Summary
Aging is commonly accompanied by a decline in cognitive functions such as learning and memory. In social insects, aging is tightly linked to social role. The honeybee (Apis mellifera L.) offers the unique opportunity to separate chronological age from social role. In the present paper, we tested whether chronological age, social role and the duration of performing this role affect tactile learning in honeybees. We compared acquisition, retention and discrimination between foragers with short and long foraging durations and age-matched nurse bees. Our data show that chronological age is of minor importance for tactile learning, retention and discrimination whereas social role has a decisive impact. Tactile acquisition is severely impaired in bees that have foraged for more than two weeks but not in nurse bees of the same chronological age. Interestingly, neither discrimination nor retention appear to be impaired by long foraging duration. The complex associations between acquisition, discrimination and retention in bees of different social roles open up rich possibilities for future studies on the neuronal correlates of behavioural performance and underline that the honeybee has great potential as a model system in the biology of aging.
Keywords: tactile conditioning, retention, discrimination, aging, division of labour, PER
INTRODUCTION
Many animals, ranging from worms to humans, show a decline in cognitive function with increasing chronological age (Marriott and Abelson, 1980; Grotewiel et al., 2005; Mell et al., 2005; Murakami and Murakami, 2005; Yu et al., 2006; Soei and Daum, 2008; Woodruff-Pak et al., 2008). In contrast to most animal species, honeybees normally show a strong relationship between chronological age and social role. The female caste of worker honeybees displays a temporal progression through a distinct set of social tasks. Younger bees typically work in the centre of the colony and provide the queen and larvae with food (`nurse bees'), they repair the combs or are involved in the production of honey from incoming nectar. Later, when workers reach about their third week of adult life, they begin to forage outside to provide the colony with nectar, pollen, water and propolis (Winston, 1987; Seeley, 1995). After the transition from nursing to foraging tasks, remaining life expectancy is short. Foragers normally die after a few weeks due to high mortality risks (Visscher and Dukas, 1997). Although there is no clear-cut definition for physiological age in honeybees, it is assumed that foragers are not only chronologically older than nurse bees but also physiologically older. One indicator of increased physiological age in bees is oxidative stress damage. Foragers can show increased levels of oxidative carbonylation in the optic lobes and, to a smaller extent, in the mushroom bodies and antennal lobes (Seehuus et al., 2006). In addition, Neukirch demonstrated a relationship between increased foraging activity and decreased life span (Neukirch, 1982).
Division of labour in a honeybee colony is very plastic. Even when bees of identical chronological age are placed together to form a social unit (single-cohort colony), they rapidly segregate into nurse bees and foragers (Robinson et al., 1989). In single-cohort colonies, thereby, foragers start their activities much earlier in life than in normal colonies and soon display signs of increased physiological age, although they are not chronologically older than nurse bees of the same colony. Thus, this setup allows us to separately study the effects of social role and chronological age on behaviour.
In a previous study (Behrends et al., 2007), this setup was used to demonstrate that a long foraging duration (>15 days) results in impaired olfactory acquisition. However, it is unclear if the impaired olfactory learning performance in honeybee foragers is caused by a general impairment of central integration processes or if the pattern is related to a decline in specific peripheral functions such as the perception of olfactory stimuli. In addition, it is unclear whether only classical forms of learning, such as olfactory proboscis extension learning, are affected by long foraging duration. Finally, it is unknown whether long foraging duration can also lead to deficits in retention or discrimination.
To address these questions, in the present study, we analyse whether social role (nursing vs foraging) and the duration of performing this role affects tactile learning, retention and discrimination. In contrast to olfactory learning, this paradigm requires active antennal scanning movements of the bee and thus involves a strong operant component (Erber et al., 1998). Furthermore, the neuronal pathways of tactile and olfactory learning are partly distinct. In both paradigms, sucrose is used as reward. It is perceived by contact chemoreceptors at the antennal tip and on the proboscis. The perception of tactile stimuli involves mechanoreceptors on the antennal tip, which mainly project to the dorsal lobe (Haupt, 2007). Olfactory stimuli, by contrast, are perceived by contact chemoreceptors that are not located at the antennal tip and which mainly project to the antennal lobe (Mobbs, 1985) (for a review, see Galizia and Menzel, 2000). In addition, mushroom bodies are important centres for olfactory learning (Menzel, 2001; Komischke et al., 2005; Thum et al., 2007) whereas they appear to be unimportant for tactile learning (Wolf et al., 1998; Scheiner et al., 2001c).
MATERIALS AND METHODS
Preparation of bees
Experiments were conducted at the Technische Universität Berlin, Berlin, Germany. Seven single-cohort colonies were used, each consisting of approximately 2000 marked workers of the same chronological age. To obtain bees, brood combs were placed in an incubator maintained at 34°C and 70% humidity. Newly emerged workers were marked on their abdomen with a paint mixture of shellac and colour pigments and placed in a small hive together with an inseminated queen. Pollen and honey were added to prevent starvation during the first few days before division of labour was established between nurses and active foragers.
We observed the foraging activity of each colony daily during peak foraging hours (between 12:00 h and 17:00 h, depending on weather conditions). Foragers returning from presumably their first foraging trip received an additional paint mark on their thorax. Thus, the second paint mark indicated the first day of foraging activity of an individual. We could thus determine the chronological age and the foraging duration of each forager collected for behavioural tests. Nurses were defined as bees without a second paint mark on their thorax and, in addition, were poking their head into a cell with larvae. Nurses were also required to have intact wings and hairs on their thorax [extensive wing wear and loss of body hair are hallmarks of long foraging (Catar, 1992; Page and Peng, 2001)].
For experiment 1 (tactile acquisition and retention, see below) we used five colonies. There was no effect of colony on gustatory responsiveness (ρ=0.16, P=0.76), which is an indicator of general sensory responsiveness (Scheiner et al., 2004), or on acquisition (ρ=0.14, P=0.14). The cohorts were therefore pooled. For experiment 2 (tactile acquisition and discrimination, see below) we used two single-cohort colonies that also showed no difference in responsiveness or acquisition (responsiveness, ρ=0.01, P=0.95; acquisition, ρ=0.03, P=0.74) and were pooled accordingly.
For behavioural comparisons, we contrasted the same groups as Behrends and colleagues (Behrends et al., 2007): foragers with short foraging durations (6–13 days), foragers with long foraging durations (>15 days) and nurse bees of the respective chronological ages. In the study by Behrends and colleagues, foragers with long foraging durations showed reduced olfactory learning performance (Behrends et al., 2007). Bees with a foraging duration of 14 or 15 days were not tested because we did not collect bees of these age groups. All bees were collected from the combs in the experimental colonies in the morning before foraging activity started. Thus, we ensured equal conditions for the behavioural groups. Workers were collected over a period of eight weeks, and their chronological age ranged from 17 to 38 days. For data analysis, both foragers and nurse bees of corresponding chronological ages were grouped according to the number of days the foragers had foraged (6–13 days and >15 days) (Behrends et al., 2007).
For testing, bees were individually placed in glass vials and stored in a refrigerator maintained at 4°C until they showed the first signs of immobility. They were then mounted in brass tubes with a strip of adhesive tape between the head and thorax and a second strip over the abdomen, as described in Bitterman et al. (Bitterman et al., 1983). We occluded the eyes of each bee with black acrylic paint to block visual inputs during antennal scanning (Erber et al., 1998). Bees rested in a humidified chamber until the experiments started one hour later.
Measuring gustatory responsiveness
We used the proboscis extension response (PER) to measure responsiveness to water and the following sucrose concentrations: 0.1%, 0.3%, 1%, 3%, 10%, 30%, which were offered in ascending order. Each bee was stimulated with either a droplet of water or one of the six different sucrose concentrations at her antennae and it was recorded whether the bee showed proboscis extension. The inter-stimulus interval was 2 min to prevent sensitisation effects. To compare gustatory responsiveness between groups, we calculated a gustatory response score (GRS). This score is composed of the sum of responses to the seven different stimuli (water and six different sucrose concentrations). The GRS has been shown to be an excellent indicator of general responsiveness in bees (Scheiner et al., 2004). Only bees that responded at least once during stimulation with water and the six different sucrose concentrations were later used for conditioning (see below) because it was unlikely that the 30% sucrose solution, used as an unconditioned stimulus, would otherwise elicit proboscis extension in them.
Experiment 1: tactile acquisition and retention
Approximately 10 min after measuring gustatory responsiveness, bees were trained to the tactile stimulus. The tactile target, which served as conditioned stimulus, consisted of a small, rectangular, copper plate (3×4 mm) in which vertical grooves were engraved (wavelength of grooves, 450 μm; width of grooves, 150–190 μm; depth of grooves, 30–40 μm). The unconditioned stimulus and reward was a droplet of 30% sucrose solution. At the beginning of the conditioning experiment, all bees were tested for their spontaneous responses to the tactile target to be used later. Whenever a bee responded spontaneously to the pattern, she was excluded from the experiment. The number of spontaneous responses was very small and was not statistically analysed.
The bees were conditioned similarly to the tactile learning paradigm of Erber and colleagues (Erber et al., 1998). In six trials, foragers with different foraging durations (6–13 days and >15 days) and respective nurse bees of the same chronological ages could scan the plate with the vertical grooves (conditioned stimulus) for approximately 3 s before the PER was elicited by touching either antenna with a droplet of 30% sucrose solution (unconditioned stimulus). Proboscis extension (unconditioned response) was rewarded by offering a droplet of sucrose to the proboscis for approximately 1 s. The inter-trial interval during conditioning was 5 min. In each conditioning trial, it was recorded whether the bee responded to the presentation of the vertical pattern by fully extending her proboscis (conditioned response). Movements of the proboscis that did not lead to its full extension were not considered to be conditioned responses. If the bee touched the target with her proboscis, the plate was subsequently cleaned with 70% ethanol and water. For quantification of acquisition, we used an acquisition score, which shows the degree of acquisition in each group. It is composed of the sum of conditioned responses during the six acquisition trials.
In each experimental group, 30 bees were conditioned. Foragers with long foraging durations were significantly older than foragers with short foraging durations in this experiment (Table 1) (Z=6.44, P⩽0.001). The respective nurse bees also differed significantly in their chronological ages (Table 1) (Z=6.21, P⩽0.001).
Table 1.
Mean chronological ages and s.e.m. of bees tested in experiments 1 and 2
|
Mean chronological age (days)
|
||
|---|---|---|
| Group | Experiment 1 | Experiment 2 |
| Foragers foraging for 6–13 days | 24.40±0.71 | 33.77±0.87 |
| Foragers foraging for >15 days | 32.43±0.52 | 28.63±0.62 |
| Nurse bees corresponding to 6–13 days | 22.70±0.73 | 33.53±0.83 |
| Nurse bees corresponding to >15 days | 32.73±0.52 | 30.80±0.78 |
After conditioning, we measured retention in the same bees at the following time points: 5 min, 1 h, 3 h, 1 day, 2 days and 3 days after conditioning. In each test, a bee was offered a tactile plate similar to that used during conditioning, and it was recorded whether the bee showed conditioned proboscis extension while scanning the plate with her antennae for approximately 8 s. Only bees that had shown conditioned PER at least once during acquisition and bees that had survived the 3 day test were used for analysis of retention. The bees were stored in a humidified chamber between tests and were fed to repletion the night before the test and the morning of the test, approximately 5 h prior to testing.
Experiment 2: tactile acquisition and discrimination
Conditioning to the tactile plate with vertical grooves was similar to that described for acquisition and retention. However, we also tested spontaneous responses to an alternative plate with horizontal grooves (wavelength of grooves, 450 μm; width of grooves, 150–190 μm; depth of grooves, 30–40 μm) before conditioning. Whenever a bee responded spontaneously to either pattern, she was excluded from the experiment. As before, the number of spontaneous responses was very small and was not statistically analysed.
In each group, 30 bees were tested. In this experiment, foragers with long foraging durations were significantly younger than foragers with short foraging durations and in contrast to experiment 1 (Table 1) (Z=4.52, P⩽0.001). The respective nurse bees also differed significantly in their chronological ages (Table 1) (Z=2.71, P⩽0.05).
After six conditioning trials, bees were tested for tactile discrimination. Only bees that had at least shown one conditioned response during acquisition were analysed. We exposed the bees to the two patterns in five unrewarded choice tests for each pattern in the following order: horizontal, vertical, vertical, horizontal, horizontal, vertical, vertical, horizontal, horizontal, vertical. The inter-trial interval was 5 min. Proboscis extensions were counted as before. The copper plate with vertical grooves used in the discrimination tests was different from that used in the conditioning trials but it had the same pattern. To quantify the discrimination of the bees between the conditioned vertical pattern and the unrewarded horizontal pattern, a discrimination index (DI) was defined and calculated for each group as follows (Scheiner et al., 2001a):
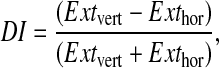 |
(1) |
where Extvert = sum of conditioned responses to the conditioned vertical pattern (`extinction' to vertical pattern), Exthor = sum of responses to the alternative horizontal pattern (`extinction' to horizontal pattern). This DI ranges from –1 to 1. If DI=0, no discrimination takes place. If DI>0, the bees prefer the conditioned pattern. If DI<0, they prefer the alternative pattern. Bees that did not respond in any of the discrimination tests received a DI of 0.
Statistics
Chronological age, GRS, acquisition scores and discrimination indices of different groups were compared using two-tailed Mann–Whitney U-tests (SPSS 15.0, Chicago, IL, USA) because the data of some of the groups did not follow a normal distribution. To test for correlations between colony, GRS, acquisition scores and discrimination indices, we used Spearman rank correlations (SPSS 15.0). The number of bees showing the conditioned PER in the last conditioning trial and in the different retention tests was compared between groups with Fisher exact probability tests (GraphPad Instat 3.06, San Diego, CA, USA). The course of acquisition was compared by fitting exponential saturating functions of the type f(x)=a[1–exp(–bx)] to the acquisition curves (Sigma Plot 2001, parameters in Table 2). The slopes of the regression functions were compared between groups using two-tailed Welsh's t-tests (GraphPad Instat 3.06). For all comparisons between the four groups, we used Bonferroni corrections to avoid type I errors.
Table 2.
Values for variables a and b of the exponential saturating functions fitting the acquisition curves for all four behavioural groups in experiments 1 and 2
| Group | Parameter a, ± s.e.m. and significance level | Parameter b, ± s.e.m. and significance level |
|---|---|---|
| Foragers foraging for 6–13 days (experiment 1) | a=82.98±0.69 | b=0.64±0.02 |
| P⩽0.001 | P⩽0.001 | |
| Foragers foraging for 6–13 days (experiment 2) | a=91.08±1.09 | b=1.41±0.09 |
| P⩽0.001 | P⩽0.001 | |
| Nurse bees corresponding to 6–13 days (experiment 1) | a=86.22±1.35 | b=1.13±0.08 |
| P⩽0.001 | P⩽0.001 | |
| Nurse bees corresponding to 6–13 days (experiment 2) | a=72.24±2.92 | b=0.79±0.11 |
| P⩽0.001 | P⩽0.01 | |
| Foragers foraging for >15 days (experiment 1) | a=45.99±3.14 | b=0.52±0.09 |
| P⩽0.001 | P⩽0.01 | |
| Foragers foraging for >15 days (experiment 2) | a=117.51±32.94 | b=0.18±0.07 |
| P⩽0.05 | P=0.07 | |
| Nurse bees corresponding to >15 days (experiment 1) | a=66.38±1.44 | b=1.88±0.29 |
| P⩽0.001 | P⩽0.01 | |
| Nurse bees corresponding to >15 days (experiment 2) | a=88.10±1.14 | b=1.50±0.11 |
| P⩽0.001 | P⩽0.001 |
Bold font indicates significant results
RESULTS
Experiment 1: tactile acquisition and retention
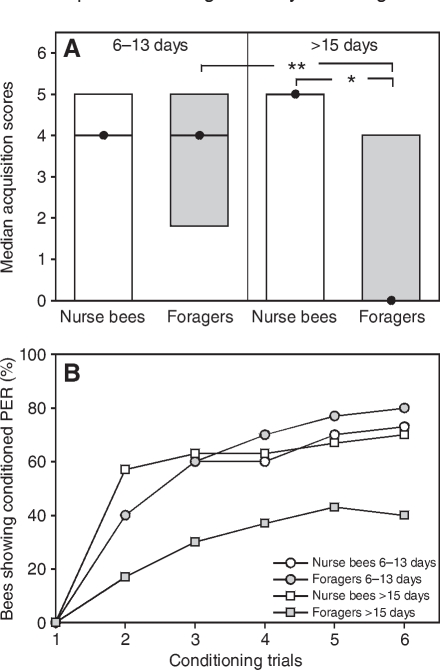
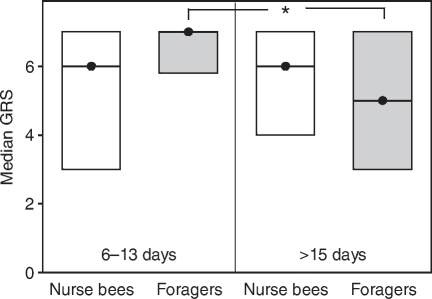
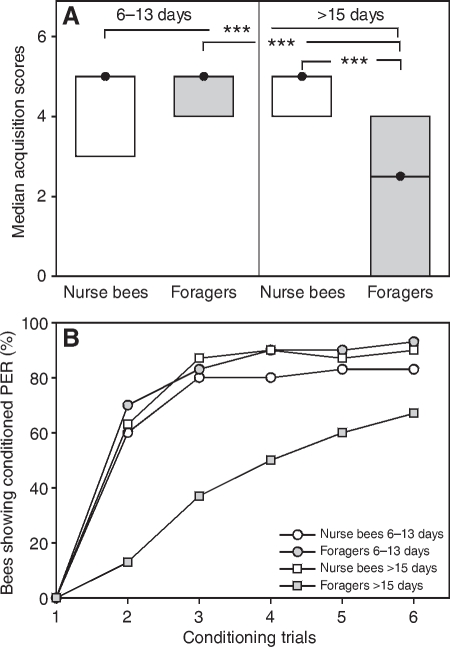
Chronological age did not correlate with tactile acquisition scores (ρ=0.10, P=0.30), i.e. bees with high vs low chronological age did not differ in their acquisition scores. Gustatory responsiveness is a decisive determinant of tactile acquisition (Scheiner et al., 1999; Scheiner et al., 2001a; Scheiner et al., 2001b; Scheiner et al., 2003). Bees with high gustatory responsiveness, i.e. bees with high GRS, learn quickly whereas bees with low GRS generally perform poorly. In this experiment, the GRS of the four behavioural groups did not differ (Fig. 1; Table 3). We therefore expected that these groups would also not differ in their tactile acquisition. However, foragers with long foraging durations (>15 days) had significantly lower acquisition scores than age-matched nurse bees (Fig. 2A) (Z=2.68, P⩽0.05) and foragers with short foraging durations (6–13 days) (Fig. 2A) (Z=3.00, P⩽0.01). This demonstrates an effect of foraging duration on tactile acquisition. The two nurse bee groups, by contrast, did not differ from each other (Fig. 2A) (Z=0.74, P=0.91). This shows that learning impairment only occurs in one of the two social roles analysed. Foragers with short foraging durations (6–13 days) did not differ in their tactile acquisition scores from age-matched nurse bees (Fig. 2) (Z=0.44, P=0.99). The tactile acquisition curves of the four groups are shown in Fig. 2B. For the tactile acquisition curves, the slope of the fitting saturating function (see Materials and methods) was significantly less steep in foragers with long foraging durations (>15 days) compared with age-matched nurse bees (see Table 2 for parameters of functions: comparison of slopes, t=4.48, d.f.=5, P⩽0.01). This result implies that foragers with long foraging durations (>15 days) learned more slowly than age-matched nurse bees. In addition, they reached a significantly lower level of acquisition than foragers with short foraging durations (6–13 days) (Fig. 2B) (P⩽0.01) but they did not differ from age-matched nurse bees (P=0.14). The lower acquisition scores of foragers with long foraging durations (>15 days) are therefore a result of slow acquisition and a low level of conditioned responses at the end of the conditioning phase.
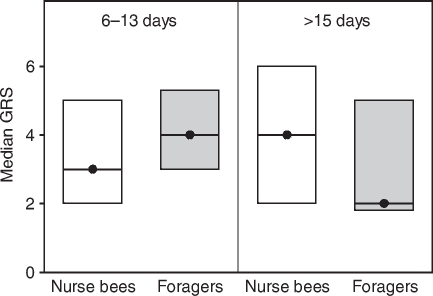
Fig. 1.
Median gustatory response scores (GRS, closed circles) and quartiles (upper and lower lines) of bees tested for tactile acquisition in experiment 1. We analysed foragers with short foraging durations (6–13 days) and age-matched nurse bees as well as foragers with long foraging durations (>15 days) and age-matched nurse bees. Note that in this experiment, foragers with long foraging durations (>15 days) and age-matched nurse bees were significantly older than foragers with short foraging durations (6–13 days) and age-matched nurse bees (see Table 1). There were no significant differences in the GRS between the different groups (see Table 3). In each group, 30 bees were tested.
Table 3.
Comparison of gustatory response scores (GRS) of foragers with different foraging durations and age-matched nurse bees tested for tactile acquisition in experiments 1 and 2
| Group comparisons | Experiment 1 GRS | Experiment 2 GRS |
|---|---|---|
| Foragers foraging for 6–13 days vs nurse bees corresponding to 6–13 days | Z=1.12 P=0.70 | Z=1.04 P=0.76 |
| Foragers foraging for 6–13 days vs foragers foraging for >15 days | Z=1.82 P=0.25 | Z=3.00 P⩽0.01 |
| Foragers foraging for >15 days vs nurse bees corresponding to >15 days | Z=1.72 P=0.31 | Z=0.56 P=0.97 |
| Nurse bees corresponding to 6–13 days vs nurse bees corresponding to >15 days | Z=1.17 P=0.67 | Z=1.57 P=0.40 |
Bold font indicates significant results
Fig. 2.
(A) Median acquisition scores (closed circles) and quartiles (upper and lower lines) for tactile acquisition in bees with different behavioural roles (experiment 1). We analysed foragers with short foraging durations (6–13 days) and age-matched nurse bees as well as foragers with long foraging durations (>15 days) and age-matched nurse bees. Note that in this experiment, foragers with long foraging durations (>15 days) and age-matched nurse bees were significantly older than foragers with short foraging durations (6–13 days) and age-matched nurse bees (see Table 1). Significant differences in the acquisition scores of groups are marked with asterisks. *P⩽0.05; **P⩽0.01. (B) Tactile acquisition curves of bees in experiment 1 with different behavioural roles. The percentage of bees showing conditioned proboscis extension response (PER) during presentation of the tactile object in each conditioning trial is shown for each behavioural group. See text for statistics. In each group, 30 bees were tested.
Of each of the four behavioural groups, only bees with an acquisition score >0 that survived the last retention test three days after conditioning were selected for retention analysis. Mortality was low: three bees in the group of nurse bees (6–13 days) and four bees in the group of foragers (6–13 days) died before the 3 day test. There was no relationship between survival rate and acquisition as the mean acquisition score of surviving bees was not different from that of non-survivors [mean acquisition score of surviving nurse bees 6–13 days, 4.00±0.26 (±s.e.m.), N=19; mean acquisition score of non-surviving nurse bees 6–13 days, 5.00±0.00 (±s.e.m.), N=3; comparison, Z=1.71, P=0.13; mean acquisition score of surviving foragers 6–13 days, 3.72±0.33 (±s.e.m.), N=22; mean acquisition score of non-surviving foragers 6–13 days, 4.00±0.41 (±s.e.m.), N=4; comparison, Z=0.11, P=0.92)].
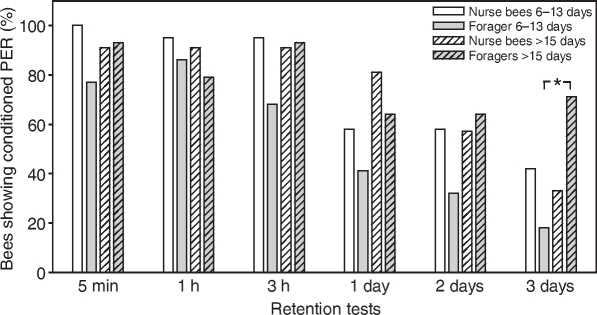
In this subset of workers, GRS did not differ (Fig. 3; Table 4). Foragers with long foraging durations (>15 days) had significantly lower acquisition scores than age-matched nurse bees but they did not differ from foragers with short foraging durations (6–13 days) (Table 4). In the three tests up to 3 h after conditioning, the number of bees showing conditioned PER was similarly high in all groups (Fig. 4). Retention one day and two days after conditioning also did not differ between groups. In the final test, three days after conditioning, foragers with long foraging durations (>15 days), interestingly, showed significantly more conditioned responses than foragers with short foraging durations (6–13 days) (P⩽0.05). These data show that retention is not impaired in the subset of foragers with long foraging durations (>15 days) that survived the final test and, in addition, showed some acquisition in the conditioning phase.
Fig. 3.
Median gustatory response scores (GRS, closed circles) and quartiles (upper and lower lines) of bees tested for tactile retention three days after conditioning in experiment 1. We analysed foragers with short foraging durations (6–13 days) and age-matched nurse bees as well as foragers with long foraging durations (>15 days) and age-matched nurse bees. Only bees that had an acquisition score of 1 or higher and that survived the last retention test were analysed. There were no significant differences in the GRS between the different groups (see Table 4). The numbers of bees in each group are: nurse bees 6–13 days, 19; foragers 6–13 days, 22; nurse bees >15 days, 22; foragers >15 days, 14.
Table 4.
Comparison of gustatory response scores (GRS) and acquisition scores of bees tested for retention in experiment 1 and for discrimination in experiment 2
|
Experiment 1
|
Experiment 2
|
|||
|---|---|---|---|---|
| Group comparisons | GRS | Acquisition scores | GRS | Acquisition scores |
| Foragers foraging for 6–13 days vs nurse bees corresponding to 6–13 days | Z=0.28 P=1.00 | Z=0.19 P=1.00 | Z=2.08 P=0.15 | Z=0.78 P=0.90 |
| Foragers foraging for 6–13 days vs foragers foraging for > 15 days | Z=0.17 P=1.00 | Z=0.50 P=0.98 | Z=2.20 P=0.19 | Z=3.81 P⩽0.001 |
| Foragers foraging for > 15 days vs nurse bees corresponding to > 15 days | Z=1.29 P=0.61 | Z=2.53 P⩽0.05 | Z=1.00 P=0.81 | Z=3.91 P⩽0.001 |
| Nurse bees corresponding to 6–13 days vs nurse bees corresponding to > 15 days | Z=1.57 P=0.41 | Z=2.11 P=0.25 | Z=1.22 P=0.64 | Z=1.69 P=0.32 |
Bold font indicates significant results
Fig. 4.
Retention after tactile conditioning in bees of different behavioural groups (experiment 1). Percentage of bees showing the conditioned proboscis extension response (PER) at different time points after conditioning is presented. The only significant difference between groups is indicated: *P⩽0.05. The numbers of bees in each group are: nurse bees 6–13 days, 19; foragers 6–13 days, 22; nurse bees >15 days, 22; foragers >15 days, 14.
Experiment 2: tactile acquisition and discrimination
As in experiment 1, we found no correlation between chronological age and tactile acquisition scores (ρ=0.12, P=0.21). In this experiment, the GRS of foragers with long foraging durations (>15 days) were significantly lower than those of bees with shorter foraging durations (6–13 days) (Fig. 5; Table 3). The other groups did not differ in their gustatory responsiveness (Table 3). Foragers with long foraging durations (>15 days) had significantly lower acquisition scores than age-matched nurse bees (Fig. 6A) (Z=4.21, P⩽0.001) and foragers with short foraging durations (6–13 days) (Fig. 6A) (Z=4.46, P⩽0.001). By contrast, the two respective groups of nurse bees did not differ (Fig. 6A) (Z=0.47, P=0.98). Foragers with short foraging durations (6–13 days) did not differ in their tactile acquisition scores from age-matched nurse bees (Fig. 6A) (Z=0.91, P=0.84).
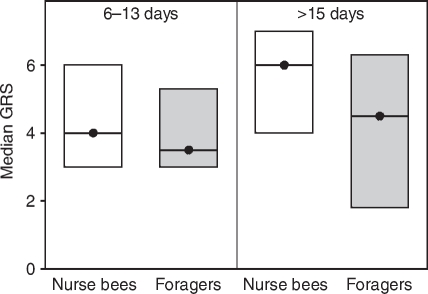
Fig. 5.
Median gustatory response scores (GRS, closed circles) and quartiles (upper and lower lines) of bees tested for tactile acquisition in experiment 2. We analysed foragers with short foraging durations (6–13 days) and age-matched nurse bees as well as foragers with long foraging durations (>15 days) and age-matched nurse bees. Note that in this experiment, foragers with long foraging durations (>15 days) and age-matched nurse bees were significantly younger than foragers with short foraging durations (6–13 days) and age-matched nurse bees (see Table 1). Foragers with long foraging durations (>15 days) had significantly lower GRS than foragers with short foraging durations (6–13 days) (Table 3). This significant difference is marked with an asterisk (*P⩽0.05). The other groups did not differ in their GRS. In each group, 30 bees were tested.
Fig. 6.
(A) Median acquisition scores (closed circles) and quartiles (upper and lower lines) for tactile antennal acquisition in bees with different behavioural roles (experiment 2). We analysed foragers with short foraging durations (6–13 days) and age-matched nurse bees as well as foragers with long foraging durations (>15 days) and age-matched nurse bees. Note that in this experiment, foragers with long foraging durations (>15 days) and age-matched nurse bees were significantly younger than foragers with short foraging durations (6–13 days) and age-matched nurse bees (see Table 1). Significant differences in the acquisition scores of groups are marked with asterisks. ***P⩽0.001. (B) Tactile acquisition curves of bees in experiment 2 with different behavioural roles. The percentage of bees showing conditioned proboscis extension response (PER) during presentation of the tactile object in each conditioning trial is shown for each behavioural group. See text for statistics. In each group, 30 bees were tested.
These results support the findings of experiment 1 and demonstrate that tactile acquisition in foragers with long foraging durations (>15 days) is impaired. Similar to experiment 1, the low acquisition scores of foragers with long foraging durations (>15 days) were partly a result of slow acquisition (Fig. 6B). The slope of the exponential satiation function fitting the acquisition curve (see Table 2 for parameters) was significantly less steep for foragers with long foraging durations (>15 days) than for age-matched nurse bees (t=10.20, d.f.=8, P⩽0.001) and foragers with short foraging durations (6–13 days) (t=10.86, d.f.=9, P⩽0.001). The percentage of bees showing the conditioned response in the final acquisition trial did not differ between foragers with long foraging durations (>15 days) and foragers with short foraging durations (6–13 days) (P=0.018; Bonferroni corrected significance level for 5% probability of type I error, 0.017) or between foragers with long foraging durations (>15 days) and age-matched nurse bees (P=0.21). Part of the learning differences can be explained by differences in GRS because GRS generally correlate with performance during acquisition, with highly responsive bees showing higher acquisition scores than unresponsive bees (Scheiner et al., 1999; Scheiner et al., 2001a; Scheiner et al., 2001b; Scheiner et al., 2001c; Scheiner et al., 2003; Scheiner et al., 2005). Foragers with long foraging durations (>15 days) were less responsive to sucrose than foragers with short foraging durations (6–13 days) and might therefore have displayed lower acquisition scores. By contrast, the learning differences between foragers (>15 days) and age-matched nurse bees are not related to different GRS because these groups do not differ in their GRS.
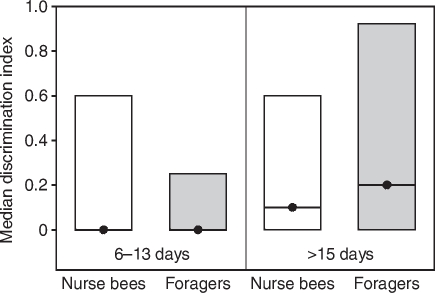
Only bees with an acquisition score >0 were analysed for tactile discrimination. In this subset of bees, all of which survived the discrimination test, the GRS of the four behavioural groups did not differ from each other (Fig. 7; Table 4). Foragers with long foraging durations (>15 days) had significantly lower acquisition scores than age-matched nurse bees and foragers with short foraging durations (6–13 days) (Table 4). For analysis of discrimination, we calculated a DI (see Materials and methods). There was no correlation between chronological age and tactile DI (ρ=0.14, P=0.17). Foragers with long foraging durations (>15 days) did not differ in their DI from age-matched nurse bees (Fig. 8) (Z=0.39, P=0.97) or foragers with short foraging durations (6–13 days) (Fig. 8) (Z=1.81, P=0.25). Foragers with short foraging durations (6–13 days) also did not differ from age-matched nurse bees (Fig. 8) (Z=1.00, P=0.79). Nurse bees corresponding to foragers with short foraging durations (6–13 days) did not differ from nurse bees corresponding to foragers with long foraging durations (>15 days) (Z=0.55, P=0.97).
Fig. 7.
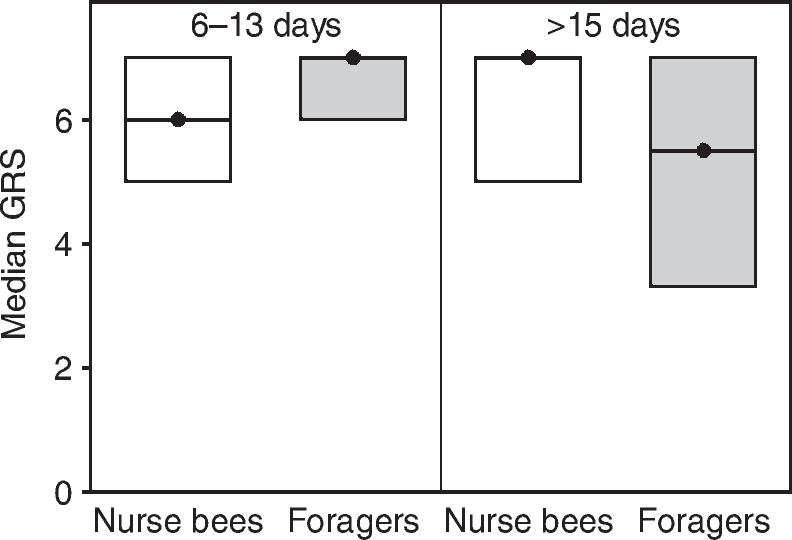
Median gustatory response scores (GRS, closed circles) and quartiles (upper and lower lines) of bees tested for tactile discrimination in experiment 2. We analysed foragers with short foraging durations (6–13 days) and age-matched nurse bees as well as foragers with long foraging durations (>15 days) and age-matched nurse bees. Only bees that had an acquisition score of 1 or higher were analysed for discrimination. The numbers of bees in each group are: nurse bees 6–13 days, 27; foragers 6–13 days, 28; nurse bees >15 days, 27; foragers >15 days, 20.
Fig. 8.
Median discrimination indices (closed circles) and quartiles (upper and lower lines) of bees tested for discrimination after tactile conditioning (experiment 2). There were no significant differences in the discrimination indices of the four different groups. The numbers of bees in each group are: nurse bees 6–13 days, 27; foragers 6–13 days, 28; nurse bees >15 days, 27; foragers >15 days, 20.
Acquisition scores correlated negatively with discrimination indices (ρ=0.36, P⩽0.001). Individuals with high acquisition scores showed poor discrimination whereas bees with low acquisition scores discriminated well between the two tactile patterns. In addition, discrimination indices correlated positively with extinction scores (ρ=0.30, P⩽0.01). Bees with high discrimination indices showed little extinction. These experiments demonstrate that foragers with long foraging durations (>15 days) do not show an impaired discrimination.
DISCUSSION
Tactile acquisition
Our results show an impairment of associative tactile acquisition in honeybees that is linked to social role and the duration of performing this role but not to chronological age. Specifically, foragers with long foraging durations (>15 days) displayed significantly poorer tactile acquisition than age-matched nurse bees and foragers with short foraging durations (6–13 days). This finding suggests that with increasing foraging duration, bees have increasing problems in acquiring new information. If the bees spent the same amount of time in the hive working as nurse bees, they did not display any deficits in tactile acquisition. Unlike in many other studies (Scheiner et al., 1999; Scheiner et al., 2001a; Scheiner et al., 2001b; Scheiner et al., 2003), differences in gustatory responsiveness are not the main reason for the poor acquisition performance in foragers with long foraging durations because GRS mostly did not differ between groups (see Table 3).
It is also conceivable that foragers with long foraging durations (>15 days) displayed a poorer acquisition than foragers with short foraging durations (6–13 days) in experiment 2 because they scanned the tactile stimuli differently (i.e. less effectively). Tactile scanning activity was shown to correlate with GRS (Scheiner et al., 2005). Because foragers with long foraging durations (>15 days) had significantly lower GRS than foragers with short foraging durations in this experiment, the learning differences could be related to differences in scanning behaviour. However, GRS between foragers with long foraging durations (>15 days) and all of the other groups did not differ significantly in either experiment 1 or experiment 2. These learning differences are therefore unlikely to be related to differences in scanning behaviour, although we have not tested scanning behaviour directly in this experiment.
Our current data are well in line with the findings of Behrends and colleagues who showed an impairment of olfactory acquisition in foragers with long foraging durations compared with foragers with short foraging durations (Behrends et al., 2007). Our data also support the findings of Rueppell and colleagues who showed that age per se has no effect on olfactory acquisition learning (Rueppell et al., 2007). In other experiments analysing the effect of chronological age and behavioural role on associative olfactory learning in honeybees, no learning differences were detected between normal-aged nurse bees (5–7 days old) and foragers (>21 days old) under similar conditions as in our experiments (Ben-Shahar and Robinson, 2001). However, like Rueppell and colleagues (Rueppell et al., 2007), the authors did not measure how long the workers had been foraging. The same applies to the study by Bhagavan and colleagues who failed to detect effects of age and behavioural role on olfactory learning using a different experimental setup (Bhagavan et al., 1994). Due to the short life expectancy of bees after foraging onset, it is likely that old foragers were represented at such a low frequency in these sample populations that the average learning performance of the foragers was not affected. Furthermore, the slow acquisition of foragers with long foraging durations in our present experiments compares nicely with the foraging behaviour of bees during their lifetime. Tofilski showed that foragers needed significantly more time for handling flowers shortly before they died than they did in the days before (Tofilski, 2000).
However, this increased handling time of flowers, combined with our results on slow acquisition and intact long-term memory of foragers with long foraging duration, could alternatively indicate an increased floral constancy in this group. The cost of acquiring a new floral source might lead to a slower acquisition in foragers with long foraging durations. In the cabbage white butterfly (Pieris rapae), Lewis not only demonstrated that the handling time of flowers decreased with the number of visits but also the butterflies that were forced to switch flower sources were less effective and less experienced (Lewis, 1986). However, different age groups were not compared in their study. We cannot exclude that our conditioning procedure affected foragers with short vs long foraging durations differently. Foragers with long foraging durations could learn a new odour more slowly due to trade-off costs that are not experienced by, or may even benefit, nurse bees and foragers with shorter foraging durations. Similarly, Drosophila learning experiments show a trade-off between learning ability and larval competitive ability; improved learning performance was related to a reduced larval competitive ability in finding food (Mery and Kawecki, 2003). In the future, a deeper analysis of behaviour in free-flying bees with different foraging durations should be combined with high-solution comparisons of brain compartments to help answer whether the slow acquisition of foragers with long foraging experience can reflect a life-history trade-off.
Retention after tactile conditioning
Retention in up to two days after conditioning did not differ between foragers with long foraging durations and age-matched nurse bees or foragers with shorter foraging durations. Three days after conditioning, however, foragers with long foraging durations showed more conditioned responses than foragers with short foraging durations. For this experiment, our sample sizes are small and therefore the results are suggestive. These constraints are due to the challenge of producing larger numbers of foragers with long foraging durations that display some learning in the acquisition phase and survive for three days after training. Yet in support of our results, similar data were obtained in a recent independent study on olfactory conditioning and long-term memory in foragers (D. Münch and G.V.A., unpublished).
There are several possible explanations for this finding. Firstly, it could imply that even though foragers with long foraging durations displayed poor acquisition on average, the long-term memory of the selected subset of `surviving learners', i.e. bees that survived for three days after acquisition, was very good.
Another reason for this phenomenon could be that foragers with long foraging durations show less extinction over days. In our experiments, we repeatedly measured retention performance in the same individuals, thus we cannot exclude effects of extinction on our level of responses. In the short-term extinction tests following tactile acquisition in experiment 2, foragers with long foraging durations did not differ from the other groups. This suggests that the excellent performance in the last retention test three days after conditioning of foragers with long foraging durations is related to little extinction in the time course of days but not in the time course of minutes after the training. The better retention of foragers with long foraging durations compared with those with shorter foraging durations could also imply that after a prolonged duration of foraging, bees show stronger `flower constancy' than after short periods of foraging. This interpretation would be in line with the finding by Schippers and colleagues who showed that foraging success of inexperienced foragers increased over their first foraging days (Schippers et al., 2006). Although in their experiments, the maximum foraging success was reached on day seven, it is conceivable that our bees, taken from single-cohort colonies and under different environmental conditions, experienced a similar increase in retention performance with foraging duration. In addition, the life expectancy and foraging durations in our experiment were much longer than in the experiments by Schippers and colleagues (Schippers et al., 2006).
In the fruit fly Drosophila, Brigui and colleagues showed that older flies displayed less extinction of conditioned suppression of the PER than younger flies (Brigui et al., 1990). Kane and colleagues showed that protein kinase C (PKC)-deficient flies, which failed to show immediate suppression of courtship behaviour in courtship conditioning, nevertheless displayed good memory of this behaviour afterwards (Kane et al., 1997). Thus, more experiments are clearly needed to elucidate the relationship between foraging duration, extinction and retention performance in honeybees.
Another possible explanation for the pattern of retention in our experiment is that the conditioned responses three days after conditioning could be related to the metabolic or nutritional status of the bees. It has previously been shown that the time of feeding before the conditioning experiment affects the level of olfactory PER learning and memory (Friedrich et al., 2004). Although we equalised the conditions for all behavioural groups by a uniform feeding protocol, the metabolic turnover or residual nutritional stores of foragers with long foraging durations might have been different from that of age-matched nurse bees or foragers with shorter foraging durations. Thus, the feeding regime, which meant presenting the unconditioned stimulus in the absence of the conditioned stimulus, as well as a possible difference in the sucrose metabolism and nutrient storage of the bees, might confound the retention effects outlined above. As bees have to be fed during trials that last for several days, this factor cannot be excluded from the experimental situation. However, better insight into the physiological differences between foragers with long vs short foraging durations will help to resolve these ambiguities in the future.
Tactile discrimination
Long foraging duration did not impair discrimination, in contrast to tactile acquisition. Foragers with long foraging durations did not differ from age-matched nurse bees or from foragers with short foraging durations in their DI. Our experiments on tactile discrimination of the different behavioural groups support the findings of Behrends and colleagues on olfactory discrimination (Behrends et al., 2007). In contrast to our present experiments, however, Behrends and colleagues only tested responses to a conditioned odour and to an alternative odour once (Behrends et al., 2007). In their experiments, foragers with long foraging durations also did not differ in their response level to the alternative odour from age-matched nurse bees or from foragers with short foraging durations. Interestingly, Bittermann suggests a relationship between good initial discrimination and low extinction rate (Bittermann, 1972). In our present experiments, we also found a positive correlation between tactile discrimination and extinction in all groups tested.
LIST OF ABBREVIATIONS
- DI
discrimination index
- Exthor
extinction to the alternative horizontal pattern
- Extvert
extinction to the conditioned vertical pattern
- GRS
gustatory response score
- PER
proboscis extension response
We would like to thank Benedikt Polaczek for expert knowledge and advice on beekeeping. Our thanks to Andreas Behrends, Christa Finkenwirth, Sonja Kauffmann, Anna Toteva and Jeannine Wolf for their help with the experiments. We are grateful to Daniel Münch for his input on the interpretation of our results and to Joachim Erber for many fruitful discussions on the experimental data and the manuscript. We also like to thank the three anonymous reviewers for very helpful comments on the manuscript. This work was supported by grants from the Norwegian Research Council (NFR #175413), NIH (#P01 AG22500) and the PEW Foundation to G.V.A. and a grant from the Deutsche Forschungsgemeinschaft to R.S. (SCHE 1573/1-1). Deposited in PMC for release after 12 months.
References
- Behrends, A., Scheiner, R., Baker, N. and Amdam, G. V. (2007). Cognitive aging is linked to social role in honey bees (Apis mellifera). Exp. Gerontol. 42, 1146-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar, Y. and Robinson, G. E. (2001). Satiation differentially affects performance in a learning assay by nurse and forager honey bees. J. Comp. Physiol. A 187, 891-899. [DOI] [PubMed] [Google Scholar]
- Bhagavan, S., Benatar, S., Cobey, S. and Smith, B. H. (1994). Effect of genotype but not of age or caste on olfactory learning performance in the honey bee, Apis mellifera. Anim. Behav. 48, 1357-1369. [Google Scholar]
- Bitterman, M. E. (1972). Comparative studies of the role of inhibition in reversal learning. In Inhibition and Learning (ed. R.A. Boakes and M.S. Halliday), pp. 153-176. London: Academic Press.
- Bitterman, M. E., Menzel, R., Fietz, A. and Schäfer, S. (1983). Classical conditioning extension in honeybees (Apis mellifera). J. Comp. Physiol. 97, 107-119. [PubMed] [Google Scholar]
- Brigui, N., Le Bourg, E. and Médioni, J. (1990). Conditioned suppression of the proboscis-extension response in young, middle-aged and old Drosophila melanogaster flies: acquisition and extinction. J. Comp. Psychol. 104, 289-296. [DOI] [PubMed] [Google Scholar]
- Cartar, R. V. (1992). Morphological senescence and longevity: an experiment relating wing wear and life span in foraging wild bumble bees. J. Anim. Ecol. 61, 225-231. [Google Scholar]
- Erber, J., Kierzek, S., Sander, E. and Grandy, K. (1998). Tactile learning in the honeybee. J. Comp. Physiol. A 183, 737-744. [Google Scholar]
- Friedrich, A., Thomas, U. and Müller, U. (2004). Learning at different satiation levels reveals parallel functions for the cAMP-protein kinase A cascade in formation of long-term memory. J. Neurosci. 24, 4460-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia, C. G., Menzel, R. (2000). Odour perception in honeybees: coding information in glomerular patterns. Curr. Opin. Neurobiol. 10, 504-510. [DOI] [PubMed] [Google Scholar]
- Grotewiel, M. S., Martin, I., Bhandari, P. and Cook-Wiens, E. (2005). Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 3, 372-397. [DOI] [PubMed] [Google Scholar]
- Haupt, S. S. (2007). Central gustatory projections and side-specificity of operant antennal muscle conditioning in the honeybee. J. Comp. Physiol. A 193, 523-535. [DOI] [PubMed] [Google Scholar]
- Kane, N. S., Robichon, A., Dickinson, J. A. and Greenspan, R. J. (1997). Learning without performance in PKC-deficient Drosophila. Neuron 18, 307-314. [DOI] [PubMed] [Google Scholar]
- Komischke, B., Sandoz, J. C. and Malun, D. (2005). Partial unilateral lesions of the mushroom bodies affect olfactory learning in honeybees Apis mellifera L. Eur. J. Neurosci. 21, 477-485. [DOI] [PubMed] [Google Scholar]
- Lewis, A. C. (1986). Memory constraints and flower choice in Pieris rapae. Science 232, 863-865. [DOI] [PubMed] [Google Scholar]
- Marriott, J. G. and Abelson, J. S. (1980). Age differences in short-term memory of test-sophisticated rhesus monkeys. Age 3, 7-9. [Google Scholar]
- Mell, T., Heekeren, H. R., Marschner, A., Wartenburger, I., Villringer, A. and Reischies, F. M. (2005). Effect of aging on stimulus-reward association learning. Neuopsychologia 43, 554-563. [DOI] [PubMed] [Google Scholar]
- Menzel, R. (2001). Searching for the memory trace in a mini-brain, the honeybee. Learn. Mem. 8, 53-62. [DOI] [PubMed] [Google Scholar]
- Mery, F. and Kawecki, T. J. (2003). A fitness cost of learning ability in Drosophila melanogaster. Proc. Biol. Sci. 270, 2465-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs, P. (1985). Brain structure. In Comprehensive Insect Physiology, Biochemistry and Pharmacology (ed. G. A. Kerkut and L. I. Gilbert), pp. 299-370. Oxford: Pergamon.
- Murakami, S. and Murakami, H. (2005). The effects of aging and oxidative stress on learning behavior in C. elegans. Neurobiol. Aging 26, 899-905. [DOI] [PubMed] [Google Scholar]
- Neukirch, A. (1982). Dependence of the life span of the honeybee (Apis mellifera) upon flight performance and energy consumption. J. Comp. Physiol. A 146, 35-40. [Google Scholar]
- Page, R. E. and Peng, C. Y. (2001). Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 36, 695-711. [DOI] [PubMed] [Google Scholar]
- Robinson, G. E., Page, R. E., Strambi, C. and Strambi, A. (1989). Hormonal and genetic control of behavioral integration in honeybee colonies. Science 246, 109-112. [DOI] [PubMed] [Google Scholar]
- Rueppell, O., Christine, S., Mulcrone, C. and Groves, L. (2007). Aging without functional senescence in honey bee workers. Curr. Biol. 17, R274-R275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner, R., Erber, J. and Page, R. E. (1999). Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.). J. Comp. Physiol. A 185, 1-10. [DOI] [PubMed] [Google Scholar]
- Scheiner, R., Page, R. E. and Erber, J. (2001a). Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav. Brain Res. 120, 67-73. [DOI] [PubMed] [Google Scholar]
- Scheiner, R., Page, R. E. and Erber, J. (2001b). The effects of genotype, foraging role and sucrose perception on the tactile learning performance of honey bees (Apis mellifera L.). Neurobiol. Learn. Mem. 76, 138-150. [DOI] [PubMed] [Google Scholar]
- Scheiner, R., Weiß, A., Malun, D. and Erber, J. (2001c). Learning in honey bees with brain lesions: how partial mushroom-body ablations affect sucrose responsiveness and tactile learning. Anim. Cogn. 4, 227-235. [Google Scholar]
- Scheiner, R., Barnert, M. and Erber, J. (2003). Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie 34, 67-72. [Google Scholar]
- Scheiner, R., Page, R. E. and Erber, J. (2004). Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 35, 133-142. [Google Scholar]
- Scheiner, R., Kuritz-Kaiser, A., Menzel, R. and Erber, J. (2005). Sensory responsiveness and the effects of equal subjective rewards on tactile learning and memory of honey bees. Learn. Mem. 12, 626-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers, M. P., Dukas, R., Smith, R. W., Wang, J., Smolen, K. and McClelland, G. B. (2006). Lifetime performance in foraging honeybees: behaviour and physiology. J. Exp. Biol. 209, 3828-3836. [DOI] [PubMed] [Google Scholar]
- Seehuus, S. C., Krekling, T. and Amdam, G. V. (2006). Cellular senescence in honey bee brain is largely independent of chronological age. Exp. Gerontol. 41, 1117-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, T. D. (1995). The Wisdom of the Hive. Cambridge, MA: Harvard University Press.
- Soei, E. and Daum, I. (2008). Course of relational and non-relational recognition memory across the adult lifespan. Learn. Mem. 15, 21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum, A. S., Jenett, A., Ito, K., Heisenberg, M. and Tanimoto, H. (2007). Multiple memory traces for olfactory reward learning in Drosophila. J. Neurosci. 24, 11132-11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofilski, A. (2000). Senescence and learning in honeybee (Apis mellifera) workers. Acta Neurobiol. Exp. 60, 35-39. [DOI] [PubMed] [Google Scholar]
- Visscher, P. K. and Dukas, R. (1997). Survivorship of foraging honey bees. Insectes Soc. 44, 1-5. [Google Scholar]
- Winston, M. L. (1987). The Biology of the Honey Bee. Cambridge, MA: Harvard University Press.
- Wolf, R., Wittig, T., Liu, L., Wustman, G., Eyding, D. and Heisenberg, M. (1998). Drosophila mushroom bodies are dispensable for visual, tactile, and motor learning. Learn. Mem. 5, 166-178. [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak, D. S., Seta, S. E., Roker, L. A. and Lehr, M. A. (2008). Effects of paradigms and inter-stimulus interval on age differences in eyeblink classical conditioning in rabbits. Learn. Mem. 14, 287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L., Tucci, V., Kishi, S. and Zhdanova, I. V. (2006). Cognitive Aging in Zebrafish. PLoS ONE 1, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]