Summary
Background
In many insect species, cuticular hydrocarbons serve as pheromones that can mediate complex social behaviors. In Drosophila melanogaster, several hydrocarbons including the male sex pheromone 11-cis-vaccenyl acetate (cVA) and female-specific 7,11-dienes influence courtship behavior and can function as cues for short-term memory associated with the mating experience. Behavioral and physiological studies suggest that other unidentified chemical communication cues are likely to exist. To more fully characterize the hydrocarbon profile of the D. melanogaster cuticle, we applied direct ultraviolet laser desorption/ionization orthogonal time-of-flight mass spectrometry (UV-LDI-o-TOF MS) and analyzed the surface of intact fruit flies at a spatial resolution of approximately 200 μm.
Results
We report the chemical and spatial characterization of 28 species of cuticular hydrocarbons, including a new major class of oxygen-containing compounds. Using UV-LDI MS, pheromones previously shown to be expressed exclusively by one sex, e.g. cVA, 7,11-heptacosadiene, and 7,11-nonacosadiene, appear to be found on both male and female flies. In males, cVA co-localizes at the tip of the ejaculatory bulb with a second acetylated hydrocarbon named CH503. We describe the chemical structure of CH503 as 3-O-acetyl-1,3-dihydroxy-octacosa-11,19-diene and show one behavioral role for this compound as a long-lived inhibitor of male courtship. Like cVA, CH503 is transferred from males to females during mating. Unlike cVA, CH503 remains on the surface of females for at least 10 days.
Conclusions
Oxygenated hydrocarbons comprise one major previously undescribed class of compounds on the Drosophila cuticular surface. In addition to cVA, a newly-discovered long chain acetate, CH503, serves as a mediator of courtship-related chemical communication.
Introduction
In many species of insects, chemical communication is mediated by hydrocarbons found on the surface of the cuticle [1]. For example, ants, bees, termites, and some wasps rely on such chemical cues for kin recognition, aggregation, and to trigger alarm [1]. In the fruit fly Drosophila melanogaster, several features of courtship behavior are influenced by specific cuticular hydrocarbons (CH) [2–6]. For example, the characteristic female compounds 7,11-heptacosadiene and 7,11-nonacosadiene serve as attractants for males [7]. In addition, the volatile sex-pheromone, 11-cis-vaccenyl acetate (cVA), which is reported to be synthesized exclusively by males [8, 9], plays a dual role as an aggregation factor for females and males [10, 11] and an inhibitor of male courtship [12–15]. Pheromones are detected by olfactory and gustatory receptors found in specialized sensory cells located on the antennae, maxillary palps, labellum, and forelegs (reviewed in [16]). Aside from cVA, however, no specific ligands have been identified for pheromone receptors. Behavioral observations also indicate that our understanding of chemical communication in Drosophila courtship is far from complete. For example, after successful mating, female attractiveness to males decreases and remains depressed for between 9 – 10 days [17, 18]. Initial suppression of male courtship is partly mediated by the transfer of cVA and 7-tricosene to females [2], but neither of these compounds are detected on female cuticles at significant levels beyond 24 h after mating [15]. This discrepancy between the behavioral and biochemical data indicates that prolonged suppression of female attractiveness is likely to involve other undiscovered sensory signals used by flies for mate recognition and courtship.
A major hindrance to the discovery of chemical substances from the insect cuticle is the limited detection range of conventional analytical methods. The most common technique involves extracting CHs with an organic solvent followed by analysis of the extract by gas chromatography (GC) in combination with mass spectrometry (MS). GC/MS allows detection and some structural elucidation of sufficiently volatile, non-polar hydrocarbons like alkanes (composed of single bonds) and alkenes (composed of one or more double bonds). However, the standard GC/MS conditions used for Drosophila analysis have two limitations: first, spatial information about CH expression is lost due to the extraction procedure; and second, larger and more polar cuticular compounds are likely to be missed [1].
To more fully characterize the chemical profile of the D. melanogaster cuticle, we applied direct ultraviolet laser desorption/ionization orthogonal time-of-flight mass spectrometry (UV-LDI-o-TOF MS). Using a 200 μm diameter laser beam, molecules from the cuticular surface are brought into the gas phase and detected as ions with a cation adduct. This method, used here for the first time to analyze intact individual fruit flies, allows spatially-resolved chemical profiling of fine anatomical features and broadens the detection range to include more polar CHs, many of which are not detected by GC/MS. We report the chemical and spatial characterization of 28 species of CHs and the discovery of a major class of oxygen-containing compounds that has not been described before for D. melanogaster. UV-LDI MS analysis reveals that while D. melanogaster exhibit sexually dimorphic CH profiles, the differences are mostly quantitative rather than qualitative. With respect to the major CH species, the profile of female flies is largely homogenous throughout the whole body surface. Male flies also exhibit a mostly homogenous CH profile across different body parts with one important exception. The male anogenital region shows strikingly high levels of two molecules: cVA and a new male-specific sex pheromone named CH503. We determined the chemical structure of CH503 and characterized its spatial and sexually dimorphic expression. Like cVA, CH503 is transferred from male to female flies during copulation and suppresses further male courtship. Unlike cVA, CH503 persists on the cuticle of females for at least 10 days after mating.
Results
Chemical profiling of individual intact male and female Drosophila cuticles using direct UV- LDI-o-TOF mass spectrometry revealed robust, reproducible, and spatially-resolved ion signals corresponding to long-chain hydrocarbons (Figure 1). The compounds detected in the adult cuticle vary in chain length from 20 – 35 carbon atoms and contain between 0 – 3 double bonds and 0 – 3 oxygen atoms (Table 1). Males and females exhibit consistent CH profiles in terms of both content and the relative abundance of individual compounds when the same region is compared across different individual flies (a quantitative analysis is shown in Figure S1 and Table S1). Absolute quantities cannot be derived from UV-LDI analysis since the elemental composition of a compound can contribute to how easily it is ionized and detected. As a result, alkanes are not detected by this method and monoenes are detected with lower efficiency compared to GC/MS. In addition to the hydrocarbons, a few other compounds also are detected in the cuticle using UV-LDI MS, in particular triacylglycerides (Figure S2). Only the hydrocarbons will be addressed in this paper.
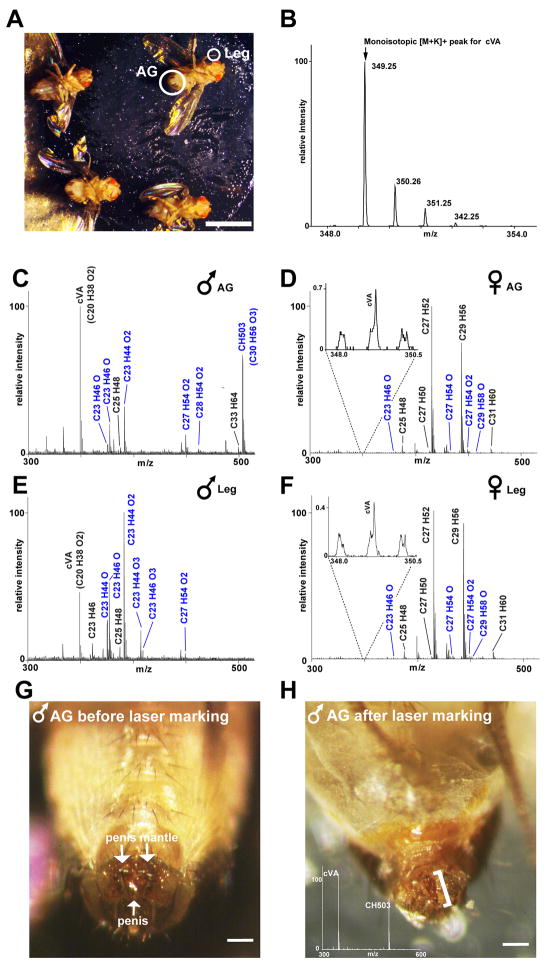
Figure 1. Cuticular hydrocarbon profiles of adult virgin male and female Drosophila melanogaster using direct UV-LDI MS analysis.
(A) Flies are mounted on a custom-made sample plate using adhesive tape. A UV laser is used to scan two primary areas: the anogenital region (AG) and legs (both circled in white). The picture was taken after analysis. Some changes in the structural integrity of the abdomen and eyes occur after the flies are removed from the high vacuum conditions used during analysis. Damage to the cuticle by the UV-laser is not visible. Scale bar: 2 mm.
(B) Each CH is detected bearing a potassium adduct and with a series of resolved isotopes. The signal for cVA is shown as an example. For each species, only the monoisotopic [M+K]+ peak is used to calculate intensity (indicated by arrow).
(C, E) Representative CH profiles of the AG (C) and legs (E) of male flies. Males show large differences in CH expression when comparing the two regions. Novel oxygen-containing CH species are highlighted in blue. Major ion signals are labeled with the proposed elemental composition. All labeled signals correspond to potassiated molecules [M+K]+. Peaks attributed to sodiated species are not labeled.
(D, F) Representative CH profiles of the AG (D) and legs (F) of female flies. Both regions appear similar in terms of content and relative abundances of individual CHs. Profiles of socially naïve females show low levels of signals corresponding to the male pheromone cVA (inset). The accompanying peaks in the inset represent non-specific chemical noise.
(G, H) In-situ MS profiling followed by carbonization with a second laser was used to mark a region of the male terminalia expressing the highest levels of cVA and CH503. Prior to laser ablation, the penis mantle is darkly pigmented (G). The site of interest was located first using the standard UV-laser with a 200 μm flat top beam profile (no visible damage to the cuticle is induced by this laser). A second, more tightly-focused high-intensity UV-laser beam with ca. 100 μm diameter was aimed at the site illuminated by the first UV-laser, thereby causing carbonization of the cuticle. After laser-ablation, the penis mantle pigmentation is no longer present and carbonization of the penis is evident (H). The marked site is indicated by a bracket and the inset shows a mass spectrum taken from this region. Scale bar: 100 μm.
Table 1.
Proposed elemental composition of hydrocarbons detected by direct UV-LDI-o-TOF mass spectrometry in the cuticle of socially naïve adult male and female Drosophila melanogaster.
| Elemental compositiona | Proposed structure and functional groupsb | Calculated mass of [M+K]+ ionc | Male AGd (n=13) | Male leg (n=12) | Female AG (n=12) | Female leg (n=12) |
|---|---|---|---|---|---|---|
| C20 H38 O2 (cVA)* | C20:1 (OAc) | 349.25 | ++++ | ++ | (+) | (+) |
| C23 H44 (tricosadiene)* | C23:2 | 359.31 | + | ++ | + | + |
| C23 H44 O | C23:2 (OH) | 375.30 | ++ | +++ | + | ++ |
| C23 H44 O2 | C23:2 (2 OH) | 391.30 | +++ | ++++ | + | + |
| C23 H44 O3 | C23:2 (3 OH) | 407.29 | (+) | +++ | (+) | (+) |
| C23 H46 (tricosene)* | C23:1 | 361.32 | + | ++ | (+) | (+) |
| C23 H46 O | C23:1 (OH) | 377.32 | +++ | ++++ | ++ | ++ |
| C23 H46 O3 | C23:1 (3 OH) | 409.31 | + | ++ | n.d. | (+) |
| C25 H48 (pentacosadiene)* | C25:2 | 387.34 | ++ | +++ | ++ | ++ |
| C25 H48 O | C25:2 (OH) | 403.33 | (+) | + | + | + |
| C25 H50 (pentacosene)* | C25:1 | 389.36 | (+) | + | − | − |
| C25 H50 O | C25:1 (OH) | 405.35 | + | ++ | + | + |
| C27 H50 (heptacosatriene) | C27:3 | 413.35 | + | + | + | + |
| C27 H52 (heptacosadiene)* | C27:2 | 415.37 | + | + | ++++ | ++++ |
| C27 H54 (heptacosene)* | C27:1 | 417.39 | + | + | − | − |
| C27 H54 O | C27:1 (OH) | 433.38 | + | ++ | ++ | ++ |
| C27 H54 O2 | C27:1 (2 OH) | 449.38 | (+) | ++ | ++ | + |
| C29 H54 (nonacosatriene) | C29:3 | 441.39 | n.d. | n.d. | + | + |
| C29 H56 (nonacosadiene)* | C29:2 | 443.40 | + | + | ++++ | ++++ |
| C29 H58 (nonacosene)* | C29:1 | 445.42 | + | + | − | − |
| C29 H58 O | C29:1 (OH) | 461.41 | (+) | (+) | + | + |
| C30 H56 (triacontatriene) | C30:3 | 455.40 | n.d. | n.d. | (+) | (+) |
| C30 H58 (triacontadiene) | C30:2 | 457.42 | (+) | (+) | + | + |
| C30 H56 O3 (CH503) | C30:2 (OAc + OH) | 503.39 | ++++ | + | n.d. | n.d. |
| C31 H60 (hentriacontadiene) | C31:2 | 471.43 | + | + | ++ | ++ |
| C31 H62 (hentriacontene) | C31:1 | 473.45 | + | + | − | − |
| C33 H64 ( (tritriacontadiene) | C33:2 | 499.46 | ++ | ++ | + | + |
| C35 H68 (pentatriacontadiene) | C35:2 | 527.50 | ++ | ++ | + | + |
Based on exact mass measurements within a 20 ppm mass accuracy; previously unreported CH species are highlighted in gray;
indicates compounds detected in extracts from males or females in previous GC/MS studies [Ref. 20].
Proposed structures are listed according to the number of carbon atoms followed by the number of double bonds within the carbon chain. The assignment of functional groups is based on tandem ESI-MS of cuticular extract. The occurrence of other isomeric compounds (e.g., in the form of ketones or epoxides) cannot be excluded; these species would typically not produce characteristic fragment ions under the tandem ESI-MS conditions.
Potassiated species [M+K]+ form the base peaks in all UV-LDI-o-TOF mass spectra; mass values correspond to the monoisotopic mass.
The relative abundance of each CH species is calculated by dividing the area under the monoisotopic peak by the total area of all CH peaks detected in the same experiment: ++++: >10% of the total area; +++: 5–10%; ++: 1–5%; +: <1%; (+): detected in some but not all flies; n.d.: not detected; −: unable to detect due to overlapping signals from other hydrocarbons. See Figure S1 and Table S1 for averaged values. AG: anal-genital region; cVA: 11-cis-vaccenyl acetate; OAc: acetate; OH: hydroxyl.
Cuticular hydrocarbon profile of adult male flies
In adult male flies, the anogenital region expresses a very different CH profile from other parts of the body (Figure 1C, E; see Table S2 for exact mass values). Within this area, two major signals are observed: one corresponds to cVA (C20H38O2); the other to a previously unreported compound with the elemental composition C30H56O3. This CH is detected with a mass-to-charge ratio (m/z) of 503.39 (corresponding to the mass of the molecule bearing a potassium adduct) and is hereafter called CH503. Both cVA and CH503 are detected with high intensity only in the anogenital region, though cVA also is found at lower levels in other areas of the body (Figure 1E). To more precisely identify the source of these signals, a second tightly focused high-intensity UV laser was used to mark the site of maximum expression by inducing visible carbonization (Supplemental experimental procedures). This experiment shows that the tip of the fly penis is one major site of expression for these two molecules (Figure 1G, H). Structural identification of CH503 and evidence for its role as a courtship-mediating pheromone is described further below.
The CH profiles of other regions of male flies are largely homogenous (Figure S3). We measured CH profiles primarily from the legs since this region is representative of other body parts and shows high intensity signals with low chemical background. Previous GC/MS studies had concluded that male cuticles contain linear and branched alkanes and alkenes with 20 – 33 carbon atoms [19, 20]. In particular, shorter length CHs such as 7-tricosene (C23H46), n-tricosane (C23H48), and 7-pentacosene (C25H48) were identified as the most abundant species. Using UV-LDI MS, we detected numerous novel oxygen-modified compounds in addition to most of the previously described alkenes (Figure 1E, Table 1). The most intense signals found in the legs correspond to oxygenated tricosene (C23H46O) and oxygenated tricosadiene (C23H44O), compounds with 23 carbon atoms and 1 – 3 oxygen atoms. Fully saturated aliphatic CHs such as tricosane (C23H48) and pentacosane (C25H52) are not detected using UV-LDI MS due to the limits of the method. A more detailed comparison of CH species detected from the cuticle of the male fly using several different MS methods including GC/MS is provided in Table S2.
Cuticular hydrocarbon profile of adult female flies
The profiles of adult virgin females show little spatial variation when comparing the legs, anogenital region, wing, and head (Figure 1D, F, Table 1, and Figures S1 and S4 for profiles of different body regions). The aliphatic dienes heptacosadiene (C27H52) and nonacosadiene (C29H56) are the most prominent components found in female profiles. Previous GC/MS studies also identified these compounds as being the most abundant in females[19, 21]. Heptacosene (C27H54) and nonacosene (C29H58), two other well-characterized female CHs, could not be unambiguously identified due to overlapping signals from other CHs. As with the male profiles, oxygen-containing CHs are detected that exhibit the same numbers of carbon atoms as these monoenes (Figure 1D, F, Table 1).
Sex-specific differences in cuticular hydrocarbon profile of adult flies
Previous reports using GC/MS have shown that D. melanogaster female cuticles are characterized by the presence of long chain CHs containing up to 2 double bonds whereas males express shorter chain CHs with primarily single double bonds [9, 19, 20]. In contrast to these studies, analysis by UV-LDI MS shows mainly quantitative differences between the sexes. In particular, signals corresponding to all of the characteristic female CHs, including pentacosadiene (C25H48), heptacosadiene (C27H52), and nonacosadiene (C29H56; see inset to Figure 3A, B), are detected in low abundance on male cuticles. Other studies have also reported that cVA is expressed exclusively by males [8, 9]. To our surprise, analysis by LDI-MS revealed the presence of a signal corresponding to cVA in trace quantities in 10 out of 15 socially naïve virgin females (Figure 1D, F, inset). Clear sexual dimorphism in CH expression is evident when comparing the intensities of oxygen-containing forms of the C23-hydrocarbons. Specifically, C23H46O and C23H44O2 are major components of male leg profiles but only minor components of female profiles. The long chain acetate CH503 was not detected on any of the virgin females examined. These results demonstrate that male and female fruit flies are capable of producing a wide range of similar hydrocarbons. However, differences in relative quantities of certain of these compounds and chemical modification with the addition of oxygen-containing functional groups generate sexually dimorphic profiles. UV-LDI MS cannot differentiate between isobaric species so it is unclear how many isoforms of each CH species are expressed and whether these could be different between the sexes.
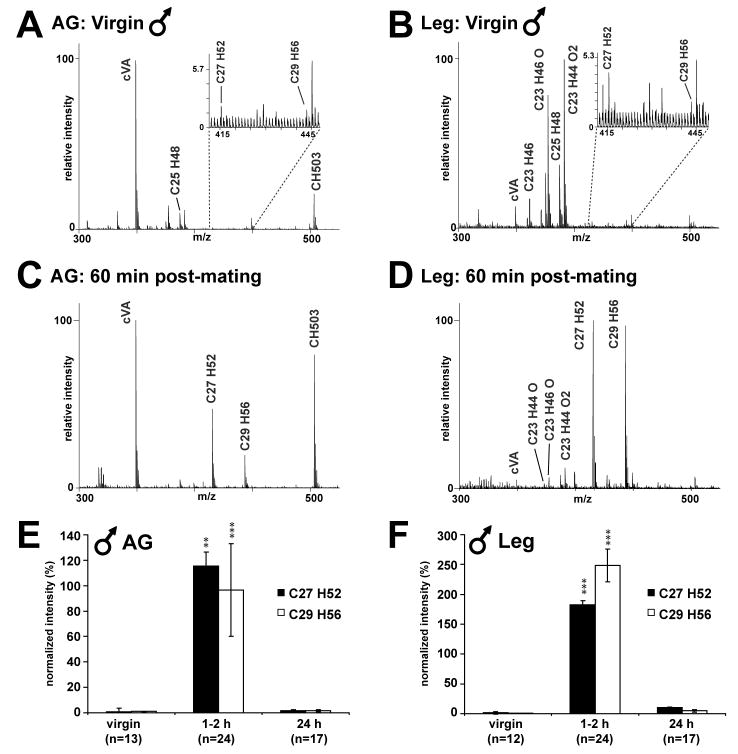
Figure 3. Male Drosophila exhibit time-dependent changes in the CH profile for up to 24 h after successful copulation. All assigned peaks correspond to potassiated molecules [M+K]+.
(A, B) Profiles of the anogenital region (AG) and legs of virgin males show low levels of the characteristic female hydrocarbons heptacosadiene (C27H52) and nonacosadiene (C29H56) (insets).
(C, D) 60 min after mating, the major female CHs heptacosadiene (C27H52) and nonacosadiene (C29H56) are detected above normal intensity levels in both the AG and legs of males.
(E, F) Intensity values for heptacosadiene (C27H52) and nonacosadiene (C29H56) in the AG and legs before and after mating are shown normalized to the sum of the values for the major male hydrocarbons (see Experimental Procedures; see Supplemental table 5 for values). Increases in the abundance of these compounds appear greater in the legs than in the AG. In both regions, the highest intensities for these compounds are found 1–2 h after mating and drop precipitously by 24 h. Levels of the major female CHs are no longer significant at 24 h post-mating in the AG and legs. **p < 0.01; ***p < 0.001 compared to virgin levels (Dunn’s multiple comparison test).
Detection of male-specific hydrocarbons on female cuticles after successful mating
Based on the observations that CH503 is found on male but not on virgin female flies, and that CH503 is co-expressed with cVA in males, we hypothesized that this molecule might function as a communication cue involved in courtship behavior. To investigate this possibility, mated females were examined by UV-LDI MS at several time points from 15 min up to 15 d following successful copulation (Figure 2, Table S4). Increased levels of cVA, hydroxylated tricosene (C23H46O) and tricosadiene (C23H44O), and CH503 are found on female cuticles after courtship, each displaying a different time course of expression. The pheromone cVA is detected with the highest intensity between 15 min – 3 d after mating. By 6 d post-mating, cVA was only observed at elevated levels in 3 out of 10 females. In contrast, significant increases in hydroxylated tricosene (C23H46O) and hydroxylated tricosadiene (C23H44O) take place between 24 h – 2 d post-mating. Lastly, CH503 expression is detected in all mated females for at least 10 d, with the highest abundances found between 15 min – 6 d post-mating. By 15 d, the signal could still be observed in 5 out of 12 females. CH503, therefore, was the only detectable change on the cuticular surface of mated females at this late time point.
Figure 2. Female Drosophila exhibit changes in CH profile for at least 10 d after successful copulation.
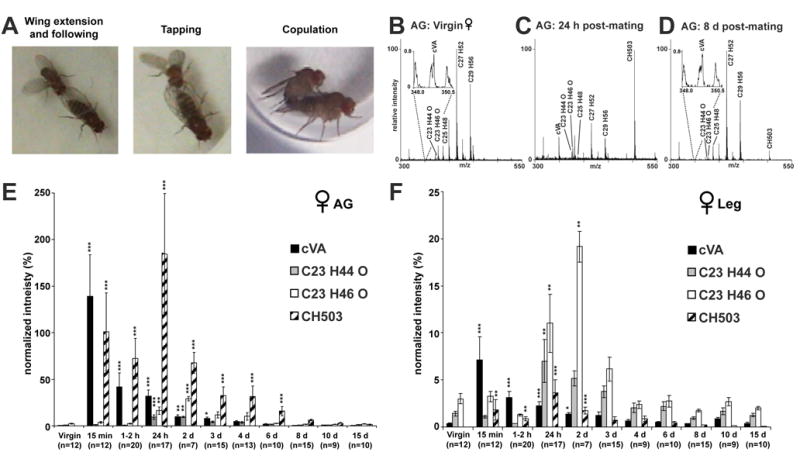
(A) Male flies exhibit several stereotyped behaviors during courtship such as wing extension (indicative of singing), following the female, tapping the female with the forelegs, and copulation.
(B–D) Representative UV-LDI mass spectra from the anogenital region (AG) of females before and after mating. 24 h after mating, levels of cVA and CH503 are significantly higher. Increases in hydroxylated tricosene (C23H46O) and hydroxylated tricosadiene (C23H44O) abundance also are observed. By 8 d, cVA on the cuticle is found near baseline quantities while the signal for CH503 is still evident. All assigned peaks correspond to potassiated molecules [M+K]+.
(E, F) Intensity values for cVA, hydroxylated tricosene and tricosadiene, and CH503 in the AG and legs before and after mating are shown normalized to the sum of the values for the major female hydrocarbons (see Experimental Procedures; see Table S4 for values). Changes observed in the AG are greater in both magnitude and duration than those found in the legs. Some variation can be attributed to the method (which samples over a small surface) and pair-to-pair differences in copulation time and intensity. In the AG, cVA exhibits a monotonic decrease in abundance over time. Hydroxylated tricosene (C23H46O) and tricosadiene (C23H44O) increase in relative intensity between 24 h – 2 d. CH503 expression persists for at least 10 d. In the legs, the most striking increases are seen in the levels of hydroxylated tricosene and tricosadiene between 24 h – 2 d after mating. *p < 0.05; **p < 0.01; ***p < 0.001 compared to virgin levels (Dunn’s multiple comparison test). Relative intensity values are not representative of absolute abundances since ionization efficiency can depend partly on chemical structure.
A different dynamic of mating-related chemical changes is observed in the legs of mated females. Increases in cVA and CH503 expression in the legs are substantially smaller in magnitude than in the anogenital region (Figure 2F, Table S4). Significant changes are detected only up to 2 d post-mating for both compounds. Increases in the signals for hydroxylated tricosene (C23H46O) and tricosadiene (C23H44O) are found between 24 – 48 h with a magnitude of change similar to that observed in the anogenital region.
Detection of female-specific hydrocarbons on male cuticles after successful mating
Male flies exhibit striking changes in their CH profiles after mating. At 2 h after mating, major male hydrocarbons (e.g., C23H46O and C23H44O2) comprise a relatively small proportion of the total profile in the legs compared to the characteristic female compounds. The primary female hydrocarbons, heptacosadiene (C27H52) and nonacosadiene (C29H56), appear to be transferred to the anogenital region and to a greater extent, the legs of males during mating. In contrast to mated females, these changes in CH are not significant beyond 2 h post-mating (Figure 3, Table S5).
Purification and structural characterization of CH503
Two observations about the spatial expression of CH503 before and after mating suggest a putative role as a sex pheromone: (i) CH503 co-localizes with cVA in the ejaculatory bulb of male flies and (ii) CH503 appears to be transferred with cVA to female flies during copulation. In order to determine the chemical structure of CH503 and to directly test its function in courtship behavior, we purified the compound by preparative high-performance thin-layer chromatography (HPTLC; Figure 4A). Analysis by electrospray ionization (ESI) mass spectrometry confirmed that CH503 is the major component within the HPTLC-isolated fraction (Figure 4B).
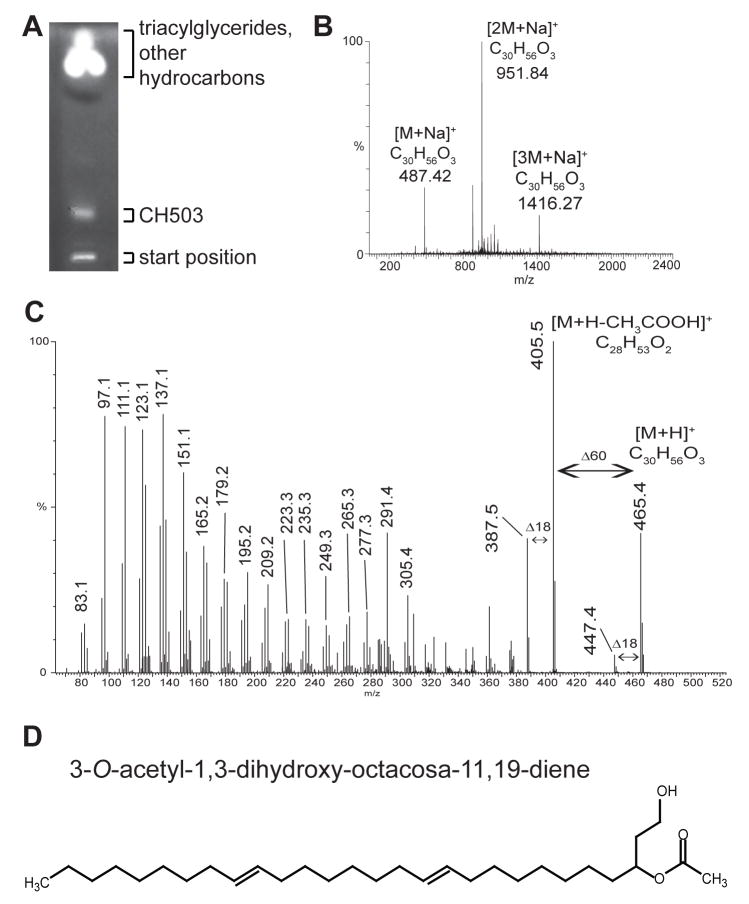
Figure 4. Structural characterization of CH503.
(A) HPTLC of crude cuticular extract from 10 g of flies separated CH503 from triacylglycerides, phospholipids, and other hydrocarbons. GC/MS analysis did not detect any other hydrocarbons in this fraction (data not shown).
(B) NanoESI-QTOF mass analysis of extracts from the HPTLC band containing CH503 shows that CH503 is the major component in the fraction. Due to the high concentration of the compound in the solution, CH503 is detected primarily as a mixture of sodiated monomers and oligomers. Other unassigned signals correspond to the protonated and potassiated forms of CH503 and residual amounts of triacylglycerides.
(C) The protonated molecule [M+H]+ of CH503 at m/z 465.5 was analyzed from crude cuticular extract using nanoESI-QTOF MS and low-energy CID tandem mass spectrometry. The product mass spectrum reveals the presence of: (i) an acetate group, indicated by the loss of acetic acid (CH3COOH, MW 60.0 Da); (ii) a hydroxyl group, indicated by the loss of a water molecule (MW 18.0 Da); and (iii) a linear hydrocarbon chain indicated by a series of fragment ions separated by mass increments of 14.0 Da.
(D) The proposed chemical structure of CH503 as 3-O-acetyl-1,3-dihydroxy-octacosa-11,19-diene and is shown in a (Z, Z) 11,19 configuration. The actual (Z/E) configuration of the double bonds remains to be determined.
Tandem MS analysis of CH503 by ESI mass spectrometry revealed the presence of an acetate and an additional hydroxyl group in the molecule (Figure 4C). Analysis by nuclear magnetic resonance spectroscopy of the HPTLC-purified fraction confirmed the presence of the functional groups and indicated that the molecule does not contain a ring structure or conjugated double bonds (Supplemental experimental procedures). In order to more fully elucidate the chemical structure of CH503, we performed a series of chemical derivatization experiments using the HPTLC-purified fraction and, as a reference, a mixture of synthetic acetate esters. These studies provided (i) the position of the hydroxyl and the acetate group; and (ii) the positions of the two carbon-carbon double bonds (Supplemental experimental procedures; Figure S5 – S7). Based on these results, the proposed structure for CH503 is 3-O-acetyl-1,3-dihydroxy-octacosa-11,19-diene (Figure 4D).
CH503 inhibits male courtship behavior
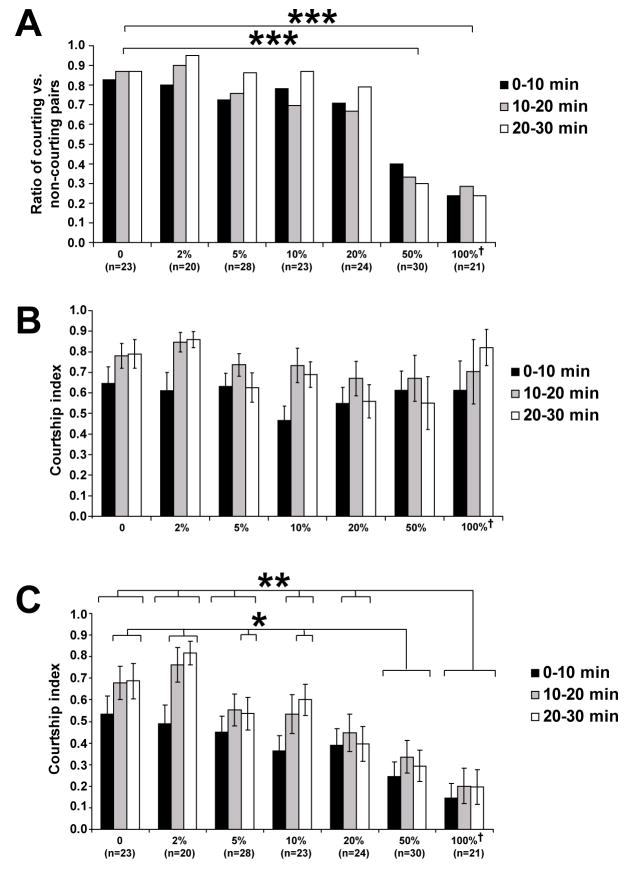
To test the function of CH503 in courtship behavior, we measured the courtship activity of CantonS male flies towards immobilized CantonS virgin female targets perfumed with CH503. When virgin female targets are perfumed with CH503, the likelihood of male flies to initiate courtship is reduced in a dose-dependent manner (Figure 5A). When using a target female perfumed with a dose approximately equal to the amount found on 7 males, only 36% of the males display any kind of courtship behavior by the end of the 30 min trial. In contrast, 87–95% of males placed with a non-perfumed or low-dose target exhibit courtship activity at the end of 30 min. Of the trials in which courtship did occur, the amount of time the males spend courting females is not significantly different across all concentrations (Figure 5B). Overall, the courtship performance of males is reduced in a dose-dependent manner and the anti-aphrodisiac effect persisted for at least 30 min (Figure 5C). UV-LDI MS analysis of female targets perfumed at maximum dosage showed that the amounts of CH503 deposited was comparable to the levels normally detected on mated female cuticles (Figure S8). Female targets perfumed with extracts of HPTLC plates containing no cuticular extract do not inhibit male courtship behavior (n=6, data not shown). These results indicate that within a certain concentration range of the compound, courtship initiation is suppressed while the drive of male flies to court and to continue to court after initiation remains unaffected. Other possible functional roles of the compound, e.g., as a female aphrodisiac or as an aggregation pheromone, were not examined.
Figure 5. Male courtship behavior is inhibited in the presence of CH503.
(A) Courtship initiation is suppressed in a dose-dependent manner with increasing concentration of CH503. The number of trials for each condition is indicated in parentheses; †equivalent to the amount extracted from 7 male flies; ***: p<0.001 (Chi-Squared test).
(B) The courtship index is not significantly different over time and across dosage concentrations in the trials during which courtship was seen (p>0.05, ANOVA); †equivalent to the amount extracted from 7 male flies.
(C) The male courtship index decreases in a dose-dependent manner with increasing concentration of CH503. Male courtship indices (CI) were calculated in 10 min time bins for each 30 min trial. CI is defined as the amount of time the male spends following, extending and vibrating one wing, tapping, licking, and copulating divided by the total length of the trial. The number of trials for each condition is indicated in parentheses; †equivalent to the amount extracted from 7 male flies; *: p<0.01 (Tukey-Kramer post-hoc analysis); **: p<0.001 (Tukey-Kramer post-hoc analysis).
Discussion
A novel class of D. melanogaster cuticular compounds: oxygen-containing hydrocarbons
Prior to this study, cVA and its hydrolyzed product, cis-vaccenol, have been the only oxygen-containing cuticular hydrocarbons identified in D. melanogaster [13, 22]. By using direct UV-LDI MS analysis, we detected 11 additional oxygenated hydrocarbon species on the cuticle. In addition, we also show that cVA is occasionally found on the cuticles of virgin female flies and that the female-expressed aphrodisiacs heptacosadiene (C27H52) and nonacosadiene (C29H56) [7] are observed on the cuticles of virgin males. Although these characteristic male and female substances are detected on the cuticles of the opposite sex in very low abundances, these results clearly indicate that both sexes can synthesize a wider variety of CHs than previously believed. Rather than qualitative “all-or-none” differences, it appears that large disparities in the quantities of certain CH species serve to distinguish the CH profiles between males and females.
The presence of oxygen-containing hydrocarbons might be explained in several different ways. First, the laser used to desorb and ionize the compounds might have sampled molecules from beneath the cuticular surface. We believe this possibility to be unlikely for three reasons: (i) there is no visible sign of disruption of the cuticle even after exposure to several thousand laser shots (data not shown); (ii) continuous irradiation of the same spot for 10 min with the flat-top laser beam profile (corresponding to approximately 18,000 laser shots) did not produce additional ion signals in the spectrum (data not shown); and (iii) after washing fly cuticles with a single drop of hexane, most signals corresponding to hydrocarbons, including those of oxygen-containing substances, were no longer detected (data not shown). These observations confirm that only the surface layer is sampled by the UV laser used and that the likely source of the hydrocarbon signals is the outermost layer of the cuticle. A second possible explanation for the detection of oxygen-containing hydrocarbons could be that these compounds are LDI-induced artifacts. When UV-LDI MS was used to analyze synthetic alkene reference compounds, however, laser-induced fragmentation of ions or oxidation of hydrocarbon ions was not observed (data not shown). In addition, alternative desorption/ionization methods (ESI-MS and Direct Analysis in Real Time (DART) -MS) employed for the analysis of CHs from cuticular extracts and intact animals confirmed the presence of all oxygenated hydrocarbons detected by UV-LDI MS (Table S2).
Taken together, these lines of evidence indicate that oxygen-containing hydrocarbons are naturally occurring molecular entities. In addition to D. melanogaster, oxygen-containing hydrocarbons are common in other insects. For example, in the Dipteran [23], Leptidopteran [24] and Coleopteran [25] orders, ketone-modified hydrocarbons and blends of straight chain alkenes and corresponding epoxides are used as sex-pheromones. Notably, in the housefly, (Z)-9-tricosene is converted to the corresponding C23 epoxide and ketone on the surface of the cuticle[23]. Quantitative and qualitative differences in pheromone profiles have been implicated as one genetic mechanism driving sexual isolation and speciation of insects (reviewed in [26]). In Drosophila, CHs that are excitatory courtship cues for conspecifics can serve as inhibitory cues for sibling species and thus serve to reinforce reproductive isolation [27]. It is intriguing to consider whether the pheromone function of CH503 or cVA are evolutionarily conserved and can influence the courtship of sibling species such as D. simulans and D. maurutiana, both of which are monomorphic in their CH profile. Furthermore, it will be important to determine whether any of the oxygenated compounds could mediate intra- and inter-species courtship, particularly in combination with the corresponding non-oxygenated alkene. The disparity in the abundance of oxygenated C23-compounds between the sexes hints at the possibility that these molecules, either acting singly or in various chemical cocktails, may play a role in the chemical communication of Drosophila. Future work using UV-LDI MS to profile related sibling species will allow us to ask (i) whether oxygen-modification of hydrocarbons is widely used among drosopholids and (ii) if species in which males and females have identical CH profiles (as assessed by GC/MS) exhibit quantitative or qualitative differences in the expression of other species of CHs. Because UV-LDI MS has a lower sensitivity for monoenes and alkanes, we cannot at this point compare absolute quantities of many of the previously characterized CHs to the oxygenated CHs. Determining the ionization efficiency of these different classes of CHs with synthetic standards will be necessary in order to make a quantitarive assessment.
Why have CH503 and other oxygen-containing hydrocarbons not been reported before in Drosophila? Interestingly, the existence of polar lipid compounds on Drosophila cuticles was mentioned by Butterworth in the original publication describing cVA [12]. However, the identities of the unknown compounds were not elucidated in that work. We speculate that due to the relatively high molecular weight of CH503 and the comparatively polar nature of the oxygen-containing compounds, these molecules could have been missed using standard GC/MS conditions that are optimized for the detection of lower molecular weight alkanes and alkenes [1]. Further structural elucidation will be needed to determine the E and Z isomer geometries of these compounds and whether additional isomers exist. Ultimately, it will be necessary to perform GC/MS analysis in parallel with other desorption/ionization methods like LDI-MS, ESI-MS, and DART-MS in order to more fully characterize the chemical composition of fly cuticles.
The contribution of CH503 to the current model of Drosophila courtship
The suppression of female attractiveness to other males following successful mating is well-documented in many species of insects [28]. This phenomenon is thought to have a reproductive benefit: the sperm-investment of the male is protected from other males and females are allowed more energy and time to devote to laying eggs and finding advantageous oviposition sites [29–31]. In Drosophila, a number of signaling molecules that facilitate this effect are transferred from males to females during copulation. These compounds include peptides [32, 33] and enzymes [22, 34] found in the seminal fluid [30], and cVA and other hydrocarbons found on the cuticular surface [2, 8, 13].
Despite some discrepancies in earlier studies [13, 35, 36], recent work strongly implicates cVA as a short-term mediator of courtship suppression since the amount of cVA found on females at 24 h post-mating can significantly suppress courtship behavior [15]. Studies investigating other CHs such as 7-tricosene (C23H46) or 7,11-heptacosadiene (C27H52) have not agreed with each other or have failed to find significant changes in the levels of these compounds 24 h after mating [2, 15, 30, 35, 36]. It could be that spatial-specific changes occur but are obviated when analyzing extracts from whole cuticles.
Here we show that another transferred CH also can inhibit male courtship. At 10 d post-mating, CH503 is the only detectable change on female cuticular surfaces (although the possibility that some cVA is held in the vaginal tract cannot be excluded). Taken together with the finding that CH503 inhibits courtship initiation, these results strongly implicate CH503 as a long-lived mediator of male-courtship suppression. Both the oxygen modification and long chain length of CH503 may reduce its volatility suggesting that it may be active only at short range. It may also be possible that CH503 and cVA interact synergistically via cVA-related olfactory receptors [10, 15, 37] or via gustatory receptors. More refined behavioral analysis will be necessary to determine whether individual features of the courtship ritual (e.g. singing, tapping) are affected by CH503. It should be noted that in contrast to the localized distribution of CH503 observed in mated females, the targets used here for courtship assays have been perfumed with CH503 over the entire body. Such spatial differences might further suppress male courtship by masking endogenous female cues from other body regions. Future experiments using spatially-resolved methods of perfuming should help to elucidate the biological efficacy of CH503 and the concentration range over which it can suppress courtship.
We also found that levels of hydroxylated tricosene (C23H46O) and tricosadiene (C23H44O) are more abundant between 24 – 48 h post-mating. Unlike the patterns observed for cVA and CH503, this increase was detected in similar magnitude across the anogenital region and legs, consistent with the explanation that females systemically upregulate expression of this compound. Scott observed a similar increase in tricosene at 24 h post-mating in mated females [2] (though other studies have not found the same effect [15]). While the behavioral relevance of the hydroxylated compounds remains to be determined, it is intriguing to consider the possibility that females actively upregulate CHs or oxygen modification of tricosene as part of a post-mating mechanism to discourage male courtship.
Consistent with previous observations, a bi-directional transfer of CHs was observed during copulation [2, 31, 36]. One effect of the “cross-perfuming” between males and females during copulation is that the hydrocarbon profiles of males and females no longer appear to be chemically distinct for at least 60 min following copulation. It may simply be the case that CHs are sticky and easily transferred between animals during bodily contact. Determining whether mating-induced cross-perfuming has a behavioral role in both dimorphic and monomorphic Drosophila species could provide some insight into a possible function for this phenomenon.
Conclusion
Using direct UV-LDI-o-TOF mass spectrometry, we characterized the spatial expression of cuticular hydrocarbons from the surface of intact male and female fruit flies and found major quantitative differences in CH expression. These studies resulted in the discovery of a new major class of oxygen-containing hydrocarbons. Structural and behavioral characterization of one of these compounds led to the identification of a new sex pheromone, CH503. CH503 adds to the roster of identified pheromones involved in fly courtship behavior and may help to explain some of the long-term behavioral changes observed after mating. Elucidating the full compliment of CHs, identifying their cognate receptors and relevance to social behavior, and characterizing their biochemical regulation are important next steps towards a more complete understanding of chemical communication in Drosophila and the contribution of pheromone-mediated behavior to speciation and evolution.
Experimental Procedures
Drosophila stocks and husbandry
CantonS flies were raised on autoclaved cornmeal-yeast-sucrose-agar food at 25°C. To prevent exposure to adult flies of the opposite sex, flies were isolated at the pupal stage and placed individually or with 3 – 4 other pupae of the same sex in glass culture tubes (16 × 100 mm; VWR Scientific) filled with 2 ml of standard fly food.
Preparation of flies for UV-LDI mass spectrometry analysis
Individual flies were anesthetized and mounted with fine forceps onto adhesive tape (G304, Plano, Wetzlar, Germany; Figure 1) attached to a glass coverslip. The coverslip was attached to a milled-out custom-built sample plate with adhesive tabs. To prevent potential cross-contamination, separate forceps were used for male and for female flies. Up to 18 flies were typically placed on the sample plate at once (Figure 1A). The flies remain intact during analysis in the mass spectrometer.
Laser desorption/ionization orthogonal time-of-flight mass spectrometry
The mass spectrometer has been described in detail recently [38]. This instrument is equipped with an 200 μm-diameter N2 laser emitting 3 ns long pulses at a wavelength of 337 nm and a repetition rate of 30 Hz. Ions are generated in a buffer gas environment using 2 – 4 mbar of Argon gas. The elevated pressure was found to enhance the detection of hydrocarbons. No chemical matrix is applied to the flies. For acquisition of mass spectra, either 900 or 1800 laser pulses were applied over 30 or 60 sec, respectively. Laser fluence (light energy per pulse and area) was adjusted to values moderately above the ion detection threshold, corresponding to values between 100 – 200 J/m2. At present, the mechanisms underlying laser energy deposition, desorption, and ion generation are not clear [39]. The position of the sample plate was adjusted by 10 μm steps during measurements in order to optimize signal intensity. Overall signal intensity can vary from sample to sample due to individual biological variation as well as the position of the fly on the sample plate. Mass resolution (full width at half maximum) was about 10,000, sufficient to distinguish between two neighboring hydrocarbon species differing in mass by about 50 mDa. Mass accuracy was about 10 – 20 ppm through all measurements. All LDI MS data were acquired in positive ion mode.
Mass spectra were processed using the MoverZ software (v. 2001.02.13, Genomic Solutions, Ann Arbor, MI). Potassiated molecules formed the dominant peaks for signals corresponding to hydrocarbons in all recorded LDI mass spectra.
Quantification of MS measurements
The signal intensity for each CH is defined as the area under the monoisotopic peak of the potassiated ion species, as calculated by MoverZ software. For spectra of virgin males and females, the normalized intensity of each CH is calculated by dividing the signal by the sum of all detected CH signals in the same experiment. 12 – 13 individuals were analyzed for each sex. For spectra of mated males, the signals for C25H48, C27H52, or C29H56 are normalized to the sum of signals for the major male CH species found in the anogenital region (cVA and CH503) or in the legs (C23H44O, C23H46O, C25H44O2). For spectra of mated females, the signals for cVA, C23H44O, C23H46O, and CH503 are normalized to the sum of signals for the major female CH species C25H48, C27H52, and C29H56.
Elemental composition assignment
Elemental composition assignments are based on the assumption that the observed and theoretical mass values agree within +/− 0.02 Da and that the neutral CH molecules contain only C, H, and O atoms (thus neglecting the unlikely occurrence of N and S). Exact mass values were corroborated by ESI MS measurements of CH extract using a micrOTOF instrument (Bruker Daltonik, Bremen, Germany) that provided a mass accuracy within 2 ppm.
Electrospray ionization (ESI) mass spectrometry
Cuticular extracts were prepared by placing 30–40 flies in hexane for 1 h at room temperature. Prior to analysis, the extract was dissolved in a solution of 49.5% methanol, 49.5% acetonitrile, 0.1% trifluoroacetic acid, and 10 mM aqueous ammonium acetate. Three different ESI MS instruments were used to record CH profiles of extracts and to apply collision-induced dissociation (CID) for partial structural characterization of selected ions: 1) a micrOTOF I mass spectrometer (Bruker Daltonik, Bremen, Germany), 2) a quadrupole time-of-flight (QTOF) mass spectrometer (Waters/Micromass, Manchester, UK), and 3) a triple-quadrupole mass spectrometer (QuattroLC; Waters/Micromass, Manchester, UK). All ESI data were acquired in positive ion mode. Specific details about measurement parameters and CID conditions can be found in Supplemental experimental procedures.
Chemical isolation of CH503 using high-performance thin-layer chromatography (HPTLC)
The cuticular lipids of 10 g of male and female flies of mixed Drosophila melanogaster genotype were extracted with 150 ml of chloroform/methanol (2:1, v/v) overnight at room temperature. The lipids were re-extracted with hexane in order to remove co-extracted triacylglycerides and saccharides. HPTLC separation was performed on glass-backed silica gel plates (10 × 10 cm, coated with 0.2 mm of silica gel 60; Merck, Darmstadt, Germany, catalog no. 1.05633.0001) using a running solvent consisting of hexane/diethyl ether/methanol (85:5:10, each by volume) and visualized under UV illumination after spraying with a primulin solution (0.001% in 20% acetone). The CH503-containing band was located with direct infrared (IR)-LDI-o-TOF MS analysis employing an Er:YAG laser (previously described in [38]). The silica gel of corresponding positions on unstained parallel bands was scraped and the compounds extracted from the gel with chloroform/methanol (2:1, v/v). Analysis by ESI-QTOF MS confirmed that CH503 is the major component. Negative control fractions for behavioral experiments were prepared from HPTLC plates that contained no extract and had been subjected to the same solvent conditions and purification procedure.
Structural analysis of CH503
Chemical derivatization procedures (silylation and ozonolysis) and nuclear magnetic resonance spectroscopy are described in Supplemental experimental procedures.
Courtship behavior assays of flies used for UV-LDI MS analysis
3 – 5 d old CantonS flies were used for all courtship experiments. Single virgin males were aspirated into a glass isolation vial containing a live virgin female and were visually inspected.
Following successful copulation, females were separated from males and analyzed with UV-LDI MS at the following time intervals: 15 min, 60 min, 24 h, 2 d, 3 d, 4 d, 6 d, 8 d, 10 d and 15 d.
Courtship behavior assays with perfumed targets
Perfuming of courtship targets was performed using a method described by Kent et al [21]. Briefly, 6 – 8 live female flies were placed in a vial coated with lyophilized HPTLC-extracted CH503 or blank fractions (see above) and gently vortexed for 3 × 20 sec with a 20 sec pause between each bout. Details regarding the dosage preparation and estimation of the concentration are provided in Supplemental experimental procedures.
Courtship assays were performed in chambers of 10 mm diameter and 3 mm depth. The heads of perfumed flies were crushed immediately prior to behavioral testing. The perfumed target and a 3 – 5 d old socially naïve male were placed in each chamber and videotaped for 30 min. Behavioral scoring began 2 min after the males were loaded into the chambers. Courtship indices for the males were calculated as the amount of time the males spent licking, tapping, extending and vibrating a wing, and/or attempting to copulate with the females during each of three 10 min intervals [5].
Statistical analysis
Significant differences in CH levels were performed with a Kruskall-Wallis test followed by a Dunn’s multiple comparison test. Bars in the figures represent means ± standard error of the mean (S.E.M.). For mating behavior, regression analysis was performed using a generalized least squares model with time and dose concentration as the main effect (JMP Software, v. 7.0.2; SAS Institute, Cary, NC). The distribution of courted versus non-courted pairs was analyzed with a chi-square test (http://faculty.vassar.edu/lowry/VassarStats.html). Courtship indices were assessed by ANOVA followed by posthoc analysis with a Tukey-Kramer HSD test.
Supplementary Material
Acknowledgments
JYY and KD designed and carried out the LDI MS experiments. JYY, HL, JM, GP, and KD designed and performed the biochemical purification, chemical analysis, and other MS analyses. JYY carried out the behavioral analysis. JYY, KD, and EAK wrote the manuscript. We wish to thank members of the Kravitz laboratory (Drs. Olga Alekseyenko, Sarah Certel, Yick-Bun Chan, Maria de la Paz Fernandez, Adelaine Leung, Jill Penn) and members of the Biomedical Analysis and Laser Mass Spectrometry groups at the University of Münster (Ute Distler, Marcel Hülsewig, Ewald Kalthoff, Stephan Kirsch, Alexander Pirkl, Jens Soltwisch, Jamal Souady, and Mostafa Zarei) for helpful discussions and excellent technical support. We are grateful to Dr. Stefan Berkenkamp and Sequenom GmbH for use of the UV-LDI-o-TOF instrument. We also thank Prof. Jasna Peter-Katalini, Prof. Christian Klämbt, and Dr. Robert Cody for graciously allowing use of laboratory facilities. We would like to acknowledge Dr. Klaus Bergander for generously providing NMR measurements and help in data interpretation, Dr. Michael Mormann and Dr. Simone König for additional MS measurements, and Dr. Alo Basu for help with statistical analysis. Finally, we thank Dr. Aki Ejima, Prof. Stephen Goodwin, Prof. Franz Hillenkamp, Prof. Joel Levine, Dr. Dennis Mathew, Prof. Peter O’Connor, and Prof. Kathleen Siwicki for stimulating discussions and helpful insights on various aspects of this manuscript.
This work was supported by a Human Frontier Science Program short-term fellowship (JYY), a National Institute of Mental Health National Research Service Award (JYY) and research grants from the National Institute of General Medical Sciences (GM074675 and GM067645 to EAK), the National Science Foundation (IDS-075165 to EAK), and the Deutsche Forschungsgemeinschaft (DR416/5-1 to KD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howard RW, Blomquist GJ. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. [DOI] [PubMed] [Google Scholar]
- 2.Scott D. Sexual mimicry regulates the attractiveness of mated Drosophila melanogaster females. Proc Natl Acad Sci USA. 1986;83:8429–8433. doi: 10.1073/pnas.83.21.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 4.Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu Rev Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 5.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 6.Ferveur JF, Sureau G. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc Biol Sci. 1996;263:967–973. doi: 10.1098/rspb.1996.0143. [DOI] [PubMed] [Google Scholar]
- 7.Antony C, Davis TL, Carlson DA, Pechine JM, Jallon JM. Compared behavioral responses of male Drosophila melanogaster (Canton-S) to natural and synthetic aphrodisiacs. J Chem Ecol. 1985;11:1617–1629. doi: 10.1007/BF01012116. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth FM. Lipids of Drosophila: a newly detected lipid in the male. Science. 1969;163:1356–1357. doi: 10.1126/science.163.3873.1356. [DOI] [PubMed] [Google Scholar]
- 9.Ferveur JF, Savarit F, O’Kane CJ, Sureau G, Greenspan RJ, Jallon JM. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997;276:1555–1558. doi: 10.1126/science.276.5318.1555. [DOI] [PubMed] [Google Scholar]
- 10.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 11.Bartelt RJ, Schaner AM, Jackson LL. cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- 12.Jallon JM, Antony C, Benamar O. Un antiaphrodisiac que produit par les males de Drosophila melanogaster et transfere aux femelles lors de la copulation. Cr Acad Sci Paris. 1981;292:1147–1149. [Google Scholar]
- 13.Zawistowski S, Richmond RC. Inhibition of courtship and mating of Drosophila melanogaster by the male-produced lipid, cis-vaccenyl acetate. J Insect Physiol. 1986;32:189–192. [Google Scholar]
- 14.Mane SD, Tepper CS, Richmond RC. Studies of esterase 6 in Drosophila melanogaster. XIII Purification and characterization of the two major isozymes. Biochem Genet. 1983;21:1019–1040. doi: 10.1007/BF00483957. [DOI] [PubMed] [Google Scholar]
- 15.Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amrein H. Pheromone perception and behavior in Drosophila. Curr Opin Neurobiol. 2004;14:435–442. doi: 10.1016/j.conb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Tompkins L, Hall JC. The different effects on courtship of volatile compounds from mated and virgin Drosophila females. J Insect Physiol. 1981;27:17–21. [Google Scholar]
- 18.McRobert SP, Adams CR, Wuttke M, Frank J, Jackson LL. A comparison of female postcopulatory behavior in Drosophila melanogaster and Drosophila biarmipes. J Insect Behav. 1997;10:761–770. [Google Scholar]
- 19.Antony C, Jallon J. The chemical basis for sex recognition in Drosophila melanogaster. J Insect Physiol. 1982;28:873–880. [Google Scholar]
- 20.Foley B, Chenoweth SF, Nuzhdin SV, Blows MW. Natural genetic variation in cuticular hydrocarbon expression in male and female Drosophila melanogaster. Genetics. 2007;175:1465–1477. doi: 10.1534/genetics.106.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent C, Azanchi R, Smith B, Chu A, Levine J. A model-based analysis of chemical and temporal patterns of cuticular hydrocarbons in male Drosophila melanogaster. PLoS ONE. 2007;2:e962. doi: 10.1371/journal.pone.0000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mane SD, Tompkins L, Richmond RC. Male esterase 6 catalyzes the synthesis of a sex pheromone in Drosophila melanogaster females. Science. 1983;222:419–421. doi: 10.1126/science.222.4622.419. [DOI] [PubMed] [Google Scholar]
- 23.Blomquist GJ, Dillwith JW, George Pomonis J. Sex pheromone of the housefly: Metabolism of (Z)-9-tricosene to (Z)-9,10-epxytricosane and (Z)-14-tricosen-10-one. Insect Biochem. 1984;14:279–284. [Google Scholar]
- 24.Millar JG. Polyene hydrocarbons and epoxides: a second major class of Lepidopteran sex attractant pheromones. Annu Rev Entomol. 2000;45:575–604. doi: 10.1146/annurev.ento.45.1.575. [DOI] [PubMed] [Google Scholar]
- 25.Allison JD, Borden JH, Seybold SJ. A review of the chemical ecology of the Cerambycidae (Coleoptera) Chemoecology. 2004;14:123–150. [Google Scholar]
- 26.Smadja C, Butlin RK. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity. 2009;102:77–97. doi: 10.1038/hdy.2008.55. [DOI] [PubMed] [Google Scholar]
- 27.Coyne JA, Crittenden AP, Mah K. Genetics of a Pheromonal Difference Contributing to Reproductive Isolation in Drosophila. Science. 1994;265:1461–1464. doi: 10.1126/science.8073292. [DOI] [PubMed] [Google Scholar]
- 28.Ringo J. Sexual receptivity in insects. Annual Review of Entomology. 1996;41:473–494. doi: 10.1146/annurev.en.41.010196.002353. [DOI] [PubMed] [Google Scholar]
- 29.Manning A. Control of sexual receptivity in female Drosophila. Anim Behav. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- 30.Tram U, Wolfner MF. Seminal fluid regulation of female sexual attractiveness in Drosophila melanogaster. Proc Natl Acad Sci USA. 1998;95:4051–4054. doi: 10.1073/pnas.95.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott D, Richmond RC, Carlson DA. Pheromones exchanged during mating - a mechanism for mate assessment in Drosophila. Anim Behav. 1988;36:1164–1173. [Google Scholar]
- 32.Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richmond RC, Gilbert DG, Sheehan KB, Gromko MH, Butterworth FM. Esterase 6 and reproduction in Drosophila melanogaster. Science. 1980;207:1483–1485. doi: 10.1126/science.6767273. [DOI] [PubMed] [Google Scholar]
- 35.Vander Meer RK, Obin MS, Zawistowski S, Sheehan KB, Richmond RC. A reevaluation of the role of cis-vaccenyl acetate, cis-vaccenol and esterase 6 in the regulation of mated female sexual attractiveness in Drosophila melanogaster. J Insect Physiol. 1986;32:681–686. [Google Scholar]
- 36.Scott D, Richmond RC. Evidence against an antiaphrodisiac role for cis-vaccenyl acetate in Drosophila melanogaster. J Insect Physiol. 1987;33:363–369. [Google Scholar]
- 37.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dreisewerd K, Müthing J, Rohlfing A, Meisen I, Vukelic Z, Peter-Katalinic J, Hillenkamp F, Berkenkamp S. Analysis of gangliosides directly from thin-layer chromatography plates by infrared matrix-assisted laser desorption/ionization orthogonal time-of-flight mass spectrometry with a glycerol matrix. Anal Chem. 2005;77:4098–4107. doi: 10.1021/ac048373w. [DOI] [PubMed] [Google Scholar]
- 39.Dreisewerd K. The desorption process in MALDI. Chem Rev. 2003;103:395–426. doi: 10.1021/cr010375i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.