Abstract
Objective
To determine the incidence rates of hospital acquired infections (HAI) during the first 14 days after ICU discharge after treatment during ICU-stay with Selective Decontamination of the Digestive tract (SDD), Selective Oropharyngeal Decontamination (SOD) or Standard Care (SC).
Design
Prospective observational study.
Setting
ICUs in two tertiary care hospitals.
Patients
Patients discharged from the ICU to the ward.
Interventions
None.
Measurements and results
Post-ICU incidences of HAI per 1,000 days at risk were 11.2, 12.9 and 8.3 for patients that had received SDD (n = 296), SOD (n = 286) or SC (n = 289) respectively in ICU, yielding relative risks, as compared to SC, of 1.49 (CI95 0.9–2.47) for SOD and 1.44 (CI95 0.87–2.39) for SDD. Incidences of surgical site infections (per 100 surgical procedures) were 4 after SC and 11.8 and 8 after SOD and SDD (p = 0.04). Among patients that succumbed in the hospital after ICU-stay (n = 58) eight (14%) had developed HAI after ICU discharge; 3 of 21 after SDD, 3 of 15 after SOD and 2 of 22 after SC.
Conclusions
Incidences of HAI in general wards tended to be higher in patients that had received either SDD or SOD during ICU-stay, but it seems unlikely that these infections have an effect on hospital mortality rates.
Keywords: SDD, SOD, Critically ill patients, Intensive care, Hospital acquired infections
Introduction
Prophylactic antibiotic regimens, such as Selective Decontamination of the Digestive tract (SDD) and Selective Oropharyngeal Decontamination (SOD), reduce the incidence of respiratory tract infections (RTI) in Intensive Care Unit (ICU) patients and improve survival [1–6]. The concept of SDD, developed in the 1980s [7, 8] consists of prevention of secondary colonization with Gram negative bacteria, S. aureus, and yeasts through application of non-absorbable antimicrobial agents in the oropharynx and gastrointestinal tract. Further it consists of pre-emptive treatment of possible infections due to commensal respiratory tract bacteria through systemic administration of cephalosporins during the first four days in ICU and maintaining the anaerobic intestinal flora through the use of antibiotics (both topically and systemically) not active against anaerobic bacteria [8]. In meta-analyses, three single center randomized studies and a recent multi-center trial, SDD was associated with improved patient survival [1, 4, 6, 9–11]. SOD (application of topical antibiotics in the oropharynx only) might be an alternative to SDD, as they are both effective in reducing day 28 mortality in a recent multi-center study [6].
SDD (and to lesser extent SOD) aim to extensively modulate the microbial ecology of patients. It is unknown how discontinuation of these interventions at ICU discharge changes the patients’ microbial ecology and whether this influences their immediate risk of infections. The current study was motivated by the findings from de Jonge et al. [4]. In their SDD study the observed relative risk reduction (RRR) in ICU mortality of 35% reduced to 22% at hospital discharge. This reduction in survival benefit after ICU discharge might have been related to an increased incidence of hospital acquired infection (HAI) in those patients that had received SDD in ICU. Nested within a multi-center SDD–SOD trial we prospectively monitored the occurrence of HAI during the first 14 days after ICU discharge in all patients transferred to regular wards in two university hospitals.
Patients and methods
Setting
The study was conducted in two tertiary care hospitals: the University Medical Center Utrecht and the Leiden University Medical Center. Nested within a multi-center SDD–SOD trial [6], the occurrence of HAI during the first 14 days after ICU discharge was prospectively monitored in all patients transferred to regular wards.
Study design, data collection and definitions
In ICU, patients with an expected stay ≥72 h, or with an expected duration of mechanical ventilation ≥48 h, had received either SDD, SOD or standard care (SC), which rotated in 6-month periods, as described previously [6].
Data were collected from patient records for a maximum of 14 days post-ICU. The following data were recorded for each patient: gender, age, length of stay at the ICU, mechanical ventilation and APACHE II score at the ICU. At the wards, medical records were prospectively reviewed twice weekly by an Infection Control Professional for HAI according to the Centers for Disease Control and Prevention (CDC) definitions [12, 13].
In the surveillance period the following HAIs (and day of diagnosis) were registered in both hospitals: surgical site infections (SSI), bloodstream infections (BSI), and RTI. In one of the hospitals, oropharyngeal infections were also registered. Infection control policies (other than the subject of the study) did not change during the period of the study in either hospital.
Data analysis
The incidence of HAI was expressed per 1,000 days at risk, i.e. days until first HAI, day of discharge or end of observation period. The proportion of patients with HAI was expressed as the total number of patients with HAI per 100 patients surveyed post-ICU, with 95%-confidence interval (CI95). The total number of SSIs was expressed per 100 patients with surgical procedures. Statistical analysis was performed with SPSS for Windows 12.0.1 (SPSS, Inc., Chicago, IL, USA). Differences in continuous variables between groups were determined by Student’s t test. Differences in proportions of HAI (with CI95) in the successive study periods were determined. Statistical significance was defined as a p value of less than 0.05.
Results
Patients
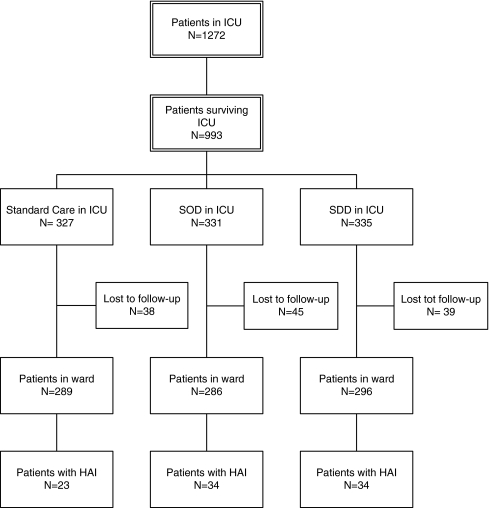
Between May 2004 and July 2006, 871 patients were included; 289 after SC, 286 after SOD and 296 SDD (Fig. 1). Reasons for patients being lost to follow-up (n = 122) mainly included their transfer to other hospitals after ICU discharge. Although fewer patients in the SC group had received mechanical ventilation in ICU (84% vs. 96% and 94% in SOD and SDD, respectively), other characteristics (such as age, sex, Apache II-score on ICU admission and surgical or non-surgical reasons for admission) were comparable for all three groups (Table 1).
Fig. 1.
Flowchart
Table 1.
Patients’ characteristics
| Standard care | SOD | SDD | |
|---|---|---|---|
| No. of patients | 289 | 286 | 296 |
| Sex (male/female) | 187/102 | 181/105 | 180/116 |
| Age | |||
| Mean (median) | 56.7 (59) | 57.9 (61) | 57.0 (60) |
| SD | 18 | 17.1 | 17.5 |
| Range | 16–93 | 12–87 | 16–87 |
| APACHE II at ICU admission | |||
| Mean (median) | 18.9 (18) | 19.8 (19) | 20.1 (19) |
| SD | 7.85 | 7.86 | 7.98 |
| Range | 4–48 | 4–45 | 0–45 |
| LOS in ICU | |||
| Mean (median) | 13.6 (8) | 12.6 (8) | 14.2 (9) |
| SD | 15 | 11.8 | 15.1 |
| Range | 1–141 | 1–93 | 1–121 |
| Mechanical ventilation in ICU | |||
| Yes (%) | 243 (84)* | 274 (96)* | 279 (94)* |
| No | 46 | 12 | 17 |
| Specialty | |||
| Cardiology | 9 | 15 | 10 |
| Cardiothoracic surgery | 13 | 27 | 16 |
| Surgery | 109 | 94 | 117 |
| Medical | 61 | 54 | 61 |
| Pulmonology | 8 | 8 | 7 |
| Neurosurgery | 38 | 49 | 43 |
| Neurology | 28 | 26 | 23 |
| Other | 23 | 13 | 19 |
| No. of surgical patients | 126 | 127 | 137 |
LOS Length of stay
* Standard care versus SOD and SDD significantly different (p = 0.000), no difference between SOD and SDD
End points
The numbers of patients with HAI were 23, 34 and 34 from the SC, SOD and SDD groups, respectively, yielding incidences per 1,000 days at risk of 8.3, 12.9 and 11.2 for SC, SOD and SDD, respectively (Table 2). As compared to SC, the relative risks for developing HAI in the first 14 days after ICU discharge were 1.49 (CI95 0.9–2.47) after SOD and 1.44 (CI95 0.87–2.39) after SDD. Oropharyngeal infections, only registered in one hospital, occurred in one patient after SC and in four patients after SOD.
Table 2.
Infections, time until diagnosis and mortality
| Standard care (N = 289) | SOD (N = 286) | SDD (N = 296) | |
|---|---|---|---|
| Number of patients with HAI | 23 | 34 | 34 |
| Number of HAI | 26 | 39 | 37 |
| Incidence of HAI/1,000 days at risk | 8.3 | 12.9 | 11.2 |
| RR standard care versus intervention | – | 1.49 | 1.44 |
| CI95 | 0.9–2.47 | 0.87–2.39 | |
| Proportion of patients (%)with HAI | 8 | 12 | 11 |
| CI95 | 5–12 | 8–16 | 8–16 |
| Specialty of patients with HAI | |||
| Cardiology | 1 | 2 | – |
| Cardiothoracic surgery | – | 2 | – |
| Surgery | 12 | 14 | 19 |
| Medical | 2 | 7 | 4 |
| Pulmonology | – | – | – |
| Neurosurgery | 3 | 3 | 6 |
| Neurology | 2 | 4 | 3 |
| Other | 3 | 2 | 2 |
| Mortality: no. of patients (%) | 22 (7.6) | 15 (5.2) | 21 (7.1) |
| Mortality of patients with HAI: no. of patients (%) | 2 (8.7) | 3 (8.8) | 3 (8.8) |
| Mean LOS in surveillance on ward (days) | 10.1 | 10.0 | 11.0 |
| Median; range | 13; 1–14 | 14; 1–14 | 14; 1–14 |
| No. of RTI | 16 | 18 | 18 |
| Mean time until diagnosis (days) | 4.6 | 5.0 | 4.7 |
| Median; range | 4.5; 1–9 | 4.5; 1–13 | 3.5; 1–12 |
| No. of BSI | 5 | 5 | 8 |
| Mean time until diagnosis (days) | 4.8 | 5.6 | 9.0 |
| Median; range | 4.0; 1–8 | 5; 2–12 | 10; 1–12 |
| No. of SSI | 5 | 15 | 11 |
| Incidence/100 surgical procedures | 4.0 | 11.8 | 8.0 |
Differences in times until diagnosis are not significant between the three groups or between the standard care group versus SOD and SDD combined
HAI Hospital acquired infections, RR relative risk, LOS length of stay, RTI respiratory tract infection, BSI blood stream infection, SSI surgical site infection
The proportion of patients with HAI in the standard care period versus SOD and SDD combined (RR 1.47, CI95 0.935–2.305) is not significantly different
Most infections were RTI, with similar incidences and similar time until diagnosis in all three study groups (Table 2). Adjustment for the difference in number of mechanically ventilated patients in ICU did not change these observations. Incidences of BSI were also similar between the three groups, but the duration until infection tended to be longer in the post-intervention groups (means of 4.8 for SC and 7.7 days for SOD and SDD combined, p = 0.17 when comparing SC to SDD and SOD combined). Incidences of SSI, expressed per 100 surgical procedures were 4 after SC, as compared to 11.8 after SOD and 8 after SDD (p = 0.04 when comparing SC to SDD and SOD combined). Among the 26 patients with SSI in both post-intervention groups, 18 were diagnosed with superficial infections (15 patients not cultured or with a negative culture) and in 7 patients Staphylococcus aureus was documented as the cause of SSI.
Hospital mortality was 7.6% (22 patients) in the SC group, 5.2% (15 patients) in the SOD group and 7.1% (21 patients) in the SDD group. Hospital mortality among patients that developed HAI was 8.7% (n = 2), 8.8% (n = 3) and 8.8% (n = 3).
Discussion
Incidences of HAI in general wards tended to be higher in patients that had received either SDD or SOD during ICU-stay, but it seems unlikely that these infections have an important effect on hospital mortality rates. Of note, the observed differences in relative risks only approached statistical significance.
To the best of our knowledge this is the first study that has quantified timing and incidences of infections in general wards after ICU discharge related to antimicrobial infection prevention measures in the ICU. Strengths include the prospective monitoring of infections performed by a limited number (n = 3) of trained and experienced infection control professionals. The open study design in the ICU was an unavoidable limitation of the present analysis. By using objective, and internationally accepted, criteria we aimed to minimize information bias. The fact that the study was performed in only two tertiary care centers may reduce the generalizability of our findings.
The observed tendency towards a higher infection rate after an antibiotic intervention in ICU might be related to differences in patient risk factors or to changes in the colonization status between the intervention groups and the patients that received standard care. Indeed, at the time of ICU admission, a higher proportion of patients in the standard group did not receive mechanical ventilation. Yet, there were no significant differences in age, APACHE II score at the time of ICU admission or the lengths of stay in ICU or on mechanical ventilation, or in distribution of medial specialties. We therefore assume that the risk profile at the time of ICU discharge was similar for the three patient populations.
Both SDD and SOD aim to modulate the colonization status of patients, which resulted in lower colonization rates with Gram negative bacteria in the respiratory and intestinal tract [6]. After discontinuation of the prophylactic regimens, though, patients may acquire colonization with typical hospital pathogens or suppressed colonization with such bacteria may reemerge. If these phenomena are relevant, and whether they are responsible for our observations, remains to be determined.
Our study was motivated by the observation of a tendency towards higher mortality rates after ICU discharge among patients that had received SDD in a previous study [4]. In the current study, 58 patients (7%) succumbed in the hospital after ICU discharge, and eight (14%) of these patients had been diagnosed with a HAI in the first 14 days after ICU discharge. Overall mortality rates were comparable between the three study groups and the numbers of patients that died after developing a HAI was two in the standard care period and three in both the SDD and SOD period. Considering the low rates of infection, the overall low mortality rates after ICU discharge and the low prevalence of infections among those that succumbed after ICU discharge, we reject the hypothesis that an increased infection rate after ICU discharge affects the clinical outcome of patients that have received SDD or SOD in ICU, in spite of a tendency of more infections, especially superficial SSIs, in these patients after ICU discharge.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.D’Amico R, Pifferi S, Leonetti C, Torri V, Tinazzi A, Liberati A (1998) Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. BMJ 316:1275–1285 [DOI] [PMC free article] [PubMed]
- 2.Pugin J, Auckenthaler R, Lew DP, Suter PM (1991) Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia: a randomized, placebo-controlled, double-blind clinical trial. J Am Med Assoc 265:2704–2710 [DOI] [PubMed]
- 3.Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, Beysens AJ, de Leeuw PW, Stobberingh EE (2001) Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 164:382–388 [DOI] [PubMed]
- 4.de Jonge E, Schultz M, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, Kesecioglu J (2003) Effects of selective decontamination of the digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomized controlled trial. Lancet 362:1011–1016 [DOI] [PubMed]
- 5.Chan EY, Ruest A, Meade MO, Cook DJ (2007) Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 334:889–900 [DOI] [PMC free article] [PubMed]
- 6.de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ (2009) Decontamination of the digestive tract and oropharynx in intensive care patients. N Engl J Med 360:20–31 [DOI] [PubMed]
- 7.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk-van der Wees JEC (1971) Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg 69:405–411 [DOI] [PMC free article] [PubMed]
- 8.Stoutenbeek CP, van Saene HKF, Miranda DR, Zandstra DF (1984) The effect of selective decontamination of the digestive tract on colonization and infection rate in multiple trauma patients. Intensive Care Med 10:185–192 [DOI] [PubMed]
- 9.Nathens AB, Marshall JC (1999) Selective decontamination of the digestive tract in surgical patients. A systematic review of the evidence. Arch Surg 134:170–176 [DOI] [PubMed]
- 10.Krueger WA, Lenhart FP, Neeser G, Ruckdeschel G, Schreckhase H, Eissner HJ, Forst H, Eckart J, Peter K, Unertl KE (2002) Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions, and mortality in critically ill surgical patients: a prospective, stratified, randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med 166:1029–1037 [DOI] [PubMed]
- 11.de La Cal MA, Cerdá E, García-Hierro P, van Saene HK, Gómez-Santos D, Negro E, Lorente JA (2005) Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo-controlled, double-blind trial. Ann Surg 241:424–430 [DOI] [PMC free article] [PubMed]
- 12.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for hospital acquired infections. Am J Infect Control 16:128–140 [DOI] [PubMed]
- 13.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13:606–608 [PubMed]