Abstract
Objective To assess the thrombotic risk associated with oral contraceptive use with a focus on dose of oestrogen and type of progestogen of oral contraceptives available in the Netherlands.
Design Population based case-control study.
Setting Six participating anticoagulation clinics in the Netherlands (Amersfoort, Amsterdam, The Hague, Leiden, Rotterdam, and Utrecht).
Participants Premenopausal women <50 years old who were not pregnant, not within four weeks postpartum, and not using a hormone excreting intrauterine device or depot contraceptive. Analysis included 1524 patients and 1760 controls.
Main outcome measures First objectively diagnosed episodes of deep venous thrombosis of the leg or pulmonary embolism. Odds ratios calculated by cross-tabulation with a 95% confidence interval according to Woolf’s method; adjusted odds ratios estimated by unconditional logistic regression, standard errors derived from the model.
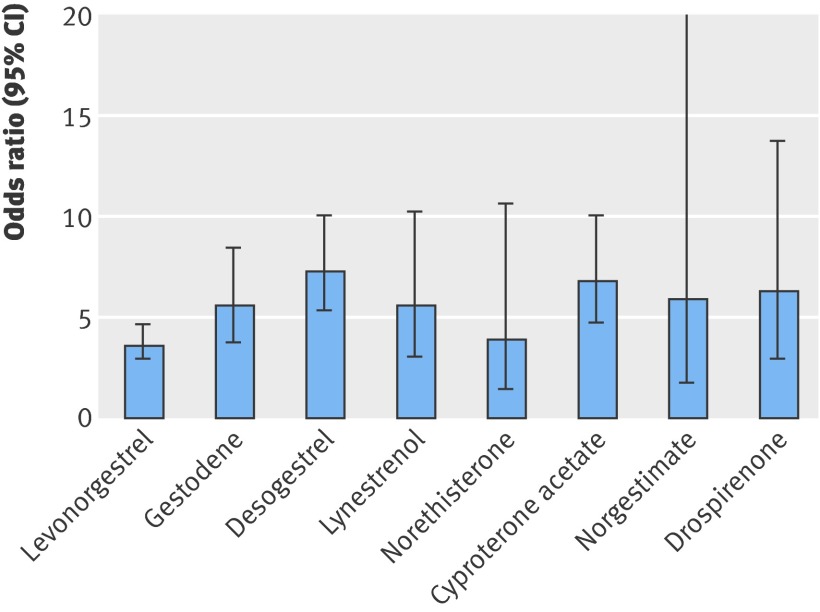
Results Currently available oral contraceptives increased the risk of venous thrombosis fivefold compared with non-use (odds ratio 5.0, 95% CI 4.2 to 5.8). The risk clearly differed by type of progestogen and dose of oestrogen. The use of oral contraceptives containing levonorgestrel was associated with an almost fourfold increased risk of venous thrombosis (odds ratio 3.6, 2.9 to 4.6) relative to non-users, whereas the risk of venous thrombosis compared with non-use was increased 5.6-fold for gestodene (5.6, 3.7 to 8.4), 7.3-fold for desogestrel (7.3, 5.3 to 10.0), 6.8-fold for cyproterone acetate (6.8, 4.7 to 10.0), and 6.3-fold for drospirenone (6.3, 2.9 to 13.7). The risk of venous thrombosis was positively associated with oestrogen dose. We confirmed a high risk of venous thrombosis during the first months of oral contraceptive use irrespective of the type of oral contraceptives.
Conclusions Currently available oral contraceptives still have a major impact on thrombosis occurrence and many women do not use the safest brands with regard to risk of venous thrombosis.
Introduction
The first report of an increased risk of venous thrombosis associated with oral contraceptives appeared in 1961.1 Since then, several large studies have confirmed a twofold to sixfold increased risk of deep venous thrombosis associated with current oral contraceptive use.2 3 4 5 To decrease the risk of thrombosis, the oestrogen dose in combined oral contraceptives was stepwise reduced over the years. A lowering of the oestrogen dose from 100 μg to 50 μg has been associated with a decreased risk of venous thrombosis.6 7 8 There is no clear evidence that the lowering of the oestrogen dose to 30 μg or 20 μg led to a further decrease of the risk of deep venous thrombosis.
Oral contraceptives may contain different types of progestogens. First generation oral contraceptives contained lynestrenol, but these are now little used. Second generation oral contraceptives, which are widely used, contain levonorgestrel or, less often, norgestrel. Third generation oral contraceptives, containing desogestrel or gestodene, which became available in the 1980s, are also widely used. Two other types of oral contraceptives are not included in this classification. Preparations containing cyproterone acetate are used for treatment of acne vulgaris, seborrhoea, or mild hirsutism and have anti-ovulatory action similar to that of a progestogen.9 10 11 Preparations containing drospirenone, which is an antimineralocorticoid, also inhibit ovulation and have been on the market since 2001.12 13
Since 1995, numerous reports have been available on the difference in thrombotic risk associated with second and third generation oral contraceptives.4 7 14 Most reported an increased risk of venous thrombosis associated with the newer third generation oral contraceptives. Some, however, did not confirm this finding or suggested that the risk difference between third and second generation oral contraceptives was overestimated because of bias or confounding such as referral or prescription bias.5 15 Kemmeren et al performed a meta-analysis on cohort and case-control studies assessing the risk of venous thrombosis among women using oral contraceptives before 1995, and found a twofold increased risk of venous thrombosis for third generation oral contraceptives compared with oral contraceptives containing levonorgestrel.16 As several authors pointed out, none of the arguments that this finding was caused by bias stood up to subsequent analyses or reasoning.17 18 19 20
Oral contraceptives containing cyproterone acetate, on the market since 1988, have been associated with a highly increased risk of venous thrombosis in some studies,21 22 23 but not all.24 25 Limited information is available for the thrombotic risk associated with the newest oral contraceptives containing drospirenone, but a series of reported cases of venous thrombosis after its introduction raised concern about an increased risk associated with this oral contraceptive.26 27 Two recent studies sponsored by the manufacturer, however, reported a similar thrombotic risk for drospirenone compared with levonorgestrel.28 29 All these studies included few cases of thrombosis and therefore had considerable uncertainty around the risk estimates.
The aim of the present study was to assess the thrombotic risk associated with current oral contraceptive use with a focus on dose of oestrogen and type of progestogen. We set out to determine which hormonal contraceptive is safest with regard to the risk of venous thrombosis using data from a large case-control study of patients with a first deep venous thrombosis and healthy controls.
Methods
Study design
This analysis was performed using data from the MEGA study (multiple environmental and genetic assessment of risk factors for venous thrombosis-study), a large, population based, case-control study on risk factors for venous thrombosis.
Between March 1999 and September 2004, consecutive patients aged <70 years with a first episode of deep venous thrombosis (leg or arm) or pulmonary embolism were included from the files of six participating anticoagulation clinics in the Netherlands (Amersfoort, Amsterdam, The Hague, Leiden, Rotterdam, and Utrecht). Information on the diagnostic procedure was obtained from hospital records and general practitioners. A deep venous thrombosis was confirmed with Doppler ultrasonography. A pulmonary embolism was confirmed by a ventilation perfusion lung scan, spiral computed tomography, or angiogram. Exclusion criteria were severe psychiatric problems and the inability to speak Dutch. Of the 6567 eligible patients, 310 died soon after the venous thrombosis. Of the remaining 6257 patients, 5184 participated (83%). Of the non-participants, 86 were in the end stage of fatal disease and 987 refused to participate or could not be located. Of the participants, 4752 (91.7%) returned a full questionnaire and 431 completed a short questionnaire by telephone.
Partners of patients <70 years old were invited to participate as controls. Of the 5184 participating patients, 3735 had an eligible partner. One partner died soon after the request for participation. Of the remaining 3734 partners, 3039 participated (81.4%). Of the non-participants, 20 were in the end stage of disease and 675 refused to participate or could not be located. A full questionnaire was returned by 2873 participating partners (94.5%), and 164 completed a short questionnaire by phone.
From January 2002 until September 2004, additional controls were recruited by random digit dialling. Phone numbers were dialled at random within the geographical inclusion area of the patients. The random controls were frequency matched to the patients with respect to age and sex. Only controls between the ages of 18 and 70 years with no history of deep venous thrombosis were included and the same exclusion criteria were applied as for the patients.
Of the 4350 eligible random controls, four died before they were able to participate. Of the remaining 4346 individuals, 3000 participated (69%). Of the non-participants, 15 were in the end stage of disease and 1331 refused to participate or could not be located. A questionnaire was returned by 2788 (93%) of the participating random controls.
For the current analyses, only women aged 18-50 years were included. Women who were postmenopausal, pregnant, or within 4 weeks postpartum at the time of the thrombotic event or index date (see below) and women using hormonal contraception other than oral contraceptives were excluded. From the total patient group, we included 1524 female patients. From the partner control group, we included 712 female partner controls. From the random digit dialling control group, we selected 1048 female controls. Of the total control group described in the current study (n=1760), 40.5% were partner controls and 59.5% were random digit dialling controls. For the analyses described in this manuscript, we pooled the two control groups into a single group and adjusted for inclusion date.
Data collection
All participants filled in a standardised questionnaire on risk factors for venous thrombosis such as family history of thrombosis, pregnancy, and oral contraceptive use in the year before the index date. The index date was the date of the thrombotic event for patients and their partners and the date of filling in the questionnaire for the random controls. The questionnaire was sent to all patients and their partners within a few weeks after the index date. For the random controls, the questionnaire was sent after their agreement to participate.
At least three months after discontinuation of the oral anticoagulation therapy, patients and their partners were invited to the anticoagulation clinic for a blood sample and an interview. During the interviews (in person or by telephone), details on current oral contraceptive use were verified.
Statistical analysis
Relative risks were assessed by calculating odds ratios and 95% confidence intervals. Risk estimates were adjusted by unconditional logistic regression, and confidence intervals were derived from the model. The odds ratios in the overall analysis of the risk associated with current oral contraceptive use were adjusted for age. When analysing the thrombotic risk associated with dose of oestrogen or type of progestogen, an additional adjustment was made for date of inclusion (divided in a total of 12 periods of 6 calendar months spanning 1999-2004). For levonorgestrel, gestodene, desogestrel, and lynestrenol, preparations can contain different doses of oestrogen and analysis was restricted to one dose—the most frequently used of 30 μg ethinyloestradiol for levonorgestrel, gestodene, and desogestrel and 37.5 μg ethinyloestradiol for lynestrenol. In all analyses, we pooled the two control groups into one group and adjusted for inclusion date. For current oral contraceptive use, the risk of venous thrombosis was calculated for all users of oral contraceptives, and separately for the different types of oral contraceptives, compared with non-users (never users and past users combined). Because a positive family history of venous thrombosis has been hypothesised to lead to preferential prescription of specific types of oral contraceptive, we took a positive family history into account. A positive family history was defined as a participant having at least one parent or sibling with a history of venous thrombosis as reported by the participants. Body mass index (weight (kg)/(height (m)2)), also a potential confounder in the association between different types of oral contraceptives and the risk of venous thrombosis, was calculated using weight and height as stated by the participants in the questionnaire. Smoking was defined as current smoking (compared with never smokers and past smokers combined).
All oral contraceptives were classified according to the dose of oestrogen and the type of progestogen. Comparisons were made relative to non-users, and between users of oral contraceptives containing different oestrogen doses and progestogen types. Because some combinations of oestrogen dose and progestogen type do not exist or were not used in this study population, we also performed stratified analyses, such as a comparison between several types of progestogens with the same dose of oestrogen (30 μg ethinylestradiol) and the effect of oestrogen dose stratified for the different types of progestogens.
Results
Table 1 shows the general characteristics of the study population. Of all 1524 patients, 859 (56.4%) had a deep venous thrombosis of the leg, 495 (32.5%) had a pulmonary embolism, 111 (7.3%) had both, and 59 (3.9%) had a deep venous thrombosis of the arm. The mean age of the 1524 patients was 37.1 years, ranging from 18 to 49. The mean age of the 1760 controls was similar to that of patients—namely, 37.4 years (range 18-49). Among the controls, women who were using hormonal contraceptives (mean age 33.6) were about six years younger than women who did not use hormonal contraceptives (mean age 39.6, mean difference 6.0, 95% CI 5.3 to 6.8).
Table 1.
General characteristics of study population. Data are number (%) unless otherwise indicated
| Thrombosis patients (n=1524) | Controls (n=1760) | |
|---|---|---|
| Deep venous thrombosis of leg only | 859 (56.4) | — |
| Pulmonary embolism only | 495 (32.5) | — |
| Deep venous thrombosis of leg + pulmonary embolism | 111 (7.3) | — |
| Deep venous thrombosis of arm | 59 (3.9) | — |
| Mean (range) age (years) | 37.1 (18-49) | 37.4 (18-49) |
| Oral contraceptive use | 1103 (72.4) | 658 (37.4) |
| Oral contraceptive use by age: | ||
| <30 years | 327 (87.2) | 266 (68.0) |
| 30-40 years | 346 (73.0) | 210 (35.5) |
| 40-50 years | 430 (63.7) | 182 (23.4) |
| Mean (range) body mass index* | 26.8 (16.0-57.8) | 24.4 (15.7-50.7) |
| Positive family history† | 309 (26.4) | 186 (14.3) |
| Smoking‡ | 380 (26.8) | 500 (30.9) |
*Data available for 1391 (91.3%) patients and 1591 (90.4%) controls.
†Defined as having one or more parents or siblings who experienced deep venous thrombosis or pulmonary embolism; information about family history of venous thrombosis available for 1171 (76.8%) patients and 1298 (73.8%) controls.
‡Defined as current smoking at index date; information on smoking available for 1418 patients (93.0%) and 1618 controls (91.9%).
Of the 1524 patients, 1103 (72.4%) were using oral contraceptives at the time of thrombosis, compared with 658/1760 (37.4%) of the controls. The percentage of women using oral contraceptives was higher in younger women than in older women (table 1).
Overall, current oral contraceptive use was associated with a fivefold increased risk of venous thrombosis (odds ratio 5.0, 95% CI 4.2 to 5.8). Additional adjustment for smoking and body mass index resulted in a relative risk of 5.4 (95% CI 4.5 to 6.4). Restriction to individuals without a positive family history of venous thrombosis resulted in a relative risk of 5.8 (4.7 to 7.2) for all oral contraceptive users combined versus non-users.
To show the absolute effect of oral contraceptive use among women in different age categories, we estimated the incidence of venous thrombosis in women not using oral contraceptives aged <30 years, 30-40 years, and 40-50 years old. The overall incidence of venous thrombosis per age category (I) can be calculated as I=(p0×I0)+(p1×I1), relative risk=I1/I0, and p1=1−p0, where I1=incidence of venous thrombosis in oral contraceptive users, I0=incidence of venous thrombosis in non-users, p1=prevalence of exposure to oral contraceptives in the general population, and p0=prevalence of non-exposure in the general population.
From the distribution of oral contraceptive use in the control group, we estimate p1 and p0. From our data, we have estimated the relative risk of venous thrombosis associated with current oral contraceptive use by age category. Using this information, together with the overall incidences of venous thrombosis in women by age category reported by Naess et al,30 we were able to estimate the absolute risk of venous thrombosis in women not using oral contraceptives by age category separately (table 2). Both the relative risk and absolute risk of venous thrombosis in women not using oral contraceptives increase with age, which indicates that the absolute risk of venous thrombosis associated with oral contraceptive use increases with age.
Table 2.
Absolute risk of venous thrombosis associated with oral contraceptive use by age category
| Age category | Incidence of venous thrombosis in non-users of oral contraceptives (I0) per 10 000 person-years* | Relative risk (95% CI) of oral contraceptive use† | Incidence of venous thrombosis in oral contraceptive users (I1) per 10 000 person-years‡ |
|---|---|---|---|
| <30 years | 1.2 | 3.1 (2.2 to 4.6) | 3.7 |
| 30-40 years | 2.0 | 5.0 (3.8 to 6.5) | 10.0 |
| 40-50 years | 2.3 | 5.8 (4.6 to 7.3) | 13.3 |
*I0 is based on incidences published by Naess et al.30
†Non-users of oral contraceptives are used as the reference category.
‡I1=I0×relative risk.
Most women used a monophasic oral contraceptive, but triphasic oral contraceptives were also used. Eighty nine patients and 72 controls were using a triphasic oral contraceptive containing levonorgestrel with 30-40 μg ethinylestradiol. The risk of venous thrombosis with this triphasic pill was similar to that with monophasic oral contraceptives that contained levonorgestrel and 30 μg ethinylestradiol (odds ratio 1.0, 95% CI 0.7 to 1.4); triphasic oral contraceptives increased the risk relative to non-users to the same extent as monophasic combined contraceptives. The risk of venous thrombosis was also similar for triphasic and monophasic preparations containing gestodene, although the number of users of a triphasic preparation was small (four patients and five controls).
Progestogen-only oral contraceptives were used by only two patients (lynestrenol) and four controls (desogestrel). No risk estimates can be inferred from these small numbers.
Oestrogen dose and type of progestogen in combined oral contraceptives
Table 3 and the figure show the risk of venous thrombosis associated with combined oral contraceptives containing different types of progestogens.
Table 3.
Risk of venous thrombosis associated with different types of progestogens in combined oral preparations. Data are numbers (percentages) unless stated otherwise
| Type of progestogen | Thrombosis patients (n=1524) | Controls (n=1760) | Odds ratio (95% CI)* |
|---|---|---|---|
| Levonorgestrel† | 485 (31.9) | 373 (21.2) | 3.6 (2.9 to 4.6) |
| Gestodene† | 119 (7.8) | 67 (3.8) | 5.6 (3.7 to 8.4) |
| Desogestrel† | 289 (19.0) | 108 (6.2) | 7.3 (5.3 to 10.0) |
| Lynestrenol† | 44 (2.9) | 19 (1.1) | 5.6 (3.0 to 10.2) |
| Norethisterone | 11 (0.7) | 7 (0.4) | 3.9 (1.4 to 10.6) |
| Cyproterone acetate | 125 (8.2) | 62 (3.5) | 6.8 (4.7 to 10.0) |
| Norgestimate | 9 (0.6) | 4 (0.2) | 5.9 (1.7 to 21.0) |
| Drospirenone | 19 (1.2) | 14 (0.8) | 6.3 (2.9 to 13.7) |
| No oral contraceptive (reference) | 421 (27.7) | 1102 (62.8) | 1 |
*Odds ratio adjusted for age and period of inclusion (categorical; divided per 6 calendar months).
†Analysis restricted to preparation with most commonly used dose of oestrogen: for levonorgestrel, gestodene, and desogestrel, 30 μg (645 patients and 385 controls); for lynestrenol 37.5 μg (42 patients and 19 controls).
Risk of venous thrombosis associated with different types of progestogens in combined oral preparations
Oral contraceptives containing levonorgestrel were the most frequently used oral contraceptives in the control group (57.0% of all users). Among the thrombosis patients the most frequently used oral contraceptives contained either levonorgestrel (44% of all users) or desogestrel (26.2%).
Compared with non-use, use of oral contraceptives was associated with increased risk of venous thrombosis—almost fourfold increase for contraceptives containing levonorgestrel (odds ratio 3.6, 95% CI 2.9 to 4.6), 5.6-fold (95% CI 3.7 to 8.4) for those containing gestodene, 7.3-fold (5.3 to 10.0) for desogestrel, 6.8-fold (4.7 to 10.0) for cyproterone acetate, and 6.3-fold (2.9 to 13.7) for drospirenone.
When we directly compared different types of oral contraceptives with those containing levonorgestrel (the most frequently used progestogen), we found an increased risk of thrombosis associated with those containing gestodene (odds ratio 1.6, 95% CI 1.0 to 2.4), desogestrel (2.0, 1.4 to 2.8), cyproterone acetate (2.0, 1.3 to 3.0), and drospirenone (1.7, 0.7 to 3.9). Among users of third generation oral contraceptives, the thrombotic risk for contraceptives containing desogestrel was mildly increased compared with oral contraceptives containing gestodene (1.3, 0.8 to 2.2).
To assess the risk of venous thrombosis associated with the dose of oestrogen, we restricted the analysis to monophasic preparations with levonorgestrel, gestodene, or desogestrel. With an oestrogen dose of 30 μg as the reference category, the thrombotic risk was 0.8 (95% CI 0.5 to 1.2) for an oestrogen dose of 20 μg and 1.9 (1.1 to 3.4) for a dose of 50 μg.
In table 4 the risk of venous thrombosis associated with different doses of oestrogen are presented by type of progestogen of the most frequently used combined oral contraceptives (monophasic)—that is, levonorgestrel, gestodene, and desogestrel. The oestrogen dose was positively associated with the risk of venous thrombosis for preparations containing desogestrel and those containing gestodene. Using the most commonly used oestrogen dose (30 μg) as the reference category, we found oral contraceptives containing 20 μg estradiol were associated with a decreased risk of thrombosis whereas those containing 50 μg estradiol were associated with an increased risk of thrombosis. For contraceptives containing levonorgestrel, no reduced thrombotic risk was seen for those containing 20 μg ethinylestradiol compared with 30 μg ethinylestradiol, but very few women used an oral contraceptive containing levonorgestrel combined with 20 μg ethinylestradiol, as shown by the wide confidence interval.
Table 4.
The risk of venous thrombosis associated with different doses of ethinylestradiol in monophasic oral contraceptives. Data are odds ratios adjusted for age (95% CI) unless stated otherwise
| Ethinylestradiol dose (μg) | Percentage use among controls* | Levonorgestrel | Gestodene | Desogestrel |
|---|---|---|---|---|
| 20 | 11.2 | 1.1 (0.4 to 3.1) | 0.3 (0.2 to 0.7) | 0.7 (0.4 to 1.2) |
| 30† | 84.4 | 1 | 1 | 1 |
| 50 | 4.4 | 2.2 (1.3 to 3.7) | — | — |
*In total, 51 women used a monophasic preparation with 20 μg ethinylestradiol, 385 women used one with 30 μg, and 20 used one with 50 μg (total 456).
†Reference category is the most commonly used dose of oestrogen among controls.
Risk associated with oral contraceptive use for different types of thrombosis
The risk of venous thrombosis associated with current oral contraceptive use was increased for deep venous thrombosis of the leg and pulmonary embolism (table 5). The relative risk was higher for women with a deep venous thrombosis of the leg (odds ratio 6.6, 95% CI 5.4 to 8.0) than for women with a pulmonary embolism (with or without deep venous thrombosis of the leg) (odds ratio 3.9, 3.2 to 4.8). The risk differences between types of progestogens which we observed for all types of thromboses combined remained essentially the same for both types of thrombosis separately.
Table 5.
Risk of different types of venous thrombosis associated with oral contraceptive use by different type of progestogen. Patients with venous thrombosis of the arm omitted from this analysis
| Type of progestogen | No of controls | Deep venous thrombosis of leg | Pulmonary embolism* | |||
|---|---|---|---|---|---|---|
| No of patients | Odds ratio (95% CI)† | No of patients | Odds ratio (95% CI)† | |||
| All | 654 | 661 | 6.6 (5.4 to 8.0) | 407 | 3.9 (3.2 to 4.8) | |
| Levonorgestrel‡ | 373 | 300 | 5.0 (3.8 to 6.5) | 171 | 2.8 (2.1 to 3.8) | |
| Gestodene‡ | 67 | 74 | 8.1 (5.2 to 12.7) | 43 | 3.8 2.2 to 6.3) | |
| Desogestrel‡ | 108 | 159 | 8.7 (6.1 to 12.4) | 122 | 7.1 (4.9 to 10.4) | |
| Lynestrenol‡ | 19 | 27 | 7.3 (3.7 to 14.2) | 15 | 4.5 (2.1 to 9.6) | |
| Norethisterone | 7 | 7 | 5.4 (1.8 to 16.6) | 4 | 3.1 (0.8 to 11.5) | |
| Cyproterone acetate | 62 | 77 | 9.4 (6.1 to 14.3) | 41 | 5.6 (3.4 to 9.2) | |
| Norgestimate | 4 | 5 | 8.7 (2.1 to 35.5) | 4 | 5.2 (1.1 to 23.7) | |
| Drospirenone | 14 | 12 | 9.1 (3.9 to 21.5) | 7 | 6.2 (2.2 to 17.6) | |
| No oral contraceptive (reference) | 1102 | 197 | 1 | 198 | 1 | |
*With or without deep venous thrombosis.
†Odds ratio per type of progestogen adjusted for age and period of inclusion (categorical; divided per 6 calendar months).
‡Analysis restricted to preparation with most commonly used dose of oestrogen. For levonorgestrel, gestodene, and desogestrel, this was 30 μg (388 patients with deep venous thrombosis of the leg, 242 patients with pulmonary embolism, and 385 controls). For lynestrenol, this was 37.5 μg (25 patients with deep venous thrombosis of the leg, 15 with pulmonary embolism, and 19 controls).
Current oral contraceptive use was also associated with a twofold increased risk of deep venous thrombosis of the arm (odds ratio 1.9, 95% CI 1.1 to 3.4), but the number of patients with this thrombosis was too small to estimate the risk of venous thrombosis associated with different types of progestogens.
Duration of oral contraceptive use
For 1005 patients with venous thrombosis and 533 control subjects information on the duration of oral contraceptive use on the index date was available. The risk of venous thrombosis was clearly highest during the first three months of use (odds ratio 12.6, 95% CI 7.1 to 22.4) (table 6). After one year, the risk of venous thrombosis for oral contraceptive users compared with non-users decreased to the overall estimate of a fivefold increased risk. In all time intervals, including those of prolonged use, both second and third generation oral contraceptives were used. When we restricted the analysis to women who were using oral contraceptives for more than two years, the thrombotic risk with oral contraceptives containing desogestrel remained increased compared with those containing levonorgestrel and was similar to the overall risk—for gestodene odds ratio 1.5 (95% CI 0.9 to 2.6) and for desogestrel 1.9 (1.3 to 2.9).
Table 6.
Risk of venous thrombosis associated with duration of use of oral contraceptive. Data are number
| Duration of use (months)* | No of thrombosis patients | No of controls | Odds ratio (95% CI)† | |||
|---|---|---|---|---|---|---|
| Total | Using levonorgestrel | Using gestodene | Using desogestrel | |||
| ≤3 | 66 | 32 | 8 | 10 | 15 | 12.6 (7.1 to 22.4) |
| >3 and ≤6 | 49 | 19 | 7 | 9 | 17 | 8.3 (4.7 to 14.5) |
| >6 and ≤12 | 63 | 31 | 4 | 9 | 26 | 7.5 (4.7 to 12.2) |
| >12 and ≤24 | 75 | 35 | 5 | 17 | 46 | 5.0 (3.4 to 7.4) |
| >24 and ≤60 | 141 | 77 | 15 | 31 | 87 | 5.0 (3.7 to 6.8) |
| >60 | 611 | 251 | 68 | 179 | 342 | 5.2 (4.3 to 6.2) |
| No oral contraceptive (reference) | 421 | — | — | — | 1102 | 1 |
*Duration of oral contraceptive use defined as time since start date of last oral contraceptive used before index date for women who used oral contraceptives at the time of the index date. For 221 users (98 patients and 125 controls) duration of use was unknown.
†Odds ratio adjusted for age.
For the newest types of oral contraceptives, we also assessed the risk of thrombosis restricted to short term users, to account for attrition of susceptibles (women at increased risk of getting deep venous thrombosis). The number of short term users for an individual type of progestogen was low, resulting in wide confidence intervals. However, for women using oral contraceptives for three months or less, the results support an increased risk for both drospirenone (odds ratio 1.9, 95% CI 0.2 to 21.3) and cyproterone acetate (1.6, 0.3 to 9.9) compared with oral contraceptives containing levonorgestrel.
Discussion
Principal findings of the study
We found that currently available oral contraceptives were associated with a fivefold increased risk of venous thrombosis. This result confirms the results of previous studies reporting a twofold to sixfold increased risk of deep venous thrombosis associated with oral contraceptive use.2 3 4 5 Several older studies report a slightly lower risk, which is probably because nowadays different types of oral contraceptives containing newer progestogens with higher risks are being used. The risk clearly differed by type of progestogen and dose of oestrogen. Oral contraceptives containing desogestrel were associated with a twofold increased risk of venous thrombosis compared with oral contraceptives containing levonorgestrel, which persisted among women who had used these oral contraceptives for many years.
The risk of thrombosis associated with oral contraceptives containing cyproterone acetate or drospirenone was similar to that associated with oral contraceptives containing desogestrel—a sixfold to sevenfold increased risk compared with non-users.
Comparison with other studies
In contrast to our findings, a large prospective cohort study found an equal risk of thrombosis associated with oral contraceptives containing drospirenone or levonorgestrel.28 However, our results of an excess risk for drospirenone are supported by previous data from laboratory studies in healthy users, where an excess in thrombin generation (endogenous thrombin potential) was found for oral contraceptives containing desogestrel, cyproterone acetate, and drospirenone that exceeded the effect of oral contraceptives containing levonorgestrel.31 Previous studies have also shown that this global test predicts risk of thrombosis, even in women not using the pill and in men.32
The size of our study allowed separate risk estimation for both desogestrel and gestodene, whereas most previous studies either included only one type or grouped these two progestogens together. The risk of deep venous thrombosis with oral contraceptives containing desogestrel was slightly higher than that with oral contraceptives containing gestodene (odds ratio 1.3, 95% CI 0.8 to 2.2). This confirmed the findings of a previous case-control study of oral contraceptives containing gestodene or desogestrel.24 Furthermore, oral contraceptives containing desogestrel have been associated with a more pronounced activated protein C resistance and higher levels of sex hormone binding globulin than those containing gestodene, which are likely to indicate thrombotic risk.33 Results of a crossover study in healthy users, including 33 women, showed different effects on the haemostatic system for third and second generation oral contraceptives.34 35 Oral contraceptives containing desogestrel were associated with more pronounced changes in both the procoagulation and anticoagulation systems compared with oral contraceptives containing levonorgestrel and activated protein C resistance (in both the activated partial thromboplastin time as well as the endogenous thrombin potential test) was more pronounced.34 35 36
We found the risk of venous thrombosis was positively associated with oestrogen dose. Lidegaard et al also showed a positive association between oestrogen dose and the risk of venous thrombosis for oral contraceptives with oestrogen doses lower than 50 μg.24 The same trend was shown earlier for oral contraceptives with higher doses of oestrogen (50-100 μg).6 7 8
Strengths and limitations of this study
This study was performed more than a decade after the introduction of oral contraceptives containing desogestrel or gestodene in the Netherlands, and data were collected 10 years after those that led to our first report on the increased thrombogeneity of third generation progestogens. Some women have used oral contraceptives containing desogestrel or gestodene for a considerable time. The large study size allowed us to study the duration of oral contraceptive use in detail. Clearly the risk of thrombosis was highest in the first year of oral contraceptive use, with a peak in the first three months of use (odds ratio 12.6, 95% CI 7.1 to 22.4). This high risk of venous thrombosis during the first months of oral contraceptive use was not the result of differences in the type of oral contraceptives, nor was the high risk conferred by third generation oral contraceptives or oral contraceptives containing cyproterone acetate the result of recent use of particular brands (“attrition of susceptibles”). We confirmed the latter in an analysis limited to women who had used oral contraceptives for more than two years. These results clearly indicate that “recent introduction” bias cannot have affected our results. Oral contraceptives containing drospirenone have been available in the Netherlands since 2002. Here we countered potential “recent introduction” bias by restricting the analysis to short term users, and we still found a similar excess risk in comparison with short term use of oral contraceptives containing levonorgestrel (1.6-fold increased risk for drospirenone).
The relative risk associated with oral contraceptive use was higher for deep venous thrombosis of the leg than for pulmonary embolism. Oral contraceptive use was also associated with a twofold increased risk of a deep venous thrombosis of the arm, as previously reported from this study.37
It could be argued that recall bias may have occurred in our study. However, patients fill in the questionnaire within a few weeks after the thrombotic event. For controls, current use of oral contraceptives is filled in on the questionnaire. The short time between thrombosis and filling in the questionnaire, as well as the fact that the questionnaire is sent to the participants’ home, where the package of the oral contraceptive is readily available, makes the occurrence of recall bias very unlikely.
Conclusions and policy implications
All currently used oral contraceptives are equally effective in preventing pregnancy. However, especially the preparations containing cyproterone acetate or drospirenone also have other indications, such as treatment of acne vulgaris, seborrhoea, or mild hirsutism. A recent Cochrane review assessed the effect of different combined oral contraceptives for the treatment of acne.38 It concluded that only minor differences were found in the effectiveness of preparations containing cyproterone acetate, desogestrel, or levonorgestrel in the treatment of acne. Also with regard to weight gain while using oral contraceptives, no major differences were found between preparations containing drospirenone or levonorgestrel.39 The effectiveness of alleviation of premenstrual symptoms by oral contraceptives containing drospirenone compared with placebo or other oral contraceptives has not been demonstrated.40
Thus, available evidence suggests that, even for acne or weight gain, there is no clear difference between most commonly used oral contraceptives. This indicates that the choice of oral contraceptive should be based on the smallest increase of side effects, such as risk of venous thrombosis. It is estimated that 100 million women use an oral contraceptive worldwide.41 With such a large number of women using oral contraceptives, even the smallest increase of side effects will affect many. Knowledge of these risks and efforts to reduce them are of crucial importance. Our results clearly show that the safest option with regard to the risk of venous thrombosis is an oral contraceptive containing levonorgestrel combined with a low dose of oestrogen.
What is already known on this topic
Current oral contraceptive use is associated with a twofold to sixfold increased risk of venous thrombosis
Limited information is available on the risk associated with the newest type of oral contraceptives containing drospirenone
What this study adds
The venous thrombotic risk clearly differed by type of progestogen and was positively associated with dose of oestrogen
The risk of thrombosis associated with oral contraceptives containing cyproterone acetate or drospirenone was similar to that associated with oral contraceptives containing desogestrel; a sixfold to sevenfold increased risk compared with non-users
The safest option with regard to the risk of venous thrombosis is an oral contraceptive containing levonorgestrel combined with a low dose of oestrogen
We thank the directors of the Anticoagulation Clinics of Amersfoort (MHH Kramer), Amsterdam (M Remkes), Leiden (FJM van der Meer), The Hague (E van Meegen), Rotterdam (AAH Kasbergen), and Utrecht (J de Vries-Goldschmeding) who made the recruitment of patients possible. The interviewers (JCM van den Berg, B Berbee, S van der Leden, M Roosen, and EC Willems of Brilman) performed the blood draws. We also thank I de Jonge, R Roelofsen, M Streevelaar, LMJ Timmers, and JJ Schreijer for their administrative support and data management. The fellows ID Bezemer, JW Blom, ER Pomp, KJ van Stralen, LW Tick took part in every step of the data collection. We express our gratitude to all individuals who participated in the MEGA study.
Funding: This research was supported by the Netherlands Heart Foundation (NHS 98.113), the Dutch Cancer Foundation (RUL 99/1992) and the Netherlands Organisation for Scientific Research (912-03-033| 2003). The funding organisations did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Competing interests: None declared
Ethical approval: All participants gave written informed consent. The study was approved by the Medical Ethics Committee of the Leiden University Medical Center, Leiden, the Netherlands.
Cite this as: BMJ 2009;339:b2921
References
- 1.Jordan WM. Pulmonary embolism. Lancet 1961;1146-7.
- 2.Vandenbroucke JP, Koster T, Briët E, Reitsma PH, Bertina RM, Rosendaal FR. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet 1994;344:1453-7. [DOI] [PubMed] [Google Scholar]
- 3.Thorogood M, Mann J, Murphy M, Vessey M. Risk factors for fatal venous thromboembolism in young women: a case-control study. Int J Epidemiol 1992;21:48-52. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1995;346:1575-82. [PubMed] [Google Scholar]
- 5.Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997;349:83-8. [DOI] [PubMed] [Google Scholar]
- 6.Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. BMJ 1986;292:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995;346:1593-6. [DOI] [PubMed] [Google Scholar]
- 8.Gerstman BB, Piper JM, Tomita DK, Ferguson WJ, Stadel BV, Lundin FE. Oral contraceptive estrogen dose and the risk of deep venous thromboembolic disease. Am J Epidemiol 1991;133:32-7. [DOI] [PubMed] [Google Scholar]
- 9.Van Vloten WA, van Haselen CW, van Zuuren EJ, Gerlinger C, Heithecker R. The effect of 2 combined oral contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis 2002;69:2-15. [PubMed] [Google Scholar]
- 10.Kuhnz W, Staks T, Jutting G. Pharmacokinetics of cyproterone acetate and ethinylestradiol in 15 women who received a combination oral contraceptive during three treatment cycles. Contraception 1993;48:557-75. [DOI] [PubMed] [Google Scholar]
- 11.Lachnit-Fixson U. The development and evaluation of an ovulation inhibitor (DIAne) containing an antiandrogen. Acta Obstet Gynecol Scand Suppl 1979;88:33-42. [DOI] [PubMed] [Google Scholar]
- 12.Muhn P, Fuhrmann U, Fritzemeier KH, Krattenmacher R, Schillinger E. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci 1995;761:311-35. [DOI] [PubMed] [Google Scholar]
- 13.Oelkers W, Foidart JM, Dombrovicz N, Welter A, Heithecker R. Effects of a new oral contraceptive containing an antimineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clin Endocrinol Metab 1995;80:1816-21. [DOI] [PubMed] [Google Scholar]
- 14.Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen components. Lancet 1995;346:1589-93. [DOI] [PubMed] [Google Scholar]
- 15.Lidegaard O, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism. A case-control study. Contraception 1998;57:291-301. [DOI] [PubMed] [Google Scholar]
- 16.Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ 2001;323:131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP, Helmerhorst FM, Bloemenkamp KW, Rosendaal FR. Third-generation oral contraceptive and deep venous thrombosis: from epidemiologic controversy to new insight in coagulation. Am J Obstet Gynecol 1997;177:887-91. [DOI] [PubMed] [Google Scholar]
- 18.Walker AM. Newer oral contraceptives and the risk of venous thromboembolism. Contraception 1998;57:169-81. [DOI] [PubMed] [Google Scholar]
- 19.Bloemenkamp KW, Rosendaal FR, Büller HR, Helmerhorst FM, Colly LP, Vandenbroucke JP. Risk of venous thrombosis with use of current low-dose oral contraceptives is not explained by diagnostic suspicion and referral bias. Arch Intern Med 1999;159:65-70. [DOI] [PubMed] [Google Scholar]
- 20.Farley TM, Meirik O, Collins J. Cardiovascular disease and combined oral contraceptives: reviewing the evidence and balancing the risks. Hum Reprod Update 1999;5:721-35. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1995;346:1582-8. [PubMed] [Google Scholar]
- 22.Vasilakis-Scaramozza C, Jick H. Risk of venous thromboembolism with cyproterone or levonorgestrel contraceptives. Lancet 2001;358:1427-9. [DOI] [PubMed] [Google Scholar]
- 23.Parkin L, Skegg DC, Wilson M, Herbison GP, Paul C. Oral contraceptives and fatal pulmonary embolism. Lancet 2000;355:2133-4. [DOI] [PubMed] [Google Scholar]
- 24.Lidegaard O, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception 2002;65:187-96. [DOI] [PubMed] [Google Scholar]
- 25.Seaman HE, de Vries CS, Farmer RD. Venous thromboembolism associated with cyproterone acetate in combination with ethinyloestradiol (Dianette): observational studies using the UK General Practice Research Database. Pharmacoepidemiol Drug Saf 2004;13:427-36. [DOI] [PubMed] [Google Scholar]
- 26.Van Grootheest K, Vrieling T. Thromboembolism associated with the new contraceptive Yasmin. BMJ 2003;326:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheldon T. Dutch GPs warned against new contraceptive pill. BMJ 2002;324:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinger JC, Heinemann LA, Kuhl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007;75:344-54. [DOI] [PubMed] [Google Scholar]
- 29.Seeger JD, Loughlin J, Eng PM, Clifford CR, Cutone J, Walker AM. Risk of thromboembolism in women taking ethinylestradiol/drospirenone and other oral contraceptives. Obstet Gynecol 2007;110:587-93. [DOI] [PubMed] [Google Scholar]
- 30.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5:692-9. [DOI] [PubMed] [Google Scholar]
- 31.Van Vliet HA, Winkel TA, Noort I, Rosing J, Rosendaal FR. Prothrombotic changes in users of combined oral contraceptives containing drospirenone and cyproterone acetate. J Thromb Haemost 2004;2:2060-2. [DOI] [PubMed] [Google Scholar]
- 32.Tans G, van Hylckama Vlieg A, Thomassen MCLGD, Curvers J, Bertina RM, Rosing J, et al. Activated protein C resistance determined with a thrombin generation-based test predicts for venous thrombosis in men and women. Br J Haematol 2003;122:465-70. [DOI] [PubMed] [Google Scholar]
- 33.van Vliet HA, Frolich M, Christella M, Thomassen MCLGD, Doggen CJM, Rosendaal FR, et al. Association between sex hormone-binding globulin levels and activated protein C resistance in explaining the risk of thrombosis in users of oral contraceptives containing different progestogens. Hum Reprod 2005;20:563-8. [DOI] [PubMed] [Google Scholar]
- 34.Tans G, Curvers J, Middeldorp S, Thomassen MCLGD, Meijers JC, Prins MH, et al. A randomized cross-over study on the effects of levonorgestrel- and desogestrel-containing oral contraceptives on the anticoagulant pathways. Thromb Haemost 2000;84:15-21. [PubMed] [Google Scholar]
- 35.Middeldorp S, Meijers JC, van den Ende AE, van Enk A, Bouma BN, Tans G, et al. Effects on coagulation of levonorgestrel- and desogestrel-containing low dose oral contraceptives: a cross-over study. Thromb Haemost 2000;84:4-8. [PubMed] [Google Scholar]
- 36.Kemmeren JM, Algra A, Meijers JC, Bouma BN, Grobbee DE. Effects of second and third generation oral contraceptives and their respective progestagens on the coagulation system in the absence or presence of the factor V Leiden mutation. Thromb Haemost 2002;87:199-205. [PubMed] [Google Scholar]
- 37.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Old and new risk factors for upper extremity deep venous thrombosis. J Thromb Haemost 2005;3:2471-8. [DOI] [PubMed] [Google Scholar]
- 38.Arowojolu AO, Gallo MF, Lopez LM, Grimes DA, Garner SE. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev 2007;(3):CD004425. [DOI] [PubMed] [Google Scholar]
- 39.Gallo MF, Lopez LM, Grimes DA, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev 2008;(4):CD003987. [DOI] [PubMed] [Google Scholar]
- 40.Lopez LM, Kaptein A, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev 2009;(2):CD006586. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Cardiovascular disease and steroid hormone contraception. Report of a WHO Technical Report Series 877. Geneva: WHO, 1998. [PubMed]