Abstract
Aims
Clinical parameters are weak predictors of outcome in patients with idiopathic dilated cardiomyopathy (IDC). We assessed the prognostic value of cardiac magnetic resonance (CMR) parameters in addition to conventional clinical and electrocardiographic characteristics.
Methods and results
One hundred and forty-one IDC patients were studied. QRS and QTc intervals were measured in 12-lead surface electrocardiogram. Patients were followed for median 1339 days, including 483 patient-years. The primary endpoint—cardiac death or sudden death—occurred in 25 (18%) patients, including 16 patients with cardiac death, 3 patients with sudden cardiac death (SCD), and 6 patients with ICD shock. Late gadolinium enhancement (LGE) was detected in 36 patients (26%). Kaplan–Meier survival analysis displayed QRS >110 ms (P = 0.010), the presence of LGE (P = 0.037), and diabetes mellitus (P < 0.001) as significant parameters for a worse outcome. Multivariable analysis revealed cardiac index (P < 0.001), right ventricular end-diastolic volume index (RVEDVI) (P = 0.006) derived from CMR imaging, the presence of diabetes mellitus (P = 0.006), and QRS >110 ms (P = 0.045) as significant predictors for the primary endpoint.
Conclusion
Cardiac index and RVEDVI derived from CMR imaging in addition to QRS duration >110 ms from conventional surface ECG and diabetes mellitus provide prognostic impact for cardiac death and SCD in patients with IDC.
Keywords: Idiopathic dilated cardiomyopathy, Magnetic resonance imaging, Late gadolinium enhancement, Prognosis
Introduction
The natural history of patients with idiopathic dilated cardiomyopathy (IDC) is variable. Some patients have minimal or no symptoms and the progression of the disease is unclear. On the other hand, symptomatic patients seem to experience progressive deterioration, and 10–50% with heart failure may succumb within 1 year.1 The annual mortality rate for a typical patient with heart failure was estimated to be 10–13%.2 Clinical predictors such as advanced age, protodiastolic gallop, failure of the myopathic ventricle to respond to inotropic stimulation, and ventricular arrhythmias have been identified as risk factors of dying from IDC.3 A prolonged QRS duration and a reduced left ventricular ejection fraction (LVEF) have been associated with a worse prognosis in patients with IDC.4–7
Cardiac magnetic resonance (CMR) imaging is a powerful tool to assess morphology and myocardial function as well as changes in tissue structure. Myocardial damage, viability, and scarring have been frequently studied in patients with coronary artery disease (CAD) post-myocardial infarction using pathological late uptake of extracellular MR contrast media by the ventricular myocardium (late gadolinium enhancement, LGE).8–11 In IDC patients, the evidence of LGE is associated with a worse prognosis compared with those without LGE.12,13 Studies on LGE behaviour in patients with IDC and their prognostic significance when compared with other CMR parameters are scarce.12,13 Furthermore, classical prognostic parameters such as QRS duration,4–7,14 right ventricular (RV) dilation,15–17 or LVEF4,5,14,18,19 have not been taken into account for prognostic impact in combination with LGE in IDC patients. However, the predictive value of any single parameter is not strong enough to assess the clinical course and outcome in an individual patient with reasonable accuracy. Therefore, the aim of this study was to assess the prognostic value of CMR parameters in addition to clinical and electrocardiographic characteristics.
Methods
Patients
One hundred and fifty-three consecutive patients with IDC according to the definition of the Word Health Organization20 were enrolled during a period of 4 years. Diagnosis was established by clinical examination, echocardiography, and normal coronary angiograms. No patient had history of previous coronary intervention or myocardial infarction. A normal coronary angiogram was defined as coronary arteries without any stenoses or occlusions. The border of the coronary arteries had to be without irregularities exceeding a luminal diameter reduction of >30%.
Cardiac magnetic resonance study was performed for evaluation of RV and LV function and LGE. All patients had chronic heart failure of at least 12-month duration and had presented with typical onset and clinical signs of heart failure. Cardiac magnetic resonance studies were performed with the patients being in a haemodynamic stable situation free from catecholamines. None of the patients showed a typical subendocardial or transmural LGE in the supplied territory of a coronary artery as a possible result of myocardial damage by coronary emboli.
Four patients did not undergo CMR imaging study because of claustrophobia resulting in a population of 149 patients. Sixty-three of 149 patients (42%) underwent myocardial biopsy for molecular analysis of virus persistence or molecular markers of chronic inflammation as described elsewhere.21 Eight patients with inflammatory cardiomyopathy due to chronic inflammation in myocardial biopsy (>10 CD2-positive cells/mm2)21–23 were excluded resulting in a study population of 141 patients.
Patients with atrial fibrillation (n = 56) were not excluded in order to enrol a real-world patient population with IDC. The study was approved by the local Ethics Committee and all patients gave their written informed consent. The investigation conforms to the principles outlined in the Declaration of Helsinki.
QRS and QTc interval measurement
At the day of CMR study, a 12-lead ECG was recorded at a paper speed of 50 mm/s on a digital ECG recorder (GE Medical Systems, Information Technologies, Freiburg, Germany). The intervals were automatically analysed (CardioSoft Version 4.2). Heart rate correction was done by the Bazett formula, prolonged QRS was defined as a QRS width of >110 ms, and prolonged QTc as a QTc interval of >440 ms.
Cardiac magnetic resonance protocol and data analysis
Cardiac magnetic resonance imaging was performed on a 1.5 T whole-body scanner (Intera CV, Philips Medical Systems, Best, The Netherlands). To define the position and axis of the left ventricle, three survey scans were performed along right–left, anterior–posterior, and foot–head orientation. Resting LV and RV function was determined with 3D cine imaging applying a multiple breath hold segmented k-space balanced FFE sequence (steady-state free precession) in short- and long-axis views aligned with the true heart axis. Parallel imaging was employed for all scans to minimize acquisition time. Depending on the field of view, in-plane resolution was between 1.5 × 1.8 and 2.3 × 1.8 mm with a slice thickness of 10 mm for the functional scans. The short-axis scans covered the whole LV and RV with 10–14 contiguous slices with a temporal resolution of 34 cardiac phases. Around 10–15 min after infusion of 0.2 mmol/kg body weight gadolinium-diethylenetriaminepentaacetate (Magnevist, Schering, Germany), a late enhancement study using a 3D spoiled turbo Gradient Echo sequence with a selective 180° inversion recovery pre-pulse was acquired in the short axis covering the whole LV (20–22 5 mm slices). Two to three long-axis views with a similar 2D sequence were additionally performed. The pre-pulse delay (range, 200–250 ms) was adjusted individually using a Look–Locker sequence.24 Cardiac magnetic resonance protocols were identical during the whole series of patient investigations.
Left ventricular and RV volumes and functional parameters were analysed off-line on a ViewForum™ Workstation (Philips) using short-axis volumetry. Papillary muscles were assigned to the myocardium. Short- and long-axis images were scrutinized by two observers for the presence of LGE. Late gadolinium enhancement was quantitatively assessed on a ViewForum Workstation.
Follow-up and endpoints
Patients were followed by a clinical visit or telephone call using a questionnaire for evaluation of NYHA functional status, actual medication, new cardiac events, worsening of disease state, and occurrence of pre-specified endpoints. The composite primary endpoint was defined as cardiac death or sudden cardiac death (SCD) from malignant ventricular arrhythmias (ventricular flutter or fibrillation). The secondary endpoint was defined as cardiac death or SCD or rehospitalization for decompensated heart failure.
Statistical analysis
Continuous variables are presented as mean ± 1 SD. Discrete variables are expressed as counts and percentages compared by means of χ2 analysis. Tests were always two-sided. Survival curves were estimated by the Kaplan–Meier method and compared by the log-rank test. Multivariable Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). We constructed two separate multivariable models to critically assess the prognostic value of the parameters, one for the primary endpoint and one for the secondary endpoint. Here, we have applied Cox proportional hazards regression and used a bootstrap approach (n= 1000), over the cases (as in Sauerbrei and Schumacher25), that has a nested selection procedure over all parameter subsets (evaluation of all parameter subsets and Akaike information criterion) to determine the most relevant parameters in each resampling step, i.e. only the most frequent variables (≥70%) from all runs are used in accordance with adjustment of the number of variables to the number of events during follow-up26,27 and collinearity is accounted for. The linearity assumption for continuous variables of the Cox model was assessed by comparing two models for each covariate, one model with the covariate as a linear term and a second model additionally including the covariate as a quadratic term. The linearity assumption could not be verified for age. The proportional hazards assumption was verified for all parameters except NYHA status with the method by Grambsch and Therneau.28 For inclusion into the multivariable model, only parameters were considered that were significant in the univariate analysis. Statistical significance was accepted for P < 0.05. All statistical analyses were conducted with Statistica version 6.1 (Statsoft Inc., Tulsa, OK, USA) and R version 2.81 (www.r-project.org).
Results
One hundred and forty-one consecutive patients with IDC were enrolled and prospectively followed. Patients were included between February 2002 and February 2006. Patient characteristics are summarized in Table 1 and are differentiated into those with and without LGE.
Table 1.
Patient characteristics
| All patients | With LGE | No LGE | |
|---|---|---|---|
| 141 | 36 | 105 | |
| Male, n (%) | 108 (76.6) | 30 (83.3) | 78 (25.7) |
| Female, n (%) | 33 (23.4) | 6 (16.7) | 27 (25.7) |
| Age (years) | 56.1 ± 13.3 | 59.7 ± 11.2 | 54.8 ± 13.8 |
| Cardiovascular risk factors, n (%) | |||
| Diabetes mellitus | 23 (16.3) | 9 (25.0) | 14 (13.3) |
| Arterial hypertension | 55 (39.0) | 13 (36.1) | 42 (40.0) |
| Hypercholesterolemia | 53 (37.6) | 14 (38.9) | 39 (37.1) |
| Smoking | 41 (27.5) | 12 (30) | 29 (26.6) |
| Familial disposition | 21 (14.9) | 8 (22.2) | 13 (12.4) |
| BMI (kg/m2) | 26.7 ± 4.9 | 26.4 ± 4.3 | 26.8 ± 5.1 |
| NYHA class I, n (%) | 17 (12.1) | 5 (13.9) | 12 (11.4) |
| NYHA class II, n (%) | 16 (11.3) | 4 (11.1) | 12 (11.4) |
| NYHA class III, n (%) | 65 (46.1) | 17 (47.2) | 48 (45.7) |
| NYHA class IV, n (%) | 43 (30.5) | 10 (27.8) | 33 (31.4) |
| QRS >110 ms | 94 (66.7) | 29 (80.6) | 65 (61.9) |
| QTc >440 ms | 65 (46.1) | 18 (50.0) | 47 (44.8) |
| ACE-inhibitor, n (%) | 123 (87.2) | 33 (91.7) | 90 (85.7) |
| Beta-blocker, n (%) | 127 (90.1) | 33 (91.7) | 94 (89.5) |
| Spironolactone, n (%) | 105 (74.5) | 28 (77.8) | 77 (73.3) |
| Diuretic, n (%) | 127 (90.1) | 32 (88.9) | 95 (90.5) |
| Phenprocoumone, n (%) | 69 (48.9) | 23 (63.9) | 46 (43.8) |
BMI, body mass index; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme.
Clinical status and risk factors
Sixty-five patients (46%) were in NYHA functional class III and 43 (31%) in class IV. Patients were set on maintenance quadruple drug therapy with beta-blockers, ACE-inhibitors/AT2-receptor blockers, aldosterone antagonists, and diuretics (Table 1). Twenty-one patients received a biventricular pacing device and 34 patients an intracardiac defibrillator (ICD) including 4 with biventricular pacing mode.
Cardiac magnetic resonance parameters
Mean values of heart rate, LV and RV end-diastolic and end-systolic volumes and indices, ejection fraction (EF), and cardiac index (CI) are listed in Table 2. Patients in NYHA class III or IV had a significantly lower LVEF (30 ± 14 vs. 39 ± 12%; P < 0.002) and a significantly lower CI (2.6 ± 0.8 vs. 3.1 ± 0.6 L/min/m2; P < 0.014) when compared with those in NYHA class I or II. Late gadolinium enhancement was present in 36 patients (26%). Distribution pattern of LGE was mid-wall, subepicardial, or diffuse. Patients with LGE showed subepicardial pattern in 14 of 36 (38.9%). Late gadolinium enhancement in relation to LV muscle mass was 5.6 ± 4.7% (range, 1.2–25.6%).
Table 2.
Cardiac magnetic resonance imaging characteristics
| All patients | With LGE | No LGE | |
|---|---|---|---|
| 141 | 36 | 105 | |
| LVEDV (mL) | 271 ± 104 | 282 ± 88 | 268 ± 108 |
| LVESV (mL) | 194 ± 103 | 209 ± 89 | 189 ± 108 |
| LVEF (%) | 32 ± 14 | 28 ± 12 | 34 ± 15 |
| CI (L/min/m2) | 2.76 ± 0.79 | 2.53 ± 0.71 | 2.84 ± 0.80 |
| LVEDVI (mL/m2) | 141 ± 56 | 150 ± 49 | 139 ± 58 |
| LVESVI (mL/m2) | 101 ± 56 | 111 ± 48 | 98 ± 59 |
| RVEDV (mL) | 166 ± 58 | 182 ± 65 | 161 ± 55 |
| RVESV (mL) | 90 ± 55 | 108 ± 62 | 84 ± 51 |
| RVEF (%) | 50 ± 16 | 43 ± 16 | 52 ± 15 |
| RVEDVI (mL/m2) | 87 ± 33 | 94 ± 32 | 85 ± 33 |
| RVESVI (mL/m2) | 47 ± 29 | 58 ± 33 | 43 ± 27 |
| Heart rate (b.p.m.) | 71 ± 14 | 69 ± 15 | 72 ± 14 |
EDV, end-diastolic volume; ESV, end-systolic volume; EDVI, end-diastolic volume index; ESVI, end-systolic volume index.
Follow-up
Follow-up data were obtained in all 141 patients. Patients were followed for a median of 1339 days (inter-quartile range, 822–1676 days). Data were collected for a total of 483 patient-years of follow-up. During follow-up, 4 patients died from non-cardiac death due to malignant tumours, 16 from cardiac death, and 3 from SCD. Additional six surviving patients with an ICD experienced shocks for ventricular flutter/fibrillation and were included in the endpoints as a surrogate parameter of SCD. Thus, the total number of cardiac deaths and SCD was 25 in 141 patients (18%). The secondary endpoint occurred in 53 patients (37.6%). Events occurred more often in patients with LGE when compared with those without LGE. Hazard ratio was 2.26 with a 95% CI of 1.03–4.99 (P = 0.043, Table 3 and Figure 1).
Table 3.
Univariate and multivariable analyses for predictors of primary endpoint
| Univariate analysis |
Multivariable analysis |
Resampling procedure | |||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | Frequency | |
| Diabetes | 4.38 (1.98–9.66) | <0.001 | 3.19 (1.40–7.29) | 0.006 | 0.90 |
| QRS >110 ms | 5.43 (1.28–23.1) | 0.022 | 4.64 (1.04–20.78) | 0.045 | 0.71 |
| QTc >440 ms | 1.57 (0.71–3.5) | 0.261 | |||
| LVEDVI | 1.00 (1.00–1.01) | 0.238 | |||
| RVEDVI | 1.01 (1.00–1.02) | 0.003 | 1.01 (1.00–1.02) | 0.006 | 0.76 |
| CI | 0.45 (0.25–0.83) | 0.010 | 0.35 (0.19–0.65) | <0.001 | 0.95 |
| LVEF | 0.95 (0.92–0.99) | 0.013 | 0.45 | ||
| RVEF | 0.97 (0.95–0.99) | 0.022 | 0.38 | ||
| LGE | 2.26 (1.03–4.99) | 0.043 | 0.54 | ||
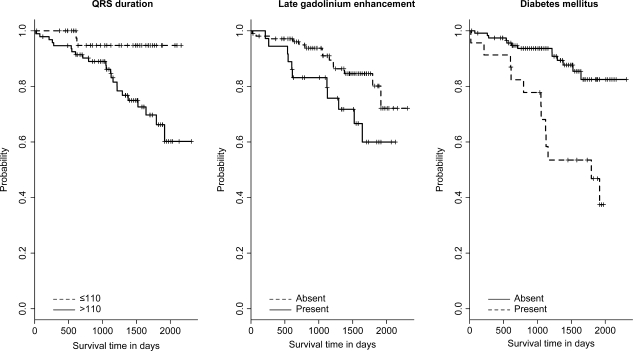
Figure 1.
Kaplan–Meier curves for the primary endpoint according to QRS duration, late gadolinium enhancement, and diabetes mellitus (panels from left to right). Survival curves were significantly different for QRS duration ≤110 vs. >110 ms (P = 0.010), the presence or absence of late gadolinium enhancement (P = 0.037), and the presence or absence of diabetes mellitus (P < 0.001).
Univariate and multivariable analyses and Kaplan–Meier curves
Among the clinical, electrocardiographic, haemodynamic, and CMR data, univariate analyses revealed diabetes mellitus, QRS duration >110 ms, right ventricular end-diastolic volume index (RVEDVI), CI, LVEF, RVEF, and the presence of LGE as significant predictors for the primary endpoint. Significant predictors for occurrence of the primary endpoint in multivariable analysis were diabetes mellitus (P = 0.006), QRS >110 ms (P = 0.045), RVEDVI (P = 0.006), and CI (P < 0.001, Table 3).
Significant predictors for the secondary endpoint from univariate analysis were QRS >110 ms, QTc >440 ms, left ventricular end-diastolic volume index (LVEDVI), RVEDVI, LVEF, and RVEF. In multivariable analysis, QRS >110 ms (P = 0.026), QTc >440 ms (P = 0.047), and RVEDVI (P = 0.010) were significant predictors for the secondary endpoint (Table 4).
Table 4.
Univariate and multivariable analyses for predictors of secondary endpoint
| Univariate analysis |
Multivariable analysis |
Resampling procedure | |||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | Frequency | |
| Diabetes | 1.50 (0.79–2.85) | 0.211 | |||
| QRS >110 ms | 2.32 (1.13–4.77) | 0.022 | 2.39 (1.11–5.12) | 0.026 | 0.86 |
| QTc >440 ms | 1.74 (1.01–2.99) | 0.046 | 1.76 (1.01–3.07) | 0.047 | 0.70 |
| LVEDVI | 1.00 (1.00–1.01) | 0.009 | 0.28 | ||
| RVEDVI | 1.01 (1.00–1.02) | 0.008 | 1.01 (1.0–1.02) | 0.010 | 0.76 |
| CI | 0.72 (0.48–1.06) | 0.096 | |||
| LVEF | 0.97 (0.94–0.99) | 0.005 | 0.61 | ||
| RVEF | 0.98 (0.96–1.00) | 0.040 | 0.32 | ||
| LGE | 1.45 (0.82–2.58) | 0.202 | |||
Kaplan–Meier analysis for the primary endpoint (Figure 1) showed that QRS >110 ms, the presence of LGE, and the presence of diabetes mellitus were associated with a significant worse outcome in IDC patients when compared with those without the corresponding criteria. Interestingly, patients with LGE in CMR imaging had significantly more often a QRS interval >110 ms when compared with patients without LGE (80.6 vs. 61.9%, P = 0.034).
Discussion
In a large cohort of 141 patients with IDC, we were able to demonstrate that in addition to CI and RVEDVI derived from CMR imaging, QRS duration >110 ms and diabetes mellitus had independent prognostic impact on the composite endpoint SCD and cardiac death.
Relevance of diagnostic workup of idiopathic dilated cardiomyopathy
With regard to prognostic aspects, the confirmation of IDC diagnosis is highly relevant, because mechanisms and incidence of cardiac death and SCD may be different in chronic inflammatory compared with non-inflammatory cardiomyopathy. Diagnosis of IDC was established by clinical examination, echocardiography, and evidence of normal coronary angiograms. In addition, with myocardial biopsy in a large number of our patients, we were able to identify individuals with inflammatory cardiomyopathy. Those patients were excluded in order to study a homogenous population with IDC.
Risk predictors
In univariate and multivariable analyses, a QRS interval of >110 ms was predictive for the primary as well as the secondary endpoint. A QTc interval of >440 ms had also prognostic impact for the secondary endpoint. A prolonged QRS complex of >110–120 ms has been shown to be predictive for a worse outcome in patients with congestive heart failure.4–7 These cohorts included patients with mixed aetiologies of cardiomyopathy with ischaemic, non-ischaemic, or valvular genesis, whereas we focused on patients with IDC. Prolonged QTc interval (>440 ms) was a strong, independent predictor for mortality in patients with heart failure and elevated B-type natriuretic peptide.29 Few reports exist on pure IDC patients, such as the study of Morgera et al.30 on 78 consecutive IDC patients using signal-averaged ECG, 24 h ECG monitoring, and electrophysiological study to assess prognostic parameters. They found an HV interval of >55 ms, a combination of frequent and repetitive ventricular ectopic beats, and poor LV function as independent predictors of death or cardiac transplantation. The association of a prolonged HV interval with a wide QRS complex (>110 ms) yielded a strong risk for future arrhythmic events. Amiya et al.14 were able to show that a QRS duration of >120 ms was a significant predictor for cardiac death or hospitalization in 78 patients with non-ischaemic dilated cardiomyopathy within a follow-up period of 35.6 ± 27.8 months. Of note, non-ischaemic dilated cardiomyopathy was carefully diagnosed by echocardiography, coronary angiography, and myocardial biopsy. Furthermore, CI was measured with Swan–Ganz thermodilution at rest. Mean EF was 26.3% by echocardiography. Cardiac index was still normal with 2.66 ± 0.84 L/min/m2. Comparable results have been obtained with another measurement technique using inert gas rebreathing.31 We derived both parameters (LVEF and CI) from CMR imaging and revealed similar results. The mean LVEF was 32% and CI was 2.76 ± 0.79 L/min/m2, both measured at rest.
Left ventricular ejection fraction has been demonstrated to be a strong predictor for cardiac mortality in patients with heart failure.4,5,14,18,19 Amiya et al.14 also analysed CI and LVEF for prognostic impact of cardiac death or hospitalization because of deterioration of heart failure, comparable to our secondary endpoint. In our population, LVEF was a univariate predictor for the primary as well as the secondary endpoint, but failed to be predictive in multivariable analysis. Other trials did not include LVEF, CI, and RV parameters for analysis of prognostic impact. With CMR imaging, those parameters can be easily and accurately measured non-invasively.32 Cardiac index was a relevant prognostic parameter for cardiac death and SCD (HR, 0.35). In our population, the haemodynamic parameter CI outperformed LVEF in determining risk for cardiac death. Very recently, two CMR studies12,13 reporting a prognostic impact of LGE in patients with non-ischaemic cardiomyopathy did not demonstrate any prognostic relevance of LVEF, whereas CI and electrocardiographic parameters were not analysed.
Interestingly, we were able to demonstrate that diabetes mellitus by univariate and multivariable analyses was a significant predictor for the primary endpoint. Our results are in line with previous reports,33,34 showing in patients with non-ischaemic cardiomyopathy a three to seven times higher risk for mortality in patients with diabetes mellitus compared with those without. Furthermore, insulin resistance has been recently discussed as a primary aetiological factor in the development of non-ischaemic heart failure,35,36 which clinically often manifests as type II diabetes. The prognostic impact of insulin resistance has been shown to be independent of LVEF.35
Furthermore, we measured RV parameters which have rarely been analysed for their prognostic impact in IDC patients so far. A preserved RVEF assessed by first-pass radionuclide ventriculography in patients with advanced heart failure referred for evaluation for cardiac transplantation was a powerful predictor for survival.15 With echocardiography, RV systolic and diastolic dysfunction was associated with a poor prognosis in 177 patients with ischaemic or IDC.16 In our IDC population, RVEDVI was a predictor of both, the primary and secondary endpoints in univariate as well as in multivariable analysis. Right ventricular ejection fraction was associated with an increased risk for both endpoints in univariate analysis. Due to ventricular interaction, an increase in RV diastolic volume and a decline in RV systolic function have been shown to impair LV systolic and diastolic function.37–39 In an IDC population including 100 patients, RV dilation determined by echocardiography was analysed for prognostic impact.17 Patients with RV dilation had a three-fold higher mortality over 4 years and more rapidly deterioration of LV function compared with patients with less initial RV dilation.
Late gadolinium enhancement was univariately associated with a significant worse outcome as recently reported.12,13 Kaplan–Meier curves significantly differed for patients with LGE when compared with those without. In multivariable analysis, LGE was not associated with an independent prognostic impact. Assomull et al.12 were able to show that mid-wall fibrosis in patients with dilated cardiomyopathy was predictive for all-cause mortality and cardiovascular hospitalization. Late gadolinium enhancement also remained relevant with inclusion of RVEF, LGE, LVEF, LVEDV, and LVESV in the multivariable model. However, RV volumes, CI, and classical electrocardiographic parameters were not integrated.
There are several important differences between our population and the group studied by Wu et al.13 The patient cohort in the study of Wu et al.13 consisted exclusively of IDC patients with an LVEF <35% selected for ICD implantation. This very homogenous but small and selected group of the sickest IDC patients does not represent the total spectrum of IDC patients, as we have studied. With myocardial biopsy in 42% of the patients, we were able to exclude patients with chronic inflammation. Several characteristics and results are consistent between both studies: CAD was excluded by angiography. In the Wu study,13 42% (n = 27/65) patients showed LGE comparable to 34% (n = 36/105) in our population. Patients with and without LGE had similar baseline characteristics. Kaplan–Meier curves showed a significant difference between patients with LGE and those without. At this point, we like to stress that in our experience and other studies,12,13 the intramural to subepicardial pattern and potentially diffuse distribution of LGE in patients with IDC is quite different from the preferred subepicardial distribution of LGE in the inferolateral wall in patients with acute myocarditis.40
In multivariable analysis, LGE remained not significant in our trial. With the intention to identify IDC patients at risk, we did not restrict our analysis to CMR parameters alone. We also integrated well-known predictors for cardiac events from 12-lead surface electrocardiography.4–7 Interestingly, the presence of LGE was associated with a significant higher frequency of a prolonged QRS interval. The QRS width in ECG is a summary of various factors. Among others, this interval is influenced by LVEF, LVEDVI, and LGE and has a strong prognostic impact in patients with IDC even in combination with CMR parameters.
No previous study combined classical electrocardiographic parameters with modern CMR data to assess prognosis in IDC patients. In our IDC population, a prolonged QRS interval outperformed LGE seen in CMR. Furthermore, we also included RV parameters into the analysis, since a depressed RV function had been previously associated with a higher event rate. In contrast, Wu et al.13 did not analyse RV parameters. Finally, we did not focus on LGE alone, since patients without LGE are not free from malignant ventricular arrhythmias. The combination of electrocardiographic and CMR parameters allows a risk stratification for patients with as well as without LGE.
Study limitations
No exercise test during CMR imaging was performed to measure the reduced increase in CI in IDC patients with reduced LVEF. Another CMR study was not performed during follow-up, because we were primarily interested in the prognostic significance of CMR parameters assessed at entry of the patients into the study. We performed MRI scans with particular attention to image quality to minimize influences of hampered image quality and artefacts in the detection of even small amounts of LGE. However, the extent of LGE in patients with IDC is typically small and quantitative assessment of diffuse LGE pattern is quite challenging limiting the accuracy of the quantitative assessment. Although myocarditis was not suspected from the clinical profile, myocardial biopsy showed the presence of virus persistence in six patients. We did not exclude these six patients from analysis since biopsy is usually not performed in those patients and the clinical course was not different with one primary and two secondary events. On the other hand, myocardial biopsy was not performed in all patients and biopsy is also limited by the possibility of false-negative results. Although we defined inflammatory cardiomyopathy as the presence of >10 infiltrating lymphocytes/mm2, other groups are using a cut-off of >741 or >14 cells/mm2.42 Another possible limitation of this study may be the number of events per variable in the initial multivariate model.
Conclusions
We were able to demonstrate that in addition to CI and RVEDVI derived from CMR imaging, a QRS duration of >110 ms and the presence of diabetes mellitus provide prognostic impact in patients with IDC. The practical advantage is that the electrocardiographic and CMR features can be derived from two non-invasive techniques with highest precision and without complicated analysis techniques.
Funding
Supported in part by an unrestricted grant of Philips Medical Systems, Best, The Netherlands, and Hamburg, Germany. Funding to pay the Open Access publication charges for this article was provided by the Department of Internal Medicine II-Research budget.
Conflict of interest: none declared.
References
- 1.Deedwania PC. The key to unravelling the mystery of mortality in heart failure: an integrated approach. Circulation. 2003;107:1719–1721. doi: 10.1161/01.CIR.0000014688.12415.C0. [DOI] [PubMed] [Google Scholar]
- 2.Konstam MA. Progress in heart failure management? Lessons from the real world. Circulation. 2000;102:1076–1078. doi: 10.1161/01.cir.102.10.1076. [DOI] [PubMed] [Google Scholar]
- 3.Drozdz J, Krzeminska-Pakula M, Plewka M, Ciesielczyk M, Kasprzak D. Prognostic value of low-dose dobutamine echocardiography in patients with idiopathic dilated cardiomyopathy. Chest. 2002;121:1216–1222. doi: 10.1378/chest.121.4.1216. [DOI] [PubMed] [Google Scholar]
- 4.Silvet H, Amin J, Padmanaban S, Pai RG. Prognostic implications of increased QRS duration in patients with moderate and severe left ventricular systolic dysfunction. Am J Cardiol. 2001;88:182–185. doi: 10.1016/s0002-9149(01)01619-8. [DOI] [PubMed] [Google Scholar]
- 5.Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. QRS duration and mortality in patients with heart failure. Am Heart J. 2002;143:1085–1091. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann M, Bauer R, Handrock R, Weidinger G, Goedel-Meinen L. Prognostic value of QRS duration in patients with heart failure: a subgroup analysis from 24 centers of ValHeFT. J Card Fail. 2005;11:523–528. doi: 10.1016/j.cardfail.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Shenkman HJ, Pampati V, Khandelwal AK, McKinnon J, Nori D, Kaatz S, Sandberg KR, McCullough PA. Congestive heart failure and QRS duration: establishing prognosis study. Chest. 2002;122:528–534. doi: 10.1378/chest.122.2.528. [DOI] [PubMed] [Google Scholar]
- 8.Kramer CM, Lima JA, Reichek N, Ferrari VA, Llaneras MR, Palmon LC, Yeh IT, Tallant B, Axel L. Regional function within noninfarcted myocardium during left ventricular remodeling. Circulation. 1993;93:1279–1288. doi: 10.1161/01.cir.88.3.1279. [DOI] [PubMed] [Google Scholar]
- 9.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–722. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 10.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 11.Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 12.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E, Tomaselli GF, Lima JAC. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiya E, Tanabe K, Ikari Y, Nakajima Y, Hara K. Prolonged QRS duration and severity of mitral regurgitation are unfavorable prognostic markers of heart failure in patients with nonischemic dilated cardiomyopathy. Circ J. 2006;70:57–62. doi: 10.1253/circj.70.57. [DOI] [PubMed] [Google Scholar]
- 15.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 16.Meluzin J, Spinarová L, Hude P, Krejcí J, Dusek L, Vítovec J, Panovsky R. Combined right ventricular systolic and diastolic dysfunction represents a strong determinant of poor prognosis in patients with symptomatic heart failure. Int J Cardiol. 2005;105:164–173. doi: 10.1016/j.ijcard.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Sun JP, James KB, Yang XS, Solankhi N, Shah MS, Arheart KL, Thomas JD, Stewart WJ. Comparison of mortality rates and progression of left ventricular dysfunction in patients with idiopathic dilated cardiomyopathy and dilated versus nondilated right ventricular cavities. Am J Cardiol. 1997;80:1583–1587. doi: 10.1016/s0002-9149(97)00780-7. [DOI] [PubMed] [Google Scholar]
- 18.Szabo BM, van Veldhuisen DJ, van der Veer N, Brouwer J, De Graeff PA, Crijns HJGM. Prognostic value of HRV in chronic congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 1997;79:978–980. doi: 10.1016/s0002-9149(97)00026-x. [DOI] [PubMed] [Google Scholar]
- 19.Zecchin M, Lenarda AD, Bonin M, Mazzone C, Zanchi C, Di Chiara C, Davanzo M, Scherl G, Sabbadini G, Sinagra G. Incidence and predictors of sudden cardiac death during long-term follow-up in patients with dilated cardiomyopathy on optimal medical therapy. Ital Heart J. 2001;2:213–221. [PubMed] [Google Scholar]
- 20.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann O, Grebe O, Merkle N, Nusser T, Kochs M, Bienek-Ziolkowski M, Hombach V, Torzewski J. Myocardial biopsy findings and gadolinium enhanced cardiovascular magnetic resonance in dilated cardiomyopathy. Eur J Heart Fail. 2006;8:162–166. doi: 10.1016/j.ejheart.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Kühl U, Seeberg B, Schultheiss HP, Strauer BE. Immunohistological characterization of infiltrating lymphocytes in biopsies of patients with clinically suspected dilated cardiomyopathy. Eur Heart J. 1994;15:62–67. doi: 10.1093/eurheartj/15.suppl_c.62. [DOI] [PubMed] [Google Scholar]
- 23.Pauschinger M, Kühl U, Dörner A, Schieferecke K, Petschauer S, Rauch U, Schwimmbeck PL, Kandolf R, Schultheiss HP. Detection of enteroviral RNA in endomyocardial biopsies in inflammatory cardiomyopathy and idiopathic dilated cardiomyopathy. Z Kardiol. 1998;87:443–452. doi: 10.1007/s003920050199. [DOI] [PubMed] [Google Scholar]
- 24.Look D, Locker D. Time saving measurement of NMR and EPR relaxation times. Rev Sci Instrum. 1970;41:250–251. [Google Scholar]
- 25.Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med. 1992;11:2093–2109. doi: 10.1002/sim.4780111607. [DOI] [PubMed] [Google Scholar]
- 26.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 27.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 28.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 29.Vrtovec B, Delgado R, Zewail A, Thomas CD, Richartz BM, Radovancevic B. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation. 2003;107:1764–1769. doi: 10.1161/01.CIR.0000057980.84624.95. [DOI] [PubMed] [Google Scholar]
- 30.Morgera T, Di Lenarda A, Sabbadini G, Rakar G, Carniel E, Driussi M, Sinagra G. Idiopathic dilated cardiomyopathy: prognostic significance of electrocardiographic and electrophysiologic findings in the nineties. Ital Heart J. 2004;5:593–603. [PubMed] [Google Scholar]
- 31.Lang CC, Karlin P, Haythe J, Tsao L, Mancini DM. Ease of noninvasive measurement of cardiac output coupled with peak VO2 determination at rest and during exercise in patients with heart failure. Am J Cardiol. 2007;99:404–405. doi: 10.1016/j.amjcard.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin SS, Neaton JD, Sengupta A, Kuller LH. Predictors of mortality from idiopathic dilated cardiomyopathy in 356,222 men screened for the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1994;139:166–172. doi: 10.1093/oxfordjournals.aje.a116978. [DOI] [PubMed] [Google Scholar]
- 34.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Witteles RM, Tang WH, Jamali AH, Chu JW, Reaven GM, Fowler MB. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol. 2004;44:78–81. doi: 10.1016/j.jacc.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 37.Feneley MP, Gavaghan TP, Baron DW, Branson JA, Roy PR, Morgan JJ. Contribution of left ventricular contraction to the generation of right ventricular systolic pressure in the human heart. Circulation. 1985;71:473–480. doi: 10.1161/01.cir.71.3.473. [DOI] [PubMed] [Google Scholar]
- 38.Kelly DT, Spotnitz HM, Beiser GD, Pierce JE, Epstein SE. Effects of chronic right ventricular volume and pressure loading on left ventricular performance. Circulation. 1971;44:403–412. doi: 10.1161/01.cir.44.3.403. [DOI] [PubMed] [Google Scholar]
- 39.Konstam MA, Cohen SR, Salem DN, Conlon TP, Isner JM, Das D, Zile MR, Levine HJ, Kahn PC. Comparison of left and right ventricular end-systolic pressure-volume relations in congestive heart failure. J Am Coll Cardiol. 1985;5:1326–1334. doi: 10.1016/s0735-1097(85)80344-2. [DOI] [PubMed] [Google Scholar]
- 40.Marholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 41.Kühl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss HP. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 42.Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]