ABSTRACT
BACKGROUND
Opioid-dependent patients often have co-occurring chronic illnesses requiring medications that interact with methadone. Methadone maintenance treatment (MMT) is typically provided separately from medical care. Hence, coordination of medical care and substance use treatment is important to preserve patient safety.
OBJECTIVE
To identify potential safety risks among MMT patients engaged in medical care by evaluating the frequency that opioid dependence and MMT documentation are missing in medical records and characterizing potential medication-methadone interactions.
METHODS
Among patients from a methadone clinic who received primary care from an affiliated, but separate, medical center, we reviewed electronic medical records for documentation of methadone, opioid dependence, and potential drug-methadone interactions. The proportions of medical records without opioid dependence and methadone documentation were estimated and potential medication-methadone interactions were identified.
RESULTS
Among the study subjects ( = 84), opioid dependence documentation was missing from the medical record in 30% (95% CI, 20%–41%) and MMT documentation was missing from either the last primary care note or the last hospital discharge summary in 11% (95% CI, 5%-19%). Sixty-nine percent of the study subjects had at least 1 medication that potentially interacted with methadone; 19% had 3 or more potentially interacting medications.
CONCLUSION
Among patients receiving MMT and medical care at different sites, documentation of opioid dependence and MMT in the medical record occurs for the majority, but is missing in a substantial number of patients. Most of these patients are prescribed medications that potentially interact with methadone. This study highlights opportunities for improved coordination between medical care and MMT.
KEY WORDS: methadone, medication interactions, patient safety, care coordination
INTRODUCTION
Both the Institute of Medicine and the Joint Commission have focused attention on the role that medication discrepancies and errors play in adverse events and decreased quality of care.1,2 For hospital, ambulatory, and behavioral healthcare, a Joint Commission goal is to “accurately and completely reconcile medications across the continuum of care.” The Institute of Medicine specifically called the improvement of communication between medical and substance use treatment providers fundamental to improving the quality of healthcare for patients with mental and substance-use conditions.3 Methadone maintenance treatment (MMT) for approximately 260,000 opioid-dependent patients in the United States is restricted to federal- and state-regulated clinics.4 Typically, MMT clinics are in locations that are separate from general medical care with increased confidentiality protections required for substance use related health information,5,6 which can be a barrier to the coordination of care.
MMT is a chronic therapy for opioid dependence, a chronic relapsing disease that often requires lifelong treatment.7 Common co-occurring conditions among opioid-dependent patients receiving MMT, that also require chronic pharmacotherapy include HIV infection,8 mood disorders,9–11 chronic pain,12 osteoporosis,13 and diabetes.14–16 Many medications for these conditions interact with methadone in potentially important ways, including prolongation of the QT interval resulting in increased risk of torsade de pointes,17–21 increased or decreased metabolism of methadone or the interacting medication by the cytochrome P450 enzyme system, and increased sedation by other opioids and benzodiazepines.22–24 Thus, medication-methadone interactions potentially contribute to clinically significant adverse events, including cardiac arrhythmias, overdoses, decreased cognitive function, opioid withdrawal symptoms, and relapses to illicit opioid use. Ideally, when patients on MMT engage in outpatient or inpatient medical care, their treating physicians are aware of the MMT and document methadone on the medication list in addition to opioid dependence on the medical problem list.
In this study of MMT patients receiving medical care, our objectives were to 1) quantify the documentation of opioid dependence in the medical record, 2) quantify the documentation of MMT in the medical record, and 3) describe the possible drug interactions that could arise from the use of methadone with other prescribed medications.
METHODS
Study Design and Population
We reviewed electronic medical records (EMRs) of patients from the Boston Public Health Commission (BPHC) MMT program who received their primary medical care at Boston Medical Center (BMC), an affiliated, but separate, medical center. Entry criteria included the following: 1) enrollment in methadone treatment on or before July 2, 2007; 2) a signed release of information permitting 2-way communication between the MMT program and primary care physician; 3) a primary care physician based at BMC; and 4) at least 1 primary care progress note or 1 discharge summary in the BMC EMR between September 1, 2002, and July 2, 2007, and during the period of MMT. This study was approved by the institutional review board of Boston University Medical Campus.
Data Collection and Measures
Methadone dose and “take home” dose information were collected from the MMT EMR for the date immediately preceding the medical center inpatient admission or primary care visit. “Take home” doses refer to unobserved methadone dosing, a privilege given only to patients who have at least 90 days of abstinence from illicit drug use. To assess whether our sample was similar to the MMT program population, we obtained information on age, gender, race/ethnicity, methadone dose, and number of days on methadone as of July 2, 2007, from the MMT EMR for all patients.
From the medical center EMR, we determined insurance status as public (e.g., Medicare or Medicaid), private, or no insurance. To gauge the burden of comorbidity, we searched the active problem list, which is edited and maintained by treating providers, for chronic conditions (e.g., HIV/AIDS), typically treated with medications that potentially interact with methadone, and substance-related conditions (e.g., alcohol dependence).
To quantify both MMT and opioid-dependence diagnosis documentation, the most recent discharge summary and primary care note in the medical center EMR between September 1, 2002, and July 2, 2007, were read and searched by one of the authors (DF). Missing documentation of MMT was defined as no listing of methadone as a medication in either the primary care progress note or the discharge summary. Subjects without any combination of “heroin, opioid, or opiate” and “use, abuse, or dependence” in the discharge summary, primary care note, or the problem list were categorized as not having documentation of opioid dependence. Both the problem list and the medication lists were cumulative, based on previous treatment episodes, and editable by each provider.
To describe possible methadone-drug interactions, 8 medication interaction categories were pre-specified prior to reviewing records and included: 1) may decrease methadone effects (e.g., efavirenz, phenytoin); 2) may increase methadone effects (e.g., fluconazole, fluvoxamine); 3) has altered metabolism with methadone (e.g., zidovudine); 4) benzodiazepines (e.g., clonazepam); 5) other opioids (e.g., oxycodone); and 3 categories of QT interval prolonging medications; 6) risk (generally accepted risk of causing torsade de pointes); 7) possible risk (associated with torsade de pointes and/or QT prolongation but lack substantial evidence); and 8) conditional risk (weakly associated with torsade de pointes and/or QT prolongation).
The first 3 categories were determined based on a published review of methadone-medication interactions.22 We created categories for benzodiazepines and other opioids because of the risks of oversedation when combined with methadone. We adopted the 3 categories of QT prolonging medications at www.qtdrugs.org to identify medications that increase the risk of torsade de pointes. www.qtdrugs.org is a website funded by the federal Agency for Healthcare Research and Quality.
Analysis
Descriptive statistics were obtained for all subject characteristics to describe the study sample. We determined the proportions with lack of opioid dependence and MMT documentation and the exact binomial 95% confidence intervals for these proportions. All analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
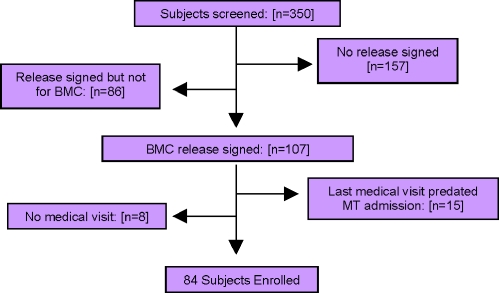
Among the 350 patients enrolled in MMT on July 2, 2007, 157 (45%) had no release of information signed for communication with a primary care physician and 86 (25%) had an existing release of information but not for BMC. Among the 107 patients with an existing release of information with a BMC primary care physician, 84 had at least 1 inpatient or primary care visit before July 2, 2007, and during the period of MMT and comprised the study sample. (Fig. 1)
Figure 1.
Study sample selection of methadone maintenance treatment patients engaged in medical care. Boston Medical Center, methadone treatment.
Characteristics of the study sample ( = 84) were mean age of 43.4 years; mean methadone dose of 85 mg; mean MMT duration of 4.4 years; 56% women; and 50% white, 30% African-American, and 20% Hispanic (Table 1). These characteristics were similar to the summary statistics available for all clinic patients ( = 350) at the time of the chart review (i.e., mean age of 39.7 years, mean methadone dose of 81 mg, MMT duration of 3.7 years, 62% women, 57% white, 24% African-American, 17% Hispanic, and 2% other).
Table 1.
Demographic and Clinical Characteristics of Methadone Maintenance Treatment Subjects Engaged in Medical Care ( = 84)
| Mean | (Standard deviation) | |
|---|---|---|
| Age, years | 43.4 | (10.8) |
| Years on MMT at last medical visit | 4.4 | (4.6) |
| Milligrams of methadone at last medical visit | 85.3 | (36.1) |
| (%) | ||
| Female | 47 | (56) |
| Race/Ethnicity | ||
| African-American | 25 | (30) |
| White | 42 | (50) |
| Hispanic | 17 | (20) |
| Insurance status | ||
| Public insurance | 72 | (86) |
| Private insurance | 7 | (8) |
| Free care/ Uninsured | 5 | (6) |
| On take homes at time of last medical visit | 25 | (30) |
| Medical problems | ||
| Hepatitis C | 50 | (60) |
| Pain condition | 37 | (44) |
| Depressive disorder | 36 | (43) |
| Anxiety disorder | 29 | (35) |
| Tobacco use | 26 | (31) |
| HIV-infection | 19 | (23) |
| Hypertension | 18 | (21) |
| Diabetes | 11 | (13) |
| Alcohol abuse or dependence | 6 | (7) |
| Cocaine use, abuse, or dependence | 6 | (7) |
| Renal insufficiency | 2 | (2) |
| Seizure disorder | 1 | (1) |
Documentation of opioid dependence diagnosis was missing from the medical record in 30% (95% CI, 20%-41%) of subjects. (Table 2) Documentation of MMT was missing from either the last discharge summary or last primary care note in 11% (95% CI, 5%-19%) of subjects. Among the 82 (98%) subjects with a primary care note, documentation of MMT was missing in 7% (95% CI, 3%–15%). Among 41 (49%) subjects with a discharge summary, documentation of MMT was missing in 10% (95% CI, 3%–23%). Among 39 subjects with both a discharge summary and primary care note, documentation of MMT was missing from both notes for 1 subject or 3% (95% CI, 0.1%-13%).
Table 2.
Proportions Without Documentation of Opioid Diagnoses and Methadone Maintenance Treatment in Medical Record
| No. | % | 95% CI | |
|---|---|---|---|
| Overall | |||
| Opioid dependence diagnosis missing from medical record ( = 84) | 25 | 30% | 20%-41% |
| Methadone as a medication missing from medical record* ( = 84) | 9 | 11% | 5%-19% |
| Subgroups | |||
| Methadone as a medication missing from: | |||
| Last primary care (PC) note ( = 82) | 6 | 7% | 3%-15% |
| Last discharge summary ( = 41) | 4 | 10% | 3%-23% |
| Both PC note and discharge summary ( = 39) | 1 | 3% | 0.1%-13% |
*Methadone not documented in either last primary care note or last discharge summary
confidence interval
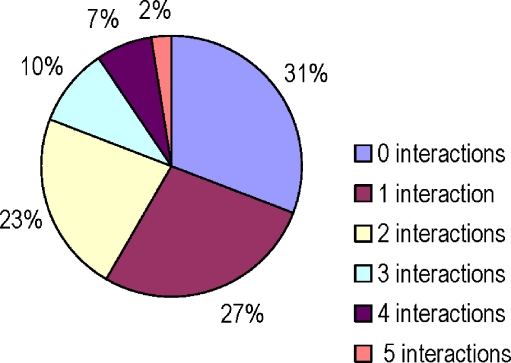
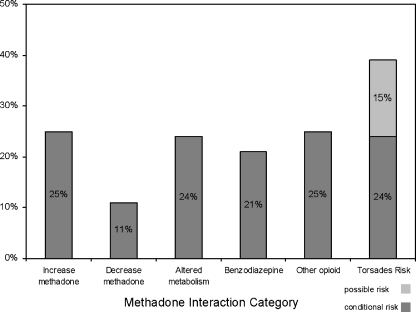
At least 1 potential methadone interaction was identified in 58 (69%) of subjects; 16 (19%) of subjects had 3 or more such medications with potential interactions. (Figure 2) The frequency of specific interaction types ranged from 9 (11%) for “may decrease methadone effects” to 33 (39%) for potentially QT-interval prolonging effect (Figure 3). For medications within the QT-prolonging subcategories, there were 13 (15%) subjects in the “possible risk” category and 20 (24%) in the “conditional risk” category. No subjects were on any medication, other than methadone, in the “risk” category.
Figure 2.
Distribution of the number of potentially harmful medication interactions with methadone among 84 subjects on methadone maintenance.
Figure 3.
Medications in each category: Increase methadone: fluoxetine, fluvoxamine, paroxetine, sertraline, omeprazole, metronidazole, ketoconazole, diltiazem Decrease methadone: efavirenz, nevirapine, lopinavir+ritonavir, butalbital, ascorbic acid Altered metabolism: didanosine, zidovudine, amytriptyline, doxepin, nortriptyline, clomethiazole., promethazine, nifedpine Benzodiazepines: clonazepam, diazepam, lorazepam Other opioids: codeine, fentanyl, morphine, oxycodone Possible risk: atazanavir, azithromycin, levofloxacin, ofloxacin, quetiapine, risperidone, venlafaxine Conditional risk: amitriptyline, nortriptyline, paroxetine, sertraline, citalopram, doxepin, fluoxetine, ketoconazole
Among the 9 subjects who had MMT documentation missing from either note, 7 (78%) had at least 1 medication that potentially interacted with methadone. Notably, 5 (56%) subjects were on other opioids, the most common interaction category among these subjects.
DISCUSSION
This study highlights two aspects in which care coordination between medical and substance use treatment providers could impact patient safety. First, even when consent for communication between substance-use treatment providers and medical providers exists, medical providers do not always document that a patient has opioid dependence or that a patient is receiving MMT. Second, most patients engaged in medical care and MMT are on 1 or more medications that potentially interact with methadone and may lead to adverse events, such as oversedation, overdose, opioid withdrawal, or cardiac dysrythmias.
This study sample likely represented a “best case” for thorough documentation, because we selected methadone clinic subjects with known primary care physicians at a medical center with comprehensive services, a standardized EMR, and an historical affiliation with the methadone clinic. The coordination problems identified in this study likely loom larger among patients receiving both MMT and primary medical care from unaffiliated providers. Limited communication between separate systems of medical care and MMT was previously documented in a Spanish study in which 89.5% of primary care physicians received no information from the methadone clinic about their patients.25
Patients receiving methadone for opioid dependence and engaged in primary medical care have substantial medical comorbidity.8–16 Thus, it is not surprising that a substantial number of subjects in this study were on medications for depression, anxiety, HIV infection, hypertension, or pain. Not only do many of these medications potentially interact with methadone, but multiple interacting medications are common. While none of the identified interacting medications are contraindicated in conjunction with methadone, prescribers should be aware when patients are taking medications that interact, so they can monitor and manage potential interactions.
Among the 9 subjects with methadone missing from a medical note, “other opioids” was the most common category. For patients with pain and opioid dependence, it may be appropriate to be treated with both methadone and other opioids, yet it is critical that both treatments are coordinated to avoid overdose.
While not a focus of our study, the recruitment of eligible subjects points to an additional challenge to care coordination; written consent for communication between medical and substance use treatment providers is commonly not present. Because of federally mandated privacy protections,5,6 the first step of care coordination between substance abuse treatment and medical providers is obtaining a signed release of information. In our study, almost half of methadone clinic patients did not have a signed release of information permitting communication with their primary care physician. The reasons for this are many: no primary care physician, difficulty for clinic staff to obtain releases because of separation of programs, and stigma among MMT patients with resulting reticence to allow communication with medical providers.
This study’s strengths include examination of the EMRs from clinical substance-use treatment and the medical care system to describe the burden of potential methadone-medication interactions and missing documentation between methadone and medical care providers that has not previously received critical attention.
This study was limited by its retrospective design and limited number of subjects. Yet, we used electronic medical records and a data collection methodology that was systematic and the subjects examined were similar to patients in the entire clinic. The generalizability of our findings is limited by focusing on patients from one methadone clinic receiving medical care at an affiliated medical center. We recognize that it is possible that physicians were aware of their patients receiving MMT or being opioid dependent, but did not document it. While methadone is routinely administered during inpatient treatment at the medical center if the treatment team is aware the patient is enrolled in MMT, we did not have access to inpatient pharmacy records to confirm this. We did not evaluate whether medication reconciliation has taken place at the MMT site. However, as a consequence of this study, the methadone clinic did refine its medication reconciliation procedures to regularly document prescribed medications. Lastly, the retrospective design precluded measurement of adverse events from methadone-medication interactions.
This study demonstrates opportunities to improve communication, care coordination, and patient safety among patients receiving medical and substance-use treatment. Patients in MMT frequently require multiple medications and these often interact with methadone and potentially lead to adverse events. Hence, communication and coordination among substance-use treatment and medical providers has room for improvement so as to mitigate and manage the potential adverse effects of methadone and interacting medications.
Acknowledgements
The authors would like to thank Eileen Brigandi, Donna Beers, RN, and the staff at Substance Abuse Prevention and Treatment Services, Boston Public Health Commission, for their support and cooperation during this study. We would also like to thank Courtney Pierce for her assistance in manuscript preparation. This project was funded in part by the National Institute on Drug Abuse R25-DA13582. Parts of this work were presented at the Society of General Internal Medicine annual meeting, April 11, 2008; the College on Problems in Drug Dependence annual meeting, June 19, 2008; the Addiction Health Services Research conference, October 21, 2008; and the Association for Medical Education and Research in Substance Abuse annual meeting, November 8, 2008.
Conflict of Interest None disclosed.
References
- 1.Kohn LT, Corrigan JM, Donaldson MS. Committee on quality of health care in america, institute of medicine. To err is human: building a safer health system. Washington, DC: National Academy Press; 2000. [PubMed]
- 2.Joint Commission. 2008 National Patient Safety Goals. Available at: www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/. Accessed May 31, 2009.
- 3.Improving the quality of healthcare for mental and substance-use conditions. Institute of Medicine. 504. 2005. Washington, DC: The National Academy Press: 2005.
- 4.Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303–5. [DOI] [PubMed]
- 5.Bureau of National Affairs. Beckerman JZ, Pritts J, Goplerud E, Leifer J, Borzi PC, Rosenbaum S. Health information privacy, patient safety, and health care quality: Issues and challenges in the context of treatment for mental health and substance use.16. Available at: ihcrp.georgetown.edu/pdfs/pritts0208.pdf. Accessed May 31, 2009.
- 6.Confidentiality of Alcohol and Drug Abuse Patient Records. Code of Federal Regulations Title 42, Volume 1, Chapter 1, Part 2. Available at: http://www.access.gpo.gov/nara/cfr/waisidx_02/42cfr2_02.html. Accessed May 31, 2009.
- 7.Leshner AI. Science-based views of drug addiction and its treatment. JAMA. 1999;282:1314–16. [DOI] [PubMed]
- 8.Gourevitch MN, Chatterji P, Deb N, Schoenbaum EE, Turner BJ. On-site medical care in methadone maintenance: associations with health care use and expenditures. J Subst Abuse Treat. 2007;32:143–51. [DOI] [PubMed]
- 9.Brienza RS, Stein MD, Chen M, et al. Depression among needle exchange program and methadone maintenance clients. J Subst Abuse Treat. 2000;18:331–7. [DOI] [PubMed]
- 10.Teesson M, Havard A, Fairbairn S, Ross J, Lynskey M, Darke S. Depression among entrants to treatment for heroin dependence in the Australian Treatment Outcome Study (ATOS): prevalence, correlates and treatment seeking. Drug Alcohol Depend. 2005;78:309–15. [DOI] [PubMed]
- 11.Milby JB, Sims MK, Khuder S, et al. Psychiatric comorbidity: prevalence in methadone maintenance treatment. Am J Drug Alcohol Abuse. 1996;22:95–107. [DOI] [PubMed]
- 12.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–8. [DOI] [PubMed]
- 13.Kim TW, Alford DP, Malabanan A, Holick MF, Samet JH. Low bone density in patients receiving methadone maintenance treatment. Drug Alcohol Depend. 2006;85:258–62. [DOI] [PMC free article] [PubMed]
- 14.Federman AD, Arnsten JH. Primary care affiliations of adults in a methadone program with onsite care. J Addict Dis. 2007;26:27–34. [DOI] [PubMed]
- 15.Umbricht-Schneiter A, Ginn DH, Pabst KM, Bigelow GE. Providing medical care to methadone clinic patients: referral vs on-site care. Am J Public Health. 1994;84:207–10. [DOI] [PMC free article] [PubMed]
- 16.Selwyn PA, Budner NS, Wasserman WC, Arno PS. Utilization of on-site primary care services by HIV-seropositive and seronegative drug users in a methadone maintenance program. Public Health Rep. 1993;108:492–500. [PMC free article] [PubMed]
- 17.Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann.Intern.Med. 2009;150:387–95. [DOI] [PubMed]
- 18.Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137:501–4. [DOI] [PubMed]
- 19.Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003;23:802–5. [DOI] [PubMed]
- 20.Krantz MJ, Lowery CM, Martell BA, Gourevitch MN, Arnsten JH. Effects of methadone on QT-interval dispersion. Pharmacotherapy. 2005;25:1523–9. [DOI] [PubMed]
- 21.Martell BA, Arnsten JH, Krantz MJ, Gourevitch MN. Impact of methadone treatment on cardiac repolarization and conduction in opioid users. Am J Cardiol. 2005;95:915–8. [DOI] [PubMed]
- 22.Leavitt SB. Methadone-drug interactions. Addiction Treatment Forum - 3rd Edition 2005; November 2005: 1–31. Available at: www.atforum.com/SiteRoot/pages/addiction_resources/Drug_Interactions.pdf. Accessed May 31, 2009.
- 23.Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006;41:563–72. [DOI] [PubMed]
- 24.Cance-Katz EF. Treatment of opioid dependence and coinfection with HIV and hepatitis C virus in opioid-dependent patients: the importance of drug interactions between opioids and antiretroviral agents. Clin Infect Dis. 2005;41(Suppl 1):S89–S95. [DOI] [PubMed]
- 25.Velarde MC, Peino AJ, Gomez de Caso Canto JA. The Methadone Maintenance Program for intravenous heroin addicts. What information do primary care physicians have? Aten Primaria. 1996;17:581–4. [PubMed]