Abstract
As impaired insulin signalling (IIS) is a risk factor for Alzheimer’s disease we crossed mice (Tg2576) over-expressing human amyloid precursor protein (APP), with insulin receptor substrate 2 null (Irs2−/−) mice which develop insulin resistance. The resulting Tg2576/Irs2−/− animals had increased tau phosphorylation but a paradoxical amelioration of Aβ pathology. An increase of the Aβ binding protein transthyretin suggests that increased clearance of Aβ underlies the reduction in plaques. Increased tau phosphorylation correlated with reduced tau-phosphatase PP2A, despite an inhibition of the tau-kinase glycogen synthase kinase-3. Our findings demonstrate that disruption of IIS in Tg2576 mice has divergent effects on pathological processes—a reduction in aggregated Aβ but an increase in tau phosphorylation. However, as these effects are accompanied by improvement in behavioural deficits, our findings suggest a novel protective effect of disrupting IRS2 signalling in AD which may be a useful therapeutic strategy for this condition.
Keywords: Alzheimer’s disease, Diabetes, Insulin signalling, Tau, APP, Abeta, GSK-3, PP2a, Transthyretin, Irs2
Introduction
Alzheimer’s disease (AD) is characterised by extracellular plaques, composed predominantly of β-amyloid (Aβ) peptides and intracellular neurofibrillary tangles (NFTs), composed of hyperphosphorylated forms of the microtubule associated protein tau. The amyloid cascade hypothesis suggests that generation and aggregation of Aβ precedes and indeed promotes tau-related pathology in AD pathogenesis. Altered IIS may be a critical modifier of these processes. For example, IIS regulates both Aβ formation and turnover [1–3] and tau phosphorylation [4,5] while in humans type 2 diabetes and variation in IIS genes are associated with AD [6–8]. Furthermore, in rodent models, brain-insulin resistance increases tau phosphorylation [9] while diet-induced insulin resistance or impaired IGF-1 signalling increases Aβ pathology [10]. Conversely, dietary restriction [11], which enhances insulin action, and IGF-1 therapy [12] ameliorate AD pathology. These observations have led to efforts to develop therapies for AD that improve IIS. However, paradoxically, impaired IIS has also been shown to reduce Aβ aggregation and toxicity in a Caenorhabditis elegans model with AD-associated pathology in muscle [13] and impaired IIS is associated with increased longevity in a variety of model organisms [14]. To determine whether toxic or protective effects of reduced IIS upon AD pathology predominate in the mammalian brain, we disrupted IIS in a mouse model of Aβ deposition by crossing plaque-prone Tg2576 mice with Irs2−/− mice, a model of type 2 diabetes.
Materials and methods
Animals
Tg2576 mice, expressing the Swedish mutant form of APP (APP—K670N, M671L) [15], were bred into Irs2−/− mice [16] as follows: male Tg2576 (C57Bl/6.SJL) were initially crossed with Irs2+/− females (C57Bl/6). We then bred the F1 Tg2576 Irs2+/− males with Irs2+/− females and the F1 Tg2576 Irs2+/− females with Irs2+/− males. These crosses generated F2 Tg2576/Irs2−/− and WT littermates that were used in all experiments. Mice were maintained on a 12-h light/dark cycle with free access to water and standard mouse chow (4% fat, RM1, Special Diet Services). Mice were handled and all in vivo studies performed in accordance with the United Kingdom Animals (Scientific Procedures) Act (1986) and University College London Ethical Review Process guidelines.
Protein extraction
Tissue was homogenised at 4 °C in 8-fold (mgs/μL) volume of PBS buffer (10 mM Phosphate, 140 mM NaCl, 2.7 mM KCl, 1 mM EDTA, 10 mM β-glycerolphosphate, 10 mM NaF, pH 7.4, and protease [Roche, UK], phosphatase inhibitor cocktails [Sigma, UK]). Homogenates were spun (100,000g, 4 °C for 30 min). The supernatant (S1) was collected and the pellet resuspended with the addition of 1% Triton-X 100, vortexed for 1 min, placed at 4 °C for 10 min and subjected to a repeat centrifugation. The supernatant (S2) was stored at −80 °C. P2 pellets were extracted with a 8-fold volume of 70% formic acid, resuspended, vortexed and placed at 4 °C over night. Following centrifugation at 100,000g (4 °C; 30 min), supernatants collected and neutralised with a19 fold volume of neutralisation buffer (1 M Tris base, 0.5 M Na2HPO4, 0.05% NaN3).
Western blotting
Phosphorylated tau. S1 and S2 samples were diluted in equal volumes of 2× reducing sample buffer (BioRad, UK), heated to 100 °C for 5 min, spun and separated on 8% SDS–polyacrylamide gels. Primary antibodies were detected with appropriate secondary antibodies conjugated to fluorophors of 700 or 800 nm, and densitometry performed, using a near infrared Odyssey imager (Licor, UK). Phospho-tau immunoreactivity values were normalized to total tau.
Detection of full-length APP and APP-CTFs. S2 fractions were used for detection of full-length holo-APP and COOH-terminal fragments of APP (APP-CTFs). Holo-APP was detected using a 6% Tris–glycine SDS–PAGE with polyclonal anti-APP antibody 369(20). APP-CTFs were detected using 4–12% Bis-Tris NuPAGE system (Invitrogen, UK). Levels of full-length APP were normalized to the levels of actin. APP-CTFβ and APP-CTFα levels were normalized to full-length APP.
Other proteins. For all other phospho-proteins, levels were normalized to total protein and non-phosphoproteins to β-actin.
Immunohistochemistry
Histological staining. Brains were dissected between 11 and 14 months of age, fixed in 10% formalin and paraffin embedded. Tissue sections of 10 μm thickness were stained with haematoxylin/eosin, Nissl or Gallyas methods or with Congo red. B19 is a rabbit polyclonal raised to adult bovine tau, reacting with all isoforms in a phosphorylation-independent manner [17]. A rabbit polyclonal antibody to human Aβ42 (Bio-Source, Belgium) was used for the detection of Aβ.
Immunocytochemistry. Immunohistochemical labelling of brain tissue was performed using the ABC method, as previously described (22). Briefly, tissue sections were treated with H2O2 and incubated with a blocking solution (10% v/v horse serum in TBS—10 mM Tris, 150 mM NaCl, pH 7.4). After overnight incubation with primary antibody, sections were incubated with horse anti-mouse antibodies conjugated to biotin followed by the ABC complex (Vector Labs, Belgium). Peroxidase activity was revealed using diaminobenzidine as chromogen. For immunolabelling with the Aβ antibodies, rehydrated tissue sections were pre-treated with 100% formic acid for 10 min before incubation with the blocking solution.
Quantification of Aβ staining. Digital images of whole brain sagittal sections taken close to the midline, were analysed with the NIH Image J program: the total area covered by Aβ positive deposits was measured using image thresholding and the total cortex surface was measured using manual selection. The area covered by Aβ deposits is expressed relative to the total cortex surface.
Aβ ELISA
Levels of human Aβ1–40 and Aβ1–42 in S1 and S2 fractions and formic acid extracted P2 pellets were measured by ELISA according to manufacturers instructions (The Genetics Company, Switzerland).
Quantitative RT-PCR
Total RNA was extracted from frontal cortex using Triazol according to manufacturers instructions (Sigma, UK). Total RNA (1 mg) was reverse transcribed using random hexamers with a Taqman RT reagent kit (Perkin Elmer, UK). Quantitative RT-PCR for transthyretin was performed as previously described [18] and using Taqman Gene Expression assay FAM/TAMRA primers (Applied Biosystems): transthyretin (Ttr) (Mm00443267_m1), Gapdh (Mm99999915_g1). Primers to mouse IDE, were designed using Universal Probe Library (Roche, UK) software. Real time PCRs were performed on a Lightcycler (Roche, UK) using QuantiTect SYBR green reagent (Qiagen, UK).
Behaviour
For contextual conditioning mice at 10–12 months were trained in a conditioning chamber (Med Associates, St. Albans, USA) in a soundproof box—after a 120 s introductory period a tone (80 dB, 3.0 kHz) was presented for 30 s, the last 2 s of which coincided with a foot-shock (0.75 mA). A further two tone/foot-shock pairings were administered at 60 s intervals and after a final 60 s period the mice were returned to their home cage. Twenty-four hours after training the mice were re-exposed to the conditioning chamber for 5 min to test for contextual fear memory. Freezing behaviour (defined as complete lack of movement, except for respiration) was scored for 2 s in every 5 s.
Results
Expression of mutant APP increases tau phosphorylation in the context of Irs2 deletion
Modest increases in tau phosphorylation were seen in 12–15 month-old Irs2−/− mice. In contrast, in Tg2576/Irs2−/− mice we detected substantially increased tau phosphorylation indicating that impaired IIS promotes tau phosphorylation which is in turn enhanced by the presence of Aβ pathology (Fig. 1). However, we found no change in tau aggregation (data not shown) but did detect reduced expression of the tau-phosphatase PP2a in animals lacking Irs2; this latter observation may, as suggested by others [9], underlie the increased tau phosphorylation seen in Irs2−/− animals even though the tau-kinase glycogen synthase kinase-3 (GSK-3) was relatively inhibited (Fig. 2).
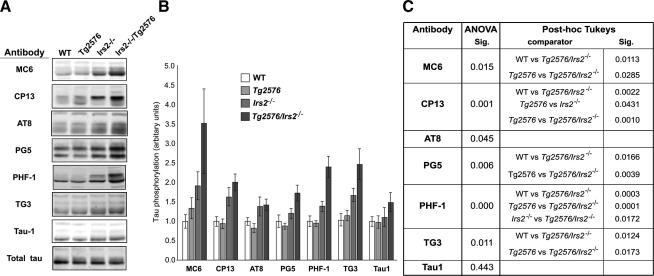
Fig. 1.
Phospho-tau immunoreactivity is increased in temporal cortex of Tg2576/Irs2−/− mice. (A) Phosphospecific anti-tau antibodies were used to probe temporal cortex of age-matched wild-type (WT); Tg2576; Irs2−/− and Tg2576/Irs2−/− animals. (B) Data shown is normalised to a phosphorylation-independent tau antibody. (C) Analysis by genotype (ANOVA with post hoc Tukeys test; n = 29) showed significant increases in tau phosphorylation in Tg2576/Irs2−/− animals relative to WT and Tg2576 animals at epitopes recognised by antibodies MC6, CP13, PG5, PHF1, and TG3 but not at the overlapping AT8 and TAU1 epitopes.
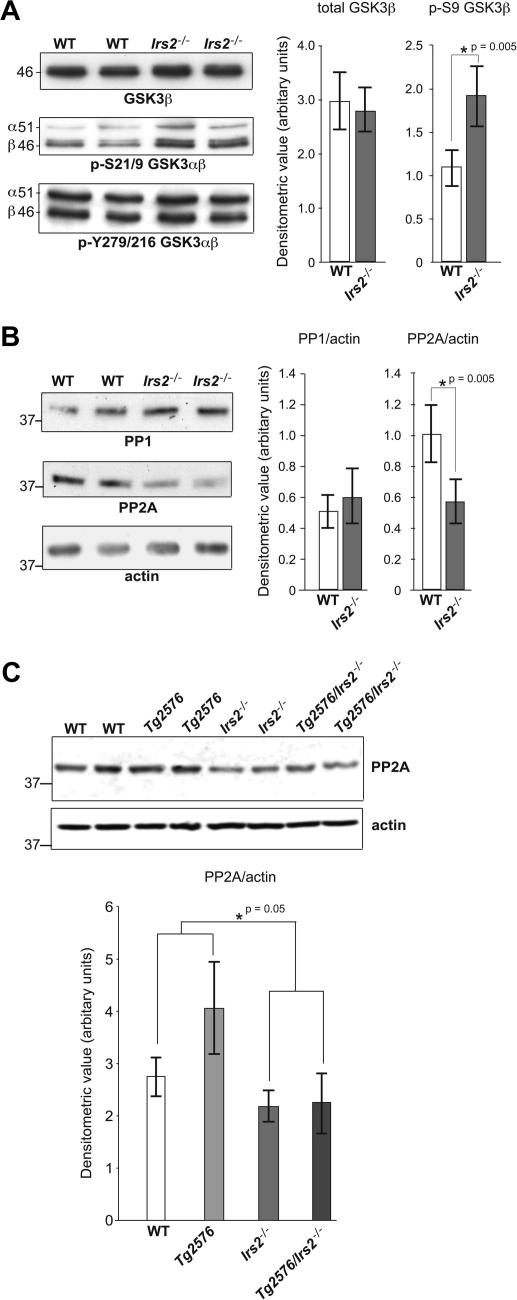
Fig. 2.
GSK-3 is inhibited and PP2a is reduced in mice lacking Irs2. The tau-kinase, GSK-3 and the protein phosphatases PP1 and PP2A were examined in wild-type (WT) and in IRS2−/− mice by Western blot. (A) Animals lacking Irs2 showed no change in total GSK-3 protein or in phosphorylation at the Tyr279 in GSK-3α/GSK-3β 216 epitope. However there was a substantial (p < 0.05) increase in GSK-3 phosphorylation at Ser 21 GSK-3α/Ser 9 GSK-3β epitope reflecting relative inhibition of GSK-3 activity in these animals. (B) There was no change in PP1 but a significant increase in PP2A in animals lacking Irs2. (C) Comparing PP2a in hippocampus across all four genotypes confirmed a reduction in PP2a in all animals lacking Irs2 but no effect of APP expression.
Deletion of Irs2 reduces amyloid burden in Tg2576 mice
Twelve month old Tg2576 mice displayed the expected numerous, large Congophilic Aβ deposits but in contrast, in age-matched Tg2576/Irs2−/− mice plaque number appeared reduced and plaques were smaller and less intensely labelled. Quantification of extracellular Aβ deposits revealed that the area covered by Aβ deposits was significantly reduced in brains of the Tg2576/Irs2−/− mice compared to littermate Tg2576 animals (p = 0.01; Fig. 3).
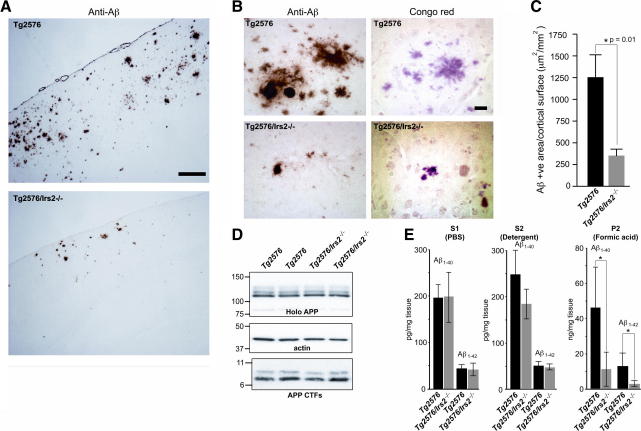
Fig. 3.
Deletion of Irs2 reduces Aβ deposition and decreases insoluble Aβ. (A) Immunocytochemical labeling with a human specific anti-Aβ antibody of whole brain frontal cortex sections of 12 month-old Tg2576 and Tg2576/Irs2−/− mice. Scale bar = 200 microns. (B) Immunocytochemical with the anti-Aβ antibody (left panels) and histochemical labeling with Congo red (right panels) of temporal cortex sections of 12 month-old Tg2576 and Tg2576/Irs2−/− mice, showing the extent of Aβ deposition in representative animals. Scale bars = 50 microns. (C) The mean surface covered by Aβ deposits in the cortex was significantly reduced in Tg2576/Irs2−/− mice compared with Tg2576 mice (n = 32; ∗p = 0.01, t-test). (D) APP processing was examined by immunoblotting for holo-APP and APP-CTFs in temporal cortex of Tg2576 and Tg2576/Irs2−/− animals. Holo-APP values were normlised to actin, CTF values were normalised to holo-APP. No differences were found between genotypes. (E) Human Aβ1–40 and Aβ1–42 were measured by ELISA in S1 (soluble) and S2 (detergent soluble) fractions and formic acid extracts of P2 pellets from temporal cortex. Significant reductions in Aβ1–40 and Aβ1–42 were found in formic acid extracts from Tg2576/Irs2−/− compared to Tg2576 animals (n = 21; ∗p = 0.0005 [Aβ1–40] and 0.002 [Aβ1–42], t-test).
Measurement of APP metabolites in the temporal cortex of age-matched Tg2576 and Tg2576/Irs2−/− mice, showed no differences in holo-APP, or β C-terminal fragments or in soluble Aβ1–40 and Aβ1–42 levels (Fig. 3). However, there was a significant reduction in insoluble, aggregated Aβ1–40 and Aβ1–42 levels in Tg2576/Irs2−/− mice compared to Tg2576 littermates, closely reflecting the reduction in Aβ load measured by immunocytochemistry. Together these results suggest that altered APP processing does not underlie the alterations in Aβ generation.
Reduced Aβ burden is associated with increased expression of transthyretin and altered membrane expression of insulin degrading enzyme
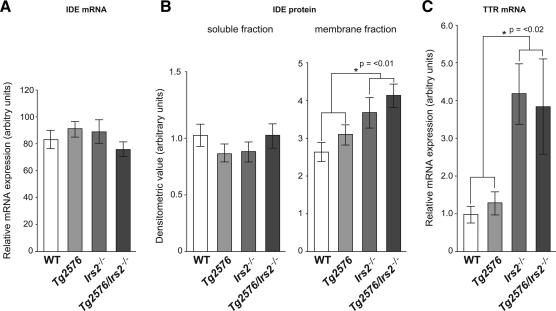
Aβ clearance is regulated by both increased proteolysis and through mechanisms dependent on binding to carrier proteins including transthyretin. Both mechanisms have been implicated in IIS—one of the key Aβ proteases, insulin degrading enzyme (IDE) or insulysin (38), is involved in both insulin and Aβ degradation and transthyretin, recently implicated in Aβ proteolysis as well as Aβ clearance [19], is elevated in both insulin resistant mice and people with type II diabetes [20,21]. We therefore examined the expression of both genes. IDE mRNA expression was not altered but TTR expression was increased 3.9-fold (p = 0.01) in animals null for Irs2 compared to wild-type animals (Fig. 4A,C). We next examined the protein levels of IDE by western blotting. As it has been recently reported that membrane-bound, but not cytosolic, IDE protein is significantly decreased in brain tissue of individuals at high risk of developing AD (40), we examined IDE in both the soluble and detergent soluble fractions. There was a significant increase in membrane associated fraction in Tg2576/Irs2−/− animals (mean 1 (SD 0.4) vs. 1.6 (SD 0.6); t-2.55; p < 0.05) (Fig. 4B).
Fig. 4.
Increase in insulin degrading enzyme and in transthyretin in mice lacking Irs2. Amyloid turnover is regulated by proteolysis through proteases such as insulin degrading enzyme (IDE) and by increased clearance following binding to proteins such as transthyretin; both known to be altered in response to insulin signalling. IDE mRNA was unaltered in IRS2−/− mice (A) but protein levels in the membrane-bound fraction were modestly increased (B). Transthyretin mRNA was increased substantially (3.9-fold; p < 0.02; SEM 1.05) in both genotypes lacking Irs2.
Deletion of Irs2 reverses behavioural deficits in Tg2576 mice
To assess the impact of disrupted Irs2 on the hippocampal learning and memory ability of the Tg2576 mice, we tested 10–12 month old mice in contextual fear conditioning, a behavioural paradigm previously shown to be impaired in the Tg2576 model [22]. One-way ANOVA showed that there was an overall significant difference between the groups (n = 32; F3, 28 = 4.6, p = 0.01) due to impairment in Tg2576 mutants (post hoc Tukey’s p < 0.05 for all groups). Thus, the deletion of the Irs2 gene in the Tg2576 mice is able to rescue the contextual fear deficit suggesting that the reduction of Aβ load and/or the inhibition of GSK-3 in these animals reversed the effects of over-expression of human APPsw.
Discussion
Considerable evidence implicates insulin resistance in the pathogenesis of AD and underlies current efforts to treat AD by improving insulin sensitivity. However, we find that disrupting Irs2 in Tg2576 mice results in improvement of both Aβ plaque burden and behaviour despite an exacerbation of tau phosphorylation and the presence of insulin resistance. Previously, over-expression of APP in mice was shown to induce transthyretin expression together with evidence of increased IIS [23]. It was suggested that these are protective mechanisms resulting in the absence of the amyloid cascade in mice. Subsequently, and supportive of this hypothesis, transthyretin protein was shown to prevent Aβ toxicity in vitro [24,25] and in mice over-expressing APP, neutralisation with antibody or deletion of the TTR gene both enhance pathology [26,27]. In addition to these roles, transthyretin has been shown recently to be, like IDE, an Aβ protease [19] and transthyretin protein levels in CSF are decreased in AD [28,29]. Our data demonstrates a substantial increase in transthyretin expression accompanying the similarly substantial amelioration of plaque pathology, Aβ fibrillisation and behavioural deficits; in line with a protective effect of transthyretin.
In marked contrast to the potentially beneficial reduction in Aβ pathology, we found increased tau phosphorylation in Tg2276 mice with disruption of Irs2. However we found no evidence of tangle formation, consistent with previous observations [9]. The increase in tau phosphorylation was observed at many but not all epitopes examined. The most pronounced changes were at epitopes positioned at the 396/404, and 235 and 231 sites, sites known to be phosphorylated in AD [30]. However, the AT8/TAU1 epitope covering Ser199/Ser202/Thr205, a key GSK-3 site which is also highly phosphorylated in AD brain, was unaffected in the Tg2576/Irs2−/− animals. When we examined tau-kinase activity in Irs2−/− mice we found no increase in the activities of GSK-3, like others previously [9], or in the GSK3 and CDK5 substrate CRMP-2 (data not shown). These data are consistent with the pattern of tau phosphorylation changes we observed, in particular the absence of an increase in phosphorylation at the key GSK-3 sites—Ser199/Ser202/Thr205. However we did find a highly significant decrease in the tau-phosphatase PP2A in Irs2−/− mice, suggesting that the mechanism of effect might be mediated not by an increase in kinase activity but by a decrease in the activity of this phosphatase.
The relative role of amyloid versus tau pathologies in influencing neuronal dysfunction and cognitive impairment has been of considerable interest and indeed controversy. The generation of a mouse model with both decreased Aβ aggregation and deposition but increased tau phosphorylation permitted us to directly address this question. Using a standard paradigm of hippocampal dependent learning, contextual fear conditioning, we observed a complete reversal of behavioural deficits in the context of Irs2 deletion. Interestingly it has been reported that the Aβ induced impairment in LTP, known to be present in the Tg2576 animals [22], is reversed by insulin [31]. The mechanism whereby insulin might restore LTP is not known but one promising candidate is GSK-3 as we and others have recently demonstrated that GSK-3 inhibition is essential for LTP [32,33]. In the Tg2576/Irs2−/− animals the reduction in Aβ and the relative inhibition of GSK-3 might both, together or separately, contribute to the reversal of the behavioural phenotype.
Although much of the current literature suggests that insulin resistance is an aetiological factor in AD, we have recently demonstrated that mice lacking Irs1 have increased lifespan and reduced age-related pathology [34] and deletion of Irs2 in the mouse brain increases longevity [35]. In C. elegans, abrogating IIS protects against a range of proteotoxic neuropathologies, including Aβ toxicity [13,36]. Our new findings demonstrate that this is also the case for mammals with specific disruption of Irs2 and suggests that for therapeutic manipulation of this pathway to be beneficial in the treatment of AD an increased understanding of the complex signalling and gene expression mechanisms downstream of IIS will be required.
Acknowledgments
This research was supported by the Wellcome Trust (S.L. and D.J.W.) the Alzheimer’s Research Trust (S.L. and D.J.W.), the Rosetrees Trust (to D.J.W.), the Biotechnology and Biological Sciences Research Council (K.P.G. and D.J.W.), the Belgian PAI P6/43, the FRSM, the SAO/FRMA, and Van Buuren and Génicot Foundations to JPB We thank Drs. P. Davies and J. Buxbaum for providing antibodies.
Contributor Information
Dominic J. Withers, Email: d.withers@ucl.ac.uk.
Simon Lovestone, Email: s.lovestone@iop.kcl.ac.uk.
References
- 1.Carro E., Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004;490:127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 2.Adlerz L., Holback S., Multhaup G., Iverfeldt K. IGF-1-induced processing of the amyloid precursor protein family is mediated by different signaling pathways. J. Biol. Chem. 2007;282:10203–10209. doi: 10.1074/jbc.M611183200. [DOI] [PubMed] [Google Scholar]
- 3.Solano D.C., Sironi M., Bonfini C., Solerte S.B., Govoni S., Racchi M. Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J. 2000;14:1015–1022. doi: 10.1096/fasebj.14.7.1015. [DOI] [PubMed] [Google Scholar]
- 4.Hong M., Lee V.M.Y. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J. Biol. Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 5.Lesort M., Johnson G.V.W. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. doi: 10.1016/s0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton G., Proitsi P., Jehu L., Morgan A., Williams J., O’Donovan M.C., Owen M.J., Powell J.F., Lovestone S. Candidate gene association study of insulin signaling genes and Alzheimer’s disease: evidence for SOS2, PCK1, and PPARgamma as susceptibility loci. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:508–516. doi: 10.1002/ajmg.b.30503. [DOI] [PubMed] [Google Scholar]
- 7.Liolitsa D., Powell J., Lovestone S. Genetic variability in the insulin signalling pathway may contribute to the risk of late onset Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2002;73:261–266. doi: 10.1136/jnnp.73.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biessels G.J., Staekenborg S., Brunner E., Brayne C., Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 9.Schubert M., Brazil D.P., Burks D.J., Kushner J.A., Ye J., Flint C.L., Farhang-Fallah J., Dikkes P., Warot X.M., Rio C., Corfas G., White M.F. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J. Neurosci. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho L., Qin W., Pompl P.N., Xiang Z., Wang J., Zhao Z., Peng Y., Cambareri G., Rocher A., Mobbs C.V., Hof P.R., Pasinetti G.M. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Ho L., Qin W., Rocher A.B., Seror I., Humala N., Maniar K., Dolios G., Wang R., Hof P.R., Pasinetti G.M. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- 12.Carro E., Trejo J.L., Gerber A., Loetscher H., Torrado J., Metzger F., Torres-Aleman I. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol. Aging. 2006;27:1250–1257. doi: 10.1016/j.neurobiolaging.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Cohen E., Bieschke J., Perciavalle R.M., Kelly J.W., Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 14.Russell S.J., Kahn C.R. Endocrine regulation of ageing. Nat. Rev. Mol. Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 16.Withers D.J., Gutierrez J.S., Towery H., Burks D.J., Ren J.M., Previs S., Zhang Y., Bernal D., Pons S., Shulman G.I., Bonner-Weir S., White M.F. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 17.Brion J.P., Hanger D.P., Bruce M.T., Couck A.M., Flament-Durand J., Anderton B.H. Tau in Alzheimer neurofibrillary tangles. N- and C-terminal regions are differentially associated with paired helical filaments and the location of a putative abnormal phosphorylation site. Biochem. J. 1991;273:127–133. doi: 10.1042/bj2730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhury A.I., Heffron H., Smith M.A., Al-Qassab H., Xu A.W., Selman C., Simmgen M., Clements M., Claret M., Maccoll G., Bedford D.C., Hisadome K., Diakonov I., Moosajee V., Bell J.D., Speakman J.R., Batterham R.L., Barsh G.S., Ashford M.L., Withers D.J. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J. Clin. Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa R., Ferreira-da-Silva F., Saraiva M.J., Cardoso I. Transthyretin protects against A-beta peptide toxicity by proteolytic cleavage of the peptide: a mechanism sensitive to the Kunitz protease inhibitor. PLoS ONE. 2008;3:e2899. doi: 10.1371/journal.pone.0002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody N., Graham T.E., Tsuji Y., Yang Q., Kahn B.B. Decreased clearance of serum retinol-binding protein and elevated levels of transthyretin in insulin-resistant ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 2008;294:E785–E793. doi: 10.1152/ajpendo.00521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundsten T., Ostenson C.G., Bergsten P. Serum protein patterns in newly diagnosed type 2 diabetes mellitus—influence of diabetic environment and family history of diabetes. Diabetes Metab. Res. Rev. 2008;24:148–154. doi: 10.1002/dmrr.789. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen J.S., Wu C.C., Redwine J.M., Comery T.A., Arias R., Bowlby M., Martone R., Morrison J.H., Pangalos M.N., Reinhart P.H., Bloom F.E. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein T.D., Johnson J.A. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J. Neurosci. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giunta S., Valli M.B., Galeazzi R., Fattoretti P., Corder E.H., Galeazzi L. Transthyretin inhibition of amyloid beta aggregation and toxicity. Clin. Biochem. 2005;38:1112–1119. doi: 10.1016/j.clinbiochem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Costa R., Goncalves A., Saraiva M.J., Cardoso I. Transthyretin binding to A-beta peptide—impact on A-beta fibrillogenesis and toxicity. FEBS Lett. 2008;582:936–942. doi: 10.1016/j.febslet.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Buxbaum J.N., Ye Z., Reixach N., Friske L., Levy C., Das P., Golde T., Masliah E., Roberts A.R., Bartfai T. Transthyretin protects Alzheimer’s mice from the behavioral and biochemical effects of Abeta toxicity. Proc. Natl. Acad. Sci. USA. 2008;105:2681–2686. doi: 10.1073/pnas.0712197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein T.D., Anders N.J., DeCarli C., Chan S.L., Mattson M.P., Johnson J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J. Neurosci. 2004;24:7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castano E.M., Roher A.E., Esh C.L., Kokjohn T.A., Beach T. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer’s disease and non-demented elderly subjects. Neurol. Res. 2006;28:155–163. doi: 10.1179/016164106X98035. [DOI] [PubMed] [Google Scholar]
- 29.Gloeckner S.F., Meyne F., Wagner F., Heinemann U., Krasnianski A., Meissner B., Zerr I. Quantitative analysis of transthyretin, tau and amyloid-beta in patients with dementia. J. Alzheimers. Dis. 2008;14:17–25. doi: 10.3233/jad-2008-14102. [DOI] [PubMed] [Google Scholar]
- 30.Hanger D.P., Byers H.L., Wray S., Leung K.Y., Saxton M.J., Seereeram A., Reynolds C.H., Ward M.A., Anderton B.H. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J. Biol. Chem. 2007;282:23645–23654. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.C., Kuo Y.M., Huang C.C., Hsu K.S. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol. Aging. 2007;30:377–387. doi: 10.1016/j.neurobiolaging.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Hooper C., Markevich V., Plattner F., Killick R., Schofield E., Engel T., Hernandez F., Anderton B., Rosenblum K., Bliss T., Cooke S.F., Avila J., Lucas J.J., Giese K.P., Stephenson J., Lovestone S. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur. J. Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 33.Peineau S., Taghibiglou C., Bradley C., Wong T.P., Liu L., Lu J., Lo E., Wu D., Saule E., Bouschet T., Matthews P., Isaac J.T., Bortolotto Z.A., Wang Y.T., Collingridge G.L. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Selman C., Lingard S., Choudhury A.I., Batterham R.L., Claret M., Clements M., Ramadani F., Okkenhaug K., Schuster E., Blanc E., Piper M.D., Al-Qassab H., Speakman J.R., Carmignac D., Robinson I.C., Thornton J.M., Gems D., Partridge L., Withers D.J. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2007;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi A., Wartschow L.M., White M.F. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 36.Florez-McClure M.L., Hohsfield L.A., Fonte G., Bealor M.T., Link C.D. Decreased insulin-receptor signaling promotes the autophagic degradation of beta-amyloid peptide in C. elegans. Autophagy. 2007;3:569–580. doi: 10.4161/auto.4776. [DOI] [PubMed] [Google Scholar]