Abstract
Aims
Atrial fibrillation (AF)-associated poor outcomes in heart failure (HF) are often attributed to older age, advanced disease, and comorbidity burden of HF patients with AF. Therefore, we examined the effect of AF on outcomes in a propensity-matched study in which patients with and without AF were well balanced on all measured baseline characteristics.
Methods and results
Of the 2708 advanced chronic systolic HF patients in the Beta-Blocker Evaluation of Survival Trial, 653 had a history of AF. Propensity scores for AF were calculated for each patient and were used to assemble a cohort of 487 pairs of patients with and without AF who were balanced on 74 baseline characteristics. Matched Cox regression analyses were used to estimate associations of AF with outcomes during 23 months of mean follow-up. All-cause mortality occurred in 187 (rate, 2046/10 000 person-years of follow-up) and 181 (rate, 1885/10 000 person-years) matched patients with and without AF, respectively [matched hazard ratio (HR) when AF was compared with no-AF 1.03, 95% confidence interval (CI) 0.79–1.33; P = 0.84]. Heart failure hospitalization occurred in 215 (rate, 3171/10 000 person-years) and 184 (rate, 2405/10 000 person-years) matched patients with and without AF, respectively (matched HR when AF was compared with no-AF 1.28, 95% CI 1.00–1.63; P = 0.049). Hazard ratios and 95% CIs for AF-associated HF hospitalization for bucindolol and placebo groups were, respectively, 1.08 (0.81–1.43) and 1.54 (1.17–2.03; P for interaction = 0.09).
Conclusion
A history of AF had no intrinsic association with mortality but was associated with HF hospitalization in chronic systolic HF.
Keywords: Heart failure, Atrial fibrillation, Mortality, Hospitalization
Introduction
Atrial fibrillation (AF) is common in heart failure (HF) and has been associated with poor outcomes.1–3 However, to what extent a history of AF is independently associated with poor outcomes has not been studied in a propensity-matched population of chronic advanced systolic HF patients.3–6 Traditional regression-based multivariable risk adjustment models are limited by strong model assumptions that may not always be appropriate and concern for residual bias and procedural transparency.7,8 However, propensity-score matching can be used to assemble two groups of patients balanced on all measured baseline covariates.9–12 Furthermore, because outcomes data are not needed during study design, it allows investigators to design observational studies while remaining blinded to study outcomes, a key feature of randomized clinical trials. Therefore, in the current study, we examined the association of a history of AF and long-term outcomes in a propensity-matched cohort of HF patients.
Methods
Source of study data
The Beta-Blocker Evaluation of Survival Trial (BEST) was a multicentre randomized clinical trial of bucindolol, a beta-blocker, in advanced systolic HF, methods and results of which have been previously published.13 Briefly, 2708 patients with advanced chronic systolic HF were enrolled from 90 different sites across the USA and Canada between May 1995 and December 1998. These patients had a mean left ventricular ejection fraction of 23% and all patients had New York Heart Association class III–IV symptoms. Over 90% of all patients were receiving angiotensin-converting enzyme inhibitors, diuretics, and digitalis. We used a public-use copy of the BEST dataset obtained from the National Heart Lung and Blood Institute that included 2707 patients (one patient did not consent to be included in the public-use copy). The current analysis focuses on a subset of 974 propensity-matched patients.
History of atrial fibrillation
Overall, 653 (24%) patients had a history of AF. Data on AF were collected by study investigators and were not centrally adjudicated. Of the 653 patients with AF, 293 (45%) had electrocardiographic (ECG) evidence of AF at baseline. Considering the similar unadjusted all-cause mortality between patients with a history of AF who had and did not have ECG evidence of AF at baseline, we chose to combine these patients into one group. Of the 2054 patients without AF, only 10 patients had ECG evidence of new AF at baseline.
Study outcomes
Primary outcomes for this propensity-matched study were all-cause mortality and HF hospitalization. Secondary outcomes were cardiovascular and HF mortality and all-cause hospitalization. Investigators blinded to the outcomes ascertained study outcomes. Patients were followed for a mean of 23 months (median follow-up, 22 months; range: 1–50 months).
Assembly of a balanced cohort
Because of significant imbalances in baseline characteristics between patients with and without AF before matching (Table 1), we used propensity-score matching to assemble a cohort of patients whereby those with and without AF would be well balanced on all measured baseline characteristics.8,14 We began by estimating propensity scores for AF for each of the 2707 participants using a non-parsimonious multivariable logistic regression model. In the model, AF was used as the dependent variable, and all clinically relevant baseline characteristics (n = 74) displayed in Figure 1 were included as covariates.
Table 1.
Baseline patient characteristics by history of atrial fibrillation before and after propensity matching
| Before propensity matching |
After propensity matching |
|||||
|---|---|---|---|---|---|---|
| No history of atrial fibrillation (n = 2054) | History of atrial fibrillation (n = 653) | P-value | No history of atrial fibrillation (n = 487) | History of atrial fibrillation (n = 487) | P-value | |
| Age (years) | 58.8 (±12.4) | 64.8 (±10.9) | <0.0001 | 62.8 (±11.7) | 63.2 (±11.2) | 0.55 |
| Female | 511 (25) | 82 (13) | <0.0001 | 68 (14) | 70 (14) | 0.92 |
| African American | 515 (25) | 112 (17) | <0.0001 | 92 (19) | 90 (19) | 0.94 |
| Current smoker | 378 (18) | 96 (15) | 0.03 | 70 (14) | 78 (16) | 0.52 |
| Past medical history | ||||||
| Duration of heart failure (months) | 46 (±46) | 60 (±54) | <0.0001 | 56 (±54) | 55 (±48) | 0.68 |
| Coronary artery disease | 1176 (57) | 417 (64) | 0.003 | 321 (66) | 313 (64) | 1.00 |
| Coronary artery bypass surgery | 552 (27) | 230 (35) | <0.0001 | 161 (33) | 157 (32) | 0.84 |
| Percutaneous coronary intervention | 325 (16) | 98 (15) | 0.62 | 78 (16) | 75 (15) | 0.86 |
| Angina pectoris | 1078 (53) | 322 (49) | 0.16 | 253 (52) | 249 (51) | 0.84 |
| Hypertension | 1205 (59) | 390 (60) | 0.63 | 271 (56) | 286 (59) | 0.37 |
| Diabetes mellitus | 747 (36) | 217 (33) | 0.15 | 178 (37) | 176 (36) | 0.95 |
| Hyperlipidaemia | 918 (44) | 252 (39) | 0.006 | 194 (40) | 195 (40) | 1.000 |
| Clinical findings | ||||||
| Body mass index (kg/m2) | 36.6 (±8.5) | 36.4 (±8.2) | 0.59 | 36.5 (±8.5) | 36.6 (±8.4) | 0.88 |
| Systolic blood pressure (mmHg) | 117 (±18) | 117 (±18) | 0.85 | 116 (±18) | 117 (±18) | 0.34 |
| Diastolic blood pressure (mmHg) | 71 (±11) | 70 (±11) | <0.0001 | 70 (±11) | 70 (±11) | 0.34 |
| Heart rate (b.p.m.) | 82 (±13) | 79 (±13) | < 0.0001 | 80 (±13) | 80 (±13) | 0.92 |
| Jugular venous distension | 1.63 (±0.85) | 1.91 (±1.01) | <0.0001 | 1.84 (±0.95) | 1.87 (±1.0) | 0.59 |
| S3 gallop | 904 (44) | 274 (42) | 0.36 | 213 (44) | 215 (44) | 0.95 |
| Pulmonary râles | 252 (12) | 107 (16) | 0.007 | 75 (15) | 77 (16) | 0.93 |
| Lower extremity oedema | 516 (25) | 214 (33) | < 0.0001 | 153 (31) | 155 (32) | 0.94 |
| NYHA class III | 1900 (93) | 581 (89) | 0.005 | 438 (90) | 433 (89) | 0.68 |
| Medications | ||||||
| ACE-inhibitors | 1890 (92) | 594 (91) | 0.40 | 447 (92) | 445 (91) | 0.91 |
| Digitalis | 1879 (92) | 615 (94) | 0.026 | 459 (94) | 459 (94) | 1.00 |
| Diuretics | 1898 (92) | 626 (96) | 0.002 | 464 (95) | 463 (95) | 1.00 |
| Vasodilators | 891 (43) | 293 (45) | 0.50 | 218 (45) | 218 (45) | 1.00 |
| Anti-arrhythmic drugs | 38 (2) | 36 (6) | <0.0001 | 23 (5) | 22 (5) | 1.00 |
| Anti-coagulants | 1063 (52) | 507 (78) | <0.0001 | 344 (71) | 347 (71) | 0.87 |
| Chest X-ray findings | ||||||
| Pulmonary oedema | 222 (11) | 86 (13) | 0.10 | 72 (15) | 70 (14) | 0.93 |
| Cardiomegaly | 1644 (80) | 560 (86) | 0.001 | 408 (84) | 403 (83) | 0.73 |
| Echocardiography findings | ||||||
| LV ejection fraction (%) | 22.9 (±7.2) | 23.5 (±7.3) | 0.05 | 22.6 (±7.4) | 23.0 (±7.2) | 0.38 |
| RV ejection fraction (%) | 34.9 (±12.1) | 33.9 (±10.5) | 0.047 | 33.2 (±11.6) | 33.4 (±10.6) | 0.83 |
| Laboratory findings | ||||||
| Haemoglobin (g/dL) | 14.0 (±1.6) | 14.0 (±1.8) | 0.98 | 13.9 (±1.6) | 14.0 (±1.7) | 0.52 |
| White blood cell (103/µL) | 7.5 (±2.2) | 7.4 (±2.1) | 0.17 | 7.4 (±2.6) | 7.5 (±2.1) | 0.75 |
| Serum creatinine (mg/dL) | 1.22 (±0.40) | 1.33 (±0.42) | <0.0001 | 1.31 (±0.43) | 1.29 (±0.41) | 0.38 |
| Blood urea nitrogen (mg/dL) | 23 (±14) | 29 (±18) | <0.0001 | 27 (±16) | 27 (±16) | 0.54 |
| Serum sodium (mEq/L) | 139 (±3) | 139 (±3) | 0.53 | 139 (±4) | 139 (±3) | 0.58 |
| Serum potassium (mEq/L) | 4.32 (±0.47) | 4.28 (±0.51) | 0.06 | 4.32 (±0.51) | 4.29 (±0.50) | 0.29 |
| Serum magnesium (mg/dL) | 1.7 (±0.24) | 1.8 (±0.27) | 0.003 | 1.8 (±0.24) | 1.8 (±0.26) | 0.63 |
| Serum glucose (mg/dL) | 136 (±77) | 129 (±67) | 0.035 | 134 (±74) | 132 (±69) | 0.66 |
| Plasma thromboplastin time (s) | 30.6 (±9.4) | 34.0 (±8.98) | < 0.0001 | 33.6 (±15.1) | 32.8 (±7.83) | 0.28 |
| Plasma norepinephrine (pg/mL) | 500 (±296) | 562 (±331) | <0.0001 | 550 (±366) | 551 (±345) | 0.98 |
Values for categorical variables are given with % values in parentheses. Values for continuous variables are given with ±standard deviation in parentheses. ACE, angiotensin-converting enzyme; LV, left ventricular; RV, right ventricular.
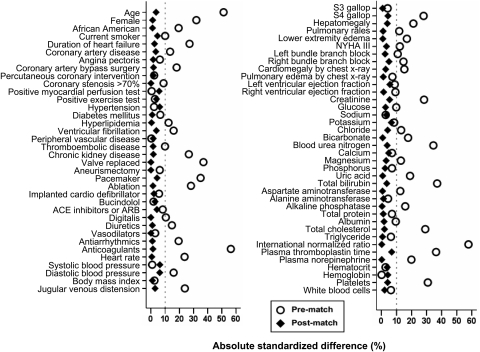
Figure 1.
Love plots for absolute standardized differences for baseline covariates between patients with and without a history of atrial fibrillation, before and after propensity score matching (ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker).
Because propensity-score models are sample-specific adjusters and are not intended to be used for out-of-sample prediction or estimation of coefficients, measures of fitness and discrimination are not important for the assessment of the model's effectiveness.9–12 The efficacy of propensity-score models is best assessed by estimating post-match absolute standardized differences between baseline covariates that directly quantifies the bias in the means (or proportions) of covariates across the groups, expressed as a percentage of the pooled standard deviations. We therefore calculated pre- and post-match absolute standardized differences and presented those findings as Love plots.9 An absolute standardized difference of 0% indicates no residual bias and < 10% is considered of inconsequential bias.
We used a greedy matching protocol to match patients with and without AF who had similar propensity scores to five, four, three, two, and one decimal places in five repeated steps.9–12 In the first step, we multiplied the raw propensity scores by 100 000. For example, propensity scores of 0.57520576 and 0.57520374 for a pair of patients with and without AF, respectively, were converted to 57520.58 and 57520.37. Then we rounded both the numbers to the nearest value divisible by 0.25 (e.g. 57520.50) and matched. All patients matched by propensity scores to the five decimal places were then removed from the file. In the second step, we multiplied the raw propensity scores by 10 000, rather than 100 000, and repeated the above process. This was then repeated three more times, each time, multiplying by 1000, 100, and finally 10. In all, we were able to match 487 of the 653 history of AF patients with 487 patients who had no history of AF, but had similar propensity for AF.
Statistical analysis
For descriptive analyses, we used Pearson χ2 and Wilcoxon rank-sum tests for the pre-match, and McNemar's test and paired sample t-test for the post-match comparisons of baseline covariates between patients with and without AF, as appropriate. Kaplan–Meier and matched Cox regression analyses were used to determine the associations of AF with various outcomes during 23 months of mean follow-up. Log-minus-log scale survival plots were used to check proportional hazards assumptions. To assess the effect of loss of participants during matching, we repeated our analysis in all 2707 pre-match patients using three different Cox regression models: (i) unadjusted, (ii) multivariable-adjusted, using all covariates used in the propensity-score model, and (iii) propensity score adjusted. To eliminate potentially inflated pre-match associations due to larger sample size, we assembled a pre-match cohort of 487 pairs of patients by pairing our matched AF patients with a randomly selected subset of 487 non-AF patients from the pool of 2054 pre-match non-AF patients.
Sensitivity analysis
Even though our matched cohort achieved excellent balance in all measured covariates between the two groups, we do not know if there was bias due to imbalances on a hidden covariate (a baseline characteristic that was not measured and thus not used to estimate propensity scores). We therefore conducted a formal sensitivity analysis to quantify the degree of hidden bias that would need to be present to invalidate any conclusions based on significant association between AF and primary outcomes among matched patients.15 Specifically, we used Rosenbaum's model and related mathematical equations to place bounds on the significance levels that would have been appropriate for our primary comparisons between patients with and without AF had a hidden bias of a particular size occurred.15
Subgroup analysis
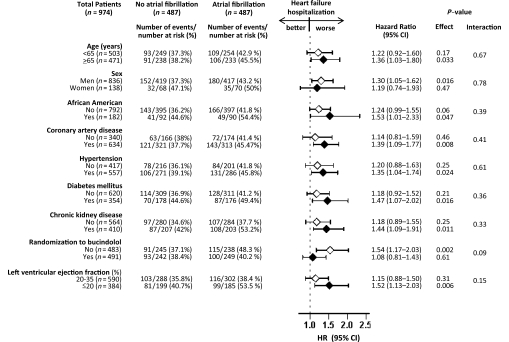
Select subgroup analyses of matched patients based on demographics (age ≥ 65 years, sex, and race), clinically relevant comorbidities and marker of disease severity (coronary artery disease, hypertension, diabetes mellitus, chronic kidney disease, and left ventricular ejection fraction < 25%), and randomization factor (bucindolol use) were conducted to determine heterogeneity of the associations of AF with HF hospitalization. We first calculated the absolute risk differences, and then estimated the effects of AF on HF hospitalization in each group using Cox regression models. Because of a lack of a significant overall association between AF and mortality, we did not perform a formal subgroup analysis for this outcome. However, considering that patients were randomized to bucindolol, we examined the association of AF and all-cause mortality in subgroups of patients receiving bucindolol and placebo. Finally, we formally tested for first-order interactions using Cox proportional hazards models, entering interaction terms for each subgroup (e.g. gender*AF for the gender subgroups). All statistical tests were two-sided, and tests with P < 0.05 were considered significant. All statistical analyses were done using SPSS for windows version 15.16
Results
Baseline characteristics
Matched patients had a mean age of 63 (±11) years, 14% were women and 19% were African American. Before matching, patients with AF were older, had a longer duration of HF and higher comorbidity burden than those without AF. These and other significant imbalances in baseline characteristics before matching and the balances achieved on all baseline characteristics after matching are displayed in Table 1 and Figure 1. After matching, absolute standardized differences for all measured covariates were <10% (most were <5%), suggesting substantial covariate balance across the groups (Figure 1).
Atrial fibrillation and mortality
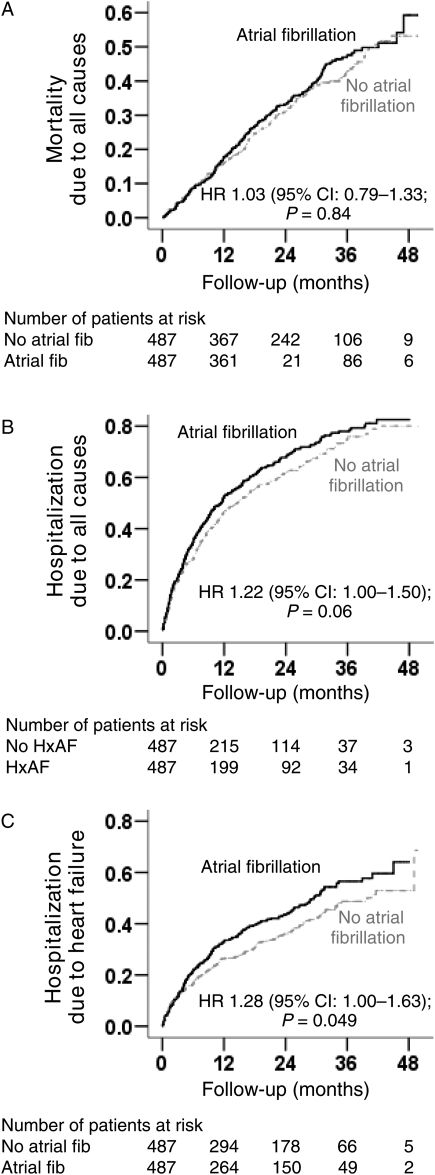
Overall, 368 (38%) matched patients died, including 314 (32%) due to cardiovascular causes and 110 (11%) due to HF during 1874 patient-years of follow-up. In the 487 matched-pair cohort, all-cause mortality occurred in 187 (rate, 2046/10 000 person-years) and 181 (rate, 1885/10 000 person-years) patients with and without AF, respectively [matched hazard ratio (HR) when AF was compared with no AF 1.03, 95% confidence interval (CI) 0.79–1.33; P = 0.84; Figure 2A and Table 2]. No formal sensitivity analysis was conducted due to lack of any significant association between AF and all-cause mortality. Atrial fibrillation-associated mortality due to all causes was of borderline significance among patients in the placebo group (HR for AF 1.30, 95% CI 0.98–1.73; P = 0.073) but not among those in the bucindolol group (HR for AF 0.90, 95% CI 0.67–1.21; P = 0.48; P for interaction = 0.08; data not shown).
Figure 2.
Kaplan–Meier plots for (A) all-cause mortality, (B) all-cause hospitalization, and (C) heart failure hospitalization by a history of atrial fibrillation (HxAF) in propensity score matched pairs (CI, confidence interval; fib, fibrillation; HR, hazard ratio).
Table 2.
Association of atrial fibrillation with all-cause mortality and heart failure hospitalization in Beta-Blocker Evaluation of Survival Trial
| Rate per 10 000 person-years (events/total follow-up years) |
Absolute rate difference (per 10 000 person-years)a | Matched hazard ratio (95% confidence interval) | P-value | ||
|---|---|---|---|---|---|
| No history of atrial fibrillation | History of atrial fibrillation | ||||
| Before matching | n = 2054 | n = 653 | |||
| All-cause mortality | 1430 (603/4216) | 2088 (256/1226) | +658 | 1.47 (1.27–1.70) | < 0.0001 |
| HF hospitalization | 2243 (750/3344) | 3209 (294/916) | +967 | 1.38 (1.21–1.58) | < 0.0001 |
| After matching | n = 487 | n = 487 | |||
| All-cause mortality | 1885 (181/960) | 2046 (187/914) | +161 | 1.03 (0.79–1.33) | 0.84 |
| HF hospitalization | 2405 (184/765) | 3171 (215/678) | +766 | 1.28 (1.00–1.63) | 0.049 |
aAbsolute differences in rates of events per 10 000 person-years of follow-up were calculated by subtracting the event rates in the atrial fibrillation group from the event rates in the no atrial fibrillation group (before values were rounded).
In the full pre-match cohort of 2707 patients, all-cause mortality occurred in 39% (rate, 2088/10 000 person-years of follow-up) and 29% (rate, 1430/10 000 person-years of follow-up) of patients with and without AF, respectively (unadjusted HR for AF 1.47; 95% CI 1.27–1.70; P < 0.0001). To determine if the significant pre-match association between AF and total mortality may have been due to its larger sample size, we assembled 487 pairs of pre-match patients by pairing 487 matched AF patients with 487 random non-AF patients from the 2054 pre-match patients without AF. Among the 487 random-pair pre-match patients, all-cause mortality occurred in 38 and 29% of patients, respectively, with and without AF (unadjusted HR 1.44; 95% CI 1.16–1.80; P = 0.001; data not shown). Multivariable-adjusted and propensity-adjusted HR's for AF were, respectively, 1.18 (95% CI 1.01–1.38; P = 0.040) and 1.12 (95% CI 0.94–1.34; P = 0.21).
In matched patients CV mortality occurred in 156 (rate, 1706/10 000 person-years) and 158 (rate, 1645/10 000 person-years) patients with and without AF, respectively (matched HR when AF was compared with no AF 0.97, 95% CI 0.74–1.28; P = 0.83; Table 3). Heart failure mortality occurred in 60 (rate, 656/10 000 person-years) and 50 (rate, 521/10 000 person-years) patients with and without AF, respectively (matched HR when AF was compared with no AF 1.10, 95% CI 0.67–1.82; P = 0.70; Table 3). Pre-match associations of AF with mortality due to cardiovascular causes and HF are displayed in Table 3.
Table 3.
Association of atrial fibrillation with other outcomes in Beta-Blocker Evaluation of Survival Trial
| Rate per 10 000 person-years (events/total follow-up years) |
Absolute rate difference (per 10 000 person-years)a | Matched hazard ratio (95% confidence interval) | P-value | ||
|---|---|---|---|---|---|
| No history of atrial fibrillation | History of atrial fibrillation | ||||
| Before matching | n = 2054 | n = 653 | |||
| Cardiovascular mortality | 1219 (514/4216) | 1762 (216/1226) | +543 | 1.45 (1.24–1.70) | < 0.0001 |
| Heart failure mortality | 403 (170/4216) | 750 (92/1226) | +347 | 1.88 (1.46–2.43) | < 0.0001 |
| All-cause hospitalization | 5020 (1255/2500) | 6493 (448/690) | +1473 | 1.25 (1.13–1.40) | < 0.0001 |
| After matching | n = 487 | n = 487 | |||
| Cardiovascular mortality | 1645 (158/960) | 1706 (156/914) | +61 | 0.97 (0.74–1.28) | 0.83 |
| Heart failure mortality | 521 (50/960) | 656 (60/914) | +136 | 1.10 (0.67–1.82) | 0.70 |
| All-cause hospitalization | 5216 (302/579) | 6271 (328/523) | +1056 | 1.22 (1.00–1.50) | 0.06 |
aAbsolute differences in rates of events per 10 000 person-years of follow-up were calculated by subtracting the event rates in the atrial fibrillation group from the event rates in the no atrial fibrillation group (before values were rounded).
Atrial fibrillation and hospitalization
Overall, 630 (65%) patients were hospitalized including 399 (41%) due to HF. Heart failure hospitalizations occurred in 215 (rate, 3171/10 000 person-years) and 184 (rate 2405/10 000 person-years) patients with and without AF, respectively (matched HR when AF was compared with no AF 1.28, 95% CI 1.00–1.63; P = 0.049; Figure 2B and Table 2). In the absence of hidden bias, a sign-score test for matched data with censoring provides evidence (P = 0.048) that HF patients with a history of AF clearly had more hospitalizations due to HF than those without a history of AF. Findings from our sensitivity analysis indicate that a hidden covariate, which is a near-perfect predictor of HF hospitalization, may potentially explain away the association between AF and HF hospitalization, if that would increase the odds of AF by only 0.205%. The associations of AF with HF hospitalization among various subgroups of patients are displayed in Figure 3. Atrial fibrillation-associated hospitalization due to HF was significant only among patients in the placebo group (HR for AF 1.54, 95% CI 1.17–2.03; P = 0.002) but not among those in the bucindolol group (HR for AF 1.08, 95% CI 0.81–1.43; P = 0.61; P for interaction = 0.09; Figure 3). Association of AF with all-cause hospitalization is displayed in Table 3.
Figure 3.
Association of a history of atrial fibrillation with hospitalization due to heart failure in subgroups of propensity score-matched patients in the BEST (CI, confidence interval; HR, hazard ratio).
Overall 918 (94%) matched patients were receiving digoxin and among these patients, AF was associated with increased HF hospitalization (HR 1.29, 95% CI 1.06–1.58; P = 0.012; data not shown). However, this association varied by the use of bucindolol. Among patient receiving digoxin without bucindolol (n = 451), AF was associated with increased HF hospitalization (HR 1.56, 95% CI 1.17–2.06; P = 0.002). On the other hand, among patients receiving both digoxin and bucindolol (n = 467), AF had no association with HF hospitalization (HR 1.08, 95% CI 0.81–1.45; P = 0.58; P for interaction = 0.09; data not shown).
In the pre-match cohort of 2707 patients, HF hospitalization occurred in 45% (rate, 3209/10 000 person-years of follow-up) and 37% (rate, 2243/10 000 person-years of follow-up) of patients with and without AF, respectively (unadjusted HR for AF 1.38; 95% CI 1.21–1.58; P < 0.0001; Table 2). Multivariable-adjusted and propensity-adjusted HR's were, respectively, 1.26 (95% CI 1.09–1.45; P = 0.002) and 1.22 (95% CI 1.04–1.44; P = 0.016).
All-cause hospitalizations occurred among 328 (rate, 6271/10 000 person-years) and 302 (rate 5216/10 000 person-years) matched patients with and without AF, respectively (matched HR when AF was compared with no AF 1.22, 95% CI 1.00–1.50; P = 0.06; Figure 2B and Table 2). Pre-match associations of AF with all-cause hospitalizations are displayed in Table 3.
Discussion
Significant bivariate associations of AF with increased risk for both all-cause, cardiovascular, and HF mortality and all-cause and HF hospitalizations in patients with advanced chronic systolic HF suggest that AF remains an important prognostic marker in these patients. However, data from propensity-matched patients, who were well balanced in 74 baseline characteristics, demonstrate that AF had no intrinsic association with total or cause-specific mortality, but had an independent association with HF hospitalization. These findings are important as hospitalization due to worsening HF is a major cause of hospitalization for patients with HF, and in the US it is the leading cause for hospitalization for Medicare beneficiaries 65 years and older. Further, the lack of an intrinsic association of AF with mortality may in part explain the lack of superiority for rhythm control strategy relative to rate control in these patients.17
Atrial fibrillation is characterized by irregular cardiac rhythm, which along with an uncontrolled heart rate, may cause significant haemodynamic abnormalities leading to worsening HF and hospitalization. Atrial fibrillation is also associated with structural and functional pathologies such as a dilated left atrium with impaired contraction, impaired left ventricular filling, and activation of vasoconstrictive neurohormones.4,18,19 However, little is known about the individual contribution of an abnormal cardiac rhythm or an uncontrolled heart rate to the haemodynamic instability in HF patients with AF that may lead to HF or all-cause hospitalizations. An irregular cardiac rhythm alone, in the presence of controlled ventricular rate, may cause acute adverse haemodynamic events in AF.20 However, whether an irregular rhythm alone, in the presence of normal heart rate, may also lead to increased HF hospitalization is unknown. Findings from a recent large randomized clinical trial of rhythm vs. rate control for AF in systolic HF suggest that fewer patients in the rate control group required hospitalization, highlighting the importance of a rate control strategy.17
Although uncontrolled heart rate may also lead to adverse haemodynamic consequences, heart rate in matched patients with AF was well controlled and was well balanced. Therefore, the observed association of AF and HF hospitalization cannot be explained by baseline uncontrolled heart rate or baseline differences in heart rate. However, it is plausible that heart rate varied between patients with and without AF during follow-up. Heart failure patients with AF may have an inappropriate tachycardic response to physical activities or other stresses which could lead to acute decompensation with resultant increases in HF hospitalization. This notion is supported by our subgroup analyses that demonstrated that AF-associated increased HF hospitalization was only observed among patients not receiving bucindolol, a beta-blocker (Figure 3). In the BEST, patients receiving bucindolol were less likely to have tachycardia.13 Beta-blockers are currently recommended as the drug of choice for control of ventricular rate in HF patients with AF.21 This is particularly important as beta-blockers, when added to digitalis, may have a synergistic effect in controlling ventricular rate both at rest and during physical activities.22–24 Over 90% of patients in our study were receiving digoxin, and among these patients, AF-associated increase in HF hospitalization was only seen in patients who were not receiving bucindolol. Interestingly, although AF had no overall association with mortality, in the subgroup of patients not receiving bucindolol, AF seemed to be associated with increased mortality, but had no significant association among those receiving bucindolol.
Despite an increased unadjusted bivariate association, findings from our study suggest that AF may not have an intrinsic association with mortality and that the bivariate association was likely due to differences in prognostically important baseline covariates. This is consistent with the findings from a recent large randomized clinical trial of rhythm vs. rate control for AF in systolic HF, where rhythm control did not reduce the primary endpoint of cardiovascular mortality.17 The authors of that study speculated that the failure of the rhythm control strategy may have been due to the fact that AF in HF may not have an intrinsic association with mortality,17 a contention which is supported by findings from our study. It was further speculated that the benefit of rhythm control may have been cancelled by harmful effects of anti-arrhythmic therapies. The proportion of patients receiving anti-arrhythmic drugs was low in our study and was well balanced in our matched cohort.
Previous studies have demonstrated a lack of an independent association between AF and mortality.4,25 However, our study is distinguished from those studies by the use of propensity-score matching in which patients with and without AF were well balanced in 74 important demographic, clinical, subclinical, and biochemical covariates. Further, our subgroup analysis provided insight into the role of beta-blockers in HF patients with AF.
Limitations
There are several limitations in the current study. Despite an excellent post-match balance in 74 baseline covariates, it is possible that there were imbalances in unmeasured covariates. In fact, our sensitivity analysis suggests that our findings may be rather sensitive to an unmeasured confounder. However, sensitivity analysis cannot determine whether an unmeasured covariate exists or not. Furthermore, for an unmeasured covariate to be a confounder, in addition to being associated with the exposure (AF), it must also be a near-perfect predictor of outcomes (HF hospitalization) and not be strongly correlated with any of the 74 measured covariates used in our propensity model. We were able to match 75% of patients with AF and any effect due to loss of participants during matching would be minimal. Further, we were able to reproduce our key findings in the pre-match dataset adjusting for propensity score.
Interestingly, despite same sample size, we observed discordant findings after risk adjustment using a traditional multivariable model and propensity score. Multivariable regression adjustments may not ensure that the distribution of the confounders is balanced between groups, which may lead to extrapolations beyond the data.7 Results of this study based on advanced systolic HF patients may not be generalizable to patients with mild to moderate HF and those with diastolic HF, who constitute half of all HF patients. It is possible that some patients without baseline history of AF may have developed AF during the follow-up period. However, this regression dilution is known to underestimate true associations.26
Conclusions
Atrial fibrillation is a marker of increased mortality and hospitalization in advanced systolic HF and remains a useful tool to identify patients at risk of poor outcomes. Atrial fibrillation seems to have an intrinsic association with hospitalization due to worsening HF, which was worse in patients not receiving bucindolol. Despite the overall lack of an intrinsic association of AF with mortality, it appears to increase mortality in those not receiving bucindolol. These data provide additional arguments that HF patients with AF should be treated with beta-blockers in the absence of an absolute contraindication.
Funding
A.A. is supported by the National Institutes of Health through a grant from the National Heart, Lung, and Blood Institute (R01-HL085561), and a generous gift from Ms Jean B. Morris of Birmingham, Alabama.
Conflict of interest: none declared.
Acknowledgements
‘The Beta-Blocker Evaluation of Survival Trial (BEST) is conducted and supported by the NHLBI in collaboration with the BEST Study Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the BEST or the NHLBI.
References
- 1.Ahmed A, Thornton P, Perry GJ, Allman RM, DeLong JF. Impact of atrial fibrillation on mortality and readmission in older adults hospitalized with heart failure. Eur J Heart Fail. 2004;6:421–426. doi: 10.1016/j.ejheart.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999;84:131R–138R. doi: 10.1016/s0002-9149(99)00713-4. [DOI] [PubMed] [Google Scholar]
- 3.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 4.Crijns HJ, Tjeerdsma G, de Kam PJ, Boomsma F, van Gelder IC, van den Berg MP, van Veldhuisen DJ. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J. 2000;21:1238–1245. doi: 10.1053/euhj.1999.2107. [DOI] [PubMed] [Google Scholar]
- 5.Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991;84:40–48. doi: 10.1161/01.cir.84.1.40. [DOI] [PubMed] [Google Scholar]
- 6.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Fitzmaurice G. Confounding: regression adjustment. Nutrition. 2006;22:581–583. doi: 10.1016/j.nut.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 9.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–1343. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Investigators B. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 15.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. 2nd ed. New York: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 16.SPSS Inc. SPSS for Windows, Rel. 15 [computer program]. Version. Chicago, IL: SPSS Inc.; 2008. [Google Scholar]
- 17.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 18.Van den Berg MP, Tuinenburg AE, Crijns HJ, Van Gelder IC, Gosselink AT, Lie KI. Heart failure and atrial fibrillation: current concepts and controversies. Heart. 1997;77:309–313. doi: 10.1136/hrt.77.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuinenburg AE, Van Veldhuisen DJ, Boomsma F, Van Den Berg MP, De Kam PJ, Crijns HJ. Comparison of plasma neurohormones in congestive heart failure patients with atrial fibrillation versus patients with sinus rhythm. Am J Cardiol. 1998;81:1207–1210. doi: 10.1016/s0002-9149(98)00092-7. [DOI] [PubMed] [Google Scholar]
- 20.Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. doi: 10.1016/s0735-1097(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 21.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda M, Matsuda Y, Yamagishi T, Takahashi T, Haraguchi M, Tada T, Kusukawa R. Effects of digoxin, propranolol, and verapamil on exercise in patients with chronic isolated atrial fibrillation. Cardiovasc Res. 1991;25:453–457. doi: 10.1093/cvr/25.6.453. [DOI] [PubMed] [Google Scholar]
- 23.David D, Segni ED, Klein HO, Kaplinsky E. Inefficacy of digitalis in the control of heart rate in patients with chronic atrial fibrillation: beneficial effect of an added beta adrenergic blocking agent. Am J Cardiol. 1979;44:1378–1382. doi: 10.1016/0002-9149(79)90456-9. [DOI] [PubMed] [Google Scholar]
- 24.Farshi R, Kistner D, Sarma JS, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304–310. doi: 10.1016/s0735-1097(98)00561-0. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol. 1996;28:1458–1463. doi: 10.1016/s0735-1097(96)00358-0. [DOI] [PubMed] [Google Scholar]
- 26.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]