering flight was attempted but failed (typically distinguished by the bee skimming across the floor of the chamber, unable to generate enough lift to hover); or (3) 3 min had elapsed, in which case the inactive bee was excluded from analysis. Bees that landed on the floor or sides of the chamber were persuaded to fly by agitating them with a small magnetic stir-bar, directed by a magnetic wand outside the chamber. Maximal flight capacity was determined as MGD, the minimal gas density that allowed hovering flight.
Honey bees hover in air and heliox using a horizontal stroke plane (Altshuler et al., 2005; Ellington, 1984); therefore, hovering flight kinematics were determined from the wing trajectories in the horizontal plane recorded by a single, high-speed (4348 frames s–1) digital video camera (Vision Research, Phantom v5.1; Wayne, NJ, USA). The camera was oriented directly above the flask and focused such that the focal plane was at the center of the flask. Hence, hovering bees in focus and viewed directly through the mouth of the flask were away from the narrow circumference(s) near the top of the flask and centered in the chamber at least five wing lengths (i.e. 50 mm) away from the chamber floor and walls. The central positioning within the chamber minimized the possibility of kinematic variation due to the boundary effect – when vortices become `trapped' between the flyer and nearby surfaces (Rayner and Thomas, 1991). Ascending, descending or maneuvering flight was ignored. The digital video sequences were analyzed using customized software (Matlab, The Mathworks; Natick, MA USA) to determine the following kinematic variables for individual bees during hovering in air (referenced by subscript `norm' in figures and following text) and hovering in the MGD (referenced by subscript `max' in figures and following text): n (in Hz) was calculated from the duration to complete 10 successive wingbeats; Φ (in deg.) was calculated as the average of the downstroke and upstroke angular displacement for each of the 10 wingbeats; and ω (in rad s–1), the average wing angular velocity, was calculated from the duration to complete the total angular displacement of one downstroke and one upstroke for each of the 10 wingbeats.
Statistical analysis
Analysis of variance (ANOVA) was used to evaluate how body mass (Mb) differed between foragers, nurses and 1 day old bees (eclosion mass). Multivariate analysis of covariance (MANCOVA; α=0.05) was used to determine the effect of behavioral caste, with Mb and age as covariates, on flight performance and kinematic variables. Our post hoc analyses consisted of evaluating specific relationships using linear or polynomial regression. Model I (least squares) linear regression was used to analyze relationships that included age or maximal flight capacity (MGD). Other relationships where both continuous variables were subject to measurement error were analyzed with model II linear (reduced major axis) regression. Because our a priori prediction was that flight capacity and kinematics in foragers would improve and then decline with age, we also used a 2nd order polynomial regression to test the effects of age on these variables.
RESULTS
Behavioral development and body mass
The honey bee Mb from a random sample of adult honey bees (exclusive of those used in flight assays) within 24 h of eclosion was 93.9±13.3 mg (mean±s.d.; N=40). The youngest age at which bees began to forage was 8 days post-eclosion. Collection of nurses and foragers for flight analyses began at this time and concluded at 27 days of age for nurses and at 40 days of age for foragers. Mb was significantly different between bees at eclosion, nurses and foragers (ANOVA: F1,94=376.9; P<0.001), with foragers (76.0±7.4 mg, N=57) being 42.9% lighter than nurses (133±19.1 mg, N=40). However, age did not significantly affect Mb for either nurses (model I linear regression: P=0.154) or foragers (model I linear regression: P=0.345).
Flight performance and kinematics
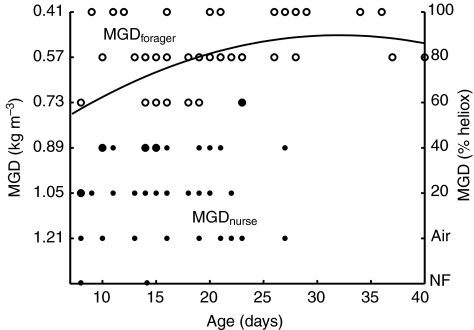
There was a significant effect of behavioral caste, mass and age on flight performance and kinematics (MANCOVA: P<0.001, P<0.001, P=0.006, respectively; see Table 1). Behavioral caste had a significant effect on MGD (MANCOVA: P<0.001), with foragers being able to fly in gas densities 34% lower than nurses could, after correcting for variation in mass and age (Table 1). Approximately 20% of foragers could hover in pure heliox, while the same fraction of nurses was capable of hovering only in normal air or could not fly at all. Age had a significant effect on MGD (MANCOVA: P<0.001). Because our hypothesis predicted that maximal flight capacity would improve with age in young foragers and senesce in older foragers, we fitted a 2nd order polynomial curve to the MGD vs forager age data (Fig. 1); this polynomial regression was significant (R2=0.26, P<0.001).
Table 1.
Multivariate analysis of covariance (MANCOVA) for the effects of caste, mass and age on flight performance
|
Parameter
estimatesa
(means ± s.d.)
|
Casteb
|
Massc
|
Aged
|
|||||
|---|---|---|---|---|---|---|---|---|
| Nurse | Forager | F1,93 | P | F1,93 | P | F1,93 | P | |
| nnorm (Hz) | 233.7±3.7 | 229.1±2.7 | 0.63 | 0.429 | 3.36 | 0.070 | 1.37 | 0.244 |
| Φnorm (deg.) | 121.1±2.9 | 108.7±2.1 | 7.21 | 0.009 | 15.3 | <0.001 | 0.45 | 0.503 |
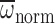 (rad s–1) (rad s–1)
|
985.1±24.4 | 866.5±18.1 | 9.28 | 0.003 | 6.33 | 0.014 | 0.01 | 0.925 |
| nmax (Hz) | 220.6±4.3 | 219.9±3.2 | 0.01 | 0.923 | 0.12 | 0.733 | 3.07 | 0.083 |
| Φmax (deg.) | 139.0±2.8 | 143.6±2.1 | 1.03 | 0.314 | 0.98 | 0.326 | 5.86 | 0.017 |
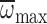 (rad s–1) (rad s–1)
|
1065.1±22.6 | 1102.6±16.8 | 1.08 | 0.302 | 0.59 | 0.446 | 15.83 | <0.001 |
| MGD (kg m–3) | 0.99±0.04 | 0.65±0.03 | 19.23 | <0.001 | 8.40 | 0.005 | 18.5 | <0.001 |
Significant values are in bold
MGD, minimal gas density (maximal flight capacity); n, wingbeat
frequency; 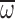 , wing angular velocity; Φ, wing
stroke amplitude; norm, flight in air; max, maximal flight in the MGD
, wing angular velocity; Φ, wing
stroke amplitude; norm, flight in air; max, maximal flight in the MGD
Least squares means evaluated at mass=100.3 mg, and age=19.9 days
MANCOVA: Pillai's trace, F7,87=5.04; P<0.001
MANCOVA: Pillai's trace, F7,87=5.18; P<0.001
MANCOVA: Pillai's trace, F7,87=3.09; P=0. 006
Fig. 1.
Maximal flight capacity (minimal gas density, MGD) vs age for foragers (MGDforager; open symbols) and nurses (MGDnurse; filled symbols). Large filled circles indicate overlapping forager and nurse data. Values of MGD (kg m–3) are inverted to reflect the increasing aerodynamic demand of flying in gas mixtures of lower density. Bees that were unable to fly in air (no flight, NF; right-hand y-axis) were plotted for descriptive purposes and were not included in the calculated MANCOVA or regressions. Second order polynomial regression for foragers: MGD=0.954+0.029age–0.0005age2, R2=0.26, P<0.001 (solid line).
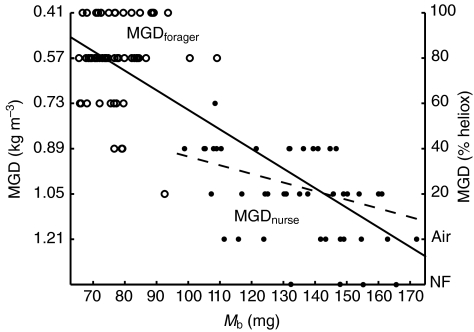
Mb also had a significant effect on MGD (MANCOVA: P=0.005). Because Mb varied greatly between the two behavioral castes, we further evaluated the relationship between Mb and MGD using linear regression (Fig. 2). MGD was independent of Mb in foragers (model II regression: MGDforager=0.619–0.001Mb, R2=0.002, P=0.772), but significantly increased with Mb in nurses (model II regression: MGDnurse=0.613+0.003Mb, R2=0.177, P=0.006). This effect was subtle, with variation in Mb explaining just 18% of variation in MGD in nurses. However, each bee in our experiment is an independent observation, and when behavioral castes were pooled, MGD significantly increased with Mb (i.e. lighter bees – primarily foragers – were better able to fly in hypodense gases), with variation in Mb explaining 66% of the variation in MGD for all bees combined (model II regression: MGDtotal=0.061+0.007Mb, R2=0.660, P<0.001).
Fig. 2.
Maximal flight capacity (minimal gas density, MGD) vs body mass (Mb) for foragers (MGDforager; open symbols) and nurses (MGDnurse; filled symbols). Values of MGD (kg m–3) are inverted to reflect the increasing aerodynamic demand of flying in gas mixtures of lower density. Bees that were unable to fly in air (no flight, NF; right-hand y-axis) were plotted for descriptive purposes and were not included in the calculated MANCOVA or regressions. Model II regression: MGDforager=0.619–0.001Mb, R2<0.01, P=0.772; MGDnurse=0.613+0.003Mb, R2=0.18, P=0.006 (broken line). MGDtotal=0.061+0.007Mb, R2=0.66, P<0.001 (solid line).
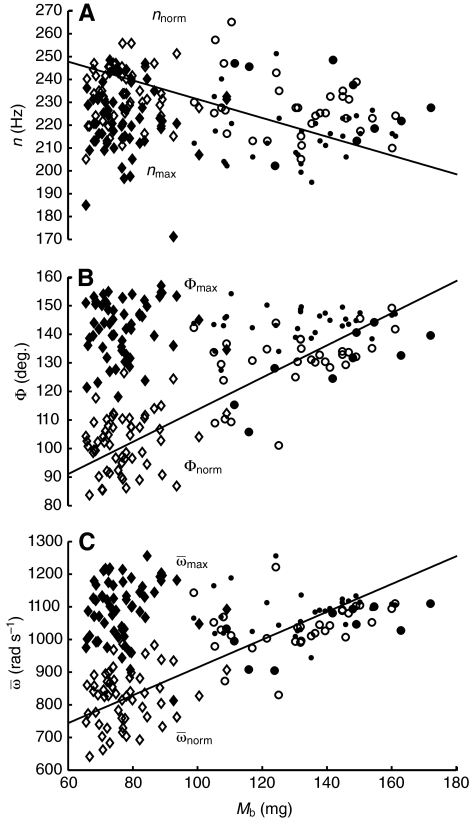
For bees hovering in air, nnorm tended to decrease
across Mb, but this trend was not significant (MANCOVA:
P=0.070). However, Mb significantly affected
Φnorm and 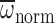 (MANCOVA: P<0.001, P=0.014, respectively). During
hovering in air, Φnorm significantly increased with
Mb (model II regression: P<0.001), with
variation in Mb explaining 67% of the variation in
Φnorm (Fig. 3).
The heaviest bees had Φnorm values approximately 45% higher
than the lightest bees. Likewise,
(MANCOVA: P<0.001, P=0.014, respectively). During
hovering in air, Φnorm significantly increased with
Mb (model II regression: P<0.001), with
variation in Mb explaining 67% of the variation in
Φnorm (Fig. 3).
The heaviest bees had Φnorm values approximately 45% higher
than the lightest bees. Likewise,
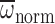 significantly increased with
Mb during hovering in air (model II regression:
P<0.001), with variation in the latter explaining 58% of the
variation in the former. During hovering in the MGD, Mb
did not affect nmax, Φmax or
significantly increased with
Mb during hovering in air (model II regression:
P<0.001), with variation in the latter explaining 58% of the
variation in the former. During hovering in the MGD, Mb
did not affect nmax, Φmax or
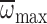 (MANCOVA: P=0.733,
P=0.326, P=0.446, respectively). Behavioral caste had a
significant effect on MGD, Φnorm and
(MANCOVA: P=0.733,
P=0.326, P=0.446, respectively). Behavioral caste had a
significant effect on MGD, Φnorm and
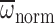 (MANCOVA:
P<0.001, P=0.009, P=0.003, respectively;
Fig. 3). The effects of
behavioral caste are similar to those of Mb
(Table 1), in large part due to
the significant difference in Mb between the nursing and
foraging castes.
(MANCOVA:
P<0.001, P=0.009, P=0.003, respectively;
Fig. 3). The effects of
behavioral caste are similar to those of Mb
(Table 1), in large part due to
the significant difference in Mb between the nursing and
foraging castes.
Fig. 3.
Wingbeat frequency (n; A), wing stroke amplitude (Φ; B) and
average wing angular velocity (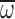 ; C) vs body
mass (Mb) for foragers (diamonds) and nurses (circles)
during flight in air (norm; open symbols) and maximal flight in the MGD (max;
filled symbols). Model II regression for n:
nnorm=272.18–0.411 Mb,
R2=0.07, P=0.008 (solid line);
nmax=267.71–0.474Mb,
R2=0.01, P=0.401. Model II regression for Φ:
Φnorm=56.85+0.568Mb,
R2=0.67, P<0.001 (solid line);
Φmax=173.54–0.318 Mb,
R2<0.01, P=0.823. Model II regression for
ω:
; C) vs body
mass (Mb) for foragers (diamonds) and nurses (circles)
during flight in air (norm; open symbols) and maximal flight in the MGD (max;
filled symbols). Model II regression for n:
nnorm=272.18–0.411 Mb,
R2=0.07, P=0.008 (solid line);
nmax=267.71–0.474Mb,
R2=0.01, P=0.401. Model II regression for Φ:
Φnorm=56.85+0.568Mb,
R2=0.67, P<0.001 (solid line);
Φmax=173.54–0.318 Mb,
R2<0.01, P=0.823. Model II regression for
ω:
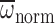 =488.49+4.256Mb,
R2=0.58, P<0.001 (solid line);
=488.49+4.256Mb,
R2=0.58, P<0.001 (solid line);
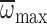 =1356.80–2.689Mb,
R2=0.01, P=0.347.
=1356.80–2.689Mb,
R2=0.01, P=0.347.
Age did not affect nnorm, Φnorm and
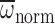 during hovering in air
(Table 1). Although age did not
affect nmax during hovering in the MGD, age had a
significant effect on Φmax and
during hovering in air
(Table 1). Although age did not
affect nmax during hovering in the MGD, age had a
significant effect on Φmax and
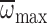 (MANCOVA: P=0.017,
P<0.001, respectively). However, the MANCOVA is a linear model and
thus cannot reveal the predicted parabolic relationships between kinematic
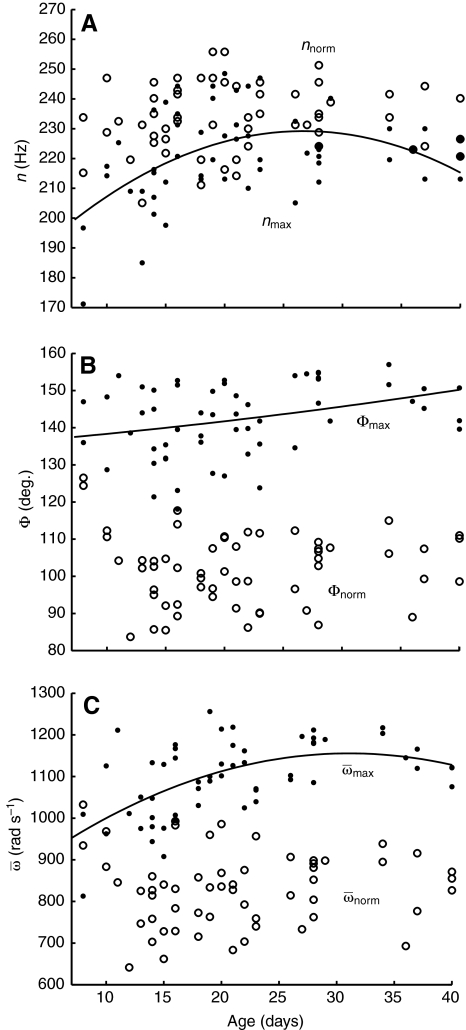
capacity and age. To test whether maximal kinematic capacities peaked in
middle-aged foragers, we fitted a 2nd order polynomial curve to the forager
data. The polynomial regression for nmax vs age
was significant (R2=0.24, P<0.001) for
foragers hovering in the MGD (Fig.
4A). For Φmax vs age, the 2nd order
polynomial regression curve fitted for foragers hovering in MGD was
significant but explained only a small percentage of the variation in
Φmax across age (R2=0.11, P=0.040;
Fig. 4B). The 2nd order
polynomial curve fitted to
(MANCOVA: P=0.017,
P<0.001, respectively). However, the MANCOVA is a linear model and
thus cannot reveal the predicted parabolic relationships between kinematic
capacity and age. To test whether maximal kinematic capacities peaked in
middle-aged foragers, we fitted a 2nd order polynomial curve to the forager
data. The polynomial regression for nmax vs age
was significant (R2=0.24, P<0.001) for
foragers hovering in the MGD (Fig.
4A). For Φmax vs age, the 2nd order
polynomial regression curve fitted for foragers hovering in MGD was
significant but explained only a small percentage of the variation in
Φmax across age (R2=0.11, P=0.040;
Fig. 4B). The 2nd order
polynomial curve fitted to 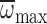 vs age was significant (R2=0.34,
P<0.001) for foragers hovering in MGD
(Fig. 4C). Hence,
nmax and
vs age was significant (R2=0.34,
P<0.001) for foragers hovering in MGD
(Fig. 4C). Hence,
nmax and 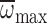 increased with age in precocious foragers, reached a plateau in middle-aged
foragers, and senesced to a small degree in older foragers. In foragers,
nmax was less than nnorm, while
Φmax and
increased with age in precocious foragers, reached a plateau in middle-aged
foragers, and senesced to a small degree in older foragers. In foragers,
nmax was less than nnorm, while
Φmax and 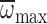 were
greater than Φnorm and
were
greater than Φnorm and
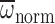 , respectively (paired
t-test: P<0.001 in each comparision). There were no
significant regressions of n, Φ or
, respectively (paired
t-test: P<0.001 in each comparision). There were no
significant regressions of n, Φ or 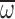 across age for nurses hovering in air or MGD. In nurses,
nmax was slightly, but significantly, less than
nnorm, while Φmax and
across age for nurses hovering in air or MGD. In nurses,
nmax was slightly, but significantly, less than
nnorm, while Φmax and
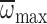 were significantly greater
than Φnorm and
were significantly greater
than Φnorm and
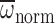 (Fig. 5; paired
t-test: P<0.001 in each comparison). In order to better
understand how kinematic performance might affect the caste-specific flight
performance, we performed an ANCOVA to investigate the effects of caste on MGD
with
(Fig. 5; paired
t-test: P<0.001 in each comparison). In order to better
understand how kinematic performance might affect the caste-specific flight
performance, we performed an ANCOVA to investigate the effects of caste on MGD
with 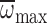 as a covariate
(Fig. 6). There was a
significant interaction between caste and
as a covariate
(Fig. 6). There was a
significant interaction between caste and
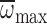 on MGD (ANCOVA:
F1,93=5.38, P=0.023). In foragers, MGD
significantly increased with
on MGD (ANCOVA:
F1,93=5.38, P=0.023). In foragers, MGD
significantly increased with 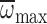 ,
with variation in
,
with variation in 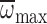 explaining
62% of the variation in MGD. However, there was no relationship between
explaining
62% of the variation in MGD. However, there was no relationship between
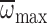 and MGD in nurses.
and MGD in nurses.
Fig. 4.
Wingbeat frequency (n; A), wing stroke amplitude (Φ; B) and
average wing angular velocity (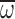 ; C) vs age
for foragers. Second order polynomial regression for nmax:
nmax=173.27+4.19age–0.079age2,
R2=0.24, P<0.001. Second order polynomial
regression for Φmax:
Φmax=135.57+0.252age–0.003age2,
R2=0.11, P<0.001. Second order polynomial
regression for
; C) vs age
for foragers. Second order polynomial regression for nmax:
nmax=173.27+4.19age–0.079age2,
R2=0.24, P<0.001. Second order polynomial
regression for Φmax:
Φmax=135.57+0.252age–0.003age2,
R2=0.11, P<0.001. Second order polynomial
regression for 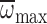 :
:
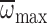 , R2=0.34,
P<0.001 (solid line).
, R2=0.34,
P<0.001 (solid line).
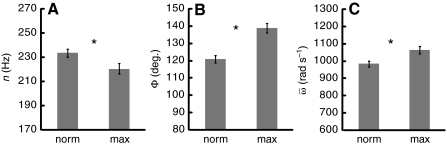
Fig. 5.
Wingbeat frequency (n; A), wing stroke amplitude (Φ; B) and
average wing angular velocity (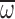 ; C) for nurses during
hovering in air (norm) and hovering in the MGD (max). Asterisks indicate
significant differences between normal and maximal hovering for n,
Φ and ω (paired t-test: P<0.001 in each
case).
; C) for nurses during
hovering in air (norm) and hovering in the MGD (max). Asterisks indicate
significant differences between normal and maximal hovering for n,
Φ and ω (paired t-test: P<0.001 in each
case).
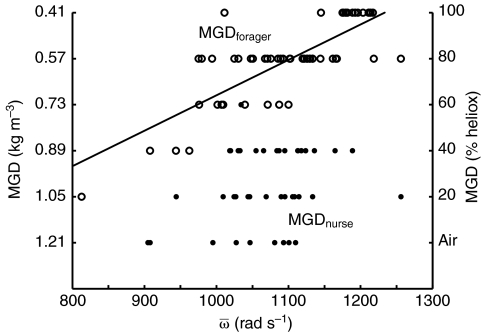
Fig. 6.
Maximal flight capacity (minimal gas density: MGD) vs maximal wing
angular velocity (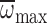 ) for
foragers (MGDforager; open symbols) and nurses (MGD; filled
symbols). Values of MGDnurse (kg m–3) are inverted
to reflect the increasing aerodynamic demand of flying in gas mixtures of
lower density. Model I regression:
) for
foragers (MGDforager; open symbols) and nurses (MGD; filled
symbols). Values of MGDnurse (kg m–3) are inverted
to reflect the increasing aerodynamic demand of flying in gas mixtures of
lower density. Model I regression:
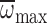 , R2=0.62,
P<0.001 (solid line);
, R2=0.62,
P<0.001 (solid line);
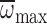 , R2=0.08,
P=0.083.
, R2=0.08,
P=0.083.
DISCUSSION
Using SCCs and variable-density gas mixtures, we were able to show that both age and behavioral development affect the flight performance of honey bees. To our knowledge this is the first study to experimentally segregate these factors and test their effects on the locomotor capacity of a free-living organism over a lifetime. The ability to fly in hypodense atmospheres greatly improves at the transition from nursing to foraging behaviors, and this improvement is facilitated predominantly by a large decrease in body mass that accompanies this transition. Although precocious (8–14 day old) foragers had greater flight capacity than age-matched nurses, flight capacity generally improved with age in young (15–21 days old) and typical-aged (22–28 day old) foragers. Peak kinematic performance was lowest in precocious (7–14 day old) foragers, highest in normal-aged (15–28 day old) foragers and intermediate in foragers older than 29 days. Kinematic performance and flight ability strongly increased following the transition to foraging (although this improvement was not complete if the behavioral transition occurred too early), and also showed modest, but perhaps ecologically important, signs of senescence in the oldest foragers in the study.
Body mass and flight performance
The primary basis for improved flight ability in foragers was the large (∼43%) decrease in Mb that occurred prior to the transition to foraging behavior, regardless of age. The reduction in Mb prior to the behavioral transition is restricted to tissues of the abdomen and is primarily due to gut emptying; hence, thoracic mass remains constant (but relative thorax mass increases) across the behavioral transition (Harrison, 1986). The strong effect of Mb on flight capacity was not apparent by comparing the two variables within each behavioral caste, as flight capacity was unaffected by Mb in foragers and only weakly correlated with Mb in nurses. However, when the two behavioral castes were pooled, yielding a much broader range of independent observations of mass and flight capacity in honey bees as a general group, a strong inverse relationship between Mb and MGD was revealed (Fig. 2).
Nurse bees had a very limited reserve capacity for kinematic and
aerodynamic performance due predominantly to their heavy bodies but also to
their immature flight muscles. While hovering in air, the Φnorm
and 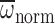 of heavier, younger bees
(nurses) were at or just below maximal attainable levels. Moreover, nurses
were unable to sustain normal n when challenged to hover in hypodense
gases – to the extent that
of heavier, younger bees
(nurses) were at or just below maximal attainable levels. Moreover, nurses
were unable to sustain normal n when challenged to hover in hypodense
gases – to the extent that
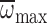 exceeded
exceeded
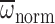 by only 8% (after adjusting
for the effects of age and mass). Precocious foragers and very old foragers
were similarly unable to maintain n when challenged with hypodense
gases, but their ability to strongly increase Φ still offered greatly
elevated
by only 8% (after adjusting
for the effects of age and mass). Precocious foragers and very old foragers
were similarly unable to maintain n when challenged with hypodense
gases, but their ability to strongly increase Φ still offered greatly
elevated 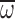 during maximal hovering performance. When
challenged with hypodense gases, only middle-aged foragers were able to
increase Φ and maintain n. Hummingbirds
(Altshuler and Dudley, 2003;
Chai et al., 1997), euglossine
bees (Dudley, 1995) and
carpenter bees (Roberts et al.,
2004) similarly increase Φ and maintain (or even slightly
increase) n during maximal hovering flight. Although there is no
information on the age dependence of flight performance in these taxa, it
seems plausible that kinematic performance might be similarly affected in very
young or old individuals.
during maximal hovering performance. When
challenged with hypodense gases, only middle-aged foragers were able to
increase Φ and maintain n. Hummingbirds
(Altshuler and Dudley, 2003;
Chai et al., 1997), euglossine
bees (Dudley, 1995) and
carpenter bees (Roberts et al.,
2004) similarly increase Φ and maintain (or even slightly
increase) n during maximal hovering flight. Although there is no
information on the age dependence of flight performance in these taxa, it
seems plausible that kinematic performance might be similarly affected in very
young or old individuals.
Across closely related hovering insects, n decreases with Mb during hovering flight (Dillon and Dudley, 2004; Dudley, 2000), but this negative relationship does not always hold true for the few available datasets allowing intraspecific comparisons of n and Mb. In honey bees, there is a slight negative relationship between nnorm and Mb, although this is unlikely to be due to resonance issues and an increase in the induced power required to move a larger wing (factors typically associated with the negative relationship between n and Mb across similar species) because neither wing size nor thorax dimensions differ between foragers and nurses (J.T.V., unpublished observation). Instead, the heaviest honey bees (nurses) require elevated Φ just to fly in air, but their immature flight muscles do not allow them to reach n values attainable by many (particularly middle-aged) foragers, which are much lighter than nurses. For carpenter bees (Xylocopa varipuncta) hovering in air, heavier individuals have higher Φ (as do honey bees; Fig. 3B) and n due to disproportionately heavier abdomens and high wing loading (Roberts et al., 2004), although peak kinematic performance and Mb are independent of each other in both of these species. This is not the case during flight in heliox and maximal load lifting across several species of euglossine bees whose Mb span over an order of magnitude, in which case Φmax is highly conserved near 140 deg., but nmax decreases with Mb (Dudley, 1995; Dillon and Dudley, 2004).
Variation in Mb was smallest in foragers, and
Mb had no effect on MGD in this group. This is not so for
X. varipuncta, in which body mass varies by 3-fold, with lighter
individuals capable of hovering in lower gas densities than heavier
individuals due to lower wing loading, relatively larger flight muscles and
smaller abdomens (Roberts et al.,
2004). The ability of honey bee foragers to fly in hypodense gases
was positively correlated with
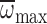 (Fig. 6). In several species of
Drosophila, aerodynamic forces scale to the square of wing
translational velocity (Lehmann and
Dickinson, 1998), which is determined by ω, and hence it is
not surprising that the honey bees capable of generating the highest values of
(Fig. 6). In several species of
Drosophila, aerodynamic forces scale to the square of wing
translational velocity (Lehmann and
Dickinson, 1998), which is determined by ω, and hence it is
not surprising that the honey bees capable of generating the highest values of
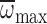 were also the ones capable of
hovering in the lowest gas densities. To our knowledge this is the only study
to date linking individual variation in kinematic capacity (in this case
largely due to age plus random effects) to peak flight performance.
were also the ones capable of
hovering in the lowest gas densities. To our knowledge this is the only study
to date linking individual variation in kinematic capacity (in this case
largely due to age plus random effects) to peak flight performance.
The development and senescence of flight performance
The improvement of flight muscle performance at the transition to foraging and during foraging (if the transition is premature) is likely to be due to a suite of biochemical and structural changes in the flight muscle that occur during honey bee maturation and behavioral development. For example, young honey bees (∼3 days old) that have acquired the ability to fly express an isoform of TnT similar to the 46 kDa TnT localized only to the mature flight muscle of adult Drosophila (Domingo et al., 1998). This TnT isoform is absent in juvenile stages in Drosophila as well as in 1–2 day old bees that are unable to fly, suggesting that the muscle function necessary for flight is dependent upon the expression of specific TnT isoforms. Furthermore, honey bee foragers express more TnT 10A (>2-fold increase) in their flight muscles than younger hive bees (Schippers et al., 2006). The effects of the differential TnT isoform expression on honey bee flight are unknown, but in the dragonfly Libellula pulchella the differential expression of TnT isoforms affects flight muscle calcium sensitivity and is correlated with an increase in wingbeat frequency and amplitude as the dragonflies progress from the teneral stage to sexual maturity (Fitzhugh and Marden, 1997; Fitzhugh et al., 1999; Marden et al., 2001; Marden et al., 1998; Marden et al., 1999). Elevated TnT 10A expression may contribute to the age- and behavior-dependent increase in maximal wingbeat frequency in honey bees, and attempts to determine whether the expression of TnT isoforms (and other flight-motor proteins) is similarly affected by age and behavioral development are ongoing in our laboratories.
The reduction in maximal kinematic and flight capacity in the older foragers likely reflects senescence via oxidative stress within the flight muscles. The intense aerobic metabolism of forager flight muscle (over 2000 W kg–1 muscle) yields high levels of reactive oxygen species, the effects of which are mitigated by the upregulation of stress and antioxidant proteins such as Hsp 70, catalase and CuZn superoxide dismutase (Schippers et al., 2006; Williams et al., 2008; Wolschin and Amdam, 2007). However, resistance to oxidative stress declines with age, as old (30–32 days) honey bee foragers express less catalase and have lower total antioxidative capacity than precocious foragers (Williams et al., 2008). Cytochrome c oxidase activity also decreases in aged honey bee flight muscle (Schippers, 2006), but other cellular pathologies of honey bee flight muscle senescence are unknown. In Drosophila and other dipterans, such pathologies include depressed actin transcription, decreased sarcomere length, enlarged/degraded mitochondria, depressed mitochondrial respiration and depressed aconitase activity (Ferguson et al., 2005; Labuhn and Brack, 1997; Miller et al., 2008; Yarian and Sohal, 2005).
The mechanical wear of wings has also been implicated as an important
factor contributing to the senescence of flight performance and mortality in
eusocial bees (Cartar, 1992;
Dukas, 2008;
Hedenstrom et al., 2001;
Higginson and Barnard, 2004).
These authors hypothesize that degraded wings in older bees limit flight
performance with consequences for foraging ability and predator evasion. Wing
wear was not a factor contributing to senescence of flight performance in our
study because in our experiments we only assayed bees that possessed intact,
unworn wings. However, our finding of impaired nmax in
very old foragers may compound the problems of worn wings. For example,
bumblebees increase n in response to wing clipping
(Hedenstrom et al., 2001), and
such compensation may be unavailable to older honey bee foragers. We have no
information about the foraging history of the bees in our study (i.e. we know
the absolute age of foragers, but not how long they had been foraging), but we
believe that the declines in nmax,
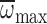 and maximal flight capacity
in the older foragers probably mark the onset of senescence in the flight
muscle. The pace of senescence of overall flight ability is still unknown but
should be a function of both flight muscle and wing degradation.
and maximal flight capacity
in the older foragers probably mark the onset of senescence in the flight
muscle. The pace of senescence of overall flight ability is still unknown but
should be a function of both flight muscle and wing degradation.
The ecological significance of honey bee flight performance
A honey bee colony can shift worker demographics in response to a deficiency of workers in a particular caste (Huang and Robinson, 1992; Robinson et al., 1989) or worker effort in response to a shortage of pollen stores (Fewell and Winston, 1992). Such shifts might involve precocious or very old foragers, both of which have reduced maximal flight capacity, and negatively affect foraging loads and rate of foraging intake (Higginson and Barnard, 2004; Schippers et al., 2006), with potential consequences for colony-scale economy and energy flux (Schmid-Hempel et al., 1985). Likewise, colony-level intake should be higher when, all else being equal, the foraging caste is represented by middle-aged individuals. Indeed, the amount of food collected per trip increases by over 300% throughout a bee's first week of foraging behavior (Schippers et al., 2006). Finally, precocious and aged foragers may be subject to a higher predation risk due to their limited burst flight capacities (Cartar, 1992; Dukas, 2008). There are no data to confirm this linkage in honey bees, although wing damage resulting from male–male combat in the burrowing bee Amegilla dawsoni increases the risk of predation by birds and shortens longevity (Alcock, 1996).
Certain honey bee genotypes are predisposed to early or late initiation of foraging (Calderone and Page, 1988; Giray and Robinson, 1994), and it is possible that the trajectory of the age-dependent development of maximal flight capacity varies genetically as well. For colonies genetically predisposed to begin foraging at an earlier age, any potential colony-level costs of precocious foraging may be mitigated by a faster rate of development and shorter periods of sub-optimal maximal flight capacity. Conversely, in colonies predisposed to a later onset of foraging, the costs of precocious foraging may be prolonged by a slower rate of development, or foraging onset may be temporally coordinated with slower development of flight capacity. Experiments addressing the temporal kinetics of foraging initiation and flight capacity among such genotypes would be valuable to test these possibilities.
Conclusion
The development of the flight capacity necessary for effective foraging in honey bees depends upon the sharp reduction in body mass at the transition from nursing to foraging behavior. Following this transition, the age-dependent development and senescence of maximal flight capacity in foragers reflects the ability, when aerodynamically challenged, to increase Φ while simultaneously maintaining n. Importantly, our experiment does not allow us to determine whether the timing of the initiation of foraging affects the onset and pace of senescence (which would require lifetime ethography of individual bees), although precocious foraging does shorten lifespan (Rueppell et al., 2007). Even so, our results suggest that variation in honey bee flight capacity across age is an important factor explaining known life-history patterns of foraging behavior and mortality rates. However, future research is needed to directly link the ontogeny of flight capacity to foraging efficacy, predation risk and mortality.
LIST OF ABBREVIATIONS
- Mb
body mass
- MGD
minimal gas density
- n
wingbeat frequency
- SCC
single-cohort colony
- Φ
wing stroke amplitude
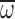
wing angular velocity
Funding was provided by Nevada NASA Space Grant Fellowship to J.T.V, National Institutes of Health Grant 1F32AR055033-01 to J.B.W. and National Science Foundation IOS-0725030 to M.M.E. and S.P.R. Deposited in PMC for release after 12 months.
References
- Agarwal, S. and Sohal, R. S. (1994). Aging and protein oxidative damage. Mech. Ageing Dev. 75, 11-19. [DOI] [PubMed] [Google Scholar]
- Alcock, J. (1996). Male size and survival: the effects of male combat and bird predation in Dawson's burrowing bees, Amegilla dawsoni. Ecol. Entomol. 21, 309-316. [Google Scholar]
- Altshuler, D. L. and Dudley, R. (2003). Kinematics of hovering hummingbird flight along simulated and natural elevational gradients. J. Exp. Biol. 206, 3139-3147. [DOI] [PubMed] [Google Scholar]
- Altshuler, D. L., Dickson, W. B., Vance, J. T., Roberts, S. P. and Dickinson, M. H. (2005). Short-amplitude high-frequency wing strokes determine the aerodynamics of honeybee flight. Proc. Natl. Acad. Sci. USA 102, 18213-18218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam, G. V. and Omholt, S. W. (2002). The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 216, 209-228. [DOI] [PubMed] [Google Scholar]
- Calderone, N. W. and Page, R. E. (1988). Genotypic variability in age polyethism and task specialization in the honey bee, Apis mellifera (Hymenoptera, Apidae). Behav. Ecol. Sociobiol. 22, 17-25. [Google Scholar]
- Carey, J. R., Papadopoulos, N., Kouloussis, N., Katsoyannos, B., Miller, H. G., Wang, J. L. and Tseng, Y. K. (2006). Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp. Gerontol. 41, 93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartar, R. V. (1992). Morphological senescence and longevity: an experiment relating wing wear and life span in foraging wild bumble bees. J. Anim. Ecol. 61, 225-231. [Google Scholar]
- Chai, P., Chen, J. S. C. and Dudley, R. (1997). Transient hovering performance of hummingbirds under conditions of maximal loading. J. Exp. Biol. 200, 921-929. [DOI] [PubMed] [Google Scholar]
- Dillon, M. E. and Dudley, R. (2004). Allometry of maximal vertical force production during hovering flight of neotropical orchid bees (Apidae: Euglossini). J. Exp. Biol. 207, 417-425. [DOI] [PubMed] [Google Scholar]
- Domingo, A., Gonzalez-Jurado, J., Maroto, M., Diaz, C., Vinos, J., Carrasco, C., Cervera, M. and Marco, R. (1998). Troponin-T is a calcium-binding protein in insect muscle: in vivo phosphorylation, muscle-specific isoforms and developmental profile in Drosophila melanogaster. J. Muscle Res. Cell Motil. 19, 393-403. [DOI] [PubMed] [Google Scholar]
- Dudley, R. (1995). Extraordinary flight performance of orchid bees (Apidae: Euglossini) hovering in heliox (80 percent He/20 percent O2). J. Exp. Biol. 198, 1065-1070. [DOI] [PubMed] [Google Scholar]
- Dudley, R. (2000). The evolutionary physiology of animal flight: paleobiological and present perspectives. Annul. Rev. Physiol. 62, 135-155. [DOI] [PubMed] [Google Scholar]
- Dukas, R. (2008). Mortality rates of honey bees in the wild. Insectes Soc. 55, 252-255. [Google Scholar]
- Ellington, C. P. (1984). The aerodynamics of hovering insect flight. III. Kinematics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 305, 41-78. [Google Scholar]
- Ferguson, M., Mockett, R. J., Shen, Y., Orr, W. C. and Sohal, R. S. (2005). Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 390, 501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell, J. H. and Harrison, J. F. (2001). Variation in worker behavior of African and European honey bees. In Proceedings of the Second International Conference on Africanized Honeybees and Bee Mites (ed. R. E. Page and E. Erickson). Medina, OH: A. I. Root.
- Fewell, J. H. and Winston, M. L. (1992). Colony state and regulation of pollen foraging in the honey bee Apis mellifera. L. Behav. Ecol. Sociobiol. 30, 387-393. [Google Scholar]
- Fitzhugh, G. H. and Marden, J. H. (1997). Maturational changes in troponin T expression Ca2+-sensitivity and twitch contraction kinetics in dragonfly flight muscle. J. Exp. Biol. 200, 1473-1482. [DOI] [PubMed] [Google Scholar]
- Fitzhugh, G. H., Wolf, M. R. and Marden, J. H. (1999). Adjusting muscle power and optimal frequency: strong effects of calcium sensitivity and troponin T expression on flight muscle of the dragonfly L. pulchella. Am. Zool. 39, 72a. [Google Scholar]
- Giray, T. and Robinson, G. E. (1994). Effects of intracolony variability in behavioral development on plasticity of division of labor in honey bee colonies. Behav. Ecol. Sociobiol. 35, 13-20. [Google Scholar]
- Golden, T. R., Hinerfeld, D. A. and Melov, S. (2002). Oxidative stress and aging: beyond correlation. Aging Cell 1, 117-123. [DOI] [PubMed] [Google Scholar]
- Grotewiel, M. S., Martin, I., Bhandari, P. and Cook-Wiens, E. (2005). Functional senescence in Drosophila melanogaster. Aging Res. Rev. 4, 372-397. [DOI] [PubMed] [Google Scholar]
- Harrison, J. M. (1986). Caste specific changes in honeybee flight capacity. Physiol. Zool. 59, 175-187. [Google Scholar]
- Harrison, J. M. and Fewell, J. H. (2002). Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp. Biochem. Physiol. A 133, 323-333. [DOI] [PubMed] [Google Scholar]
- Hedenstrom, A., Ellington, C. P. and Wolf, T. J. (2001). Wing wear, aerodynamics and flight energetics in bumblebees (Bombus terrestris): an experimental study. Funct. Ecol. 15, 417-422. [Google Scholar]
- Herold, R. C. and Borei, H. (1963). Cytochrome changes during honey bee flight muscle development. Dev. Biol. 8, 67-79. [DOI] [PubMed] [Google Scholar]
- Higginson, A. D. and Barnard, C. J. (2004). Accumulating wing damage affects foraging decisions in honeybees (Apis mellifera L.) Ecol. Entomol. 29, 52-59. [Google Scholar]
- Huang, Z. Y. and Robinson, G. E. (1992). Honey bee colony integration: worker worker interactions mediate hormonally regulated plasticity in division of labor. Proc. Natl. Acad. Sci. USA 89, 11726-11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuhn, M. and Brack, C. (1997). Age-related changes in the mRNA expression of actin isoforms in Drosophila melanogaster. Gerontology 43, 261-267. [DOI] [PubMed] [Google Scholar]
- Leffelaar, D. and Grigliatti, T. (1984). Age-dependent behavior loss in adult Drosophila melanogaster. Dev. Genet. 4, 211-227. [Google Scholar]
- Lehmann, F. O. and Dickinson, M. H. (1998). The control of wing kinematics and flight forces in fruit flies (Drosophila spp.). J. Exp. Biol. 201, 385-401. [DOI] [PubMed] [Google Scholar]
- Marden, J. H., Fitzhugh, G. H. and Wolf, M. R. (1998). From molecules to mating success: integrative biology of muscle maturation in a dragonfly. Am. Zool. 38, 528-544. [Google Scholar]
- Marden, J. H., Fitzhugh, G. H., Wolf, M. R., Arnold, K. D. and Rowan, B. (1999). Alternative splicing, muscle calcium sensitivity, and the modulation of dragonfly flight performance. Proc. Natl. Acad. Sci. USA 96, 15304-15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden, J. H., Fitzhugh, G. H., Girgenrath, M., Wolf, M. R. and Girgenrath, S. (2001). Alternative splicing, muscle contraction and intraspecific variation: associations between troponin T transcripts, Ca2+ sensitivity and the force and power output of dragonfly flight muscles during oscillatory contraction. J. Exp. Biol. 204, 3457-3470. [DOI] [PubMed] [Google Scholar]
- Martin, I. and Grotewiel, M. S. (2006). Oxidative damage and age-related functional declines. Mech. Aging Dev. 127, 411-423. [DOI] [PubMed] [Google Scholar]
- Miller, M. S., Lekkas, P., Braddock, J. M., Farman, G. P., Ballif, B. A., Irving, T. C., Maughan, D. W. and Vigoreaux, J. O. (2008). Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys. J. 95, 2391-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner, J. M. V. and Thomas, A. L. R. (1991). On the vortex wake of an animal flying in a confined volume. Philos. Trans. R. Soc. Lond. B Biol. Sci. 334, 107-117. [Google Scholar]
- Ricklefs, R. E. and Wikelski, M. (2002). The physiology/life-history nexus. Trends Ecol. Evol. 17, 462-468. [Google Scholar]
- Roberts, S. P. and Elekonich, M. M. (2005). Commentary: behavioral development and the ontogeny of flight capacity in honey bees. J. Exp. Biol. 208, 4193-4198. [DOI] [PubMed] [Google Scholar]
- Roberts, S. P. and Harrison, J. F. (1999). Mechanisms of thermal stability during flight in the honeybee Apis mellifera. J. Exp. Biol. 202, 1523-1533. [DOI] [PubMed] [Google Scholar]
- Roberts, S. P., Harrison, J. F. and Dudley, R. (2004). Allometry of kinematics and energetics in carpenter bees (Xylocopa varipuncta) hovering in variable-density gases. J. Exp. Biol. 207, 993-1004. [DOI] [PubMed] [Google Scholar]
- Robinson, G. E., Page, R. E., Strambi, C. and Strambi, A. (1989). Hormonal and genetic control of behavioral integration in honey bee colonies. Science 246, 109-112. [DOI] [PubMed] [Google Scholar]
- Roff, D. A. (2007). Contributions of genomics to life-history theory. Nat. Rev. Genet. 8, 116-125. [DOI] [PubMed] [Google Scholar]
- Rose, M. R., Rauser, C. L., Benford, G., Matos, M. and Mueller, L. D. (2007). Hamilton's forces of natural selection after forty years. Evolution 61, 1265-1276. [DOI] [PubMed] [Google Scholar]
- Rueppell, O., Bachelier, C., Fondrk, M. K. and Page, R. E. (2007). Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.). Exp. Gerontol. 42, 1020-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers, M. P., Dukas, R., Smith, R. W., Wang, J., Smolen, K. and McClelland, G. B. (2006). Lifetime performance in foraging honeybees: behaviour and physiology. J. Exp. Biol. 209, 3828-3836. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel, P., Kacelnik, A. and Houston, A. I. (1985). Honeybees maximize efficiency by not filling their crop. Behav. Ecol. Sociobiol. 17, 61-66. [Google Scholar]
- Seehuus, S. C., Norberg, K., Gimsa, U., Krekling, T. and Amdam, G. V. (2006). Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 103, 962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal, R. S. and Buchan, P. B. (1981). Relationship between physical activity and life span in the adult housefly, Musca domestica. Exp. Gerontol. 16, 157-162. [DOI] [PubMed] [Google Scholar]
- Sohal, R. S. and Dubey, A. (1994). Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic. Biol. Med. 16, 621-626. [DOI] [PubMed] [Google Scholar]
- Sun, J. T. and Tower, J. (1999). FLP recombinase mediated induction of Cu/Zn superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol. Cell. Biol. 19, 216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, C., Pasyukova, E. G., Zenf, Z. B., Hackett, J. B., Lyman, R. F. and Machay, T. F. (2000). Genotype-environment interaction for quantitative trait loci affecting life in Drosophila melanogaster. Genetics 154, 213-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J. B., Roberts, S. P. and Elekonich, M. M. (2008). Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Exp. Gerontol. 43, 538-549. [DOI] [PubMed] [Google Scholar]
- Wolschin, F. and Amdam, G. V. (2007). Comparative proteomics reveal characteristics of life-history transitions in a social insect. Proteome Sci. 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. J. and Sohal, R. S. (1998). Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. USA 95, 12896-12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. J. and Sohal, R. S. (2000). Prevention of flight activity prolongs the life span of the housefly, Musca domestica, and attenuates the age-associated oxidative damage to specific mitochondrial proteins. Free Radic. Biol. Med. 29, 1143-1150. [DOI] [PubMed] [Google Scholar]
- Yan, L. J., Levine, R. L. and Sohal, R. S. (1997). Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. USA 94, 11168-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarian, C. S. and Sohal, R. S. (2005). In the aging housefly aconitase is the only citric acid cycle enzyme to decline significantly. J. Bioenerg. Biomembr. 37, 91-96. [DOI] [PubMed] [Google Scholar]
- Yoon, S. O., Yun, C. H. and Chung, A. S. (2002). Dose effect of oxidative stress on signal transduction in aging. Mech. Ageing Dev. 123, 1597-1604. [DOI] [PubMed] [Google Scholar]
- Yu, B. P. and Chung, H. Y. (2006). Adaptive mechanisms to oxidative stress during aging. Mech. Ageing Dev. 127, 436-443. [DOI] [PubMed] [Google Scholar]