Summary
Accurate recognition of individuals is a foundation of social cognition. The remarkable ability of humans to distinguish among thousands of similar faces depends on sensitivity to unique configurations of facial features, including subtle differences in the relative placement of the eyes and mouth [1, 2]. Determining whether similar perceptual processes underlie individual recognition in nonhuman primates is important for both the study of cognitive evolution and the appropriate use of primate models in social cognition research. In humans, some of the best evidence for a keen sensitivity to the configuration of features in faces comes from the “Thatcher Effect”. This effect shows that it is difficult to detect changes in the orientation of the eyes and mouth in an image of an inverted face, even though identical changes are unmistakable in an upright face [3, 4]. Here, we demonstrate for the first time that a nonhuman primate species also shows the Thatcher Effect. This direct evidence of configural face perception in monkeys, collected under testing conditions that closely parallel those used with humans, indicates that perceptual mechanisms for individual recognition have been conserved through primate cognitive evolution.
Results and Discussion
Look briefly at Figure 1, which contains two pictures of the same person. Now turn the page upside down and look again. While one face may look unusual in both orientations, the difference between the faces is especially striking when they are viewed upright (i.e. when the page is upside down). This phenomenon is called the Thatcher Effect because it was first demonstrated using an image of the face of Margaret Thatcher [3]. Note that the two images share the same facial features placed in the same regions of the face. The images differ in the relations among these features; the orientation of the eyes and mouth is altered in the “thatcherized” face. The fact that we can more easily detect manipulation of the configuration of features in upright faces demonstrates two properties of human face perception: 1) we normally perceive faces configurally, which promotes sensitivity to the relative placement of facial features, and 2) configural perception is disrupted when a face is viewed upside down [3-7]. Because faces share many similar features they are difficult to differentiate based on features alone. Distinguishing among a large number of faces is enhanced by sensitivity to unique configurations of facial features, including subtle differences in the relative placement of the eyes and mouth [1, 2]. Thus, the Thatcher Effect demonstrates a critical perceptual process supporting individual recognition.
Fig. 1. Example of the Thatcher Effect.
The face on the left is unaltered while the face on the right has been “thatcherized” by inverting the mouth and eyes relative to the rest of the face. Contrast your perception of the faces viewed inverted, as shown, and after rotating the page to make the faces upright. Thatcherization is most obvious when faces are viewed upright.
Consistent with the impaired perception of inverted faces demonstrated by the Thatcher Effect, many studies of human perception have shown that faces are more easily recognized when upright than when inverted [1, 8, 9]. Investigators of nonhuman primate perception have also compared recognition and discrimination of upright and inverted faces, but with inconsistent results. Some studies show superior perception of upright faces like that found in humans (cotton-top tamarins [10]; pigtail macaques [11]; chimpanzees [12-15]; Japanese macaques [16]; rhesus macaques [17, 18]). However, in other studies no difference in accuracy with inverted and upright faces was found (cotton-top tamarins [19]; longtail macaques [20, 21]; rhesus monkeys [22, 23]; baboons [24]). The cause of the inconsistency is not clear, but there are at least two reasons to be cautious in using these studies to evaluate the role of configural perception in primate face recognition. First, configural face perception was not directly assessed in these studies because the relations among facial features were not manipulated (but see [18], where low and high pass filtering was used in an effort to isolate configural processing). Second, most of these studies involved extensive training with a small set of images. Such training may encourage subjects to discriminate faces by memorizing individual salient features (e.g., a dark spot on the chin on one face that is absent from others) rather than by perceiving the configuration of facial features, as monkeys might do in nature where they are confronted with the many faces in their social group. Because findings have been inconsistent, and the methodologies used to date may artificially encourage nonconfigural processing, the extent to which configural perception underlies natural nonhuman primate face recognition is difficult to determine from the existing literature.
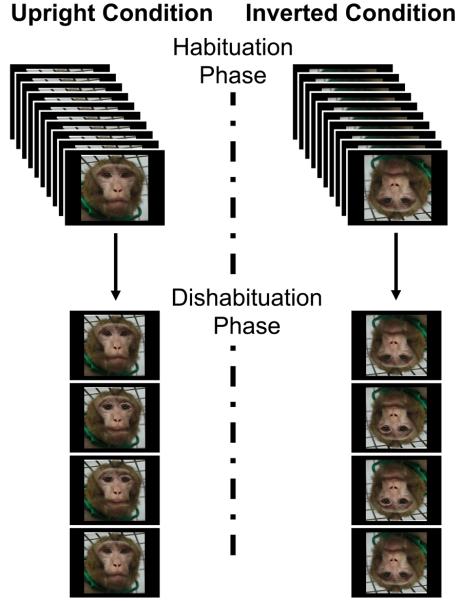
We used the Thatcher Effect to directly assess configural face perception in rhesus monkeys without explicit training. Because thatcherization involves manipulation of the configural properties of faces, and the Thatcher Effect is revealed by comparing perception of upright and inverted faces, this approach allows us to clearly evaluate the effect of face orientation on configural face perception, should it occur in monkeys. Monkeys saw thatcherized and normal monkey faces in a habituation-dishabituation paradigm. During the habituation phase of each test, we presented one of six unaltered images of monkey faces either upright (Upright condition) or inverted (Inverted condition) 10 times consecutively. The dishabituation phase followed, in which the original (intact) and the thatcherized versions of the habituated face were presented in the same orientation used in the habituation phase (Figure 2). The order of presentation of the normal and the thatcherized images in the dishabituation phase was counterbalanced across the subjects, and across tests with the two orientations of the six different stimulus monkey images. Thus, twelve tests (six unfamiliar monkey faces, each presented in both the Upright and Inverted orientation) were administered to each subject monkey. During both the habituation and the dishabituation phases, a “beep” from a speaker located behind the monitor indicated to the subject when an image was displayed. Each image was presented for 30 seconds with a 10 s interval between images, during which the screen was black. Subjects' looking behavior was video recorded and quantified by a coder blind to test condition later.
Fig. 2. Schematic of the habituation-dishabituation paradigm.
Half of tests used upright images (left side) and the other half inverted images (right side). Each image was presented for 30 seconds, separated from the next presentation by 10 seconds with no image. Each presentation was cued by a “beep.” Ten presentations of a given image constituted the habituation phase (top). The habituated (intact) and thatcherized faces were presented twice each in the dishabituation phase in an ABBA sequence (bottom). Whether an intact or thatcherized face was shown first in the dishabituation phase was counter-balanced across tests and monkeys.
We expected a decrease in the time monkeys spent looking at a face over the course of the habituation phase of each trial. Based on the results of studies of the Thatcher Effect in humans, we hypothesized that if monkeys perceive faces configurally they should be surprised by the unusual manipulation of the eyes and mouth in the thatcherized faces. Such surprise would manifest in monkeys looking longer at thatcherized than intact faces during the dishabituation phase of trials. Furthermore, if monkey face perception follows the pattern found in humans, such dishabituation should be much more pronounced for upright than for inverted faces.
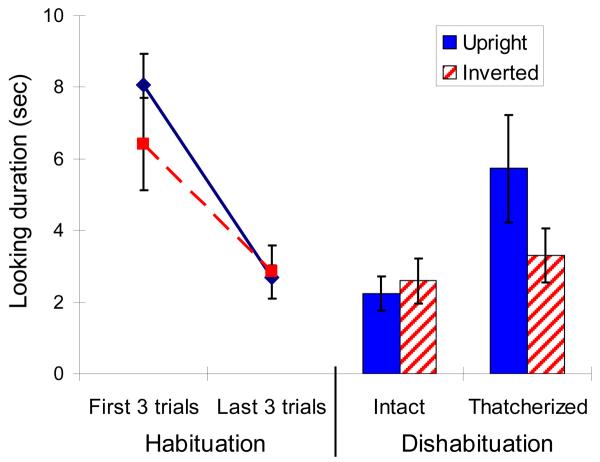
As expected, the monkeys showed decreased interest in both the upright and inverted images of faces across the habituation trials, indicated by reduction in time spent looking at the images (Figure 3, line graphs on the left side). From this habituated state, monkeys showed significantly more dishabituation to the upright thatcherized faces than to the inverted thatcherized faces (Figure 3, bar graphs on the right side). The difference in dishabituation demonstrates that the manipulation of the orientation of the eyes and mouth was more salient in the upright faces, constituting a Thatcher Effect in monkeys that parallels that seen in humans.
Fig. 3. Monkeys look longer at upright than at inverted thatcherized faces.
Mean time spent looking at the monitor in the habituation phase (left side, line graphs) and the dishabituation phase (right side, bar graphs). The upright condition is shown in solid blue; the inverted condition is shown in dashed or hatched red. Error bars are standard error. Looking times were calculated in milliseconds (based on frame by frame analysis of digital video) and then log transformed to approximate normality. Monkeys habituated indistinguishably to upright and inverted faces during the habituation phase (Repeated measures ANOVA: Trial Block, F1,3 = 51.384, p = 0.006; Orientation, F1,3 = 0.976, p = 0.396; Trial Block X Orientation, F1,3 = 4.483, p= 0.125). The Thatcher Effect is evident in the dishabituation phase by the significant interaction between face type (thatcherized or intact) and orientation (Repeated measures ANOVA: Face Type X Orientation, F1,3 = 64.714, p = 0.004; Face Type, F1,3 =12.964, p = 0.037; Orientation, F1,3 = 7.946, p = 0.067). To confirm that the significant Face Type X Orientation interaction was caused by longer looking times for upright thatcherized faces, we conducted two posthoc tests. Monkeys looked significantly longer at upright than inverted thatcherized faces but looked equally long at upright and inverted intact faces (two-tailed paired t-test, thatcherized faces: t3 = 7.167, p = 0.006; intact faces: t3 = 1.227, p = 0.307).
Because we used identical images in the upright and inverted conditions, the differences in dishabituation cannot be explained by any idiosyncratic characteristics of our stimulus materials. The orientation of the faces was the only difference between the two conditions. Our subjects showed similar initial interest in upright and inverted faces and habituated equivalently to the two types of stimuli (compare blue and red lines in Figure 3). The lack of significant dishabituation in the inverted condition cannot, therefore, be explained by unsuccessful habituation during the habituation phase. Instead, these results provide direct behavioral evidence that, 1) monkeys perceive faces configurally and, 2) this configural processing is disrupted when the face is inverted. Humans are likely to describe an upright thatcherized human face as “gruesome.” While we cannot be certain whether or not the monkeys had similar phenomenological experience while viewing the upright thatcherized monkey faces, the behavioral results presented clearly demonstrate that the changes brought about by thatcherization were more readily detected by the monkeys in upright faces. Future studies using heart rate, pupil size, or other physiological measures might begin to address whether monkeys, like humans, perceive thatcherized faces as alarming or gruesome.
We know of only two other studies of nonhuman species that have used thatcherized faces. In apparent conflict with the present results, thatcherization of stimulus faces did not affect accuracy in tests of perceptual competence in either study (pigeons, Columba livia [25]; baboons, Papio papio [26]). However, both of these studies used a matching-to-sample paradigm that required extensive pre-training. Extensive pre-training, particularly with a small set of images, may cause subjects to use a few salient cues, rather than the configuration of facial features, to identify stimulus faces. In contrast to the techniques used in the pigeon and baboon studies cited above, in humans the Thatcher Effect is normally demonstrated as a spontaneous reaction, outside the context of any explicit recognition or matching test [3, 4]. Such spontaneous reactions likely better reflect normal face perception than do trained discriminations. According to this analysis, failure to find the Thatcher Effect in earlier studies does not represent a discontinuity between humans and nonhumans in the mechanisms of normal face perception, but rather indicates changes in perception or attention brought about by extensive training with specific stimuli. The present study is a direct test of configural face perception, and better matches the spontaneous conditions under which the Thatcher Effect is observed in humans. It also directly shows that configural perception is disrupted by face inversion in monkeys. Because we did not train discrimination of the images we used, the behavior of our monkeys likely reflects the same perceptual processes used in natural face perception.
This first demonstration of the Thatcher Effect in nonhuman animals is important because it indicates conservation of configural face perception across primate species and suggests that this mechanism for distinguishing among many similar faces may have evolved in an ancestor common to humans and rhesus monkeys 30 million or more years ago [27]. It is likely that previous findings that appear inconsistent with configural processing, such as the lack of an “inversion effect”, are training artifacts and do not reflect true species differences in face perception among primates. However, it will be of interest to determine the extent to which the Thatcher Effect reflects species-specific specializations of face perception. This question can best be addressed by “crossed” comparative studies in which two different species are tested with thatcherized faces of both their own and the other species. Our behavioral evidence reinforces recent comparative neuroimaging results showing similar specialized neural substrates for face perception between monkeys and humans [28] (but see also [29], for a different view). It will be necessary to repeat behavioral tests on other species to determine how widespread configural face perception is phylogenetically, and whether it has evolved only in species for which individual recognition is critical.
Experimental Procedures
We studied four 4-year-old male rhesus macaque monkeys (Macaca mulatta) raised for 2 to 3 years in large social groups at the Yerkes National Primate Research Center. In the lab they were pair-housed, permitted full social contact with their cagemates outside of testing periods, and had visual and auditory contact with additional monkeys living in the same room. All procedures used were approved by the Institutional Animal Care and Use Committee at Emory University.
Monkeys were tested in a cage (60cmW*72.5cmL*81.3cmH), placed 80 cm away from a 19 inch LCD color monitor inside a sound attenuating booth. A camera was attached to the monitor to record the looking behavior of subjects. Stimuli were color frontal views of six unfamiliar male rhesus monkey faces and the thatcherized versions of these faces shown on a black background (450 pixel * 550 pixels, Figure 4). Testing was controlled by custom software written using Presentation© (Neurobehavioral Systems, Albany, CA).
Fig. 4. Intact and thatcherized monkey faces used.

The left hand column shows all six intact monkey faces used; the right column shows the thatcherized version of each of these faces.
Videos of subjects were analyzed after all testing was complete. The first author separated the 14 image presentations from each test (10 presentations in the habituation phase and 4 from the dishabituation phase) into separate digital video files, resulting in 168 files for each subject (14 presentations per test * 6 stimulus monkeys * two orientations). Each clip was arbitrarily named and the order of the clips was randomized. Because videos were taken from the position of the display monitor, they did not reveal which image was presented to the subject monkey, permitting blind coding by the second author. The video files were examined frame by frame and the monkey was coded as looking at the monitor when a pupil was directed at the camera, irrespective of head and body orientation. One 10 s presentation of an intact inverted face was not captured on video due to a technical problem and was not included in the calculation of average looking times.
Acknowledgements
This study was supported by a grant from the James S. McDonnell Foundation, by Yerkes Center base grant No. RR-00165 awarded by the Animal Resources Program of the National Institutes of Health, and by the Center for Behavioral Neuroscience under the STC Program of the National Science Foundation under Agreement No. IBN-9876754. The work of the first author was supported by a Postdoctoral Fellowship for Research Abroad awarded by the Japan Society for the Promotion of Science. We thank Ben Basile, Emily Brown, Regina Paxton, Victoria Templer, and Hillary Rodman for comments on an earlier draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Searcy JH, Bartlett JC. Inversion and processing of component and spatial-relational information in faces. J. Exp. Psychol. Hum. Percept. Perform. 1996;22:904–915. doi: 10.1037//0096-1523.22.4.904. [DOI] [PubMed] [Google Scholar]
- 2.Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychol. Rev. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- 3.Thompson P. Thatcher,Margaret - a New Illusion. Perception. 1980;9:483–484. doi: 10.1068/p090483. [DOI] [PubMed] [Google Scholar]
- 4.Murray JE, Yong E, Rhodes G. Revisiting the perception of upside-down faces. Psychol. Sci. 2000;11:492–496. doi: 10.1111/1467-9280.00294. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett JC, Searcy J. Inversion and Configuration of Faces. Cognit. Psychol. 1993;25:281–316. doi: 10.1006/cogp.1993.1007. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes G, Brake S, Taylor K, Tan S. Expertise and Configural Coding in Face Recognition. Br. J. Psychol. 1989;80:313–331. doi: 10.1111/j.2044-8295.1989.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 7.Boutsen L, Humphreys GW, Praamstra P, Warbrick T. Comparing neural correlates of configural processing in faces and objects: An ERP study of the Thatcher illusion. Neuroimage. 2006;32:352–367. doi: 10.1016/j.neuroimage.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Yin RK. Looking at Upside-Down Faces. J. Exp. Psychol. 1969;81:141. &. [Google Scholar]
- 9.Valentine T. Upside-Down Faces - a Review of the Effect of Inversion Upon Face Recognition. Br. J. Psychol. 1988;79:471–491. doi: 10.1111/j.2044-8295.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 10.Neiworth JJ, Hassett JM, Sylvester CJ. Face processing in humans and new world monkeys: the influence of experiential and ecological factors. Anim. Cogn. 2007;10:125–134. doi: 10.1007/s10071-006-0045-4. [DOI] [PubMed] [Google Scholar]
- 11.Overman WH, Doty RW. Hemispheric-Specialization Displayed by Man but Not Macaques for Analysis of Faces. Neuropsychologia. 1982;20:113. doi: 10.1016/0028-3932(82)90002-1. &. [DOI] [PubMed] [Google Scholar]
- 12.Parr LA, Dove T, Hopkins WD. Why faces may be special: Evidence of the inversion effect in chimpanzees. J. Cogn. Neurosci. 1998;10:615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- 13.Parr LA, Heintz M. The perception of unfamiliar faces and houses by chimpanzees: Influence of rotation angle. Perception. 2006;35:1473–1483. doi: 10.1068/p5455. [DOI] [PubMed] [Google Scholar]
- 14.Tomonaga M. Inversion effect in perception of human faces in a chimpanzee (Pan troglodytes) Primates. 1999;40:417–438. [Google Scholar]
- 15.Tomonaga M. Visual search for orientation of faces by a chimpanzee (Pan troglodytes): face-specific upright superiority and the role of facial configural properties. Primates. 2007;48:1–12. doi: 10.1007/s10329-006-0011-4. [DOI] [PubMed] [Google Scholar]
- 16.Tomonaga M. How Laboratory-Raised Japanese Monkeys (Macaca-Fuscata) Perceive Rotated Photographs of Monkeys - Evidence for an Inversion Effect in Face Perception. Primates. 1994;35:155–165. [Google Scholar]
- 17.Parr LA, Winslow JT, Hopkins WD. Is the inversion effect in rhesus monkeys face-specific? Anim. Cogn. 1999;2:123–129. [Google Scholar]
- 18.Gothard KM, Brooks KN, Peterson MA. Multiple perceptual strategies used by macaque monkeys for face recognition. Anim. Cogn. 2009;12:155–167. doi: 10.1007/s10071-008-0179-7. [DOI] [PubMed] [Google Scholar]
- 19.Weiss DJ, Kralik JD, Hauser MD. Face processing in cotton-top tamarins (Saguinus oedipus) Anim. Cogn. 2001;4:191–205. [Google Scholar]
- 20.Bruce C. Face Recognition by Monkeys - Absence of an Inversion Effect. Neuropsychologia. 1982;20:515–521. doi: 10.1016/0028-3932(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 21.Dittrich W. Representation of Faces in Longtailed Macaques (MacacaFascicularis) Ethology. 1990;85:265–278. [Google Scholar]
- 22.Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Anim. Cogn. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld SA, Vanhoesen GW. Face Recognition in the Rhesus-Monkey. Neuropsychologia. 1979;17:503. doi: 10.1016/0028-3932(79)90057-5. &. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Malivel J, Fagot J. Perception of pictorial human faces by baboons: Effects of stimulus orientation on discrimination performance. Anim. Learn. Behav. 2001;29:10–20. [Google Scholar]
- 25.Jitsumori M, Yoshihara M. Categorical discrimination of human facial expressions by pigeons: A test of the linear feature model. Q. J. Exp. Psychol. B. 1997;50:253–268. [Google Scholar]
- 26.Parron C, Fagot J. Baboons (Papio papio) spontaneously process the first-order but not second-order configural properties of faces. Am. J. Primatol. 2008;70:415–422. doi: 10.1002/ajp.20503. [DOI] [PubMed] [Google Scholar]
- 27.Steiper ME, Young NM. Primate molecular divergence dates. Mol. Phylogenet. Evol. 2006;41:384–394. doi: 10.1016/j.ympev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parr LA, Heintz M, Pradhan G. Rhesus Monkeys (Macaca mulatta) Lack Expertise in Face Processing. J. Comp. Psychol. 2008;122:390–402. doi: 10.1037/0735-7036.122.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]