Abstract
Gene transfer into cells of the cochlea is useful for both research and therapy. Bovine adeno-associated virus (BAAV) is a novel viral vector with potential for long term gene expression with little or no side effects. In this study we assessed transgene expression using BAAV with ß-actin-GFP as a reporter gene, in cochleae of normal and deafened guinea pigs. We used two different routes to inoculate the cochlea: scala media (SM) or scala tympani (ST). Auditory brainstem response assessments were done prior to inoculation, 7 days after inoculation and immediately prior to sacrifice, to assess the functional consequences of the treatment. We observed threshold shifts due to the surgical invasion, but no apparent pathology associated with the virus. Fourteen days after the injection, animals were sacrificed and cochleae assessed histologically. Epi-fluorescence showed that BAAV transduced the supporting cells of both normal and deafened animals through SM and ST inoculations. Transgene expression in cells of the membranous labyrinth following ST inoculation is an important outcome because of the greater feasibility of this route for future clinical application. BAAV facilitates efficient transduction of the membranous labyrinth epithelium with minimum pathogenicity and may become clinically applicable for inner ear gene therapy.
Keywords: Scala media, scala tympani, bovine adeno-associated virus, gene therapy
Introduction
Sensorineural hearing loss is one of the most common disorders in our society today, and although it is not a life threatening impairment, it can have a substantial influence on the patient’s daily life. A common cause of this disorder is the degeneration of the sensory hair cells in the organ of Corti. The loss of these cells is irreversible in humans and other mammals, leading to permanent functional impairment1, and treatments of this impairment have limited efficacy.
Gene transfer is a powerful technique to introduce potentially therapeutic genes into target cells. Using this technique, future otological therapies may be able to prevent hair cell loss or replace missing hair cells, and consequently reduce functional impairment. Side effects and other technical obstacles still make gene transfer in the inner ear clinically unfeasible. To produce safe and practical applications of this technique, improvements in both viral vectors and delivery routes are necessary. Previous studies on inner ear gene transfer demonstrate that the ear is a viable target for gene therapy2–6. The most efficient way to induce forced expression of transgenes in vivo is by viral mediated vectors, but the very nature of these vectors raises concerns about safety and potential side effects. In recent years, AAV has been found to have desirable characteristics such as low pathogenicity, high infectious efficiency in non-dividing cells and sustained gene expression; which could make it one of the most effective virus vectors7–9. However, one of the disadvantages of this vector is that the effect of AAV gene therapy could be diminished in patients who possess AAV neutralizing antibodies. AAV infection is common in humans and neutralizing anybodies against AAV-1, -2, and -3 are reported to be up-regulated in childhood10. To overcome this fundamental deficiency, investigation of other distinct AAV serotypes is necessary. Once such vector is the bovine AAV (BAAV), which has been shown to be serologically distinct from AAV serotypes 1 to 411 and has been recently cloned and characterized12. Furthermore, limited inhibition by pool human immunoglobulins suggest minimal neutralizing antibody levels in the general human population12. In a recent study, BAAV showed significantly higher efficiency than AAV serotypes -2,-4, and -5 for transducing explants of rat inner ear epithelia in vitro13. Thus BAAV presents several attractive features that could overcome potential side effects and make the virus a novel candidate for gene therapy.
In this study, we evaluated BAAV cell tropism and transduction efficiency in vivo, with β-actin-GFP as a reporter gene, in the inner ear of normal hearing and deafened guinea pigs. We evaluated both the efficiency of transduction and its side effects for two different routes of inoculation: the scala tympani (ST) and scala media (SM). ST inoculation is feasible for clinical application and is minimally invasive. SM inoculation, while not feasible clinically in human ears, may be of importance for investigating protein function by forced gene expression in the membranous labyrinth. We compared BAAV-mediated gene expression and side effects between normal hearing and deafened guinea pigs. Our data indicate that BAAV transduces the auditory epithelia with low pathogenicity, through both SM and ST injection, in both normal hearing and deaf models, suggesting that BAAV is a useful gene carrier for both therapeutic and basic inner ear research.
Results
1. Effects of innoculation on auditory physiology of normal guinea pigs
To evaluate the effect of BAAV inoculation on the auditory physiology of normal hearing guinea pigs, we assessed the ABRs to clicks at 4, 12, and 20 kHz at 3 time points: prior to surgery, 1 and 2 weeks after surgery. To separate effects of injection route from effects of the BAAV activity, the same tests were performed on animals receiving BAAV by each injection route (SM or ST). In addition, control groups were inoculated with 5µl of artificial endolymph or perilymph via SM or ST, respectively, and were assessed by ABR audiometry at the same frequencies and time points as BAAV treated animals. The contralateral ears (right ear) served as a second control.
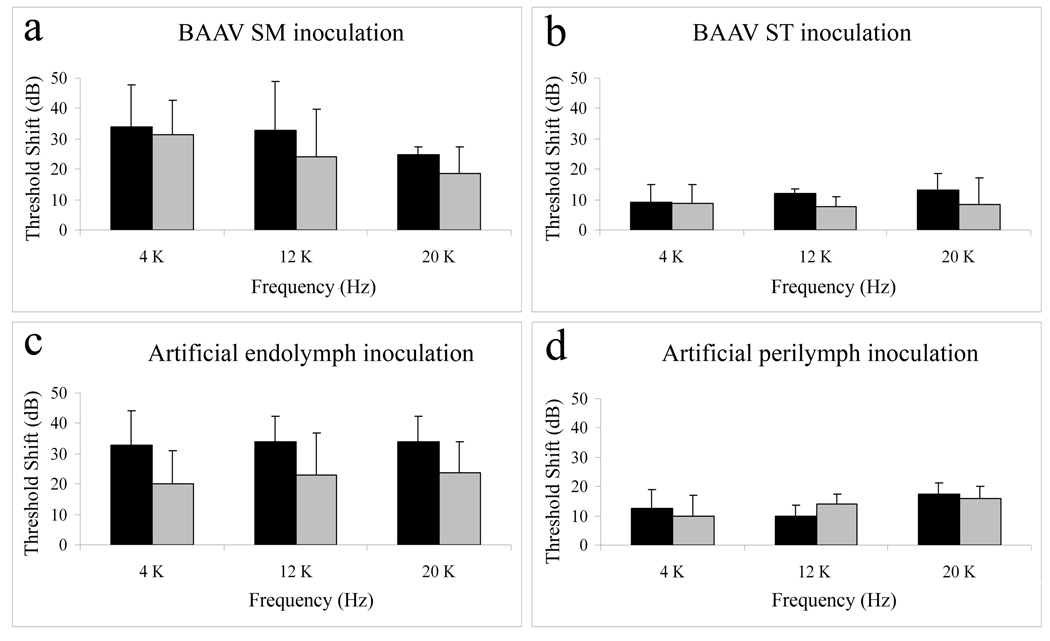
Baseline thresholds were similar across all groups. At one week, the threshold shifts at all frequencies were significantly larger, indicating greater hearing loss, in SM inoculated animals (Fig. 1a and c) than in ST inoculated animals (Fig. 1b and d), whether the animal received BAAV or the appropriate control serum (p < 0.05). In all groups, the ABR thresholds appeared to improve slightly by week 2, but the differences between weeks were not significant. Threshold shifts in BAAV SM and ST inoculated animals were not significantly different from their respective control groups. The contralateral ears in all groups showed no change in thresholds across all time points and frequencies (data not shown).
Fig. 1. Threshold shifts (Mean and standard deviation) measured 1 week (black bars) or 2 weeks (gray bars) after inoculation, at 4, 12, and 20 kHz.
(a) SM inoculation with BAAV (n=4). (b) ST inoculation in with BAAV (n=4). (c) SM inoculation with artificial endolymph (n=3). (d) ST inoculation with artificial perilymph (n=3). Threshold shifts were significantly larger at all frequencies in both SM inoculated animals compared to ST inoculated animals (p < 0.05). Threshold shifts were comparable between the BAAV inoculated animals and the sterile endolymph or perilymph injected animals.
2. Effect of BAAV SM and ST inoculation on histology of normal ears
We performed SM or ST inoculation in normal guinea pigs with 5µl of BAAV-ß-actin-GFP and sacrificed the animals 2 weeks after surgery. Whole mounts of the auditory sensory epithelium were stained to show distributions of GFP and F-actin by epi-fluorescence to evaluate quantity and location of BAAV transgene expression. The distribution of GFP in the different cell types in each cochlear turn following BAAV inoculation into SM or ST is summarized in Table 1.
Table 1. The number of BAAV treated ears with positive β-actin-GFP transgene expression and the proportional range of positive cells in normal and deafened ears.
The presence and distribution of the transgene was assessed in the 2nd and basal turn of SM inoculated guinea pigs and in the basal turn for ST inoculated guinea pigs. The transgene expression efficiency is higher following SM inoculation compared to the ST inoculation for both normal and deafened ears. The transgene expression is mainly observed in the supporting cells.
| SM Normal (n=4) | ST Normal (n=4) | SM Deafened (n=3) | ST Deafend (n=10) | |||||
|---|---|---|---|---|---|---|---|---|
| Distribution of expression | ||||||||
| 2nd turn Basal turn | 3 2 |
0 2 |
3 2 |
0 2 |
||||
| Site of expression | GPs with transgene expression | Proportion of positive cells (%) | GPs with transgene expression | Proportion of positive cells (%) | GPs with transgene expression | Proportion of positive cells (%) | GPs with transgene expression | Proportion of positive cells (%) |
| Organ of Corti | ||||||||
| Deiters cell | 1 | 4 | 1 | 5 | 2 | 10–12 | 0 | 0 |
| Pillar cell | 1 | 20 | 0 | 0 | 1 | 30 | 0 | 0 |
| Hensen cell | 3 | 28–68 | 2 | 10–38 | 3 | 18–34 | 2 | 6–10 |
| Inner sulculs cell | 3 | 30–38 | 2 | 8–17 | 3 | 25–70 | 1 | 5 |
| Interdental cells | 3 | 20–34 | 2 | 12–21 | 3 | 20–34 | 2 | 7–10 |
| Outer hair cells | 0 | 0 | 1 | 7 | 0 | 0 | 0 | 0 |
| Inner hair cells | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reissner's membrane | 3 | 50–70 | 0 | 0 | 3 | 53–67 | 0 | 0 |
Values described in the “Proportion of positive cells (%)” represent the ranges of positive cells among the GPs with GFP transgene expression.
Transgene expression of GFP in SM inoculated animals was robust near the inoculation site (2nd turn), less prevalent in the basal turn and absent in the higher (3rd and 4th) turns. Transgene expression was abundant in the supporting cells in the area of inner and outer hair cells, and in Reissner’s membrane (Fig. 2a–d). Among supporting cells, interdental, inner sulcus and Hensen cells were consistently transduced, but Deiters and pillar cells showed much less transgene expression. Hair cells were not transduced.
Fig. 2. Whole-mounts of the 2nd turn or basal turn auditory epithelium of normal (non-deafened) guinea pigs after SM inoculation (a–d) or ST inoculation (e–h) viewed with epi-fluorescence.
β-actin-GFP positive cells (green) indicate transduction and phalloidin (red) represents the actin. (a) Interdental cells (IDC) and (b) inner sulcus cells (ISC) with β-Actin-GPF expression. (c) Hensen cells (H) show robust expression, and pillar cells (P) and phalangeal scars (PS) can be observed in place of outer hair cells. (d) Reissner’s membrane (RM) is broadly transduced. (e) Interdental cells and (f) Hensen cells are transduced. (g) Transduced Hensen cells and Deiters cells (arrow). (h) Outer hair cell rows (1, 2, and 3) with phalangeal scars (white arrow), residual hair cells (arrow head) and a transduced hair cell (black arrow). Bar = 20µm, for all images.
An important outcome of the SM inoculation was hair cell loss. The severity of the loss was substantial near the inoculation site, but it decreased further away from the site14. The contralateral (right) ears showed no transgene expression or pathological hair cell loss in any animals (data not shown).
In guinea pigs that received BAAV in ST, the gene expression was restricted to the vicinity of the inoculation site (basal turn). Cell types presenting gene expression in the basal turn epithelium were interdental, inner sulcus and Hensen cells, and rarely, outer hair cells (Fig. 2e–f). Outer hair cell transduction could only be seen in the ST inoculation; however pillar cells and Reissner’s membrane, which were transduced through the SM route, were not affected through the ST route. Partial hair cell loss was also apparent in the basal turn of the ST inoculated organ of Corti (Fig. 2h). In contrast, hair cell loss was not observed in the corresponding control group.
3. Effect of BAAV inoculation on histology of deafened guinea pigs
Animals were deafened by a combination of subcutaneous kanamycin and intravenous ethacrynic acid; subsequently, hair cells degenerated and were replaced by scars15,16. Three days later the deafened guinea pigs were inoculated with the same vector and concentration, by the same routes as normal guinea pigs and sacrificed 2 weeks later. In order to assess the transduction of BAAV in deafened ears, we counted the number of ears with positive transgene expression, as in BAAV inoculated normal guinea pigs (Table 1).
In SM injected deaf animals, GFP was seen in the 2nd and basal turn, with strong transgene expression near the inoculation site (Fig. 3). Transgene expression was especially robust in the inner sulcus cells (Fig. 3c) appearing more efficient than that seen in normal SM injected guinea pigs (Fig. 2b). Transgene expression was seen in the interdental, Hensen, and Deiters cells with apparently similar intensity and frequency to that seen in normal SM injected guinea pigs (Fig. 3a, b, and d compared to 2a and c).
Fig. 3. Whole-mounts of the 2nd turn or basal turn auditory epithelium of SM inoculated (a–d) or ST inoculated (e) deafened guinea pigs.
(a) Interdental cells (IDC) show efficient transduction. (b) Hensen cells (H) are transduced, as are cells constituting the phalangeal scars (PS, arrows). (c) Inner sulcus cells (ISC) are transduced at high efficiency. (d) Deiters cells (arrow head) are transduced in moderate efficiency. (e) Efficiency of transduction of interdental cells is low compared to SM inoculation (compare to (a)). Bar = 20µm, for all images.
In ST inoculated deafened guinea pigs, transgene expression was seen in interdental (Fig. 3e) and Hensen cells. Positive cells were seen in the basal turn but were much less abundant than in normal ST injected guinea pigs. Pillar cells were not infected by BAAV, which is consistent with normal ST injected guinea pigs. The efficiency of the transduction was notably lower in the deafened ST inoculated guinea pigs, where numbers of transfected ears and frequencies of positive cells (%) were generally lower than in normal ST injected guinea pigs.
Discussion
This study characterizes the transgene expression of BAAV with ß-actin-GFP injected into the normal hearing and deafened guinea pig inner ear through two different routes: the SM and ST inoculation. We show that in vivo inoculation through both routes transduces hair cells in normal guinea pigs and a variety of supporting cells in both normal and deafened guinea pigs, with minimal side effects.
BAAV effects on the auditory system
The ABR results from our control group showed a significantly higher (worse) thresholds in the artificial endolymph inoculated SM animals (Fig. 1c) compared to the artificial perilymph inoculated ST animals (Fig. 1d) at both 1 and 2 week time points. The elevated threshold in artificial endolymph inoculated SM animals is thought to be the consequence of a mechanical disruption of the organ of Corti, possibly involving rupture of the Reissner’s membrane and/or mixing of perilymph and endolymph fluids around the inoculation site. Thus, hair cell loss is most severe in this area and gradually diminishes away from the inoculation site, as described in previous literature using this method in guinea pigs14. Animals inoculated with artificial perilymph into ST showed a threshold shift no more than 13 dB at the 1st week and 8 dB at the 2nd week which is also thought to be caused by the surgical procedure. ST inoculation caused little hair cell loss in any cochlear turn; most of the damage was again near the site of injection. For both surgical approaches, the threshold shifts seen in BAAV inoculated guinea pigs were comparable to those of the control animals (Fig. 1a and 1b), suggesting that the injection of the BAAV had little or no side effects on auditory function. In addition, after the initial threshold shift, the ABR thresholds improved between the 1st and 2nd week, supporting the inference that the functional effect of the procedure is due to the surgery, rather than the presence of the virus or the expression of the transgene. Together, these results indicate that BAAV has minimal detrimental effects on cell survival and function of the inoculated ears.
Distribution of transgene expression in normal guinea pigs
The distribution of transgene expression among cell types is shown in Table 1. Transduction of Hensen, interdental and inner sulcus cells was demonstrated in all groups receiving BAAV. The efficiency was high in the SM inoculated guinea pigs, with almost all ears inoculated with BAAV showing transgene expression. The ST inoculated guinea pigs typically did not show efficient gene expression, although a few outer hair cells did appear positive. The transgene efficiency was low in the ST inoculated guinea pigs in both normal and deafened conditions. Therefore, these results suggest that the cell types such as Hensen, interdental, and inner sulcus cells can be transduced consistently by BAAV through both routes but transduction of hair cells and Reissner’s membrane is dependent on the inoculation route. Additionally, the proportion of transduced cells is much higher through the SM inoculation compared to the ST inoculation.
In cultures of rat cochleae exposed to BAAV, an estimated 100% of Hensen cells; 40% of interdental and phalangeal cells; 10% of inner and outer hair cells; and 48% of vestibular cells were transduced13. The frequencies of Hensen, interdental, and outer hair cell transduction is consistent with our data. In contrast, transduction of inner sulcus, Deiters cells, pillar cells and Reissner’s membrane in the rat cultures was not observed. These discrepancies may be caused by differences in susceptibility to the effects of BAAV in organ of Corti among species and by differences between culture and in vivo conditions.
Transfer of genes into hair cells is of importance for research issues such as understanding function of gene products, and for clinical applications such as protection or phenotypic rescue for hereditary disease. However, work with adenovirus vectors in mature normal mammals has shown that the transduction was restricted to supporting cells14,17,18. The reasons for the lack of hair cell transduction are not clear, especially considering that these cells do express the CAR receptor17,18. As such, our current finding about BAAV transduction of hair cells is a positive first step toward work on protection and phenotypic rescue. However, means for increasing the efficiency of hair cell transduction need to be identified.
It appears that different viral vectors have different patterns of transduction in the cochlea. Inoculation with various serotypes of AAV (1, 2, 3, 5, 7, 8, and 9), into the murine or guinea pig cochlea in vivo, produced stronger transgene expression in the inner hair cells but weaker expression in the supporting cells. Inoculation with adenovirus showed robust transgene expression in the supporting cells when inoculated through the SM route14 and in the mesothelial cells when inoculated through the ST route19. Therefore, the BAAV has similar transgene expression to adenovirus by showing high transduction efficiency in the supporting cells through SM route and differs from other AAV serotypes by transducing mainly the supporting cells through the ST route.
High transduction efficiency of supporting cells with BAAV through the SM route is an encouraging result for inducing transdifferentiation of supporting cells to hair cells, especially in research work. Such studies of regeneration are based on forced expression of genes that encode the hair cell phenotype during development, resulting in a phenotypic change of supporting cells20,21. Our data suggest that BAAV would be an appropriate gene vector for SM inoculation leading to forced expression of Atoh1 and other genes that may induce regeneration in the organ of Corti. For clinical application, however, SM approach is not feasible due to access limitation in the human ear. In contrast, the ST route is accessible, via the oval or round window. Our results on low efficiency transduction of non-sensory cells via the ST constitutes a proof for the principle that BAAV can accomplish such transduction, but higher efficiency will be needed for robust biological activity.
Distribution of transgene expression in deafened guinea pigs
The deafened animals exhibited scars that replaced missing hair cells. SM inoculation resulted in similar pattern of supporting cell transduction in normal and deafened animals. Following ST inoculation, the pattern of supporting cell transduction was also comparable between deafened and normal animals. However, the staining intensity in the transduced cells of the deafened animals was lower and the efficiency of transduction was notably lower compared to normal guinea pigs.
The reason for reduced transduction in traumatized ears as compared with normal ears is unclear. We speculate that the scarring process that follows hair cell loss leads to changes in supporting cells that reduce their affinity for transduction by BAAV after SM inoculation. Areas with no scarring, such as the inner sulcus, did not show a reduction in efficiency of transgene expression following a lesion. Changes seen after ST inoculation could be caused by structural change (thickening) of the basilar membrane, as seen after amikacin treatment22. Similar thickening of the basilar membrane can be observed in presbycusis in human23. This structural change of the basilar membrane may alter the permeability and allow less BAAV to reach the baso-lateral domains of membranous labyrinth cells. Changes of efficiency of virus uptake in the inner ear epithelium after induced lesions have been shown to occur, at times leading to increased efficiency 24,25. Because the traumatized auditory epithelium will be the target for clinical reparative procedures, it is important to characterize the optimal vector designs, promoters and inoculation methods that would yield the desirable outcome in terms of efficiency and safety in deafened ears.
Because the efficiency of transgene expression of BAAV is relatively low in deafened animals, practical application will require some means to enhance transgene expression. One important task is to elucidate the specific receptors for BAAV in the cochlea. Once receptors are identified, it would be possible to alter virus construct for augmenting the affinity of these receptors, thereby leading to better transgene expression efficiency. Gangliosides have been reported act as receptors for BAAV in vitro26. Therefore, co-inoculation BAAV and gangliosides into the ST may enhance the transgene efficiency.
Pattern of GFP distribution within a cell
The intracellular distribution of the fusion β-actin–GFP protein was determined to be throughout the cytoplasm. This was expected given that G-actin is distributed throughout the cytoplasm. Over-expression of this fusion protein mediated by the BAAV is unregulated and as such excess gene product may even spread into the nucleus, but this was not observed in cochlear cells transduced with BAAV β-actin–GFP, demonstrating that the cells were able to contain the protein in the cytoplasm.
Time course of gene expression
The BAAV showed transgene expression most efficiently 2 weeks after inoculation. This onset of gene expression is slower compared to adenovirus which peaks several days after the inoculation, both in the ear4 and in other tissues27. The delay in gene expression of BAAV is related to its molecular structure. BAAV is a single stranded DNA virus; therefore conversion into a double stranded DNA in the nucleus is necessary in order for transgene expression. The late onset of gene expression makes this vector less desirable for goals related to rescue of cells after an insult has occurred. However, the long duration of gene expression (over several years) makes the vector attractive for long term protective effects or for substitution of a mutated gene7.
Side effects of BAAV in the cochlea
In our in vivo study it was possible to test toxicity as well as the extent of immune response. We determined that the auditory system was largely preserved and morphology of the middle ear was intact 2 weeks after the inoculation. All guinea pigs were healthy and had no signs of inflammation (discharge or contamination of fluids in the inner ear) 2 weeks after the surgery. Thus, no particular side effects from the BAAV were apparent in most of the animals. However, in ST inoculated guinea pigs with BAAV, few hair cells were transduced and there was sporadic hair cell loss (Fig. 2h). This may suggest possible cytotoxicity of the virus. Hair cells may take up the virus and possibly degenerate. For adenovirus, the volume of 5µl has been mentioned to be a safe volume with no apparent side effects28. However, the adenovirus injected into ST does not transduce cells of the membranous labyrinth, so comparison of side effects on hair cells and hearing are difficult. It is likely that the volume of 5µl may not be optimal for BAAV transduction. Modifying the virus volume or concentration of the BAAV may significantly reduce side effects.
BAAV delivery through SM and ST route
For future clinical intervention, the feasibility of inoculation surgery is a crucial factor. Because of the difficulty of reaching the endolymphatic space of human temporal bone, the SM inoculation is not readily adapted for humans, although other methods of reaching the endolymphatic space are possible (e.g. endolymphatic sac29). An important outcome in this study was transduction of cells in the membranous labyrinth through ST inoculation, which is more feasible for clinical intervention. These data indicate that the BAAV may be a potential candidate for future clinical application, especially once means are found to enhance to efficiency of transgene expression in deafened animals.
Conclusions
We have shown that BAAV can shuttle genes into cells of the membranous labyrinth via endolymph or perilymph inoculation. Onset of gene expression is slow but once expressed, it is sustained over a log time, with little or no side effects. Once efficiency can be increased, this vector has the potential for becoming useful for a wide range of applications for inner ear research and future clinical intervention.
Material and Methods
1. Animals & Groups
Animal care and handling were approved by the University of Michigan Institutional Committee on the Use of Care of Animals and performed using accepted veterinary standards. Twenty male pigmented guinea pigs weighing 300–350g were purchased from Elm Hill, Chelmsford, MA USA. Each animal was tested for normal Preyer’s reflex before being included in the study. Normal hearing animals were divided into 4 groups, according to the inoculation site and injected reagent. One group of animals had BAAV ST inoculation (n=4). The second group had BAAV SM inoculation (n=4). The third group of animals had artificial perilymph (NaCl 145mM, KCl 2.7mM, MgSO4 2.0mM, CaCl2·2H2O, HEPES, HPLC grade water, and HPLC grade MeOH) ST inoculation (n=3). The last group had artificial endolymph (NaCl 1mM, KCl 126mM, KHCO3 25mM, MgCl2 0.025mM, CaCl2 0.025mM and K2HPO4 1.4mM) SM inoculation (n=3). Deafened animals were divided into 2 groups. One was inoculated in the ST with BAAV (n=3) and the other in the SM with BAAV (n=10). All cochleae in each group were processed for whole-mount (surface preparation) analysis.
2. Auditory brainstem response assessment
Auditory brainstem responses (ABRs) were assessed for each animal in both ears. The thresholds were measured by each ear for frequencies at 4k, 12k, and 20 kHz (tone bursts, 15ms duration, 1ms cos2-shaped rise-fall times) as described previously30. ABRs were assessed prior to the inoculation surgery (baseline), 7 and 14 days after the inoculation surgery. We compared the threshold shift from baseline at 7 and 14 days. Furthermore, we compared the threshold shifts between the SM and ST inoculation for both BAAV and corresponding controls. Statistical analyses of ABR threshold shifts were performed by ANOVA in SYSTAT. A P value < 0.05 was considered statistically significant.
3. BAAV-ß-actin-GFP vector construction, preparation, and quantification
293T (human kidney) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The media contained 2 mM L-glutamine, 100 U of penicillin/ml, and 0.1 mg of streptomycin/ml. Cells were maintained at 37°C under a 5% CO2 humidified atmosphere. Recombinant BAAV expressing β-actin–GFP was produced using a four-plasmid procedure previously described13. Briefly, semiconfluent 293T cells were transfected by calcium phosphate with four plasmids: an adenovirus helper plasmid (pAd12) containing the VA RNA, E2, and E4; Two AAV helper plasmids containing the AAV2 Rep and BAAV Cap genes respectively and a vector plasmid containing AAV2 inverted terminal repeats flanking CMV–β-actin–GFP fusion expression cassette. The AAV2 vector plasmid containing the AAV2 inverted terminal repeats (ITRs) flanking the CMV–β-actin–GFP fusion expression cassette was constructed by subcloning of the CMV promoter β-actin–GFP cassette from the β-actin–GFP plasmid (BD Biosciences) into the AAV2 RSV–GFP expression plasmid and replacement of the RSV–GFP cassette with the CMV β-actin–GFP. Forty-eight hours post-transduction the cells were harvested by scraping in TD buffer (140 mM NaCl, 5 mM KCl, 0.7 mM K2 HPO4, 25 mM Tris–HCl, pH 7.4) and the cell pellet was concentrated by low-speed centrifugation. Cells were lysed in TD buffer by three cycles of freeze–thaw. The clear lysate was treated with 0.5% deoxicolic acid (DOC) and 100 U/ml DNase (Benzonase) for 30 min at 37°C. Then the virus was purified using CsCl gradients. Particle titers were determined by QPCR. Amplification was detected using an ABI 7700 sequence detector (ABI). Specific primers for CMV were designed by using the Primer Express program (ABI): CMV forward 5'-CATCTACGTATTAGTCATCGCTATTACCAT-3', CMV reverse 5'-TGGAAATCCCCGTGAGTCA-3'. Following denaturation at 96°C for 10 min, cycling conditions were 96°C for 15 s, 60°C for 1 min for 40 cycles. The viral DNA in each sample was quantified by comparing the fluorescence profiles with a set of DNA standards. The BAAV particle titers were in the range of 1012 DNAse resistant particles (DRP)/ml.
4. Inoculation and Deafening surgeries
Under general anesthesia, the ST inoculation surgery was carried out as described previously24. We inoculated the left ear with 5µl BAAV and repeated the same procedure for the artificial perilymph for the control group. SM inoculation surgery was performed as described previously14 except that we performed cochleostomy in the second turn of the cochlea. We injected 5µl of BAAV and repeated likewise with artificial endolymph for the control group.
For the deafening surgery, we used a single systemic dose of kanamycin (500mg/kg, subcutaneously) followed by an intravenous injection of ethacrynic acid (50mg/kg)31. Deafness in each animal was verified by ABR 4 days after the surgery. Typically, animals showed a threshold shift of 80–90db. We excluded any animal with threshold shift less than this value after surgery. Excluded animals are not counted in the total number of animals reported here.
5. Immunohistochemistry
Fourteen days after the inoculation, animals had ABR assessment and were decapitated under general anesthesia. Both temporal bones were extracted and the cochleae were locally perfused with 4% paraformaldehyde (PFA). After 2 hrs of fixation with 4% PFA, the cochleae were rinsed in phosphate buffered saline (PBS).
The cochleae which were used for whole mounts in all groups were stained for reporter gene β-actin-GFP detection in both ears (including contralateral ear). Tissues were permeabilized with 0.3% Triton X-100 for 10min, blocked against non-specific binding of secondary antibody by incubation in 5% normal donkey serum for 30min. We incubated the tissues in mouse monoclonal antibody to GFP (Chemicon, International), diluted 1:200 in PBS, for 1 hr. After rinsing with PBS twice, we used fluorescent-labeled donkey anti-mouse IgG (Alexafluor 488 Molecular Probes) as secondary antibodies diluted in 1:200 in PBS. We counterstained the tissue for F-actin with rhodamine-phallodin for 2 min diluted to 1:300 in PBS. After the tissues were washed with PBS they were whole mounted on slides, cover-slipped with Gel/Mount (Biomeda, Foster City, CA, USA) and observed with a Leica DMRB epi-fluorescence microscope (Leica, Eaton, PA, USA).
6. Quantification of β-actin–GFP positive transgene expression in normal and deafened guinea pigs
Images obtained from the epi-fluorescence microscope were processed by Photoshop CS2 to meet equal imaging conditions. Through the assessment of 200µm radius of every whole mount image, the guinea pigs with five or more β-actin–GFP transfected cells were considered positive (Table 1). Among the guinea pigs ears with positive expression, the β-actin–GFP transfected cells were counted within a 100µm2 box, in each morphological segment (e.g. Hensen cell area, interdental area). Counting was performed in Image J, which was also used to estimate the size (apical surface area) of each positive cell. The areas of the positive cells fitting within the 100µm2 box were summed and converted to percentage. The ranges of these percentages among positive guinea pigs are reported in Table 1.
Acknowledgements
We thank Donald Swiderski and Agnieszka Rzadzinska for assistance. Work supported by the A. Alfred Taubman Medical Research Institute, the Berte and Alan Hirschfield Foundation, the R. Jamison and Betty Williams Professorship, and NIH/NIDCD Grants R01-DC01634, R01-DC05401, R01-DC03685, T32DC005356 and P30-DC05188.
References
- 1.Hawkins JE., Jr Comparative otopathology: aging, noise, and ototoxic drugs. Adv Otorhinolaryngol. 1973;20:125–141. doi: 10.1159/000393093. [DOI] [PubMed] [Google Scholar]
- 2.Bedrosian JC, Gratton MA, Brigande JV, Tang W, Landau J, Bennett J. In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther. 2006;14:328–335. doi: 10.1016/j.ymthe.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–18. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG, et al. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16:224–236. doi: 10.1038/sj.mt.6300351. [DOI] [PubMed] [Google Scholar]
- 5.Holt JR. Viral-mediated gene transfer to study the molecular physiology of the Mammalian inner ear. Audiol Neurootol. 2002;7:157–160. doi: 10.1159/000058302. [DOI] [PubMed] [Google Scholar]
- 6.Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N, et al. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Ther. 1998;5:277–281. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- 7.Coura Rdos S, Nardi NB. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol J. 2007;4:99. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 9.Bueler H. Adeno-associated viral vectors for gene transfer and gene therapy. Biol Chem. 1999;380:613–622. doi: 10.1515/BC.1999.078. [DOI] [PubMed] [Google Scholar]
- 10.Blacklow NR, Hoggan MD, Rowe WP. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 11.Luchsinger E, Strobbe R, Wellemans G, Dekegel D, Sprecher-Goldberger S. Haemagglutinating adeno-associated virus (AAV) in association with bovine adenovirus type 1. Brief report. Arch Gesamte Virusforsch. 1970;31:390–392. doi: 10.1007/BF01253774. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Katano H, Bossis I, Chiorini JA. Cloning and characterization of a bovine adeno-associated virus. J Virol. 2004;78:6509–6516. doi: 10.1128/JVI.78.12.6509-6516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Pasquale G, Rzadzinska A, Schneider ME, Bossis I, Chiorini JA, Kachar B. A novel bovine virus efficiently transduces inner ear neuroepithelial cells. Mol Ther. 2005;11:849–855. doi: 10.1016/j.ymthe.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173:187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- 15.Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hear Res. 1991;51:173–183. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- 16.Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- 17.Excoffon KJ, Avenarius MR, Hansen MR, Kimberling WJ, Najmabadi H, Smith RJ, et al. The Coxsackievirus and Adenovirus Receptor: a new adhesion protein in cochlear development. Hear Res. 2006;215:1–9. doi: 10.1016/j.heares.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Venail F, Wang J, Ruel J, Ballana E, Rebillard G, Eybalin M, et al. Coxsackie adenovirus receptor and alpha nu beta3/alpha nu beta5 integrins in adenovirus gene transfer of rat cochlea. Gene Ther. 2007;14:30–37. doi: 10.1038/sj.gt.3302826. [DOI] [PubMed] [Google Scholar]
- 19.Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J Gene Med. 2008 doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- 20.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladrech S, Wang J, Simonneau L, Puel JL, Lenoir M. Macrophage contribution to the response of the rat organ of Corti to amikacin. J Neurosci Res. 2007;85:1970–1979. doi: 10.1002/jnr.21335. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt KA, Liberman MC, Nadol JB., Jr Morphometric analysis of age-related changes in the human basilar membrane. Ann Otol Rhinol Laryngol. 2001;110:1147–1153. doi: 10.1177/000348940111001212. [DOI] [PubMed] [Google Scholar]
- 24.Stover T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 25.Weiss MA, Frisancho JC, Roessler BJ, Raphael Y. Viral-mediated gene transfer in the cochlea. Int J Dev Neurosci. 1997;15:577–583. doi: 10.1016/s0736-5748(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Chiorini JA. Gangliosides are essential for bovine adeno-associated virus entry. J Virol. 2006;80:5516–5522. doi: 10.1128/JVI.02393-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park CY, Chuck RS, Cano M, Yew M, Nguyen V, Parker J, et al. Periocular triamcinolone enhances intraocular gene expression after delivery by adenovirus. Invest Ophthalmol Vis Sci. 2008;49:399–406. doi: 10.1167/iovs.07-0619. [DOI] [PubMed] [Google Scholar]
- 28.Stover T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7:377–383. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- 29.Yamasoba T, Yagi M, Roessler BJ, Miller JM, Raphael Y. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther. 1999;10:769–774. doi: 10.1089/10430349950018526. [DOI] [PubMed] [Google Scholar]
- 30.Yamasoba T, Dolan DF. Chronic strychnine administration into the cochlea potentiates permanent threshold shift following noise exposure. Hear Res. 1997;112:13–20. doi: 10.1016/s0378-5955(97)00092-0. [DOI] [PubMed] [Google Scholar]
- 31.West BA, Brummett RE, Himes DL. Interaction of kanamycin and ethacrynic acid. Severe cochlear damage in guinea pigs. Arch Otolaryngol. 1973;98:32–37. doi: 10.1001/archotol.1973.00780020036009. [DOI] [PubMed] [Google Scholar]