Abstract
Objective
Paired-associative stimulation (PAS) is a transcranial magnetic stimulation technique inducing Hebbian-like synaptic plasticity in the human motor cortex (M1). PAS is produced by repetitive pairing of a peripheral nerve shock and a transcranial magnetic stimulus (TMS). Its effect is assessed by a change in size of a motor evoked response (MEP). MEP size results from excitatory and inhibitory influences exerted on cortical pyramidal cells, but no robust effects on inhibitory networks have been demonstrated so far.
Method
In 38 healthy volunteers, we assessed whether a PAS intervention influences three intracortical inhibitory circuits: short (SICI) and long (LICI) intracortical inhibitions reflecting activity of GABAA and GABAB interneurons respectively, and long afferent inhibition (LAI) reflecting activity of somatosensory inputs.
Results
After PAS, MEP sizes, LICI and LAI levels were significantly changed while changes of SICI were inconsistent. The changes in LICI and LAI lasted 45 minutes after PAS. Their direction depended on the delay between the arrival time of the afferent volley at the cortex and the TMS-induced cortical activation during the PAS.
Conclusions
PAS influences inhibitory circuits in M1.
Significance
PAS paradigms can demonstrate Hebbian-like plasticity at selected inhibitory networks as well as excitatory networks.
Keywords: plasticity, transcranial magnetic stimulation, GABAergic inhibitory interneurones, motor cortex, sensorimotor coupling
Introduction
From animal studies it is known that intracortical inhibitory circuits are involved in cortical plasticity in two different ways. (i) In vitro studies have demonstrated that decrease of local inhibitory activity accompanies and promotes the development of long-term potentiation (LTP) (Stelzer and Shi, 1994; Castro-Alamancos et al., 1995) synaptic remodeling and cortical receptive field expansion (Chowdhury and Rasmusson, 2002). (ii) Enduring changes in synaptic efficacy have been observed not only at excitatory synapses, but also at inhibitory ones (Woodin et al., 2003). In humans there is indirect evidence that a decrease of local GABAAergic inhibition in the motor cortex enhances dramatically the excitability in the intracortical circuitry during motor practice (Ziemann et al., 2001) while blockade of GABAB inhibition prevents the development of a cortical plasticity artificially induced by TMS (McDonnell et al., 2007). These results fit with the former aspect of involvement of cortical inhibition in plasticity. In this paper we do not address the role of a decrease of local inhibition in development of plasticity, we focus on the development of plasticity at the level of inhibitory synapses during artificial induction of plasticity. Various transcranial magnetic stimulation (TMS) techniques can be used to induce non-invasively “artificial” cortical plastic changes. Here we used the paired associative stimulation (PAS) technique, which may represent associative LTP - or LTD-like plasticity at a cell population level (Stefan et al., 2000; Wolters et al., 2003). PAS has not been shown so far to be accompanied by lasting changes of short-interval intracortical inhibition (SICI) involving GABAA receptors or of afferent inhibition (Stefan et al., 2002; Quartarone et al., 2003; Rosenkranz and Rothwell, 2006). Yet according to the prolongation of the silent period (SP, thought to involve GABAB inhibition) after PAS (Stefan et al., 2000; Quartarone et al., 2003), implication of GABAB inhibition in PAS-induced after effects has been suggested. This has to be confirmed as SP is a complex parameter involving spinal as well as cortical mechanisms (Fuhr et al., 1991) and evidence for a contribution of GABAB receptor activation to the SP is weak and controversial (Paulus et al., 2008).
We investigated the aftereffects of a PAS intervention on the excitability of several intracortical inhibitory circuits: those involving GABAA (SICI) and GABAB (LICI) synapses and also those fed by peripheral sensory inputs. Sensory stimulation can change motor cortex excitability. Inhibition of the MEP by peripheral stimulation has been called “long afferent inhibition” (LAI) when the delay between peripheral and TMS stimulation is from 100 to 1000 ms (Chen et al., 1999b; Abbruzzese et al., 2001; Paulus et al., 2008). Transmitters and pathways involved in LAI are unknown.
Methods
Subjects
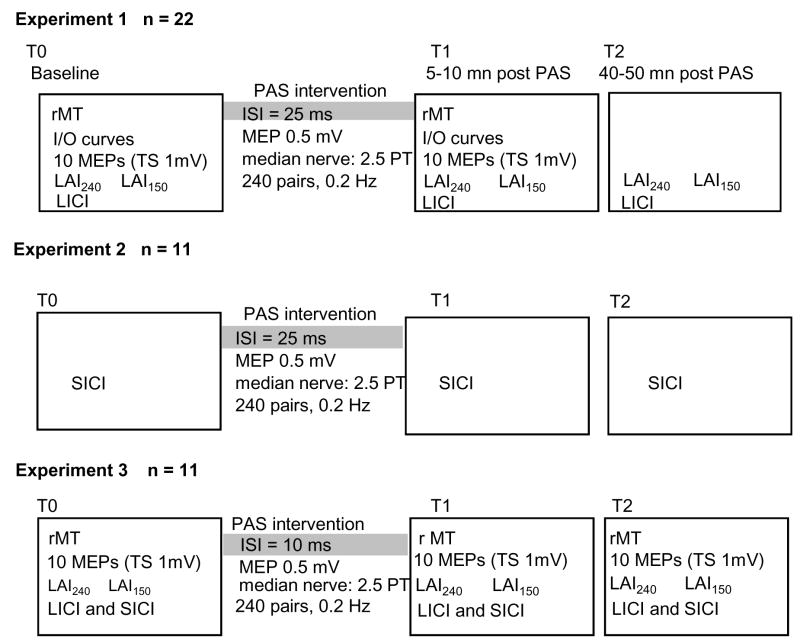
Experiments were performed on 38 healthy volunteers (19 men, 19 women) aged 19 – 67 years (mean ± SEM, 35.5 ± 6.1 years) with no history of either neurological or psychiatric disease and a normal neurological examination. Results of 3 subjects were discarded from analysis because their MEPs were highly variable due to sleepiness. The study included 3 different experiments, and each experiment included several measures. The number of subjects used in each experiment and the number used for calculating the mean value of each measure are indicated in Fig. 1 and Tables 1 – 2, respectively, as all measures were not obtained in all subjects. The experimental protocol was approved by the NINDS Institutional Review Board, and all subjects gave written informed consent. All subjects were right-handed according to the Oldfield handedness inventory (Oldfield, 1971).
Figure 1.
Experimental designs of the 3 experiments performed.
Table 1.
Mean group data (± SEM) of LICI, SICI and LAI before and after excitatory PAS (PAS25)
| EFFECT OF PAS ON LICI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FPB muscle | ADM muscle | ||||||||||
| n = 16 | LICI | post hoc | n = 16 | LICI | post hoc | ||||||
| T0 | rANOVA | F = 4.8 | p<0.01 | 42.3 ± 4 | p<0.02 | p<0.01 | T0 | rANOVA | p ns | 63.5 ± 8 | |
| T1 | 64.4 ± 7 | T1 | 77.2 ± 9 | ||||||||
| T2 | 68 ± 8 | T2 | 65.6 ± 18 | ||||||||
| EFFECT OF PAS ON SICI | |||||||||||
| FPB muscle | ADM muscle | ||||||||||
| n = 11 | SICI | post hoc | n = 8 | SICI | post hoc | ||||||
| T0 | rANOVA | p NS | 47.1 ± 4 | T0 | rANOVA | p ns | 49.3 ± 6 | ||||
| T1 | 41.1 ± 5 | T1 | 51.1 ± 11 | ||||||||
| T2 | 52.8 ± 9 | T2 | 41.3 ± 5 | ||||||||
| EFFECT OF PAS ON LAI150 | |||||||||||
| FPB muscle | ADM muscle | ||||||||||
| n = 16 | LAI150 | post hoc | n = 16 | LAI150 | post hoc | ||||||
| T0 | rANOVA | F = 9.4 | p<0.0007 | 49.7 ± 4 | p< 0.001 | p<0.0005 | T0 | rANOVA | p ns | 72 ± 10 | |
| T1 | 73.1 ± 8 | T1 | 89.7 ± 10 | ||||||||
| T2 | 74.8 ± 7 | T2 | 82.5 ± 9 | ||||||||
| EFFECT OF PAS ON LAI240 | |||||||||||
| FPB muscle | ADM muscle | ||||||||||
| n = 14 | LAI240 | post hoc | n = 11 | LAI240 | post hoc | ||||||
| T0 | rANOVA | F = 4.9 | p<0.02 | 65 ± 6 | p<0.05 | p< 0.005 | T0 | rANOVA | p ns | 87 ± 24 | |
| T1 | 53 ± 6 | T1 | 75.5 ± 11 | ||||||||
| T2 | 47.7 ± 6 | T2 | 73.8 ± 12 | ||||||||
Table 2.
Mean group data (± SEM) of LICI, SICI and LAI before and after inhibitory PAS (PAS10)
| EFFECT OF PAS ON LICI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FPB muscle | ADM muscle | |||||||||||
| n = 10 | LICI | post hoc | n = 10 | LICI | post hoc | |||||||
| T0 | rANOVA | F = 4 | p<0.04 | 65.1 ± 4 | p<0.01 | T0 | rANOVA | p ns | 87.7 ± 9 | |||
| T1 | 51 ± 10 | T1 | 81.7 ± 12 | |||||||||
| T2 | 46 ± 9 | T2 | 72 ± 12 | |||||||||
| EFFECT OF PAS ON SICI | ||||||||||||
| FPB muscle | ADM muscle | |||||||||||
| n = 12 | SICI | post hoc | n = 12 | SICI | post hoc | |||||||
| T0 | rANOVA | F = 3.6 | p<0.05 | 67.8 ± 4 | p<0.01 | T0 | rANOVA | p ns | 77.4 ± 6 | |||
| T1 | 87.2 ± 7 | T1 | 85.2 ± 10 | |||||||||
| T2 | 76.7 ± 6 | T2 | 84 ± 7 | |||||||||
| EFFECT OF PAS ON LAI150 | ||||||||||||
| FPB muscle | ADM muscle | |||||||||||
| n = 8 | LAI150 | post hoc | n = 8 | LAI150 | post hoc | |||||||
| T0 | rANOVA | p ns | 66.3 ± 8 | T0 | rANOVA | p ns | 70.6 ± 7 | |||||
| T1 | 58.4 ± 9 | T1 | 78.9 ± 12 | |||||||||
| T2 | 53.1 ± 7 | T2 | 72.1 ± 8 | |||||||||
| EFFECT OF PAS ON LAI240 | ||||||||||||
| FPB muscle | ADM muscle | |||||||||||
| n = 10 | LAI240 | post hoc | n = 10 | LAI240 | post hoc | |||||||
| T0 | rANOVA | F = 3.6 | p<0.05 | 60.5 ± 9 | p<0.02 | T0 | rANOVA | F = 3.9 | p<0.04 | 77.5 ± 9 | ||
| T1 | 86.1 ± 13 | T1 | 92.6 ± 10 | |||||||||
| T2 | 77.1 ± 12 | T2 | 86.3 ± 10 | |||||||||
EMG recording
Surface EMG activity (band-pass 10 Hz-2 kHz) was recorded from the right flexor pollicis brevis (FPB) – the target muscle- and the abductor digiti minimi (ADM) muscle, in bipolar belly-tendon arrangements, using a Nicolet Viking electromyograph (Skovlunde, Denmark). Signals were fed into an IBM compatible personal computer (486 DX) with a data acquisition system built with the Labview graphical programming language (sampling rate 5 kHz) (Kaelin-Lang and Cohen, 2000) for further off-line analysis. During the experiments, EMG activity was continuously monitored with visual and auditory feedback to ensure complete relaxation.
Transcranial magnetic stimulation
Subjects were seated in a comfortable reclining chair. A figure-of-eight shaped coil (7 cm inner diameter for each half) connected to a Bistim-module and two Magstim 200 magnetic stimulators (The Magstim Company, Dyfed, UK) was positioned on the scalp over the left M1. The hot spot for the right FPB muscle was defined as the lowest threshold site evoking a MEP response in FPB accompanied by a clear thumb flexion movement. The coil was positioned with the handle pointing backwards at an angle of 45° to the midline (Brasil-Neto et al., 1992). The hot spot was marked with a pen on the cap worn by the subject; this served as visual reference against which the coil was positioned and maintained by the experimenter.
Resting motor threshold (rMT)
The resting motor threshold (rMT) was defined as the minimum TMS intensity (measured by altering the stimulator output intensity in 1% decrements) required to elicit at least five FPB MEPs > 50 μV in 10 consecutive trials (Rossini et al., 1994; Rothwell et al., 1999). TMS stimulus intensities were then expressed as percentage of the right FPB rMT.
Input-output (I-O) curves
With the coil at the hot spot, 7 responses were recorded and averaged at each of a range of intensities. Intensity of stimulation started at the rMT and was increased by steps of 10% × rMT until the MEP size reached a plateau value. Each I-O curve was characterized by 3 parameters: (i) “slope”: the slope of a regression line fitted to the steepest part of I-O curve; (ii) “calculated” resting motor threshold (cMT), the intercept of the regression line with the × axis, and (iii) the plateau value (MEP max).
Long interval intracortical inhibition (LICI)
To evoke LICI a suprathreshold conditioning TMS stimulation (CS90) was delivered 90 ms before a test TMS stimulation (TS) (Valls-Sole et al., 1992; Nakamura et al., 1997; Chen et al., 1999a). We first created an intensity curve for LICI using CS90 intensities of 0.9, 1, 1.1, 1.2 and 1.3 × rMT while keeping the TS at 1.2 × rMT. During experiments TS intensity was adjusted to 1.2 × rMT. CS90 intensity was adjusted in each subject according to the individual intensity curve at the lowest intensity evoking an inhibition. Such a low intensity was chosen to avoid any ceiling effect.
Short interval intracortical inhibition (SICI)
To evoke SICI, a subthreshold conditioning TMS stimulation (CS2.5) was delivered 2.5 ms before a test TMS stimulation (TS) (Fisher et al., 2002). We first created an intensity curve for SICI by using CS2.5 intensities 0.5, 0.6 and 0.7 and 0.8 × rMT (Orth et al., 2003; Stinear and Byblow, 2004), while keeping the TS at 1.2 × rMT. During experiments, the intensity of the TS was adjusted to 1.2 × rMT and intensity of the CS2.5 was adjusted in each subject according to the intensity curve at the lowest intensity evoking an inhibition.
Long afferent inhibition (LAI)
Time course of LAI
The effect of a conditioning stimulation to the right median nerve on FPB and ADM MEPs was tested using 5 different interstimulus intervals (ISIs): 50, 100, 150, 200 and 240 ms. The median nerve was stimulated at wrist through bipolar surface electrodes (cathode proximal, bipolar stimulation, rectangular pulses of 0.2 ms duration) using a Grass S88 stimulator (Grass Instruments Co., Quincy, Mass., USA). Intensity was adjusted to 2.5 × the perceptual threshold (PT) (mean: 4.2 ± 1.12 mA) always below the thumb twitch threshold. TMS intensity was adjusted to 1.2 ×rMT. LAI was calculated as the ratio of the conditioned (with preceding median nerve stimulation) to the test (TS alone) MEPs and expressed as a percentage (± SEM) of the test MEP.
During the main experiments LAI was tested at only two ISIs 150 ms (LAI150) and 240 ms (LAI240), because inhibition peaked at these ISIs in the preliminary time-course experiment Median nerve stimulation and TS were adjusted as described above.
PAS intervention
Paired associative stimulation (PAS) was achieved by pairing an electrical stimulus to the right median nerve stimulation at wrist (2.5 × PT: 5.1 ± 1.67 mA, always below thumb twitch threshold), and a single TMS pulse targeting the right FPB muscle. We adjusted the TMS intensity to evoke a FPB MEP with a peak-to-peak amplitude around 0.5 mV. The original PAS technique (Stefan et al., 2000; Wolters et al., 2003) induces spike-time dependent plasticity (STD) - like effects, as direction of change in amplitude of test MEPs depends on the ISI between peripheral and TMS stimulations. Here the ISI between median nerve and TMS stimulation was set either at 25 ms or at 10 ms, because these ISIs have been shown to be optimal for inducing sustained increase/decrease in motor cortex excitability (Stefan et al., 2000; Wolters et al., 2003). Two hundred and forty pairs of stimuli were delivered at 0.2 Hz over 20 min (Muller et al., 2007). These interventions are referred in the following as PAS25 or PAS10. We asked the subjects to count the stimulations in order to maintain attention.
Effect of PAS on overall corticospinal excitability
A screening tool for the efficacy of PAS previously described in the literature is the increased amplitude of a 1 mV test MEP after PAS (Wolters et al., 2003; Stefan et al., 2004). To compare our results with literature data, we compared the mean size of 10 MEPs before and after PAS using a test MEP before PAS adjusted to 0.8 – 1 mV.
Study designs (Fig. 1)
The study designs of the 3 different experiments performed and the number of subjects are illustrated in Fig 1. Experiment 1 was designed to study PAS25-induced modulation of LAI and LICI, experiment 2 to study PAS25-induced modulation of SICI and experiment 3 to verify whether PAS-induced changes of LICI, SICI and LAI followed the rules of STDP-like plasticity. Measurements were done at baseline (T0), shortly after the PAS (T1, 5 to 10 minutes after the end of PAS) and forty to fifty minutes after the end of the PAS (T2). To measure LICI, SICI and LAI150 or LAI240, 10 test (TS) and 10 test + conditioning (CS) stimulations were randomly alternated. Intensity of TS was adjusted at T0 at 1.2 × rMT. Since the amount of LAI, SICI and LICI depends on the size of the test MEP (Sanger et al., 2001), the intensity of TS at T1 and T2 was adjusted in order that the test MEP had the same size as at T0 (0.8–1 mV).
Statistical analysis
The intensities used (CS90, CS2.5) and the different measures (LICI, SICI, LAI) done at T0 were compared between the 2 groups (the group tested for PAS25 or PAS10 respectively) using unpaired t tests.
I-O curves: values of rMT, cMT, slope and the plateau value were compared between T0 and T1 using paired t-tests. RMT and cMT were expressed in percentage of the stimulator output and the plateau values in mV. To calculate the slopes of the I-O curves measures of MEPs were normalized to the plateau value at T0 and intensities were expressed as a percentage of the rMT at T0.
Time course of LAI: conditioned and unconditioned MEP values were compared using paired t- tests at each of the tested ISIs (50, 100, 150, 200 and 240 ms).
The effects of PAS on LAI150, LAI240, LICI and SICI were evaluated by separate repeated-ANOVA (Tables 1 and 2) with the values of the measures at T0, T1 and T2 forming the repeats. Conditional on a significant F-value, post hoc paired samples t-tests (Fisher’s PLSD) were performed to explore the strength of main effects.
To compare the effects of the 2 interventions: PAS25 and PAS10 over time in the target muscle FPB versus the non-target muscle ADM, inhibitions after PAS were expressed as a percentage of the inhibitions at T0 (inhT1/inhT0 and inhT2/inhT0). Subsequently, we performed a repeated ANOVA with “TIME” (inhT1/inhT0 and inhT2/inhT0) as within-subject factor and “INTERVENTION” (PAS25 or PAS10) and “MUSCLE “(FPB or ADM) as between-subject factors. Conditional on a significant F-value, post hoc unpaired t-tests were performed to explore each muscle and each time.
To look for a relationship between PAS-induced changes of MEP size and LICI or LAI changes we used linear regression analysis. PAS-induced changes of MEPs or inhibitions were expressed as a percentage of their values at T0 (see above).
A p-value of < 0.05 was considered significant. All data are given as means ± SEM. Statistical analysis was performed with Statview 5.1 software.
Results
For clarity we do not present results of the 3 experiments separately but we present together results of “excitatory” (PAS25) and “inhibitory” (PAS10) PAS for each tested measure: rMT, I-O curves, LICI, SICI, LAI.
Subjects did not report any adverse side effects during the study.
Motor Threshold (rMT)
Neither PAS25 nor PAS10 modified the mean value of the FPB rMT (PAS25: 46.1 ± 1.4 % of stimulator output at T0 versus 45.4 ± 1.4 % at T1; p ns; n = 19) (PAS10: 44.8 ± 5.6 % at T0, 44.5 ± 6 % at T1 and 44.1 ± 5.7 % at T2; p ns; n = 11).
Input-Output curves
cMT was very close to rMT and was not modified after PAS25 (46.9 ± 2.4 % at T0 and 45.2 ± 2.2 % at T1; p ns; n = 15). The slope of the I-O curve was significantly enhanced after PAS25 (1.7 ± 0.2 at T0 versus 2.2 ± 0.3 at T1, p< 0.03; n = 15) while there was a trend for the plateau value to be increased (2.4 ± 0.4 mV at T0 versus 3.2 ± 0.4 mV at T1, p = 0.08; n = 15).
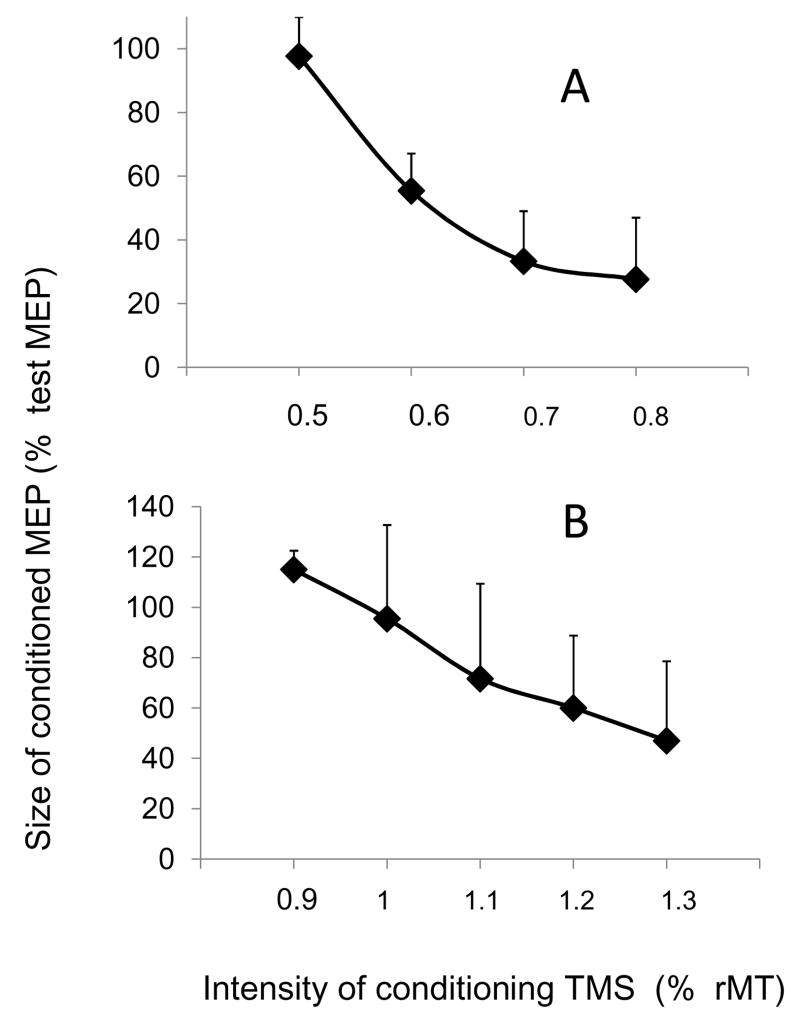
Effect of PAS on LICI
The averaged intensity curve for LICI, obtained in 15 subjects, is presented on Fig. 2B. The lowest intensity evoking an inhibition was 1.1 × rMT in all but 2 subjects. The mean CS90 was 1.12 ± 0.06 × rMT and 1.10 ± 0.03 × rMT for the PAS25 and the PAS10 group, respectively (p ns). Despite these similar intensities of CS90 in the 2 groups, baseline LICI was quite different between the 2 groups (FPB: 42.3 ± 4 % and 65.1 ± 4 % of the test MEP in PAS25 and PAS10 group respectively; p < 0.005; ADM: 63.5 ± 8 % and 87.7 ± 9 % of test MEP in PAS25 and PAS10 group, respectively; p ns).
Figure 2. Intensity curves of SICI (A) and LICI (B).
The effect of a conditioning TMS pulse (CS) is tested on a 1 mV test MEP from FPB. In A: the ISI between CS and test stimulation was 2.5 ms; each dot represents the mean of 120 measurements (12 subjects). B: the ISI between CS and test stimulation was 90 ms; each dot represents the mean of 150 measurements (15 subjects).
LICI was reduced in the target muscle immediately and 45 minutes after PAS25 while it was only slightly modified in the non-target ADM (Table 1). Contrasting with the decrease of LICI after PAS25, PAS10 led to a significant increase of the LICI in the target FPB without significant change in the non-target muscle ADM (Table 2).
Effect of PAS on SICI
The averaged intensity curve for SICI, obtained in 12 subjects, is presented on Fig. 2A. The lowest intensity evoking an inhibition was 0.6 × rMT in all but 2 subjects. The mean CS2.5 was 0.65 ± 0.07 × rMT and 0.60 ± 0.07 × rMT in the PAS25 and the PAS10 group, respectively. Despite these similar intensities of CS2.5 in the 2 groups, baseline SICI was quite different between the 2 groups (FPB: 47.1 ± 3 % and 67.8 ± 4 % of the test MEP in PAS25 and PAS10 group, respectively; p < 0.001; ADM: 49.3 ± 6 % and 77.4 ± 6 % of the test MEP in PAS25 and PAS10 group, respectively; p < 0.007).
Effects of PAS on SICI among subjects were more variable than those on LICI or LAI. After PAS25 mean SICI was not modified (Table 1). After PAS10 mean SICI was significantly decreased in FPB (Table 2).
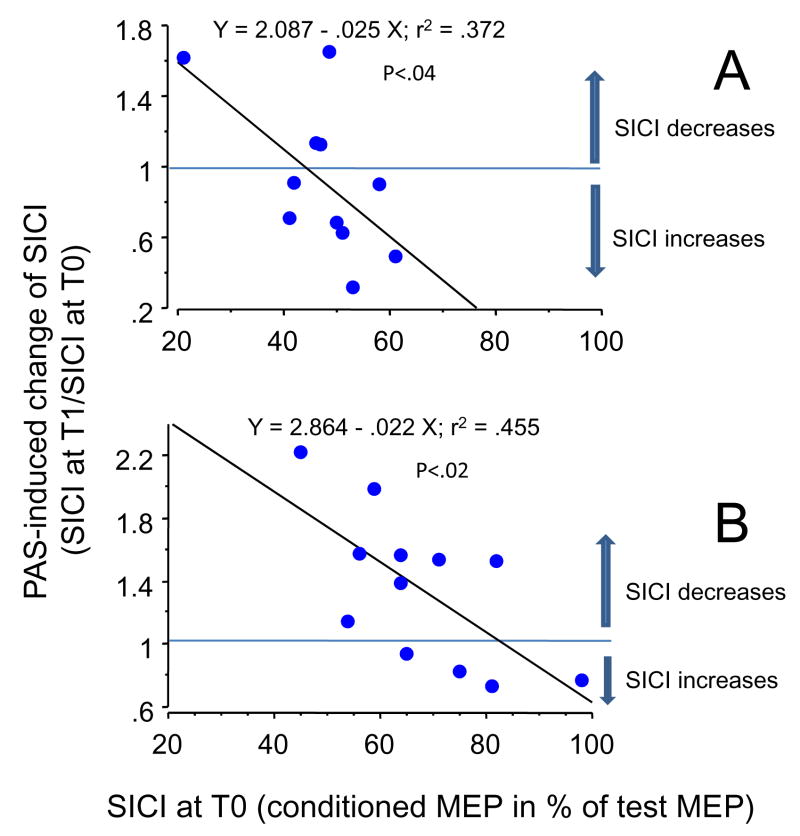
We noticed that for subjects with a small baseline SICI, the net effect of PAS25 was most of the time a SICI increase while when working on the whole group, who had a stronger baseline SICI, PAS25 did not induce any significant effect. An explanation for such a discrepancy is illustrated in Fig. 3: the amount of baseline SICI influenced the PAS - induced effect. All subjects with small baseline SICIs (more than 50%) exhibited a SICI increase after PAS25 (Fig. 3A). The amount of baseline SICI also influenced the PAS10-induced effect (Fig. 3B). Neither amount of baseline LICI, nor amount of baseline LAI influenced PAS-induced effects.
Figure 3. Relationship between the magnitude of baseline SICI in FPB and the PAS-induced change at T2.
Each point represents one subject. The x-axis represents the magnitude of the baseline inhibition expressed as the size of the conditioned MEP in percentage of the size of the test MEP. The y-axis represents the PAS-induced changes in inhibition calculated as “(inhibition at T2/inhibition at T0)”. Thus ratios greater than 1 represent a decrease in inhibition and ratios smaller than 1: an increase in inhibition.
The changes in SICI were correlated with the magnitude of baseline SICI after PAS25 (A) and also after PAS10 (B).
Effect of PAS on LAI
Time course of LAI
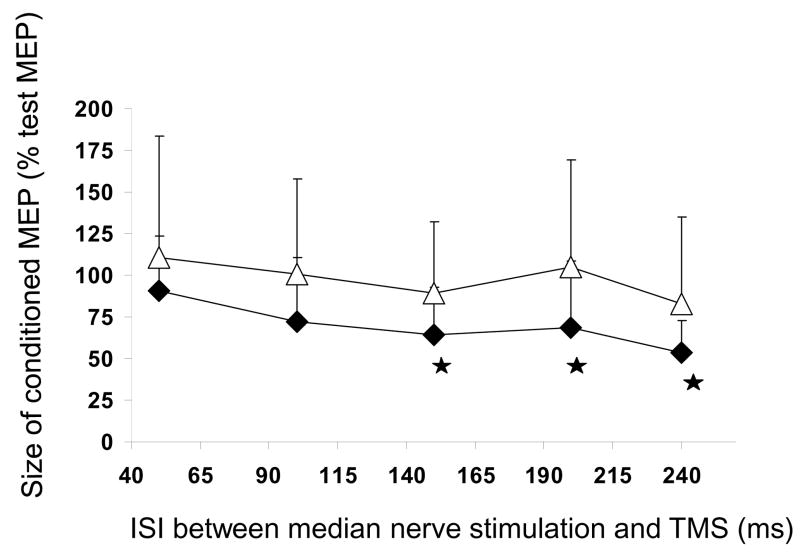
Eight subjects participated in this preliminary experiment. FPB MEP was inhibited at all but the 50 ms and the 100 ms ISI (50 ms: p ns, 100 ms p = ns, 150 ms: p< 0.02, 200 ms p< 0.05, 240 ms p<0.004) (Fig. 4, black diamonds). Inhibition reached similar maxima for ISI 150 ms (59.5 ± 15 % of the test MEP) and 240 ms (68.3 ± 15 %). Median nerve stimulation induced a small inhibition of ADM MEP at 150 ms (89.2±14 %) and at 240 ms (82.8 ± 14 %) (p ns at all ISIs) (Fig. 4, white triangles).
Figure 4. Time course of long afferent inhibition (LAI).
The effect of median nerve stimulation (2.5 × PT) is tested on a 1mV test MEP from FPB (black diamonds) and ADM (white triangles). Each square or diamond represents the mean of 80 measures (8 subjects). Median nerve stimulation inhibits the FPB MEPs at all but the 50 ms and the 100 ms ISI. Stars indicate the ISIs at which the mean conditioned MEP was statistically different from the mean test MEP (paired t-tests).
LAI150
At T0, LAI150 was evoked in FPB in all 24 tested subjects but one (16 subjects for PAS25 and 8 for PAS10), and in 18 out of the 24 tested in ADM. There was a clear topographic specificity as median nerve stimulation induced a stronger inhibition in the target FPB (LAI150 at T0 = 49.7 ± 4 % and 66.3 ± 8 % of the test MEP in PAS25 and PAS10 group, respectively; p < 0.04) than in the non-target ADM (LAI150 at T0 = 72 ± 10 % and 70.6 ± 9 % in PAS25 and PAS10 group respectively, p ns).
After PAS25 LAI150 was significantly decreased in the target muscle FPB and to a lesser (non significant) extent in the ADM muscle (Table 1). After PAS10, LAI150 showed a trend to be increased but it did not reach statistical significance (Table 2).
LAI240
At T0, LAI240 was evoked in FPB in 21 out of the 24 tested subjects (14 subjects for PAS25 and 10 for PAS10), and in 15 out of the 21 tested in ADM. There was a clear topographic specificity as median nerve stimulation induced a stronger inhibition in the target FPB (LAI240 at T0 = 65 ± 6 % and 60.5 ± 9 % of test MEP in PAS25 and PAS10 group, respectively; p ns) than in the non-target ADM (LAI240 at T0 = 87 ± 24 % and 77.5 ± 9 % of test MEP in PAS25 and PAS10 group, respectively, p ns).
Contrasting with the decrease of LAI150 after PAS25, LAI240 was significantly increased after PAS25 at T1 and T2 (Table 1) while it was significantly decreased after PAS10 (Table 2). PAS-induced changes in the non-target ADM were of similar direction than those observed in FPB; these changes were not significant after PAS25, but significant after PAS10.
Comparison of the 2 types of PAS interventions
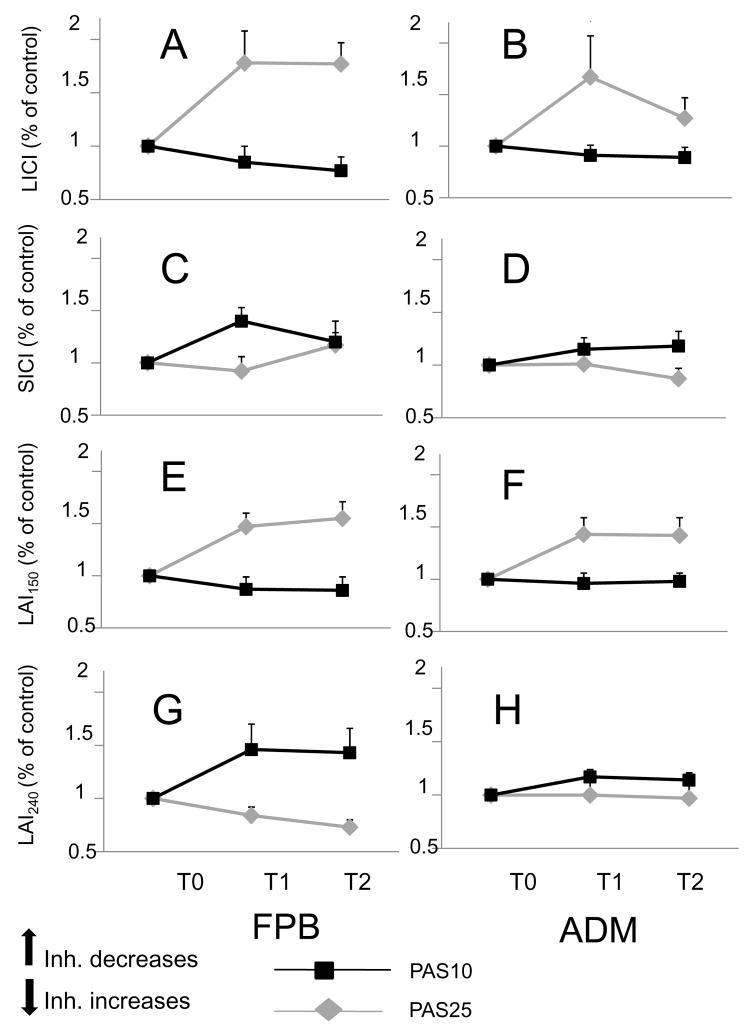
Baseline inhibitions were different between the groups tested for PAS25 and PAS10, so to compare the effects of the 2 interventions over time and across muscles, inhibitions after PAS were normalized to their baseline values. Figure 5 illustrates the normalized inhibitions in FBP and ADM after PAS25 (gray lines) and PAS10 (black lines). Changes of LICI (Fig. 5A–B), LAI150 (Fig. 5E–F) and LAI240 (Fig. 5.G–H) had the same direction in both muscles, and were not different at T1 and T2, but had reverse directions after PAS25 and PAS10, respectively. This was confirmed by the repeated ANOVA (see statistical analysis) showing a significant effect of INTERVENTION (LICI: F = 9.9, p< 0.003; LAI150: F = 13.1, p< 0.0008; LAI240: F = 10.4, p< 0.003), no effect of MUSCLE or TIME and no significant interaction. For SICI (Fig. 5C–D) the only consistent change was a decrease of SICI after PAS10 at T1 in FPB while changes after PAS25 were not substantial. This was confirmed by the repeated ANOVA with no significant effect of INTERVENTION (p = 0.06), but a significant interaction TIME*MUSCLE*INTERVENTION (F = 5.1, p< 0.03).
Figure 5. Differential effects of PAS25 and PAS10 on inhibitions.
Graph of interactions between “TIME” (PAS induced change of inhibition at T1 and T2), “MUSCLE” (target muscle: FPB [left column] versus non-target muscle: ADM [right column]) and “INTERVENTION” (PAS25 [gray diamonds and lines] versus PAS10 [black squares and lines]). The y-axis represents the PAS-induced changes of inhibition (inhibition at T1/inhibition at T0 and inhibition at T2/inhibition at T0) with, in A-B: LICI, in C-D: SICI, in E-F: LAI150 and in G-H: LAI240. There was a significant effect of ”INTERVENTION” for LICI, LAI240 and LAI150 as the effects of PAS25 and those of PAS10 go in opposite directions. There was a significant interaction “TIME”*”INTERVENTION”*”MUSCLE” for SICI. The stars indicate a statistically significant result for the post-hoc analysis (unpaired t-tests).
Effect of PAS on overall corticospinal excitability (Fig. 6)
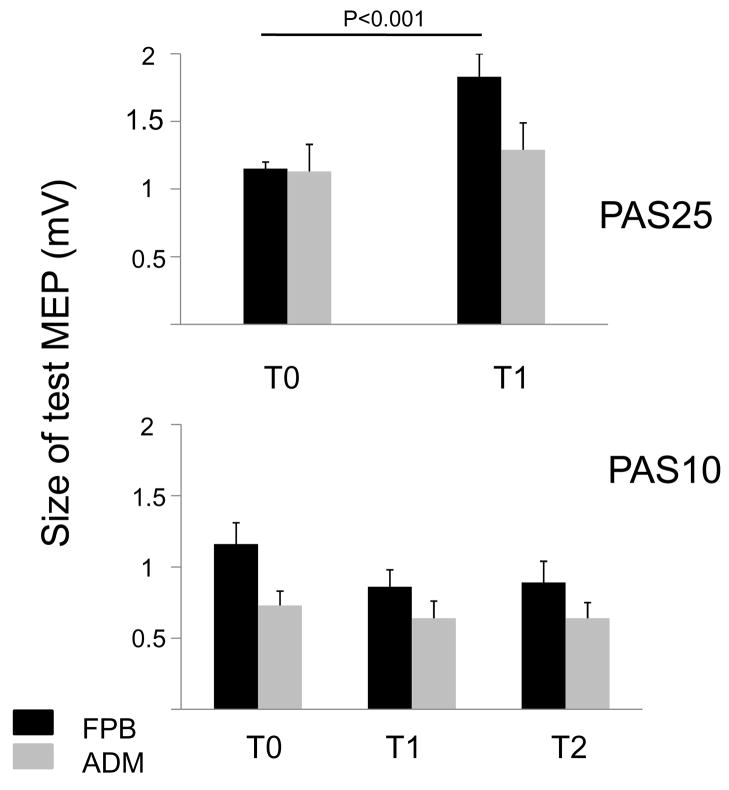
Figure 6. Effects of PAS25 and PAS10 on overall corticospinal excitability.
Upper graph: each column represents the mean of 160 measurements (16 subjects), bottom graph: each column represents the mean of 110 measurements (11 subjects). The size of a test MEP is expressed in mV for the FPB (black columns) and the ADM (gray columns). Intensity was adjusted in order to obtain a 1 mV MEP at baseline (T0). Target muscle was the FPB. Immediately after PAS25 (T1) size of the test MEP is increased for FPB, but not for ADM (upper graph). Immediately after PAS10 (T1), but also 40 to 50 minutes later (T2), the size of the test MEP is decreased for FPB but not for ADM.
The mean overall corticospinal excitability to FPB after PAS25 was significantly enhanced at T1 (not tested at T2) (T0: 1.02 ± 0.07 mV; T1: 1.76 ± 0.17 mV, p < 0.002) and not modified to ADM (T0: 1.02 ± 0.17 mV; T1: 1.05 ± 0.18 mV, p ns). After PAS10, the mean overall corticospinal excitability to FPB was significantly decreased to a same extent at T1 and T2 (T0: 1.16 ± 0.04 mV, p< 0.04; T1: 0.86 ± 0.12 mV; T2: 0.89 ± 0.15 mV, p ns) while corticospinal excitability to ADM was not modified (T0: 0.73 ± 0.11 mV; T1: 0.64 ± 0.12 mV, p ns; T2: 0.64 ± 0.11 mV, p ns).
The question then arose to know whether the subjects who exhibited the largest MEP increase or decrease after PAS are those in whom the PAS-induced decrease/increase of LICI or LAI150 were the largest. Neither after PAS25 nor PAS10 did we found a correlation between the percentage of change of the test MEP (MEP size at T1/MEP size at T0) and the LICI change (LICI at T1/LICI at T0) or the LAI150 change (LAI at T1/LAI at T0). We also did not find any correlation between percentage of change of the test MEP and SICI changes.
Discussion
This work shows that a PAS intervention inducing lasting changes of the overall corticospinal excitability, induces concomitant changes in amount of LICI and LAI. Changes in LICI and LAI have opposite directions according to the interval (10 or 25 ms) used between median nerve and cortical TMS stimulations during PAS. Changes in SICI are more difficult to interpret as direction of the changes depends on the baseline SICI.
Are PAS-induced changes of inhibitions related to a bi-directional associative synaptic plasticity of inhibitory synapses?
A first possibility to explain the bi-directional PAS-induced decrease/increase of LICI and LAI would be a change in the “gain” of the corticospinal system. Such a change was demonstrated by the increase of the slope of the I-O curve after PAS25 (also shown by (Ridding and Taylor, 2001). We did not study the I-O curves after PAS10 but a decrease of the slope of the I-O curve after PAS10 has already been described (Rosenkranz et al., 2007). As illustrated in Fig. 7, even in absence of any change of synaptic efficacy in intracortical inhibitory pathways, an increase in the slope of the input-output relationship after PAS may induce an apparent increase in inhibitions. Conversely a decrease of the slope may induce an apparent decrease of inhibitions. In our results LICI and LAI150 were decreased after PAS25 while the slope of the I-O curve was increased and they were increased after PAS10 while the slope was presumably decreased, so these changes cannot be ascribed to the changes of the slope.
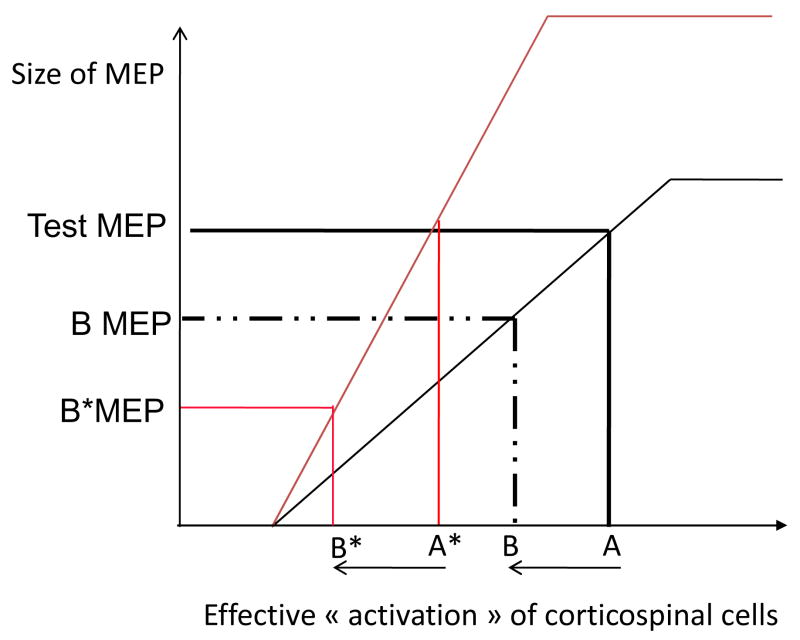
Figure 7. Interpretation of PAS-induced changes of intracortical inhibitions in presence of a change of the input-output curve (I-O curve).
Hypothetical recruitment curve for FPB corticospinal cells is represented before PAS (black thin line) and after PAS (red thin line) under the assumption that PAS has induced a change of the recruitment gain (increase of the slope).
In abscissa: the “final” net input arriving at the corticospinal cells (which is the sum of excitatory and inhibitory inputs); in ordinate: the size of the corresponding MEP.
Before PAS an input “A” results in a test MEP, if the input decreases from A to B due, for example, to activation of inhibitory GABAergic neurons, then the corresponding MEP becomes “B MEP” which is smaller than the test MEP. What would happen if PAS causes an increase of the I-O curve is illustrated. After PAS in order to get a test MEP of the same size as before PAS, a smaller input “A*” is necessary. The same activation of inhibitory interneurones as before PAS occurs then and the final input to corticospinal cells becomes “B*” with A-B = A*-B*. The corresponding MEP is “B*MEP” and it appears clearly than “B*MEP” is smaller than “B MEP”. Such an apparent post-PAS “increase” of the amount of the inhibition reflects only the change of the slope of the I-O curve and not a change of the inhibitory drive to corticospinal cells.
A confounding factor to interpret PAS-induced changes of inhibitions would be a PAS-induced change of the corticospinal volley evoked by the TMS test stimulation. If PAS favours the development of lasting changes of excitability in cortical circuits responsible for the early recruited I waves it may be that the descending volleys evoking the 1 mV test MEPS be different before and after PAS. An increase of the relative weight of early components versus the latter ones after PAS might decrease by itself the amount of LICI as LICI acts by decreasing the later components of the corticospinal volley (I3 waves) and has no effect on early components (D wave and I1 waves) (Chen et al., 1999a; Di Lazzaro et al., 2002). Epidural recordings of the descending volleys have recently brought a direct demonstration that PAS enhances the amplitude of later descending I waves of the test volley whereas the I1 wave was not modified (Di Lazzaro et al., 2009). Is such a conclusion applicable to our data? Di Lazzaro et al (Di Lazzaro et al., 2009) used a standard PAS intervention with 1 mV MEPs during both the PAS and the testing of excitability of the corticospinal volley. We used lower intensities of TMS during the PAS intervention (0.5 mV) than before and after the intervention (1 mV test MEPs). Using also 1 mV test MEPs and very weak (subthreshold) stimulation during PAS, Kujirai et al (Kujirai et al., 2006) observed an increased effectiveness of PAS when they used anterior-posterior directed currents (known to increase the recruitment of late I waves) instead of posterior-anterior directed ones. It suggests that, even with the use of low TMS intensities, PAS after-effects are due to a change in the excitability of neurons responsible for recruiting the I3 waves in the corticospinal tract and not the early waves.
The third and most plausible explanation is that PAS-induced changes of LICI and LAI are related to enduring changes in excitability of the corresponding pathways. Arguments for attributing bidirectional changes of a test MEP after a PAS25 or a PAS10 intervention to long-term facilitation/long-term depression-like phenomena governed by temporal Hebbian rules have been developed in several papers (Stefan et al., 2000; Stefan et al., 2002; Wolters et al., 2003; Weise et al., 2006; Rosenkranz et al., 2007). The same arguments can be used here for PAS-induced changes of LICI and LAI150: topographic specificity (changes are prominent in the targeted muscle FPB and absent or less in the control ADM), long duration (>30 minutes), reversibility, direction of the change depending whether the peripheral volley arrives at the cortical level before or after the triggering of the cortical stimulation. However, we did not demonstrate here that changes in inhibitions are NMDA dependant, a characteristic of LTP/LTD.
Such reasoning cannot apply for SICI since the only consistent effect of PAS on SICI was the decrease of SICI after PAS10. Such a change may be caused, at least partly, by the decrease of the slope of the I-O curve after PAS10 (see above). The direction of the PAS25-induced changes in SICI depended on the baseline SICI. Baseline SICI has already been described to influence the effect of an ongoing 1Hz rTMS intervention (Bagnato et al., 2005; Daskalakis et al., 2006). In the light of recent findings (Paulus et al., 2008; Peurala et al., 2008) subjects with large baseline SICI might be not tested in the descending part of the intensity curve where SICI is less contaminated by SICF (and especially at the 2.5 ms interval that we used (Ni et al., 2007)) but later when SICI and SICF are superimposed (see Peurala et al 2008, their Fig. 2 (Peurala et al., 2008)). Contrastingly, subjects with low baseline SICI might be tested in the descending part of the SICI curve, and, in those subjects, PAS-induced SICI changes might reflect more faithfully changes in GABAergic interneurones. If we look at Fig 3, it appears that, for moderate baseline SICI, PAS25 induced mainly an increase in inhibition while PAS10 induced a decrease. This suggests a possible bi-directional effect according whether the peripheral volley arrives before or after the cortical one at the target cells. Such an interpretation fits with the bi-directional changes of SICI recently demonstrated after theta-burst stimulation of M1: if theta-burst stimulation is applied in an intermittent (excitatory) mode SICI is increased, whereas if applied in a continuous (inhibitory) mode SICI is decreased (Huang et al., 2005; Murakami et al., 2008). Interpretation of SICI changes is weakened by the difference in baseline SICIs between the groups.
LAI240 versus LAI150
It was unexpected to find opposite changes of LAI when using 2 different ISIs: 150 ms and 240 ms. As discussed above, as they have an opposite direction to the changes of the slope of the I-O curve, PAS-induced change of LAI150 can be reasonably attributed to the development of a LTP/LTD - like bidirectional plasticity. Oppositely, as they follow the direction of the changes of slope of the I-O curve (increase after PAS25, decrease after PAS10) changes of LAI240 may be due to the changes of the I-O relationship or at least be masked by it. In such a case PAS-induced changes of LAI150 and LAI240 would be of same direction but of different amount. The large changes of LAI150 would overcome the changes due to the change of the slope while the small changes of LAI240 would be masked by it. Another possible explanation comes from the strong inhibitory interaction described between LICI and LAI (Sailer et al., 2002). Then a PAS-induced decreased/increased excitability of interneurones shared by LICI and LAI pathways might explain the increase/decrease of AI240, while a 150-ms delay would be too short for the inhibitory interaction to develop.
Link with previous data in the literature
SP and LICI
Conventional PAS (suprathreshold stimulations, 0.05 to 0.25 Hz) fail to modulate SICI (Stefan et al., 2000; Stefan et al., 2002; Quartarone et al., 2003; Rosenkranz and Rothwell, 2006), yet induced an increase in duration of the silent period (SP) (Morgante et al. 2006; Stefan et al. 2000; Stefan et al. 2004). As LICI and SP (at least its second part) have been considered so far as related phenomena (Wassermann et al., 1996), reflecting activation of GABAB receptors (Roick et al. 1993; Siebner et al. 1998; Werhahn et al. 1999), the increase of duration of SP after PAS was thought to reflect increased GABAB activity. The results obtained here question the relationship between SP and LICI; indeed what we found, after excitatory PAS, is a decrease of LICI and not the increase that should go with a prolongation of the SP (see above). Several papers have recently showed a dissociation between SP and LICI modulation: during voluntary contraction (Hammond and Vallence, 2007) during fatiguing hand exercise (Benwell et al., 2007) during treatment by a GABAB receptor agonist (McDonnell et al., 2006).
Different PAS protocols
We used a PAS protocol similar to that used by Mueller et al. (Muller et al., 2007) but different from the “conventional” one. It is possible that by changing the relative “weight” of cortical stimulation with respect to the peripheral one (during PAS, TMS was adjusted to evoke a MEP of 0.5 mV instead of the 1mV used in the original protocol) and by delivering a larger number of pairs (240 instead of 90) we favor the development of plasticity in the inhibitory circuits. Indeed when using rTMS, for a given frequency and a given intensity, the longer the duration of stimulation the more substantial are the induced effects on interneurone excitability (Jung et al., 2008) (see also Fitzgerald et al 2006 (Fitzgerald et al., 2006)). Also while 1 Hz subthreshold rTMS of M1 has no effect on the final motor output (no change in MEP size) it induces effects on interneuronal excitability suggesting that the “lowest” threshold system activated by TMS in the hand area of human motor cortex is inhibitory (Bagnato et al., 2005).
Conclusion
The present data show that PAS paradigms can demonstrate Hebbian-like plasticity in the inhibitory networks responsible for LICI (a GABAB mediated inhibition) and LAI as well as excitatory networks. Despite some clues indicating that enduring changes of LICI might occur at a presynaptic level on GABAA interneurones and induce changes of SICI in the opposite direction, further experiments are needed to elucidate the complex relationship between PAS-induced changes of GABAA and GABAB mediated inhibitions.
Acknowledgments
We thank Devera Schoenberg for skillful editing.
This research was supported (in part) by the Intramural Research Program of the NIH, NINDS.
Heike Russmann was funded by the Swiss National Funds PBSKB-104264, the Swiss Parkinson Society and NIH intramural. Jean-Charles Lamy was funded by Fondation pour la Recherche Medicale and NIH intramural.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C, Sanes JN, Donoghue JP, Ridding MC, Taylor JL, Chen R, Chen R, Wassermann EM, Canos M, Hallett M. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–545. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Bagnato S, Curra A, Modugno N, Gilio F, Quartarone A, Rizzo V, Girlanda P, Inghilleri M, Berardelli A. One-hertz subthreshold rTMS increases the threshold for evoking inhibition in the human motor cortex. Exp Brain Res. 2005;160:368–374. doi: 10.1007/s00221-004-2020-0. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Mastaglia FL, Thickbroom GW. Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res. 2007;179:255–262. doi: 10.1007/s00221-006-0790-2. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:132–136. [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999a;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999b;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Effect of GABAB receptor blockade on receptive fields of raccoon somatosensory cortical neurons during reorganization. Exp Brain Res. 2002;145:150–157. doi: 10.1007/s00221-002-1130-9. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res. 2002;147:108–113. doi: 10.1007/s00221-002-1223-5. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F, Tonali PA. Associative Motor Cortex Plasticity: Direct Evidence in Humans. Cereb Cortex. 2009 doi: 10.1093/cercor/bhn255. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Hammond G, Vallence AM. Modulation of long-interval intracortical inhibition and the silent period by voluntary contraction. Brain Res. 2007;1158:63–70. doi: 10.1016/j.brainres.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jung SH, Shin JE, Jeong YS, Shin HI. Changes in motor cortical excitability induced by high-frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clin Neurophysiol. 2008;119:71–79. doi: 10.1016/j.clinph.2007.09.124. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Cohen LG. Enhancing the quality of studies using transcranial magnetic and electrical stimulation with a new computer-controlled system. J Neurosci Methods. 2000;102:81–89. doi: 10.1016/s0165-0270(00)00284-3. [DOI] [PubMed] [Google Scholar]
- Kujirai K, Kujirai T, Sinkjaer T, Rothwell JC. Associative plasticity in human motor cortex during voluntary muscle contraction. J Neurophysiol. 2006;96:1337–1346. doi: 10.1152/jn.01140.2005. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABA(B) receptor agonist baclofen. Exp Brain Res. 2007;180:181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- Muller JF, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci. 2007;25:3461–3468. doi: 10.1111/j.1460-9568.2007.05603.x. [DOI] [PubMed] [Google Scholar]
- Murakami T, Sakuma K, Nomura T, Nakashima K, Hashimoto I. High-frequency oscillations change in parallel with short-interval intracortical inhibition after theta burst magnetic stimulation. Clin Neurophysiol. 2008;119:301–308. doi: 10.1016/j.clinph.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498 (Pt 3):817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583:971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen G, Large C, Lazzaro VD, Nitsche M, Pascual-Leone A, Rosenow F, Rothwell J, Ziemann U. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimulation. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Müller-Dahlhaus JMF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008 doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer’ cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL. Mechanisms of motor-evoked potential facilitation following prolonged dual peripheral and central stimulation in humans. J Physiol. 2001;537:623–631. doi: 10.1111/j.1469-7793.2001.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci. 2006;23:822–829. doi: 10.1111/j.1460-9568.2006.04605.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Kacar A, Rothwell JC. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 2007;27:12058–12066. doi: 10.1523/JNEUROSCI.2663-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI, Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544:617–629. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer A, Shi H. Impairment of GABAA receptor function by N-methyl-D-aspartate-mediated calcium influx in isolated CA1 pyramidal cells. Neuroscience. 1994;62:813–828. doi: 10.1016/0306-4522(94)90479-0. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Elevated threshold for intracortical inhibition in focal hand dystonia. Mov Disord. 2004;19:1312–1317. doi: 10.1002/mds.20160. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, Classen J. The two sides of associative plasticity in writer’ cramp. Brain. 2006;129:2709–2721. doi: 10.1093/brain/awl221. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]